Submitted:
19 December 2023
Posted:
20 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
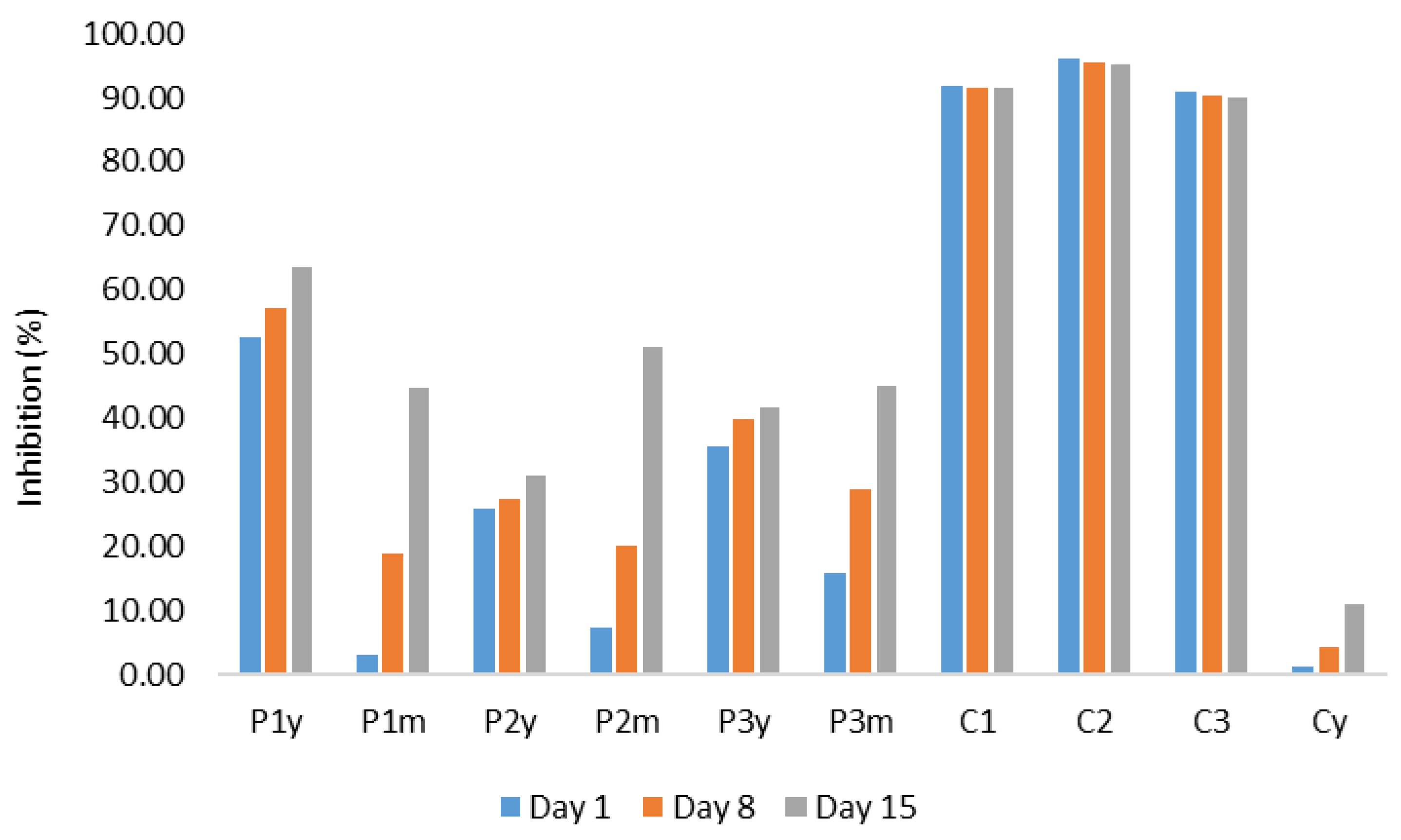
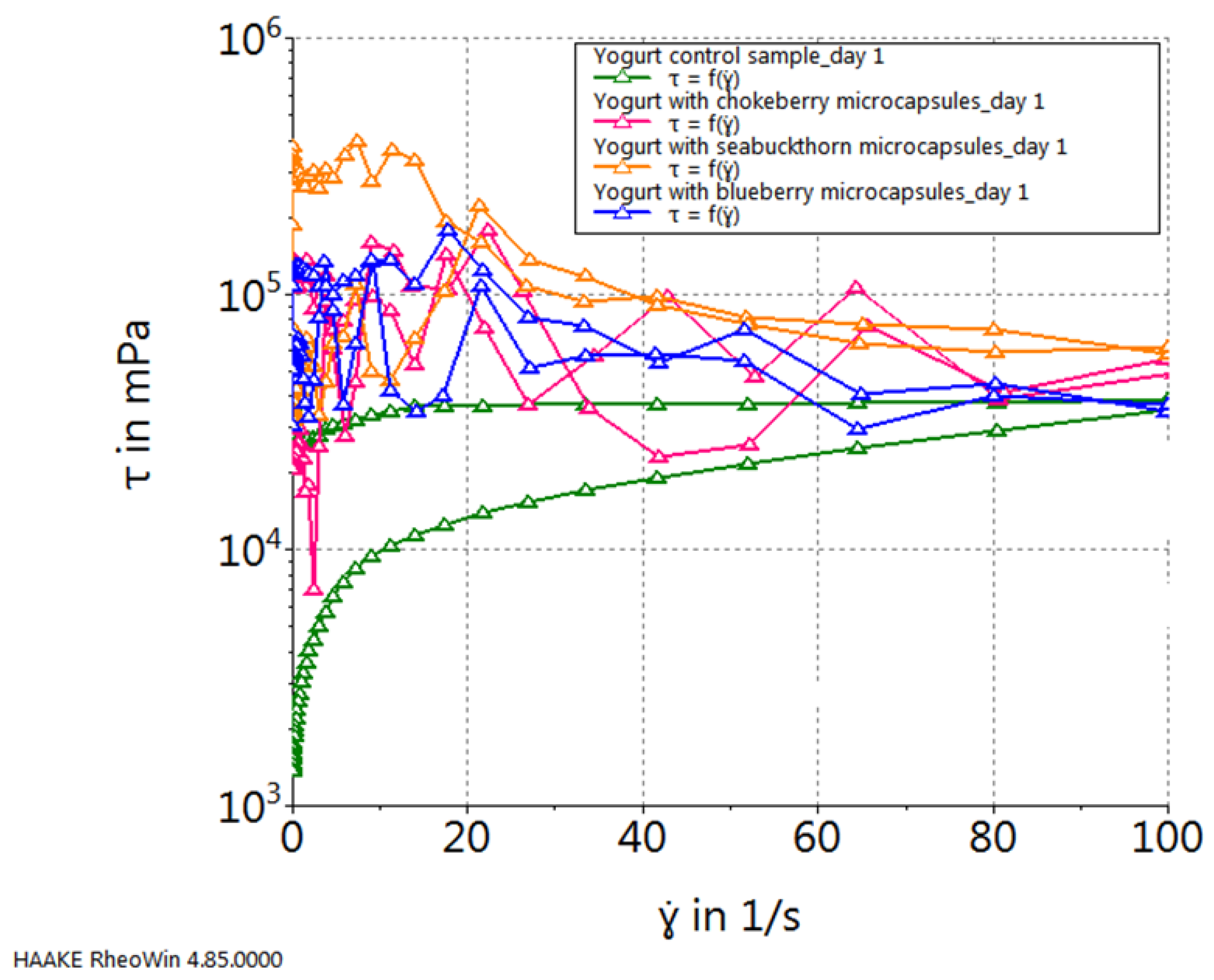
