1. Introduction
The Qinghai-Tibetan plateau area (QTPA), boasting the highest average elevation globally, harbors a diverse range of ungulate species, comprising approximately 42% of the total ungulate species in China [
1]. The significant variations in altitude and the diverse climatic conditions of the plateau have fostered a multitude of ecological environments, which, in turn, have created ecological niches for a wide range of high-altitude species, including different kinds of domestic animals [
2]. Nonetheless, the sensitivity of the Qinghai-Tibetan plateau to the effects of global climate change has been noted [
3].
The QTPA is home to a diverse range of livestock species that have adapted to survive in the high altitude and cold climate conditions [
4]. Among these unique species are Tibetan sheep (
Ovis aries), goats (
Capra hircus), yaks (
Bos grunniens), cattle (
Bos taurus domestica), horses (
Equus ferus caballus), camels (
Camelus bactrianus), and donkeys (
Equus asinus) [
5]. Within the QTPA, particular livestock species coexist alongside thriving ticks that exhibit adaptability to various environmental niches, characterized by their sensitivity to temperature and humidity conditions [
6]. Ticks serve as primary vectors for various pathogens worldwide, impacting both wildlife and domestic animals. While the majority of these pathogens are specific to animals, certain tick-borne diseases are zoonotic, posing significant health risks to humans and, in some cases, leading to severe or fatal consequences [
7]. Previous studies have identified the presence of 2 families, 6 genera, and 31 distinct species of ticks in Qinghai plateau of the QTPA. Notably,
Haemaphysalis qinghaiensis and
Dermacentor nuttalli are the predominant tick species found in Qinghai province and are widespread across most regions [
8]. Consequently, the presence of tick-borne bacterial pathogens including
Anaplasma,
Borrelia,
Rickettsia,
Coxiella poses a significant concern in this region.
Anaplasmosis is caused by a group of important tick-borne bacteria in the genus
Anaplasma. In China, anaplasmosis is generally found in livestock.
A. platys was detected in Bactrian camels in China [
9]. Furthermore, the occurrence of
A. bovis in sheep, goats and yaks,
A. ovis in sheep and goats,
A. phagocytophilum in yaks, as well as
A. marginale in cattle, has been reported within China [
10,
11,
12,
13]. Notably,
A. bovis and
A.ovis can cause anaplasmosis in cattle and sheep, with a high infection rate among livestock; the infection rate of sheep in Haixi and Haibei reached 58% [
14].
Lyme disease is a global natural zoonotic disease caused by
Borrelia burgdorferi [
15]. In China, at least 14 species of
B. burgdorferi sensu lato have been indentified in ticks, animals, and humans, providing evidence of the wide distribution and diversity of
B. burgdorferi s.l. across China [
16]. Results of an epidemiological survey conducted in the forested region of Qinghai plateau revealed a higher prevalence of Lyme disease in the agricultural area as compared to the pastoral area [
17].
Earlier study has indicated the presence of
B. burgdorferi s.l. in tick hosts, including humans, yaks, cattle, mice, and Tibetan sheep. [
18].
Rickettsia spp., categorized under the spotted fever group (SFG), have been identified as causative agents of zoonotic diseases affecting humans, domestic animals, and wildlife, thus emerging as a significnt global health concern [
19]. In China, several species including
R. heilongjiangensis,
R. sibirica subsp. sibirica BJ-90,
R. raoultii,
R. japonica,
Rickettsia sp. XY99, and
Candidatus Rickettsia tarasevichiae have been reported [
20,
21,
22,
23,
24,
25,
26]. While
Rickettsia is predominantly present in ticks within Qinghai plateau [
19,
27,
28,
29], there is limited information regarding its incidence in domestic animals.
Coxiella burnetii, the causative agent of Q fever, is a significant zoonotic pathogen with a wide distribution [
30]. Infection of
C. burnetii in goats has been identified as a major reservoir for human infection. Furthermore, infections in cattle have been associated with cases of abortion and have shown higher prevalence rates in older animals [
31]. A study conducted in the Qinghai plateau region reported the detection of
C. burnetii within ticks [
14].
Previous research has primarily focused on tick-borne pathogens that cause diseases in ticks. However, limited information is available regarding the incidence of these pathogens in domestic animals, particularly goats, cattle, horses, and donkeys in the QTPA. In order to gain a comprehensive understanding of bacterial microorganisms associated with ticks in various domestic animals in Qinghai-Tibetan plateau area, China, we performed targeted molecular screening to identify the presence of bacterial pathogens. Therefore, the current study aims to investigate the incidence of tick-borne bacterial pathogens that infect both ticks and seven distinct types of livestock within the Qinghai-Tibetan Plateau area, providing a comprehensive understanding of the prevalence and distribution of pathogens within the study area.
2. Materials and Methods
2.1. Blood sample collection
A comprehensive collection of 366 randomly obtained blood samples was assembled, representing a diverse range of livestock species, including 52 Tibetan sheep (
Ovis aries), 67 goats (
Capra hircus), 43 yaks (
Bos grunniens), 49 cattle (
Bos taurus domestica), 40 donkeys (
Equus ferus caballus), 50 camels (
Camelus bactrianus), and 65 horses (
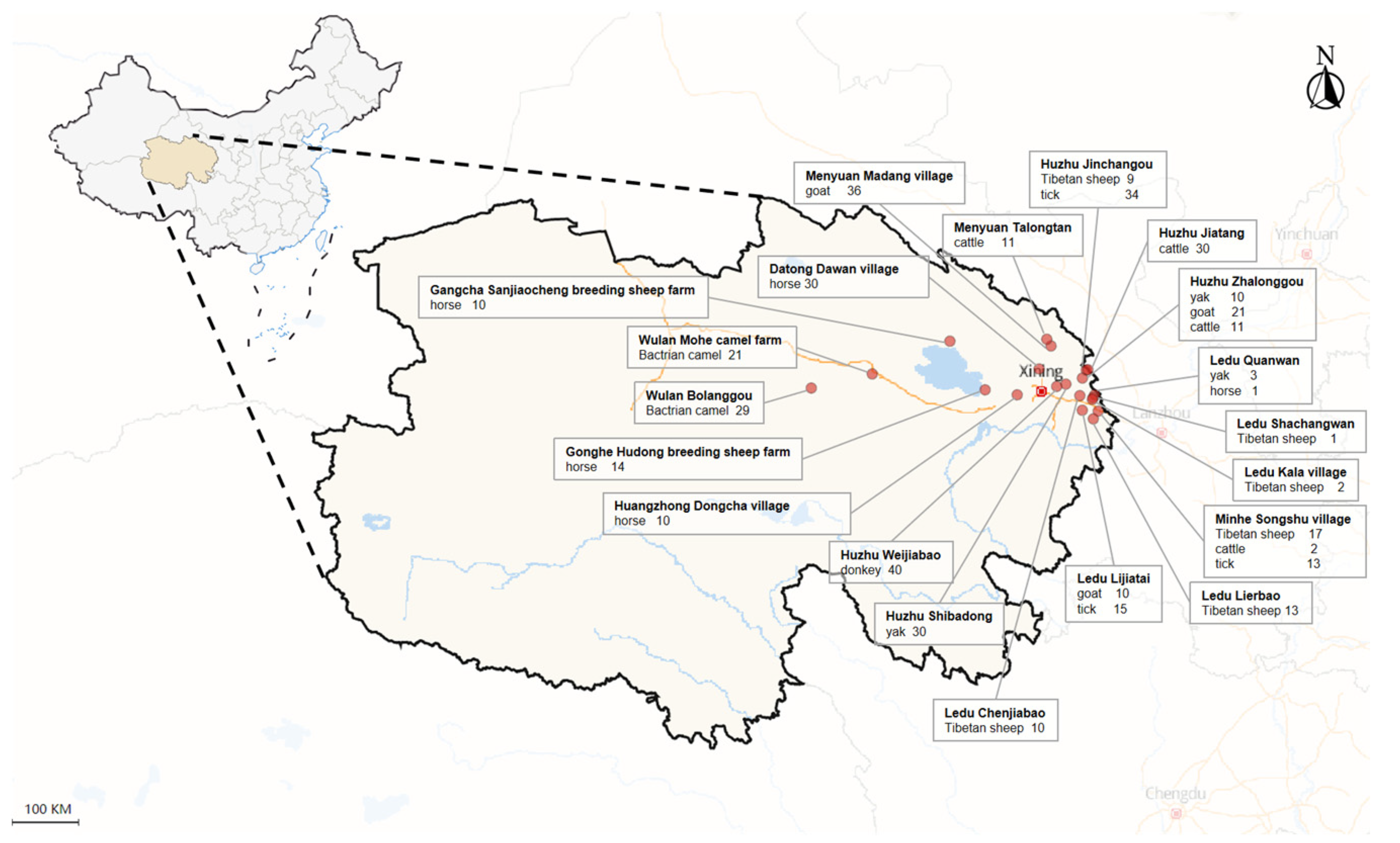
Equus asinus). These samples were gathered from 20 distinct locations situated within the Qinghai plateau of QTPA (
Figure 1). Prior to including livestock in this study, the farm owner was asked for their willingness to participate in the research activity and verbal consent was obtained.
