Submitted:
11 December 2023
Posted:
18 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
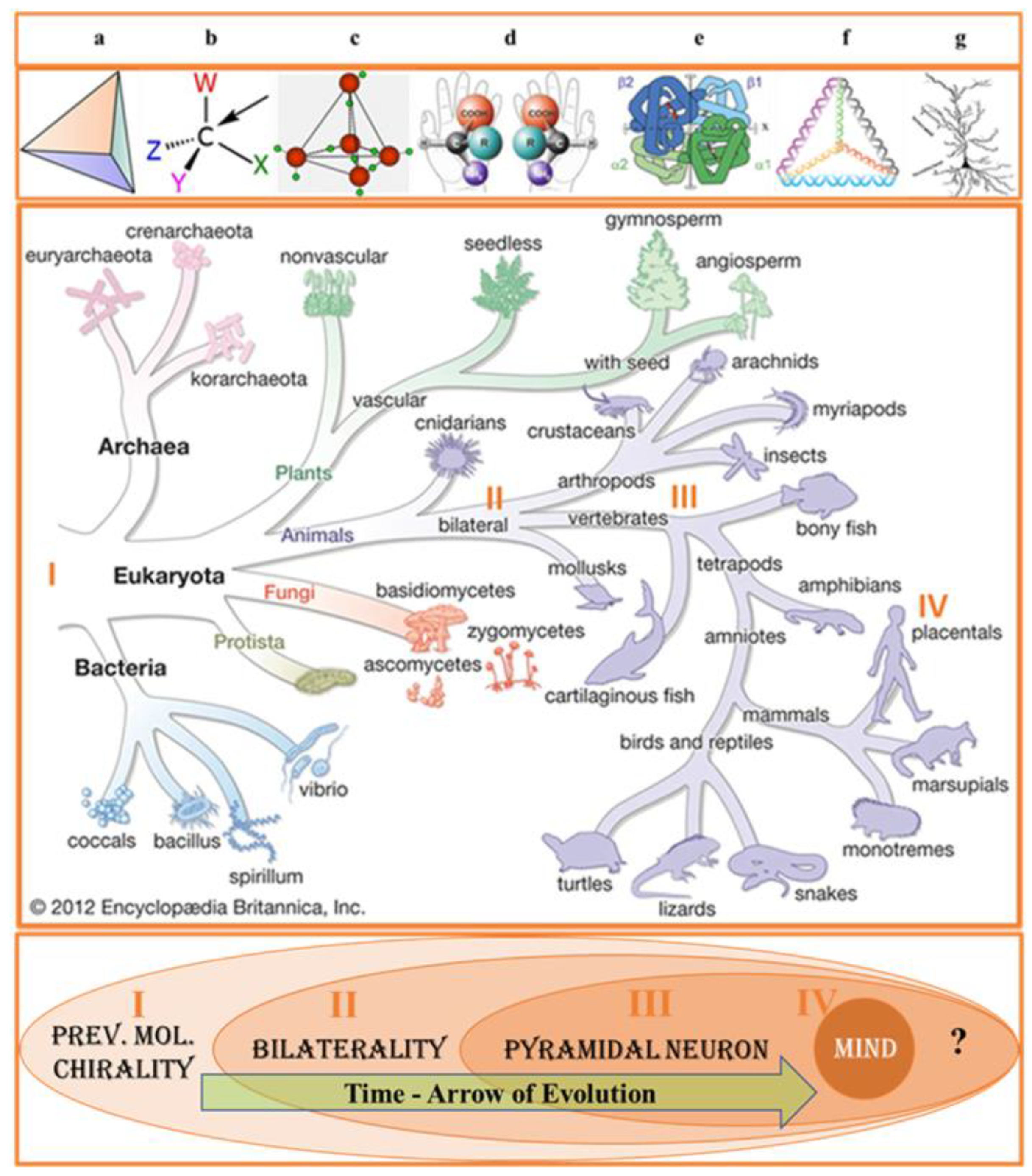
2. Hierarchical Chain of Chirality Transfer
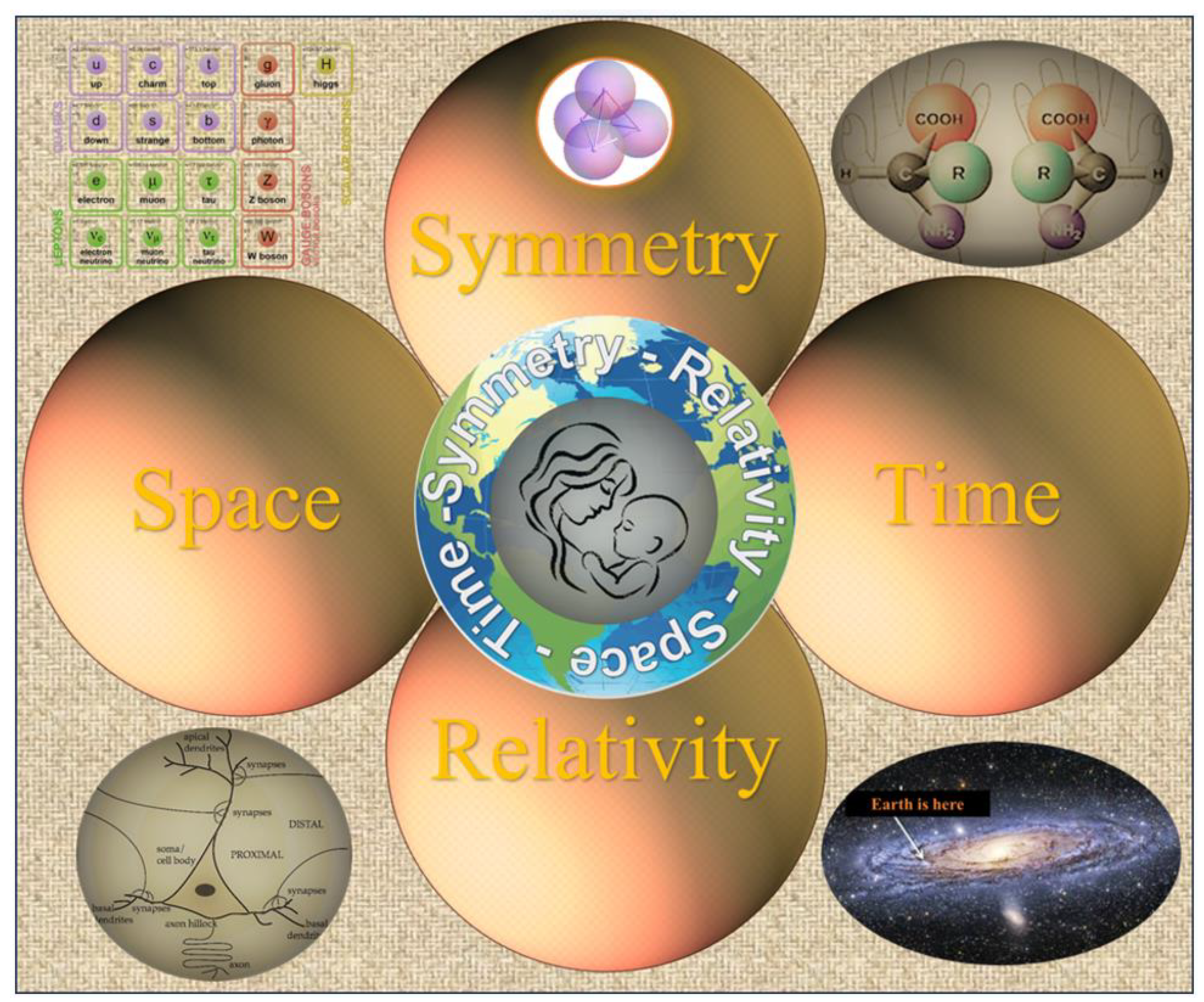
3. Space, Time, Symmetry, and Relativity
4. Biological Evolution
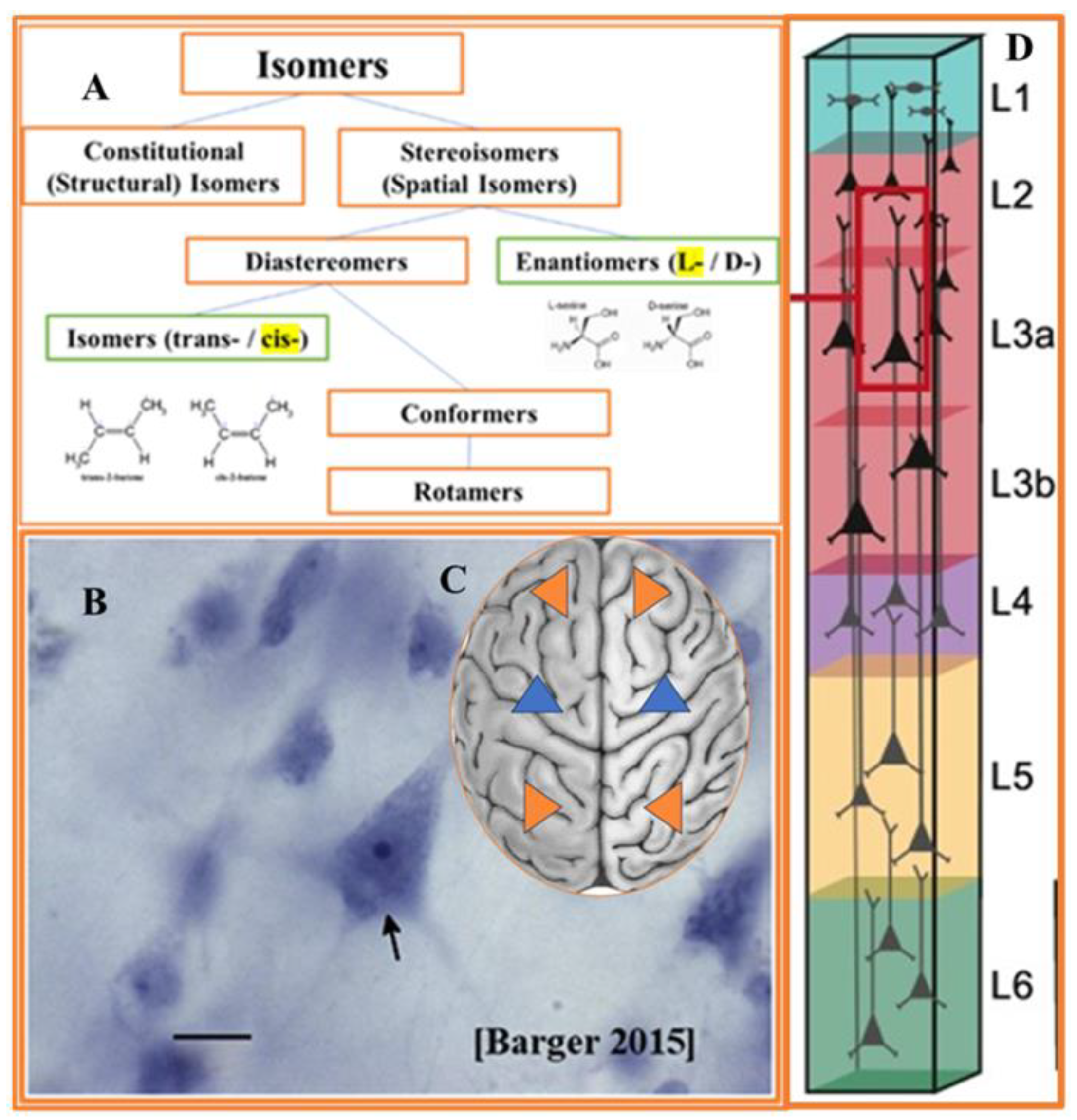
4.1. Molecular Chirality
4.2. Link of Physiological and Psychological Functions
4.3. Bilaterians: Symmetry-Function Interplay
4.4. Cell Chirality
4.4.1. Cell Evolution
4.4.2. Pyramidal Neurons
4.4.3. From Molecular to Cell Chirality
5. Self-Assembly of Biomolecules
Molecular-Cellular Co-Evolution
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Michael, G.; John, S. and Witten, Edward (2012). Superstring theory. Vol. 2: Loop amplitudes, anomalies and phenomenology. Cambridge University Press. 2019.