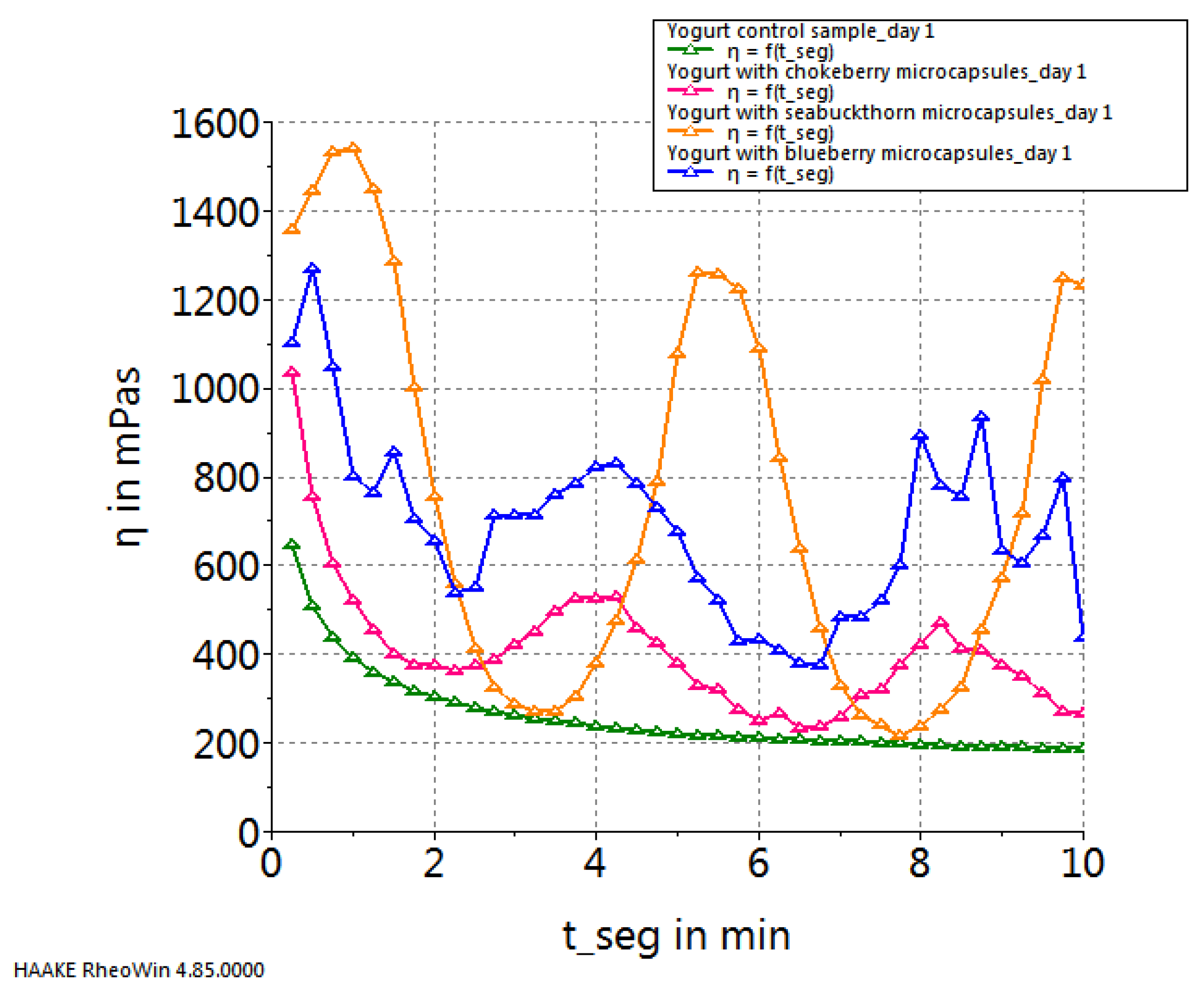
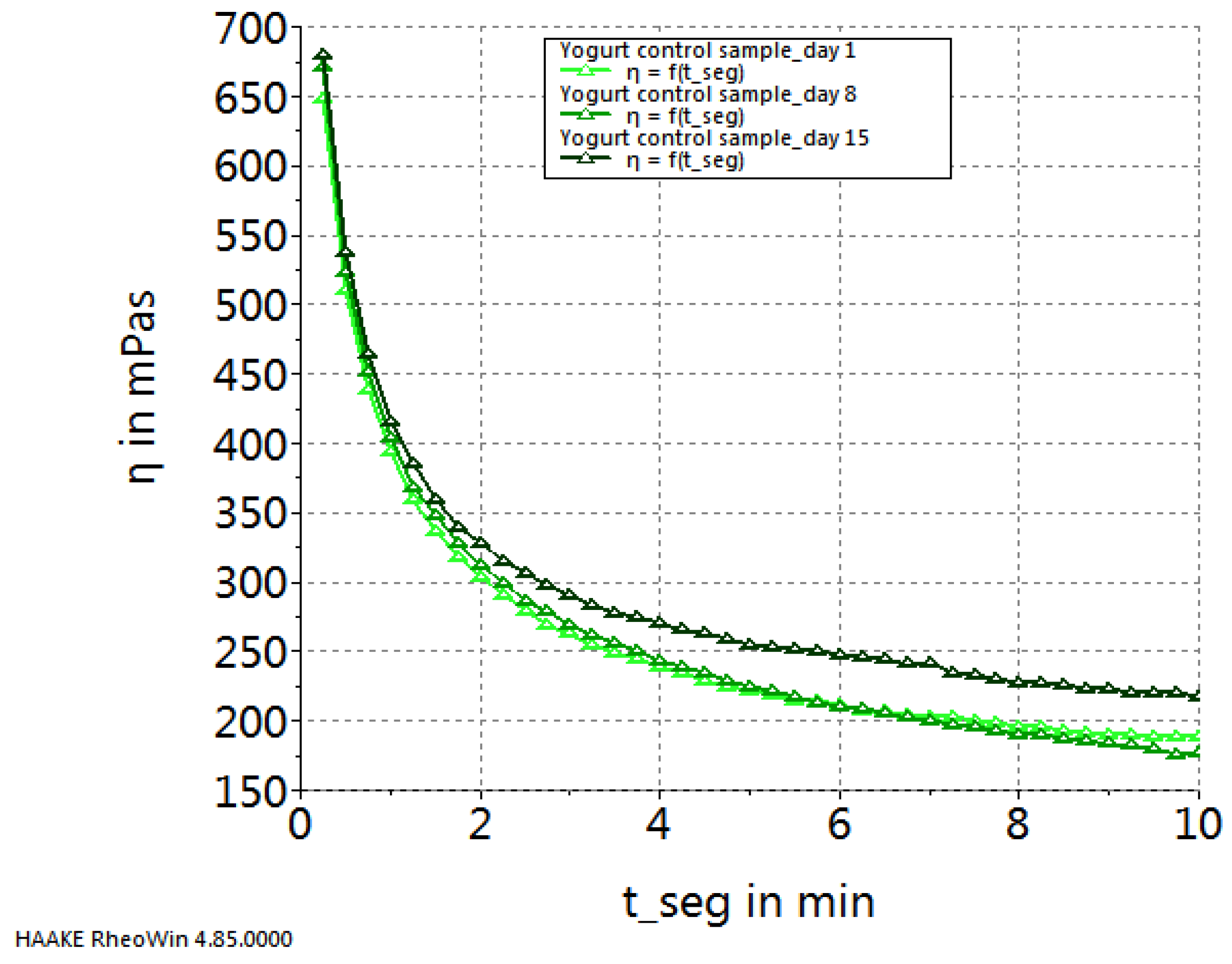
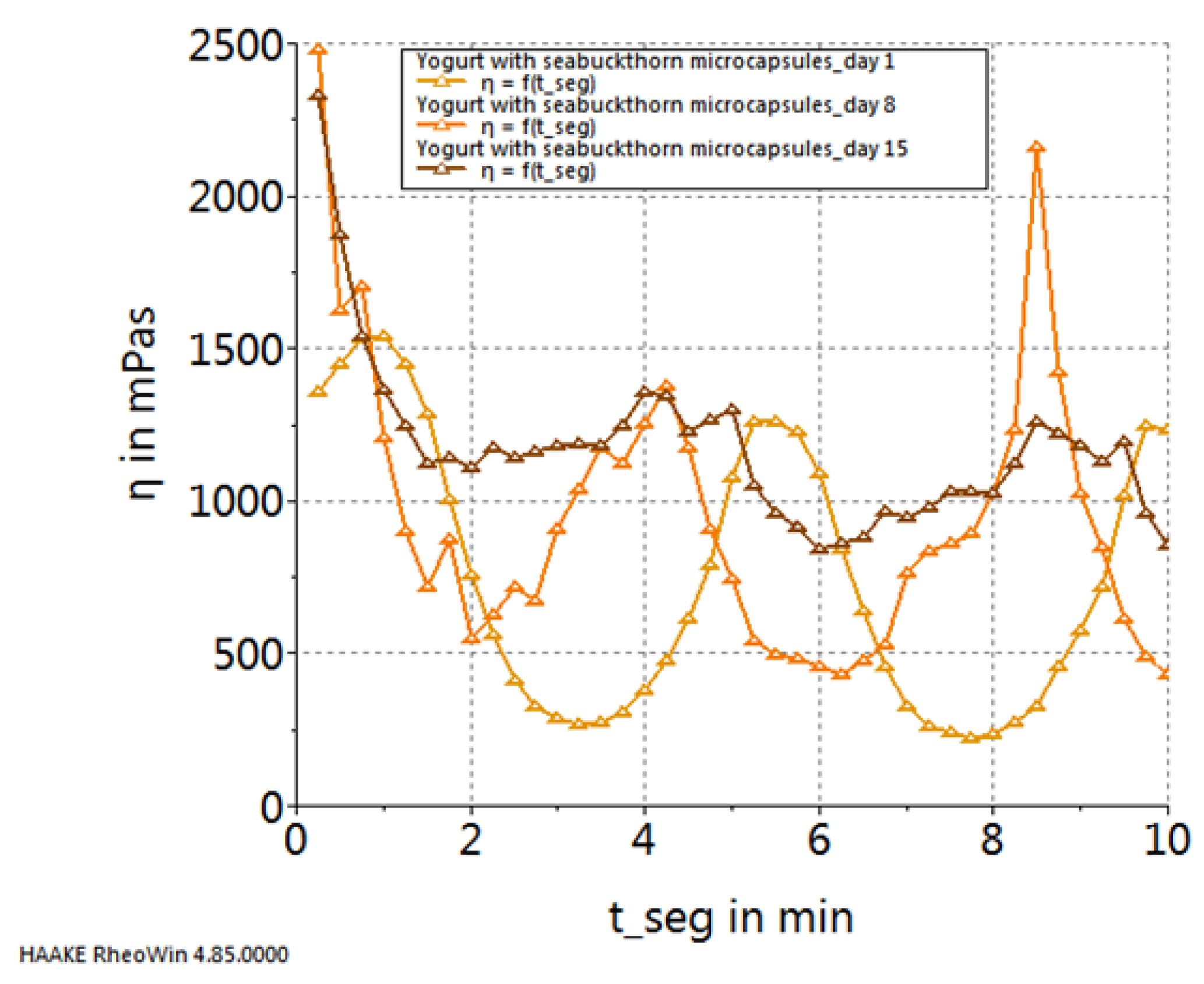
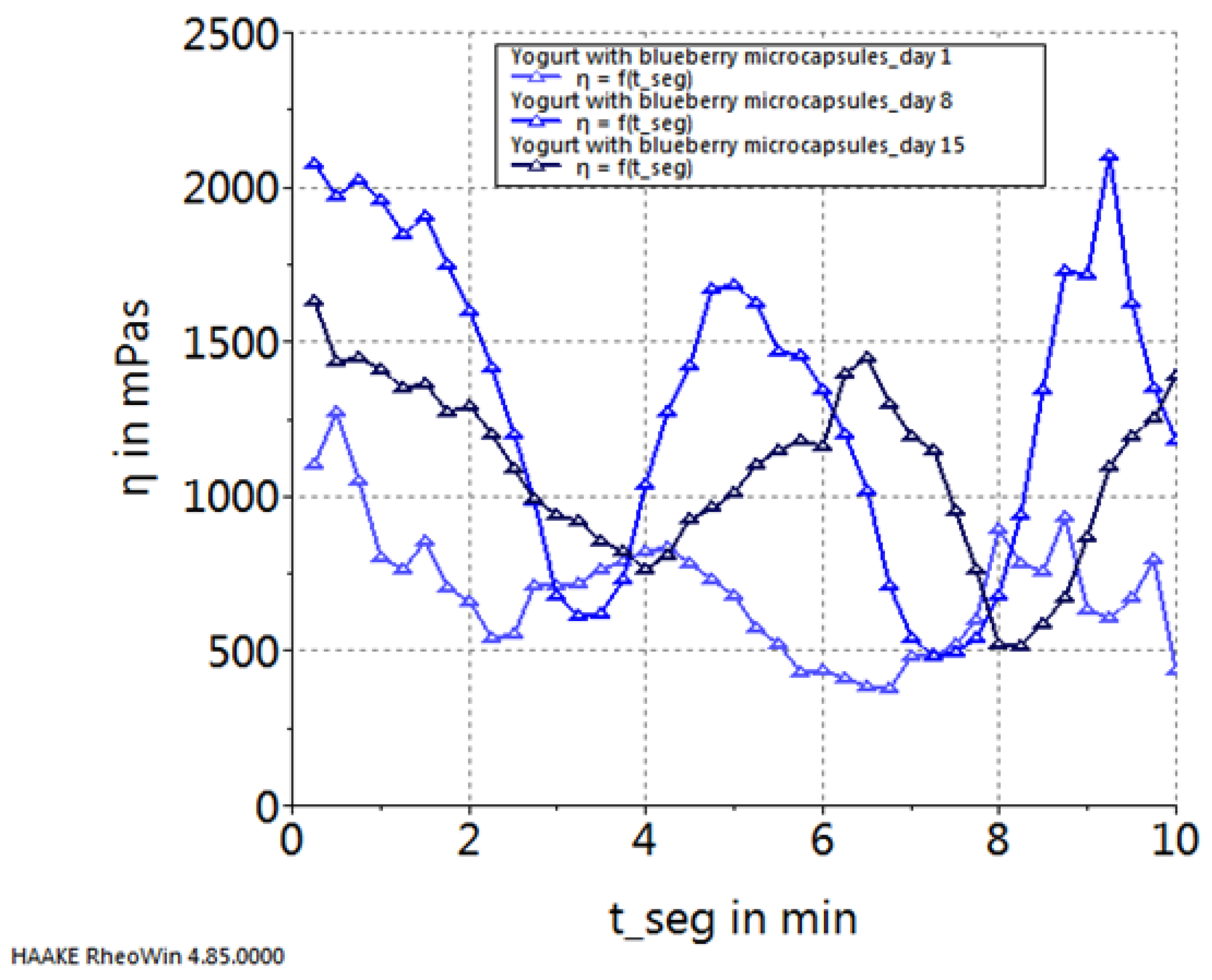
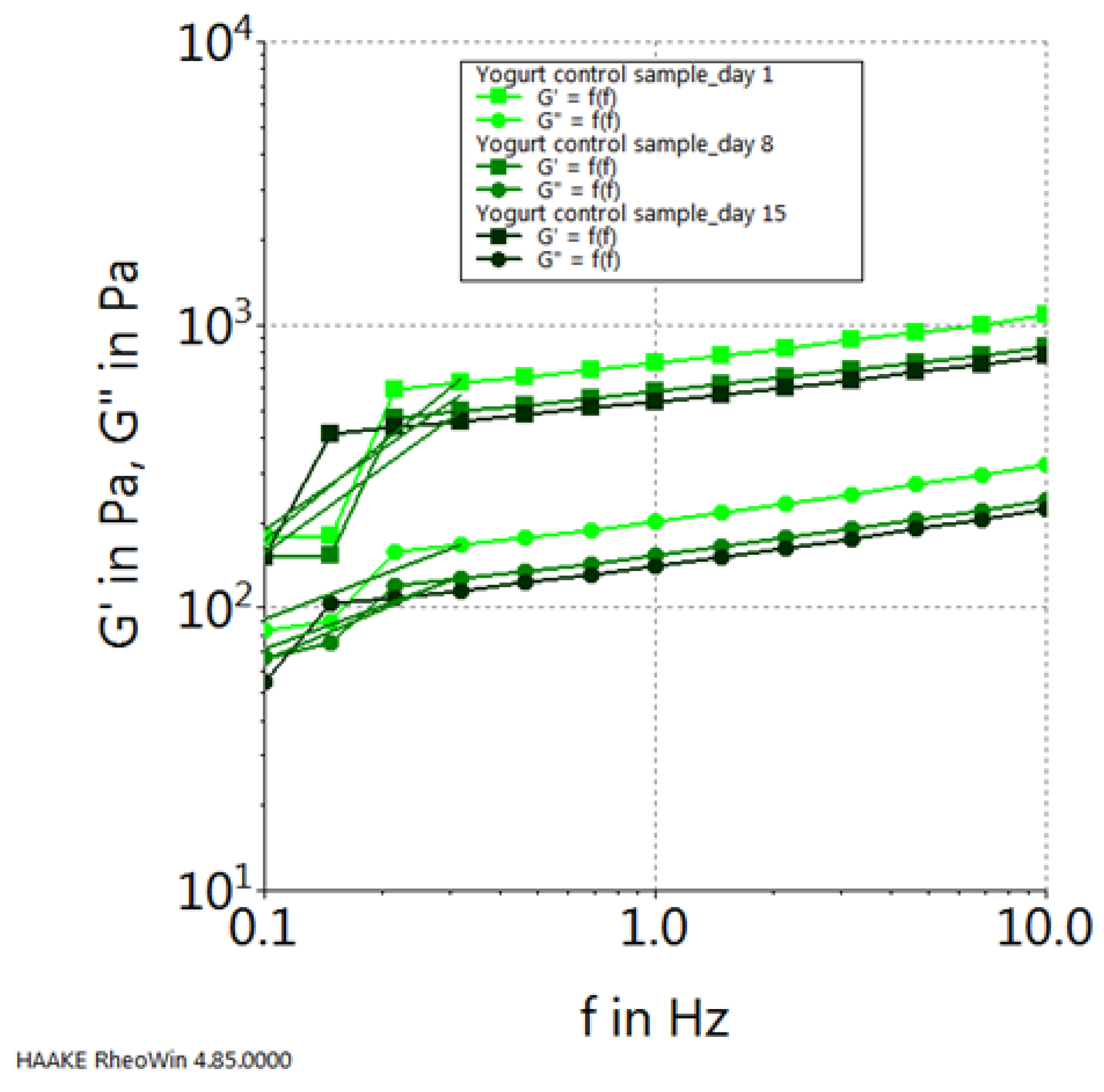