2.2. Morphological and molecular identification of ticks
A total of 62 tick samples were collected from three distinct locations within the Qinghai plateau of the Qinghai-Tibetan Plateau Area (QTPA), specifically Jinchangou in Huzhu County, Lijiatai Village in Ledu County, and Songshu Village in Minhe County (
Figure 1). Tick samples were obtained from the field and external surfaces of domestic animals. All tick specimens were carefully preserved in sampling vials and promptly transported to the laboratory for detailed analyses. The identification of each tick specimen was conducted based on morphological criteria, following the methodology outlined by Chen et al. [
32]. Additionally, the identification was further confirmed through sequence analyses of a partial fragment of the cytochrome c oxidase subunit I (
coxI) gene. A fragment of approximately 850 base pairs (bp) within the
coxI gene was amplified through polymerase chain reaction (PCR) using the primers
coxI F (5'-GGAACAATATATTTAATTTTTGG-3') and
coxI R (5'-ATCTATCCCTACTGTAAATATATG-3') [
33].
2.3. DNA extraction
The genomic DNA was extracted from whole blood, which included an anticoagulant (EDTA), utilizing the TIANamp Genomic DNA Kit (TIANGEN, China) following the guidelines provided by the manufacturer. The concentration of DNA was determined using a Biochrom WPA Biowave DNA Life Science Spectrophotometer (Biochrom, UK), and the DNA samples were subsequently stored at -20℃ until their future utilization.
2.4. Molecular identification of tick-borne bacterial pathogens
Tick-borne bacterial pathogens were detected and charaterized by PCR in Tibetan sheep, goats, yaks, cattle, donkeys, horses, Bactrian camels, and ticks from the 20 sites of Qinghai plateau, using the primers listed in
Table 1. The PCR reaction was performed with a total volume of 10 μL, containing 1 μL of the DNA template, 0.5 μL each of the forward and reverse primer (100 μM), 0.2 μL of deoxyribonucleotide triphosphate (200 μM; New England BioLab, USA), 1 μL of 10×ThermoPol Reaction Buffer (New England BioLab, USA), 0.1 μL of Taq polymerase (0.5 U; New England BioLab, USA), and double-distilled water up to 10 μL [
34]. A negative control was included, using double-distilled water instead of DNA template. The PCR products were visualized by UV transillumination in a 1.5% agarose gel following electrophoresis and staining with ethidium bromide.
2.5. Sequencing analyses of detected tick-borne bacterial pathogens
For the detection of tick-borne bacterial pathogens, at least one positive sample per animal species within each sampling area was selected for sequencing and subsequent molecular characterization. The PCR product obtained from the positive sample was purified using the EasyPure Quick Gel Extraction Kit (TransGen Biotech, China) and subsequently cloned into E. coli DH5ɑ via the pMD™18-T Vector Cloning Kit (Takara, Japan). At a minimum, three positive clones were sent for sequencing services provided by Genewiz (USA) company.
2.6. Sequencing analyses of detected tick-borne bacterial pathogens
The acquired sequences were validated through a BLASTn search in GenBank (
https://blast.ncbi.nlm.nih.gov/Blast.cgi). Subsequently, phylogenetic trees were constructed using the maximum likelihood statistical method and 1000 replications of bootstrap analyses through MEGA X: Molecular Evolutionary Genetics Analysis version X, designed for larger datasets [
45].
2.7. Statistical analyses
The proportion of samples tested positive between different regions and different animals was compared by Epitools online (
https://epitools.ausvet.com.au). The chi-squared test for contingency table was applied to the original data to quantify the prevalence of each pathogen's infection rate, along with a corresponding 95% confidence interval (CI). Observed differences were considered statistically significant when the resulting p-values were lower than 0.05.
3. Results
3.1. Morphological and molecular identification of ticks
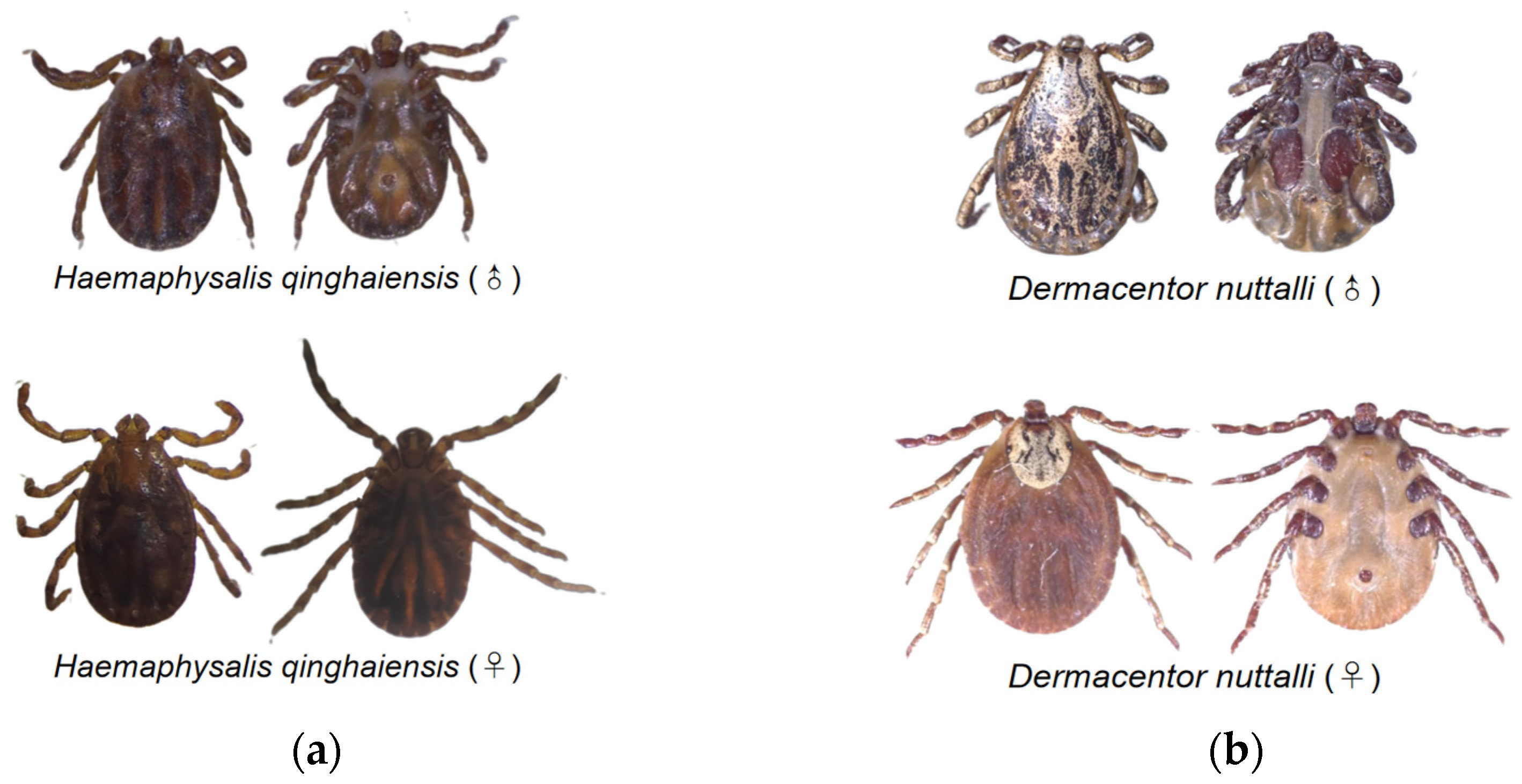
A light microscope was utilized to facilitate the observation of tick species. The results of the morphological identification of ticks revealed the detection of two primary species within the Qinghai plateau, namely
H. qinghaiensis and
D. nuttalli (