- Ma, Y.; Shi, L.; Yue, H. and Gao, G. Recognition at chiral interfaces: From molecules to cells. Colloids and Surfaces B: Biointerfaces. 2020, 195, 111268. [CrossRef]
- Li, R. and Bowerman, B. Symmetry Breaking in Biology Cold Spring Harb Perspect Biol. 2010, 2(3). a003475. [CrossRef]
- Reasoning for Symmetry in Biological Systems. SCE Scholary Community Encyclopedia, 2023. [CrossRef]
- Hollo, G. A new paradigm for animal symmetry. Interface Focus 2015, 5, 20150032. [CrossRef]
- Xin, Z.; Cai, Y.; Dang, L.T.’ Burke, H.M.S.; Revote, J.; Charitakis, N.; Bienroth, D.; Nim, H.T.; Li, Y-F. & Ramialison, M. Mona, GO: a novel gene ontology enrichment analysis visualisation system. BMC Bioinformatics 2022, 23(1), 69. [CrossRef]
- Dyakin V.V. Fundamental Cause of Bio-Chirality: Space-Time Symmetry—Concept Review. Symmetry 2023, 15(1), 79. [CrossRef]
- Palladino, P. Stereochemistry and the Nature of Life: Mechanist, Vitalist, and Evolutionary Perspectives. JSTOR, 1990, 81(1), 44-67, https://www.jstor.org/stable/234082.
- Kushchayev, S.V.; Moskalenko, V.F.; Wiener, P.C.; Tsymbaliuk, V.I.; Cherkasov, V.G.; Dzyavulska, I.V.; Kovalchuk, O.I.; Sonntag, V.K.H.; Spetzler, R.F. and Preul, M.C. The discovery of the pyramidal neurons: Vladimir Betz and a new era of neuroscience. Braim. A Journal of Neurology 2012, 135; 285–300. [CrossRef]
- Radler, M.R.; Liu, X.; Peng, M.; Doyle, B.; Toyo-Oka, K. and Spiliotis, E.T. Pyramidal neuron morphogenesis requires a septin network that stabilizes filopodia and suppresses lamellipodia during neurite initiation. Current Biology 2023, 33(3), 434-448.e8. [CrossRef]
- Sousa, A.M.M.; Meyer, K.A.; Santpere, G.; Gulden, F.O. and Sestan, N. Evolution of the Human Nervous System Function, Structure, and Development. Cell. 2017 Jul 13; 170(2): 226–247. [CrossRef]
- Dahanayake, J.N. and Mitchell-Koch, K.R. Entropy connects water structure and dynamics in protein hydration layer. Phys.Chem.Chem.Phys., 2018, 20, 14765. [CrossRef]
- Marvan, T.; Polák, M.; Bachmann, T. and Phillips, W.A. Apical amplification—a cellular mechanism of conscious perception? Neuroscience of Consciousness, 2021, 2021(2), 2021. [CrossRef]
- Kumar, P.; Buldyrev, S.V. and H. Eugene, H. A tetrahedral entropy for water. PNAS. 2009, 106 (52) 22130-22134. [CrossRef]
- Wang, Y.; Ye, M.; Kuang, X.; Li, Y. and Hu, S. A simplified morphological classification scheme for pyramidal cells in six layers of primary somatosensory cortex of juvenile rats. IBRO Rep. 2018, 74–90. [CrossRef]
- Luine, V. and Frankfurt, M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience. 2013, 239, 34-45. [CrossRef]
- Mason, S.F. The development of concepts of chiral discrimination. Chirality 1989, 1(3), 183-191. [CrossRef]
- Bastings, J.J.A.J.; van Eijk, H.M.; Damink. S.W.O and Sander S. Rensen, S.S. D-amino Acids in Health and Disease: A Focus on Cancer. Nutrients 2019, 11(9), 2205. [CrossRef]
- Kondepudi, D. Chiral Asymmetry in Nature, Ch. 1 in Chiral Analysis (Second Edition). Advances in Spectroscopy, Chromatography and Emerging Methods 2018, pg. 3-28. [CrossRef]
- Cristadoro, G., Degli Esposti, M. & Altmann, E.G. The common origin of symmetry and structure in genetic sequences. Sci Reports 2018, 8, 15817. [CrossRef]
- Book by Ibanez, L.E and Uranga, A.M. String theory and particle physics: An introduction to string phenomenology. 688 pages. Cambridge University Press 2012.
