Submitted:
18 December 2023
Posted:
19 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Regulation of FOSL1
2.1. Transcriptional regulation
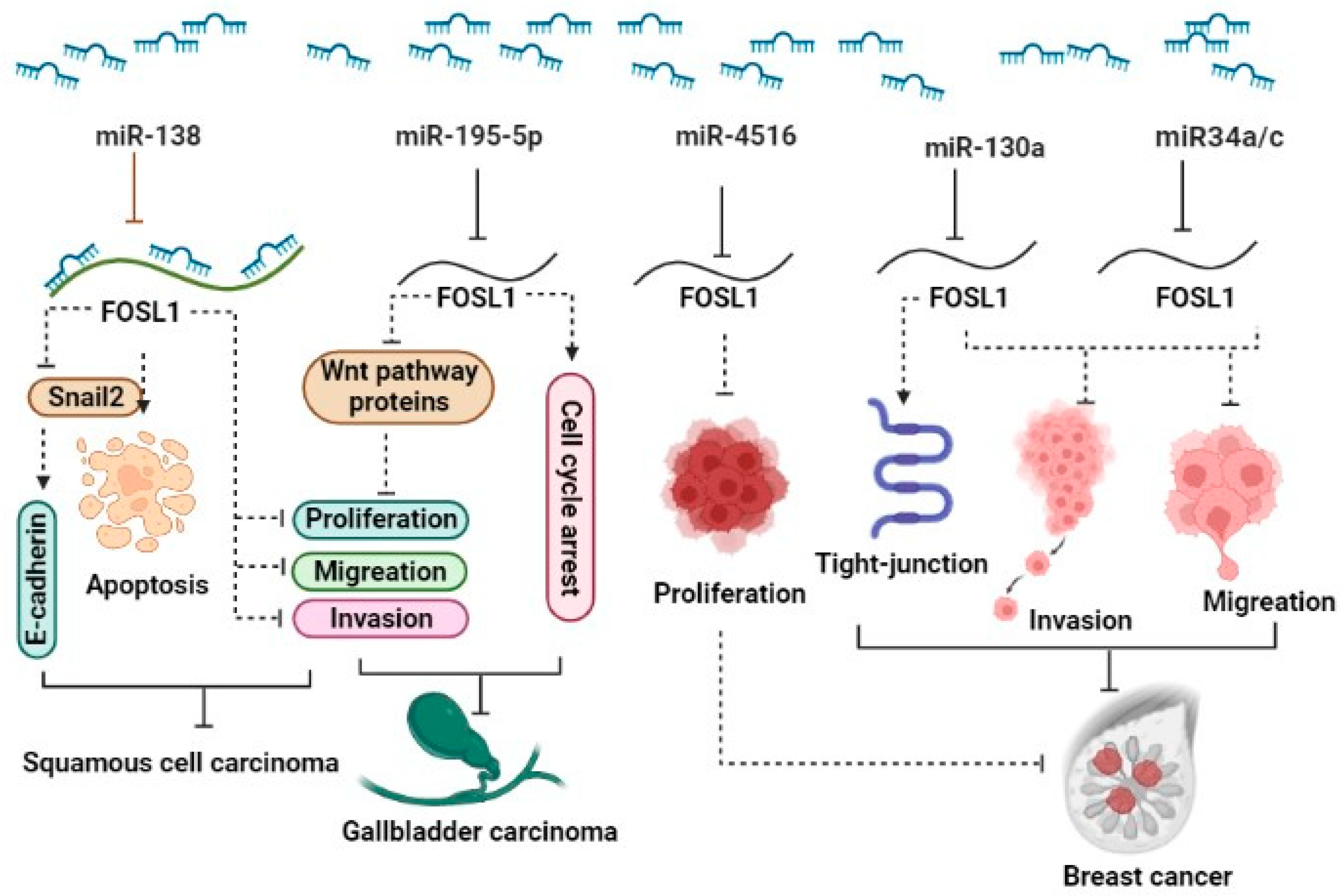
2.2. Non-coding RNAs regulate FOSL1
2.2.1. miRNAs and FOSL1
2.2.2. LncRNA and FOSL1
2.3. Post-translational regulation
3. The general roles of FOSL1 in glioma and other tumor types
| Cancer type | Clinical samples | Assessed cell line | FOSL1 expression | Effects: in vitro/ in vivo | Regulatory mechanism | Ref |
|---|---|---|---|---|---|---|
| BC |
51 paired tissues | MDA-MB-231, Hs578T | Up | ∆ miR34a/c: ↓Invasion, ↓Migration | miR-34a/c-Fra1 |
19 |
| 30 paired tissues | MDA-MB-231, MCF7, BT-474, SK-BR-3, BT-20, NF, CAF | UP | ∆ miR4516: ↓Proliferation | miR4516/FOSL1 | 20 | |
| 33 TNBC and 34 non-TNBC patients | MDA-MB-231, Hs578T | UP | ∆ miR-130a: ↑Tight-junction protein, ↓Invasion, ↓Migration | miR-130a/ FOSL1/ ZO1 | 18 | |
| - | 4T1, 4T07, RAW264.7 | UP | ∆ miR-19a-3p: ↓Metastasis, ↓Invasion, ↓Migration, ↑Macrophage polarization, ↓Tumor growth | miR-19a-3p /Fra-1/STAT3 | 21 | |
| MCF7, 4T1, ZR-75-1 | UP | ∆ FOSL1: Invasion ↓, Migration ↓ | MLK3–FRA-1–MMP-1 | 50 | ||
| MDA-MB-231, BT549 | UP | ∆ FOSL1: Invasion ↓, Migration ↓ | ERK1/2, SPAK | 51 | ||
| MDA-MB-231, MDA-MB-436, MCF-7 | UP | ∆ FOSL1: Invasion ↓, Migration ↓ | HMGA1 | 53 | ||
| fourth node mice | BRC-17, BRC-31, BRC-32, BRC-36, BRC- | UP | ∆ FOSL1: Invasion ↓, Migration ↓ | Integrin-uPAR | 53 | |
| 4T1 | UP | ∆ FOSL1: Invasion ↓, Migration ↓ | IL-6/JAK/Stat3 | 60 | ||
| CRC |
- | CRL-1831, LoVo, RKO, HCT15, HCT28, HCT116, and SW480 | UP | ∆ miR-497: ↓EMT, ↓Migration, ↓Invasion | miR-497/ Fra-1 | 27 |
| 40 paired tissues | HEK293T, RKO, HCT116, HCT116 | UP | ▼Fra-1 or ∆ miR-34a: ↓Migration, ↓Invasion | miR-34a/p53/ Fra-1 | 26 | |
