Submitted:
28 March 2024
Posted:
28 March 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
- - Characterising the effect of subregional intra-tumoural heterogeneity on grading in ccRCC;
- - Comparing the diagnostic accuracy of radiomics and ML through image-guided biopsy in determining the grade of renal masses, using resection (partial or complete) histopathology as a reference standard.
1.1. Key Contributions
- - Clinical Application:This research offers a practical application of radiomics and machine learning techniques in the field of oncology, specifically in the diagnosis and grading ccRCC which could potentially aid clinicians in early detection, accurate and precise treatment planning due to its higher diagnostic accuracy in comparison to traditional methods.
- - Subregion Heterogeneity Analysis: The inclusion of intra-tumoural subregion heterogeneity analysis highlights the depth of the research beyond simple tumour delineation and delves into the spatial distribution and variations within the tumour. This provides deeper insights into tumour biology and behaviour, leading to more personalised treatment approaches.
- - Potential for Non-invasive Assessment:Radiomics–machine learning algorithms, has the potential to extract valuable information from medical images non-invasively reducing the need for invasive procedures for tumour characterisation and grading, improving patient comfort and reducing healthcare costs.
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Cohorts
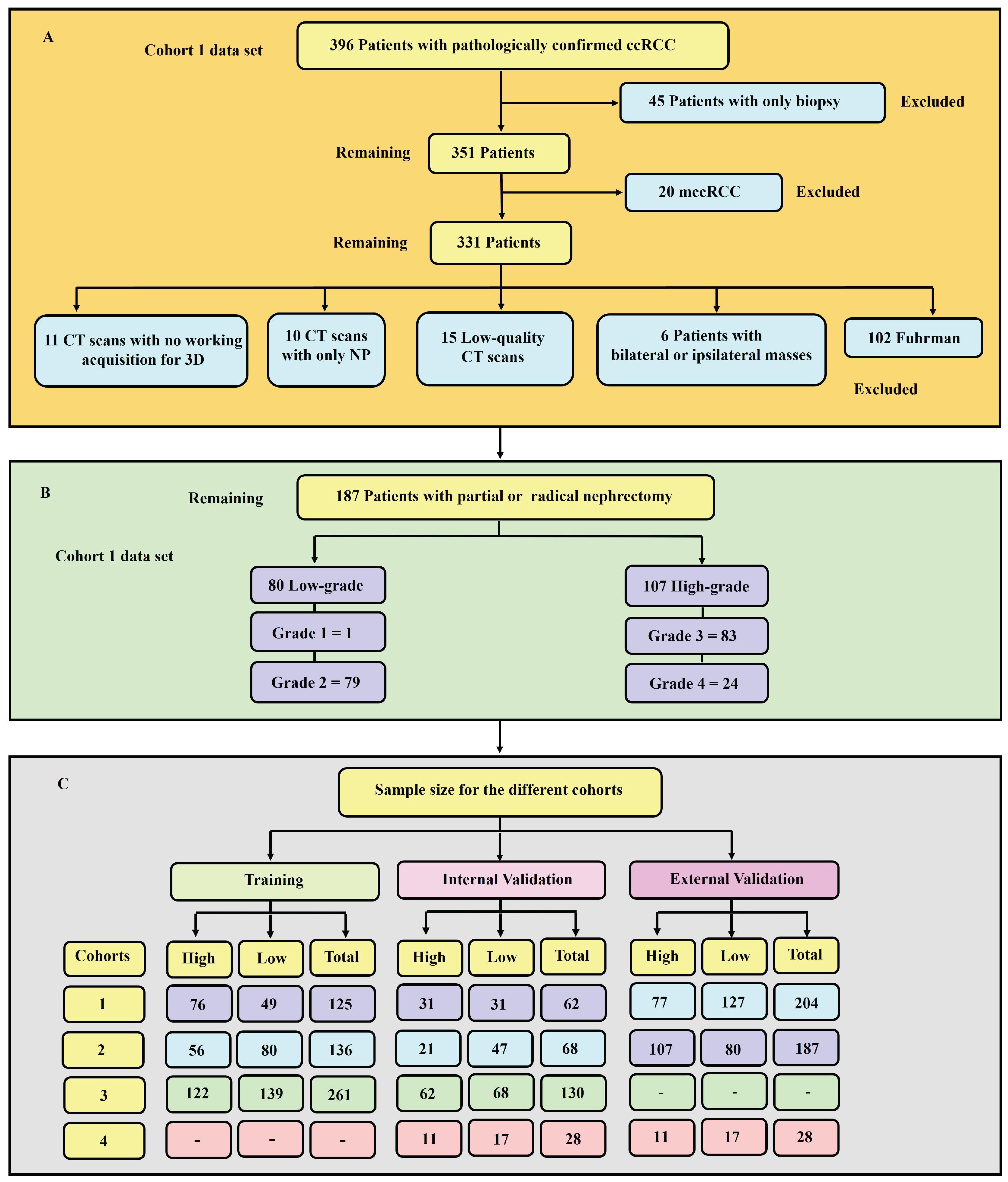
2.2.1. Inclusion Criteria
- - Availability of protocol-based pre-operative contrast-enhanced CT scan in the arterial phase. The selection of the arterial phase is justified due to its widespread adoption across medical centres. Moreover, this phase, characterised by its enhancement pattern and hypervascularity, has demonstrated promising capabilities in distinguishing between low- and high-grade ccRCC [33].
- - Confirmed histopathology from partial or radical nephrectomy with grades reported by a uro-pathologist according to the WHO/ISUP grading system.
- - CT scans with data to achieve a working acquisition for 3D image reconstruction.
2.2.2. Exclusion Criteria
- - Patients with only biopsy histopathology.
- - Metastatic clear cell renal cell carcinoma (mccRCC).
- - Patients with bilateral or ipsilateral multiple tumours, primarily due to the ambiguity of the database in distinguishing between the exact tumour grades.
2.3. CT Acquisition Technique
2.4. Hardware and Software Consideration
2.5. Data Curation
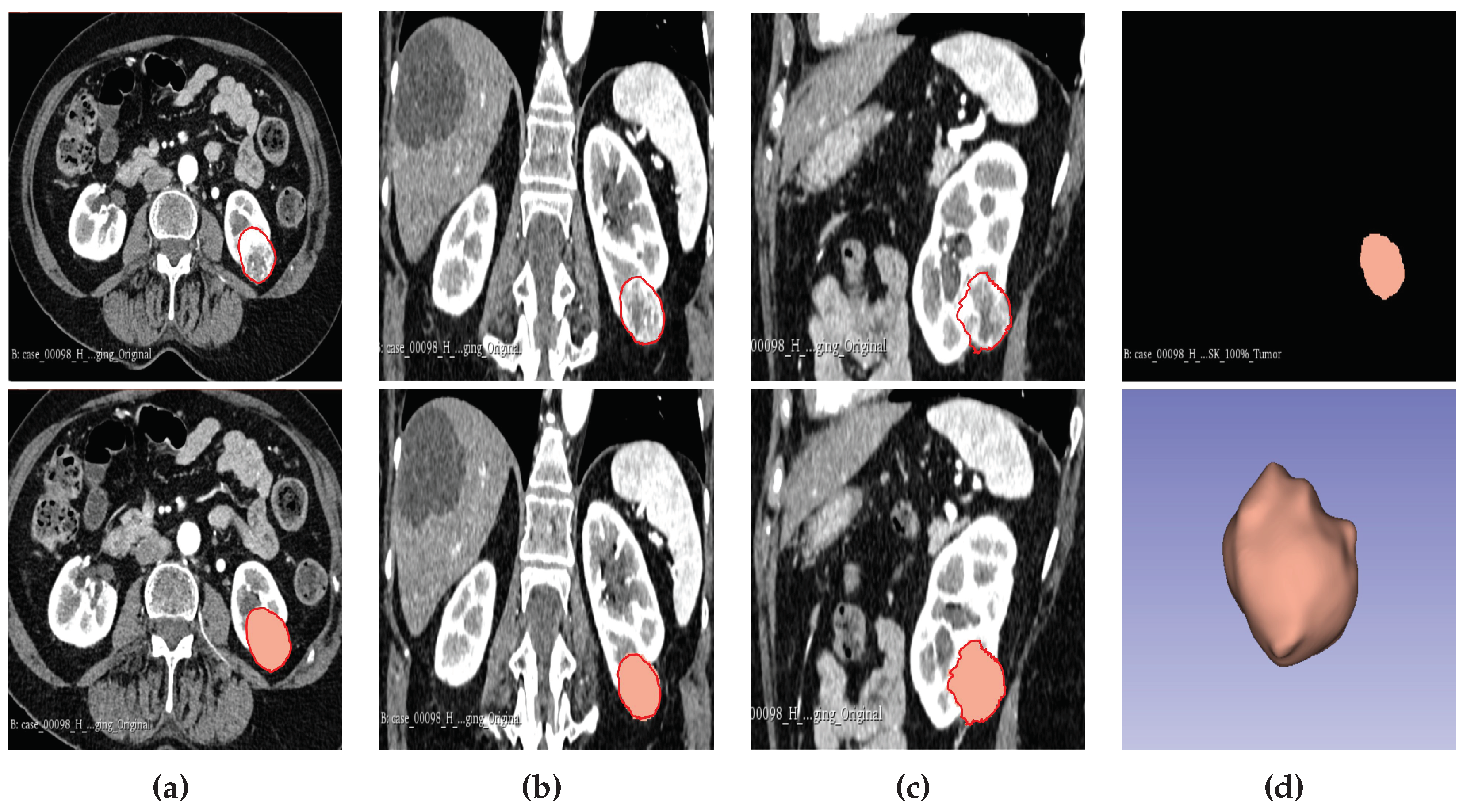
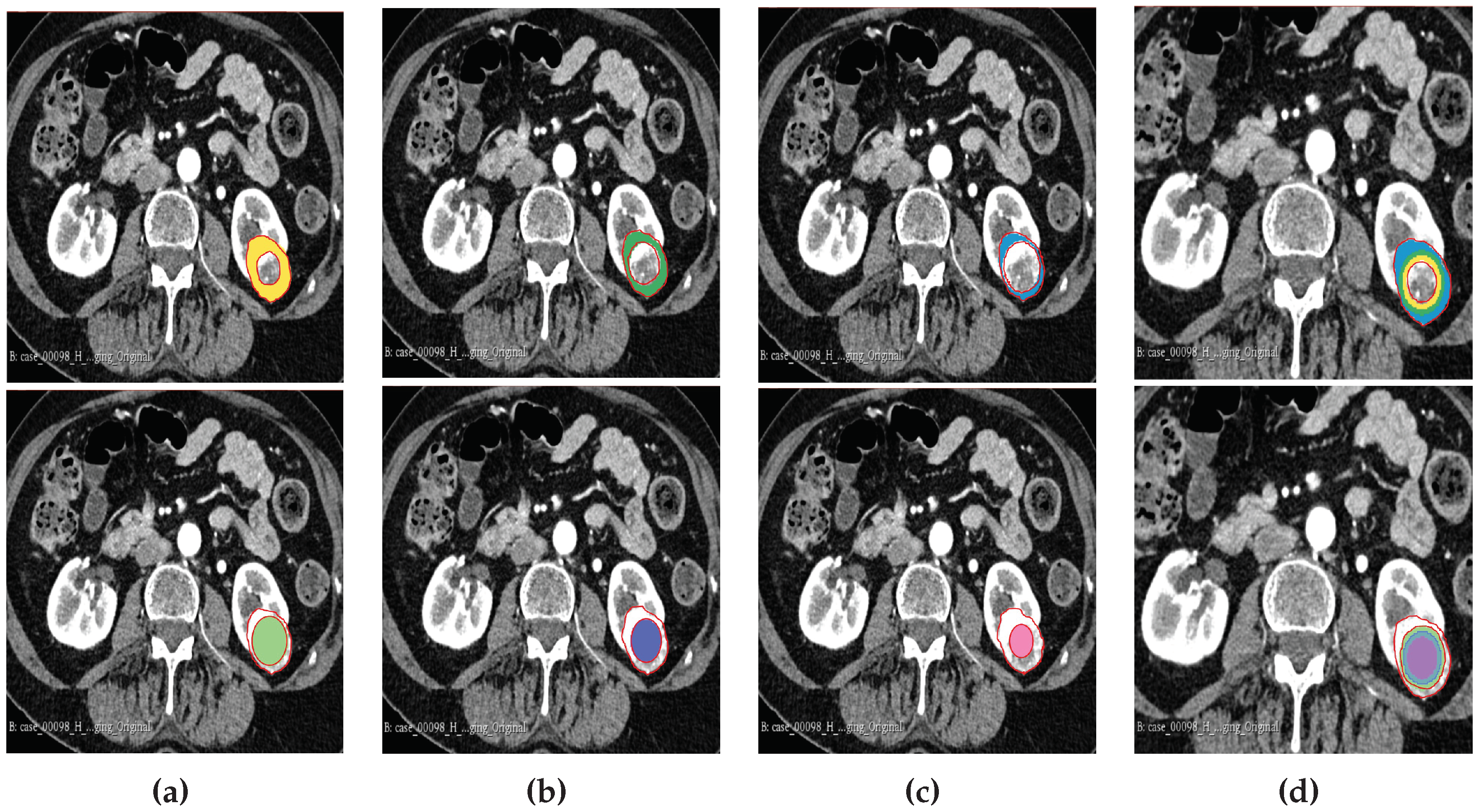
2.6. Tumour Sub-Volume Segmentation Technique
2.7. Radiomics Feature Computation
2.8. Feature Processing and Feature Selection
- Z: Value after scaling the feature.
- x: The feature.
- : Mean of all features in the training set.
- : Standard deviation of the training set.