- Inaki, M.; Liu, J. and Matsuno, K. Cell chirality: its origin and roles in left–right asymmetric development. Philos Trans R Soc Lond B Biol Sci. 2016, 371(1710). [CrossRef]
- Lin, YM. Creating chirality. Nat Chem Biol. 2008, 4, 330. [CrossRef]
- Takaoka, K.; Yamamoto, M.; and Hamada, H. Origin of body axes in the mouse embryo. Current Opinion in Genetics & Development 2007, 17(4}44-350. [CrossRef]
- Dyakin, V.V.; Lucas, J.; Dyakina-Fagnano, N.V.; Posner, E.P.; Vadasz, C. The Chain of Chirality Transfer as Determinant of Brain Functional Laterality. Breaking the Chirality Silence: Search for New Generation of Biomarkers; Relevance to Neurodegenerative Diseases, Cognitive Psychology, and Nutrition Science. Neurology and Neuroscience Research. 2017, 1(1),2. [CrossRef]
- Duan, P.; Cao, H.; Zhang, L. and Liu, M. Gelation induced supramolecular chirality: chirality transfer, amplification and application. Soft Matter. 2014, 10, 5428. [CrossRef]
- Vallortigara G. The evolutionary psychology of left and right: costs and benefits of lateralization Dev Psychobiol. 2006 Sep;48(6):418-27. [CrossRef]
- Francks, C. Exploring human brain lateralization with molecular genetics and genomics Annals of The New York Academy of Sciences Issue: The Year in Cognitive Neuroscience. 2015, 1359, 1-13. [CrossRef]
- Stacho, M. and Manahan-Vaughan, D. Mechanistic flexibility of the retrosplenial cortex enables its contribution to spatial cognition. Trends in Neuroscience. 2022, 45(4), P284-296. [CrossRef]
- Lee, T-W; Dolan, R.J.; and Critchley, H.D. Controlling Emotional Expression: Behavioral and Neural Correlates of Nonimitative Emotional Responses. Cerebral Cortex, 2008, 18(1), 104–113. [CrossRef]
- Yuan, J.; Lu, X.; Zhang, S.; Zheng, F.; Deng, Q.; Han, L. and Lu, Q. Molecular Chirality and Morphological Structural Chirality of Exogenous Chirality-Induced Liquid Crystalline Block Copolymers. Macromolecules 2022, 55, 5, 1566–1575. [CrossRef]
- Book by C.G. Jung. Psychological Types (The Collected Works of C. G. Jung, Vol. 6) (Bollingen Series XX). (Part of the Jung’s Collected Works (#6) Series and Dzieła (#2) Series). Publisher: Princeton University Press. 1976. 1977.
- Assagioli, A. Dynamic Psychology and Psychosynthesis. Publisher: Psychosynthesis Research Foundation, inc 1958. Roberto Assagioli.
- Myers, S. The five functions of psychological type. Analytical Psychology 2016, 61(2), 183-202. [CrossRef]
- Pillai, A.S. and Jirsa, V.K. Perspective. Symmetry Breaking in Space-Time Hierarchies Shapes Brain Dynamics and Behavior. Neuron 2017, 94(5), 1010-1026. [CrossRef]
- Jirsa, V. and Sheheitli, H. Entropy, free energy, symmetry and dynamics in the brain. Journal of Physics: Complexity 2022 3(1), 015007. [CrossRef]
- Dyakin, V.V. and Uversky, V.N. Arrow of Time, Entropy, and Protein Folding: Holistic View on Biochirality. Int J Mol Sci. 2022, 23(7):3687. [CrossRef]
- Iohnston, I.G.; Dingle, K.; Greenbury, S.F.; Camargo, C.Q.; Doye, J.P.K.; Ahnert, S.E. and Louis, A.A. Symmetry and simplicity spontaneously emerge from the algorithmic nature of evolution. Proc Natl Acad Sci U S A. 2022, 119(11), e2113883119. [CrossRef]
- Jammer, M. Concepts of Space: The history of Theories of Space in Physics, 3rd ed.; Dover: New York, NY, USA, 1993.
- Capecchi, D. Development of the Concept of Space up to Newton Encyclopedia 2022, 2(3), 1528-1544. [CrossRef]
- Nederbr, H. Hierarchical Organization of Biological Systems and the Structure of Adaptation in Evolution and Tumorigenesis. Journal of Theoretical Biology 1997, 184(2), 149-156. [CrossRef]
- Eronen, M.I. Levels of Organization in Biology. Sranf. Encycl. of Philos. 2023. {https://plato.stanford.edu/entries/levels-org-biology/}.