Figure 2). A total 23 of ticks were submitted to the Genebank. After conducting a rigorous sequence alignment analyses, our investigation has provided evidence of the presence of two distinct tick species,
H. qinghaiensis and
D. nuttalli, in the Qinghai plateau region, with nucleotide consistency levels ranging from 97.53% -100% (
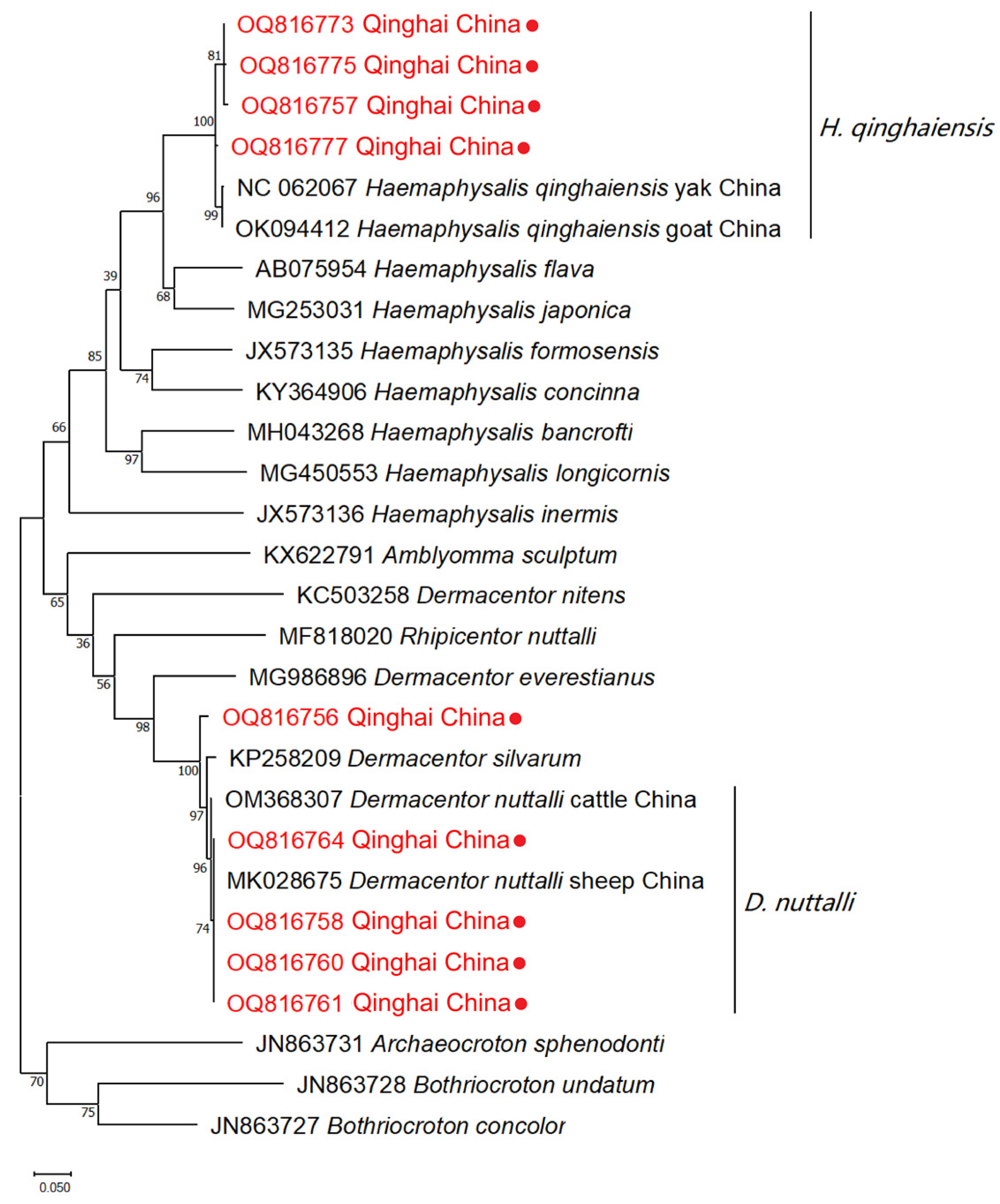
Table 2). The results of the phylogenetic analyses indicate that based on the
coxI gene, the tick species obtained in this study can be mainly classified into two clades: One clade is closely related to
H. qinghaiensis, while the other is closely related to
D. nuttalli from China (
Figure 3). In this investigation, we have observed that OQ816756 exhibits notable dissimilarity when compared to the
D. nuttalli and
D. silvarum clade. It is suggested that OQ816756 could potentially represent novel variants within the
Dermacentor genus.
3.2. Identification of tick-borne bacterial pathogens
A. bovis,
A. capra,
A. marginale,
A. ovis,
A. phagocytophilum,
B. burgdorferi s.l.,
C. burnetii, and
Rickettsia spp. transmitted by ticks were investigated. As presented in
Table 3, the prevalence of infection was found to be 16.4% (70/428) for
A. bovis, 4.7% (20/428) for
A. capra, 5.8% (25/428) for
A. ovis, 6.3% (27/428) for
B. burgdorferi s.l., 0.7% (3/428) for
C. burnetii, and 0.5% (2/428) for
Rickettsia spp. No cases of infections caused by
A. marginale and
A. phagocytophilum were detected. Additionally, no instances of tick-borne bacterial pathogen infections were found in yaks, Bactrian camels, donkeys, and horses among the livestock examined. The prevalence of tick-borne bacterial pathogens in the dominant animals showed no statistically significant difference (chi-squared test, p >0.05).
3.3. Sequencing and Comparative Analyses
The sequences obtained in the present investigation were analyzed using the the Basic Local Alignment Search Tool (BLAST) at the National Center for Biotechnology Information (NCBI). Thirteen sequences of
Anaplasma bovis, two of
A. ovis, eight of
A. capra, two of
Rickettsia spp., two of
B. burgdorferi s.l., and two sequences of
C. burnetii were submitted to the Genebank. The results presented in this study include 24 distinctive sequences of tick-borne bacterial pathogens (
Table 4).
The investigation into tick-borne bacterial pathogens has revealed a high degree of sequence similarity among the identified species of Anaplasma, ranging from 99.27% to 100% for A. bovis, 99.71% to 100% for A. ovis, and 97.58% to 99.62% for A. capra. For the identified species of Rickettsia spp. (R. raoultii), the sequence similarity was 100%. The identified species of Borrelia (KY284014) exhibited sequence similarity ranging from 99.52% to 99.84%, and the identified species of C. burnetii (MK416231) showed a sequence similarity of 99.69%.
3.3. Phylogenetic analyses
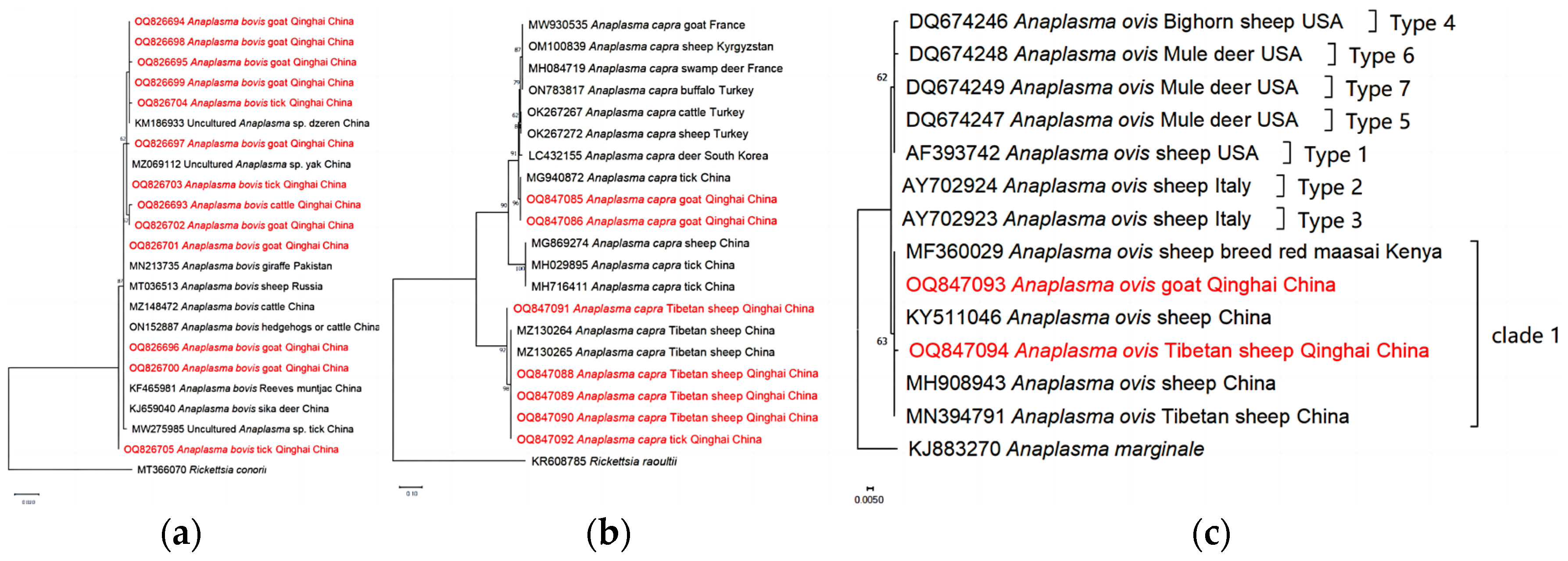
Based on our analyses of the 16S rRNA gene, the isolates of
A. bovis obtained from goats, cattle, and ticks exhibited a close genetic relationship with previously reported
A. bovis isolates. However, it is noteworthy that the isolate OQ826705 displayed distinct dissimilarity compared to
A. bovis, suggesting its potential classification as a novel variant within the species
A. bovis (
Figure 4a). Additionally, our phylogenetic analyses based on partial sequences of the citrate synthase gene (
gltA) revealed interesting findings. They showed that
A. capra isolates obtained from goats in our study are genetically related to
A. capra isolates previously detected from ticks in China [
46]. Moreover, the analyses demonstrated that
A. capra isolates derived from Tibetan sheep and ticks are positioned within the same clade as Tibetan sheep isolates obtained from Qinghai, as reported by He et al. [
47]. These results suggest a genetic similarity and potential association between
A. capra variants found in Tibetan sheep, ticks, and the Tibetan sheep population from the Qinghai region (
Figure 4b). Moreover, our phylogenetic analyses of
A. ovis, based on the Major surface protein 4 (
msp4) partial sequences obtained from Tibetan sheep and goats, revealed the presence of a new clade, distinct from those previously identified in a study conducted by de la Fuente et al. [
48] (Figure 4c).
