1. Introduction
Stroke is the second cause of death in Europe, with 1.4 million people experiencing a stroke every year [
1]. Acute ischemic stroke accounts for roughly 80% of all strokes, 20% of which originate in vulnerable carotid plaques [
2]. The identification of those asymptomatic carotid plaques that will become vulnerable and symptomatic remains unclear, despite its value for earlier intervention. Several biomarkers have been explored to identify asymptomatic carotid patients who could benefit from prophylactic carotid intervention [
3]. Recognizing the importance of predicting which plaques will become symptomatic, the European Society for Vascular Surgery Guidelines have emphasized “the need for a validated algorithm for identifying high risk for stroke on best medical therapy asymptomatic patients in whom to target carotid endarterectomy and carotid artery stenting” [
1].
Studies conducted in cardiology reported that changes in coronary perivascular adipose tissue (PVAT) are associated with vulnerable coronary plaques and can be identified on computed tomography [
4,
5,
6].
PVAT is the adipose tissue that supports the vessels and produces several molecules which affect the function and structure of the vessels [
7,
8]. The relationship between the PVAT and the vessels is complex and bidirectional [
9]. The PVAT exerts anti-inflammatory and vasodilatory functions, which have a protective effect [
8,
10]. However, under pathological conditions, the PVAT can become dysfunctional, leading to an increase of vasoconstriction agents and to a proinflammatory phenotype, and may ultimately be involved in the initiation and progression of atherosclerosis [
8,
10].
The amount of PVAT that surrounds the common carotid artery can be easily measured in clinical practice using ultrasound, with carotid artery extra-media thickness (EMT) [
11]. The main determinants of EMT are arterial adventitia and PVAT [
7,
11].
The primary aim of this study was to analyze whether the amount of the peri-carotid adipose tissue, estimated through EMT, is associated with the atherosclerotic characteristics at the carotid bifurcation.
2. Materials and Methods
2.1. Study Type, Inclusion / Exclusion Criteria
An observational, prospective, unicenter study was conducted from January 2018-December 2022. Patient recruitment rate was reduced during 2020 due to the COVID-19 pandemic. We included consecutive patients who were attending the Vascular Surgery consultations of the first author (JF) with varicose veins, peripheral arterial disease and carotid artery plaque to a minimum number of 106. These patients were followed for six months by the first author (JF). Bedridden individuals, subjects who refused to participate in the protocol or patients with any disease that could potentially change body composition or pro-inflammatory state were excluded.
2.2. Ethical Considerations
Ethics approval was obtained from the Ethics Committee of the local hospital (Ref:75/2017). All participants signed the informed consent.
2.3. Demographic and Clinical Characteristics
Patient’s age, gender and medication were registered. The definitions of arterial hypertension, diabetes, dyslipidemia and smoking habits were considered according to other publications [
12]. Obesity was defined as waist circumference (WC) > 102 cm in men and > 88 cm in women [
13]. “Acute culprit” carotid stenosis was defined as the presence of symptomatic plaques identified within the first 15 days after stroke [
14].
2.4. Lipid Profile and HbA1c
To determine the levels of hemoglobin A1c (HbA1c), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglycerides, a phlebotomy was performed in the morning, after a 10–12 hours fast. Blood samples were taken into appropriate Vacutainer, centrifuged for 5min at 4000 cycle/minute rate, and serum was separated. The tests were performed by routine procedures in the department of clinical chemistry.
2.5. Evaluation of the Carotid Bifurcation with Doppler-ultrasound
The carotids were characterized using a SIEMENS (Siemens Medical Solutions, Inc., Mountain View, CA, USA) ACUSON X300 ultrasound, with a 5–10 Hz linear scan head. Two-dimensional grey scale was used to measure the intima-media thickness (IMT), the EMT, and the presence and morphology of the atherosclerotic plaque [
7].
To determine the IMT, the probe surface was placed parallel to the common carotid artery, in a longitudinal position [
15]. Two bright lines were identified: the interface between blood and the intima and the layer between intima and media [
15], with the distance between these two lines being defined as the IMT [
15]. The IMT was measured according to the Mannheim Consensus guidelines on a 10 mm -length segment starting 5 mm proximally to the carotid bulb. Three measures were taken in each common carotid artery and the mean value was the IMT [
16].
