Submitted:
19 December 2023
Posted:
20 December 2023
You are already at the latest version
Abstract
Keywords:
Introduction
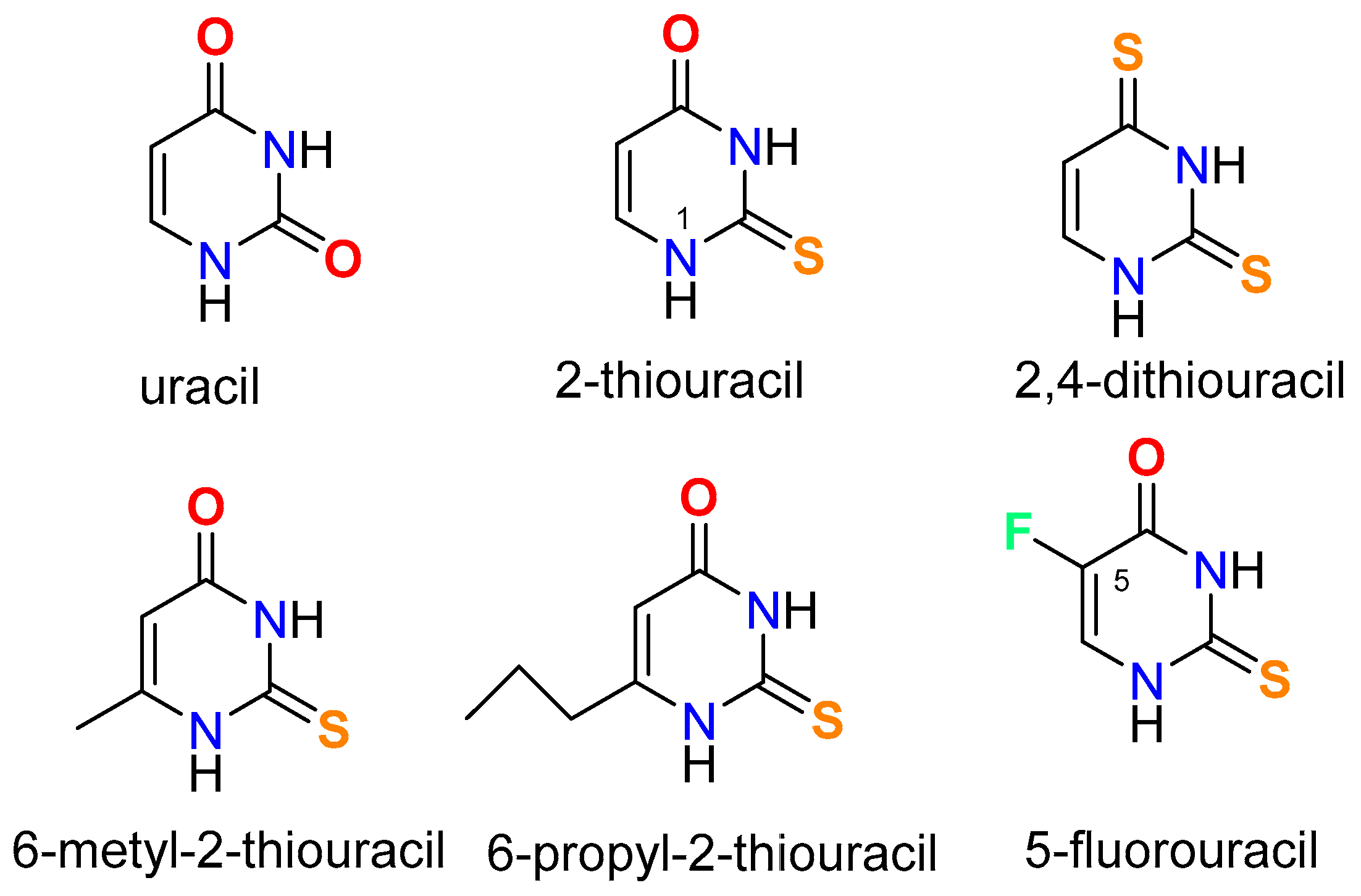