The 16S rRNA sequences of
B. burgdorferi s.l. obtained from Tibetan sheep and goats in this study were found to cluster with homologous sequences from camels in China (
Figure 5).
Furthermore, the phylogenetic analyses of
Rickettsia spp. based on the
gltA gene revealed that the tick isolates obtained in this study belong to the
R. raoultii clade along with ticks from China and Russia (
Figure 6).
Moreover, the
C. burnetii based on heat shock protein B (
htpB) was found to be in the same clade as those from ticks and humans in other countries (
Figure 7). The detection of similar clade membership across diverse geographic locations underscores the potential for transnational transmission.
4. Discussion
The Qinghai-Tibetan plateau region is characterized by vast grasslands, towering mountains, and serene lakes, constituting a unique geographical environment. The landscape of this region is intricately intertwined with diverse livestock species that rely on the grasslands for grazing, establishing a symbiotic relationship with the natural surroundings. Moreover, ticks, known to inhabit grasslands, forests, and shrubbery, are also prevalent in this ecosystem. Ticks play a crucial role as carriers of human and animal pathogens globally. Notable examples of these tick-borne bacterial pathogens, with implications for both veterinary and public health, include
Rickettsia spp.,
Anaplasma phagocytophilum,
Borrelia burgdorferi sensu lato (s.l.),
Coxiella burnetii. These pathogens have the capacity to infect a diverse array of hosts, encompassing humans as well as wild and domestic animals [
49]. While various studies have investigated the prevalence of tick-borne pathogens in different regions [
50,
51,
52,
53], limited research has been conducted specifically focusing on the Qinghai-Tibetan plateau [
14,
18,
19]. To date, this study represents the first comprehensive investigation into the presence of tick-borne bacterial pathogens in this unique geographical region.
The investigation of tick-borne diseases may depend significantly on the distribution and diversity of tick species. Individuals bitten by
Haemaphysalis ticks carrying bacterial infections face increased risk of bacterial infection [
54]. Some tick species have successfully adapted to the dry and cold climate of the plateau, such as
H. qinghaiensis [
55], which is the predominant tick species in Qinghai province, China. Our study confirmed the presence of
H. qinghaiensis and
D. nuttalli, aligning with previous research findings [
8]. To date, there has not been a comprehensive and organized investigation, leading to a lack of knowledge about various pathogens in Qinghai province. This study employed molecular detection methods to investigate the prevalence of these pathogens among major livestock species in Qinghai province. The primary objective of this study was to ascertain the presence of tick-borne pathogens and acquire a more profound comprehension of their biological characteristics and classification within the province. Furthermore, it facilitates research into the biological traits of potential novel tick-borne pathogens.
In the context of
Anaplasma spp., this investigation has provided valuable insights into the geographical distribution of diverse tick-borne bacterial pathogens in China. Importantly, it marks the first identification of
A. bovis,
A. capra, and
A. ovis in goats, as well as the detection of
A. bovis in cattle within the Qinghai plateau. Notably, Tibetan sheep and goats displayed a high susceptibility to
Anaplasma infections, with a remarkable 65.7% of goats found to be infected with
A. bovis. Among the five different
Anaplasma species tested, the highest infection rate was observed for
A. bovis, with 16.4% of the samples testing positive. The presence of
A. phagocytophilum has been previously documented in Gansu province [
56], while
A. marginale has been detected in both Sichuan province [
57] and the Tibet Autonomous Region [
58], which shares a border with the Qinghai plateau. Additionally, ticks from Qinghai have also tested positive for both
A. marginale and
A. phagocytophilum [
46,
61]. However, contrary to these findings, the present study observed the absence of these pathogens.
Based on the results of this study, only two instances of
Rickettsia spp. infection were detected in the ticks. Detailed analyses of the genetic sequences unveiled 100% similarity between the positive sequences and the previously identified
R. raoultii isolates originating from Russia and China. It is worth noting that earlier studies had already established the presence of
R. raoultii in ticks from the Qinghai province [
19,
27,
28], further confirming our findings.
The findings regarding to
C. burnetii reveal that Tibetan sheep and ticks in Qinghai demonstrate a seropositivity rate of 0.5% (2/428). It is noteworthy that the sequencing analyses revealed a remarkable 99.69% similarity to the tick sample obtained from Tunisia (MK416231). This finding differs from the strain of
C. burnetii previously detected in ticks from the Qilian area of Qinghai province [
13]. Moreover, phylogenetic tree analyses further establish that these organisms belong to the same clade as those found in foreign nations, strongly suggesting the possibility of pathogen importation from other countries. The identification of
C. burnetii in neighboring provinces [
62,
63,
64] and the Qinghai plateau implies the widespread presence of this bacterium in diverse geographical regions, indicating its potential for transmission to both animals and humans across a broader geographic scope.
In this study, the presence of
B. burgdorferi s.l. was detected in Tibetan sheep and goats. It is worth mentioning that while previous studies have reported various animal species being infected with
B. burgdorferi s.l. [
18], this study represents the first documented instance of its detection in goats within the Qinghai region.
In summary, this study represents the inaugural exploration of tick-borne pathogens in goats, horses, and donkeys within the QTPA. It offers crucial insights into the prevalence and genetic diversity of Anaplasma spp., Rickettsia spp., Coxiella spp., and Borrelia spp. throughout China. The significance of these pathogens lies in their zoonotic potential, indicating the risk of transmission from animals to humans. The intricate interplay between animal and human ecosystems underscores the imperative for robust surveillance and prevention strategies to alleviate the impact of these zoonotic tick-borne bacteria on health and productivity. This study reveals the zoonotic risk of tick-borne pathogens in the Qinghai-Tibetan plateau, emphasizing the need for public health awareness, veterinary vigilance, and conservation efforts. The findings underscore international health security concerns and advocate for a comprehensive One Health approach, urging further research and collaborative strategies. Nevertheless, it is essential to acknowledge certain limitations inherent in this study, including its focused scope, potential diagnostic biases, and the absence of long-term monitoring capabilities, which may impact its generalizability or applicability beyond specific contexts or regions. Consequently, future research initiatives should conscientiously address these constraints to foster a more comprehensive understanding of tick-borne infections, not only within the QTPA but potentially extending to broader geographical contexts as well.
Author Contributions
Conceptualization, X.X. and L.M.; methodology, Y.M.; software, Y.M. and Y.J.; validation, Y.J. and G.W.; formal analysis, Y.J.; investigation, X.L., G.W., Y.H.; resources, L.M.; data curation, X.L.; writing—original draft preparation, Y.M.; writing—review and editing, J.L., I.Z., H.L., M.L., and N.Y.; visualization, Y.M.; supervision, L.M.; project administration, X.X.; funding acquisition, X.X. and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study received generous support from several esteemed funding sources in China, including the Transformation of Scientific and Technological Achievements of Qinghai province (Grant No. 2021-SF-146), the China Agriculture Research System (Grant No. CARS-39-16), and the National Foreign Experts Program of China (Grant No. G2022043003L). Additionally, we would like to express our sincere appreciation for the support received from Japan, including the AMED project (Grant No. JP23WM02250317), the JSPS Core-to-Core program, and a grant from the Strategic International Collaborative Research Project (JPJ008837) promoted by the Ministry of Agriculture, Forestry, and Fisheries of Japan. Their financial support has played a crucial role in facilitating the successful execution of this research endeavor.
Institutional Review Board Statement
All protocols were carried out according to the ethical guidelines approved by the Obihiro University of Agriculture and Veterinary Medicine (Permit for the animal experiment: 22-23).