To determine the EMT, the carotid artery and jugular vein were both scanned in a longitudinal position with the probe parallel to the vessels [
7]. The distance between the carotid media-adventitia interface and the jugular lumen was determined along the 7 mm segment, starting 3 mm proximal to the bulb [
7]. Five values were recorded at the end-diastole, from five consecutive beats. The mean of these values indicated the EMT [
16]. For each carotid bifurcation, right and left EMT were determined, to evaluate the mean EMT (calculated as the mean between the right and the left EMT), and the EMT ipsilateral to the carotid bifurcation. To determine if the amount of peri-carotid adipose tissue was correlated with the ipsilateral carotid atherosclerotic characteristics, we compared the EMT on the left and right sides to the ipsilateral and contralateral characteristics of atherosclerosis at the carotid bifurcation.
The number of atherosclerotic plaques at the carotid bifurcation was recorded. The morphology and the area of the largest plaque were described. The plaque surface was classified as smooth, irregular, or ulcerated. A depression of the plaque surface by more than 2 mm indicates ulceration [
16]. The plaque area was calculated based on the length and height of the plaque16. The plaque echogenicity was classified according to the Gray-Weale’s classification modified by Geroulakos [
17].
Colour and pulse Doppler ultrasonography was applied to determine the grade of carotid artery stenosis, according to the Guidelines of the European Society of Vascular Surgery [
1,
16].
2.6. Power Consideration
To determine the differences in the amount or the peri-carotid adipose tissue, estimated through EMT between patients with carotid plaques and those without carotid plaques (a marker of atherosclerosis at the carotid bifurcation) a minimum sample of 106 patients is necessary (with a significant level of 5%, a power of 80% and an effect size of 0.5).
2.7. Statistical Analysis
Continuous variables were expressed as the mean ± standard deviation, while percentages were presented for categorical variables. The Shapiro–Wilk test was used to assess all continuous variables for normality. Between-group differences in continuous variables were assessed with the Mann-Whitney U test, and the effect size r was determined by calculating the ratio between test statistics Z and the square root of the number of pairs n. Associations between parameters were assessed using the non-parametric Spearman correlation analysis since variables were not normally distributed. Wilcoxon Signed Rank test was used to study the evolution of EMT, comparing 2 time points (t = 0 and t = 6 months). A p-value of less than .05 was considered statistically significant. Statistical analysis was performed using the SPSS Statistics software, version 20.0 (IBM SPSS Statistics, IBM Corporation Chicago, IL, USA).
3. Results
3.1. General description of the population
We included 177 subjects (mean age: 67.16 ± 9.87 years-old; 80.23% males). Cardiovascular risk factors and comorbidities of the studied population, as well as the atherosclerotic characteristics at the right and left carotid bifurcation are presented in
Supplementary Materials (
Tables S1 and S2).
3.2. Peri-carotid adipose tissue and cardiovascular risk factors
The majority of the included patients had at least three cardiovascular risk factors (34.6% had three and 24.0% had four risk factors).
Patients with hypertension and patients with obesity had a higher mean EMT, whereas patients on fibrates had lower mean EMT (
Table 1). Age and the number of cardiovascular risk factors showed a weak positive correlation with mean EMT (rs=.21; p=.006 and rs =.24; p = .002).
3.3. Peri-carotid adipose tissue and chronic atherosclerosis at the carotid bifurcation
3.3.1. Presence of carotid plaques
Mean EMT: The presence of carotid plaques was associated with a statistically significant increase in mean EMT [present: Median=1.14, IQR=0.66; versus non-present: Median=0.97, IQR=0.40; p=.001, r =0.26].
Ipsilateral EMT: Patients with plaques at right carotid arteries were associated with a significantly higher ipsilateral EMT [Median=1.02; IQR=0.58; versus Median=0.96; IQR=0.48; p<.001]. This association was not found on left side. The left EMT was also significantly higher in patients with right carotid plaques [Median=0.93; IQR=1.60; versus Median=0.80; IQR=1.20; p=.010].
3.3.2. Area of highest carotid plaque
Mean EMT: A positive correlation was found between mean EMT and area of highest carotid plaque, at right and left side (ρ=017; p=.036; ρ=.22; p=.004, respectively).
Ipsilateral EMT: A positive correlation was found between left EMT and ipsilateral area of carotid plaque (ρ=.22; p=.004). This association was not found on right side. There was no correlation between right EMT and the area of contralateral carotid plaque.
3.3.3. IMT
Mean EMT: A positive correlation was found between mean EMT and IMT (right and left) (right: rs=.20; p=.010; left: rs=.21; p=.007).
Ipsilateral EMT: A positive correlation was found between ipsilateral EMT and ipsilateral IMT (right: ρ=.19; p=.017; left: ρ=.19; p=.012). Right EMT was positively correlated with left IMT (ρ=.18; p=.023). Left EMT was not correlated with right IMT.