2.9. Sub-Sampling
2.10. Statistical Analysis
2.11. Model Construction, Validation, and Evaluation
| MODELS | PARAMETERS |
|---|---|
| SVM | kernel=rbf, probability=True, random_state=42, gamma=0.2, C=0.01 |
| RF | n_estimators=401, random_state=42, max_depth=3 |
| XGBoost | random_state=42, learning_rate=0.01, n_estimators=401, gamma=0.52 |
| NB | GaussianNB |
| MLP | hidden_layer_sizes=(401,201), activation=relu, solver=adam, max_iter=5 |
| LSTM | loss=binary_crossentropy, optimizer=Adam, lr=0.01, metrics=accuracy, epochs=1000, batch_size=16 |
| LR | random_state=42, max_iter=4 |
| QDA | reg_param=0.05 |
| LightGBM | random_state=42, n_estimators=9 |
| CatBoost | random_state=42, verbose=False, iterations=50 |
| AdaBoost | base_estimator=rf, n_estimators=201, learning_rate=0.01, random_state=42 |
3. Results
3.0.1. Study Population and Statistical Analysis
3.0.2. Feature Extraction, Pre-processing, and Selection
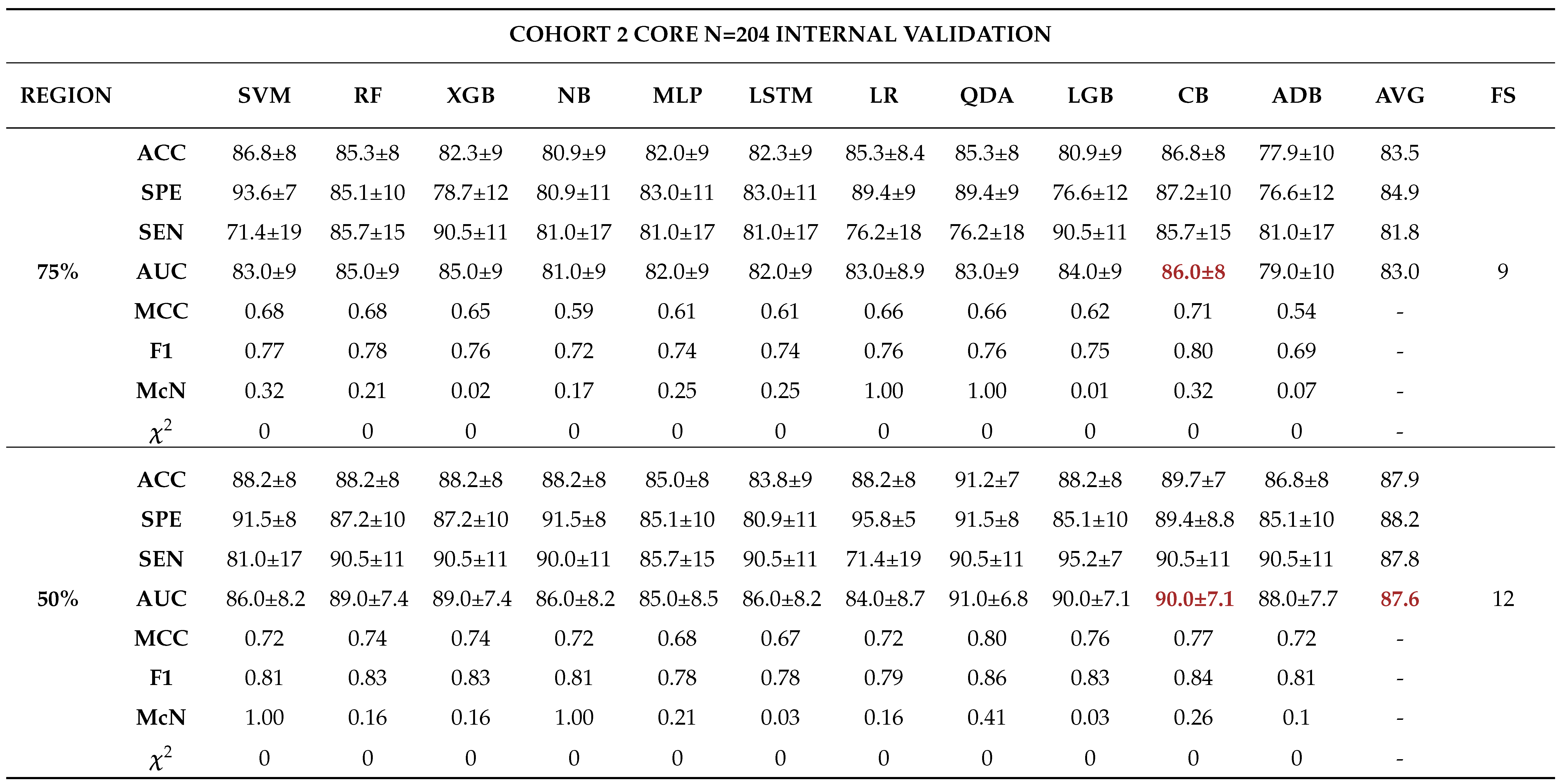
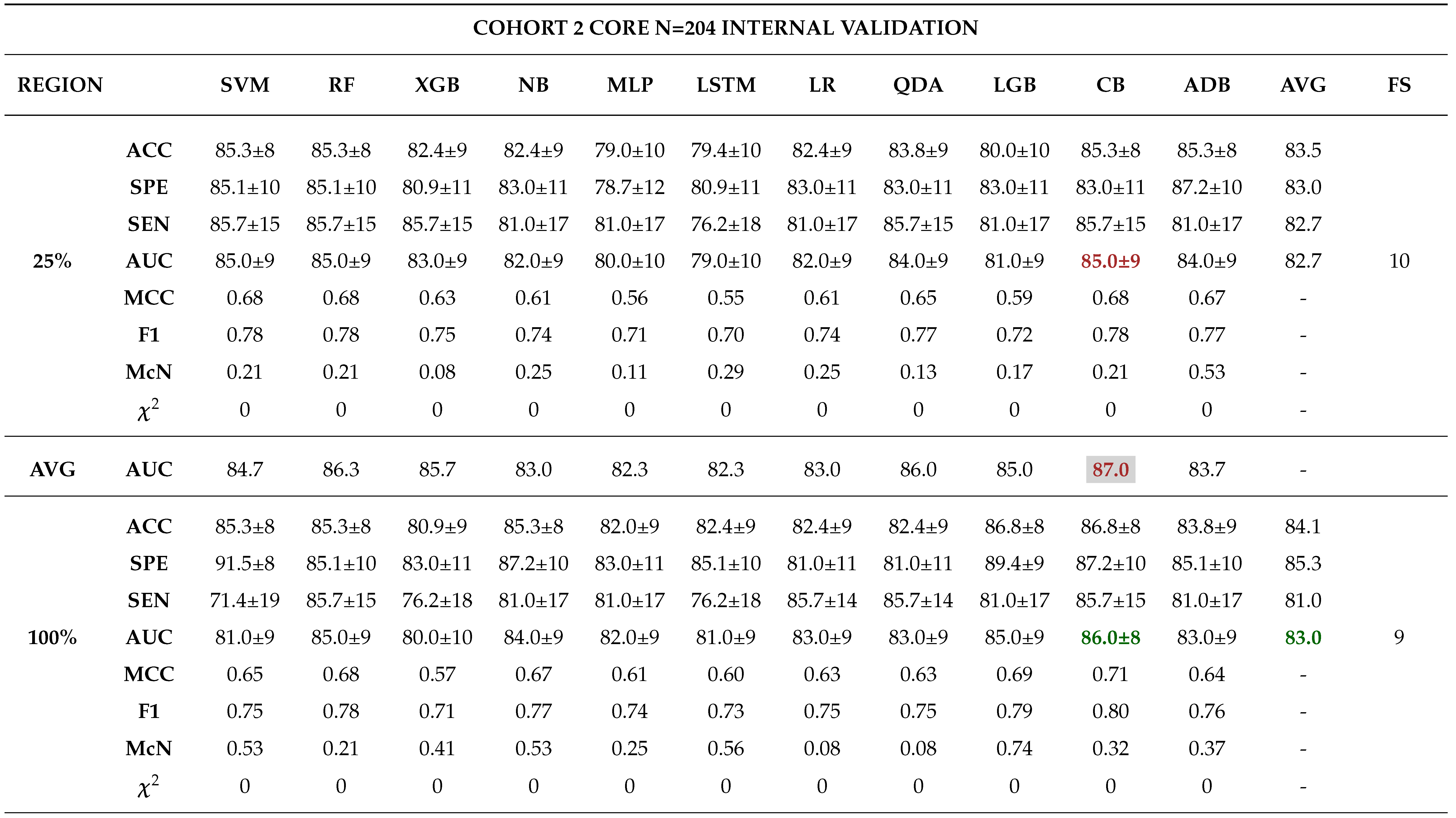
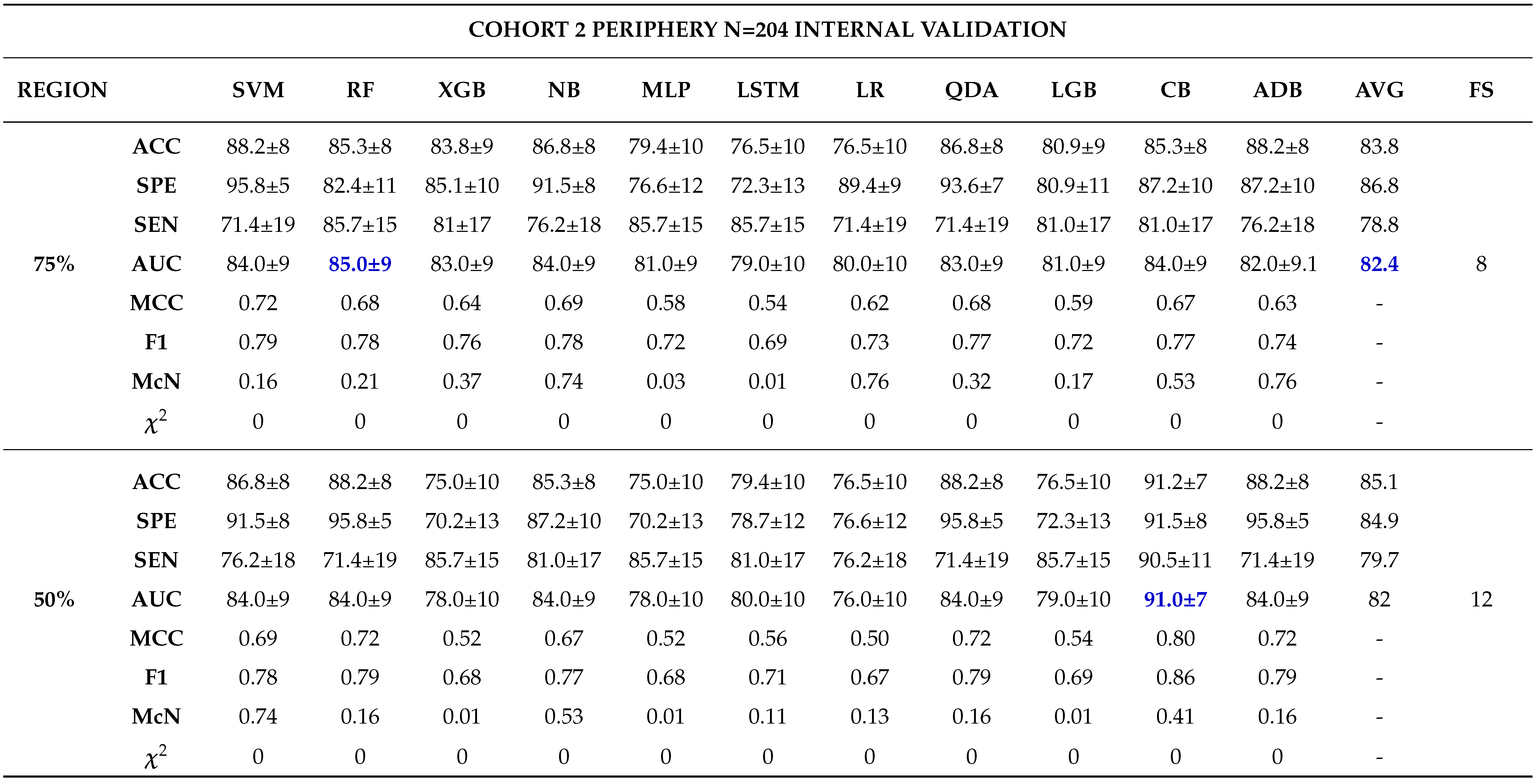
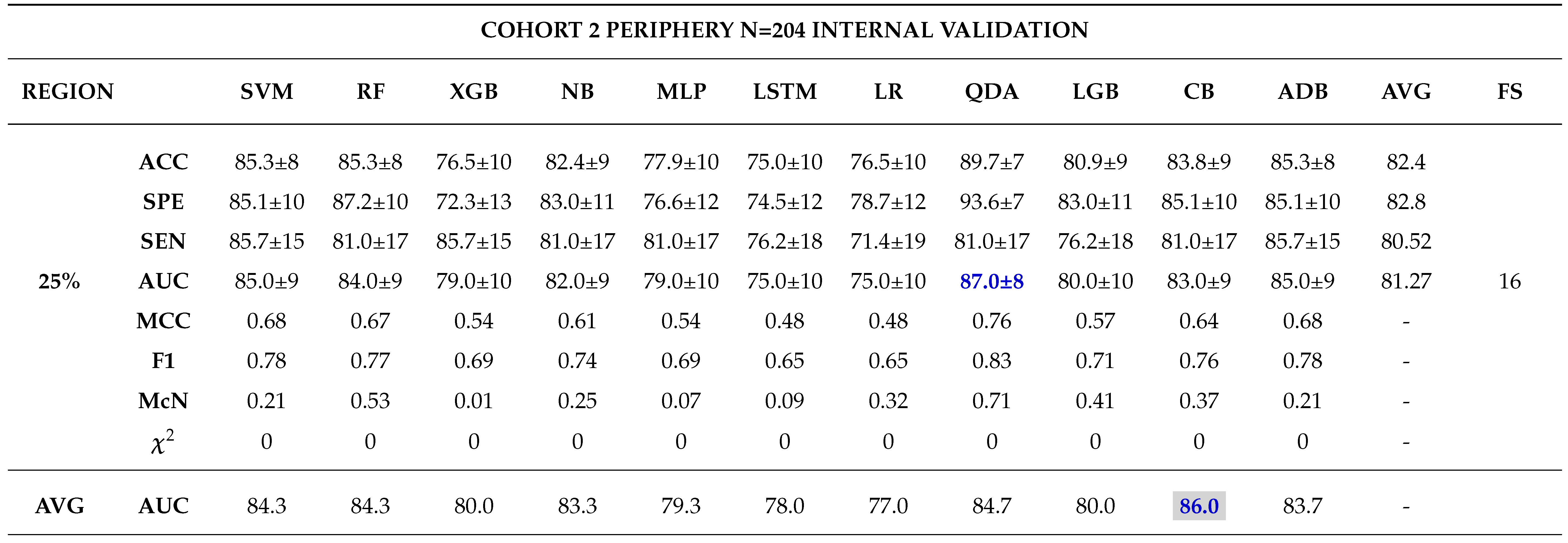
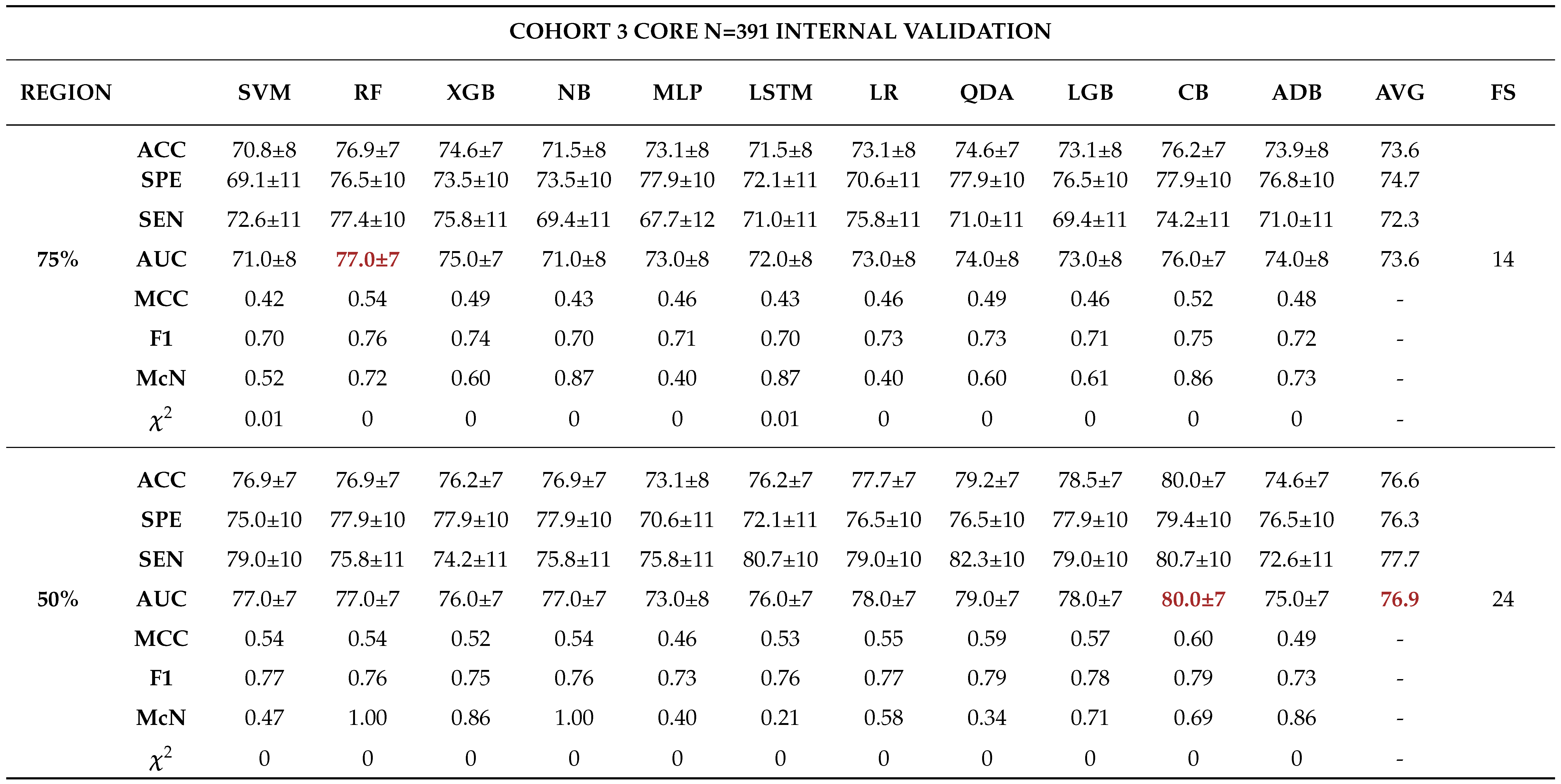
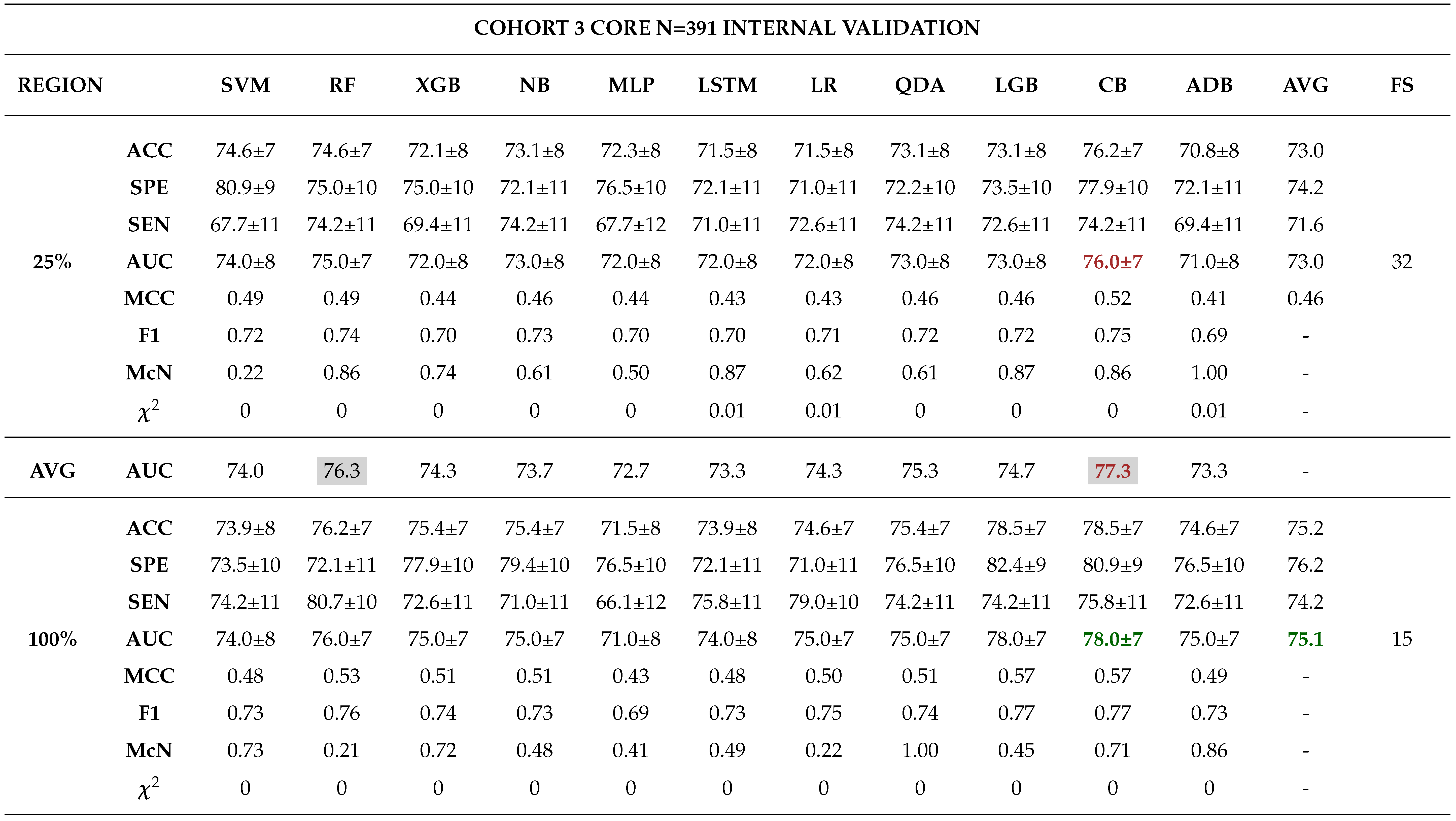
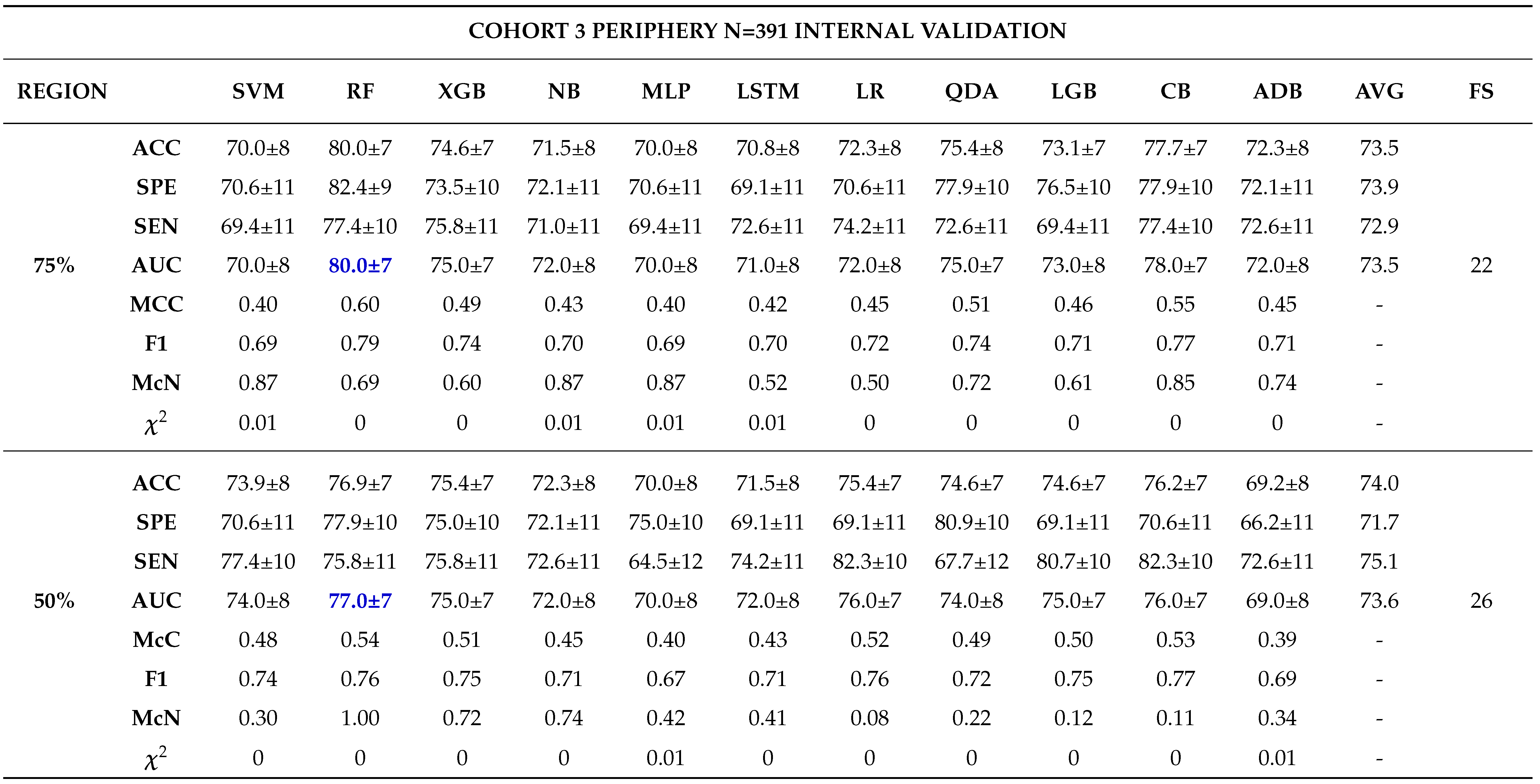
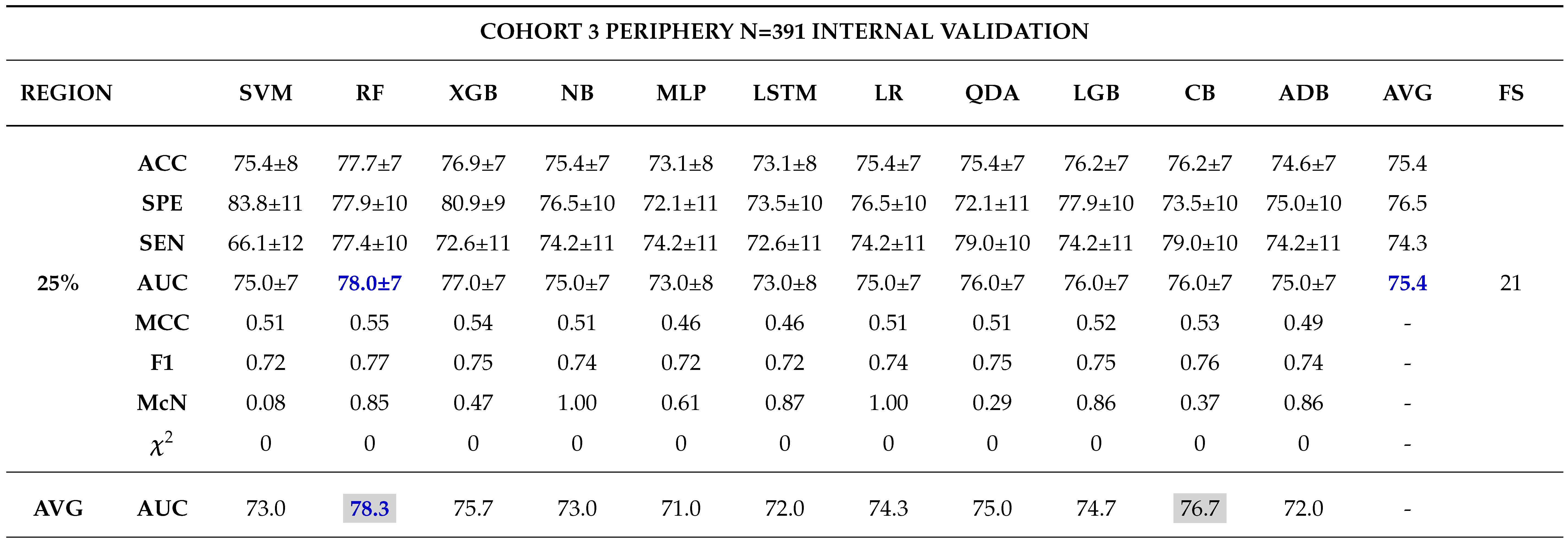
3.0.3. Model Validation and Evaluation
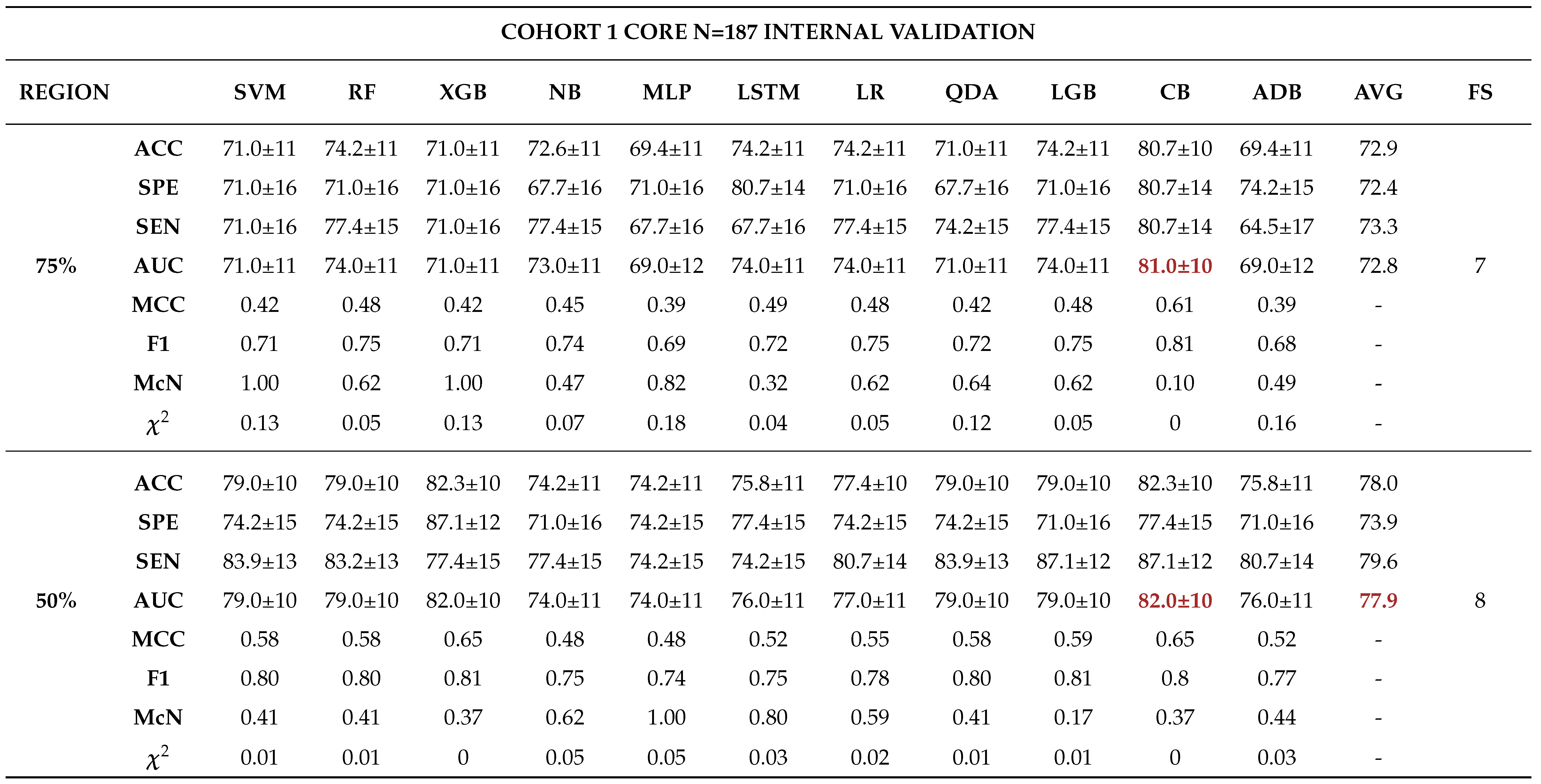
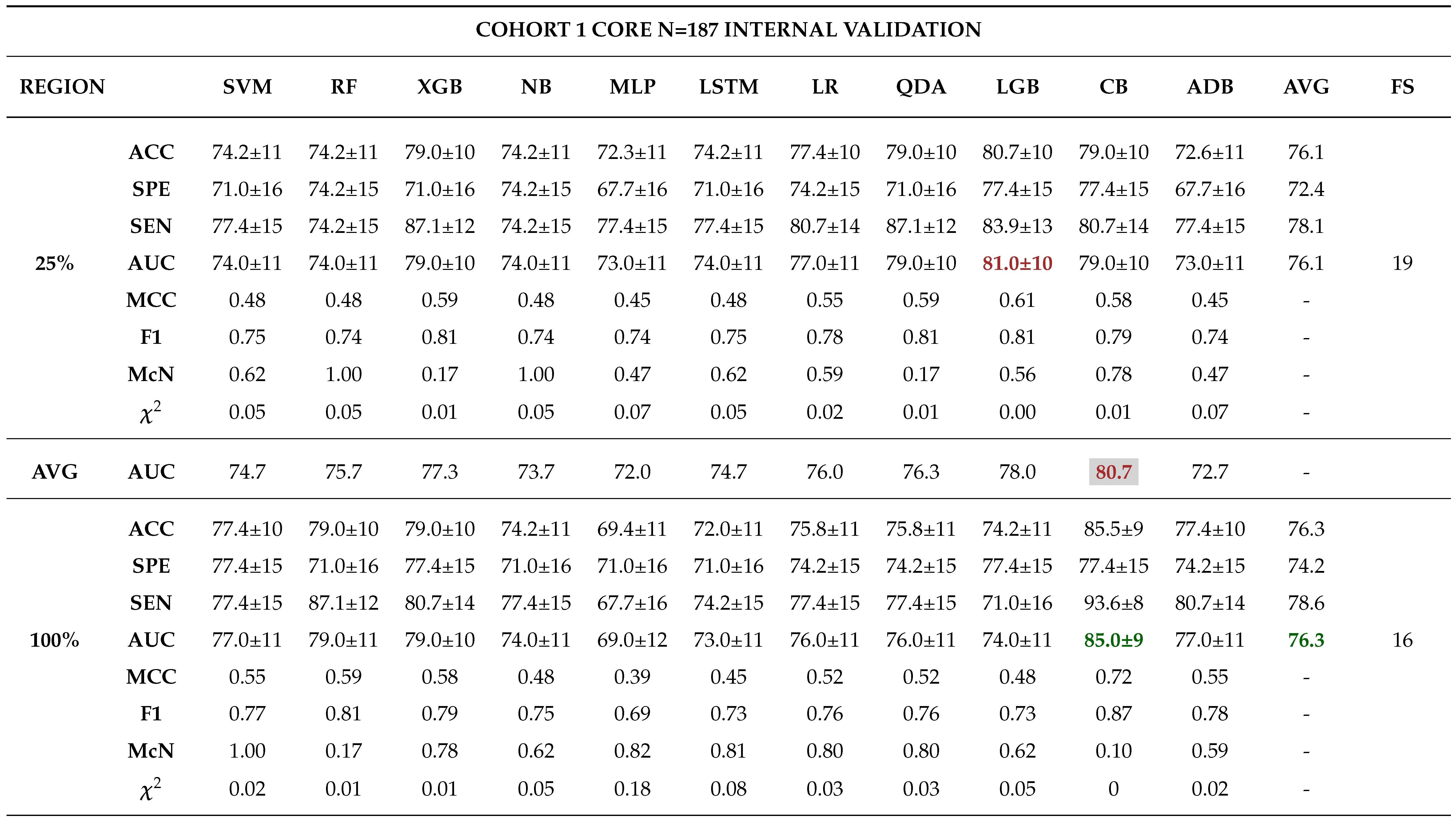
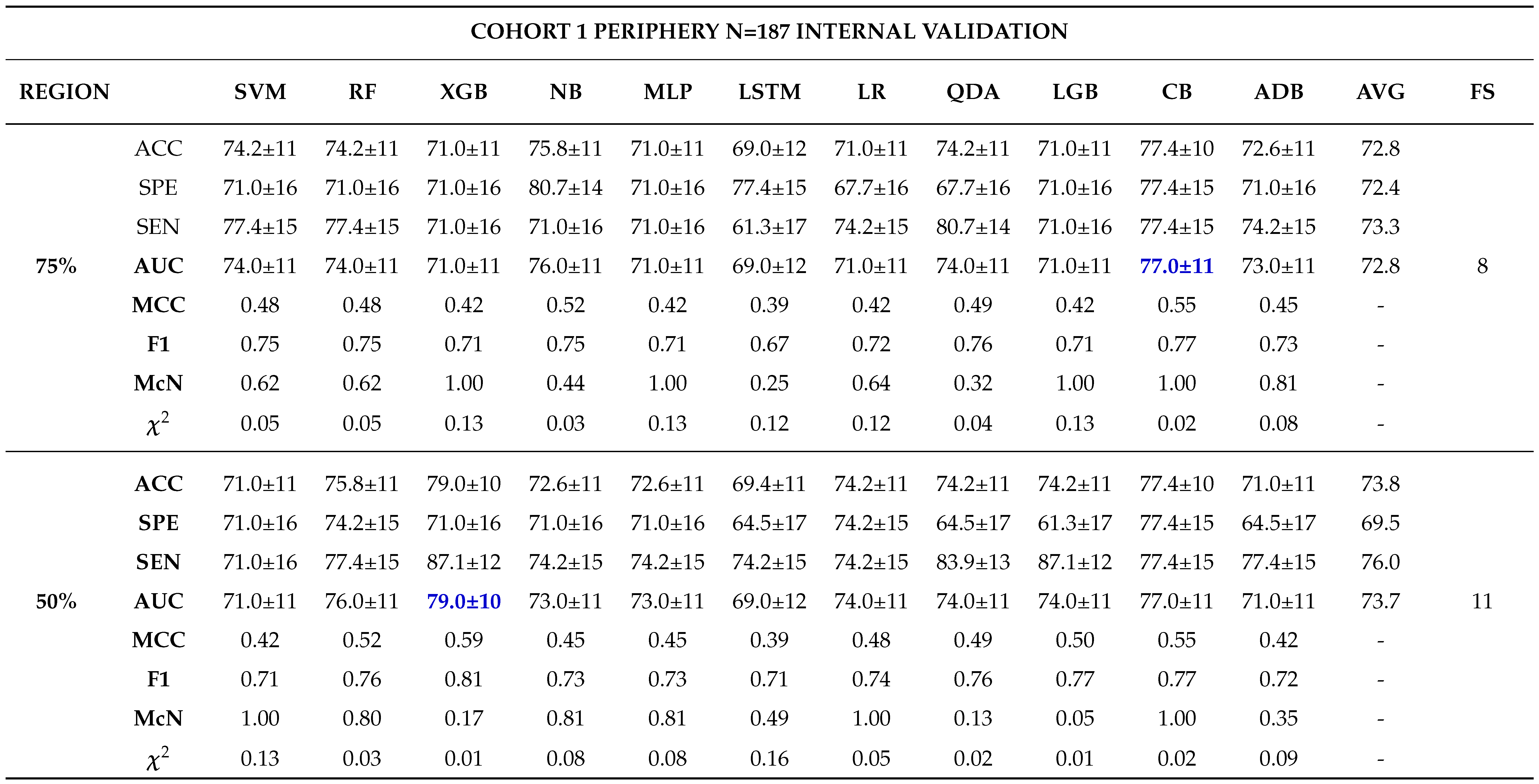
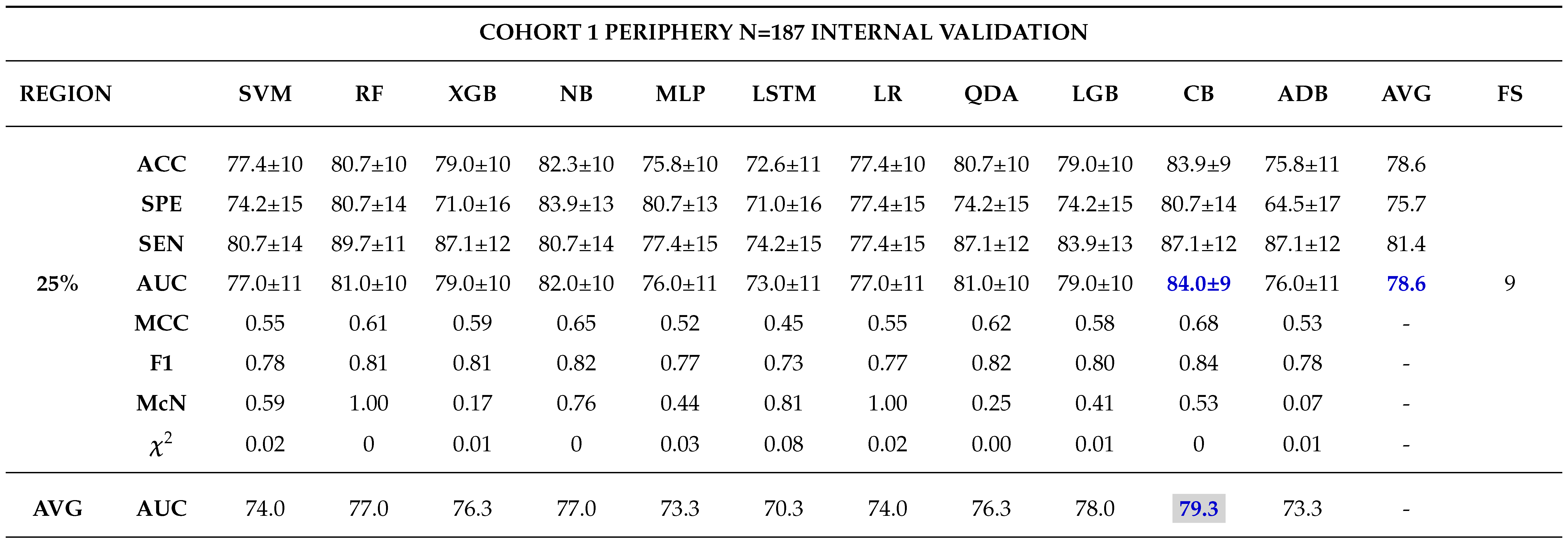
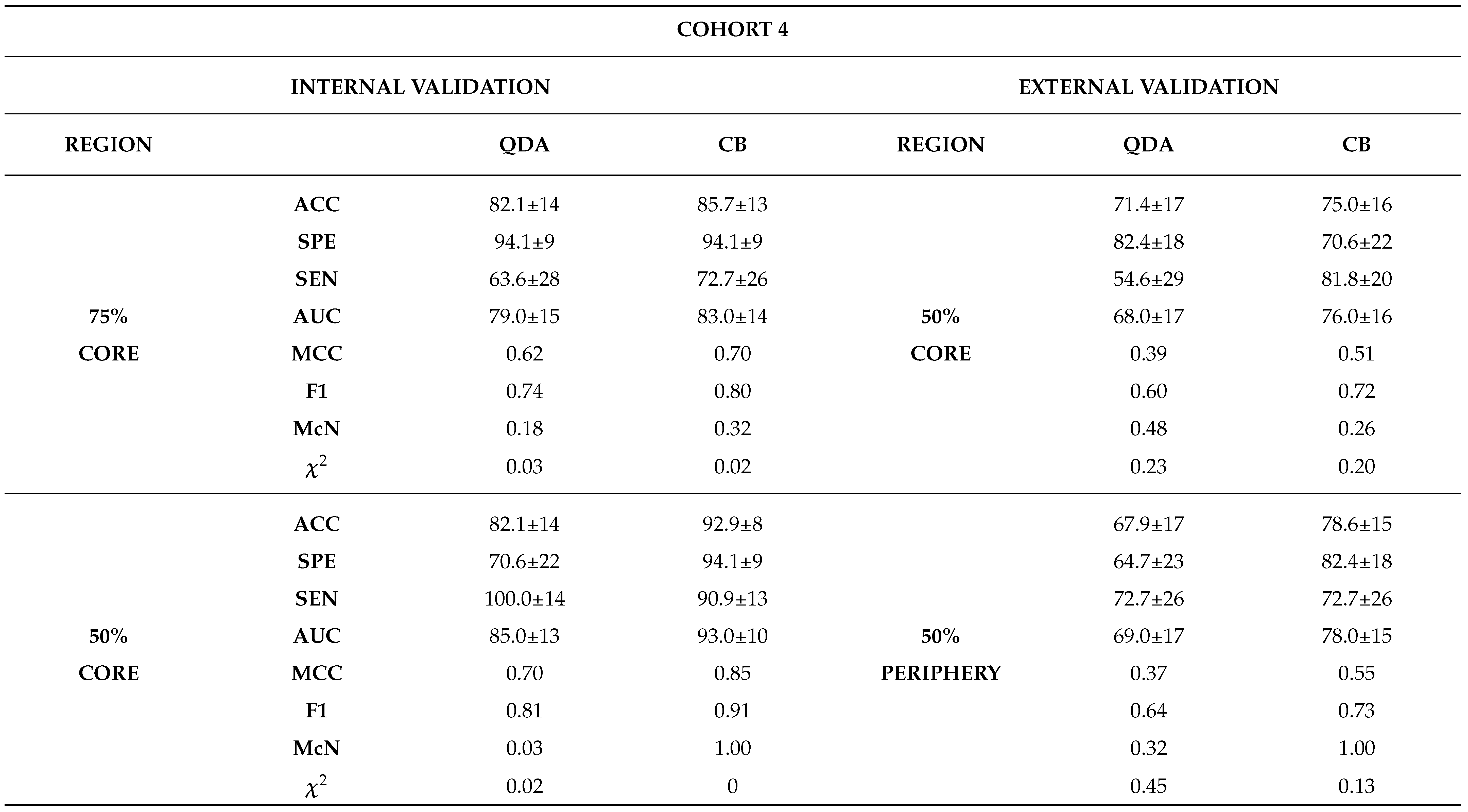
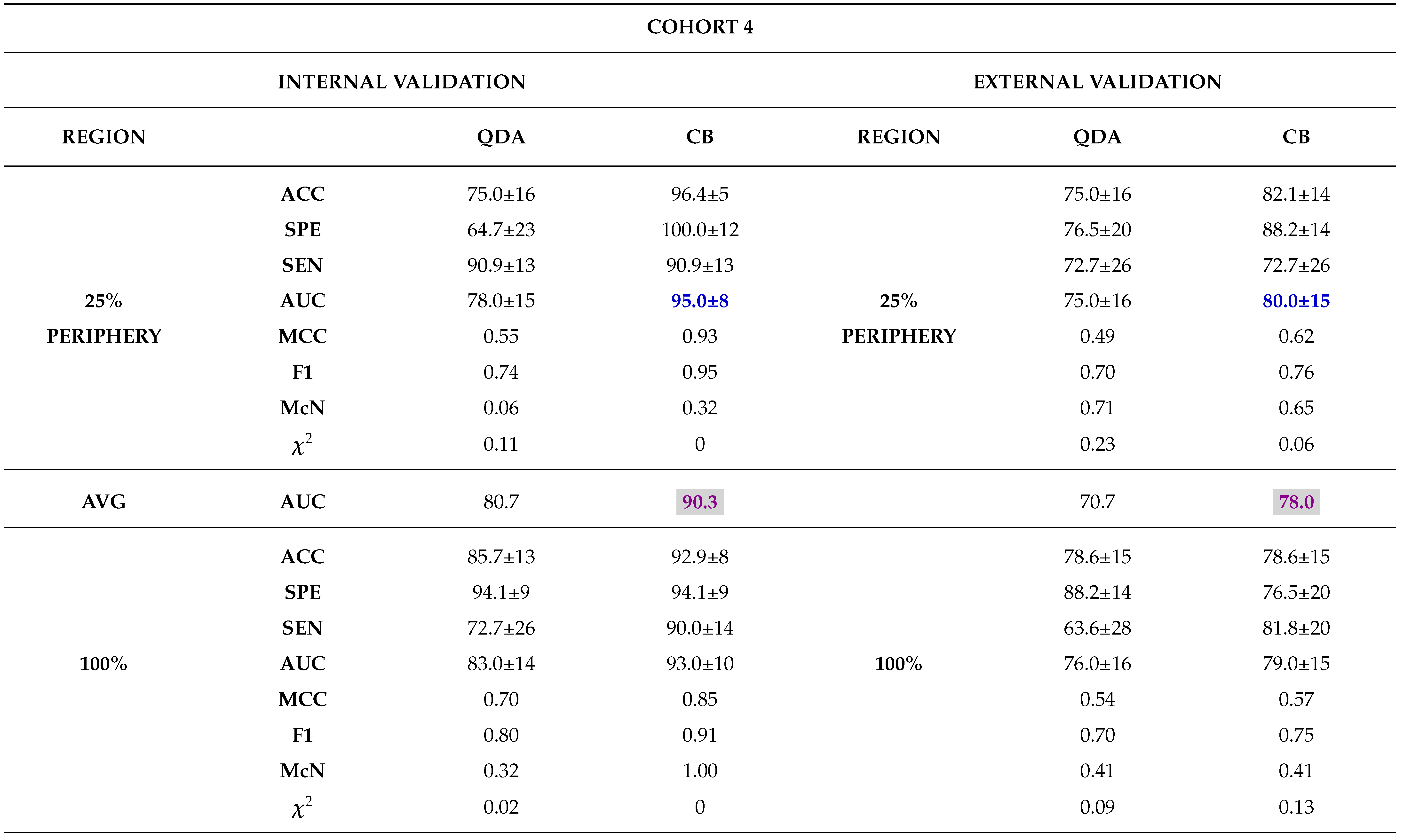
Internal Validation
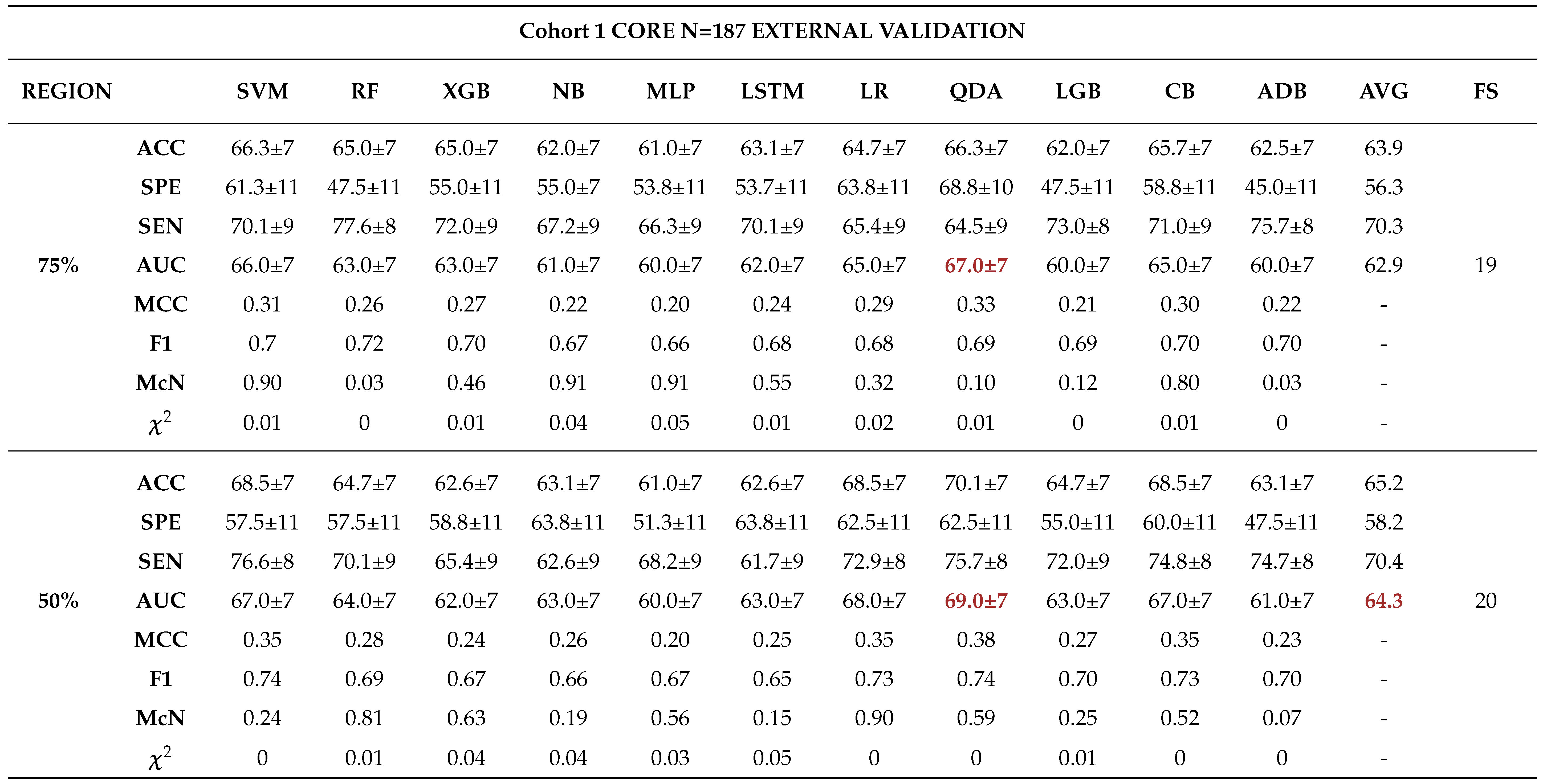
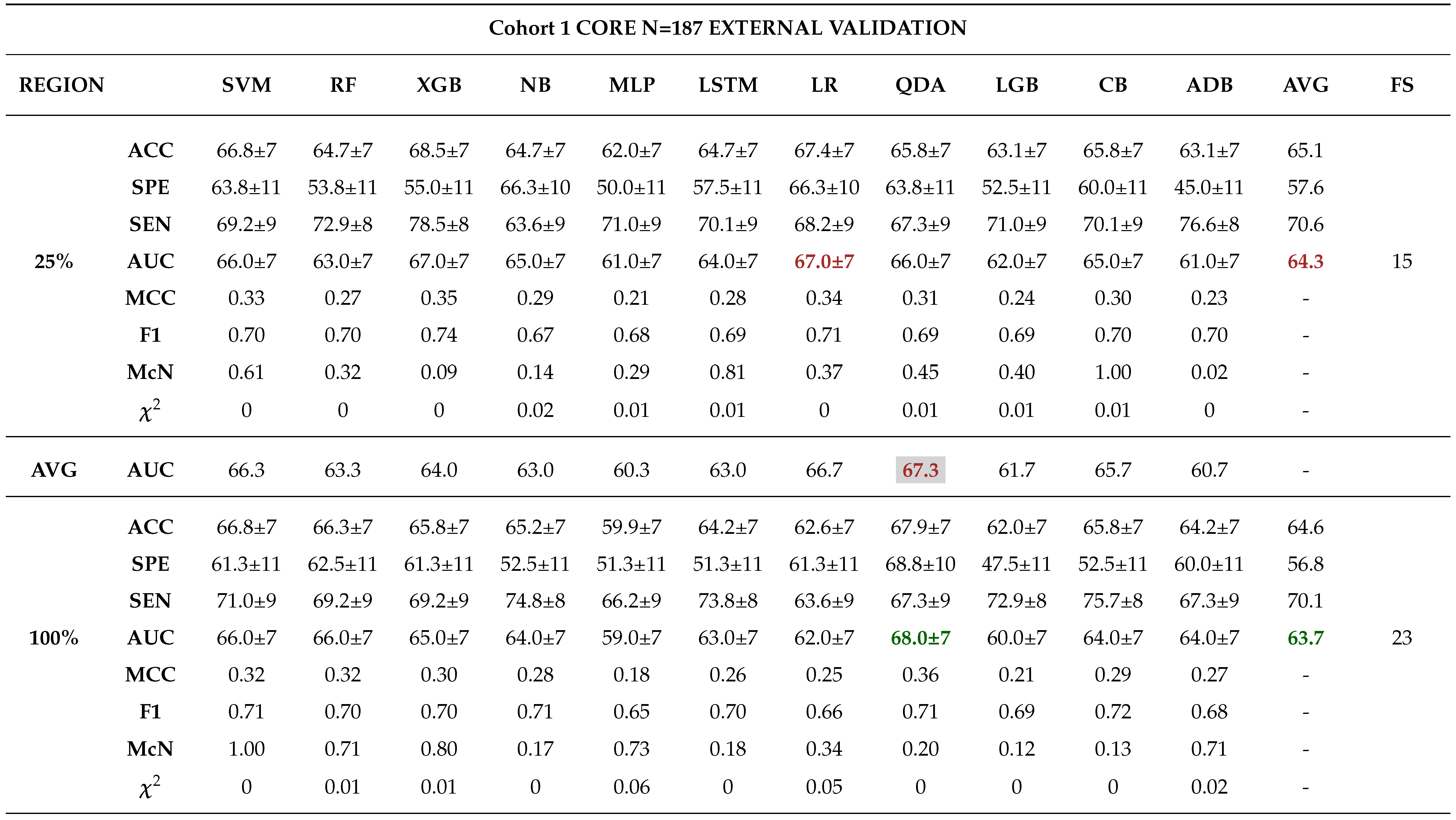
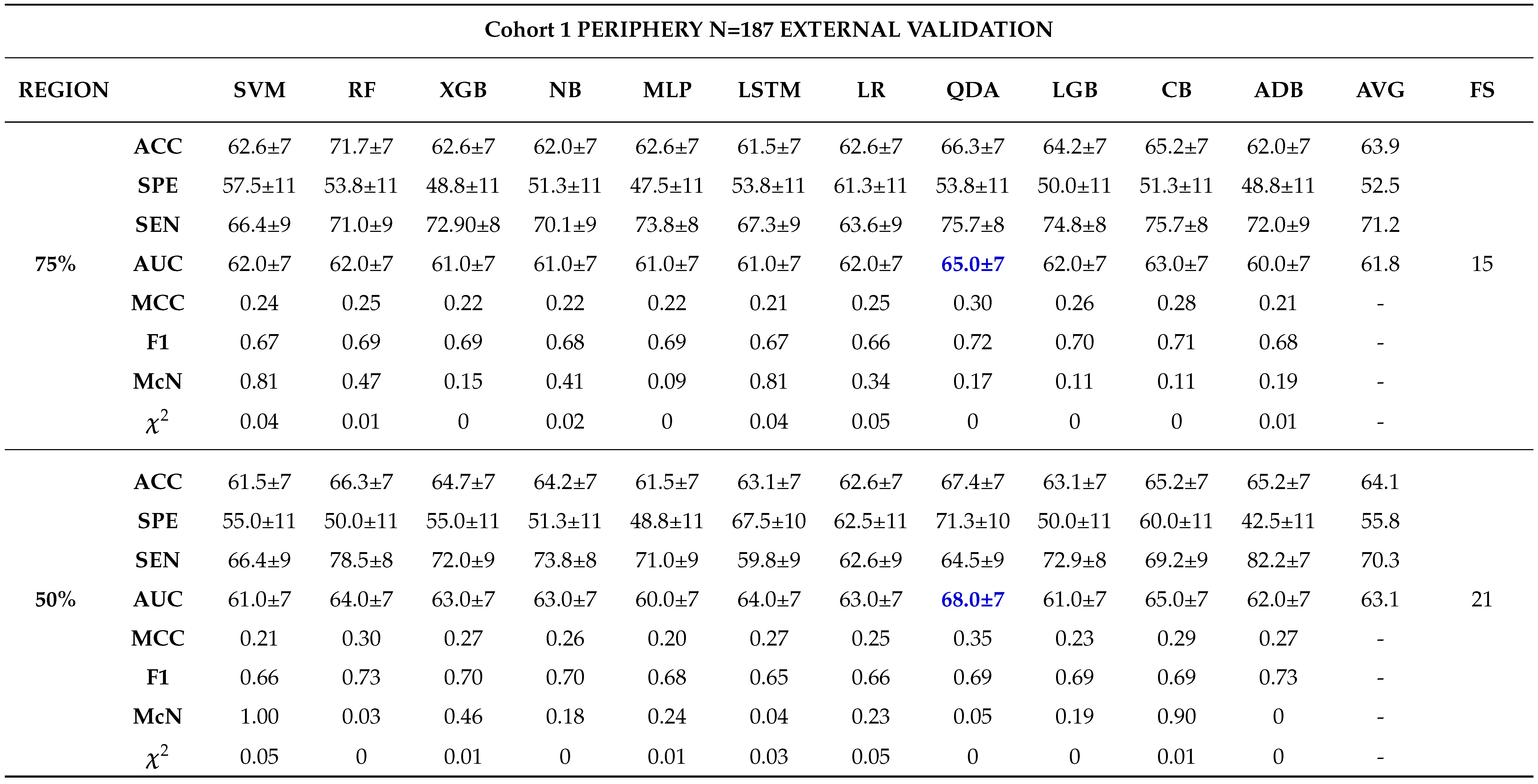
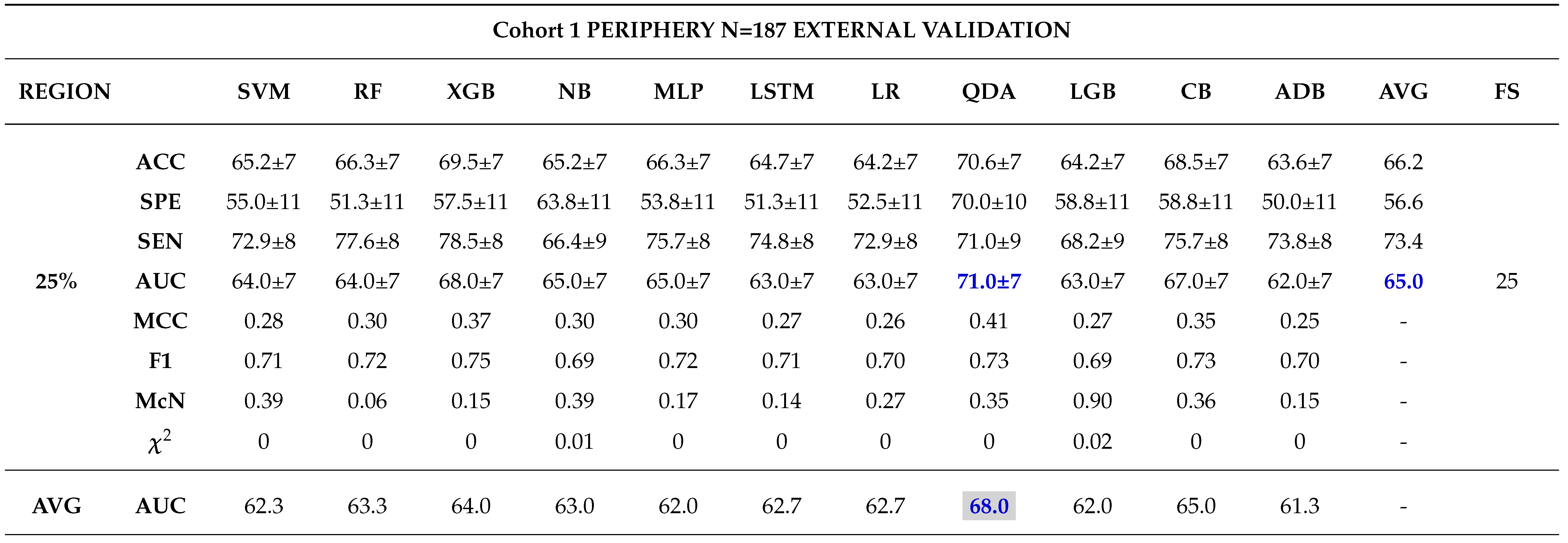
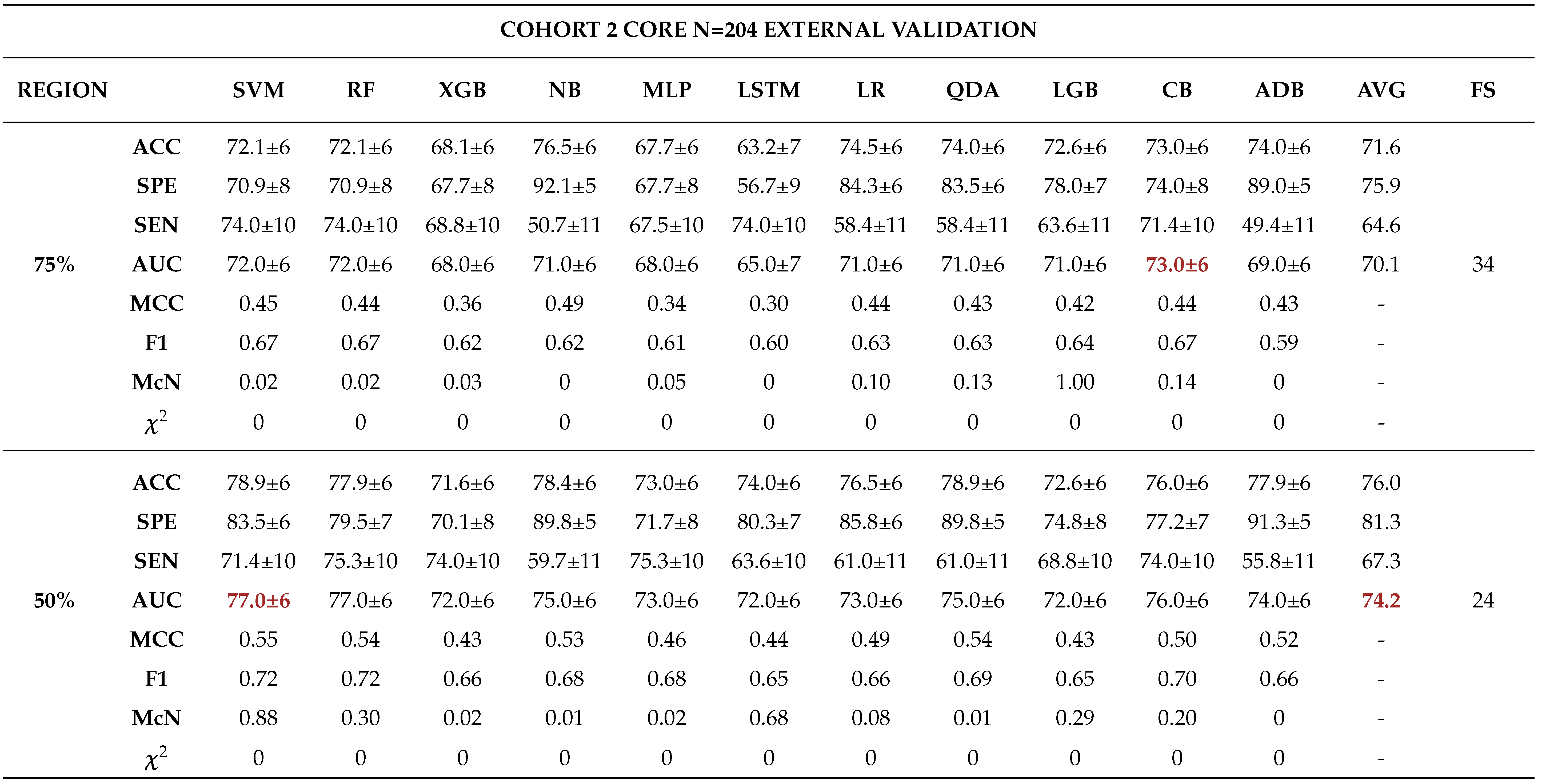
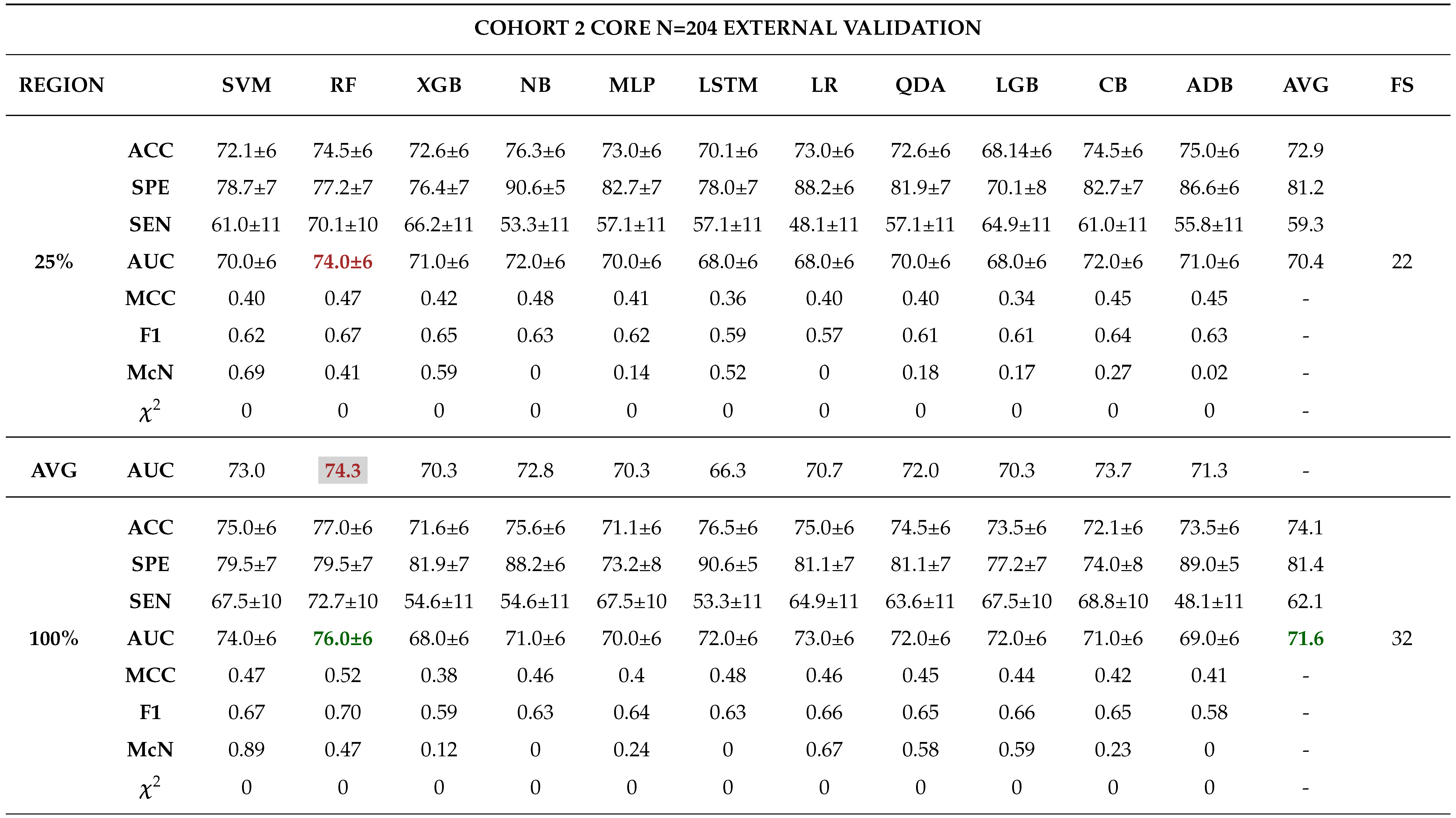
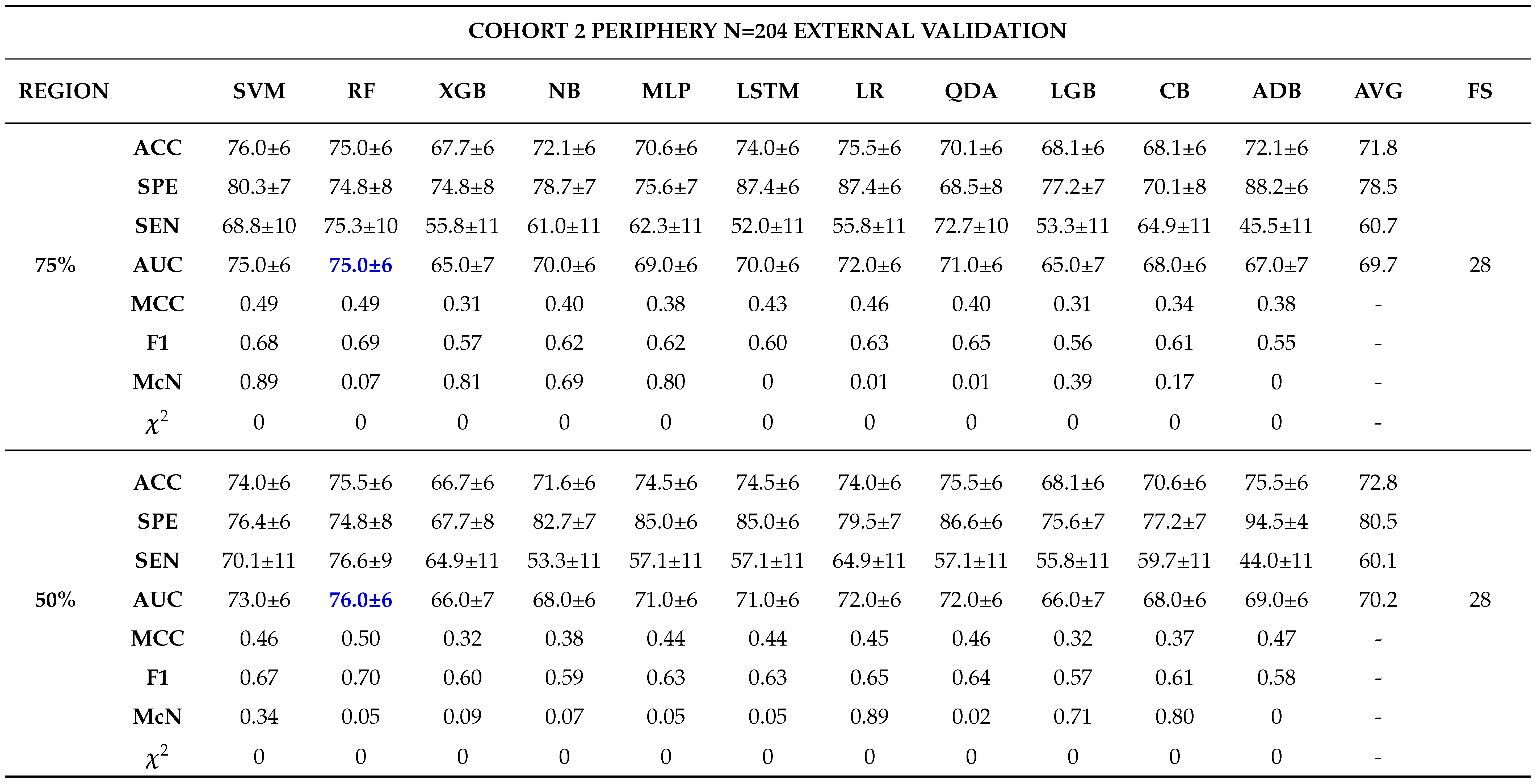
