1. Introduction
COVID-19 pandemic has significantly impacted the healthcare systems across the world in the past few years. The COVID-19 pandemic has infected more than 760 million and led to more than 6.7 million deaths worldwide.[
1]
In multicenter retrospective study involving 3000 patients with confirmed SARS-CoV-2 infection, prevalence of heart failure was 10.1% [
2]. Multicenter study in Italy has reported HF as an independent predictor of mortality and risk factor for in-hospital complications including acute renal failure (28.1% vs. 12.9%,
P < 0.001), multi-organ failure(15.9% vs. 5.8%,
P = 0.004) and sepsis (18.4% vs. 8.9%,
P = 0.006) Acute heart failure has been reported in 9.1% of patients with almost 50% of them having newly diagnosed heart failure in this study[
3].
Heart failure has been reported to be one of the strongest predictor for in-hospital admission [odds ratio(OR), 4.43; 95% confidence interval(CI), 2.59-8.04;p<0.001] and critical illness(OR, 1.9;95% CI, 1.4-2.5;p<0.001) in a prospective cohort study with confirmed SARS-CoV-2 infected patients[
4].In a study published in JACC, including 442 patients with history of heart failure with spectrum ranging from HFr EF, HFm EF to HFpEF has shown patients with history of HF experienced increased risk of mechanical ventilation( 22.8 % vs 11.9%), longer length of stay( 8 days vs 6 days; p<0.001) and mortality of (40.o% vs 24.9%) compared to the patients without history of heart failure.[
5].
1.1. COVID 19 & Cardiac Arrest
Critically ill patients with COVID-19 have severe hypoxia and are at risk of cardiac arrest. In a multicenter study from the United States with more than 5000 critically ill patients with COVID-19, the Incidence of cardiac arrest was reported to be around 14%. Pulseless electrical activity(49.8%) and asystole(23.8%) were reported to be the most common rhythms among cardiac arrest patients in this analysis.[
6] Incidence of in-hospital cardiac arrest varies from 3% to 7% among patients admitted to hospital with COVID-19 [
7,
8].In another study from Germany, the Incidence of IHCA increased from 4.6% to 6.6% compared to pre-pandemic time.[
9] Overall, the Incidence of in-hospital cardiac arrest in the U.S.A., based on the Get with the Guidelines Registry(GWTG-R), is 9.7 per 1000 hospital admissions. In a registry data-based analysis of COVID-19 patients, 32% have a-systole, 55% have P.E.A. cardiac arrest, and 9% have ventricular tachycardia/ ventricular fibrillation.[
10] In another study published in JAMA Cardiology, among 52 patients with cardiac arrest, 81.5% have pulse-less electrical activity & 14.8% have a-systole, and 3.7% developed pulse-less ventricular tachycardia.[
11]
1.2. Pathophysiology of Cardiac Arrest
Sudden cardiac arrest (S.C.A.) and sudden cardiac death (S.C.D.) refer to the sudden cessation of organized cardiac electrical activity with hemodynamic collapse. The major cause of S.C.D. is ventricular tachycardia (V.T.), ventricular fibrillation (V.F.), P.E.A., and Asystole.
Ventricular tachycardia can be driven by acute precipitating events, including myocardial infarction, catecholamine surge, imbalance of electrolytes, and sometimes without precipitating events. This may manifest in the form of electrical or mechanical failure. Polymorphic VT is most often the result of underlying ischemia, short Q.T. syndrome, acquired or congenital Q.T. prolongation, or congenital short Q.T. interval and is rarely linked with a genetic abnormality associated with catecholaminergic polymorphic V.T.[
12]In approximately one-third of cases, the tachyarrhythmia is initiated by an early R on T premature ventricular complex/contraction; the rest are initiated by late coupled P.V.C. Mono-morphic and polymorphic Ventricular tachycardia can degenerate into Ventricular fibrillation. Ventricular fibrillation results from multiple localized areas of micro reentry without any organized electrical activity. The most likely mechanism is rotating spiral waves.[
13]
P.E.A. is defined as any one of a heterogeneous group of organized E.C.G. rhythms without sufficient mechanical contraction of the heart to produce a palpable pulse or measurable B.P. in the absence of ventricular arrhythmia. This form of cardiac arrest does not respond to defibrillation.[
14]β-agonists are the mainstay of treatment for P.E.A. cardiac arrest and help in improving the myocardial contraction by phosphorylating L-type Ca channels, sarcoplasmic reticulum CA-ATPase regulator increases the calcium entry into the cell and synchronizing the calcium release from the sarcoplasmic reticulum and improving myofilament Ca-responsiveness. [
15].Common causes of P.E.A. are Pericardial tamponade, tension pneumothorax, Aortic dissection or in the setting of hypoxia, acidosis, and increased vagal tone. [
16].