3.3.4. Carotid Stenosis ≥ 70%
Mean EMT: The presence of left carotid stenosis ≥ 70% was associated with a statistically significant increase in mean EMT [present: Median=1.55, IQR=0.68 versus non-present: Median=1.00, IQR=0.46; p=.019, r=0.61].
Ipsilateral EMT: Patients with left carotid stenosis ≥ 70% were associated with a statistically significantly higher ipsilateral EMT (
Table 2).
3.3.5. Plaque echogenicity
There were no differences in EMT (mean or ipsilateral) in respect to carotid plaques echogenicity (
Tables S3 and S4).
3.4. The particular case of “acute culprit” carotid stenosis
We found that patients with “acute culprit” carotid stenosis (n=11) had a significantly higher mean EMT and ipsilateral EMT, when compared with non-acute carotid stenosis patients. This finding revealed a large effect size and was valid for both left and right carotid stenosis (
Table 3). This association was not found in the contra-altera carotid bifurcation.
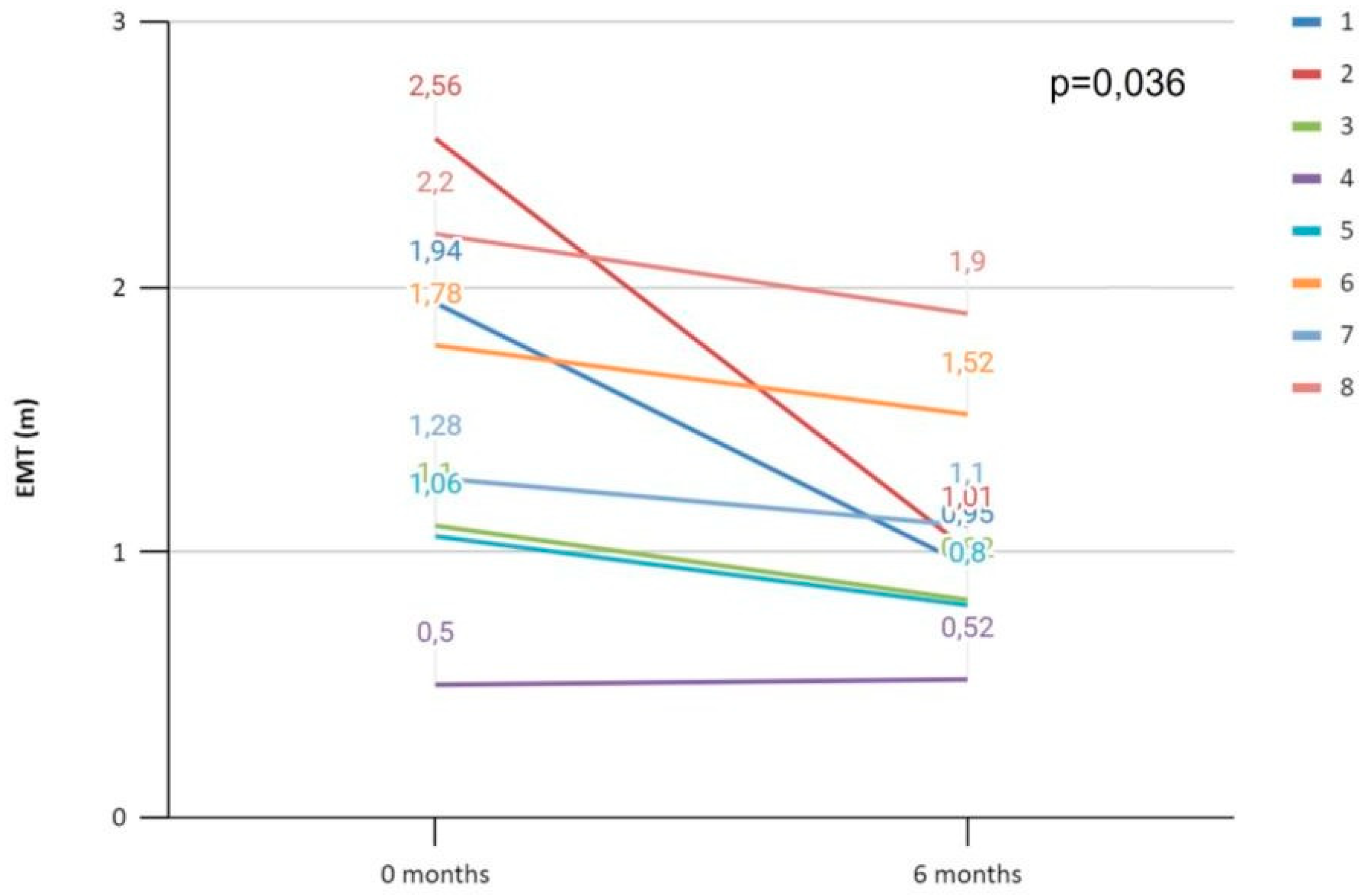
Furthermore, we evaluated the ipsilateral peri-carotid adipose tissue six months after the neurologic event, by measuring a follow-up EMT, in 8 of 11 patients with “acute culprit” carotid stenosis and we found a significant decrease in ipsilateral EMT (
Figure 1).