| HCT116 |
UP | ∆ FOSL1: Invasion ↓, Migration | miR-34a/Fra1 |
|||
| HT-29, HCT116 |
UP | ∆ FOSL1: Invasion ↓, Migration ↓ |
USP21 |
47 | ||
| SW620, HCT116, DLD1 HT29 |
UP | ∆ FOSL1: Invasion ↓, Migration ↓ | RAS | 48 | ||
| paired tissues, PDX mouse model |
UP |
∆ FOSL1: Proliferation↓, Invasion ↓, Migration ↓, |
EGFRMAPK-FRA-1 |
49 | ||
| GC | 20 paired tissues |
AGS | UP | ∆ FOSL1: Proliferation↓, apoptosis | PI3K/Akt ,p53 |
35 |
| NPC |
20 NPC and 16 non-NPC patients | NP69, N2-Tert, C666-1, CNE1, CNE2, HK1, HNE1, HONE1, SUNE1, 5–8F and 6–10B | UP | ▼LINC01503: ↓Proliferation, ↓Invasion , ↓Migration , ↓Metastasis, ↓Tumor growth | LINC01503/ SFPQ/ FOSL1 | 24 |
| 53 paired tissues | CNE1 | UP | ∆ FOSL1: Proliferation↓, Growth tumors ↓ | MSK1, LMP1 | 38 | |
| LC | 55 paired tissues | H460 | UP | ∆ FOSL1: apoptosis↓ | p53 |
40 |
| OS | Saos2 , MG63 | UP | ∆ FOSL1: Proliferation↓, Invasion ↓, Migration ↓, |
ERK/AP-1 |
37 | |
| GBC | - | NOZ , GBC-SD | UP | ∆ miR-195-5p: ↓Proliferation, ↑G0/G1 phase , ↓Invasion , ↓Migration , ↓Tumor growth | miR-195-5p /FOSL1/Wnt pathway | 17 |
| TC |
FRTL-5K-Ra | UP | ∆ FOSL1: Proliferation↓ |
cyclin A, Jun B |
38 | |
| paired tissues | BHP10-3, BCPAP, SNU790 | UP | ∆ FOSL1: Invasion ↓, Migration ↓ | ERK/Fra-1/ZEB1 | 42 | |
| HSCC | 40 paired tissues | FaDu, Detroit 562, NP69 | UP | ▼HOXA11-AS1: ↓Immune escape, ↓ Metastasis, ↓Proliferation, ↓Tumor growth | HOXA11-AS1/FOSL1/PTBP1/PD-L1 | 25 |
| EC | 53 paired tissues | E70, KYSE-510, EC9706, HEECs | UP | ▼AGAP2-AS1: ↓Proliferation, ↓Invasion , ↓Migration , ↑Apoptosis, ↑G0/G1 phase, ↓Tumor growth | AGAP2-AS1/ miR-195-5p/FOSL1 | 23 |
| Glioma | - | A172, T98G, U87MG | UP | ▼FOSL1: ↑Apoptosis, ↓Tumor growth | FOSL1/ miR-28-5p | 16 |