Data Availability Statement
All data are disclosed in the paper.
Acknowledgments
The authors would like to express their heartfelt gratitude to all individuals who played a crucial role in the successful completion of this project. In particular, we extend our sincere appreciation to the dedicated technical staff at the Animal Husbandry and Veterinary Station for their invaluable assistance in collecting blood and tick samples from various regions, including Minhe, Ledu, Huzhu, Menyuan, Huangyuan, Datong, and other counties of Qinghai province, China. Their unwavering commitment and expertise have significantly contributed to the achievements of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang, Z.; Li, L.; Hu, Y.; Hu, H.; Li, C.; Ping, X.; Luo, Z. Diversity and endemism of ungulates on the Qinghai-Tibetan plateau: evolution and conservation. Biodivers. Sci. 2018, 26, 158–170. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Li, Q.; Tseng, Z.J.; Takeuchi, G.T.; Deng, T.; Xie, G.; Chang, M.; Wang, N. Cenozoic vertebrate evolution and paleoenvironment in Tibetan plateau: progress and prospects. Gondwana Res. 2015, 27, 1335–1354. [Google Scholar] [CrossRef]
- Jin, Z.; Zhuang, Q.; He, J.-S.; Luo, T.; Shi, Y. Phenology shift from 1989 to 2008 on the Tibetan plateau: an analysis with a process-based soil physical model and remote sensing data. Clim. Change 2013, 119, 435–449. [Google Scholar] [CrossRef]
- Spicer, R.A.; Su, T.; Valdes, P.J.; Farnsworth, A.; Wu, F.-X.; Shi, G.; Spicer, T.E.V.; Zhou, Z. Why ‘the Uplift of the Tibetan Plateau’ Is a Myth? Natl. Sci. Rev. 2020, 8, nwaa091. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Ai, J.; Yang, J.; Zhu, H.; Zhou, Y.; Zhu, Y.; Zhang, H.; Qin, Q.; Kang, M.; Sun, Y.; et al. Seroepidemiology of neosporosis in various animals in the Qinghai-Tibetan plateau. Front. Vet. Sci. 2022, 9, 953380. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.F.; Magnarelli, L.A. Biology of ticks. Infect. Dis. Clin. North Am. 2008, 22, 195–215. [Google Scholar] [CrossRef]
- Moraga-Fernández, A.; Muñoz-Hernández, C.; Sánchez-Sánchez, M.; de Mera IG, F.; de la Fuente, J. Exploring the diversity of tick-borne pathogens: The case of bacteria (Anaplasma, Rickettsia, Coxiella and Borrelia) protozoa (Babesia and Theileria) and viruses (Orthonairovirus, tick-borne encephalitis virus and louping ill virus) in the European continent. Vet. Microbiol. 2023, 286, 109892. [Google Scholar] [CrossRef]
- Wang, Y.; Han, S.; He, H. Research status of tick distribution and tick-borne diseases in Qinghai province. Prev. Vet. Med. 2022, 43, 115–119. [Google Scholar]
- Li, H.; Zheng, Y.C.; Ma, L.; Jia, N.; Jiang, B.G.; Jiang, R.R.; Huo, Q.B.; Wang, Y.W.; Liu, H.B.; Chu, Y.L.; et al. Human infection with a novel tick-borne Anaplasma species in China: a surveillance study. Infect. Dis. 2015, 15, 663–670. [Google Scholar] [CrossRef]
- Zhou, Z.; Nie, K.; Tang, C.; Wang, Z.; Zhou, R.; Hu, S.; Zhang, Z. Phylogenetic analysis of the genus Anaplasma in southwestern China based on 16S rRNA sequence. Res. Vet. Sci. 2010, 89, 262–265. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, M.; Wang, Z.; Wang, J.; Peng, Y.; Li, Y.; Guan, G.; Luo, J.; Yin, H. Molecular survey and genetic identification of Anaplasma species in goats from central and southern China. Appl. Environ. Microbiol. 2012, 78, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Liu, Z.; Liu, J.; Niu, Q.; Ren, Q.; Chen, Z.; Guan, G.; Luo, J.; Yin, H. Molecular detection and characterization of Anaplasma spp. in sheep and cattle from Xinjiang, northwest China. Parasites Vectors 2015, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Z.; Niu, Q.; Liu, J.; Guan, G.; Xie, J.; Luo, J.; Wang, S.; Wang, S.; Yin, H. First molecular survey and identification of Anaplasma spp. in white yaks (Bos grunniens) in China. Parasitology 2016, 143, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Zhang, X.; Li, X.; Wang, G.; Wang, G.; Ma, L. Identification of ticks and tick-borne pathogens in different areas of Haibei of Qinghai province. Chin. Qinghai J. Anim. Vet. Sci. 2020, 50, 35. [Google Scholar]
- Wang, G.; van Dam, A.P.; Schwartz, I.; Dankert, J. Molecular Typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin. Microbiol. Rev. 1999, 12, 633–653. [Google Scholar] [CrossRef]
- Hao, Q.; Hou, X.; Geng, Z.; Wan, K. Distribution of Borrelia burgdorferi sensu lato in China. J. Clin. Microbiol. 2011, 49, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Shi, Y. Epidemiological investigation of Lyme disease in parts of forest areas in Qinghai province. Chin. J. Vector. Bio. Control 2009, 20, 358–359. [Google Scholar]
- Han, R. Studies on species diversity of ticks and gene polymorphism of tick-borne pathogens in Qinghai province. Ph.D., Chinese Academy of Agricultural Sciences, China, 2018.
- Jian, Y.; Li, J.; Adjou Moumouni, P.F.; Zhang, X.; Tumwebaze, M.A.; Wang, G.; Cai, Q.; Li, X.; Wang, G.; Liu, M.; et al. Human spotted fever group Rickettsia infecting yaks (Bos grunniens) in the Qinghai-Tibetan plateau area. Pathogens 2020, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jin, Y.; Fan, M.; Xu, G.; Liu, Q.; Raoult, D. Genotypic and antigenic identification of two new strains of spotted fever group rickettsiae isolated from China. J. Clin. Microbiol. 1993, 31, 83–88. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.; Jiang, X.; Guo, X.; Garnier, M.; Raoult, D.; Parola, P. Molecular identification of spotted fever group rickettsiae in ticks collected in central China. Clin. Microbiol. Infect. 2009, 15, 279–280. [Google Scholar] [CrossRef]
- Tian, Z.C.; Liu, G.Y.; Shen, H.; Xie, J.R.; Luo, J.; Tian, M.Y. First Report on the occurrence of Rickettsia slovaca and Rickettsia raoultii in Dermacentor silvarum in China. Parasites Vectors 2012, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, G.; Kelly, P.; Zhang, Z.; Wei, L.; Yu, D.; Kayizha, S.; Wang, C. First report of Rickettsia felisin China. BMC Infect. Dis. 2014, 14, 682. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.Q.; Guo, L.P.; Wang, A.D.; Mu, L.M.; Zhang, K.; Chen, C.F.; Zhang, W.J.; Wang, Y.Z. The first detection of Rickettsia aeschlimannii and Rickettsia massiliae in Rhipicephalus turanicus ticks, in northwest China. Parasites Vectors 2015, 8, 631. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Yin, H.; Rikihisa, Y.; Pan, W.; Yin, H. Molecular detection of tick-borne Rickettsiales in goats and sheep from southeastern China. Vector. Borne. Zoonotic. Dis. 2016, 16, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Q.; Zhang, X.; Li, Z.; Wang, Z.; Song, M.; Wei, F.; Wang, S.; Liu, Q. Characterization of Rickettsiae in ticks in northeastern China. Parasites Vectors 2016, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Yang, J.; Niu, Q.; Liu, Z.; Chen, Z.; Kan, W.; Hu, G.; Liu, G.; Luo, J.; Yin, H. Molecular prevalence of spotted fever group Rickettsiae in ticks from Qinghai province, northwestern China. Infect. Genet. Evol. 2018, 57, 1–7. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Wang, L.; Wei, F. Epidemiological investigation of tick-transmitted Rickettsia in Qinghai province. Heilongjiang Anim. Sci. and Vet. Med. 2019, 65–69. [Google Scholar]
- Li, J.; Jian, Y.; Jia, L.; Galon, E.M.; Benedicto, B.; Wang, G.; Cai, Q.; Liu, M.; Li, Y.; Ji, S.; et al. Molecular characterization of tick-borne bacteria and protozoans in yaks (Bos grunniens), Tibetan sheep (Ovis aries) and Bactrian camels (Camelus bactrianus) in the Qinghai-Tibetan plateau area, China. Ticks. Tick. Borne. Dis. 2020, 11, 101466. [Google Scholar] [CrossRef]
- Maurin, M.; Raoult, D. Q fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef]