In a prospective observational study on more than 50,000 hospital cardiac arrests between 1999 and 2005, P.E.A. was reported in 37%, asystole in 39%, V.F. in 17%, and VT in 7% of the patients. Survival to hospital discharge was similar for VF/VT(37%) and much lower in P.E.A. and systole (12% and 11%).[
16]
1.3. COVID-19 & Myocardial Dysfunction
COVID-19 can be associated with endothelial activation, dysfunction and pro-thrombotic states resulting in macro and micro-vascular coronary thrombosis with subsequent cardiac dysfunction.(17,18) Fever and sympathetic surge can result in the higher metabolic demands with increased myocardial oxygen consumption resulting in higher oxidative stress. Higher oxidative stress with reactive oxygen species production can lead to intracellular acidosis, mitochondrial damage and cellular death.(19,20)
Dys regulated immuno-inflammatory response to COVID-19 and cytokine storm has been linked with cardiac dysfunction. The systemic inflammatory response has an end-organ impact, including myocardial complications.[
21] Cytokine storm is distinguished by a significant production of pro-inflammatory cytokines like TNF-Alpha, Interferon-gamma, IL-1, and IL-6 as dysregulated host immune response to the SARS-Cov 2 virus.[
22,
23] Cytokines can depress the myocardial function by multiple pathways, including nitric oxide-mediated blunting of beta-adrenergic signaling.[
24] Cytokine storm may result in atrial fibrillation, the development of conduction abnormalities, and cardiac injury reflected by elevated BNP and cardiac markers in plasma.[
25]
There is a controversy around the incidence of myocarditis among COVID-19 patients. In a series published on 104 Electron microscopy biopsies among COVID-19 patients with suspected myocarditis or unexplained HF, only 5 cases were reported to have SARS-CoV 2 virus in myocardium assessed directly by RT-PCR[
26].Prevalence of myocarditis was reported to be around 1.4% from 277 cardiac autopsies from 22 studies. Myocardial fibrosis (80-100%) of cases is the most common histo-pathological finding. A specific interstitial macrophage infiltration has been reported in 86% of cases and 14% of patients have multifocal lymphocytic myocarditis.[
27] These studies are suggestive of fact that direct invasion by virus is not that frequent compared to dys-regulated inflammatory response resulting in myocardial inflammation and myocarditis.
Cardiac arrest among COVID-19 patients with heart failure can be multi-factorial including QTc interval prolongation resulting in Torsades de pointes, result of multi-organ dysfunction, hypoxia resulting in P.E.A, Hyper-coagulable status resulting in macrovascular thrombosis resulting in ACS and V fib arrest. QT interval prolongation among COVID patients depends on various factors including the use of QT prolonging medications like Hydroxy-choloroquine, Azithromycin, electrolyte disturbances, older age, risk of cardiovascular disease and bradycardia.[
28]
There is no direct study available on literature review evaluating the patho-physiology of Cardiac arrest among COVID 19 patients with underlying heart failure. Various studies have shown the impact of heart failure on COVID 19 mortality, length of mechanical ventilator stay and influence of multi organ failure, renal dysfunction and sepsis on the mortality among COVID 19 patients.
We have designed our study to evaluate these clinical predictors along with few other chronic co morbidities as risk factors for cardiac arrest among patients admitted to the hospital with COVID 19 and heart failure.
2. Methods
The study does not require I.R.B. approval, and informed consent was waived due to the de-identified nature of this administrative dataset. International Classification of Diseases, 10th Revision, Clinical Modification(ICD-10 CM) codes( provided in supplementary appendix) were used to identify all patients over the age of 18 years with a discharge diagnosis of heart failure, COVID-19, cardiac arrest and other co morbidities.
2.1. Main Data Source
We have used the National Readmission Data from 2020 for the analysis of data. The database contains discharge-level data without any patient identifier and has around 100 clinical and non-clinical variables for each hospital stay. Major limitation of the study is dependency on ICD 10 codes for the diagnoses and inability to verify the severity of disease by reviewing the labs, charts and may not have complete list of all the co-morbidities which can influence the outcome.
2.2. Study Population
This observational study was conducted by extracting the data for the year 2020. We have used the following ICD 10 codes for the heart failure:I50.20,I50.21,I50.22,I50.23 & I50.30,I50.31,I50.32,I50.33 and got 2882435 discharge data after applying these codes.
After getting this data of 2882435 discharges, we have used U07.1 & U07.3 for inpatient COVID 19 admissions and got 148916 admissions for our analysis. There was uncertainty regarding accurately diagnosing the inpatient COVID 19 admissions during the early pandemic. We have reviewed the literature and came across a Canadian study reviewing the admissions between March 1, 2020 to February 28, 2021 and has shown that U07.1 & U07.3 has PPV of 92.9% in accurately identifying the inpatient and ER admissions with COVID 19. [
29]
There were 148916 patient admissions with COVID-19 using above mentioned ICD codes out of which 17 patients had age 18 or less than 18, and we used 148899 patient admissions for analysis. We have used I46.2, I46.8, I46.9 codes for identifying the cardiac arrest. Out of 148899, 6703 patients were found to have cardiac arrest, and 5783 died.
Study outcome: The primary objective is to find a correlation between end-organ dysfunction and co-morbidities with cardiac arrest from COVID-19 in heart failure patients. The secondary objectives are to evaluate the cardiac arrest and mortality percentage with respect to age groups, time on mechanical ventilator, and total length of stay among various groups.
2.3. Statistical Analysis
Categorical data are represented by numbers and percentages. Continuous variables are expressed as means with S.D.s or medians and inter-quartile ranges. For each analysis, the null hypothesis was evaluated at a 2-sided significance level of 0.05 and calculated 95% C.I.s using standard errors. The statistical significance of the model was determined by Chi-Square and p < 0.05, suggesting that it could distinguish between patients with cardiac arrest and non cardiac arrest patients. Binary Logistic Regression was used to examine whether predictors were associated with the likelihood of having cardiac arrest among COVID 19 patients. We have used SAS 9.4 and SPSS statistics-28 for the analysis.
3. Results
Total 148899 patients were found to be admitted to hospitals with COVID-19. 6703 patients had cardiac arrest and 5783 died during the hospitalization. Incidence of Cardiac arrest was 4.5% consistent with previous studies reporting the incidence of cardiac arrest ranging between of 3% to 7% as discussed above.[
7,
8].