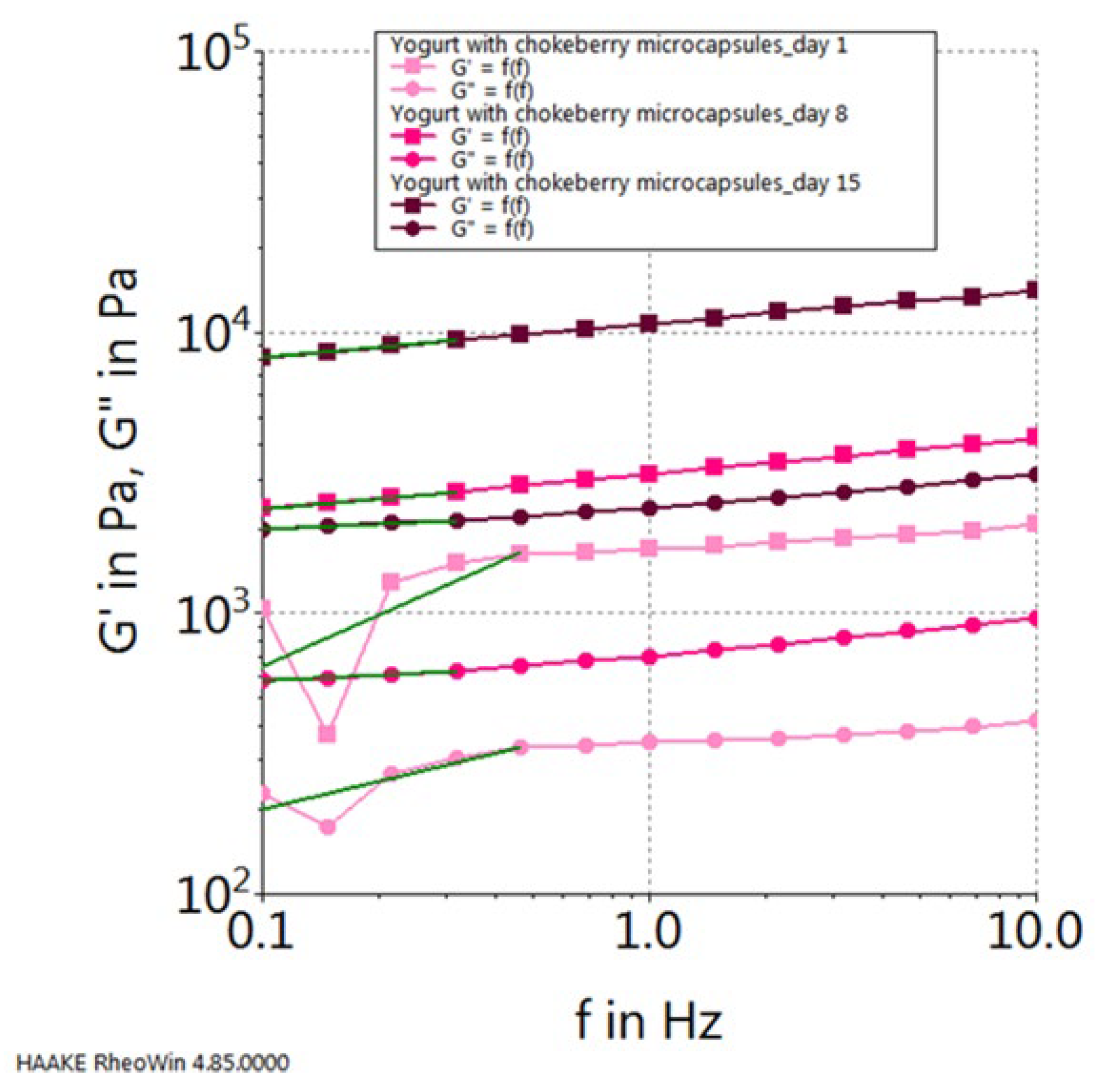
2. Results and Discussion
3. Conclusions and Future Perspectives
4. Materials and Methods
- Goal: Evaluate the overall sensorial quality of yogurt samples.