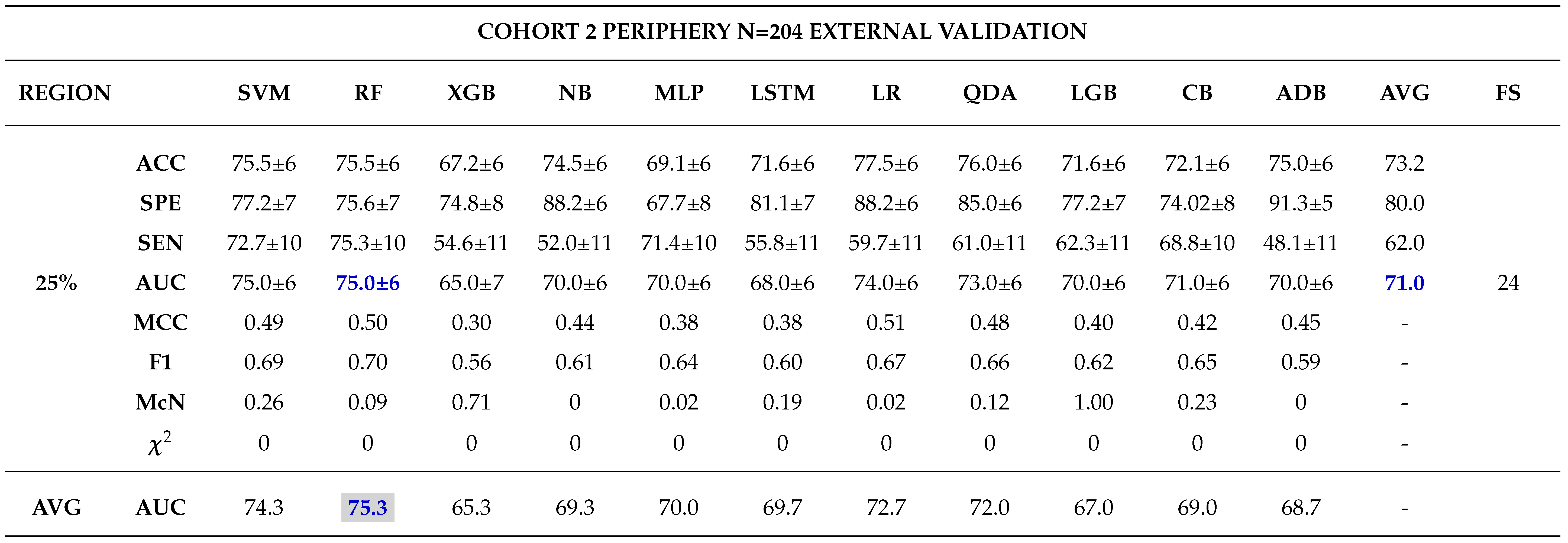
External Validation
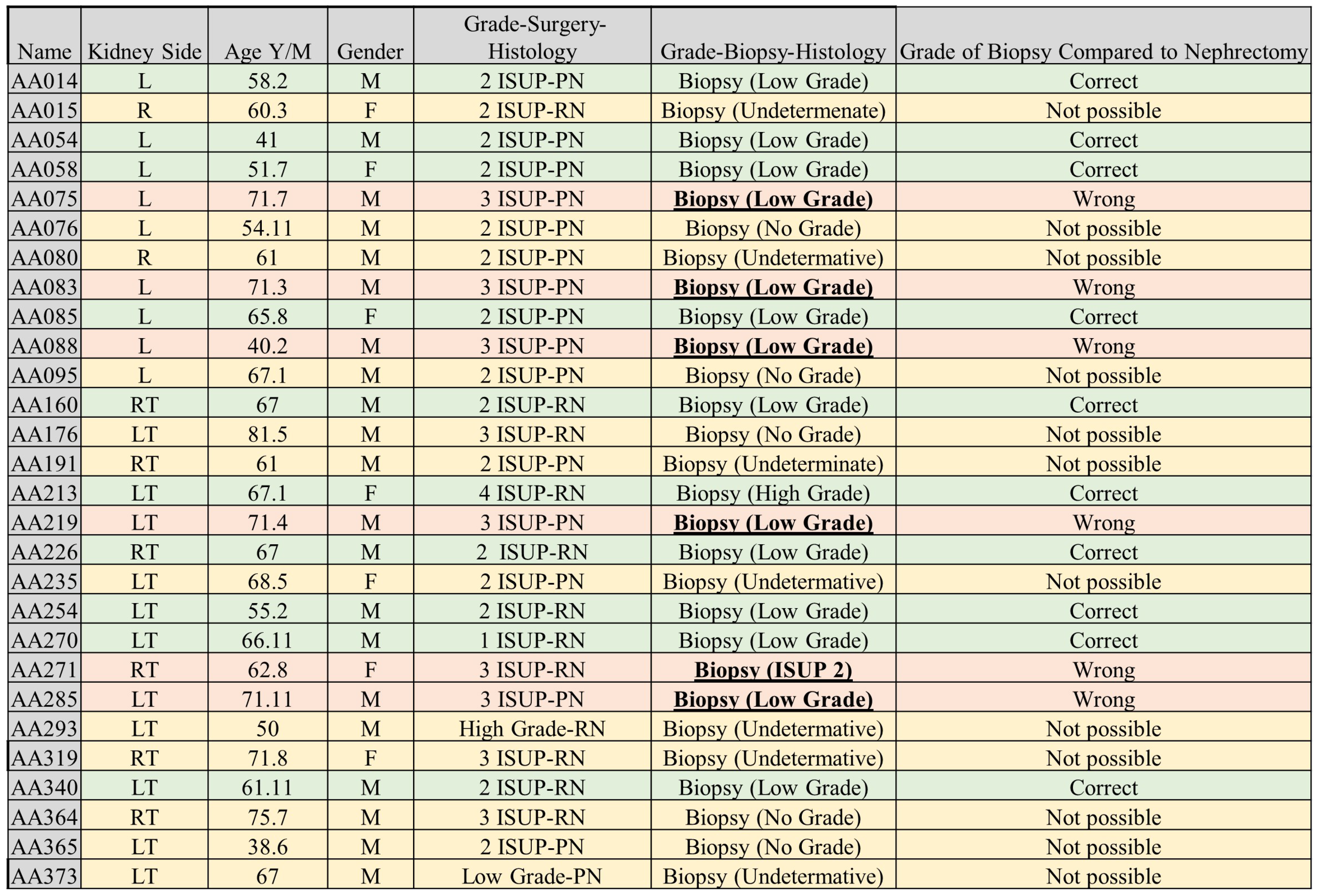
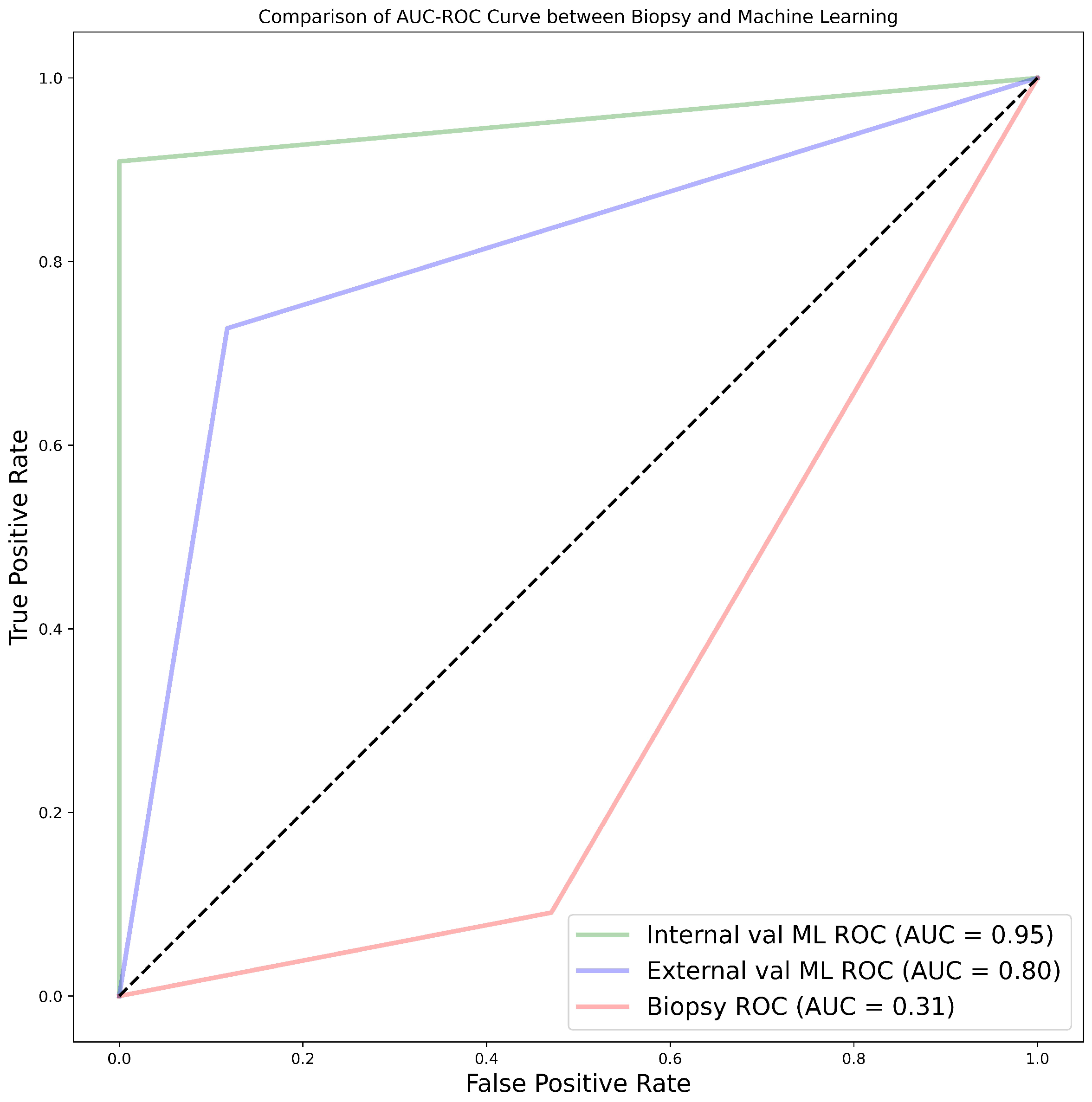
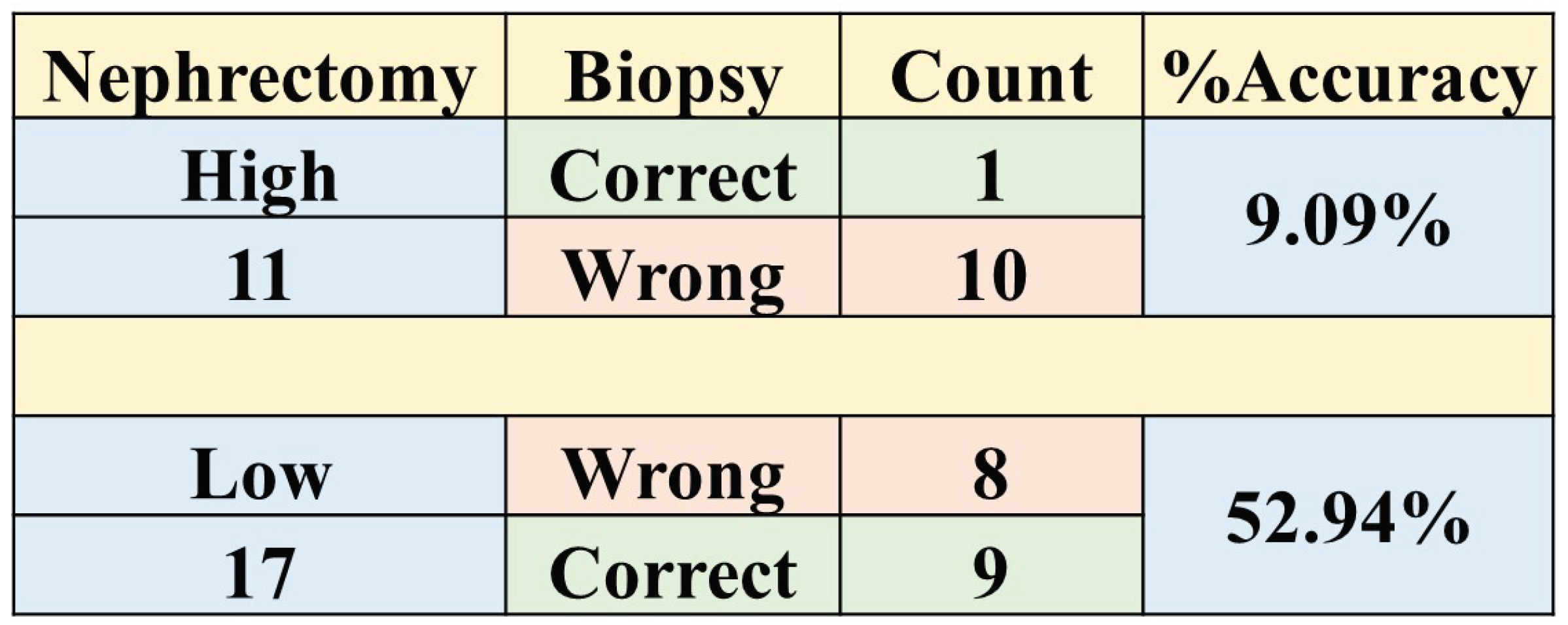
3.0.4. Comparison between Biopsy and Machine Learning Classification
4. Discussion
4.0.1. Literature Related to Methodological Proposal
4.0.6. Biopsy Grading and Its Comparison with ML
5. Summary and Conclusions
5.0.1. Limitations and Future research
5.0.2. Take-Home Messages
- - Radiomics features combined with ML algorithms have the potential to predict the WHO/ISUP grade of ccRCC more accurately than pre-operative biopsy.
- - Analysing different tumor subregions, such as the 50% tumor core and 25% tumor periphery, provides valuable information for determining tumor grade.
- - Analysing different cohorts from both single and multi-centre studies represented the effect of data heterogeneity on the model’s performance. This underscores the importance of implementing a robust model that generalizes well for real-world applications in grading ccRCCs.
- - The study highlighted the promising application of advanced imaging techniques and ML in oncology for precise tumor grading.
5.0.3. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Delahunt, B.; Eble, J.N.; Egevad, L.; Samaratunga, H. Grading of renal cell carcinoma. Histopathology 2019, 74, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Delahunt, B.; Sika-Paotonu, D.; Bethwaite, P.B.; Jordan, T.W.; Magi-Galluzzi, C.; Zhou, M.; Samaratunga, H.; Srigley, J.R. Grading of clear cell renal cell carcinoma should be based on nucleolar prominence. The American journal of surgical pathology 2011, 35, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, S.A.; Lasky, L.C.; Limas, C. Prognostic significance of morphologic parameters in renal cell carcinoma. The American journal