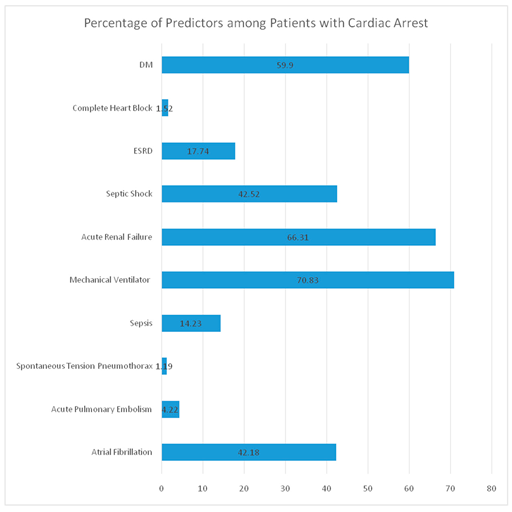
Mechanical Ventilator use was found to be the most significant determinant for cardiac arrest with an odd ratio of 14.443(Confidence Interval 13.67-15.26) & P value( <.001), followed by septic shock with an odd ratio of 5.62( Confidence Interval 5.34-5.92 ) & P value( <.001).
Spontaneous tension pneumothorax has an odd ratio of 4.92( Confidence Interval 3.82-6.34) & P value( <.001). Acute renal failure has an odd ratio of 2.45(CI 2.32-2.57) & P value(<.001).
Sepsis has an odd ratio of 2.27(CI 2.11-2.44) & P value(<.001), Complete heart block has an odd ratio of 1.90(CI 1.55-2.33) & P value(<.001). ESRD has an odd ratio of 1.59( CI 1.49-1.71) & P value(<.001), followed by acute pulmonary embolism with an odd ratio of 1.53( CI 1.35-1.73) & P value(<.001).
Type 2 MI with an odd ratio of 1.50(1.37-1.63) & P value(<.001). Diabetes Mellitus is associated with an increased risk of cardiac arrest and an odd ratio of 1.29(CI 1.23-1.36) & P value(<0.001).Atrial fibrillation with an odd ratio of 1.13(CI 1.08-1.19) & P value(<.001).
These results have been shown in the
Table 1.
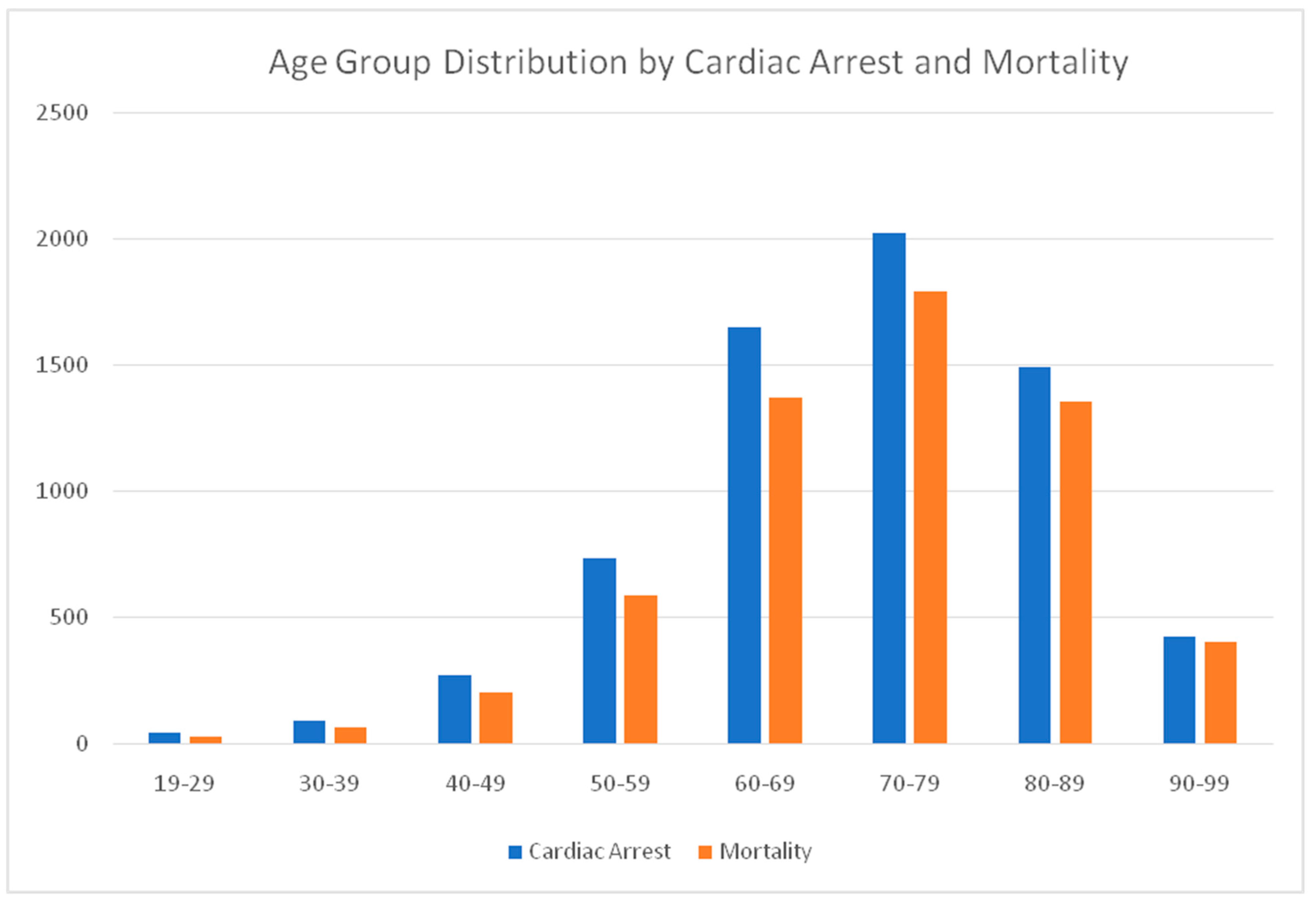
We have reported the mortality & cardiac arrest distribution across the age groups among COVID-19 patients. There is 57.14% mortality in the age group between 19-29, 70.11% for 30-39, 73.98% for the age group 40-49 with a steady increase in mortality with 50-59 reporting 80.39%, 60-69 reporting 83.19%,70-79 reporting 88.70%, 80-89 reporting 90.92% and 90-99 reporting 94.55% mortality among cardiac arrest patients with Acute COVID 19. These results have been shown in
Table 2 and
Figure 1
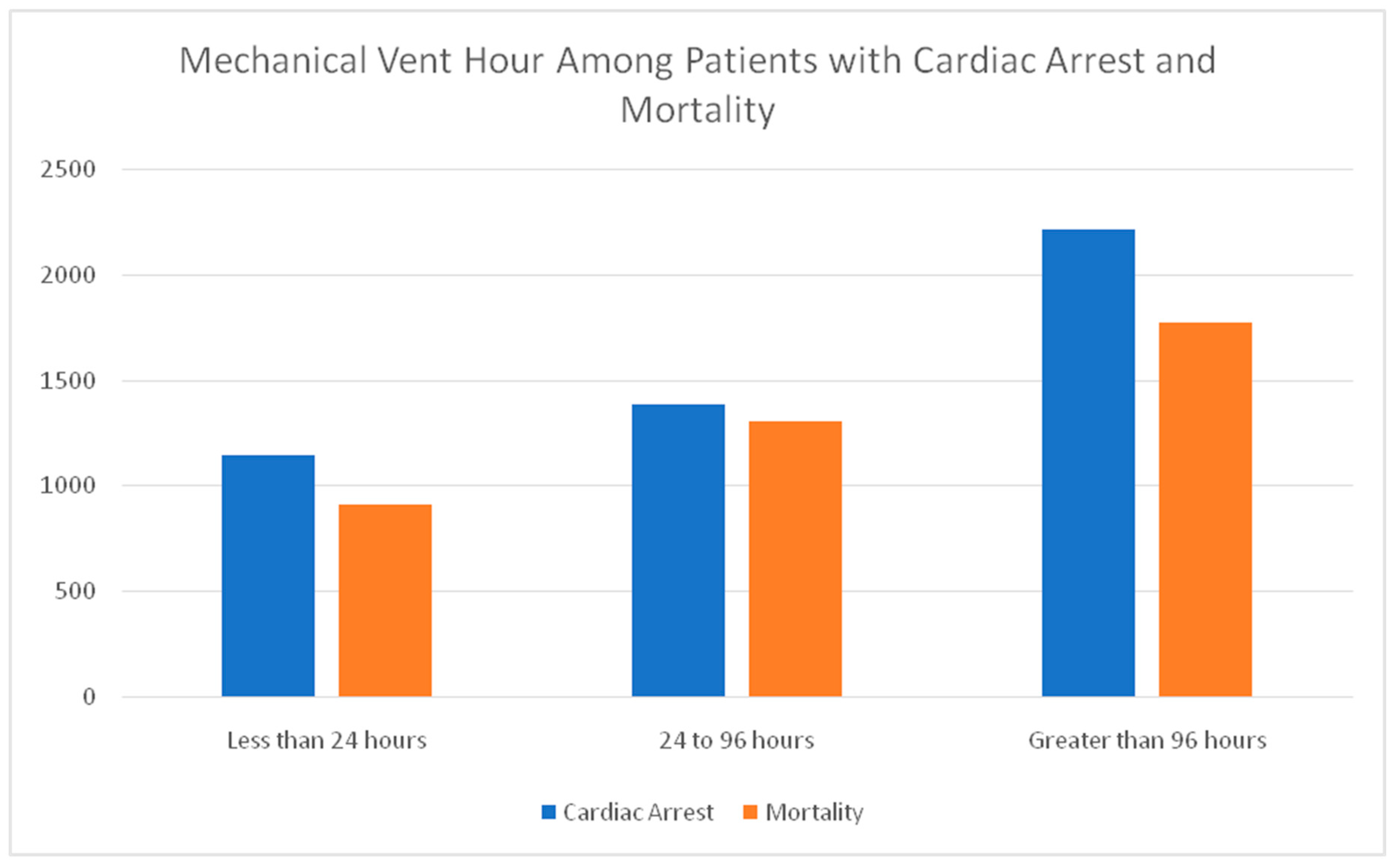