| 22 | U251 | UP | miR-33a/FOSL1 | 51 |
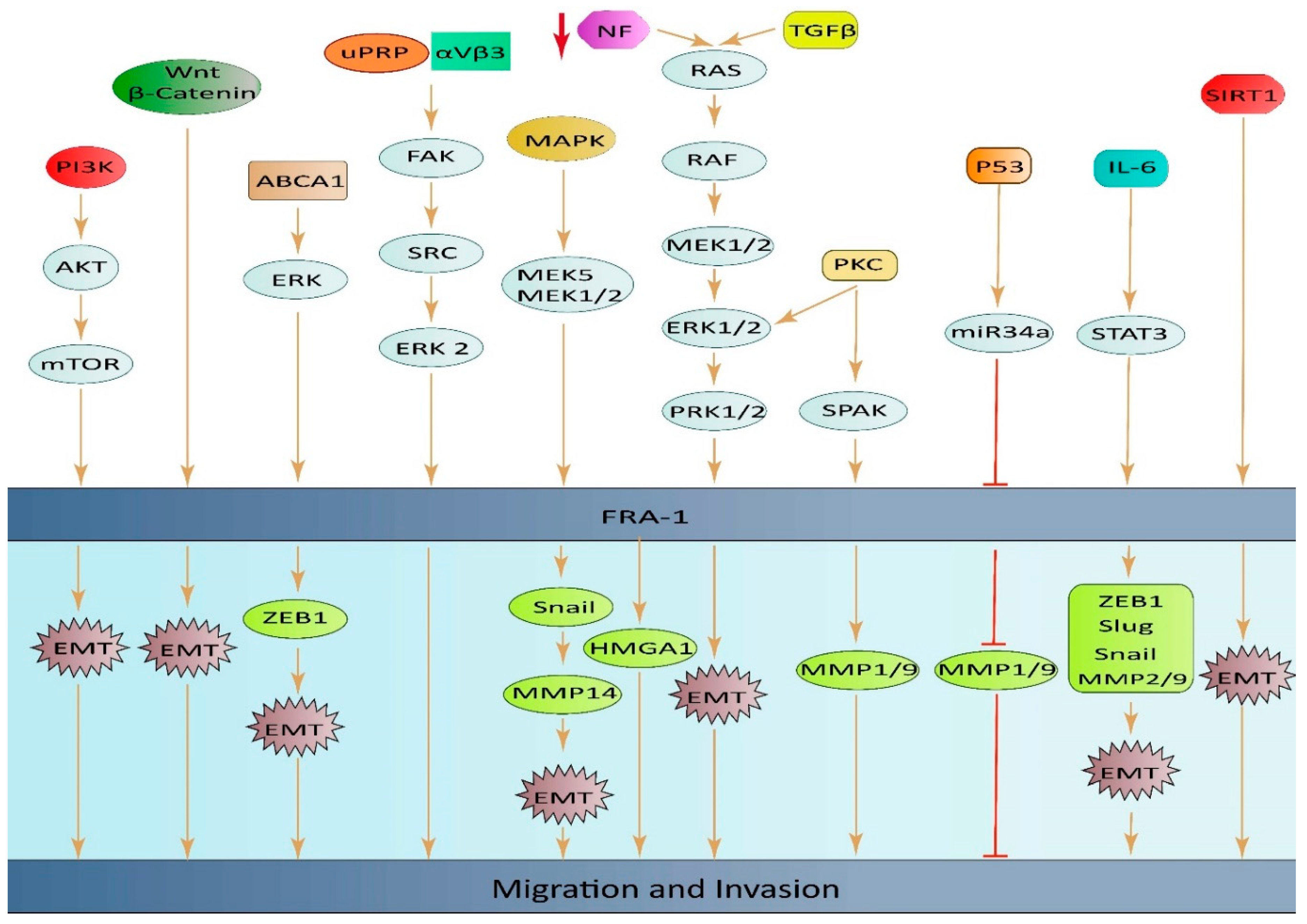
3.1. The role of FOSL1 in apoptosis and proliferation and its associated signaling pathways.
3.2. The role of FOSL1 in migration and its associated signaling pathways.
3.2.1. CRC
3.2.2. Breast cancer
3.2.3. Other cancers
3.3. The involvement of FOSL1 in the tumor microenvironment.
3.4. Cancer stem cells
4. FOSL1 serves as an independent and prognostic factor
5. The role of FOSL1 in drug resistance
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rong, L.; Li, N.; Zhang, Z. Emerging therapies for glioblastoma: Current state and future directions. Journal of Experimental & Clinical Cancer Research 2022, 41, 1–18. [Google Scholar]
- Pecce, V.; Verrienti, A.; Fiscon, G.; Sponziello, M.; Conte, F.; Abballe, L.; Durante, C.; Farina, L.; Filetti, S.; Paci, P. The role of FOSL1 in stem-like cell reprogramming processes. Scientific reports 2021, 11, 14677. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.R.; Curran, T. fra-1: a serum-inducible, cellular immediate-early gene that encodes a fos-related antigen. Molecular and Cellular Biology 1988, 8, 2063–2069. [Google Scholar] [PubMed]
- Talotta, F.; Casalino, L.; Verde, P. The nuclear oncoprotein Fra-1: a transcription factor knocking on therapeutic applications’ door. Oncogene 2020, 39, 4491–4506. [Google Scholar] [CrossRef] [PubMed]
- Curran, T.; Franza Jr, B.R. Fos and Jun: the AP-1 connection. Cell 1988, 55, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Angel, P.; Imagawa, M.; Chiu, R.; Stein, B.; Imbra, R.J.; Rahmsdorf, H.J.; Jonat, C.; Herrlich, P.; Karin, M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell 1987, 49, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Eferl, R.; Wagner, E.F. AP-1: a double-edged sword in tumorigenesis. Nature Reviews Cancer 2003, 3, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; He, J.; Jin, X.; Liao, Q.; Chen, Z.; Peng, H.; Zhou, Y. FRA-1: A key factor regulating signal transduction of tumor cells and a potential target molecule for tumor therapy. Biomedicine & Pharmacotherapy 2022, 150, 113037. [Google Scholar]
- Jiang, X.; Xie, H.; Dou, Y.; Yuan, J.; Zeng, D.; Xiao, S. Expression and function of FRA1 protein in tumors. Molecular Biology Reports 2020, 47, 737–752. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, L.; He, J.; He, Z.; Yue, C.; Jin, X.; Gao, M.; Xiao, S.; Zhou, Y. Fra-1 inhibits cell growth and the Warburg effect in cervical cancer cells via STAT1 regulation of the p53 signaling pathway. Frontiers in cell and developmental biology 2020, 8, 579629. [Google Scholar] [CrossRef]
- Basbous, J.; Chalbos, D.; Hipskind, R.; Jariel-Encontre, I.; Piechaczyk, M. Ubiquitin-independent proteasomal degradation of Fra-1 is antagonized by Erk1/2 pathway-mediated phosphorylation of a unique C-terminal destabilizer. Molecular and cellular biology 2007, 27, 3936–3950. [Google Scholar] [CrossRef] [PubMed]
- Hnisz, D.; Abraham, B.J.; Lee, T.I.; Lau, A.; Saint-André, V.; Sigova, A.A.; Hoke, H.A.; Young, R.A. Super-enhancers in the control of cell identity and disease. Cell 2013, 155, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Lovén, J.; Hoke, H.A.; Lin, C.Y.; Lau, A.; Orlando, D.A.; Vakoc, C.R.; Bradner, J.E.; Lee, T.I.; Young, R.A. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 2013, 153, 320–334. [Google Scholar] [CrossRef]
- Casalino, L.; Talotta, F.; Cimmino, A.; Verde, P. The Fra-1/AP-1 Oncoprotein: From the “Undruggable” Transcription Factor to Therapeutic Targeting. Cancers 2022, 14, 1480. [Google Scholar] [CrossRef]
- Liu, M.; Inoue, K.; Leng, T.; Guo, S.; Xiong, Z.G. TRPM7 channels regulate glioma stem cell through STAT3 and Notch signaling pathways. Cell Signal 2014, 26, 2773–2781. [Google Scholar] [CrossRef]
- Guo, S.; Ramar, V.; Guo, A.A.; Saafir, T.; Akpobiyeri, H.; Hudson, B.; Li, J.; Liu, M. TRPM7 transactivates the FOSL1 gene through STAT3 and enhances glioma stemness. Cell Mol Life Sci 2023, 80, 270. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; He, X.; Jia, L.; Zhang, X. Circular RNAs in glioma: Molecular functions and pathological implications. Noncoding RNA Res 2024, 9, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Mallela, V.R.; Rajtmajerová, M.; Trailin, A.; Liška, V.; Hemminki, K.; Ambrozkiewicz, F. miRNA and lncRNA as potential tissue biomarkers in hepatocellular carcinoma. Noncoding RNA Res 2024, 9, 24–32. [Google Scholar] [CrossRef]
- He, S.W.; Xu, C.; Li, Y.Q.; Li, Y.Q.; Zhao, Y.; Zhang, P.P.; Lei, Y.; Liang, Y.L.; Li, J.Y.; Li, Q.; et al. AR-induced long non-coding RNA LINC01503 facilitates proliferation and metastasis via the SFPQ-FOSL1 axis in nasopharyngeal carcinoma. Oncogene 2020, 39, 5616–5632. [Google Scholar] [CrossRef]