of surgical pathology 1982, 6, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Dagher, J.; Delahunt, B.; Rioux-Leclercq, N.; Egevad, L.; Srigley, J.R.; Coughlin, G.; Dunglinson, N.; Gianduzzo, T.; Kua, B.; Malone, G.; others. Clear cell renal cell carcinoma: validation of World Health Organization/International Society of Urological Pathology grading. Histopathology 2017, 71, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO classification of tumours of the urinary system and male genital organs—part A: renal, penile, and testicular tumours. European urology 2016, 70, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Coy, H.; Douek, M.; Young, J.; Brown, M.S.; Goldin, J.; Sayre, J.; Raman, S. Differentiation of low grade from high grade clear cell renal cell carcinoma neoplasms using a CAD algorithm on four-phase CT., 2016.
- Jeon, H.G.; Seo, S.I.; Jeong, B.C.; Jeon, S.S.; Lee, H.M.; Choi, H.Y.; Song, C.; Hong, J.H.; Kim, C.S.; Ahn, H.; others. Percutaneous kidney biopsy for a small renal mass: a critical appraisal of results. The Journal of urology 2016, 195, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Delahunt, B.; Cheville, J.C.; Martignoni, G.; Humphrey, P.A.; Magi-Galluzzi, C.; McKenney, J.; Egevad, L.; Algaba, F.; Moch, H.; Grignon, D.J.; others. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. The American journal of surgical pathology 2013, 37, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Remzi, M.; Marberger, M. Renal tumor biopsies for evaluation of small renal tumors: why, in whom, and how? European urology 2009, 55, 359–367. [Google Scholar] [CrossRef]
- Lane, B.R.; Samplaski, M.K.; Herts, B.R.; Zhou, M.; Novick, A.C.; Campbell, S.C. Renal mass biopsy—a renaissance? The Journal of urology 2008, 179, 20–27. [Google Scholar] [CrossRef]