Mechanical ventilator uses duration and mortality percentage has a direct correlation with mechanical vent hours with (< 24 hours) use, reported a 17.07% cardiac arrest and 15.79% mortality and increased to 20.68% cardiac arrest and 22.65% mortality with 24-96 hours of mechanical ventilator use. Patients on mechanical ventilators for more than 96 hours have 33.09% cardiac arrest and 30.75% mortality. Results have been shown in the
Table 3 & figure 2 as well.
Incidence of Cardiac arrest has impacted the length of stay for patients as well with 13.47 days vs 10.25 days. We have shown the frequency distribution of all the co-morbidities among cardiac arrest patients in
Table 4.
4. Discussion
In our study, we have tried to reveal the correlation and relevance of the following clinical variables in determining the impact on cardiac arrest, although no prior studies have been done to establish the causal relationship of these clinical predictors with cardiac arrest among COVID 19 and heart failure.
4.1. Mechanical Ventilator & Cardiac Arrest
Critically ill COVID-19 patients have severe lung parenchyma injuries requiring mechanical ventilator support. Hypoxia and hypercapnia are well-known risk factors for cardiac arrest. Hypoxic cardiac arrest patients usually have poor outcomes.[
30] Hypoxia has a detrimental impact on cardiac function and can lead to sodium-potassium (Na+/K+) ATP ase dysfunction and mitochondrial damage triggering energy failure and cell apoptosis.[
31]
In one of the rare studies done to understand peri mortem pathophysiology and co-correlation of hypoxia and cardiac arrest, it was found that after reaching threshold hypoxia, blood pressure starts falling almost 8 minutes prior to arrest, followed by a drop in heart rate 4 minutes prior to terminal circulatory arrest.[
32] suggesting the critical role of hypoxia in cardiac arrest.