- El-mahallawy, H.S.; Lu, G.; Kelly, P.; Xu, D.; Li, Y.; Fan, W.; Wang, C. Q fever in China: a systematic review, 1989–2013. Epidemiol. Infect. 2014, 143, 673–681. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Liu, Z.; Ren, Q.; Ma, M.; Luo, J.; Yin, H. Scanning electron microscopy of all parasitic stages of Haemaphysalis qinghaiensis Teng, 1980 (Acari: Ixodidae). Parasitol. Res. 2014, 113, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Chitimia, L.; Lin, R.-Q.; Cosoroaba, I.; Wu, X.-Y.; Song, H.-Q.; Yuan, Z.-G.; Zhu, X.-Q. Genetic characterization of ticks from southwestern Romania by sequences of mitochondrial cox1 and nad5 genes. Exp. Appl. Acarol. 2010, 52, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, L.; Moumouni, P.F.A.; Jian, Y.; Wang, G.; Zhang, X.; Li, X.; Wang, G.; Lee, S.-H.; Galon, E.M.; et al. Molecular survey and characterization of tick-borne pathogens in sheep from Qinghai, China. Small Rumin. Res. 2019, 175, 23–30. [Google Scholar] [CrossRef]
- Barlough, J.E.; Madigan, J.E.; DeRock, E.; Bigornia, L. Nested polymerase chain reaction for detection of Ehrlichia equi genomic DNA in horses and ticks (Ixodes pacificus). Vet. Parasitol. 1996, 63, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Rikihisa, Y.; Lin, Q.; Isogai, E.; Tahara, K.; Itagaki, A.; Hiramitsu, Y.; Tajima, T. Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia sp. in wild deer and ticks on two major islands in Japan. Appl Environ Microbiol 2006, 72, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Torina, A.; Agnone, A.; Blanda, V.; Alongi, A.; D’Agostino, R.; Caracappa, S.; Marino, A.M.F.; Di Marco, V.; de la Fuente, J. Development and validation of two PCR tests for the detection of and differentiation between Anaplasma ovis and Anaplasma marginale. Ticks. Tick. Borne. Dis. 2012, 3, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Massung, R.F.; Slater, K.; Owens, J.H.; Nicholson, W.L.; Mather, T.N.; Solberg, V.B.; Olson, J.G. Nested PCR assay for detection of granulocytic Ehrlichiae. J. Clin. Microbiol. 1998, 36, 1090–1095. [Google Scholar] [CrossRef]
- Ni, X.-B.; Jia, N.; Jiang, B.-G.; Sun, T.; Zheng, Y.-C.; Huo, Q.-B.; Liu, K.; Ma, L.; Zhao, Q.-M.; Yang, H.; et al. Lyme borreliosis caused by diverse genospecies of Borrelia burgdorferi sensu lato in northeastern China. Clin. Microbiol. Infect. 2014, 20, 808–814. [Google Scholar] [CrossRef]
- Zhai, B.; Niu, Q.; Yang, J.; Liu, Z.; Liu, J.; Yin, H.; Zeng, Q. Identification and molecular survey of Borrelia burgdorferi sensu lato in sika deer ( Cervus nippon ) from Jilin province, north-eastern China. Acta Trop. 2017, 166, 54–57. [Google Scholar] [CrossRef]
- To, H.; Kako, N.; Zhang, G.Q.; Otsuka, H.; Ogawa, M.; Ochiai, O.; Nguyen, S.V.; Yamaguchi, T.; Fukushi, H.; Nagaoka, N.; et al. Q fever pneumonia in Children in Japan. J. Clin. Microbiol. 1996, 34, 647–651. [Google Scholar] [CrossRef]
- Kidd, L.; Maggi, R.; Diniz, P.P.V.P.; Hegarty, B.; Tucker, M.; Breitschwerdt, E. Evaluation of conventional and real-time PCR assays for detection and differentiation of spotted fever group Rickettsia in dog blood. Vet. Microbiol. 2008, 129, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Roux, V.; Raoult, D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (OmpB). Int. J. Syst. Evol. Microbiol. 2000, 50, 1449–1455. [Google Scholar] [CrossRef]
- Labruna, M.B.; Whitworth, T.; Horta, M.C.; Bouyer, D.H.; McBride, J.W.; Pinter, A.; Popov, V.; Gennari, S.M.; Walker, D.H. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of São Paulo, Brazil, where Brazilian spotted fever is endemic. J. Clin. Microbiol. 2004, 42, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Yang, J.-F.; Mukhtar, M.U.; Chen, Z.; Niu, Q.-L.; Lin, Y.-Q.; Liu, G.-Y.; Luo, J.-X.; Yin, H.; Liu, Z.-J. Molecular detection of Anaplasma Infections in Ixodid ticks from the Qinghai-Tibet plateau. Infect. Dis. Poverty 2019, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, W.; Ma, P.; Wei, Y.; Li, R.; Chen, Z.; Tian, S.; Qi, T.; Yang, J.; Sun, Y.; et al. Molecular detection of Anaplasma spp., Babesia spp. and Theileria spp. in yaks (Bos grunniens) and Tibetan sheep (Ovis aries) on the Qinghai-Tibetan Plateau, China. Parasites Vectors 2021, 14, 613. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Atkinson, M.W.; Naranjo, V.; Fernández de Mera, I.G.; Mangold, A.J.; Keating, K.A.; Kocan, K.M. Sequence analysis of the msp4 gene of Anaplasma ovis strains. Vet. Microbiol. 2007, 119, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Del Cerro, A.; Oleaga, A.; Somoano, A.; Barandika, J.F.; García-Pérez, A.L.; Espí, A. Molecular identification of tick-borne pathogens (Rickettsia spp., Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, Coxiella burnetii and piroplasms) in questing and feeding hard ticks from North-Western Spain. Ticks and tick-borne diseases 2022, 13, 101961. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.P.; Wang, Y.X.; Fan, Z.W.; Ji, Y.; Liu, M.; Zhang, W.H.; Li, X.L.; Zhou, S.X.; Li, H.; Liang, S.; et al. Mapping ticks and tick-borne pathogens in China. Nat Commun 2021, 12, 1075. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Q.; Liu, J.Q.; Xu, B.L.; Lv, S.; Xia, S.; Zhou, X.N. Tick-borne pathogens and associated co-infections in ticks collected from domestic animals in central China. Parasites Vectors 2014, 7, 1–8. [Google Scholar] [CrossRef]
- Wu, X.B.; Na, R.H.; Wei, S.S.; Zhu, J.S.; Peng, H.J. Distribution of tick-borne diseases in China. Parasites Vectors 2013, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Q.; Liu, J.Q.; Xu, B.L.; Lv, S.; Xia, S.; Zhou, X.N. Tick-borne pathogens and associated co-infections in ticks collected from domestic animals in central China. Parasites Vectors 2014, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hirunkanokpun, S.; Ahantarig, A.; Baimai, V.; Pramual, P.; Rakthong, P.; Trinachartvanit, W. Spotted fever group Rickettsia, Anaplasma and Coxiella-like endosymbiont in Haemaphysalis ticks from mammals in Thailand. Vet Res Commun 2022, 46, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Chen, Z.; Robbins, R.G. Haemaphysalis qinghaiensis (Acari: Ixodidae), a correct original species name, with notes on Chinese geographical and personal names in zoological taxa. Syst. Appl. Acarol. 2016, 21, 267. [Google Scholar]
- Yang, J.; Liu, Z.; Guan, G.; Liu, Q.; Li, Y.; Chen, Z.; Ma, M.; Liu, A.; Ren, Q.; Luo, J.; et al. Prevalence of Anaplasma phagocytophilum in ruminants, rodents and ticks in Gansu, north-western China. J. Med. Microbiol. 2013, 62, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Han, R.; Liu, Z.; Niu, Q.; Guan, G.; Liu, G.; Luo, J.; Yin, H. Insight into the Genetic Diversity of Anaplasma marginale in cattle from ten provinces of China. Parasites Vectors 2017, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Jian, R.; Zhang, Y.; Chen, R. Simultaneous detection of Anaplasma marginale and a new Ehrlichia species closely related to Ehrlichia chaffeensis by sequence analyses of 16S ribosomal DNA in Boophilus microplus ticks from Tibet. J Clin Microbiol 2002, 40, 3286–3290. [Google Scholar] [CrossRef] [PubMed]
- Beare, P.A.; Unsworth, N.; Andoh, M.; Voth, D.E.; Omsland, A.; Gilk, S.D.; Williams, K.P.; Sobral, B.W.; Kupko, J.J.; Porcella, S.F.; et al. Comparative genomics reveal extensive transposon-mediated genomic plasticity and diversity among potential effector proteins within the genus Coxiella. Infect Immun 2009, 77, 642–656. [Google Scholar] [CrossRef]
- Selmi, R.; Ben Said, M.; Mamlouk, A.; Ben Yahia, H.; Messadi, L. Molecular detection and genetic characterization of the potentially pathogenic Coxiella burnetii and the Endosymbiotic candidatus midichloria mitochondrii in ticks infesting camels (Camelus dromedarius) from Tunisia. Microb. Pathog. 2019, 136, 103655. [Google Scholar] [CrossRef]
- Gao, Y. Distribution of tick species and epidemiological investigation of 3 species of bacterial and protozoal in Qinghai province. Master, Jilin Agricultural University, China, 2018.