- Dhaun, N.; Bellamy, C.O.; Cattran, D.C.; Kluth, D.C. Utility of renal biopsy in the clinical management of renal disease. Kidney international 2014, 85, 1039–1048. [Google Scholar] [CrossRef]
- Andersen, M.; Norus, T. Tumor seeding with renal cell carcinoma after renal biopsy. Urology Case Reports 2016, 9, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Mattar, K.; Finelli, A.; Kachura, J.R.; Evans, A.J.; Geddie, W.R.; Jewett, M.A. Contemporary results of percutaneous biopsy of 100 small renal masses: a single center experience. The Journal of urology 2008, 180, 2333–2337. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gong, G.; Cui, Y.; Li, R. Intratumor partitioning and texture analysis of dynamic contrast-enhanced (DCE)-MRI identifies relevant tumor subregions to predict pathological response of breast cancer to neoadjuvant chemotherapy. Journal of Magnetic Resonance Imaging 2016, 44, 1107–1115. [Google Scholar] [CrossRef]
- Synnott, N.C.; Poeta, M.L.; Costantini, M.; Pfeiffer, R.M.; Li, M.; Golubeva, Y.; Lawrence, S.; Mutreja, K.; Amoreo, C.; Dabrowska, M.; others. Characterizing the tumor microenvironment in rare renal cancer histological types. The Journal of Pathology: Clinical Research 2022, 8, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Aguilera, T.; Shultz, D.; Gudur, M.; Rubin, D.L.; Loo Jr, B.W.; Diehn, M.; Li, R. Early-stage non–small cell lung cancer: quantitative imaging characteristics of 18F fluorodeoxyglucose PET/CT allow prediction of distant metastasis. Radiology 2016, 281, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Serganova, I.; Doubrovin, M.; Vider, J.; Ponomarev, V.; Soghomonyan, S.; Beresten, T.; Ageyeva, L.; Serganov, A.; Cai, S.; Balatoni, J.; others. Molecular imaging of temporal dynamics and spatial heterogeneity of hypoxia-inducible factor-1 signal transduction activity in tumors in living mice. Cancer Research 2004, 64, 6101–6108. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.Y.; Jiang, H.; Song, X.H.; Wu, J.K.; Ma, H. Four-phase computed tomography helps differentiation of renal oncocytoma with central hypodense areas from clear cell renal cell carcinoma. Diagn Interv Radiol 2022. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, Q.; Song, X.; Jiang, H.; Ma, H.; Li, W.; Wang, X. CT differentiation of the oncocytoma and renal cell carcinoma based on peripheral tumor parenchyma and central hypodense area characterisation. BMC Medical Imaging 2023, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Teifke, A.; Behr, O.; Schmidt, M.; Victor, A.; Vomweg, T.W.; Thelen, M.; Lehr, H.A. Dynamic MR imaging of breast lesions: correlation with microvessel distribution pattern and histologic characteristics of prognosis. Radiology 2006, 239, 351–360. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: images are more than pictures, they are data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; Van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; others. Radiomics: extracting more information from medical images using advanced feature analysis. European journal of cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Alhussaini, A.J.; Steele, J.D.; Nabi, G. Comparative Analysis for the Distinction of Chromophobe Renal Cell Carcinoma from Renal Oncocytoma in Computed Tomography Imaging Using Machine Learning Radiomics Analysis. Cancers 2022, 14, 3609. [Google Scholar] [CrossRef] [PubMed]
- Demirjian, N.L.; Varghese, B.A.; Cen, S.Y.; Hwang, D.H.; Aron, M.; Siddiqui, I.; Fields, B.K.; Lei, X.; Yap, F.Y.; Rivas, M. ; others. CT-based radiomics stratification of tumor grade and TNM stage of clear cell renal cell carcinoma. European Radiology, 2022, pp. 1–12.