- Blackmond, D.G. The Origin of Biological Homochirality Cold Spring Harb Perspect Biol. 2010, 2(5): a002147. [CrossRef]
- Ocklenburg & Mundorf. Ocklenburg S. and Mundorf, A. Symmetry and asymmetry in biological structures Proc. Natl. Acad. Sci. U.S.A. PNAS. 2022, 119 (28), e2204881119. [CrossRef]
- Tasson. J.D. What Do We Know About Lorentz Invariance? Rep. Prog. Phys. 2014, 77, 062901. [CrossRef]
- Comte, C. Symmetry, relativity and quantum mechanics. Nuov Cim B 1996, 111, 937–956. [CrossRef]
- Field, J.N. Space-Time Exchange Invariance: Special Relativity as a Symmetry Principle. American Journal of Physics 2001, 69, 569. [CrossRef]
- Ajaltouni Z.J. Symmetry and relativity: From classical mechanics to modern particle physics. Natural Science. 2014, 6(4), Article ID:43343,7 pages. [CrossRef]
- Auffray, C. and Nottale, L. Review. Scale relativity theory and integrative systems biology: 1: Founding principles and scale laws. Progress in Biophysics and Molecular Biology 2008, 97(1), 79-114. [CrossRef]
- Noble, R.; Tasaki, K.; Noble, P.J. and Noble, D. Biological Relativity Requires Circular Causality but Not Symmetry of Causation: So, Where, What and When Are the Boundaries? Front. Physiol. Sec. Integrative Physiology 2019, 10. [CrossRef]
- Devínsky, F. Chirality and the Origin of Life. Symmetry 2021, 13(12), 2277. [CrossRef]
- Piñeros, W.D. and Tlusty, T. Spontaneous chiral symmetry breaking in a random driven chemical system. Nat Commun 2022, 13, 2244. [CrossRef]
- Cornelissen, J.J.L.M.; Rowan, A.E.; Nolte, R.J.M. and Sommerdijk, N.A.J.M. Chiral Architectures from Macromolecular Building Blocks Chem. Rev. 2001, 101, 12, 4039–4070. [CrossRef]
- Todoroff, N.; Kunze, J.; Schreuder, H.; Hessler, G.; Baringhaus, K-H. and Schneider, G. (2014). Fractal Dimensions of Macromolecular Structures. Mol Inform. 2014, 33(9): 588–596. [CrossRef]
- Wang, J., Panagiotou, E. The protein folding rate and the geometry and topology of the native state. Sci Rep 12, 6384. [CrossRef]
- Pandey, S., Mandal, S., Danielsen, M.B. et al. Chirality transmission in macromolecular domains. Nat Common 2022, 13, 76. [CrossRef]
- Kim, N.H., Choi, H., Shahzad, Z.M. et al. Supramolecular assembly of protein building blocks: from folding to function. Nano Convergence 2022, 9(4). [CrossRef]
- MacKenzie, L.E. and Stachelek, P. The twists and turns of chiral chemistry. Nat. Chem. 2021, 13, 521–522. [CrossRef]
- Dhanavade, M.J. and Sonawane K.D. Amyloid beta peptide-degrading microbial enzymes and its implication in drug design. 3 Biotech. 2020, 10(6), 247. [CrossRef]
- Reetz, M.T. and Garcia-Borràs, M. The Unexplored Importance of Fleeting Chiral Intermediates in Enzyme-Catalyzed Reactions. J. Am. Chem. Soc. 2021, 143, 37, 14939–14950. [CrossRef]
- Dyakin, V.V.; Dyakina-Fagnano, N.V.; Mcintire, L.B. and Uversky, V.N. Fundamental Clock of Biological Aging: Convergence of Molecular, Neurodegenerative, Cognitive and Psychiatric Pathways: Non-Equilibrium Thermodynamics Meet Psychology. Int J Mol Sci. 2022, 23(1), 285. [CrossRef]
- Dyakin, V.V.; Wisniewski T.M. and Lajtha, A. Chiral Interface of Amyloid Beta (Aβ): Relevance to Protein Aging, Aggregation and Neurodegeneration. Symmetry 2020, 12(4), 585. [CrossRef]
- Stansley, B. L and Yamamoto, B.K. L-dopa-induced dopamine synthesis and oxidative stress in serotonergic cells. Neuropharmacology. 2013, 67:243-51. [CrossRef]
- van Hooff, J.J.E. Towards unraveling the origins of eukaryotic nuclear genome organization. Trends in Cell Biology 2023, 33(10), 820-823. [CrossRef]
- Caforio, A. and Driessen, A.J.M. Archaeal phospholipids: Structural properties and biosynthesis. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2017, 1862 (11), 1325–1339. [CrossRef]
- Fujii, N.; Takata, T.; Fujii,N.; Aki K. & Sakaue, H. D-Amino Acid Residues in Proteins Related to Aging and Age-Related Diseases and a New Analysis of the Isomers in Proteins Chapter in the book (pg 241-245) by Yoshimura, T., Nishikawa, T., Homma, H. (eds) D-Amino Acids Springer, Tokyo. [CrossRef]
- Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Kitagishi,Y. and Matsuda, S. Omar Cauli, Academic Editor and Soraya L. Valles, Academic Editor D-Amino Acids as a Biomarker in Schizophrenia. Diseases. 2022, 10(1), 9. [CrossRef]
- Jacco J.A.J. Bastings, Hans M. van Eijk, Steven W. Olde Damink,and Sander S. Rensen. D-amino Acids in Health and Disease: A Focus on Cancer. Nutrients. 2019, 11(9): 2205. [CrossRef]
- Murtas, G. and Pollegioni, L. D-Amino Acids and Cancer: Friends or Foes? Int. J. Mol. Sci. 2023, 24(4), 3274. [CrossRef]
- Wolosker, H., Balu, D.T. D-Serine as the gatekeeper of NMDA receptor activity: implications for the pharmacologic management of anxiety disorders. Transl Psychiatry 2020 10, 184. [CrossRef]
- Li, Y.; Han, H.; Yin, J.; Li, T.and Yina, Y. Role of D-aspartate on biosynthesis, racemization, and potential functions: A mini-review. Anim Nutr. 2018, 4(3): 311–315. [CrossRef]