Hypercapnia reduces the sensitivity of adrenergic receptors and adversely affects the expression of these receptors. Hypercapnia can induce metabolic acidosis, which can depress the cardiac contractility and response of the left ventricle to catecholamine.[
33] In the literature, so far, no clear causal mechanism has been discovered to explain cardiac arrest among ventilated patients with COVID-19, but hypoxia and hypercapnia can partly explain the possible mechanism of cardiac arrest. In our sub-analysis, the mortality has been shown to worsen with a longer mechanical ventilator duration.
4.2. Pulmonary Embolism and Cardiac Arrest
Venous thromboembolism, including pulmonary embolism, leads to significant morbidity and mortality in COVID-19 patients. This may be due to a combination of a hypercoagulable state due to epitheliopathy and a hyper-inflammatory state due to cytokine storm, which leads to venous and arterial thrombosis. [
34] COVID-19 patients have a seven times higher risk of pulmonary embolism than non-COVID patients. Pulmonary embolism is seen in 2.6-8.9% of hospitalized COVID-19 patients, with one-third of them requiring intensive care unit admission, despite thromboprophylaxis use.[
35]
A propensity-matched analysis of the National Inpatient Sample (N.I.S.) database from 2020 showed COVID-19 patients with pulmonary embolism needed more vasopressor, mechanical ventilation, longer hospital stays, and higher in-hospital mortality when compared to COVID-19 patients without pulmonary embolism.[
36]In anecdotal cases, acute pulmonary embolism has been seen in young asymptomatic females with COVID-19 presenting as sudden death.[
37]Pulmonary embolism is also reported as a sequela of long COVID-19, presenting as paroxysmal supra-ventricular tachycardia followed by cardiac arrest in a 31-year-old lady.[
38]
4.3. Type 2 MI in COVID-19 & Cardiac Arrest
Incidence of Type 2 MI incidence has been relatively high among COVID-19 patients, and a study based on a Nationwide inpatient sample database concluded that T 2MI had higher in-hospital mortality with an odds ratio of 1.4, sudden cardiac arrest with an odd ratio of 1.3 and cardiogenic shock with an odd ratio of 2.16 compared to one without T2MI in COVID 19 patients. [
39] Type 2 MI in various analyses has shown poor outcomes in the long term. A prospective study with follow-up for 1. 8 years reported In-hospital mortality of 10% and 30% all-cause mortality for Type 2 MI.[
40] In a multicenter study on COVID-19, myocardial injury was associated with a 2.31 times higher risk of mortality, although after adjusting for age, multi-organ dysfunction, and vaso-pressor requirement, the association was attenuated.[
41]
4.4. Spontaneous Tension Pneumothorax, COVID-19 & Cardiac Arrest
Critically ill COVID-19 patients require mechanical ventilation, and there is a high risk of tension pneumothorax. Tension pneumothorax can lead to compression of mediastinal structures, lung parenchyma, and a decrease in cardiac output, possibly by compression of great vessels, hypoxia, and poor venous return. It manifests clinically in the form of a progressive decrease in Spo2 and reduction in cardiac output, which can precipitate cardiac arrest. [
42]In the retrospective investigation of 21 COVID patients, there is (42.9%) mortality rate among patients with spontaneous tension pneumothorax. [
43]
4.5. Sepsis & Septic Shock, COVID-19 & Cardiac Arrest
Sepsis is one of the leading causes of death in critically ill patients [
44]. In a large meta-analysis based on 170 studies across North America, Europe, and Australia, septic shock has a mortality of 34.7% over 30 days time period. [
45] The most common cause of cardiac arrest includes pulseless electrical cardiac activity triggered by hypoxemia, hypovolemia, hypokalemia, hyperkalemia, and acidosis, which can occur concomitantly with sepsis.[
46] Finally, Altered cardiac contractility and pre and after-load changes can induce pulse-less electrical activity.[
47]
In a retrospective analysis of 30 patients with COVID-19 septic shock, the mortality rate was reported to be 96.7%(48), whereas another study reported in the European Heart Journal reported a mortality rate of 56% among septic shock patients and 4.44% among sepsis patients with COVID-19.[
49]Multiple co-morbidities and end-organ dysfunction impact cardiac arrest. Acute Respiratory failure with an odd ratio of 2.96, G.I. bleeding with a ratio of 1.26, RCRI index of 1.07, and male gender with a ratio of 1.32 are significant determinants of In hospital cardiac arrest with sepsis noticed in a retrospective study.[
50]
4.6. Diabetes Mellitus, COVID-19 & Cardiac Arrest
A large meta-analysis of 18 studies to evaluate the impact of D.M. on COVID-19 severity and mortality reported 2.5 fold increase in mortality among diabetic patients. [
51]There are limited studies in the literature elucidating the pathophysiological impact of diabetes mellitus on COVID-19.