4. Discussion
To the best of our knowledge, our group is the first trying to elucidate the role of ipsilateral peri-carotid adipose tissue versus total amount of such tissue in atherosclerosis at the carotid bifurcation. Our research has revealed a correlation between the total amount of peri-carotid adipose tissue, estimated with mean EMT, and the presence of atherosclerosis in the carotid arteries (presence of carotid plaque, IMT, area of the highest plaque and left carotid stenosis ≥ 70%). This association also extends to cases of “acute culprit” carotid stenosis. However, in “acute culprit” carotid stenosis the link is even higher with ipsilateral peri-carotid adipose tissue, potentially serving as a marker of acute events.
4.1. Peri-carotid adipose tissue and cardiovascular risk factors
We found that patients with obesity or with hypertension had a higher amount of peri-carotid adipose tissue, estimated by mean EMT (which is in accordance with the literature). Various authors have demonstrated that EMT is associated with major parameters of obesity, including abdominal obesity [
7,
8,
18]. Pacifico et al have highlighted the expansion of peri-carotid adipose tissue in obesity and emphasized the role of visceral obesity in vascular disease at distant site [
8]. Obesity increases the amount of PVAT and causes functional changes, leading to vascular remodeling [
7,
19].
The association between a higher amount of EMT and hypertension is also well-known, and is independent of obesity [
8,
18,
20]. EMT is a measure of carotid PVAT, which produces molecules that modulate the vascular tone [
21].
We also found that greater mean EMT was correlated with aging and with a larger number of cardiovascular risk factors, in accordance with previous reports [
7,
18,
20,
22]. However, contrary to all studies, we did not find any association between EMT and diabetes or between EMT and lipid profile [
7,
18]. These differences can be explained by the fact that the studies analyzed different populations [
7,
18]. We studied patients from the Vascular Surgery department, while Haberka et al. studied patients from the Cardiology department. The physiopathologic explanation for the possible association between diabetes and an increase in EMT (a measure of PVAT) is that an increase in PVAT causes microvascular dysfunction in muscle and consequently insulin resistance [
23].
Remarkably, our study revealed that patients on fibrates had lower mean EMT, which had not been previously described. This finding aligns with documented evidence indicating that fibrates inhibit adipocyte hypertrophy, offering a potential explanation for our results [
24]. Further studies are needed to support this novel finding that suggests a potential protective effect of fibrates on diseases with abnormally increased EMT. Additionally, literature supports that statins can limit the adventitial neovascularization and EMT progression [
22,
25]. In our study, however, we observed no effect of statins on mean EMT.
4.2. Peri-carotid adipose tissue and chronic atherosclerosis at the carotid bifurcation
We found that the total amount of peri-carotid adipose tissue, estimated with mean EMT was associated with the presence of carotid plaques and with IMT. Haberka et al also observed a significant, albeit weak association, between EMT and IMT, suggesting that both indexes may share underlying factors, with EMT being more related to cardiometabolic risk [
7].
Patients with left carotid stenosis ≥ 70% had a statistically significantly higher mean EMT, with a large effect size. While consistent with the literature, our study is unique in separately analyzing stenosis at the right and left bifurcation [
7,
11]. We identified only one study that also analyzed the association between EMT and carotid stenosis, which included a population scheduled for elective coronary angiography [
11]. That study demonstrated that mean EMT was a strong and independent predictor of the severity of internal carotid stenosis, surpassing the predictive power of other cardiovascular risk factors [
11]. The authors suggested that mean EMT may be a systemic marker of atherosclerosis [
11]. While the literature analyzing the peri-carotid adipose tissue is still scarce, evidence on the peri-coronary adipose tissue is more elucidative. Several studies have demonstrated that the volume of peri-coronary epicardial adipose tissue was significantly associated with the extent and severity of coronary atherosclerosis [
26,
27].