- Saitoh, Y.; Katane, M.; Miyamoto, T.; Sekine, M.; Sakai-Kato, K. and Homma, H. D-Serine and D-Alanine Regulate Adaptive Foraging Behavior in Caenorhabditis elegans via the NMDA Recepto,r Journal of Neuroscience 2020, 40 (39) 7531-7544. [CrossRef]
- Kera, Y.; Aoyama, H.; Matsumura, H.; Hasegawa, Hisae Nagasaki, H. and Yamada, R. Presence of free D-glutamate and D-aspartate in rat tissues. Biochimica et Biophysica Acta (BBA) 1995, 1243(2), 282-286. [CrossRef]
- Mangas, A.; Coveñas,R.; Bodet, D.; Geffard, M.; Aguilar, L.A. and Yajeya, J. Immunocytochemical visualization of d-glutamate in the rat brain. Neuroscience 2007, 144(2), 654-664. [CrossRef]
- Lin CH, Yang HT, Lane HY (2019) d-glutamate, D-serine, and d-alanine differ in their roles in cognitive decline in patients with Alzheimer’s disease or mild cognitive impairment. Pharmacol Biochem Behav 2019,185: 172760. [CrossRef]
- Luo, Q.; Hou, C.; Bai, Y.; Wang, R. and Liu, J. Protein Assembly: Versatile Approaches to Construct Highly Ordered Nanostructures. Chem. Rev. (American Chemical Society) 2016, 116, 22, 13571–13632. [CrossRef]
- Riccio, A.; Vitagliano, L.; di Prisco, G.; Zagari, A. and Mazzarella, L. The crystal structure of a tetrameric hemoglobin in a partial hemichrome state. Proc Natl Acad Sci U S A. 2002, 99(15), 9801–9806. [CrossRef]
- Ha, C-E. and Bhagavan, N.V. Hemoglobin Chapter in Essentials of Medical Biochemistry. eBook ISBN: 9780124166974. Sec Ed. Elsevier 2015.
- Goldstein, E. B. Crosstalk between psychophysics and physiology in the study of perception. In E. B. Goldstein (Ed.). Blackwell handbook of perception (pp. 1–23). Blackwell Publishing. 2001.
- Xu, X.; Hanganu-Opatz,I.L. and Bieler, M. Cross-Talk of Low-Level Sensory and High-Level Cognitive Processing: Development, Mechanisms, and Relevance for Cross-Modal Abilities of the Brain. Front. Neurorobot., 2020, 14. [CrossRef]
- Logothetis, N.K. and Pauls, J. Psychophysical and Physiological Evidence for Viewer-centered Object Representations in the Primate. Cerebral Cortex 1995, 3, 270-288, 1047-3211/95/S4.OO. [CrossRef]
- Mascalzoni, E.; Osorio, D.; Regolinm L. and Giorgio Vallortigara, G. Symmetry perception by poultry chicks and its implications for three-dimensional object recognition. Proc Biol Sci. 2012. 7;279(1730), 841-6. [CrossRef]
- Pizlo, Z. and de Barros, J. A. The Concept of Symmetry and the Theory of Perception Front. Comput. Neurosci., 2021, 15. [CrossRef]
- Nakade, Y.; Iwata,Y.; Furuichi, K.; Mita, M.; Hamase, K.; Konno, R. et al. Gut microbiota-derived D-serine protects against acute kidney injury. The Journal of Clinical Investigation Insight 2018 18, 3(20), e97957. [CrossRef]
- Freud S. An outline of psychoanalysis. Std. Edn. Vol. 23. London: Vintage. 1940.
- Wada, K.; Yamamoto, M. and Nakashima, K. Psychological function in aging. Nihon Rinsho (Janan) 2013, 71(10).1713-9.
- Bottaccioli, A.G.; Bologna, M. and Bottaccioli, F. Psychic Life-Biological Molecule Bidirectional Relationship: Pathways, Mechanisms, and Consequences for Medical and Psychological Sciences—A Narrative Review. Int. J. Mol. Sci. 2022, 23, 3932. [CrossRef]
- Corballis, M. C., & Beale, I. L. (1970). Bilateral symmetry and behavior. Psychological Review, 77(5), 451–464. [CrossRef]
- Delvenne, J-F.; Castronovo, J.; Demeyere, N. and umphreys, G.W. Bilateral Field Advantage in Visual Enumeration PLOS 2011. [CrossRef]
- Tamaki Amano and Motomi Toichi. Zoi Kapoula, Editor. The Role of Alternating Bilateral Stimulation in Establishing Positive Cognition in EMDR. Therapy: A Multi-Channel Near-Infrared Spectroscopy Study. PLoS One. 2016; 11(10): e0162735. [CrossRef]
- Boukezzi, S.; Silva, C.; Nazarian, B.; Rousseau, P-F.; Guedj, E. and Valenzuela-Moguillansky, C. Bilateral Alternating Auditory Stimulations Facilitate Fear Extinction and Retrieval. Front. Psychol. Sec. Psychology for Clinical Settings 2017, 8. [CrossRef]
- Kasprian G, Langs G, Brugger PC, Bittner M, Weber M, Arantes M, et al. The prenatal origin of hemispheric asymmetry: an in-utero neuroimaging study. Cereb Cortex. 2011, 21(5), 1076–83. pmid:20851852. [CrossRef]
- Mitter, C.; Prayer, D.; Brugger, P.C.; Weber, M. and Kaspria, G. In Vivo Tractography of Fetal Association Fibers. PLOS. ONE 2015, 10(3), e011953. [CrossRef]
- Nakamura, M. and Hashimoto, T. Mechanistic Insights into Plant Chiral Growth. Symmetry 2020, 12(12), 2056. [CrossRef]
- Zagórska-Marek, B.; Sokołowska, K. and Turzańska, M. Chiral events in developing gametophores of Physcomitrella patens and other moss species are driven by an unknown, universal direction-sensing mechanism. Am. J. of Botany 2018. 105(12), 1986-1994. [CrossRef]
- Than, K. 2005. Symmetry in Nature: Fundamental Fact or Human Bias. Live Science. 2005.{https://www.livescience.com/4002-symmetry-nature-fundamental-fact-human-bias.html}.