- Criteria: Different sensory attributes such as aspect, color, texture, smell and taste.
- Alternatives: 3 yogurt samples being analyzed P1 (aronia), P2 (sea buckthorn) and P3 (chokeberries).

Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From food industry to biomedical applications and management of metabolic disorders. Polymers 2020, 12, 1–30. [Google Scholar] [CrossRef]
- Adinepour, F.; Pouramin, S.; Rashidinejad, A.; Jafari, S.M. Fortification/enrichment of milk and dairy products by encapsulated bioactive ingredients. Food Res. Int. 2022, 157, 111212. [Google Scholar] [CrossRef] [PubMed]
- Ephrem, E.; Najjar, A.; Charcosset, C.; Greige-Gerges, H. Encapsulation of natural active compounds, enzymes, and probiotics for fruit juice fortification, preservation, and processing: An overview. J Funct Foods 2018, 48, 65–84. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, L. Encapsulated natural antimicrobials: A promising way to reduce microbial growth in different food systems. Food Control 2021, 123, 107678. [Google Scholar] [CrossRef]
- de Boer, F.Y.; Imhof, A.; Velikov, K.P. Encapsulation of colorants by natural polymers for food applications. Color. Technol. 2019, 135, 183–194. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Li, C.; Zheng, S.; Li, H.; Yu, J. Development of a microencapsulated synbiotic product and its application in yoghurt. LWT 2020, 122, 109033. [Google Scholar] [CrossRef]
- Silva, M.P.; da S, M.; Fernanda, F.T.; Thomazini, M.; Favaro-Trindade, C.S. Fortification of yoghurt drink with microcapsules loaded with Lacticaseibacillus paracasei BGP-1 and guaraná seed extract. Int Dairy J 2022, 125, 105230. [Google Scholar] [CrossRef]
- Khair, A.G.A.-E.; Soliman, T.N.; Hashim, A.F. Development of composite nanoemulsion gels as carriers for co-delivery of wheat germ oil and probiotics and their incorporation in yoghurt. Food Biosci 2023, 55, 103001. [Google Scholar] [CrossRef]
- El-Kholy, W.M.; et al. Evaluation of date palm pollen (Phoenix dactylifera L.) encapsulation, impact on the nutritional and functional properties of fortified yoghurt. PLoS ONE, 2019; 14. [Google Scholar] [CrossRef]
- Akgün, D.; et al. Stirred-type yoghurt incorporated with sour cherry extract in chitosan-coated liposomes. Food Hydrocoll 2020, 101, 105532. [Google Scholar] [CrossRef]
- Popescu, L.; et al. The Effect of Aromatic Plant Extracts Encapsulated in Alginate on the Bioactivity, Textural Characteristics and Shelf Life of Yogurt. Antioxidants 2023, 12, 893. [Google Scholar] [CrossRef]
- de F, F.J.; Clemente, H.A.; Santana, A.L.B.D.; da S, M.A. Stability of encapsulated and non-encapsulated anthocyanin in yogurt produced with natural dye obtained from Solanum melongena L. Bark. Bark. Rev Bras Frutic 2020. 42, e-137. [CrossRef]
- Acevedo-Fani, A.; Ochoa-Grimaldo, A.; Loveday, S.M.; Singh, H. Digestive dynamics of yoghurt structure impacting the release and bioaccessibility of the flavonoid rutin. Food Hydrocoll 2021, 111, 106215. [Google Scholar] [CrossRef]
- Diep, T.T.; Yoo, M.J.Y.; Rush, E. Tamarillo Polyphenols Encapsulated-Cubosome: Formation, Characterization, Stability during Digestion and Application in Yoghurt. Antioxidants 2022, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.; Fredes, C. The Encapsulation of Anthocyanins from Berry-Type Fruits. Trends in Foods. Trends in Foods. Molecules 2015 20, 5875–5888. [CrossRef]
- Šeregelj, V.; et al. New concept of fortified yogurt formulation with encapsulated carrot waste extract. LWT 2021, 138, 110732. [Google Scholar] [CrossRef]
- Šeregelj, V.; et al. Application of encapsulated natural bioactive compounds from red pepper waste in yogurt. J Microencapsul 2019, 36, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, C.; Li, G.; Chrubasik, S. The clinical effectiveness of chokeberry: A systematic review. Phytother. Res. 2010, 24, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Drożdżyńska, A.; Gramza-Michałowska, A. Black chokeberry (Aronia melanocarpa) and its products as potential health-promoting factors - An overview. Trends Food Sci Technol 2019, 89, 45–60. [Google Scholar] [CrossRef]
- Zhang, Y.; et al. Chokeberry (Aronia melanocarpa) as a new functional food relationship with health: An overview. J. Future Foods 2021, 1, 168–178. [Google Scholar] [CrossRef]
- Thi, N.D.; Hwang, E.S. Effects of drying methods on contents of bioactive compounds and antioxidant activities of black chokeberries (Aronia melanocarpa). Food Sci Biotechnol 2016, 25, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Hires, C.; Baker, C.; Keenan, L.; Bush, M. Daily supplementation with aronia melanocarpa (chokeberry) reduces blood pressure and cholesterol: A meta analysis of controlled clinical trials. J Diet Suppl 2021, 18, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Gill, N.K.; et al. Anticancer Effects of Extracts from Three Different Chokeberry Species. Nutr Cancer, 2021. [Google Scholar] [CrossRef]
- Kim, D.-H.; et al. Antibacterial Activity of Crude Aronia melanocarpa (Black Chokeberry) Extracts against Bacillus cereus, Staphylococcus aureus, Cronobacter sakazakii, and Salmonella Enteritidis in Various Dairy Foods: Preliminary Study. J Dairy Sci Biotechnol 2018, 36, 155–163. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black Chokeberry Aronia Melanocarpa L.—A Qualitative Composition, Phenolic Profile and Antioxidant Potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Fernando, W.M.A.D.B.; Durham, R.; Stockmann, R.; Jayasena, V. Nutritional Value, Health-promoting Benefits and Food Application of Sea Buckthorn. Food Rev. Int. 2023, 39, 2122–2137. [Google Scholar] [CrossRef]
- Tereshchuk, L.V.; Starovoitova, K.V.; Vyushinsky, P.A.; Zagorodnikov, K.A. The Use of Sea Buckthorn Processing Products in the Creation of a Functional Biologically Active Food Emulsion. Foods 2022, 11, 2226. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; et al. Development of a food preservative from sea buckthorn together with chitosan: Application in and characterization of fresh-cut lettuce storage. Front Microbiol 2023, 14, 1080365. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Destandau, E.; Le Floch, G.; Lucchesi, M.E.; Elfakir, C. Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophaë rhamnoides L.) leaf, stem, root and seed. Food Chem 2012, 131, 754–760. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, F.; Wei, P.; Chai, X.; Hou, G.; Meng, Q. Phytochemistry, health benefits, and food applications of sea buckthorn (Hippophae rhamnoides L.): A comprehensive review. Front Nutr 2022, 9, 1036295. [Google Scholar] [CrossRef]
- Wani, T.A.; Wani, S.M.; Ahmad, M.; Ahmad, M.; Gani, A.; Masoodi, F.A. Bioactive profile, health benefits and safety evaluation of sea buckthorn (Hippophae rhamnoides L.): A review. Cogent Food Agric 2016, 2. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Z.; Liao, X. Bioactive compounds, health benefits and functional food products of sea buckthorn: A review. Crit Rev Food Sci Nutr 2022, 62, 6761–6782. [Google Scholar] [CrossRef]
- Tereshchuk, L.V.; Starovoitova, K.V.; Vyushinsky, P.A.; Zagorodnikov, K.A. The Use of Sea Buckthorn Processing Products in the Creation of a Functional Biologically Active Food Emulsion. Foods 2022, 11, 2226. [Google Scholar] [CrossRef]