Synthesis of metal complexes with uracil and its derivatives
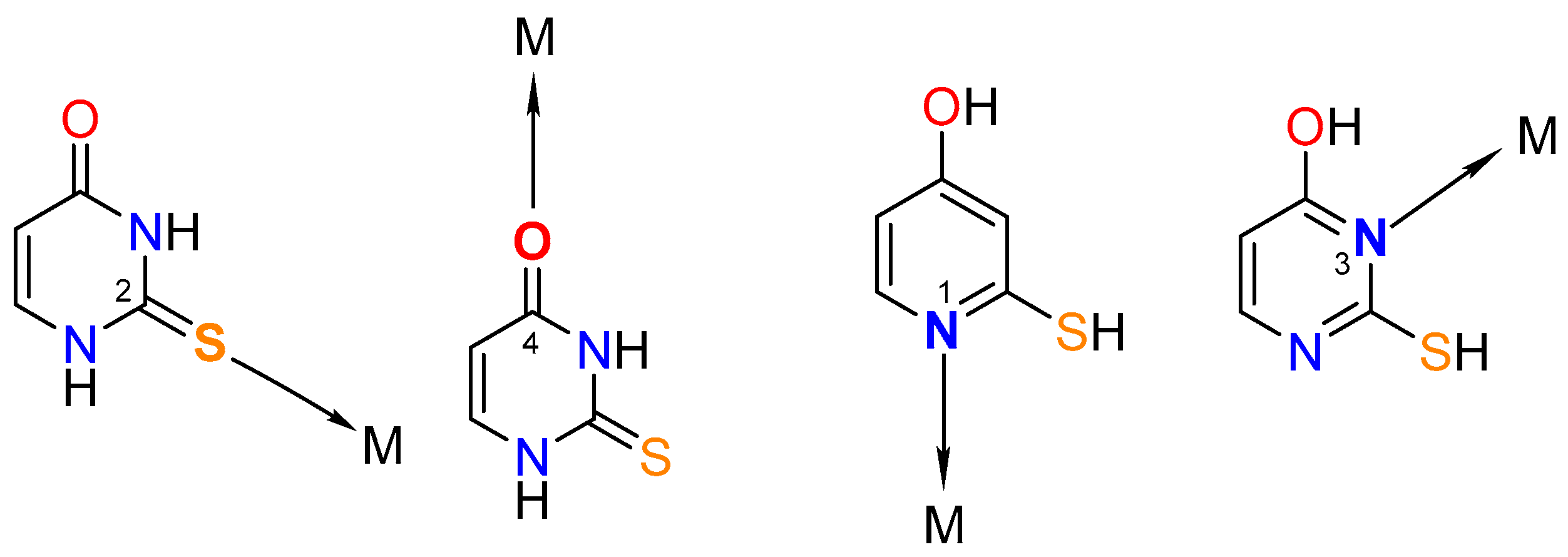
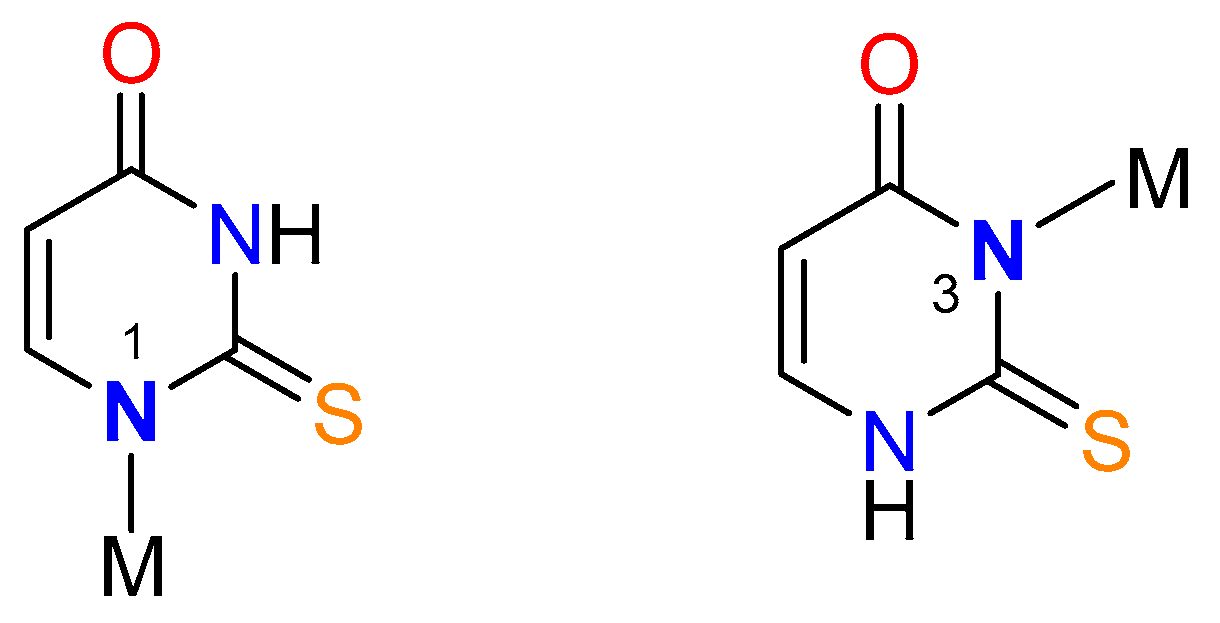
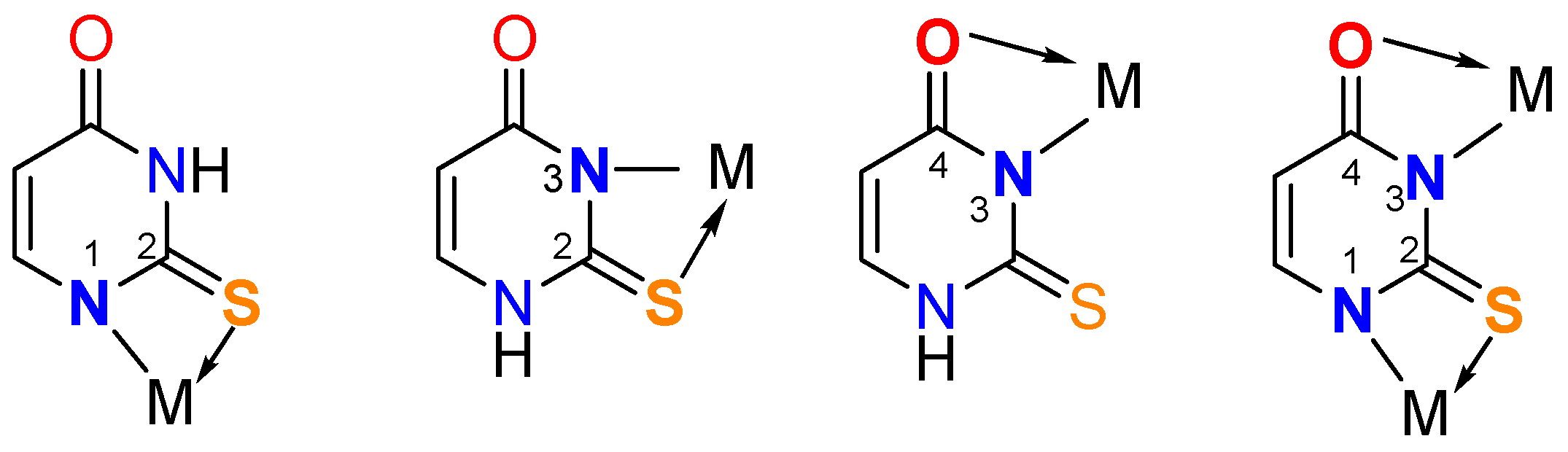