- Ni, J.; Lin, H.; Xu, X.; Ren, Q.; Aizezi, M.; Luo, J.; Luo, Y.; Ma, Z.; Chen, Z.; Tan, Y.; et al. Coxiella burnetii is widespread in ticks (Ixodidae) in the Xinjiang areas of China. BMC Vet Res 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Yin, M.Y.; Qin, S.Y.; Tan, Q.D.; Feng, S.Y.; Liu, G.X.; Zhou, D.H.; Zhu, X.Q. First Report of Coxiella burnetii seroprevalence in Tibetan sheep in China. Vector. Borne. Zoonotic. Dis. 2015, 15, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Shurong, Y. Coxiella burnetii in China. Q Fever 1991, 2, 327–341. [Google Scholar]
Figure 1.
A map of the Qinghai plateau, highlighting the various sampling sites and animals included. The figure was created and adjusted using map data in Excel.
Figure 1.
A map of the Qinghai plateau, highlighting the various sampling sites and animals included. The figure was created and adjusted using map data in Excel.
Figure 2.
Morphological identification of ticks in QTPA. (a) H. qinghaiensis detected in QTPA; (b) D. nuttalli from QTPA in this study.
Figure 2.
Morphological identification of ticks in QTPA. (a) H. qinghaiensis detected in QTPA; (b) D. nuttalli from QTPA in this study.
Figure 3.
Phylogenetic tree of ticks using Maximum Likelihood method. The numbers indicated at the nodes represent the percentage of occurrence of clades based on 1000 bootstrap replications of the data. The sequences of isolates obtained in this study, along with their corresponding accession numbers, are highlighted in red.
Figure 3.
Phylogenetic tree of ticks using Maximum Likelihood method. The numbers indicated at the nodes represent the percentage of occurrence of clades based on 1000 bootstrap replications of the data. The sequences of isolates obtained in this study, along with their corresponding accession numbers, are highlighted in red.
Figure 4.
Phylogenetic trees of Anaplasma spp. constructed using Maximum Likelihood method in MEGA X, employing the Kimura 2-parameter model. (a) The phylogenetic tree of A. bovis based on 16s rRNA gene. (b) The phylogenetic tree of A. capra based on gltA genes. (c) The phylogenetic of A. ovis based on msp4 gene. The numbers assigned to the nodes represent the percentage of occurrence of clades, determined through 1000 bootstrap replications of the data. Isolates from this study, along with their corresponding accession numbers, are highlighted in red. .
Figure 4.
Phylogenetic trees of Anaplasma spp. constructed using Maximum Likelihood method in MEGA X, employing the Kimura 2-parameter model. (a) The phylogenetic tree of A. bovis based on 16s rRNA gene. (b) The phylogenetic tree of A. capra based on gltA genes. (c) The phylogenetic of A. ovis based on msp4 gene. The numbers assigned to the nodes represent the percentage of occurrence of clades, determined through 1000 bootstrap replications of the data. Isolates from this study, along with their corresponding accession numbers, are highlighted in red. .
Figure 5.
The phylogenetic tree of B. burgdorferi s.l., based on 16s rRNA partial sequences obtained from Tibetan sheep and goats in this study, as well as sequences retrieved from the GenBank database, was constructed using the Maximum Likelihood method in MEGA X and Kimura 2-parameter model. The numbers assigned to the nodes indicate the percentage of occurrence of clades, determined through 1000 bootstrap replications of the data. Isolates from this study along with their corresponding accession numbers, are highlighted in red.
Figure 5.
The phylogenetic tree of B. burgdorferi s.l., based on 16s rRNA partial sequences obtained from Tibetan sheep and goats in this study, as well as sequences retrieved from the GenBank database, was constructed using the Maximum Likelihood method in MEGA X and Kimura 2-parameter model. The numbers assigned to the nodes indicate the percentage of occurrence of clades, determined through 1000 bootstrap replications of the data. Isolates from this study along with their corresponding accession numbers, are highlighted in red.
Figure 6.
The phylogenetic tree of Rickettsia spp., constructed using the Maximum Likelihood method in MEGA X and employing the Tamura 3-parameter model, includes numbers assigned to the nodes representing the percentage of occurrence of clades, determined through 1000 bootstrap replications of the data. Isolates from this study, along with their corresponding accession numbers, are highlighted in red.
Figure 6.
The phylogenetic tree of Rickettsia spp., constructed using the Maximum Likelihood method in MEGA X and employing the Tamura 3-parameter model, includes numbers assigned to the nodes representing the percentage of occurrence of clades, determined through 1000 bootstrap replications of the data. Isolates from this study, along with their corresponding accession numbers, are highlighted in red.
Figure 7.
The phylogenetic tree of C. burnetii constructed using the Maximum Likelihood method in MEGA X and employing the Kimura 2-parameter model, includes numbers assigned to the nodes representing the percentage of occurrence of clades, determined through 1000 bootstrap replications of the data. Isolates from this study, along with their corresponding accession numbers, are highlighted in red. .
Figure 7.
The phylogenetic tree of C. burnetii constructed using the Maximum Likelihood method in MEGA X and employing the Kimura 2-parameter model, includes numbers assigned to the nodes representing the percentage of occurrence of clades, determined through 1000 bootstrap replications of the data. Isolates from this study, along with their corresponding accession numbers, are highlighted in red. .
Table 1.
The list contains the sequences of the PCR primers used in tick-borne bacterial pathogens.
Table 1.
The list contains the sequences of the PCR primers used in tick-borne bacterial pathogens.
| Species |
Target gene |
Method |
Primer sequence |
Annealing temperature
(℃)
|
Amplicon size
(bp)
|
Reference |
| A. bovis |
16S rRNA |
PCR |
F |
TCCTGGCTCAGAACGAACGCTGGCGGC |
55 |
1433 |
[35] |
| R |
AGTCACTGACCCAACCTTAAATGGCTG |
| nPCR |
nF |
CTCGTAGCTTGCTATGAGAAC |
55 |
551 |
[36] |
| nR |
TCTCCCGGACTCCAGTCTG |
| A. capra |
gltA |
PCR |
F |
GCGATTTTAGAGTGYGGAGATTG |
55 |
1031 |
[9] |
| R |
TACAATACCGGAGTAAAAGTCAA |
| nPCR |
nF |
GGGTTCMTGTCYACTGCTGCGTG |
55 |
793 |
| nR |
TTGGATCGTARTTCTTGTAGACC |
| A. marginale |
msp4 |
PCR |
F |
CTGAAGGGGGAGTAATGGG |
60 |
344 |
[37] |
| R |
GGTAATAGCTGCCAGAGATTCC |
| A. ovis |
msp4 |
PCR |
F |
TGAAGGGAGCGGGGTCATGGG |
62 |
347 |
[37] |
| R |
GAGTAATTGCAGCCAGGCACTCT |
| A. phagocytophilum |
16S rRNA |
PCR |
F |
CACATGCAAGTCGAACGGATTATTC |
55 |
932 |
[38] |
| R |
TTCCGTTAAGAAGGATCTAATCTCC |
| nPCR |
nF |
AACGGATTATTCTTTATAGCTTGCT |
55 |
546/565 |
| nR |
GGCAGTATTAAAAGCAGCTCCAGG |
|
B. burgdorferi s.l. |
23S rRNA |
PCR |
F |
GCGAACGGGTGAGTAACG |
50 |
1360 |
[39] |
| R |
CCTCCCTTACGGGTTAGAA |
| 16S rRNA |
PCR |
F |
GAGGCGAAGGCGAACTTCTG |
60.2 |
622 |
[40] |
| R |
CTAGCGATTCCAACTTCATGAAG |
| C. burnetii |
htpB |
PCR |
F |
GCGGGTGATGGTACCACAACA |
57 |
501 |
[41] |
| R |
GGCAATCACCAATAAGGGCCG |
| nPCR |
nF |
TTGCTGGAATGAACCCCA |
52 |
325 |
| nR |
TCAAGCTCCGCACTCATG |
|
Rickettsia spp. |
ompB |
PCR |
F |
AAACAATAATCAAGGTACTGT |
55 |
212/209 |
[42] |
| R |
TACTTCCGGTTACAGCAAAGT |
| ompA |
nPCR |
nF |
GCTTTATTCACCACCTCAAC |
811 |
[43] |
| nR |
TR(g/a)ATCACCACCGTAAGTAAAT |
| gltA |
nPCR |
nF |
GCAAGTATCGGTGAGGATGTAAT |
401 |
[44] |
| nR |
GCTTCCTTAAAATTCAATAAATCAGGAT |
Table 2.