- Xu, Y.J.; Kaur, M.; Dhillon, R.S.; Tappia, P.S.; Dhalla, N.S. Health benefits of sea buckthorn for the prevention of cardiovascular diseases. J Funct Foods 2011, 3, 2–12. [Google Scholar] [CrossRef]
- Dupak, R.; et al. The consumption of sea buckthorn (Hippophae rhamnoides L.) effectively alleviates type 2 diabetes symptoms in spontaneous diabetic rats. Res Vet Sci 2022, 152, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Gâtlan, A.M.; Gutt, G. Sea Buckthorn in Plant Based Diets. An Analytical Approach of Sea Buckthorn Fruits Composition: Nutritional Value, Applications, and Health Benefits. Int. J. Environ. Res. Public Health 2021, 18, 8986. [Google Scholar] [CrossRef]
- Schubertová, S.; Krepsová, Z.; Janotková, L.; Potočňáková, M.; Kreps, F. Exploitation of Sea Buckthorn Fruit for Novel Fermented Foods Production: A Review. Processes 2021, 9, 749. [Google Scholar] [CrossRef]
- Sun, L.Q.; et al. Antioxidant anthocyanins screening through spectrum–effect relationships and DPPH-HPLC-DAD analysis on nine cultivars of introduced rabbiteye blueberry in China. Food Chem 2012, 132, 759–765. [Google Scholar] [CrossRef]
- Pap, N.; et al. Berry polyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr Opin Food Sci 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Popović, T.; et al. Potential health benefits of blueberry and raspberry pomace as functional food ingredients: Dietetic intervention study on healthy women volunteers. Front Nutr 2022, 9, 969996. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, M.; Yang, F.; Liu, J. Antioxidant activity of high purity blueberry anthocyanins and the effects on human intestinal microbiota. LWT 2020, 117, 108621. [Google Scholar] [CrossRef]
- Jiao, X.; et al. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J Nutr Biochem 2019, 64, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Whyte, A.R.; Williams, C.M. Effects of a single dose of a flavonoid-rich blueberry drink on memory in 8 to 10 y old children. Nutrition 2015, 31, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.L.; Tran, T.T.D. Blueberry Supplementation in Neuronal Health and Protective Technologies for Efficient Delivery of Blueberry Anthocyanins. Biomolecules 2021 11, 102. [CrossRef]
- Wu, Y.; Han, T.; Yang, H.; Lyu, L.; Li, W.; Wu, W. Known and potential health benefits and mechanisms of blueberry anthocyanins: A review. Food Biosci 2023, 55, 103050. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Chai, Z.; Beta, T.; Feng, J.; Huang, W. Blueberry anthocyanins: An updated review on approaches to enhancing their bioavailability. Trends Food Sci Technol 2021, 118, 808–821. [Google Scholar] [CrossRef]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Phillips, D.R.; Kong, F. In vitro release properties of encapsulated blueberry (Vaccinium ashei) extracts. Food Chem 2015, 168, 225–232. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, J.R.; et al. Microencapsulation of anthocyanin compounds extracted from blueberry (Vaccinium spp.) by spray drying: Characterization, stability and simulated gastrointestinal conditions. Food Hydrocoll 2019, 89, 742–748. [Google Scholar] [CrossRef]
- Kazan, A.; Sevimli-Gur, C.; Yesil-Celiktas, O.; Dunford, N.T. In vitro tumor suppression properties of blueberry extracts in liquid and encapsulated forms. Eur. Food Res. Technol. 2017, 243, 1057–1063. [Google Scholar] [CrossRef]
- Sivapragasam, N.; Neelakandan, N.; Rupasinghe, H.P.V. Potential health benefits of fermented blueberry: A review of current scientific evidence. Trends Food Sci Technol 2023, 132, 103–120. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, F.; Chai, Z.; Liu, M.; Battino, M.; Meng, X. Mixed fermentation of blueberry pomace with L. rhamnosus GG and L. plantarum-1: Enhance the active ingredient, antioxidant activity and health-promoting benefits. Food Chem. Toxicol. 2019, 131, 110541. [Google Scholar] [CrossRef]
- Neuenfeldt, N.H.; de Moraes, D.P.; de Deus, C.; Barcia, M.T.; de Menezes, C.R. Blueberry Phenolic Composition and Improved Stability by Microencapsulation. Food Bioprocess Technol. 2021, 15, 750–767. [Google Scholar] [CrossRef]
- Tifrea, A.; Tiţa, O.; Máthé, E.; Ketney, O. Physicochemical parameters of probiotic yoghurt with bioactive natural products from sea buckthorn. Acta Univ. Cibiniensis - Ser. E: Food Technol. 2013, 17, 27–38. [Google Scholar] [CrossRef]
- Gunenc, A.; Khoury, C.; Legault, C.; Mirrashed, H.; Rijke, J.; Hosseinian, F. Seabuckthorn as a novel prebiotic source improves probiotic viability in yogurt. LWT - Food Sci. Technol. 2016, 66, 490–495. [Google Scholar] [CrossRef]
- Brodziak, A.; et al. Effect of Sea Buckthorn (Hippophae rhamnoides L.) Mousse on Properties of Probiotic Yoghurt. Appl. Sci. 2021, 11, 545. [Google Scholar] [CrossRef]
- Najgebauer-Lejko, D.; Liszka, K.; Tabaszewska, M.; Domagała, J. Probiotic Yoghurts with Sea Buckthorn, Elderberry, and Sloe Fruit Purees. Molecules 2021, 26, 2345. [Google Scholar] [CrossRef] [PubMed]
- Bidchol, A.M.; Wilfred, A.; Abhijna, P.; Harish, R. Free Radical Scavenging Activity of Aqueous and Ethanolic Extract of Brassica oleracea L. var. italica. Food Bioproc Tech 2011, 4, 1137–1143. [Google Scholar] [CrossRef]
- Olas, B. Berry phenolic antioxidants - implications for human health? Front Pharmacol 2018, 9, 320038. [Google Scholar] [CrossRef] [PubMed]
- Muniandy, P.; Shori, A.B.; Baba, A.S. Influence of green, white and black tea addition on the antioxidant activity of probiotic yogurt during refrigerated storage. Food Packag Shelf Life 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Zhu, F.; Li, J.; Ma, Z.; Li, J.; Du, B. Structural identification and in vitro antioxidant activities of anthocyanins in black chokeberry (Aronia melanocarpa lliot). eFood 2021, 2, 201–208. [Google Scholar] [CrossRef]
- Hwang, S.J.; Yoon, W.B.; Lee, O.H.; Cha, S.J.; Kim, J.D. Radical-scavenging-linked antioxidant activities of extracts from black chokeberry and blueberry cultivated in Korea. Food Chem 2014, 146, 71–77. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Bober, I. The effect of addition of chokeberry, flowering quince fruits and rhubarb juice to strawberry jams on their polyphenol content, antioxidant activity and colour. Eur. Food Res. Technol. 2008, 227, 1043–1051. [Google Scholar] [CrossRef]
- Gani, A.; Jan, R.; Ashwar, B.A.; Ashraf, Z.U.; Shah, A.; Gani, A. Encapsulation of saffron and sea buckthorn bioactives: Its utilization for development of low glycemic baked product for growing diabetic population of the world. LWT 2021, 142, 111035. [Google Scholar] [CrossRef]
- Kim, S.-H.; Chon, J.-W.; Song, K.-Y.; Jeong, D.; Seo, K.-H. Sensory Attributes of Market Milk, Yogurt, and Kefir Supplemented with Various Concentrations of Aronia melanocarpa (black chokeberry) Powder: A Preliminary Study. J Dairy Sci Biotechnol 2019, 37, 108–114. [Google Scholar] [CrossRef]
- Laaksonen, O.; Knaapila, A.; Niva, T.; Deegan, K.C.; Sandell, M. Sensory properties and consumer characteristics contributing to liking of berries. Food Qual Prefer 2016, 53, 117–126. [Google Scholar] [CrossRef]
- “Articol Bacau”.