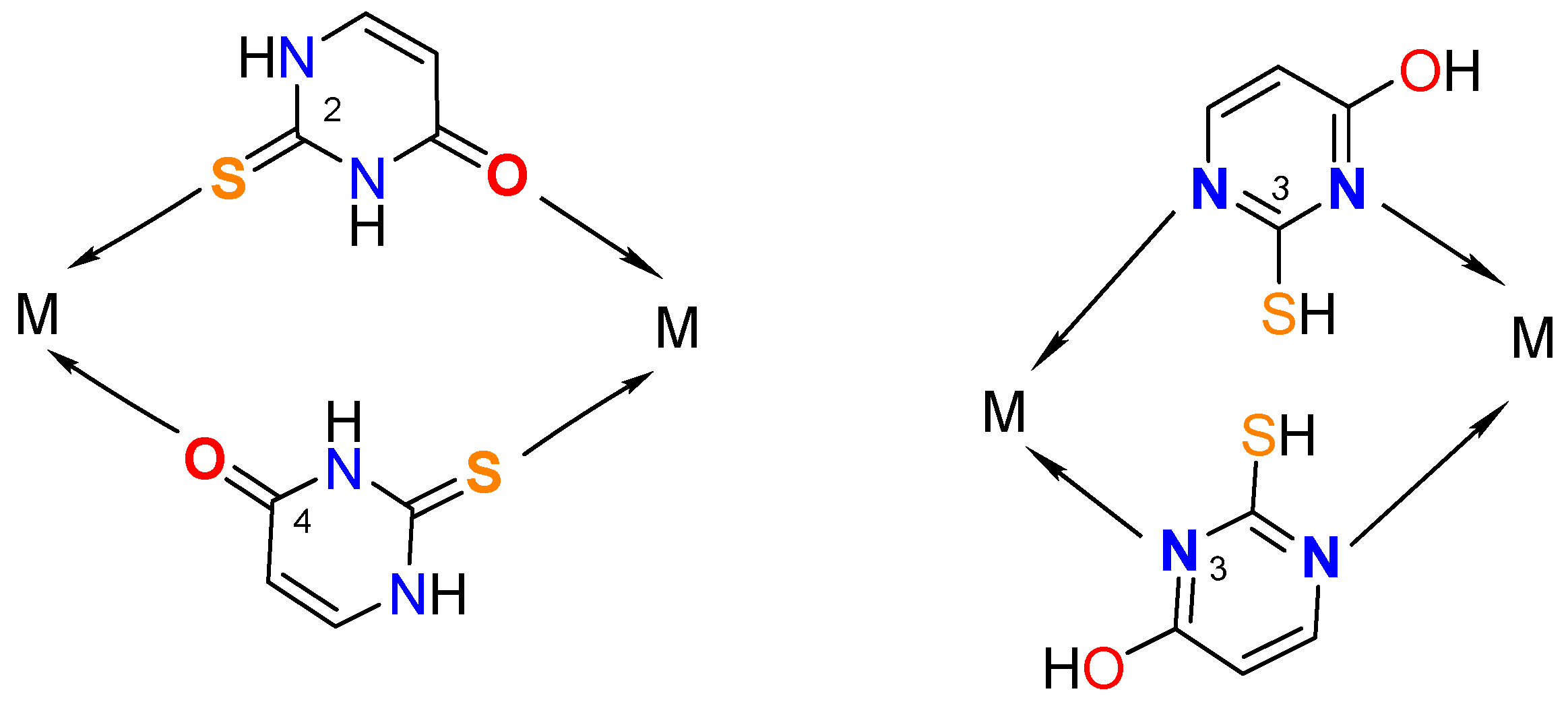
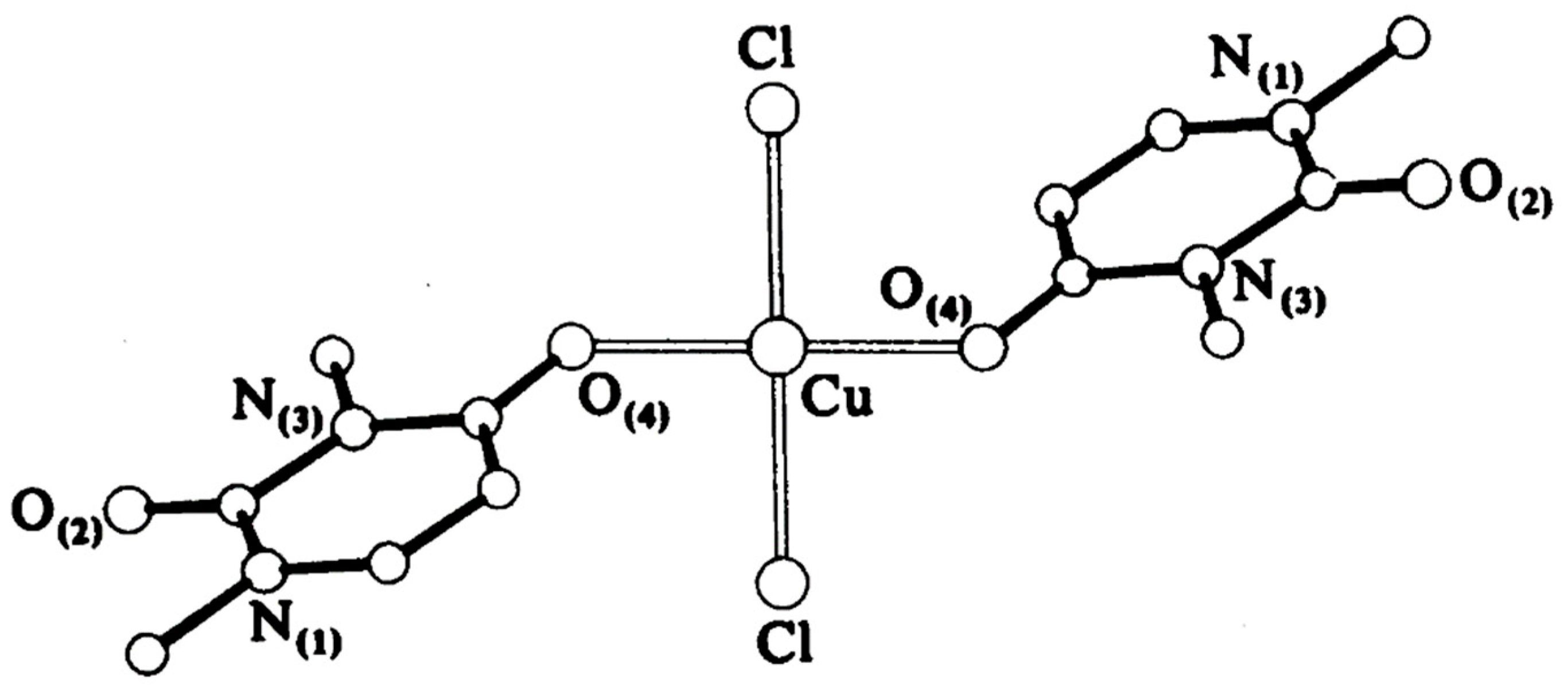
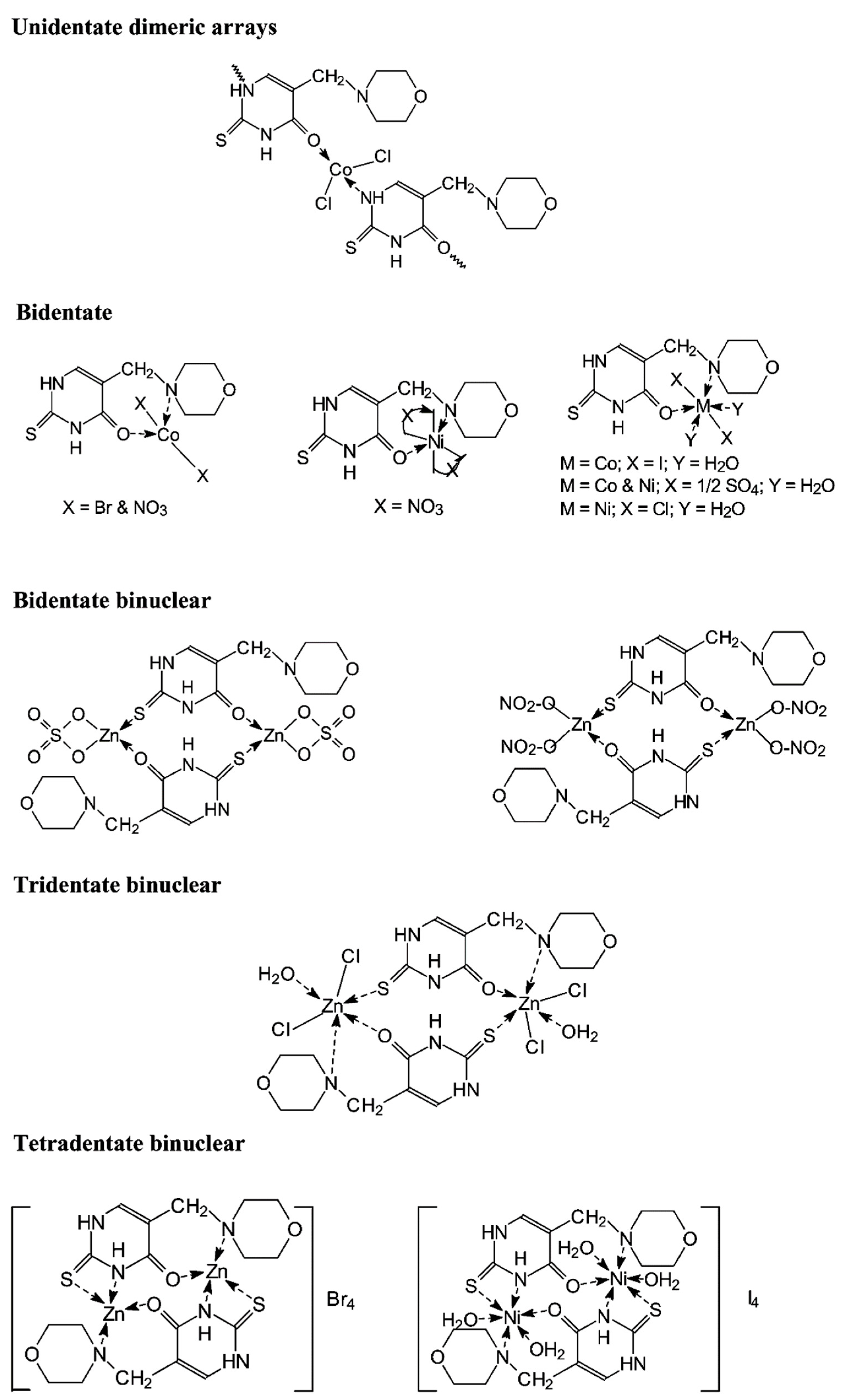
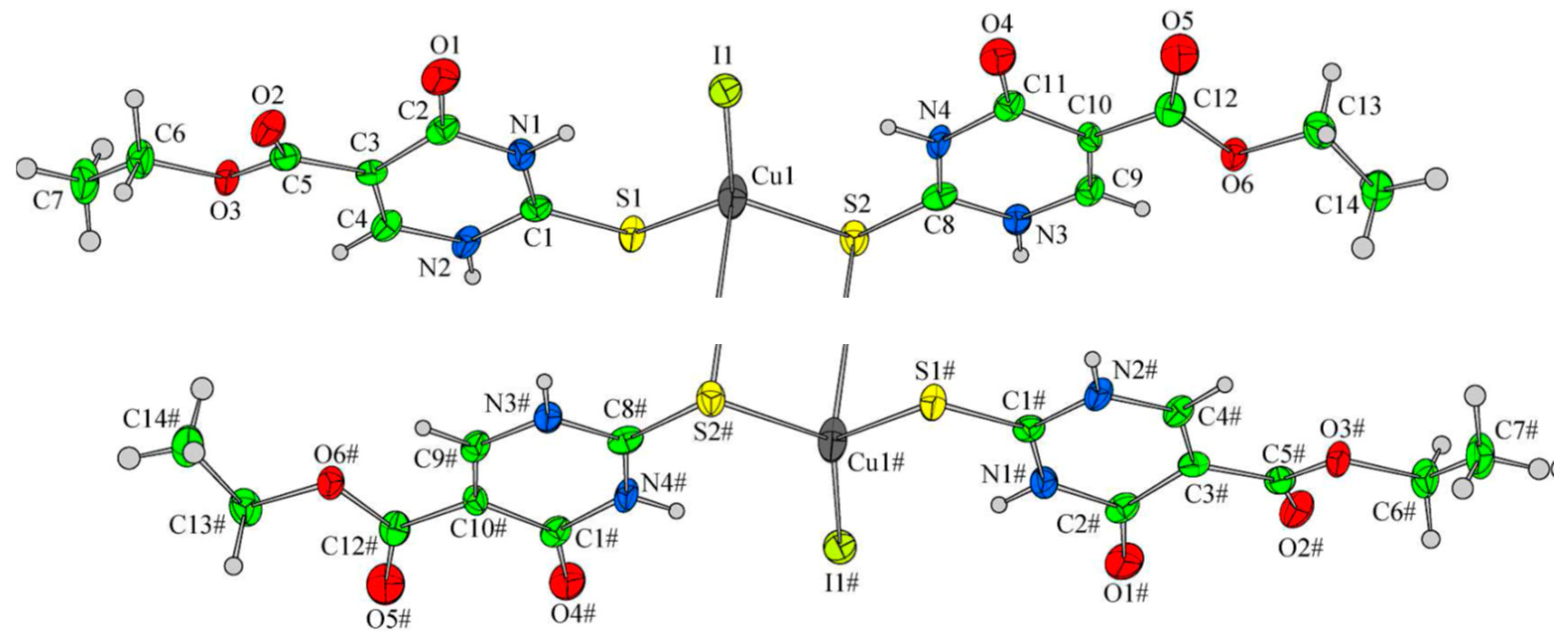
Synthesis of metal complexes with 2-thiouracil and its derivatives
Biological Activities
Antibacterial Activity
Antifungi Activity
Antitumor and Cytotoxic activities
Conclusions
Funding
Conflicts of Interest
References
- Garrett, R. H.; Grisham, C. M. Principles of Biochemistry with a Human Focus. Brooks/Cole Thomson Learning: USA, Pacific Grove, CA, 2001, 939, ISBN 0-03-097369-4.