- Shen, S.; Li, K.; Liu, Y.; Liu, X.; Liu, B.; Ba, Y.; Xing, W. Silencing lncRNA AGAP2-AS1 Upregulates miR-195-5p to Repress Migration and Invasion of EC Cells via the Decrease of FOSL1 Expression. Molecular therapy. Nucleic acids 2020, 20, 331–344. [Google Scholar] [CrossRef]
- Guo, S.; King, P.; Liang, E.; Guo, A.A.; Liu, M. LncRNA HOTAIR sponges miR-301a-3p to promote glioblastoma proliferation and invasion through upregulating FOSL1. Cell Signal 2022, 94, 110306. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Che, W.; Liang, N.; Deng, S.; Song, Z.; Yang, L. Silent FOSL1 enhances the Radiosensitivity of glioma stem cells by Down-regulating miR-27a-5p. Neurochemical Research 2021, 46, 3222–3246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, Z.; Yu, J.; Wu, J.; Zhuo, X.; Chen, Q.; Liang, Y.; Li, G.; Wan, Y. MiR-195-5p suppresses the proliferation, migration, and invasion of gallbladder cancer cells by targeting FOSL1 and regulating the Wnt/β-catenin pathway. Annals of Translational Medicine 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, M.; Huang, J.; Li, Y.; Wang, S.; Harrington, C.A.; Qian, D.Z.; Sun, X.X.; Dai, M.S. MicroRNA-130a suppresses breast cancer cell migration and invasion by targeting FOSL1 and upregulating ZO-1. Journal of cellular biochemistry 2018, 119, 4945–4956. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Y.; Gao, J.; Zhang, T.; Li, S.; Luo, A.; Chen, H.; Ding, F.; Wang, X.; Liu, Z. MicroRNA-34 suppresses breast cancer invasion and metastasis by directly targeting Fra-1. Oncogene 2013, 32, 4294–4303. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, B.G.; Jang, Y.; Kang, S.; Lee, J.H.; Cho, N.H. The stromal loss of miR-4516 promotes the FOSL1-dependent proliferation and malignancy of triple negative breast cancer. Cancer letters 2020, 469, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Z.; Chen, C.; Liu, Y.; Si, Q.; Chuang, T.; Li, N.; Gomez-Cabrero, A.; Reisfeld, R.; Xiang, R. MicroRNA-19a-3p inhibits breast cancer progression and metastasis by inducing macrophage polarization through downregulated expression of Fra-1 proto-oncogene. Oncogene 2014, 33, 3014–3023. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, G.; Lv, L.; Ren, Y.F.; Zhang, X.J.; Xue, Y.F.; Li, G.; Lu, X.; Sun, Z.; Tang, K.F. MicroRNA-34a inhibits migration and invasion of colon cancer cells via targeting to Fra-1. Carcinogenesis 2012, 33, 519–528. [Google Scholar] [CrossRef]
- Zhang, N.; Shen, Q.; Zhang, P. miR-497 suppresses epithelial-mesenchymal transition and metastasis in colorectal cancer cells by targeting fos-related antigen-1. OncoTargets and therapy 2016, 9, 6597–6604. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, C.; Liu, X.; Mu, W.; Chen, Z.; Yu, D.; Wang, A.; Dai, Y.; Zhou, X. Molecular characterization of the microRNA-138-Fos-like antigen 1 (FOSL1) regulatory module in squamous cell carcinoma. Journal of Biological Chemistry 2011, 286, 40104–40109. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Q.; Zhang, G.; Mohammed, D.; Amadou, S.; Tan, G.; Zhang, X. HOXA11-AS1 Promotes PD-L1-Mediated Immune Escape and Metastasis of Hypopharyngeal Carcinoma by Facilitating PTBP1 and FOSL1 Association. Cancers 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Li, K.; Liu, Y.; Liu, X.; Liu, B.; Ba, Y.; Xing, W. Silencing lncRNA AGAP2-AS1 upregulates miR-195-5p to repress migration and invasion of EC cells via the decrease of FOSL1 expression. Molecular Therapy-Nucleic Acids 2020, 20, 331–344. [Google Scholar] [CrossRef] [PubMed]
- He, S.-W.; Xu, C.; Li, Y.-Q.; Li, Y.-Q.; Zhao, Y.; Zhang, P.-P.; Lei, Y.; Liang, Y.-L.; Li, J.-Y.; Li, Q. AR-induced long non-coding RNA LINC01503 facilitates proliferation and metastasis via the SFPQ-FOSL1 axis in nasopharyngeal carcinoma. Oncogene 2020, 39, 5616–5632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Q.; Zhang, G.; Mohammed, D.; Amadou, S.; Tan, G.; Zhang, X. HOXA11-AS1 promotes PD-L1-mediated immune escape and metastasis of hypopharyngeal carcinoma by facilitating PTBP1 and FOSL1 association. Cancers 2022, 14, 3694. [Google Scholar] [CrossRef] [PubMed]
- Casalino, L.; De Cesare, D.; Verde, P. Accumulation of Fra-1 in ras-transformed cells depends on both transcriptional autoregulation and MEK-dependent posttranslational stabilization. Molecular and cellular biology 2003, 23, 4401–4415. [Google Scholar] [CrossRef] [PubMed]