- DABIJA, A.; CODINĂ, G.G.; GÂTLAN, A.-M.; SĂNDULEAC, E.T.; RUSU, L. EFFECTS OF SOME VEGETABLE PROTEINS ADDITION ON YOGURT QUALITY. Sci. Study Res. : Chem. Chem. Eng. Biotechnol. Food Ind. 2018, 19, 181–192. [Google Scholar]
- Sidor, A.M.; Gutt, G.; Dabija, A.; Sanduleac, E.T.; Sidor, V. The effect of yogurt enrichment with sea buckthorn powder on its sensory acceptance, rheological, textural and physicochemical properties. Int. Multidiscip. Sci. GeoConference Surv. Geol. Min. Ecol. Manag. 2017, 17, 1117–1128. [Google Scholar] [CrossRef]
- Dabija, D.D. Research on the use of the AHP method in the sensory analysis of buckwheat and sorghum beer. Sci. Study; Research. Chem. ; Chem. Eng. Biotechnol. Food Ind. 2023, 24, 291–300. [Google Scholar]
- Baviera-Puig, A.; García-Melón, M.; López-Cortés, I.; Ortolá, M.D. Combining sensory panels with Analytic Hierarchy Process (AHP) to assess nectarine and peach quality. Cogent Food Agric 2023, 9. [Google Scholar] [CrossRef]
- Gurmeric, V.E.; Dogan, M.; Toker, O.S.; Senyigit, E.; Ersoz, N.B. Application of Different Multi-criteria Decision Techniques to Determine Optimum Flavour of Prebiotic Pudding Based on Sensory Analyses. Food Bioproc Tech 2013, 6, 2844–2859. [Google Scholar] [CrossRef]
- de J, E.; et al. Analytic hierarchy process as an alternative for the selection of vocabularies for sensory characterization and consumer preference. Analytic hierarchy process as an alternative for the selection of vocabularies for sensory characterization and consumer preference. J Sens Stud 2020. 35, e12547. [CrossRef]
- Mogbojur, A.O.; Olanrewaju, O.A.; Ogunleye, T.O. Evaluation of inventory management practice in food processing industries in Lagos: Analytical hierarchy process approach. Niger. J. Technol. 2022, 41, 236–246. [Google Scholar] [CrossRef]


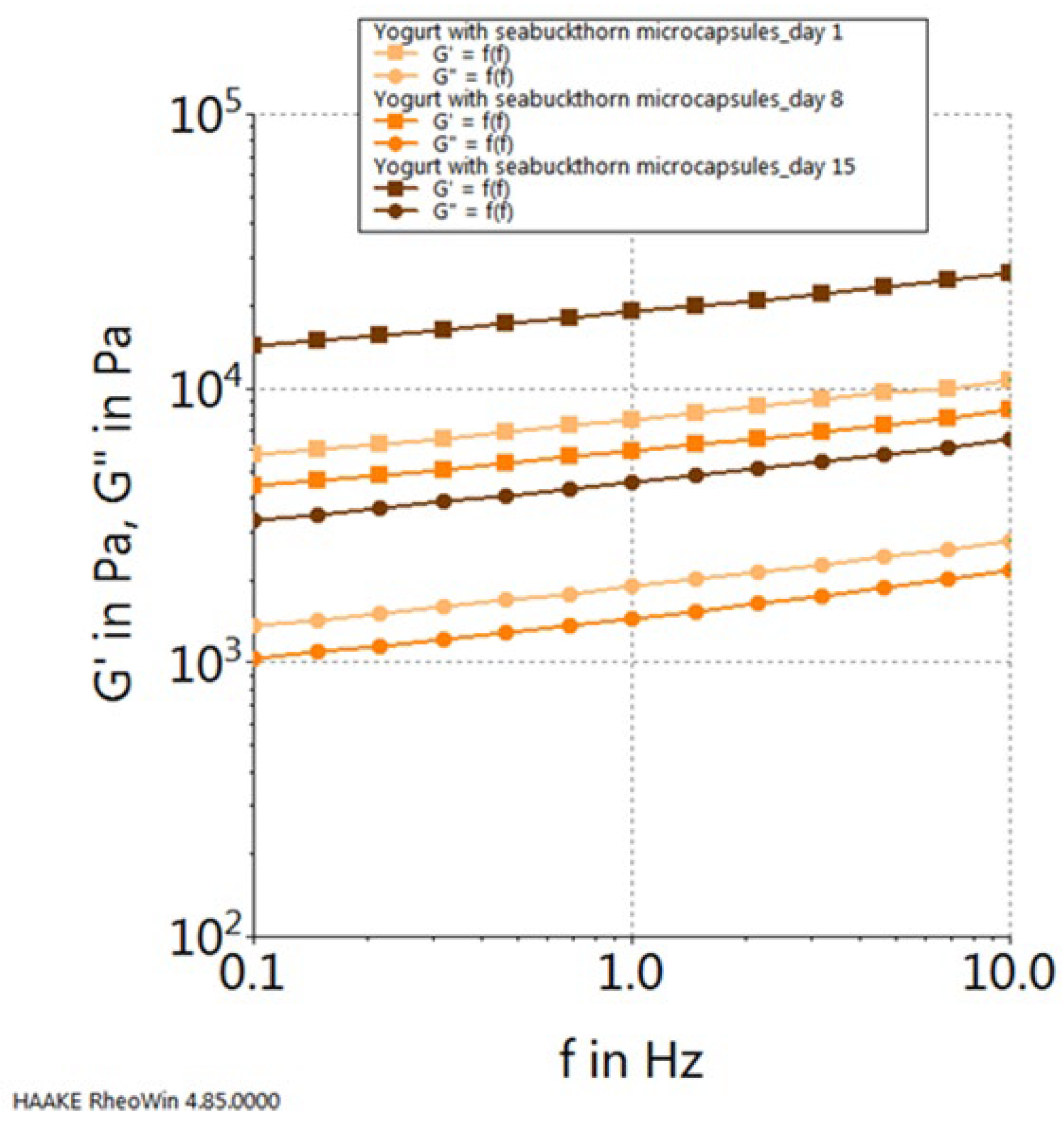