- Astwood, E.B. The chemical nature of compounds which inhibit the function of the thyroid gland. J. Pharmacol. Exp. Ther. 1943, 78(1), 79–89. [Google Scholar]
- Mao, X.-M.; Li, H.-Q.; Li, Q.; Li, D.-M.; Xie, X.-J.; Yin, G.-P.; Zhang, P.; Xu, X.-H.; Wu, J.-D.; Chen, S.-W.; Wang, S.-K. Prevention of Relapse of Graves’ Disease by Treatment with an Intrathyroid Injection of Dexamethasone. J. Clin. Endocrinol. Metab. 2009; 94, 12, 4984–4991. [Google Scholar] [CrossRef]
- Rosenfeld, H.; Ornoy, A.; Shechtman, S.; Diav-Citrin, O. Pregnancy outcome, thyroid dysfunction, and fetal goiter after in utero exposure to propylthiouracil: a controlled cohort study. B. J. Clin. Pharmacol. 2009, 68(4), 609–617. [Google Scholar] [CrossRef]
- Cooper, D. S. Antithyroid Drugs. N. Eng. J. Med. 2005, 352, 905–917. [Google Scholar] [CrossRef]
- Volpé, R. The Immunomodulatory Effects of Anti-thyroid Drugs are Mediated via Actions on Thyroid Cells, Affecting Thyrocyte-immunocyte Signalling: A Review. Curr. Pharm. Des. 2001, 7, 451–460. [Google Scholar] [CrossRef]
- Burch, H. B.; Cooper, D.S. Antithyroid drug therapy: 70 years later. Eur. J. Endocrinol. 2018, 179(5), R261–R274. [Google Scholar] [CrossRef]
- Fernandez, M.G. Hyperthyroidism and pregnancy. Endocrinol Nutr. 2013, 60(9), 535–543. [Google Scholar] [CrossRef]
- Oladipo, M.A.; Isola, K.T. Coordination Possibility of Uracil and Applications of Some of Its Complexes: A Review. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 386–394. [Google Scholar] [CrossRef]
- Masoud, M. S.; Ramadana, M. Sh.; Ramadana, A. M.; Al-Saify, M. H. Complexing Properties and Applications of Some Biologically Active Nucleic Acid Constituents. Int. J. Innov. Res. Technol. Sci. Eng. 2020, 6(6), 23–39, https://ijisset.org/storage/Volume6/Issue6/IJISSET-060524.pdf. [Google Scholar]
- Narang, K.K.; Singh, V.P; Bhattacharya, D. Synthesis, characterization and antitumor activity of uracil and uracil–histidine complexes with metal(III) ions. Trans. Metal. Chem. 1997, 22, 333–337. [Google Scholar] [CrossRef]
- Cartwright, B. A.; Goodgame, M.; Johns, K. W.; Skapski, A. C. Strong Metal-Oxygen Interaction in Uracils. X-ray crystal structure of bis-(1,3-dimethyluracil)dichlorocopper(II). Biochem J. 1978, 175, 337–339. [Google Scholar] [CrossRef]
- Masoud, M. S.; Ibrahim, A. A.; Khalil, E. A.; El-Marghany, A. Spectral properties of some metal complexes derived from uracil–thiouracil and citrazinic acid compounds. Spectrochimica Acta Part A 2007, 67, 662–668. [Google Scholar] [CrossRef]
- Ghosh, P.; Mukhopadhyay, T. K.; Sarkar A., R. Interaction of Divalent Metal Ions with Uracil III. Complexes of MnII, FeII, CoII, NiII and Cull with Uracil Acting as Bidentate Ligand. Trans. Metal Chem. 1984, 9, 46–48. [Google Scholar] [CrossRef]
- Koz, G.; Kaya, H.; Astley, D.; Yaşa, İ.; Astley, S. T. Synthesis, Characterization and Antimicrobial Screening of Ni(II), Cu(II) and Co(II) Complexes of Some Schiff Base Ligands Derived from 5-Aminouracil. Gazi University J. Sci. 2011, 24(3), 407–413, https://hdl.handle.net/11454/19239. [Google Scholar]
- Kufelnicki, A.; Jaszczak, J.; Kalinowska-Lis, U.; Wardak, C.; Ochocki, J. Complexes of Uracil (2,4-Dihydroxypyrimidine) Derivatives Part III. pH-Metric, ISE, and Spectrophotometric Studies on Co(II), Ni(II), and Zn(II) Complexes. J. Solution Chem. 2006, 35(5), 739–751. [Google Scholar] [CrossRef]
- Tyagi, S.; Singh, S. M.; Gencaslan, S.; Sheldrick, W. S.; Singh, U. P. Metal-5-fluorouracil-histamine complexes: solution, structural, and antitumor studies. Metal Based Drugs 2002, 8(6), 337–345. [Google Scholar] [CrossRef]
- Abdullah, A. A. Synthesis, structural studies of some nucleic acids metal complexes. Basrah J. Sci 2006, 24(1), 115–128, https://iasj.net/iasj/download/22c868262f2d5d19. [Google Scholar]