- Doehn, U.; Hauge, C.; Frank, S.R.; Jensen, C.J.; Duda, K.; Nielsen, J.V.; Cohen, M.S.; Johansen, J.V.; Winther, B.R.; Lund, L.R. RSK is a principal effector of the RAS-ERK pathway for eliciting a coordinate promotile/invasive gene program and phenotype in epithelial cells. Molecular cell 2009, 35, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Belguise, K.; Cherradi, S.; Sarr, A.; Boissière, F.; Boulle, N.; Simony-Lafontaine, J.; Choesmel-Cadamuro, V.; Wang, X.; Chalbos, D. PKCθ-induced phosphorylations control the ability of Fra-1 to stimulate gene expression and cancer cell migration. Cancer Letters 2017, 385, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Gruda, M.C.; Kovary, K.; Metz, R.; Bravo, R. Regulation of Fra-1 and Fra-2 phosphorylation differs during the cell cycle of fibroblasts and phosphorylation in vitro by MAP kinase affects DNA binding activity. Oncogene 1994, 9, 2537–2547. [Google Scholar] [PubMed]
- Terasawa, K.; Okazaki, K.; Nishida, E. Regulation of c-Fos and Fra-1 by the MEK5-ERK5 pathway. Genes to Cells 2003, 8, 263–273. [Google Scholar] [CrossRef]
- Tower, G.B.; Coon, C.I.; Belguise, K.; Chalbos, D.; Brinckerhoff, C.E. Fra-1 targets the AP-1 site/2G single nucleotide polymorphism (ETS site) in the MMP-1 promoter. European journal of biochemistry 2003, 270, 4216–4225. [Google Scholar] [CrossRef]
- Basbous, J.; Jariel-Encontre, I.; Gomard, T.; Bossis, G.; Piechaczyk, M. Ubiquitin-independent-versus ubiquitin-dependent proteasomal degradation of the c-Fos and Fra-1 transcription factors: is there a unique answer? Biochimie 2008, 90, 296–305. [Google Scholar] [CrossRef]
- Marques, C.; Unterkircher, T.; Kroon, P.; Oldrini, B.; Izzo, A.; Dramaretska, Y.; Ferrarese, R.; Kling, E.; Schnell, O.; Nelander, S.; et al. NF1 regulates mesenchymal glioblastoma plasticity and aggressiveness through the AP-1 transcription factor FOSL1. Elife 2021, 10. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, S.; Li, H.L.; Luo, H.; Wu, X.; Lu, J.; Wang, H.W.; Chen, Y.; Chen, D.; Wu, W.T.; et al. FOSL1 promotes proneural-to-mesenchymal transition of glioblastoma stem cells via UBC9/CYLD/NF-κB axis. Mol Ther 2022, 30, 2568–2583. [Google Scholar] [CrossRef] [PubMed]
- Geer, L.Y.; Marchler-Bauer, A.; Geer, R.C.; Han, L.; He, J.; He, S.; Liu, C.; Shi, W.; Bryant, S.H. The NCBI BioSystems database. Nucleic Acids Res 2010, 38, D492–496. [Google Scholar] [CrossRef] [PubMed]
- Wykosky, J.; Hu, J.; Gomez, G.G.; Taylor, T.; Villa, G.R.; Pizzo, D.; VandenBerg, S.R.; Thorne, A.H.; Chen, C.C.; Mischel, P.S.; et al. A urokinase receptor-Bim signaling axis emerges during EGFR inhibitor resistance in mutant EGFR glioblastoma. Cancer research 2015, 75, 394–404. [Google Scholar] [CrossRef]
- Ramsdale, R.; Jorissen, R.N.; Li, F.Z.; Al-Obaidi, S.; Ward, T.; Sheppard, K.E.; Bukczynska, P.E.; Young, R.J.; Boyle, S.E.; Shackleton, M.; et al. The transcription cofactor c-JUN mediates phenotype switching and BRAF inhibitor resistance in melanoma. Sci Signal 2015, 8, ra82. [Google Scholar] [CrossRef]
- Kim, S.M.; Kwon, O.J.; Hong, Y.K.; Kim, J.H.; Solca, F.; Ha, S.J.; Soo, R.A.; Christensen, J.G.; Lee, J.H.; Cho, B.C. Activation of IL-6R/JAK1/STAT3 signaling induces de novo resistance to irreversible EGFR inhibitors in non-small cell lung cancer with T790M resistance mutation. Mol Cancer Ther 2012, 11, 2254–2264. [Google Scholar] [CrossRef]
- Kesanakurti, D.; Chetty, C.; Rajasekhar Maddirela, D.; Gujrati, M.; Rao, J.S. Functional cooperativity by direct interaction between PAK4 and MMP-2 in the regulation of anoikis resistance, migration and invasion in glioma. Cell death & disease 2012, 3, e445. [Google Scholar] [CrossRef]
- Saito, R.; Miki, Y.; Ishida, N.; Inoue, C.; Kobayashi, M.; Hata, S.; Yamada-Okabe, H.; Okada, Y.; Sasano, H. The Significance of MMP-1 in EGFR-TKI-Resistant Lung Adenocarcinoma: Potential for Therapeutic Targeting. International journal of molecular sciences 2018, 19. [Google Scholar] [CrossRef]
- Day, E.K.; Sosale, N.G.; Xiao, A.; Zhong, Q.; Purow, B.; Lazzara, M.J. Glioblastoma Cell Resistance to EGFR and MET Inhibition Can Be Overcome via Blockade of FGFR-SPRY2 Bypass Signaling. Cell Rep 2020, 30, 3383–3396. [Google Scholar] [CrossRef]
- Wang, W.; Liu, W.; Xu, J.; Jin, H. MiR-33a targets FOSL1 and EN2 as a clinical prognostic marker for sarcopenia by glioma. Front Genet 2022, 13, 953580. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayyat, W.; Pirkkanen, J.; Dougherty, J.; Laframboise, T.; Dickinson, N.; Khaper, N.; Lees, S.J.; Mendonca, M.S.; Boreham, D.R.; Tai, T.C.; et al. Overexpression of FRA1 (FOSL1) Leads to Global Transcriptional Perturbations, Reduced Cellular Adhesion and Altered Cell Cycle Progression. Cells 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, G.; Gao, L.; Chen, P.; Long, Y.; Liao, S.; Yi, H.; Yi, W.; Pei, Z.; Wu, M. Fra-1 is upregulated in gastric cancer tissues and affects the PI3K/Akt and p53 signaling pathway in gastric cancer. International journal of oncology 2015, 47, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhao, X.; Sun, Y.; Sui, Y.; Liu, J. Retracted: Effects of FOSL1 silencing on osteosarcoma cell proliferation, invasion and migration through the ERK/AP-1 signaling pathway. 2019.