- Nazari, M.; Shiri, I.; Zaidi, H. Radiomics-based machine learning model to predict risk of death within 5-years in clear cell renal cell carcinoma patients. Computers in biology and medicine 2021, 129, 104135. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Wynne, J.F.; Zhou, B.; Wang, T.; Lei, Y.; Curran, W.J.; Liu, T.; Yang, X. Artificial intelligence in tumor subregion analysis based on medical imaging: A review. Journal of Applied Clinical Medical Physics 2021, 22, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Arteaga, H.B.; Candamil-Cortés, M.S.; Breaux, B.; Guillen-Rondon, P.; Orozco-Arias, S.; Tabares-Soto, R. Machine learning applications on intratumoral heterogeneity in glioblastoma using single-cell RNA sequencing data. Briefings in Functional Genomics, 2023, p. elad002.
- Pan, Z.; Men, K.; Liang, B.; Song, Z.; Wu, R.; Dai, J. A subregion-based prediction model for local–regional recurrence risk in head and neck squamous cell carcinoma. Radiotherapy and Oncology 2023, 184, 109684. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yin, J. Texture analysis of breast DCE-MRI based on intratumoral subregions for predicting HER2 2+ status. Frontiers in Oncology 2020, 10, 543. [Google Scholar] [CrossRef]
- Heller, N.; Isensee, F.; Maier-Hein, K.H.; Hou, X.; Xie, C.; Li, F.; Nan, Y.; Mu, G.; Lin, Z.; Han, M.; others. The state of the art in kidney and kidney tumor segmentation in contrast-enhanced CT imaging: Results of the KiTS19 challenge. Medical image analysis 2021, 67, 101821. [Google Scholar] [CrossRef] [PubMed]
- Heller, N.; Sathianathen, N.; Kalapara, A.; Walczak, E.; Moore, K.; Kaluzniak, H.; Rosenberg, J.; Blake, P.; Rengel, Z.; Oestreich, M. ; others. The kits19 challenge data: 300 kidney tumor cases with clinical context, ct semantic segmentations, and surgical outcomes. arXiv preprint, arXiv:1904.00445 2019.