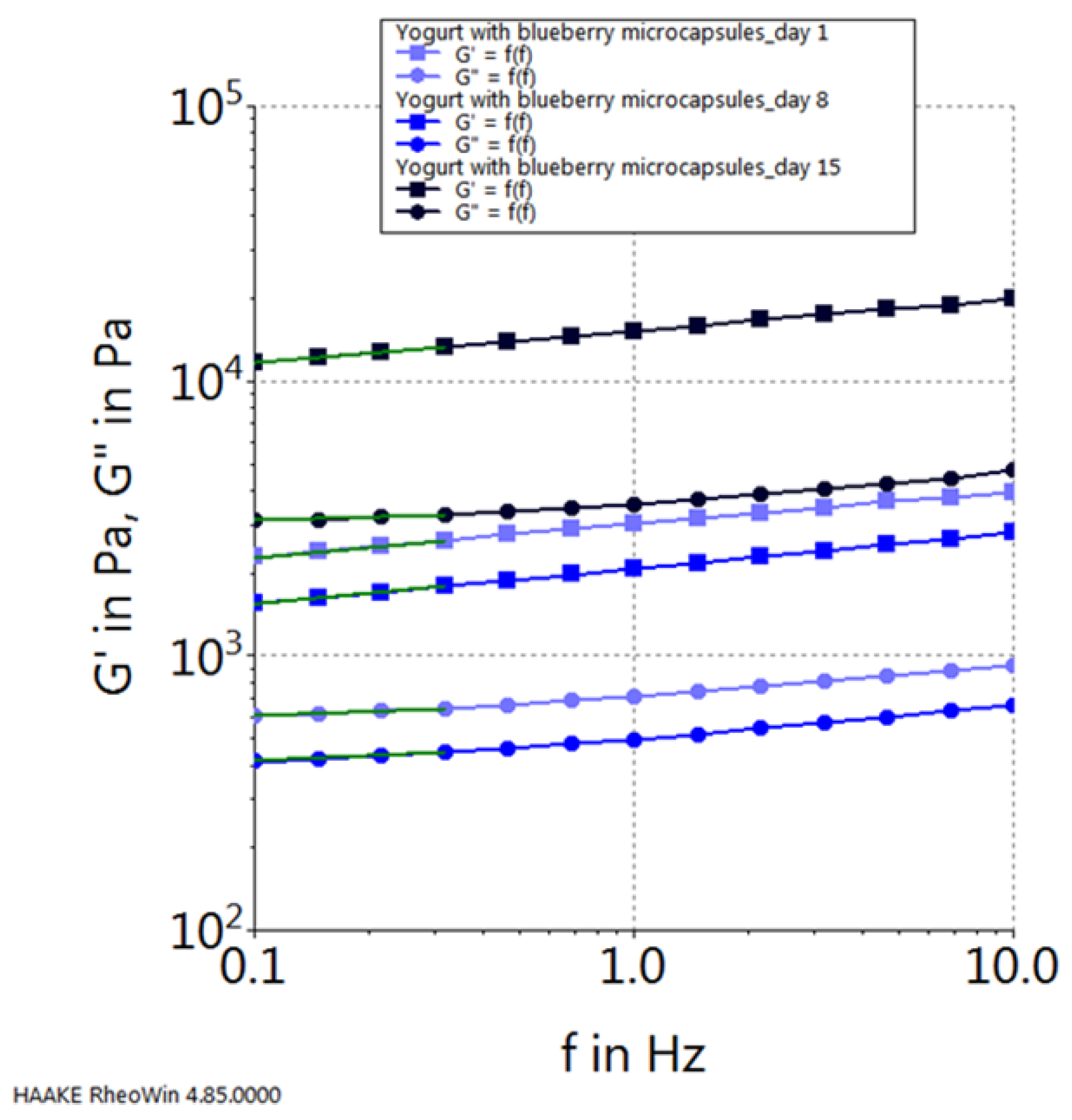
| Chokeberry Capsules | Sea Buckthorn Capsules | Blueberry Capsules | |
|---|---|---|---|
| Color Evaluation | |||
| L* | 28.89 ± 0.31 | 54.44 ± 0.32 | 28.65 ± 0.29 |
| a* | 1.53 ± 0.03 | 13.17 ± 0.08 | 1.21 ± 0.22 |
| b* | 0.82 ± 0.06 | 0.79 ± 0.14 | 0.78 ± 0.05 |
| Diameter, mm | 3.12 ± 0.33 | 3.27 ± 0.25 | 3.08 ± 0.03 |
| P1 | P2 | P3 | C | |
|---|---|---|---|---|
| pH | ||||
| Day 1 | 4.56 ± 0.02 | 4.52 ± 0.02 | 4.41 ± 0.02 | 4.34 ± 0.01 |
| Day 8 | 4.52 ± 0.01 | 4.50 ± 0.01 | 4.40 ± 0.01 | 4.32 ± 0.01 |
| Day 15 | 4.45 ± 0.01 | 4.49 ± 0.01 | 4.37 ± 0.01 | 4.31 ± 0.01 |
| Syneresis, % | ||||
| Day 1 | 47.00 ± 0.01 | 50.00 ± 0.01 | 53.50 ± 0.01 | 52.00 ± 0.01 |
| Day 8 | 45.00 ± 0.01 | 50.80 ± 0.01 | 51.30 ± 0.01 | 46.00 ± 0.01 |
| Day 15 | 44.00 ± 0.01 | 53.00 ± 0.01 | 50.50 ± 0.01 | 40.00 ± 0.01 |
| Water hold capacity, % | ||||
| Day 1 | 31.14 ± 0.01 | 38.17 ± 0.01 | 33.75 ± 0.01 | 35.84 ± 0.01 |
| Day 8 | 35.17 ± 0.01 | 41.12 ± 0.01 | 31.19 ± 0.01 | 34.12 ± 0.01 |
| Day 15 | 38.84 ± 0.01 | 44.76 ± 0.01 | 39.04 ± 0.01 | 30.86 ± 0.01 |
| P1 | P2 | P3 | C | |
|---|---|---|---|---|
| L* | ||||
| Day 1 | 71.92 ± 1.93 | 77.94 ± 0.64 | 74.70 ± 0.76 | 79.71 ± 0.08 |
| Day 8 | 72.49 ± 0.29 | 78.47 ± 0.24 | 74.85 ± 0.46 | 78.74 ± 0.21 |
| Day 15 | 73.22 ± 0.06 | 78.62 ± 0.29 | 74.04 ± 0.40 | 79.17 ± 0.47 |
| a* | ||||
| Day 1 | 3.13 ± 0.24 | 1.47 ± 0.02 | 0.49 ± 0.09 | -2.45 ± 0.03 |
| Day 8 | 3.32 ± 0.13 | 1.50 ± 0.01 | 0.91 ± 0.04 | -2.51 ± 0.02 |
| Day 15 | 3.84 ± 0.02 | 1.54 ± 0.01 | 1.29 ± 0.19 | - 2.52 ± 0.02 |
| b* | ||||
| Day 1 | 2.45 ± 0.19 | 8.22 ± 0.10 | 4.79 ± 0.39 | 8.68 ± 0.02 |
| Day 8 | 3.81 ± 0.07 | 8.24 ± 0.02 | 4.73 ± 0.02 | 8.64 ± 0.02 |
| Day 15 | 4.20 ± 0.02 | 8.29 ± 0.03 | 4.68 ± 0.41 | 8.56 ± 0.08 |
| ΔEs | ||||
| Day 1 | 9.99 | 2.07 | 6.63 | - |
| Day 8 | 7.94 | 1.11 | 5.74 | - |