- Gunturkun, O and Ocklenburg, S. Ontogenesis of Lateralization. Neuron, 2017, 94(2), 249–263. [CrossRef]
- Mehler, M.F. Epigenetic Principles and Mechanisms Underlying Nervous System Functions in Health and Disease. Prog Neurobiol. 2008, 86(4): 305–341. [CrossRef]
- Zion, E. and Chen, X. Breaking Symmetry: The Asymmetries in Epigenetic Inheritance. Biochem (Lond). 2021. 43(1): 14–19. [CrossRef]
- Wan, L.Q.; Chin, A.S.; Worley, K.E. and Ray, P. Cell chirality: emergence of asymmetry from cell culture. Philos Trans R Soc Lond B Biol Sci. 2016, 371(1710), 20150413. [CrossRef]
- Sun. Na.; Dou, X.; Tang, Z.; Zhang, D.; Ni, N.; Wang, J.; Gao, H.; Ju, Y.; Dai, X.; Zhao, C.; Gu, P.; Ji, J. and Feng, C. Bio-inspired chiral self-assemblies promoted neuronal differentiation of retinal progenitor cells through activation of metabolic pathway. Bioactive Materials 2021, 6(4), 990-997. [CrossRef]
- Dong, C., Madar, A.D. & Sheffield, M.E.J. Distinct place cell dynamics in CA1 and CA3 encode experience in new environments. Nat Commun 2021, 12, 2977. [CrossRef]
- Graham, H.K. and Duan, X. Molecular mechanisms regulating synaptic specificity and retinal circuit formation. Wiley Interdiscip Rev Dev Biol. 2021, 10(1), e379. [CrossRef]
- Striedter, G.F. Review. Special Issue: Cortical Evolution. Evolution of the hippocampus in reptiles and birds. JCN (Res. in System Neuroscience) 2016, 524(3), 496-517. [CrossRef]
- Matho,K.S; Huilgol, D.; Galbavy, W.; He, M.; Kim, G.; Xu An, Xu.; Lu, J. et al. Genetic dissection of the glutamatergic neuron system in cerebral cortex. Nature. 2021, 598, 7879):182-187. [CrossRef]
- Zhang, H. and Wan, L.Q. Cell Chirality as A Novel Measure for Cytotoxicity 2022, 6(1): e2101088. [CrossRef]
- Bekkers. J.M. Pyramidal neurons. Current biology Curr Biol. 2011, 21(24), R975. [CrossRef]
- Banovac, I.; Sedmak, D.; Džaja, D.; Jalšovec, D.; Milošević, N.J.; Rašin, [M.R. and Petanjek, Z. Somato-dendritic morphology and axon origin site specify von Economo neurons as a subclass of modified pyramidal neurons in the human anterior cingulate cortex. Journal of Anatomy 2019, 235, 3, 651-666. [CrossRef]
- Johns, P. Neurons and glial cells. Chapter 5 in Clinical Neuroscience, 2014, 61-69. [CrossRef]
- Barger, N.; Sheley, MF. and Schumann, C.M. Stereological study of pyramidal neurons in the human superior temporal gyrus from childhood to adulthood. J Comp Neurol. 2015, 523(7), 054–1072. [CrossRef]
- Sha, Z.; Schijven, D.; Carrion-Castillo, A.; Joliot, M.; Mazoyer, B.; Fisher, S.E.; Crivello, F. and Francks, C. The genetic architecture of structural left-right asymmetry of the human brain. Nature Human Behaviour 2021, 5, 1226–1239. [CrossRef]
- Ottersen, O.P.; Zhang, N. and Walberg, F. Metabolic compartmentation of glutamate and glutamine: morphological evidence obtained by quantitative immunocytochemistry in rat cerebellum. Neuroscience. 1992, 46(3),519-34. [CrossRef]
- Sanghai, N. and Tranmer, G.K. Biochemical and Molecular Pathways in Neurodegenerative Diseases: An Integrated View. Cell 2023, 12(18), 2318. [CrossRef]
- Rabinowitz, J.D. and Enerbäck, S. Lactate: the ugly duckling of energy metabolism Nat Metab. 2020, 2(7): 566–571. [CrossRef]
- [Sultan et al. 2015] Sultan, S.; Li, L.; Moss, J.; Petrelli, F.; Cassé, F.; Gebara, E.; Lopatar, J.; Pfrieger, F.W.; Bezzi, P.; Bischofberger, J.; et al. Synaptic Integration of Adult-Born Hippocampal Neurons Is Locally Controlled by Astrocytes. Neuron 2015, 88, 957–972. [CrossRef]
- Krupic, J.; Bauza, M.; Burton, S.; Barry,C. and O’Keefe, J. Grid cell symmetry is shaped by environmental geometry. Nature. 2015, 518(7538): 232–235. [CrossRef]
- Oh, M.M.; Simkin, D. and Disterhoft, J.F. Intrinsic Hippocampal Excitability Changes of Opposite Signs and Different Origins in CA1 and CA3 Pyramidal Neurons Underlie Aging-Related Cognitive Deficits. Front. Syst. Neurosci. 2016, 10. [CrossRef]
- Constant M. and Mellet, E. The Impact of Handedness, Sex, and Cognitive Abilities on Left–Right Discrimination: A Behavioral Study. Front. Psychol. 2018, 9, 405. [CrossRef]