No previous study has analysed the relationship between the ipsilateral/contralateral EMT and the atherosclerotic characteristics at the carotid bifurcation, limiting our ability to discuss our results. It seems that the peri-carotid adipose tissue, estimated with EMT, may not have a direct local ipsilateral effect in a chronic atherosclerotic lesion at the carotid bifurcation. This aligns with Haberka et al.’s argument that EMT, which is measured in the distal segment of the common carotid artery, may be a systemic marker of atherosclerotic and not have an ipsilateral action in the carotid bulb/proximal internal carotid artery [
11].
4.3. The particular case of “Acute culprit” carotid stenosis
We observed a higher amount of mean EMT and also ipsilateral EMT to an “acute culprit” carotid stenosis at the left and right carotid bifurcations (when compared to patients with non-acute carotid stenosis or to the contra-lateral side). Six months after the neurologic event, the amount of EMT ipsilateral to an “acute culprit” carotid stenosis decreased, which suggests a resolution mechanism following the acute phase.
To our knowledge, this is the first time that these results are described with Doppler ultrasound. Baradan et al. demonstrated an increase in density of the peri-carotid adipose tissue surrounding stenotic internal carotid arteries in patients with a history of ipsilateral stroke or transitory ischemic attack, using CT scans [
28]. Higher fat density on CT scan was associated with histopathologic markers of inflammation [
10,
28]. The authors suggested that the inflammation associated with “culprit” carotid plaques extends beyond the vessel lumen [
28].
As previously mentioned, a larger number of publications is found on coronary disease, with Balcer et al. reporting a higher volume of peri-coronary adipose tissue surrounding a culprit coronary stenosis [
29]. Inflammatory changes in the fat surrounding coronary arteries have been associated with coronary artery disease and with high-risk, rupture-prone “culprit” plaques [
28]. Culprit lesions in patients with acute coronary syndrome exhibit an increase in inflammatory activity [
30]. Moreover, peri-vascular adipose tissue depots expand through differentiation or in response to the infiltration of inflammatory cells and have a complex bidirectional relationship with the vascular wall [
11]. Thus, we hypothesize that our observation of augmented EMT ipsilateral to an “acute culprit” carotid stenosis could reflect increased inflammation in peri-carotid adipose tissue associated with “acute culprit” carotid plaques. The question of whether the inflammation in PVAT is a cause or consequence of atherosclerosis is remains unknown [
31].
4.4. Peri-carotid adipose tissue as a marker of plaque instability in clinical practice
The interest in identifying features related to carotid plaque instability is growing, along with the investigation of several biomarkers [
32]. Although markers of systemic inflammation, such as C-reactive protein, have been associated with an increased risk of plaque instability, they cannot specifically identify unstable or higher-risk vascular beds [
28]. Peri-carotid adipose tissue can serve as a local biomarker of higher stroke risk, similarly to adipose tissue surrounding the coronary arteries, which has been associated with an increased risk of fatal heart attacks [
9]. Furthermore, cross-sectional imaging techniques can assess peri-carotid tissue inflammation and identify possible "culprit" carotid stenosis [
9,
28]. However, these imaging methods are time-consuming, expensive and less readily available in clinical practice. Ultrasound, in turn, is an attractive, non-invasive method that can be easily performed at the patient’s bedside.
Our results suggest that EMT may be able to help predicting carotid events, aid in clinical risk stratification and monitor the therapeutic effect of anti-atherosclerotic medication. As the EMT ipsilateral to an “acute culprit” carotid stenosis decreased throughout time, reflecting a decrease in inflammatory activity, this may point to the optimal timing for carotid endarterectomy.
4.5. Strengths and Limitations
This paper has several strengths. It is a prospective, longitudinal study that includes 177 patients. It is one of the few investigations analyzing the association between peri-carotid adipose tissue and atherosclerosis at the carotid bifurcation. It conducts a distinct analysis between total and ipsilateral PVAT. To the best of our knowledge it is the first study demonstrating a significant association between increased ipsilateral peri-carotid adipose tissue, estimated with EMT, and "acute culprit" carotid stenosis. This finding is clinically significant as the measurement of peri-carotid adipose tissue using Doppler ultrasound is easily obtainable in daily practice and can have implications on personalized treatment approaches, such as determining the need for carotid endarterectomy in borderline cases. This paper also reports a previously undocumented observation that patients on fibrates had lower EMT, suggesting a potential protective effect of fibrates on carotid diseases, although further research is needed to confirm this finding.