- Li, B.; Wan, Z.; Huang, G.; Huang, Z.; Zhang, X.; Liao, D.; Luo, S.; He, Z. Mitogen-and stress-activated Kinase 1 mediates Epstein-Barr virus latent membrane protein 1-promoted cell transformation in nasopharyngeal carcinoma through its induction of Fra-1 and c-Jun genes. BMC cancer 2015, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Casalino, L.; Bakiri, L.; Talotta, F.; Weitzman, J.B.; Fusco, A.; Yaniv, M.; Verde, P. Fra-1 promotes growth and survival in RAS-transformed thyroid cells by controlling cyclin A transcription. The EMBO journal 2007, 26, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Vallone, D.; Battista, S.; Pierantoni, G.M.; Fedele, M.; Casalino, L.; Santoro, M.; Viglietto, G.; Fusco, A.; Verde, P. Neoplastic transformation of rat thyroid cells requires the junB and fra-1 gene induction which is dependent on the HMGI-C gene product. The EMBO journal 1997, 16, 5310–5321. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, G.; Tallini, G.; De Biasio, M.C.; Pentimalli, F.; de Nigris, F.; Losito, S.; Fedele, M.; Battista, S.; Verde, P.; Santoro, M. FRA-1 expression in hyperplastic and neoplastic thyroid diseases. Clinical Cancer Research 2000, 6, 4300–4306. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, C.; Hua, Y.; Cheng, K.; Zhang, Y.; Liu, J.; Han, Y.; Liu, S.; Zhang, G.; Xu, S. Psoralen induced cell cycle arrest by modulating Wnt/β-catenin pathway in breast cancer cells. Scientific reports 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Belguise, K.; Kersual, N.; Galtier, F.; Chalbos, D. FRA-1 expression level regulates proliferation and invasiveness of breast cancer cells. Oncogene 2005, 24, 1434–1444. [Google Scholar] [CrossRef]
- Ibrahim, S.A.E.; Abudu, A.; Johnson, E.; Aftab, N.; Conrad, S.; Fluck, M. The role of AP-1 in self-sufficient proliferation and migration of cancer cells and its potential impact on an autocrine/paracrine loop. Oncotarget 2018, 9, 34259–34278. [Google Scholar] [CrossRef]
- Xiao, S.; Zhou, Y.; Yi, W.; Luo, G.; Jiang, B.; Tian, Q.; Li, Y.; Xue, M. Fra-1 is downregulated in cervical cancer tissues and promotes cervical cancer cell apoptosis by p53 signaling pathway in vitro. International journal of oncology 2015, 46, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Chen, X.; Fang, X.; Wang, D.; Xie, M.; Chen, Q. Fra-1 is upregulated in lung cancer tissues and inhibits the apoptosis of lung cancer cells by the P53 signaling pathway. Oncology reports 2016, 35, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liang, L.; He, J.; He, Z.; Yue, C.; Jin, X.; Gao, M.; Xiao, S.; Zhou, Y. Fra-1 Inhibits Cell Growth and the Warburg Effect in Cervical Cancer Cells via STAT1 Regulation of the p53 Signaling Pathway. Front Cell Dev Biol 2020, 8, 579629. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Chen, X.; Fang, X.; Wang, D.; Xie, M.; Chen, Q. Fra-1 is upregulated in lung cancer tissues and inhibits the apoptosis of lung cancer cells by the P53 signaling pathway. Oncol Rep 2016, 35, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Tulchinsky, E. FRA-1 as a driver of tumour heterogeneity: a nexus between oncogenes and embryonic signalling pathways in cancer. Oncogene 2015, 34, 4421–4428. [Google Scholar] [CrossRef] [PubMed]
- Bakiri, L.; Macho-Maschler, S.; Custic, I.; Niemiec, J.; Guío-Carrión, A.; Hasenfuss, S.; Eger, A.; Müller, M.; Beug, H.; Wagner, E. Fra-1/AP-1 induces EMT in mammary epithelial cells by modulating Zeb1/2 and TGFβ expression. Cell Death & Differentiation 2015, 22, 336–350. [Google Scholar]
- Zhao, C.; Qiao, Y.; Jonsson, P.; Wang, J.; Xu, L.; Rouhi, P.; Sinha, I.; Cao, Y.; Williams, C.; Dahlman-Wright, K. Genome-wide profiling of AP-1–regulated transcription provides insights into the invasiveness of triple-negative breast cancer. Cancer research 2014, 74, 3983–3994. [Google Scholar] [CrossRef]
- Liu, H.; Ren, G.; Wang, T.; Chen, Y.; Gong, C.; Bai, Y.; Wang, B.; Qi, H.; Shen, J.; Zhu, L. Aberrantly expressed Fra-1 by IL-6/STAT3 transactivation promotes colorectal cancer aggressiveness through epithelial–mesenchymal transition. Carcinogenesis 2015, 36, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yue, M.; Li, Z. FOSL1 promotes tumorigenesis in colorectal carcinoma by mediating the FBXL2/Wnt/β-catenin axis via Smurf1. Pharmacological Research 2021, 165, 105405. [Google Scholar] [CrossRef]
- Wu, J.; Wu, G.; Lv, L.; Ren, Y.-F.; Zhang, X.-J.; Xue, Y.-F.; Li, G.; Lu, X.; Sun, Z.; Tang, K.-F. MicroRNA-34a inhibits migration and invasion of colon cancer cells via targeting to Fra-1. Carcinogenesis 2012, 33, 519–528. [Google Scholar] [CrossRef]
- Yun, S.-I.; Hong, H.K.; Yeo, S.-Y.; Kim, S.-H.; Cho, Y.B.; Kim, K.K. Ubiquitin-specific protease 21 promotes colorectal cancer metastasis by acting as a Fra-1 deubiquitinase. Cancers 2020, 12, 207. [Google Scholar] [CrossRef]
- Andreolas, C.; Kalogeropoulou, M.; Voulgari, A.; Pintzas, A. Fra-1 regulates vimentin during Ha-RAS-induced epithelial mesenchymal transition in human colon carcinoma cells. International journal of cancer 2008, 122, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Wei, W.; Qian, M.R.; Tian, R.F.; Fu, X.; Li, H.W.; Nan, G.; Yang, T.; Lin, P.; Chen, X. PDGFA-associated protein 1 is a novel target of c-Myc and contributes to colorectal cancer initiation and progression. Cancer Communications 2022, 42, 750–767. [Google Scholar] [CrossRef]