Accession numbers assigned to the isolates of ticks in Qinghai plateau of QTPA.
Table 2.
Accession numbers assigned to the isolates of ticks in Qinghai plateau of QTPA.
| Tick species |
Target gene |
GenBank accession number |
Length (bp) |
Identity (%) |
Accession number (host, country) |
| D. nuttalli |
coxI |
OQ816756 |
849 |
97.76% |
OM368307 cattle China |
| H. qinghaiensis |
coxI |
OQ816757 |
849 |
97.76% |
NC_062067 yak China |
| D. nuttalli |
coxI |
OQ816758 |
849 |
100% |
MK028675 sheep China |
| H. qinghaiensis |
coxI |
OQ816759 |
849 |
99.18% |
NC_062067 yak China |
| D. nuttalli |
coxI |
OQ816760 |
849 |
100% |
MK028675 sheep China |
| D. nuttalli |
coxI |
OQ816761 |
849 |
100% |
MK028675 sheep China |
| D. nuttalli |
coxI |
OQ816762 |
849 |
100% |
MK028675 sheep China |
| D. nuttalli |
coxI |
OQ816763 |
849 |
100% |
MK028675 sheep China |
| D. nuttalli |
coxI |
OQ816764 |
849 |
99.88% |
MK028675 sheep China |
| D. nuttalli |
coxI |
OQ816765 |
849 |
100% |
MK028675 sheep China |
| D. nuttalli |
coxI |
OQ816766 |
849 |
100% |
MK028675 sheep China |
| D. nuttalli |
coxI |
OQ816767 |
849 |
100% |
MK028675 sheep China |
| D. nuttalli |
coxI |
OQ816768 |
849 |
100% |
MK028675 sheep China |
| D. nuttalli |
coxI |
OQ816769 |
849 |
100% |
MK028675 sheep China |
| D. nuttalli |
coxI |
OQ816770 |
849 |
100% |
MK028675 sheep China |
| D. nuttalli |
coxI |
OQ816771 |
849 |
99.88% |
MK028675 sheep China |
| D. nuttalli |
coxI |
OQ816772 |
849 |
100% |
MK028675 sheep China |
| H. qinghaiensis |
coxI |
OQ816773 |
849 |
98.12% |
OK094412 goat China |
| D. nuttalli |
coxI |
OQ816774 |
849 |
98.59% |
OM368307 cattle China |
| H. qinghaiensis |
coxI |
OQ816775 |
849 |
98.23% |
OK094412 goat China |
| H. qinghaiensis |
coxI |
OQ816776 |
849 |
98.12% |
OK094412 goat China |
| H. qinghaiensis |
coxI |
OQ816777 |
849 |
98.82% |
OK094412 goat China |
| D. nuttalli |
coxI |
OQ816778 |
849 |
97.53% |
OM368307 cattle China |
Table 3.
The prevalence of tick-borne bacterial pathogens in livestock from Qinghai plateau.
Table 3.
The prevalence of tick-borne bacterial pathogens in livestock from Qinghai plateau.
| TBPs |
Livestocks |
Sheep |
goat |
Cattle |
Yak |
Camel |
Donkey |
Horse |
Tick |
p-value |
| n |
52 |
67 |
49 |
43 |
50 |
40 |
65 |
62 |
| A. bovis |
No. of positives (%) |
n. d.1 |
44 (66) |
1 (2) |
n. d. |
n. d. |
n. d. |
n. d. |
3 (5) |
0.2931 |
| A. capra |
No. of positives (%) |
8 (15) |
10 (15) |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
2 (3) |
0.2931 |
| A. marginale |
No. of positives (%) |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
- |
| A. ovis |
No. of positives (%) |
24 (46) |
1 (2) |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
0.3134 |
| A. phagocytophilum |
No. of positives (%) |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
- |
|
B. burgdorferi s.l. |
No. of positives (%) |
14 (27) |
13 (19) |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
0.3134 |
| C. burnetii |
No. of positives (%) |
2 (4) |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
1 (2) |
0.3134 |
|
Rickettsia spp. |
No. of positives (%) |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
2 (3) |
0.3326 |
Table 4.
Accession numbers for sequences of tick-borne bacterial pathogens deposited in GenBank.
Table 4.
Accession numbers for sequences of tick-borne bacterial pathogens deposited in GenBank.
| Obtained sequences |
The closest BLASTn match |
| Pathogen |
Animal |
Target gene |
GenBank accession number |
Length (bp) |
Identity (%) |
Pathogen isolate |
Accession number (host, country) |
| A. bovis |
cattle |
16s rRNA |
OQ826693 |
551 |
99.64% |
Uncultured Anaplasma sp. |
KM186933 dzeren China |
| |
goat |
16s rRNA |
OQ826694 |
551 |
99.82% |
Uncultured Anaplasma sp. |
KM186933 dzeren China |
| |
goat |
16s rRNA |
OQ826695 |
551 |
99.64% |
Uncultured Anaplasma sp. |
KM186933 dzeren China |
| |
goat |
16s rRNA |
OQ826696 |
548 |
100% |
A. bovis |
MN213735 giraffe Pakistan |
| |
goat |
16s rRNA |
OQ826697 |
551 |
99.82% |
Uncultured Anaplasma sp. |
MZ069112 yak China |
| |
goat |
16s rRNA |
OQ826698 |
551 |
99.82% |
Uncultured Anaplasma sp. |
KM186933 dzeren China |
| |
goat |
16s rRNA |
OQ826699 |
551 |
99.82% |
Uncultured Anaplasma sp. |
KM186933 dzeren China |
| |
goat |
16s rRNA |
OQ826700 |
551 |
100% |
A. bovis |
MN213735 giraffe Pakistan |
| |
goat |
16s rRNA |
OQ826701 |
551 |
100% |
A. bovis |
MN213735 giraffe Pakistan |
| |
goat |
16s rRNA |
OQ826702 |
551 |
99.82% |
Uncultured Anaplasma sp. |
MZ069112 yak China |
| |
tick |
16s rRNA |
OQ826703 |
551 |
100% |
Uncultured Anaplasma sp. |
MZ069112 yak China |
| |
tick |
16s rRNA |
OQ826704 |
551 |
99.27% |
Uncultured Anaplasma sp. |
KM186933 dzeren China |
| |
tick |
16s rRNA |
OQ826705 |
551 |
99.46% |
A. bovis |
MN213735 giraffe Pakistan |
| A. ovis |
goat |
msp4 |
OQ847093 |
347 |
100% |
A. ovis |
MN394791 Tibetan sheep China |
| |
Tibetan sheep |
msp4 |
OQ847094 |
347 |
99.71% |
A. ovis |
MN394791 Tibetan sheep China |
| A. capra |
tick |
gltA |
OQ847092 |
793 |
99.37% |
A. capra |
MZ130266 Tibetan sheep China |
| |
goat |
gltA |
OQ847085 |
793 |
98.63% |
A. capra |
MW930535 goat France |
| |
goat |
gltA |
OQ847086 |
793 |
98.49% |
A. capra |
MW930535 goat France |
| |
Tibetan sheep |
gltA |
OQ847088 |
793 |
99.75% |
A. capra |
MZ130266 Tibetan sheep China |
| |
Tibetan sheep |
gltA |
OQ847089 |
793 |
99.62% |
A. capra |
MZ130266 Tibetan sheep China |
| |
Tibetan sheep |
gltA |
OQ847090 |
793 |
99.62% |
A. capra |
MZ130264 Tibetan sheep China |
| |
Tibetan sheep |
gltA |
OQ847091 |
793 |
97.86% |
A. capra |
MZ130266 Tibetan sheep China |
|
Rickettsia spp. |
tick |
gltA |
OQ872771 |
400 |
100% |
R. raoultii |
MK304547 tick Russia |
| |
tick |
gltA |
OQ872772 |
400 |
100% |
R. raoultii |
MT178338 tick China |
|
Borrelia spp. |
goat |
16s rRNA |
OQ875206 |
623 |
99.52% |
Borrelia sp. |
KY284014 camel China |
| |
Tibetan sheep |
16s rRNA |
OQ875207 |
623 |
99.84% |
Borrelia sp. |
KY284014 camel China |
| C. burnetii |
tick |
htpB |
OQ872773 |
325 |
99.69% |
C. burnetii |
MK416231 tick Tunisia |
| |
Tibetan sheep |
htpB |
OQ872774 |
325 |
99.69% |
C. burnetii |
MK416231 tick Tunisia |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).