However, this research work as several limitations. The sample under study does not fully represent the entire population followed at our institution but consists of consecutive patients followed by the main author. Only 11 out of the total 177 patients had an "acute culprit" carotid stenosis. A larger sample size from multiple centers could provide more robust and generalizable results. Another limitation is the relatively short follow-up period of six months for "acute culprit" carotid stenosis. Furthermore, this research work is unable to establish a causal relationship between peri-carotid adipose tissue and atherosclerosis at the carotid bifurcation.
5. Conclusions
Peri-carotid adipose tissue estimated with EMT could serve as a marker for atherosclerosis at carotid bifurcation, particularly in cases of “acute culprit” carotid stenosis, that can be easily assessed in clinical practice.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Cardiovascular risk factors and comorbidities of the studied population; Table S2: Atherosclerotic characteristics at the right and left carotid bifurcation: grade of carotid stenosis, type of carotid atherosclerotic plaque and type of plaque surface; Tabel S3: Mean EMT and echogenicity of carotid plaques at the right and left carotid bifurcation and Table S4: Ipsilateral EMT at the right and left sides and echogenicity of the right and left carotid plaques.
Author Contributions
Conceptualization, J.F.; Methodology, J.F. and P.C.; Validation, M.C-N., A.L-F., A.M. and P.C.; Formal analysis, J.F., A.L-F., A.D. and P.C.; Investigation, J.F., A.L-F., A.D. and P.C.; Writing—original draft preparation, J.F. and A.D..; Writing—review and editing, M.C-N., A.M. and P.C.; Supervision, A.M. and P.C.
Funding
This work was supported by the Portuguese Society of Vascular Surgery. This work was developed under the scope of project NORTE-01-0145-FEDER- 000013, supported by the Northern Portugal Regional Operational Programme (NORTE 2020) under the Portugal Partnership Agreement, through the European Regional Development Fund (FEDER), and by National funds, through the Foundation for Science and Technology (FCT) - project UIDB/50026/2020 and UIDP/50026/2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (J.F.).
Acknowledgments
We thank Rita Alonso, for her support in the production of this article, regarding her help in organizing and collecting information.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naylor R, Rantner B, Ancetti S, de Borst GJ, De Carlo M, Halliday A, Kakkos SK, Markus HS, McCabe DJH, Sillesen H, van den Berg JC, Vega de Ceniga M, Venermo MA, Vermassen FEG, Esvs Guidelines Committee, Antoniou GA, Bastos Goncalves F, Bjorck M, Chakfe N, Coscas R, Dias NV, Dick F, Hinchliffe RJ, Kolh P, Koncar IB, Lindholt JS, Mees BME, Resch TA, Trimarchi S, Tulamo R, Twine CP, Wanhainen A, Document Reviewers, Bellmunt-Montoya S, Bulbulia R, Darling RC 3rd, Eckstein HH, Giannoukas A, Koelemay MJW, Lindström D, Schermerhorn M, Stone DH. Editor's Choice - European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur J Vasc Endovasc Surg. 2023 Jan;65(1):7-111. Epub 2022 May 20. [CrossRef] [PubMed]
- Zhang Y, Cao J, Zhou J, Zhang C, Li Q, Chen S, Feinstein S, Grayburn PA, Huang P. Plaque Elasticity and Intraplaque Neovascularisation on Carotid Artery Ultrasound: A Comparative Histological Study. Eur J Vasc Endovasc Surg. 2021 Sep;62(3):358-366. [CrossRef] [PubMed]
- Paraskevas KI, Veith FJ, Spence JD. How to identify which patients with asymptomatic carotid stenosis could benefit from endarterectomy or stenting. Stroke Vasc Neurol. 2018 Feb 24;3(2):92-100. PMCID: PMC6047337. [CrossRef] [PubMed]