- Rattanasinchai, C.; Llewellyn, B.; Conrad, S.; Gallo, K. MLK3 regulates FRA-1 and MMPs to drive invasion and transendothelial migration in triple-negative breast cancer cells. Oncogenesis 2017, 6, e345–e345. [Google Scholar] [CrossRef]
- Belguise, K.; Milord, S.; Galtier, F.; Moquet-Torcy, G.; Piechaczyk, M.; Chalbos, D. The PKCθ pathway participates in the aberrant accumulation of Fra-1 protein in invasive ER-negative breast cancer cells. Oncogene 2012, 31, 4889–4897. [Google Scholar] [CrossRef]
- Tolza, C.; Bejjani, F.; Evanno, E.; Mahfoud, S.; Moquet-Torcy, G.; Gostan, T.; Maqbool, M.A.; Kirsh, O.; Piechaczyk, M.; Jariel-Encontre, I. AP-1 signaling by Fra-1 directly regulates HMGA1 oncogene transcription in triple-negative breast cancers. Molecular cancer research 2019, 17, 1999–2014. [Google Scholar] [CrossRef] [PubMed]
- Annis, M.G.; Ouellet, V.; Rennhack, J.P.; L’Esperance, S.; Rancourt, C.; Mes-Masson, A.-M.; Andrechek, E.R.; Siegel, P.M. Integrin-uPAR signaling leads to FRA-1 phosphorylation and enhanced breast cancer invasion. Breast Cancer Research 2018, 20, 1–15. [Google Scholar] [CrossRef]
- Park, J.-H.; Myung, J.-K.; Lee, S.-J.; Kim, H.; Kim, S.; Lee, S.-B.; Jang, H.; Jang, W.-I.; Park, S.; Yang, H. ABCA1-Mediated EMT Promotes Papillary Thyroid Cancer Malignancy through the ERK/Fra-1/ZEB1 Pathway. Cells 2023, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, H.; Xu, Z.; Zhang, Y. Expression and clinical significance of FOS-like antigen 1 in gastric adenocarcinoma. Pathology-Research and Practice 2019, 215, 152394. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Mu, X.; Cui, J.; Peng, Z. Dysregulation of Fra1 expression by Wnt/β-catenin signalling promotes glioma aggressiveness through epithelial–mesenchymal transition. Bioscience reports 2017, 37. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K. Role of tumor microenvironment in tumorigenesis. Journal of Cancer 2017, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Charles, K.A.; Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer cell 2005, 7, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ni, H.; Lan, L.; Wei, X.; Xiang, R.; Wang, Y. Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2d macrophages. Cell research 2010, 20, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhou, H.; Krueger, J.; Kaplan, C.; Liao, D.; Markowitz, D.; Liu, C.; Chen, T.; Chuang, T.-H.; Xiang, R. The role of proto-oncogene Fra-1 in remodeling the tumor microenvironment in support of breast tumor cell invasion and progression. Oncogene 2010, 29, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, X.; Wang, G.; Zhai, C.; Liu, Y.; Li, H.; Zhang, Y.; Yu, W.; Zhao, Z. ITGB4 is a novel prognostic factor in colon cancer. Journal of Cancer 2019, 10, 5223. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.Y.T.; Lo, J.; Cheng, B.Y.L.; Ma, M.K.F.; Lee, J.M.F.; Ng, J.K.Y.; Chai, S.; Lin, C.H.; Tsang, S.Y.; Ma, S. Cancer-associated fibroblasts regulate tumor-initiating cell plasticity in hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell reports 2016, 15, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Jing, N.; Gao, W.-Q.; Fang, Y.-X. Regulation of formation, stemness and therapeutic resistance of cancer stem cells. Frontiers in Cell and Developmental Biology 2021, 9, 641498. [Google Scholar] [CrossRef]
- Pecce, V.; Verrienti, A.; Fiscon, G.; Sponziello, M.; Conte, F.; Abballe, L.; Durante, C.; Farina, L.; Filetti, S.; Paci, P. The role of FOSL1 in stem-like cell reprogramming processes. Scientific reports 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.L.; Lu, H.; Buikhuisen, J.; Soh, B.S.; Lim, E.; Reinhardt, F.; Wu, Z.J.; Krall, J.A.; Bierie, B.; Guo, W. Protein kinase C α is a central signaling node and therapeutic target for breast cancer stem cells. Cancer cell 2013, 24, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, S.; Li, H.-L.; Luo, H.; Wu, X.; Lu, J.; Wang, H.-W.; Chen, Y.; Chen, D.; Wu, W.-T. FOSL1 promotes proneural-to-mesenchymal transition of glioblastoma stem cells via UBC9/CYLD/NF-κB axis. Molecular Therapy 2022, 30, 2568–2583. [Google Scholar] [CrossRef]
- Wang, T.; Song, P.; Zhong, T.; Wang, X.; Xiang, X.; Liu, Q.; Chen, H.; Xia, T.; Liu, H.; Niu, Y. The inflammatory cytokine IL-6 induces FRA1 deacetylation promoting colorectal cancer stem-like properties. Oncogene 2019, 38, 4932–4947. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, V.V.; Khashukoeva, A.Z.; Evina, O.E.; Geppe, N.A.; Chebysheva, S.N.; Korsunskaya, I.M.; Tchepourina, E.; Mezentsev, A. Role of the transcription factor FOSL1 in organ development and tumorigenesis. International journal of molecular sciences 2022, 23, 1521. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, I.M.; Vaz, M.; Tamatam, C.R.; Potteti, H.R.; Reddy, N.M.; Reddy, S.P. FOSL1 promotes Kras-induced lung cancer through amphiregulin and cell survival gene regulation. American journal of respiratory cell and molecular biology 2018, 58, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Reddy, N.M.; Rajasekaran, S.; Reddy, S.P. Genetic disruption of Fra-1 decreases susceptibility to endotoxin-induced acute lung injury and mortality in mice. American journal of respiratory cell and molecular biology 2012, 46, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Iskit, S.; Schlicker, A.; Wessels, L.; Peeper, D.S. Fra-1 is a key driver of colon cancer metastasis and a Fra-1 classifier predicts disease-free survival. Oncotarget 2015, 6, 43146. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Ferrer, L.; Kürschner, M.; Labitzky, V.; Wicklein, D.; Müller, V.; Lüers, G.; Schumacher, U.; Milde-Langosch, K.; Schröder, C. Prognostic impact of transcription factor Fra-1 in ER-positive breast cancer: contribution to a metastatic phenotype through modulation of tumor cell adhesive properties. Journal of cancer research and clinical oncology 2015, 141, 1715–1726. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jin, X.; Yuan, Y.; Deng, P.; Jiang, L.; Zeng, X.; Li, X.-S.; Wang, Z.-Y.; Chen, Q.-M. Prognostic value from integrative analysis of transcription factors c-Jun and Fra-1 in oral squamous cell carcinoma: a multicenter cohort study. Scientific reports 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hoyle, R.G.; Ma, Z.; Sun, B.; Cai, W.; Cai, H.; Xie, N.; Zhang, Y.; Hou, J.; Liu, X. FOSL1 promotes metastasis of head and neck squamous cell carcinoma through super-enhancer-driven transcription program. Molecular Therapy 2021, 29, 2583–2600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, J.; Luo, S.; Lechler, T.; Zhang, J.Y. FRA1 promotes squamous cell carcinoma growth and metastasis through distinct AKT and c-Jun dependent mechanisms. Oncotarget 2016, 7, 34371. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Zhao, S.; Sun, M. FOS-like antigen 1 is a prognostic biomarker in hepatocellular carcinoma. Saudi Journal of Gastroenterology: Official Journal of the Saudi Gastroenterology Association 2019, 25, 369. [Google Scholar]
- Marques, C.; Unterkircher, T.; Kroon, P.; Oldrini, B.; Izzo, A.; Dramaretska, Y.; Ferrarese, R.; Kling, E.; Schnell, O.; Nelander, S. NF1 regulates mesenchymal glioblastoma plasticity and aggressiveness through the AP-1 transcription factor FOSL1. Elife 2021, 10, e64846. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.; Bhosale, S.D.; Tripathi, S.K.; Buchacher, T.; Biradar, R.; Rasool, O.; Moulder, R.; Galande, S.; Lahesmaa, R. Interactome Networks of FOSL1 and FOSL2 in Human Th17 Cells. ACS omega 2021, 6, 24834–24847. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resistance 2019, 2, 141. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Tyagi, A.; Vishnoi, K.; Kaur, H.; Srivastava, Y.; Roy, B.G.; Das, B.C.; Bharti, A.C. Cervical cancer stem cells manifest radioresistance: Association with upregulated AP-1 activity. Scientific reports 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, G.; Ferraro, A.; Botti, G.; Monaco, M.; Pasquinelli, R.; Vuttariello, E.; Arnaldi, L.; Di Bonito, M.; D’Aiuto, G.; Pierantoni, G.M. FRA-1 protein overexpression is a feature of hyperplastic and neoplastic breast disorders. BMC cancer 2007, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, N.; Xiong, Y.; Guo, G.; Zhu, M.; Gu, Y. Transcription factor FOSL1 enhances drug resistance of breast cancer through DUSP7-mediated dephosphorylation of PEA15. Molecular Cancer Research 2022, 20, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zhao, M.; Ang, L.; Hu, H.; Wu, Z.; Wang, J.; Huang, J.; Zheng, L.; Dong, W. Down-regulation of FOS-like antigen 1 enhances drug sensitivity in breast cancer. International journal of clinical and experimental pathology 2020, 13, 2092. [Google Scholar] [PubMed]
- Lu, D.; Chen, S.; Tan, X.; Li, N.; Liu, C.; Li, Z.; Liu, Z.; Stupack, D.G.; Reisfeld, R.A.; Xiang, R. Fra-1 Promotes Breast Cancer Chemosensitivity by Driving Cancer Stem Cells from DormancyFra-1 Drives Breast Cancer Cells from Dormancy. Cancer research 2012, 72, 3451–3456. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.; Zhang, P.-Y.; Qian, M.; Zhang, H.; Chen, X.; Ma, D.; Xu, Y.; Chen, X.; Tang, K.-F. The Fra-1–miR-134–SDS22 feedback loop amplifies ERK/JNK signaling and reduces chemosensitivity in ovarian cancer cells. Cell death & disease 2016, 7, e2384–e2384. [Google Scholar]
- Lin, S.; Zhu, B. Exosome-transmitted FOSL1 from cancer-associated fibroblasts drives colorectal cancer stemness and chemoresistance through transcriptionally activating ITGB4. 2023.
- Endo, S.; Fujita, M.; Yamada, S.; Imadome, K.; Nakayama, F.; Isozaki, T.; Yasuda, T.; Imai, T.; Matsubara, H. Fra-1 enhances the radioresistance of colon cancer cells to X-ray or C-ion radiation. Oncology Reports 2018, 39, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Atri, P.; Nallasamy, P.; Parte, S.; Rauth, S.; Nimmakayala, R.K.; Marimuthu, S.; Chirravuri-Venkata, R.; Bhatia, R.; Halder, S. Small molecule inhibitor against onco-mucins disrupts Src/FosL1 axis to enhance gemcitabine efficacy in pancreatic ductal adenocarcinoma. Cancer Letters 2022, 551, 215922. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Ke, A.W.; Shi, G.M.; Zhang, X.; Zhang, C.; Shi, Y.H.; Wang, X.Y.; Ding, Z.B.; Xiao, Y.S.; Yan, J. αB-crystallin complexes with 14-3-3ζ to induce epithelial-mesenchymal transition and resistance to sorafenib in hepatocellular carcinoma. Hepatology 2013, 57, 2235–2247. [Google Scholar] [CrossRef] [PubMed]
- Kajanne, R.; Miettinen, P.; Tenhunen, M.; Leppä, S. Transcription factor AP-1 promotes growth and radioresistance in prostate cancer cells. International journal of oncology 2009, 35, 1175–1182. [Google Scholar]
- Halatsch, M.E.; Löw, S.; Mursch, K.; Hielscher, T.; Schmidt, U.; Unterberg, A.; Vougioukas, V.I.; Feuerhake, F. Candidate genes for sensitivity and resistance of human glioblastoma multiforme cell lines to erlotinib. Laboratory investigation. Journal of neurosurgery 2009, 111, 211–218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).