- Verma, S.; Shrivastva, S.; Rani, P. Synthesis and spectroscopic studies of mixed ligand complexes of transition and inner transition metals with a substituted benzimidazole derivative and RNA bases. J. Chem. Pharm. Res 2012, 4(1), 693–699, https://www.jocpr.com/articles/synthesis-and-spectroscopic-studies-of-mixed-ligand-complexes-of-transition-andinner-transition-metals-with-a-substitute.pdf. [Google Scholar]
- Shobana, S.; Dharmaraja, J.; Kamatchi, P.; Selvaraj, S. Mixed ligand complexes of Cu (II) / Ni (II) / Zn (II) ions with 5-Fluorouracil (5-FU) in the presence of some amino acid moieties: Structural and antimicrobial studies. J. Chem. Pharm. Res 2012, 4(12), 4995–5004, https://www.jocpr.com/articles/mixed-ligand-complexes-of-cu-ii--ni-ii--zn-ii-ions-with-5fluorouracil-5fuin-the-presence-of-some-amino-acid-moieties-str.pdf. [Google Scholar]
- Gupta, M.; Srivastava, M. N. Synthesis and characterization of complexes of copper(II), nickel(II), cobalt(II) and zinc(II) with alanine and uracil or 2-thiouracil. Synth. React. Inorg. Met.-Org. Chem. 1996, 26(2), 305–320. [Google Scholar] [CrossRef]
- Gupta, M.; Srivastava, M. N. Synthesis and characterization of mixed ligand complexes of copper(II), nickel(II), cobalt(II and zinc(II) with glycine and uracil or 2-thiouracil. Polyhedron 1985, 4, 475–479. [Google Scholar] [CrossRef]
- Gupta, M.; Srivastava, M. N. Bull Chem. Soc. Fr. 1991, 128, 859.
- Gupta, M.; Srivastava, M. N. Bull. Pol. Acad. Sci. 1992, 40, 1.
- Saxena, V. K.; Srivastava, M. N. PMR Spectral Studies of ktixed-Ligand Amino Acid Chelates of Cobalt(II), Nickel(II), Copper(II) and Zinc(II) with Nitrilotriacetic Acid and glycine, α-alanine, Valine, or Leucine. J.Inorg.Bio-Chem 1990, 38, 37. [Google Scholar] [CrossRef]
- Marinova, P.; Tsoneva, S.; Frenkeva, M.; Blazheva, D.; Slavchev, A.; Penchev, P. New Cu(II), Pd(II) and Au(III) complexes with 2-thiouracil: Synthesis, Characteration and Antibacterial Studies, Russ. J. Gen. Chem. 2022, 92(8), 1578–1584. [Google Scholar] [CrossRef]
- Marinova, P.; Hristov, M.; Tsoneva, S.; Burdzhiev, N.; Blazheva, D.; Slavchev, A.; Varbanova, E.; Penchev, P. Synthesis, Characterization and Antibacterial Studies of new Cu(II) and Pd(II) complexes with 6-methyl-2-thiouracil and 6-propyl-2-thiouracil, Appl. Sci. 2023, 13(24), 13150. [Google Scholar] [CrossRef]
- Moreno-Carretero, M.N.; Romero-Molina, M. A.; Salas-Peregrin, J. M.; Sanchez-Sanchez, M.P. Thermal analysis applied to the study of metal complexes: thermal behaviour of 6-amino-2-thiouracil and its complexes with several transition metal ions. Thermochim. Acta 1992, 200, 271–280. [Google Scholar] [CrossRef]
- Romero, M. A.; Sanchez, M. P.; Quiros, M.; Sanchez, F.; Salas, J. M.; Moreno, M.; Faure, R. Transition metal complexes of 6-amino 2-thiouracil; crystal structure of bis(6-amino-2-thiouracilato)aquazinc(II) dihydrate. Can. J. Chem. 1993, 71, 29–33. [Google Scholar] [CrossRef]
- Khullar, I. P; Agarwala, U. ; 2-Mercaptopyrimidin-4-ol (2-Thiouracil) Complexes of Copper(II), Nickel(II), Cobalt(II) and Iron(III). Aust. J. Chem. 1974, 27, 1877–1883. [Google Scholar] [CrossRef]
- Garrett, E. R.; Weber, D. J. Metal Complexes of Thiouracils II: Solubility Analyses and Spectrophotometric Investigations. J. Pharma. Sci. 1971, 60(6), 845–853. [Google Scholar] [CrossRef]
- Kamalakannan, P.; Venkappayya, D.; Balasubramanian, T. A new antimetabolite, 5-morpholinomethyl-2-thiouracil—spectral properties, thermal profiles, antibacterial, antifungal and antitumour studies of some of its metal chelates. J. Chem. Soc. Dalton Trans. 2002, 3381–3391. [Google Scholar] [CrossRef]
- Darensbourg, D. J.; Frost, B. J.; Derecskei-Kovacs, A.; Reibenspies, J. H. Coordination Chemistry, Structure, and Reactivity of Thiouracil Derivatives of Tungsten(0) Hexacarbonyl: A Theoretical and Experimental Investigation into the Chelation/Dechelation of Thiouracil via CO Loss and Addition. Inorg. Chem. 1999, 38(21), 4715–4723. [Google Scholar] [CrossRef]