Hyperglycemia can lead to increase SARS-CoV 2 replication, and glycolysis sustains this replication.[
52] Endothelial damage by inflammation, glucotoxicity, and accelerated atherosclerosis can contribute to an increased risk of thromboembolic complications impacting the end organs in patients with diabetes and contribute to adverse outcomes.[
53]In a retrospective analysis of more than 10000 hospital cardiac arrest patients, the higher non-shock-able rhythm was reported in diabetic patients compared to the rest of the cohort, and poor survival to discharge independent of other risk factors.[
54]
4.7. Renal Dysfunction, COVID-19, and Cardiac Arrest
CKD, ESRD, and A.K.I. have been found to have a significant impact on mortality among COVID-19 in various studies. In a retrospective administrative data set based on 17 million patients, a hazard ratio of 3.7 was reported for haemo-dialysis(H.D.) patients and 2.5 for CKD 4 patients and were among the top four co-morbidities with the highest mortality from COVID-19.[
55]In a large meta-analysis of 69 reviews and 66 primary studies, the pooled hazard ratio of mortality for the CKD 3 stage is 1.46, the hazard ratio of 2.84 for CKD 4, and the hazard ratio of 1.92 for ESRD patients on the H.D. among COVID-19.[
56]
The pathophysiology of the impact of COVID on renal function is still under investigation. Renal parenchyma cells, especially proximal tubular cells, express high levels of A.C.E. 2 receptors promoting the entry of SARS-CoV- 2 viral particles.[
57] In a study on 62 patients with COVID-19, all the patients were found to be tested positive for SARS-CoV-2 in the kidney specimens. It was detected in tubular cells and podocytes, and cells infected with viral R.N.A. had increased expression of genes involved in inflammation, injury, and fibrosis.[
58] The systemic impact of volume depletion, cytokine-induced systemic inflammatory response, and downstream consequences of infection, including rhabdomyolysis, ischemic thrombi, inflammation, complement dysregulation, and hemodynamic instability, may contribute towards the end organ dysfunction and mortality. [
59]
4.8. Complete Heart Block, COVID-19, and Cardiac Arrest
Cardiac arrhythmias are frequently seen in severe coronavirus disease 2019 (COVID-19). Some studies have cited arrhythmias occurring in up to 44% of patients and bradycardia (sinus bradycardia and A.V. block) occurring in up to 24.9% of patients. [
60,
61]. There are several anecdotal records of patients who have a sudden cardiac arrest from pulse-less electrical activity or complete heart block while being either evaluated for or treated for COVID-19 infection.
In certain case reports, the complete heart block was transient and recovered within 24 -48 hours.[
61]On the other hand, there are several case reports where the complete heart block resulted in C.P.R.; eventually, the rhythm converted to ventricular fibrillation(V.F.) needing cardio-version, which was mostly unsuccessful.[
62] The duration and consequences of atrioventricular (A.V.) nodal blockade were not consistent among the cases reported, analogous to most pathologies involving a spectrum of diseases.
In the literature so far, no clear causal mechanism has been discovered for the development of C.H.B. associated with this virus. Complete A.V. block can be caused by increased vagal tone, cardiac conduction system disease (fibrosis), ischemia and local inflammation, cardiomyopathies, or viral myocarditis. Some reversible causes can be hyperkalemia, thyroid disease, or medications used in the treatment of COVID. It has been suggested that direct viral infiltration of cardiomyocytes through the angiotensin-converting enzyme-2 receptors and subsequent systemic inflammation may be one mechanism of cardiac injury.[
60]
4.9. Atrial Fibrillation, COVID-19, and Cardiac Arrest
Atrial fibrillation is the most common cardiac arrhythmia among COVID-19 patients. It was found that the incidence of New-onset atrial fibrillation (AFib) was 1 in 20 among patients hospitalized with COVID-19.[
63] Using data from the American Heart Association’s COVID-19 Cardiovascular Disease Registry, researchers examined nearly 28,000 patients without a history of A Fib who were hospitalized for COVID-19. In this study, new-onset A Fib was strongly associated with increased in-hospital mortality and major adverse cardiovascular events. They also had longer hospital stays and a greater need for I.C.U. Care and intubation. Also of note, approximately 45% died in the hospital.
5. Limitation of the Study
It is based on administrative data base with limitations due to the nature of study including but not limited to accuracy and completeness of data entered by professionals with limited medical knowledge.[
64] Multivariate analysis has been done but confounding factors cannot be completely eliminated. In order to include all the heart failure admissions, we have used all the ICD 10 codes, but has led to inclusion of stable chronic heart failure diagnoses and patients with recovered ejection fraction as well without exacerbation, which may have led to dilution of severity and its impact on COVID 19 patients. We do not have access to verify the accuracy, evaluate the NYHA class of heart failure and lab values which can influence the mortality and outcome. Despite these limitations, it is one of the largest studies to evaluate the predictors of cardiac arrest among COVID 19 patients.
6. Conclusions
In this observational study, we have concluded that the use of a mechanical ventilator, a reflector of the severity of the disease, is the strongest risk factor for cardiac arrest, followed by septic shock among COVID-19 patients with history of heart failure. Future prospective studies in critically ill patients can advance our knowledge and address these risk factors for improving mortality.
Funding
This research received no external funding.
Institutional Review Board Statement
It is an administrative data set without identifiable patient information.
Informed Consent Statement
It is an administrative data set without identifiable patient information.
Data Availability Statement
The data that support the findings of this study are available from NRD 2020, but restrictions apply to the availability of the raw data in compliance with NRD policies.
Conflicts of Interest
All the authors declare that they have no competing interests.
References
- https://covid19.who.int/.
- Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef]
- Tomasoni D, Inciardi RM, Lombardi CM, Tedino C, Agostoni P, Ameri P, et al. Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID-19. Results of the Cardio-COVID-Italy multicentre study. Eur J Heart Fail. 2020, 22, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas-Lasarte M, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol. 2020, 76, 2334–2348. [Google Scholar] [CrossRef] [PubMed]
- Hayek S.S., et al. In-hospital cardiac arrest in critically ill patients with covid-19: multicenter cohort study. BMJ. 2020, 371, m3513. [Google Scholar]
- Aldabagh M., et al. Survival of in-hospital cardiac arrest in COVID-19 infected patients. Healthcare (Basel) 2021, 9. [Google Scholar]
- Miles J.A., et al. Characteristics and outcomes of in-hospital cardiac arrest events during the COVID-19 pandemic: a Single-Center experience from a New York city public hospital. Circ Cardiovasc Qual Outcomes. 2020, 13, e007303. [Google Scholar] [CrossRef]
- Roedl K., et al. Effects of COVID-19 on in-hospital cardiac arrest: incidence, causes, and outcome - a retrospective cohort study. Scand J Trauma Resusc Emerg Med. 2021, 29, 30. [Google Scholar] [CrossRef]
- Miles JA, Mejia M, Rios S, Sokol SI, Langston M, Hahn S, Leiderman E, Salgunan R, Soghier I, Gulani P, Joshi K, Chung V, Morante J, Maggiore D, Uppal D, Friedman A, Katamreddy A, Abittan N, Ramani G, Irfan W, Liaqat W, Grushko M, Krouss M, Cho HJ, Bradley SM, Faillace RT. Characteristics and Outcomes of In-Hospital Cardiac Arrest Events During the COVID-19 Pandemic: A Single-Center Experience From a New York City Public Hospital. Circ Cardiovasc Qual Outcomes. 2020, 13, e007303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thapa SB, Kakar TS, Mayer C, Khanal D. Clinical Outcomes of In-Hospital Cardiac Arrest in COVID-19. JAMA Intern Med. 2021, 181, 279–281. [Google Scholar] [CrossRef]
- Raitt MH, Dolack GL, Kudenchuk PJ, Poole JE, Bardy GH. Ventricular arrhythmias detected after transvenous defibrillator implantation in patients with a clinical history of only ventricular fibrillation. Implications for use of implantable defibrillator. Circulation 1995, 91, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Mandapati R, Asano Y, Baxter WT, Gray R, Davidenko J, Jalife J. Quantification of effects of global ischemia on dynamics of ventricular fibrillation in isolated rabbit heart. Circulation 1998, 98, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Panchal AR, Bartos JA, Cabañas JG, Donnino MW, Drennan IR, Hirsch KG, Kudenchuk PJ, Kurz MC, Lavonas EJ, Morley PT, O'; Neil BJ, Peberdy MA, Rittenberger JC, Rodriguez AJ, Sawyer KN, Berg KM; Adult Basic and Advanced Life Support Writing Group. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142 (Suppl. 2), S366–S468. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber JI, Hamilton MA. Role of inotropic agents in the treatment of heart failure. Circulation 2010, 121, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Meaney PA, Nadkarni VM, Kern KB, Indik JH, Halperin HR, Berg RA. Rhythms and outcomes of adult in-hospital cardiac arrest. Crit Care Med. 2010, 38, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Abou-Ismail, M. Y., Diamond, A., Kapoor, S., Arafah, Y. & Nayak, L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb. Res. 2020, 194, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; et al. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct. Target. Ther. 2020, 5, 293. [Google Scholar] [CrossRef] [PubMed]
- Violi F, Cangemi R, Falcone M, Taliani G, Pieralli F, Vannucchi V, et al. Cardiovascular complications and short-term mortality risk in community-acquired pneumonia. Clin Infect Dis. 2017, 64, 1486–1493. [Google Scholar] [CrossRef]
- Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013, 187, 509–517. [Google Scholar] [CrossRef]
- Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., Mou H.M., Wang L.H., Zhang H.R., Fu W.J., et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020, 49, 411–417. [Google Scholar] [CrossRef]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Costela-Ruiz, V. J., Illescas-Montes, R., Puerta-Puerta, J. M., Ruiz, C., &Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine & growth factor reviews 2020, 54, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L. Innate immunity and the failing heart: The cytokine hypothesis revisited. Circ. Res. 2015, 116, 1254–1268. [Google Scholar] [CrossRef]
- Jamal F.A., Khaled S.K. The Cardiovascular Complications of Chimeric Antigen Receptor T Cell Therapy. Curr. Hematol. Malig. Rep. 2020, 15, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Escher F, Pietsch H, Aleshcheva G, Bock T, Baumeier C, Elsaesser A, et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020, 7, 2440–2447. [Google Scholar] [CrossRef] [PubMed]
- Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021, 50, 107300. [Google Scholar] [CrossRef] [PubMed]
- Giudicessi JR, Noseworthy PA, Friedman PA, Ackerman MJ. Urgent Guidance for Navigating and Circumventing the QTc-Prolonging and Torsadogenic Potential of Possible Pharmacotherapies for Coronavirus Disease 19 (COVID-19). Mayo Clin Proc. 2020, 95, 1213–1221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu G, D'Souza AG, Quan H, Southern DA, Youngson E, Williamson T, Eastwood C, Xu Y. Validity of ICD-10 codes for COVID-19 patients with hospital admissions or ED visits in Canada: a retrospective cohort study. BMJ Open. 2022, 12, e057838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stiell IG, Nichol G, Leroux BG, et al. Early versus later rhythm analysis in patients with out-of-hospital cardiac arrest. N Engl J Med 2011, 365, 787–797. [Google Scholar] [CrossRef]
- Michiels, C. Physiological and pathological responses to hypoxia. Am J Pathol 2004, 164, 1875–1882. [Google Scholar] [CrossRef]
- Gilhooley C, Burnhill G, Gardiner D, Vyas H, Davies P. Oxygen saturation and haemodynamic changes prior to circulatory arrest: Implications for transplantation and resuscitation. J Intensive Care Soc. 2019, 20, 27–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ellison GM, Torella D, Karakikes I et al. Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. J. Biolumin. Chemilumin. 2007, 282, 11397–11409. [Google Scholar] [CrossRef]