- Clark, K.; Vendt, B.; Smith, K.; Freymann, J.; Kirby, J.; Koppel, P.; Moore, S.; Phillips, S.; Maffitt, D.; Pringle, M.; others. The Cancer Imaging Archive (TCIA): maintaining and operating a public information repository. Journal of digital imaging 2013, 26, 1045–1057. [Google Scholar] [CrossRef]
- Shu, J.; Wen, D.; Xi, Y.; Xia, Y.; Cai, Z.; Xu, W.; Meng, X.; Liu, B.; Yin, H. Clear cell renal cell carcinoma: Machine learning-based computed tomography radiomics analysis for the prediction of WHO/ISUP grade. European journal of radiology 2019, 121, 108738. [Google Scholar] [CrossRef]
- Vobugari, N.; Raja, V.; Sethi, U.; Gandhi, K.; Raja, K.; Surani, S.R. Advancements in oncology with artificial intelligence—A review article. Cancers 2022, 14, 1349. [Google Scholar] [CrossRef]
- Nazari, M.; Shiri, I.; Hajianfar, G.; Oveisi, N.; Abdollahi, H.; Deevband, M.R.; Oveisi, M.; Zaidi, H. Noninvasive Fuhrman grading of clear cell renal cell carcinoma using computed tomography radiomic features and machine learning. La radiologia medica 2020, 125, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, L.; Xu, K.; Li, W.; Huo, Z.; Liu, H.; Shen, T.; Pan, F.; Jiang, Y.; Zhang, M. Prediction of ISUP grading of clear cell renal cell carcinoma using support vector machine model based on CT images. Medicine 2019, 98. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.W. Principles of CT: radiation dose and image quality. Journal of nuclear medicine technology 2007, 35, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Van Griethuysen, J.J.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J. Computational radiomics system to decode the radiographic phenotype. Cancer research 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, B.; Goh, V.; Mandeville, H.C.; Ng, Q.S.; Hoskin, P.J.; Miles, K.A. Non–small cell lung cancer: histopathologic correlates for texture parameters at CT. Radiology 2013, 266, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Debie, E.; Shafi, K. Implications of the curse of dimensionality for supervised learning classifier systems: theoretical and empirical analyses. Pattern Analysis and Applications 2019, 22, 519–536. [Google Scholar] [CrossRef]
- Brownlee, J. How to Avoid Data Leakage When Performing Data Preparation. https://machinelearningmastery.com/data-preparation-without-data-leakage/ accessed. 27 April 2023. [Google Scholar]
- Park, J.E.; Kim, D.; Kim, H.S.; Park, S.Y.; Kim, J.Y.; Cho, S.J.; Shin, J.H.; Kim, J.H. Quality of science and reporting of radiomics in oncologic studies: room for improvement according to radiomics quality score and TRIPOD statement. European radiology 2020, 30, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Karagöz, A.; Guvenis, A. Robust whole-tumour 3D volumetric CT-based radiomics approach for predicting the WHO/ISUP grade of a ccRCC tumour. Computer Methods in Biomechanics and Biomedical Engineering: Imaging & Visualization 2023, 11, 665–677. [Google Scholar]
- Zhao, Y.; Fu, X.; Lopez, J.I.; Rowan, A.; Au, L.; Fendler, A.; Hazell, S.; Xu, H.; Horswell, S.; Shepherd, S.T.; others. Selection of metastasis competent subclones in the tumour interior. Nature ecology & evolution 2021, 5, 1033–1045. [Google Scholar]
- He, X.; Wei, Y.; Zhang, H.; Zhang, T.; Yuan, F.; Huang, Z.; Han, F.; Song, B. Grading of clear cell renal cell carcinomas by using machine learning based on artificial neural networks and radiomic signatures extracted from multidetector computed tomography images. Academic Radiology 2020, 27, 157–168. [Google Scholar] [CrossRef]
- Xv, Y.; Lv, F.; Guo, H.; Zhou, X.; Tan, H.; Xiao, M.; Zheng, Y. Machine learning-based CT radiomics approach for predicting WHO/ISUP nuclear grade of clear cell renal cell carcinoma: an exploratory and comparative study. Insights Into Imaging 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cui, E.; Li, Z.; Ma, C.; Li, Q.; Lei, Y.; Lan, Y.; Yu, J.; Zhou, Z.; Li, R.; Long, W.; others. Predicting the ISUP grade of clear cell renal cell carcinoma with multiparametric MR and multiphase CT radiomics. European Radiology 2020, 30, 2912–2921. [Google Scholar] [CrossRef]
- Wang, R.; Hu, Z.; Shen, X.; Wang, Q.; Zhang, L.; Wang, M.; Feng, Z.; Chen, F. Computed tomography-based radiomics model for predicting the WHO/ISUP grade of clear cell renal cell carcinoma preoperatively: a multicenter study. Frontiers in Oncology 2021, 11, 543854. [Google Scholar] [CrossRef] [PubMed]
- Moldovanu, C.G.; Boca, B.; Lebovici, A.; Tamas-Szora, A.; Feier, D.S.; Crisan, N.; Andras, I.; Buruian, M.M. Preoperative predicting the WHO/ISUP nuclear grade of clear cell renal cell carcinoma by computed tomography-based radiomics features. Journal of Personalized Medicine 2021, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Xiao, Q.; Zeng, F.; Yin, H.; Li, Z.; Qian, C.; Wang, C.; Lei, G.; Xu, Q.; Li, C.; others. Computed tomography radiomics for predicting pathological grade of renal cell carcinoma. Frontiers in oncology 2021, 10, 570396. [Google Scholar] [CrossRef] [PubMed]
- Paterson, C.; Ghaemi, J.; Alashkham, A.; Biyani, C.S.; Coles, B.; Baker, L.; Szewczyk-Bieda, M.; Nabi, G. Diagnostic accuracy of image-guided biopsies in small (< 4 cm) renal masses with implications for active surveillance: a systematic review of the evidence. The British Journal of Radiology 2018, 91, 20170761. [Google Scholar] [PubMed]
- Bekkar, M.; Djemaa, H.K.; Alitouche, T.A. Evaluation measures for models assessment over imbalanced data sets. J Inf Eng Appl 2013, 3. [Google Scholar]
- Shi, L.; Campbell, G.; Jones, W.; Campagne, F.; Wen, Z.; Walker, S.; Su, Z.; Chu, T.; Goodsaid, F.; Pusztai, L.; others. The MAQC-II Project: A comprehensive study of common practices for the development and validation of microarray-based predictive models. Nature biotechnology 2010, 28, 827–838. [Google Scholar] [PubMed]
- Brown, J. Classifiers and their metrics quantified. Molecular informatics 2018, 37, 1700127. [Google Scholar] [CrossRef]
- Alzahrani, S.; Al-Bander, B.; Al-Nuaimy, W. A comprehensive evaluation and benchmarking of convolutional neural networks for melanoma diagnosis. Cancers 2021, 13, 4494. [Google Scholar] [CrossRef]
- Saito, T.; Rehmsmeier, M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PloS one 2015, 10, e0118432. [Google Scholar] [CrossRef]
- Chicco, D.; Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC genomics 2020, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Muglia, V.F.; Prando, A. Carcinoma de células renais: classificação histológica e correlação com métodos de imagem. Radiologia Brasileira 2015, 48, 166–174. [Google Scholar] [CrossRef]
- Chevrier, S.; Levine, J.H.; Zanotelli, V.R.T.; Silina, K.; Schulz, D.; Bacac, M.; Ries, C.H.; Ailles, L.; Jewett, M.A.S.; Moch, H.; others. An immune atlas of clear cell renal cell carcinoma. Cell 2017, 169, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Delahunt, B.; Egevad, L.; Samaratunga, H.; Martignoni, G.; Nacey, J.N.; Srigley, J.R. Gleason and Fuhrman no longer make the grade. Histopathology 2016, 68, 475–481. [Google Scholar] [CrossRef]
- Samaratunga, H.; Gianduzzo, T.; Delahunt, B. The ISUP system of staging, grading and classification of renal cell neoplasia. Journal of kidney cancer and VHL 2014, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Delahunt, B. Advances and controversies in grading and staging of renal cell carcinoma. Modern Pathology 2009, 22, S24–S36. [Google Scholar] [CrossRef]
- Lang, H.; Lindner, V.; de Fromont, M.; Molinié, V.; Letourneux, H.; Meyer, N.; Martin, M.; Jacqmin, D. Multicenter determination of optimal interobserver agreement using the Fuhrman grading system for renal cell carcinoma: assessment of 241 patients with> 15-year follow-up. Cancer 2005, 103, 625–629. [Google Scholar] [CrossRef]
- Chartrand, G.; Cheng, P.M.; Vorontsov, E.; Drozdzal, M.; Turcotte, S.; Pal, C.J.; Kadoury, S.; Tang, A. Deep learning: a primer for radiologists. Radiographics 2017, 37, 2113–2131. [Google Scholar] [CrossRef]
- Glaßer, S.; Niemann, U.; Preim, B.; Spiliopoulou, M. Can we distinguish between benign and malignant breast tumors in DCE-MRI by studying a tumor’s most suspect region only? Proceedings of the 26th IEEE International Symposium on Computer-Based Medical Systems. IEEE, 2013, pp. 77–82.
- Karahaliou, A.; Vassiou, K.; Arikidis, N.; Skiadopoulos, S.; Kanavou, T.; Costaridou, L. Assessing heterogeneity of lesion enhancement kinetics in dynamic contrast-enhanced MRI for breast cancer diagnosis. The British journal of radiology 2010, 83, 296–309. [Google Scholar] [CrossRef]
- Milenković, J.; Hertl, K.; Košir, A.; Žibert, J.; Tasič, J.F. Characterization of spatiotemporal changes for the classification of dynamic contrast-enhanced magnetic-resonance breast lesions. Artificial intelligence in medicine 2013, 58, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Agner, S.; Rosen, M.; Englander, S.; Sobers, D.; Thomas, K.; Tomaszewski, J.; Feldman, M.; Ganesan, S.; Schnall, M.; Madabhushi, A. Distinguishing molecular subtypes of breast cancer based on computer-aided diagnosis of dce-mri. International Society for Magnetic Resonance in Medicine Annual Meeting, 2010, Vol. 2490.
- Chaudhury, B.; Zhou, M.; Goldgof, D.B.; Hall, L.O.; Gatenby, R.A.; Gillies, R.J.; Drukteinis, J.S. Using features from tumor subregions of breast dce-mri for estrogen receptor status prediction. 2014 IEEE International Conference on Systems, Man, and Cybernetics (SMC). IEEE, 2014, pp. 2624–2629.
- Mahrooghy, M.; Ashraf, A.B.; Daye, D.; Mies, C.; Feldman, M.; Rosen, M.; Kontos, D. Heterogeneity wavelet kinetics from DCE-MRI for classifying gene expression based breast cancer recurrence risk. Medical Image Computing and Computer-Assisted Intervention–MICCAI 2013: 16th International Conference, Nagoya, Japan, September 22-26, 2013, Proceedings, Part II 16. Springer, 2013, pp. 295–302.
- Zhang, L.; Wang, Y.; Peng, Z.; Weng, Y.; Fang, Z.; Xiao, F.; Zhang, C.; Fan, Z.; Huang, K.; Zhu, Y.; others. The progress of multimodal imaging combination and subregion based radiomics research of cancers. International Journal of Biological Sciences 2022, 18, 3458. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Shu, J.; Xia, Y.; Yang, R. A CT-based radiomics approach for the differential diagnosis of sarcomatoid and clear cell renal cell carcinoma. BioMed Research International 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Leng, S.; Kitajima, K.; Gomez-Cardona, D.; Thapa, P.; Carter, R.E.; Leibovich, B.C.; Sasiwimonphan, K.; Sasaguri, K.; Kawashima, A. Small (< 4 cm) renal masses: differentiation of angiomyolipoma without visible fat from renal cell carcinoma using unenhanced and contrast-enhanced CT. American Journal of Roentgenology 2015, 205, 1194–1202. [Google Scholar] [PubMed]
- Varga, T.V.; Niss, K.; Estampador, A.C.; Collin, C.B.; Moseley, P.L. Association is not prediction: a landscape of confused reporting in diabetes–a systematic review. Diabetes research and clinical practice 2020, 170, 108497. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.; Fonseca, L.M. How sample size influences research outcomes. Dental press journal of orthodontics 2014, 19, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, C.; Zhang, F.; Cheng, X.; Wei, Y.; Fan, S.; Liu, M.; He, X.; Deng, J.; Xie, T.; others. Deep learning using CT images to grade clear cell renal cell carcinoma: development and validation of a prediction model. Cancers 2022, 14, 2574. [Google Scholar] [CrossRef] [PubMed]
- Wen-Zhi, G.; Tai, T.; Zhixin, F.; Huanyu, L.; Yanqing, G.; Yuexian, G.; Xuesong, L. Prediction of pathological staging and grading of renal clear cell carcinoma based on deep learning algorithms. Journal of International Medical Research 2022, 50, 03000605221135163. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Ma, C.; Xu, J.; Lei, Y.; Li, Q.; Lan, Y.; Sun, M.; Long, W.; Cui, E. A CT-based deep learning model for predicting the nuclear grade of clear cell renal cell carcinoma. European Journal of Radiology 2020, 129, 109079. [Google Scholar] [CrossRef] [PubMed]
- Lechevallier, E.; André, M.; Barriol, D.; Daniel, L.; Eghazarian, C.; De Fromont, M.; Rossi, D.; Coulange, C. Fine-needle percutaneous biopsy of renal masses with helical CT guidance. Radiology 2000, 216, 506–510. [Google Scholar] [CrossRef]
- Volpe, A.; Finelli, A.; Gill, I.S.; Jewett, M.A.; Martignoni, G.; Polascik, T.J.; Remzi, M.; Uzzo, R.G. Rationale for percutaneous biopsy and histologic characterisation of renal tumours. European urology 2012, 62, 491–504. [Google Scholar] [CrossRef]
- Blumenfeld, A.J.; Guru, K.; Fuchs, G.J.; Kim, H.L. Percutaneous biopsy of renal cell carcinoma underestimates nuclear grade. Urology 2010, 76, 610–613. [Google Scholar] [CrossRef]
- Lebret, T.; Poulain, J.E.; Molinie, V.; Herve, J.M.; Denoux, Y.; Guth, A.; Scherrer, A.; Botto, H. Percutaneous core biopsy for renal masses: indications, accuracy and results. The Journal of urology 2007, 178, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Millet, I.; Curros, F.; Serre, I.; Taourel, P.; Thuret, R. Can renal biopsy accurately predict histological subtype and Fuhrman grade of renal cell carcinoma? The Journal of urology 2012, 188, 1690–1694. [Google Scholar] [CrossRef]
- Neuzillet, Y.; Lechevallier, E.; Andre, M.; Daniel, L.; Coulange, C. Accuracy and clinical role of fine needle percutaneous biopsy with computerized tomography guidance of small (less than 4.0 cm) renal masses. The Journal of urology 2004, 171, 1802–1805. [Google Scholar] [CrossRef]
- Schmidbauer, J.; Remzi, M.; Memarsadeghi, M.; Haitel, A.; Klingler, H.C.; Katzenbeisser, D.; Wiener, H.; Marberger, M. Diagnostic accuracy of computed tomography-guided percutaneous biopsy of renal masses. European urology 2008, 53, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, H.; Hindermann, W.; Mustafa, A.M.A.; Reichelt, O.; Junker, K.; SCHUBERT, J. The accuracy of 250 fine needle biopsies of renal tumors. The Journal of urology 2005, 174, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, D.A.; Egleston, B.L.; Uzzo, R.G. Excise, ablate or observe: the small renal mass dilemma—a meta-analysis and review. The Journal of urology 2008, 179, 1227–1234. [Google Scholar] [CrossRef]

| Tumour and Patient Characteristics | |||||
|---|---|---|---|---|---|
| Variable | Low-Grade | High-Grade | p-Value | rpb* | |
| Age (Mean ± SD) | 59.05±12.28 | 64±9.40 | 0.002* | 0.4 | |
| Cohort 1 | Size (cm) | 4.32±2.02 | 6.03±3.23 | 0.00006* | 0.6 |
| n=187 | Volume (cm) | 75.8±90.90 | 203.74±305.82 | 0* | 0.6 |
| Gender | 0.331 | ||||
| Male | 49 (26.20%) | 74 (39.57%) | |||
| Female | 31 (16.58%) | 33 (17.65%) | |||
| Age (Mean ± SD) | 57.17±12.67 | 63.68±11.14 | 0* | 0.88 | |
| Cohort 2 | Size (cm) | 3.89±2.16 | 6.81±3.55 | 0* | 0.45 |
| n=204 | Volume (cm) | 51.44±114.32 | 235.26±326.10 | 0* | 0.56 |
| Gender | 0.25 | ||||
| Male | 77 (37.75%) | 57 (27.94%) | |||
| Female | 50 (24.51%) | 20 (9.80%) | |||
| Age (Mean ± SD) | 57.89±12.55 | 63.86±10.17 | 0* | 0.56 | |
| Cohort 3 | Size (cm) | 3.89±2.16 | 6.81±3.55 | 0* | 0.2 |
| n=391 | Volume (cm) | 60.86±106.55 | 216.93±314.85 | 0* | 0.29 |
| Gender | 0.25 | ||||
| Male | 126 (32.23%) | 131 (33.50%) | |||
| Female | 81 (20.72%) | 53 (13.55%) | |||
| Variable | Low-Grade | High-Grade | p-Value | rpb* | |
| Age (Mean ± SD) | 57.12±10.25 | 62.09±9.39 | 0.22 | ||
| Cohort 4 | Size (cm) | 3.31±0.94 | 4.02±2.25 | 0.28 | |
| n=28 | Volume (cm) | 25.98±27.10 | 57.16±70.40 | 0.13 | |
| Gender | 1 | ||||
| Male | 12 (42.86%) | 8 (28.57%) | |||
| Female | 5 (17.86%) | 3 (10.71%) | |||
| * Statistical significance at the 0.05 level; * point-biserial correlation coefficient (rpb). | |||||
| BIOPSY | MACHINE LEARNING | ||
|---|---|---|---|
| METRICS | INTERNAL VALIDATION | EXTERNAL VALIDATION | |
| ACC | 35.7±18 | 96.4±5 | 82.1±14 |
| SPE | 52.9±24 | 100.0±12 | 88.2±14 |
| SEN | 9.1±13 | 90.9±13 | 72.7±26 |
| AUC | 31.0±17 | 95.0±8 | 80.0±15 |
| MCC | -0.40 | 0.93 | 0.62 |
| F1 | 0.1 | 0.95 | 0.76 |
| McN | 0.64 | 0.32 | 0.65 |
| 0.15 | 0 | 0.06 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