- Masoud, M. S.; Soayed, A. A.; El-Husseiny A., F. Coordination modes, spectral, thermal and biological evaluation of hetero-metal copper containing 2-thiouracil complexes. Spectrochim. Acta Part A: Molecular and Biomolecular Spectroscopy 2012, 99, 365–372. [Google Scholar] [CrossRef]
- Papazoglou, I.; Cox, P.J.; Hatzidimitriou, A.G.; Kokotidou, C.; Choli-Papadopoulou, T.; Aslanidis, P. Copper(I) halide complexes of 5-carbethoxy-2-thiouracil: Synthesis, structure and in vitro cytotoxicity. Eur. J. Med. Chem. 2014, 78, 383–391. [Google Scholar] [CrossRef]
- Kumar, B.; Suman, A. Synthesis, spectroscopic characterization and biological application of copper complex of 5-carbethoxy-2-thiouracil. J. Drug Deliv. Ther. 2020, 10(6), 145–148. [Google Scholar] [CrossRef]
- Kostova, I. General and inorganic chemistry, Softtrade, Sofia. 2016. [Google Scholar]
- Illán-Cabeza, N. A.; García-García, A. R.; Moreno-Carretero, M. N.; Martínez-Martos, J. M.; Ramírez-Expósito M., J. Synthesis, characterization and antiproliferative behavior of tricarbonyl complexes of rhenium(I) with some 6-amino-5-nitrosouracil derivatives: Crystal structure of fac-[ReCl(CO)3(DANU-N5,O4)] (DANU = 6-amino-1,3-dimethyl-5-nitrosouracil). J. Inorg. Biochem. 2005, 99(8), 1637–1645. [Google Scholar] [CrossRef]
- Abou-Melha, K. S. A Series of Nano-sized Metal ion-thiouracil Complexes, tem, Spectral, γ- irradiation, Molecular Modeling and Biological Studies. Orient. J. Chem. 2015, 31(4), 1897–1913. [Google Scholar] [CrossRef]
- Golubyatnikova, L. G.; Khisamutdinov, R. А.; Grabovskii, S. А.; Kabal’nova, N. N.; Murinov, Yu. I. Complexes of Palladium(II) and Platinum(II) with 6-tert-Butyl-2-thiouracil. Russ. J. Gen.Chem 2017, 87(1), 117–121. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Thanikachalam, V.; Jayamoorthy, K.; Perumal, M.V. Computational studies of 1,2-disubstituted benzimidazole derivatives. Spectrochim. Acta (A) 2012, 97, 6. [Google Scholar] [CrossRef]
- Masoud, M.S.; Amira, M.F.; Ramadan, A.M.; El-Ashry, G.M. Synthesis and characterization of some pyrimidine, purine, amino acid and mixed ligand complexes. Spectrochim. Acta Part(A) 2008, 69, 230–238. [Google Scholar] [CrossRef]
- Masoud M., S.; El-Hamid, O. H. A.; Zaki, Z. M. 2-thiouracil-based cobalt(II), nickel(II) and copper(II) complexes. Trans. Met. Chem. 1994, 19(1), 21–24. [Google Scholar] [CrossRef]
- El-Morsy, F.A.; Jean-Claude, B.J.; Butler, I.S.; El-Sayed, S.A.; Mostafa, S.I. Synthesis, characterization and anticancer activity of new zinc(II), molybdate(II), palladium(II), silver(I), rhodium(III), ruthenium(II) and platinum(II) complexes of 5,6-diamino-4-hydroxy2-mercaptopyrimidine. Inorg. Chim. Acta 2014, 423, 144–155. [Google Scholar] [CrossRef]
- Abás, E.; Pena-Martínez, R.; Aguirre-Ramírez, D.; Rodríguez-Diéguez, A.; Laguna, M.; Grasa, L. New selective thiolate gold(I) complexes inhibit the proliferation of different human cancer cells and induce apoptosis in primary cultures of mouse colon tumors. Dalton Trans. 2020, 49(6), 1915–1927. [Google Scholar] [CrossRef]
- Holowczak, M. S.; Stancl, M. D.; Wong, G. B. Trichloro( 1-metbylcytosinato)gold(III). Model for DNA interactions. J. Am. Chem. Soc. 1985, 107, 5789–5790. [Google Scholar] [CrossRef]
- Rodriguez, E. C.; Sánchez, J. R.; López-González, J. D.; Salas-Peregrin, J. M.; Olivier, M. J.; Quirós, M.; Beauchamp, A. L. Thermal Behavior and Crystal Structure of Dichloro[ 6-amino-l,3-dimethyl-5-(2-chlorophenylazo)uracilato]gold(III). Inorg. Chim. Acta 1990, 171, 151–156. [Google Scholar] [CrossRef]
- Singh U. P., Singh S., Singh S. M. Synthesis, characterization and antitumour activity of metal complexes of 5-carboxy-2-thiouracil. Metal-Based Drugs 1998, 5(1), 35–39. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