- Hanff TC, Mohareb AM, Giri J, Cohen JB, Chirinos JA. Thrombosis in COVID-19. Am J Hematol. 2020, 95, 1578–1589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sakr Y, Giovini M, Leone M, Pizzilli G, Kortgen A, Bauer M, Tonetti T, Duclos G, Zieleskiewicz L, Buschbeck S, Ranieri VM, Antonucci E. Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: a narrative review. Ann Intensive Care. 2020, 10, 124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nasrullah A, Gangu K, Shumway NB, Cannon HR, Garg I, Shuja H, Bobba A, Chourasia P, Sheikh AB, Shekhar R. COVID-19 and Pulmonary Embolism Outcomes among Hospitalized Patients in the United States: A Propensity-Matched Analysis of National Inpatient Sample. Vaccines 2022, 10, 2104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Polat, V., Bostancı, G.İ. Sudden death due to acute pulmonary embolism in a young woman with COVID-19. J Thromb Thrombolysis 2020, 50, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Talwar D, Kumar S, Acharya S, Khanna S, Hulkoti V. Paroxysmal Supraventricular Tachycardia and Cardiac Arrest: A Presentation of Pulmonary Embolism With Infarction as a Sequela of Long COVID Syndrome. Cureus 2021, 13, e18572. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sattar Y, Taha A, Patel N, Victor V, Titus A, Aziz S, Gonuguntla K, Thyagaturu H, Atti L, Micho T, Almas T, Tarun T, Alraies MC, Balla S. Cardiovascular outcomes of type 2 myocardial infarction among COVID-19 patients: a propensity matched national study. Expert Rev Cardiovasc Ther. 2023, 21, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz NR, Subramanyam P, Gianos E, Reynolds HR, Shah B, Sedlis SP. Treatment and outcomes of type 2 myocardial infarction and myocardial injury compared with type 1 myocardial infarction. Coron Artery Dis. 2018, 29, 46–52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Metkus TS, Sokoll LJ, Barth AS, Czarny MJ, Hays AG, Lowenstein CJ, Michos ED, Nolley EP, Post WS, Resar JR, Thiemann DR, Trost JC, Hasan RK. Myocardial Injury in Severe COVID-19 Compared With Non-COVID-19 Acute Respiratory Distress Syndrome. Circulation 2021, 143, 553–565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gabbott DA, Carter JA. Contralateral tension pneumothorax during thoracotomy for lung resection. Anaesthesia. 1990, 45, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Guo Q, Yang P, Barnabo Nampoukime KP, Ma K, Wang H. Spontaneous pneumothorax: a complication of coronavirus disease 2019 (COVID-19) patients. Thorac Cardiovasc Surg. 2021, 69, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M., Gerlach, H., Vogelmann, T. et al. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019— results from a systematic review and meta-analysis. Crit Care 2020, 24, 239. [Google Scholar] [CrossRef] [PubMed]
- Desbiens NA. Simplifying the diagnosis and management of pulseless electrical activity in adults: a qualitative review. Crit Care Med. 2008, 36, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Aufderheide, TP. Etiology, electrophysiology and myocardial mechanics of pulseless electrical activity. In: Paradis NA, Halperin HR, Kern KB, Wenzel V, Chamberlain DA, editors. Cardiac arrest: the science and practice of resuscitation medicine, 22. Cambridge: Cambridge University Press; 2007. p. 426.
- Chen S, Gao Z, Hu L, Zuo Y, Fu Y, Wei M, Zitello E, Huang G, Deng Y. Association of Septic Shock with Mortality in Hospitalized COVID-19 Patients in Wuhan, China. Adv Virol. 2022, 2022, 3178283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- J Cidade and others. Septic shock 3.0 in severe COVID19 patients: are we missing something clinically important? European Heart Journal 2021, 42 (Suppl. 1), ehab724.1517. [Google Scholar] [CrossRef]
- Duazo C, Hsiung JC, Qian F, Sherrod CF, Ling DA, Wu IJ, Hsu WT, Liu Y, Wei C, Tehrani B, Hsu TC, Lee CC. In-hospital Cardiac Arrest in Patients With Sepsis: A National Cohort Study. Front Med (Lausanne). 2021, 8, 731266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Almeida-Pititto, B., Dualib, P.M., Zajdenverg, L. et al. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol Metab Syndr 2020, 12, 75. [Google Scholar] [CrossRef]
- Codo, A. C. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020, 32, 437–446.e5. [Google Scholar] [CrossRef]
- Lim, S., Bae, J.H., Kwon, HS. et al. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol 2021, 17, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Parry M, Danielson K, Brennenstuhl S, Drennan IR, Morrison LJ. The association between diabetes status and survival following an out-of-hospital cardiac arrest: A retrospective cohort study. Resuscitation 2017, 113, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jdiaa SS, Mansour R, El Alayli A, Gautam A, Thomas P, Mustafa RA. COVID-19 and chronic kidney disease: an updated overview of reviews. J Nephrol. 2022, 35, 69–85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan S, Chen L, Yang CR, et al. Does SARS-CoV-2 infect the kidney? J. Am. Soc. Nephrol. 2020, 31, 2746–2748. [Google Scholar] [CrossRef] [PubMed]
- Jansen J, Reimer KC, Nagai JS, et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell 2020, 29, 217–231.e8. [Google Scholar]
- Batlle D, Soler MJ, Sparks MA, et al. COVID-19 and ACE2 in Cardiovascular, Lung, and Kidney Working Group: Acute kidney injury in COVID-19: Emerging evidence of a distinct pathophysiology. J Am Soc Nephrol 2020, 31, 1380–1383. [Google Scholar] [CrossRef]
- Ismail Z, Salabei JK, Stanger G, Asnake ZT, Frimer L, Smock A. Third-Degree Heart Block Associated With Saddle Pulmonary Embolism: A Rare Sequelae of COVID-19-Induced Hypercoagulable State. Cureus 2021, 13, e16246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar S, Arcuri C, Chaudhuri S, Gupta R, Aseri M, Barve P, Shah S. A novel study on SARS-COV-2 virus associated bradycardia as a predictor of mortality-retrospective multicenter analysis. Clin Cardiol. 2021, 44, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Kochav SM, Coromilas E, Nalbandian A, Ranard LS, Gupta A, Chung MK, Gopinathannair R, Biviano AB, Garan H, Wan EY. Cardiac Arrhythmias in COVID-19 Infection. Circ ArrhythmElectrophysiol. 2020, 13, e008719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- https://newsroom.heart.org/news/study-finds-connection-between-covid-19-and-new-onset-afib.
- Yoshihara H, Yoneoka D. Understanding the statistics and limitations of large database analyses. Spine (Phila Pa 1976) 2014, 39, 1311–1312. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).