- Zhang S, Yu X, Gu H, Kang B, Guo N, Wang X. Identification of high-risk carotid plaque by using carotid perivascular fat density on computed tomography angiography. Eur J Radiol. 2022 May;150:110269. Epub 2022 Mar 18. [CrossRef] [PubMed]
- Sugiyama T, Kanaji Y, Hoshino M, Yamaguchi M, Hada M, Ohya H, Sumino Y, Hirano H, Kanno Y, Horie T, Misawa T, Nogami K, Ueno H, Hamaya R, Usui E, Murai T, Lee T, Yonetsu T, Sasano T, Kakuta T. Determinants of Pericoronary Adipose Tissue Attenuation on Computed Tomography Angiography in Coronary Artery Disease. J Am Heart Assoc. 2020 Aug 4;9(15):e016202. Epub 2020 Jul 30. PMCID: PMC7792233. [CrossRef] [PubMed]
- Honold S, Wildauer M, Beyer C, Feuchtner G, Senoner T, Jaschke W, Gizewski E, Bauer A, Friedrich G, Stühlinger M, Plank F. Reciprocal communication of pericoronary adipose tissue and coronary atherogenesis. Eur J Radiol. 2021 Mar;136:109531. Epub 2021 Jan 11. [CrossRef] [PubMed]
- Haberka M, Gąsior Z. Carotid extra-media thickness in obesity and metabolic syndrome: a novel index of perivascular adipose tissue: extra-media thickness in obesity and metabolic syndrome. Atherosclerosis. 2015 Mar;239(1):169-77. Epub 2015 Jan 14. [CrossRef] [PubMed]
- Pacifico L, Perla FM, Tromba L, Carbotta G, Lavorato M, Pierimarchi P, Chiesa C. Carotid Extra-Media Thickness in Children: Relationships With Cardiometabolic Risk Factors and Endothelial Function. Front Endocrinol (Lausanne). 2020 Sep 24;11:574216. PMCID: PMC7541844. [CrossRef] [PubMed]
- Zhang DH, Jin JL, Zhu CF, Chen QY, He XW. Association between carotid artery perivascular fat density and cerebral small vessel disease. Aging (Albany NY). 2021 Jul 21;13(14):18839-18851. Epub 2021 Jul 21. PMCID: PMC8351687. [CrossRef] [PubMed]
- Liu Y, Xu L, Gu Y, Zhang Y, Miao C. Impact of H-Type Hypertension on Pericarotid Adipose Tissue and Plaque Characteristics Based on Computed Tomography (CT) Angiography: A Propensity Score Matching Study. Med Sci Monit. 2021 Dec 3;27:e933351. PMCID: PMC8650409. [CrossRef] [PubMed]
- Haberka M, Skilton M, Biedroń M, Szóstak-Janiak K, Partyka M, Matla M, Gąsior Z. Obesity, visceral adiposity and carotid atherosclerosis. J Diabetes Complications. 2019 Apr;33(4):302-306. Epub 2019 Jan 17. [CrossRef] [PubMed]
- Ferreira J, Carneiro A, Vila I, Silva C, Cunha C, Longatto-Filho A, Mesquita A, Cotter J, Mansilha A, Correia-Neves M, Cunha P. Inflammation and Loss of Skeletal Muscle Mass in Chronic Limb Threatening Ischemia. Ann Vasc Surg. 2023 Jan;88:164-173. Epub 2022 Aug 2. [CrossRef] [PubMed]
- Nishida C, Ko GT, Kumanyika S. Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. Eur J Clin Nutr. 2010 Jan;64(1):2-5. Epub 2009 Nov 25. [CrossRef] [PubMed]
- Ohara T, Toyoda K, Otsubo R, Nagatsuka K, Kubota Y, Yasaka M, Naritomi H, Minematsu K. Eccentric stenosis of the carotid artery associated with ipsilateral cerebrovascular events. AJNR Am J Neuroradiol. 2008 Jun;29(6):1200-3. Epub 2008 Mar 13. PMCID: PMC8118845. [CrossRef] [PubMed]
- Campos AM, Moura FA, Santos SN, Freitas WM, Sposito AC; Brasilia Study on Healthy Aging and Brasilia Heart Study. Sarcopenia, but not excess weight or increased caloric intake, is associated with coronary subclinical atherosclerosis in the very elderly. Atherosclerosis. 2017 Mar;258:138-144. Epub 2017 Jan 18. [CrossRef] [PubMed]
- Lee, W. General principles of carotid Doppler ultrasonography. Ultrasonography. 2014 Jan;33(1):11-7. Epub 2013 Dec 11. PMCID: PMC4058969. [CrossRef] [PubMed]
- Geroulakos G, Ramaswami G, Nicolaides A, James K, Labropoulos N, Belcaro G, Holloway M. Characterization of symptomatic and asymptomatic carotid plaques using high-resolution real-time ultrasonography. Br J Surg. 1993 Oct;80(10):1274-7. [CrossRef] [PubMed]