| Day 15 | 7.49 | 1.15 | 3.12 | - |
| ΔEt | ||||
| Day 8-Day 1 | 1.48 | 0.53 | 0.45 | 0.99 |
| Day 15-Day 8 | 0.83 | 0.16 | 0.89 | 0.43 |
| Day 15- Day 1 | 2.58 | 0.71 | 1.04 | 0.55 |
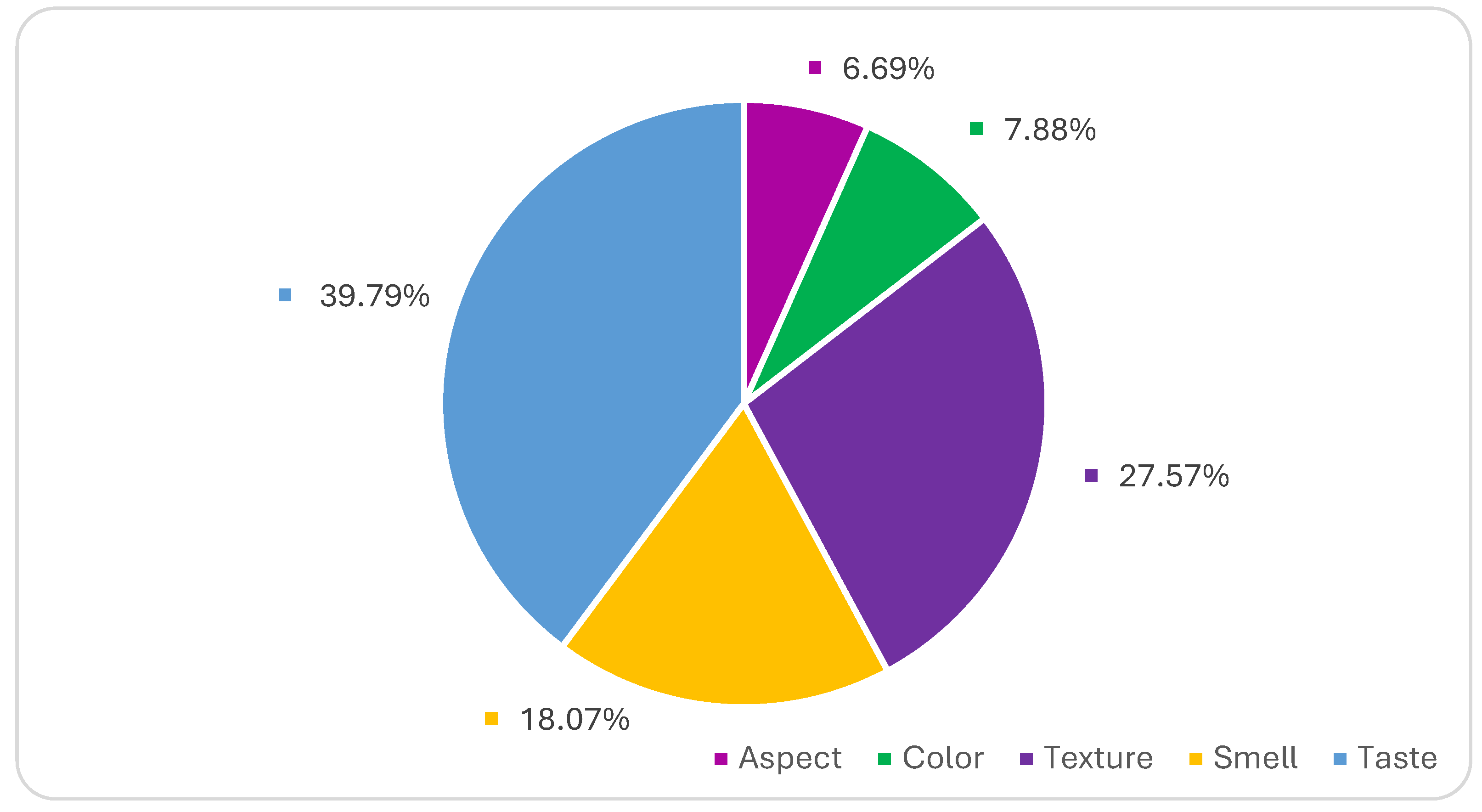
| Aspect | Color | Texture | Smell | Taste | Priorities | |
|---|---|---|---|---|---|---|
| Aspect | 1 | 0.607 | 0.252 | 0.339 | 0.226 | 0.0669 |
| Color | 1.65 | 1 | 0.24 | 0.322 | 0.21 | 0.0788 |
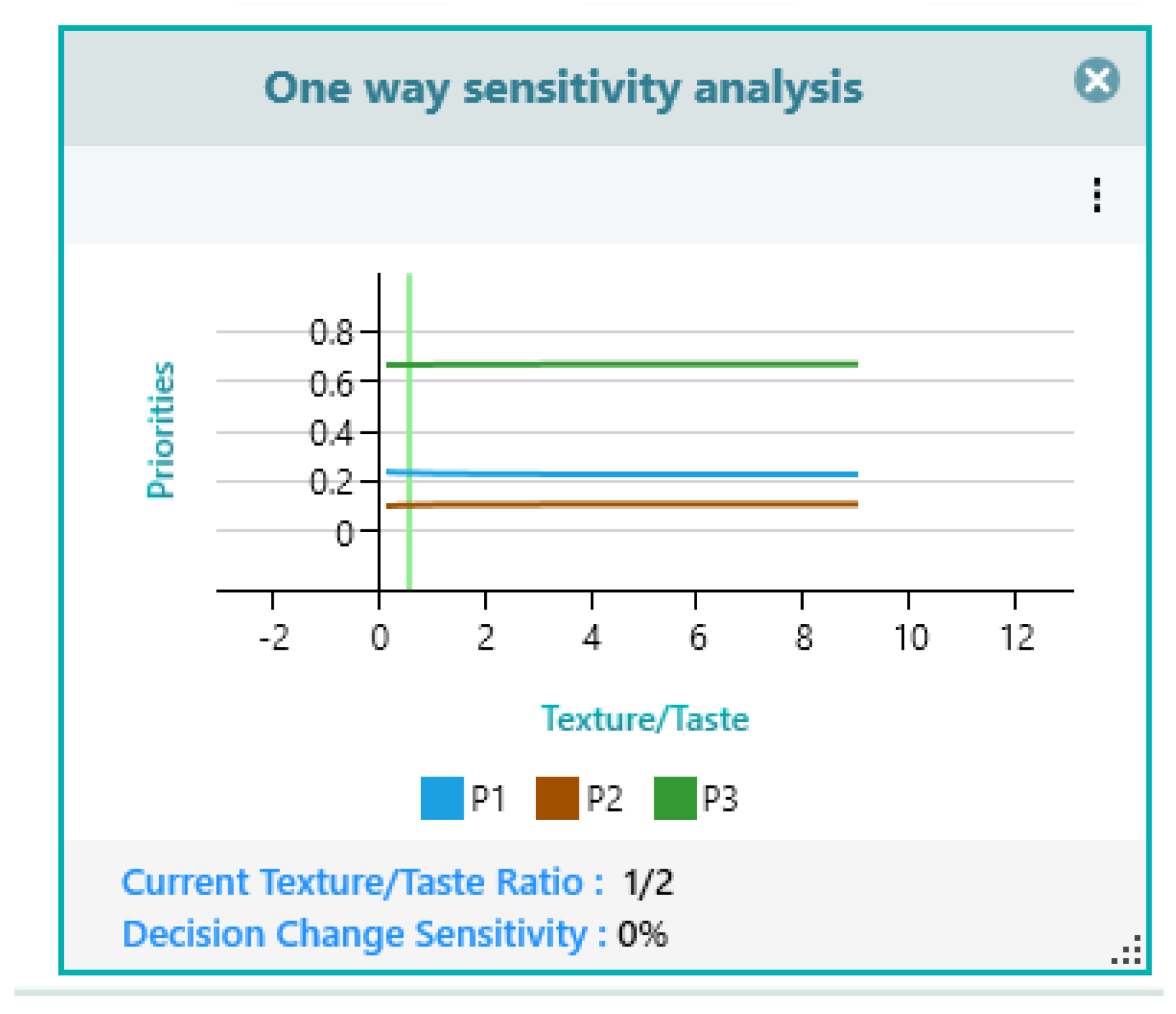
| Texture | 3.97 | 4.16 | 1 | 2.04 | 0.473 | 0.2757 |
| Smell | 2.79 | 3.11 | 0.49 | 1 | 0.441 | 0.1807 |
| Taste | 4.43 | 4.77 | 2.11 | 2.27 | 1 | 0.3979 |
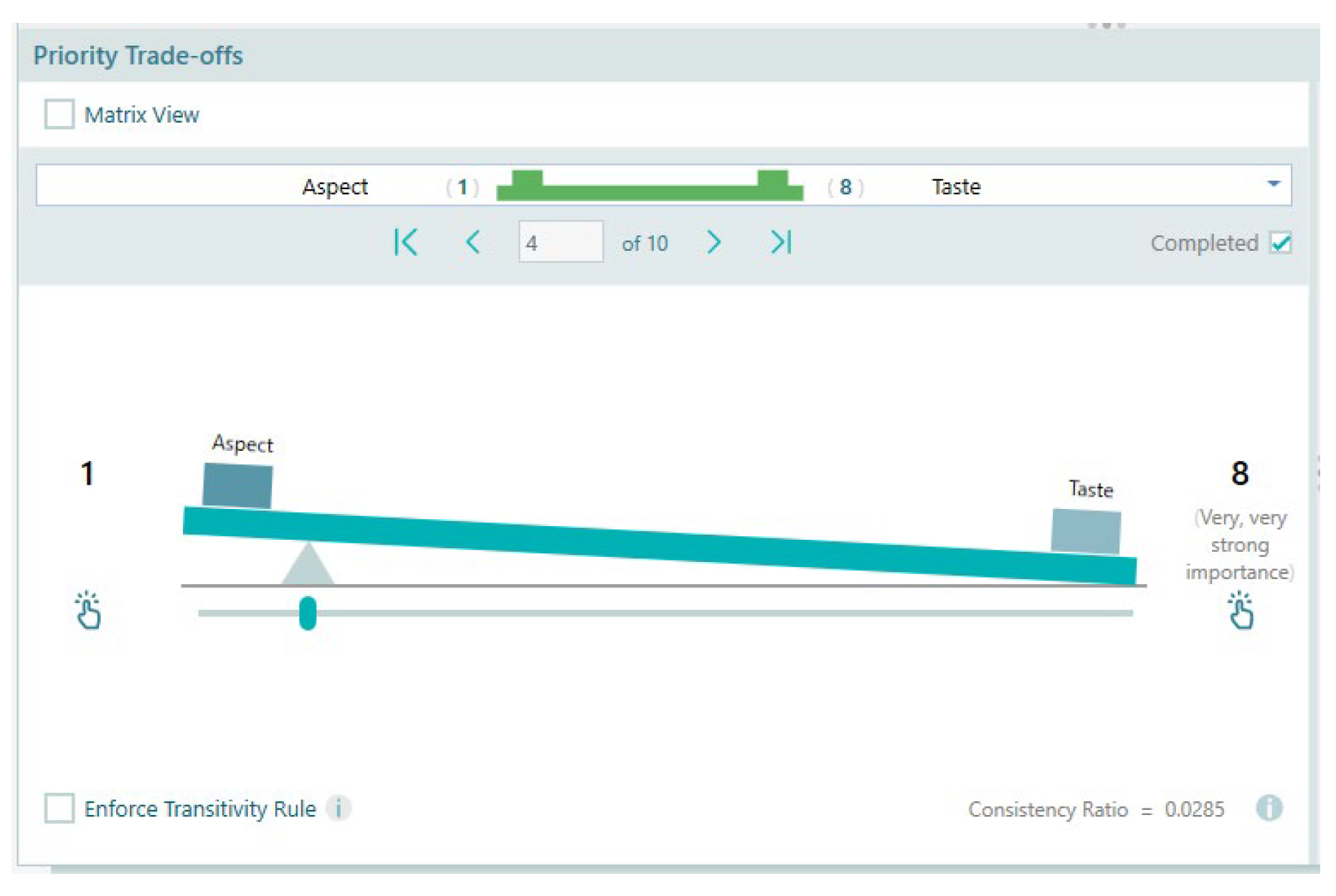
| Consistency Ratio calculated as | 0.025 | |||||
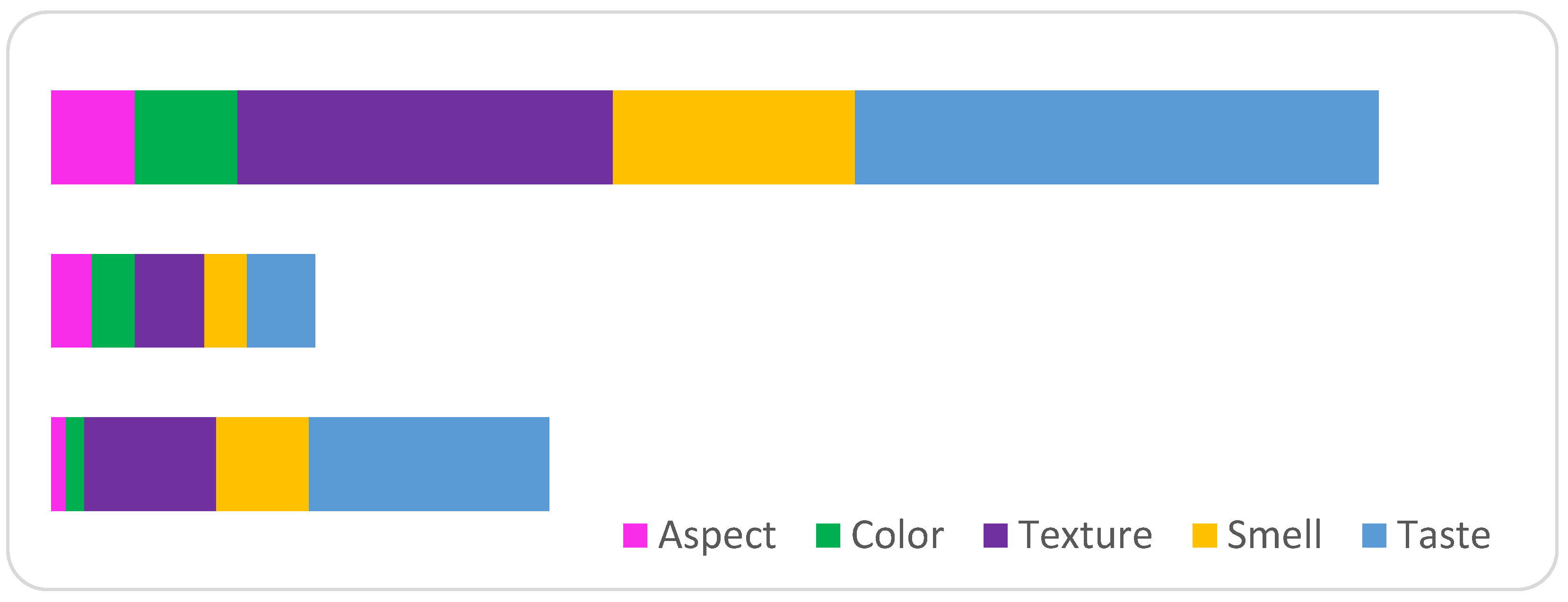
| Option | Aspect | Color | Texture | Smell | Taste |
|---|---|---|---|---|---|
| P1 | 0.0072 | 0.0089 | 0.0631 | 0.0444 | 0.1146 |
| P2 | 0.0195 | 0.0207 | 0.0332 | 0.0203 | 0.0325 |
| P3 | 0.0402 | 0.0492 | 0.1794 | 0.1159 | 0.2508 |
| From your point of view, which attribute is more important and to what extent to evaluate the QUALITY of a yogurt? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EX | MF | F | MO | = | MO | F | MF | EX | ||
| C1 Aspect | 9 | 7 | 5 | 3 | 1 | 3 | 5 | 7 | 9 | C2 Color |
| C1 Aspect | 9 | 7 | 5 | 3 | 1 | 3 | 5 | 7 | 9 | C3 Texture |
| C1 Aspect | 9 | 7 | 5 | 3 | 1 | 3 | 5 | 7 | 9 | C4 Smell |
| C1 Aspect | 9 | 7 | 5 | 3 | 1 | 3 | 5 | 7 | 9 | C5 Taste |
| C2 Color | 9 | 7 | 5 | 3 | 1 | 3 | 5 | 7 | 9 | C3 Texture |
| C2 Color | 9 | 7 | 5 | 3 | 1 | 3 | 5 | 7 | 9 | C4 Smell |
| C2 Color | 9 | 7 | 5 | 3 | 1 | 3 | 5 | 7 | 9 | C5 Taste |
| C3 Texture | 9 | 7 | 5 | 3 | 1 | 3 | 5 | 7 | 9 | C4 Smell |
| C3 Texture | 9 | 7 | 5 | 3 | 1 | 3 | 5 | 7 | 9 | C5 Taste |
| C4 Smell | 9 | 7 | 5 | 3 | 1 | 3 | 5 | 7 | 9 | C5 Taste |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