- Skilton, M. R. , Sérusclat, A., Sethu, A. H. A. U., Brun, S., Bernard, S., Balkau, B., Moulin, P., & Bonnet, F. (2009). Noninvasive Measurement of Carotid Extra-Media Thickness. JACC: Cardiovascular Imaging, 2(2), 176–182. [CrossRef]
- Costa RM, Neves KB, Tostes RC, Lobato NS. Perivascular Adipose Tissue as a Relevant Fat Depot for Cardiovascular Risk in Obesity. Front Physiol. 2018 Mar 21;9:253. PMCID: PMC5871983. [CrossRef] [PubMed]
- Carlini NA, Harber MP, Fleenor BS. Age-related carotid extra-media thickening is associated with increased blood pressure and arterial stiffness. Clin Physiol Funct Imaging. 2021 Sep;41(5):461-466. Epub 2021 Jun 12. [CrossRef] [PubMed]
- Hu H, Garcia-Barrio M, Jiang ZS, Chen YE, Chang L. Roles of Perivascular Adipose Tissue in Hypertension and Atherosclerosis. Antioxid Redox Signal. 2021 Mar 20;34(9):736-749. Epub 2020 Jun 2. PMCID: PMC7910418. [CrossRef] [PubMed]
- Choi, H. L., Au, J. S., & MacDonald, M. J. Carotid extra-media thickness increases with age, but is not related to arterial stiffness in adults. Artery Research. 2017; 21(C), 13. [CrossRef]
- Houben AJ, Eringa EC, Jonk AM, Serne EH, Smulders YM, Stehouwer CD. Perivascular Fat and the Microcirculation: Relevance to Insulin Resistance, Diabetes, and Cardiovascular Disease. Curr Cardiovasc Risk Rep. 2012 Feb;6(1):80-90. Epub 2011 Nov 22. PMCID: PMC3251783. [CrossRef] [PubMed]
- Jeong S, Yoon M. Fenofibrate inhibits adipocyte hypertrophy and insulin resistance by activating adipose PPARalpha in high fat diet-induced obese mice. Exp Mol Med. 2009 Jun 30;41(6):397-405. PMCID: PMC2705860. [CrossRef] [PubMed]
- Raggi P, Gadiyaram V, Zhang C, Chen Z, Lopaschuk G, Stillman AE. Statins Reduce Epicardial Adipose Tissue Attenuation Independent of Lipid Lowering: A Potential Pleiotropic Effect. J Am Heart Assoc. 2019 Jun 18;8(12):e013104. Epub 2019 Jun 13. PMCID: PMC6645620. [CrossRef] [PubMed]
- Hassan M, Said K, Rizk H, ElMogy F, Donya M, Houseni M, Yacoub M. Segmental peri-coronary epicardial adipose tissue volume and coronary plaque characteristics. Eur Heart J Cardiovasc Imaging. 2016 Oct;17(10):1169-77. Epub 2015 Nov 20. [CrossRef] [PubMed]
- Moradi, M., Talebi, V. Evaluation of epicardial adipose tissue by coronary multi-detector computed tomography: an independent predictor of obstructive coronary artery disease. Egypt J Radiol Nucl Med 54, 68 (2023). [CrossRef]
- H. Baradaran et al., “Association Between Carotid Artery Perivascular Fat Density and Cerebrovascular Ischemic Events,” J. Am. Heart Assoc., vol. 7, no. 24, Dec. 2018. [CrossRef]
- Balcer B, Dykun I, Schlosser T, Forsting M, Rassaf T, Mahabadi AA. Pericoronary fat volume but not attenuation differentiates culprit lesions in patients with myocardial infarction. Atherosclerosis. 2018 Sep;276:182-188. Epub 2018 May 25. [CrossRef] [PubMed]
- Kuneman JH, van Rosendael SE, van der Bijl P, van Rosendael AR, Kitslaar PH, Reiber JHC, Jukema JW, Leon MB, Ajmone Marsan N, Knuuti J, Bax JJ. Pericoronary Adipose Tissue Attenuation in Patients With Acute Coronary Syndrome Versus Stable Coronary Artery Disease. Circ Cardiovasc Imaging. 2023 Feb;16(2):e014672. Epub 2023 Feb 3. PMCID: PMC9946175. [CrossRef] [PubMed]
- Farias-Itao DS, Pasqualucci CA, Nishizawa A, da Silva LFF, Campos FM, Bittencourt MS, et al. B Lymphocytes and Macrophages in the Perivascular Adipose Tissue Are Associated With Coronary Atherosclerosis: An Autopsy Study. J Am Heart Assoc 2019;8. [CrossRef]
- Saba L, Zucca S, Gupta A, Micheletti G, Suri JS, Balestrieri A, Porcu M, Crivelli P, Lanzino G, Qi Y, Nardi V, Faa G, Montisci R. Perivascular Fat Density and Contrast Plaque Enhancement: Does a Correlation Exist? AJNR Am J Neuroradiol. 2020 Aug;41(8):1460-1465. Epub 2020 Jul 30. PMCID: PMC7658869. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).