- Merrick, C.M.; Dixon, T.C.; Breska, A.; Lin, J.; Chang, E.F.; King-Stephens, D.; Laxer, K.D.; Weber, P.B.; Carmena, J.; Knight, R.T. and Ivry, R.B. Left hemisphere dominance for bilateral kinematic encoding in the human brain. eLife 2022, 11, e69977. [CrossRef]
- Jiang, S., Guan, Y., Chen, S. et al. Anatomically revealed morphological patterns of pyramidal neurons in layer 5 of the motor cortex. Sci Rep 2020, 10, 7916. [CrossRef]
- Kawakami, R.; Shinohara, Y.; Kato, Y.; Sugiyama, H.; Shigemoto, R, and Itoauthors, I. Asymmetrical allocation of NMDA receptor ε2 subunits in hippocampal sircuitry. Science 2003, 300(5621), 990-994. [CrossRef]
- Galakhova, A.A; Hunt, A.S.; Wilbers, R.; de Kock, C.P.J.; Mansvelder, H.D.; Goriounova, N.A. et al. Evolution of cortical neurons supporting human cognition. Trands in Cognit. Science 2022. [CrossRef]
- Shinohara, Y.; Hirase, H.; Watanabe, M, Makoto Itakura, M.; Mmi Takahashi, M.and Shigemoto, R. Edited by Huganir, R.L. Left-right asymmetry of the hippocampal synapses with differential subunit allocation of glutamate receptors. PNAS. 2008,105 (49) 19498-19503. [CrossRef]
- Muller, J.F.; Mascagni, F. and McDonald. A.J. Pyramidal Cells of the Rat Basolateral Amygdala. Synaptology and Innervation by Parvalbumin-immunoreactive Interneurons. J Comp Neurol. 2006, 494(4): 635–650. [CrossRef]
- Lorente de Nó R, L. Cerebral cortex: architecture, intracortical connections and motor projections. In: Physiology of the nervous system, 3rd edn (Fulton JF, ed.), pp. 288–330. Oxford: Oxford University Press. 1949.
- Chan, C.H.; Godinho, L.N.; Thomaidou, D.; Tan, S.S.; Gulisano, M and Parnavelas J.G. Emx1 is a Marker for Pyramidal Neurons of the Cerebral Cortex. Cerebral Cortex 2001, 11(12), 1191–1198. [CrossRef]
- Hawkins, J. and Ahmad, S. Hypothesis and theory article. Why Neurons Have Thousands of Synapses, a Theory of Sequence Memory in Neocortex. Front. Neural Circuits, 2016, 10. [CrossRef]
- Jia, W.; Hu, C.; Wang, Y.; Liu, Y.; Wang, L.; Zhang, S.; Zhu, Q.; Gu, Y.; Zhang, P.; Ma, J.; Chen, et al. Identification of Single-Molecule Catecholamine Enantiomers Using a Programmable Nanopore. ACS Nano 2022, 16, 4, 6615–6624. [CrossRef]
- Parnavelas JG.; Dinopoulos A. and Davies SW. The central visual pathways. In: Handbook of chemical neuroanatomy, vol. 7. Integrated systems of the CNS, Part II (Björklund A, Hökfelt T, Swanson LW, eds), pp. 1–164. Amsterdam: Elsevier. 1989.
- Dallérac, G.; Li, X.; Lecouflet, P.; Morisot, N.; Sacchi, S.; Asselot, R.; Thu Ha Pham, T.H. et al. Dopaminergic neuromodulation of prefrontal cortex activity requires the NMDA receptor coagonist D-serine. Proc Natl Acad Sci U S A. 2021, 118(23): e2023750118. [CrossRef]
- O’Keefe J. and Krupic, J. Do hippocampal pyramidal cells respond to nonspatial stimuli? Physiological review 2021, 101(3), 1427-145. [CrossRef]
- Purpura, D.P. and Suzuki, K. Distortion of neuronal geometry and formation of aberrant synapses in neuronal storage disease. Brain Research 1976, 116(1), 1-2. [CrossRef]
- Adámek, P.; Langová, V. & Horáček, J. Early-stage visual perception impairment in schizophrenia, bottom-up and back again. Schizophrenia 2022 8, 27. [CrossRef]
- Granato, A. and De Giorgio, A. Alterations of neocortical pyramidal neurons: turning points in the genesis of mental retardation. Front. Pediatr. 2014. [CrossRef]
- Rennó-Costa, C. and Adriano Tort, A.B.L. Place and Grid Cells in a Loop: Implications for Memory Function and Spatial Coding. J Neurosci. 2017, 37(34), 8062-8076. [CrossRef]
- Kitanishi, T., Ito, H.T., Hayashi, Y. et al. Network mechanisms of hippocampal laterality, place coding, and goal-directed navigation. J Physiol Sci 2017, 67, 247–258. [CrossRef]
- Zhong, S.; He, Y.; Shu, H. and Gaolang Gong, G. Developmental Changes in Topological Asymmetry Between Hemispheric Brain White Matter Networks from Adolescence to Young Adulthood. Cerebral Cortex, 2017, 7(4), 2560–2570. [CrossRef]
- Goriounova, N.A.; Heyer, D.B.; Wilbers, R.; Verhoog, M.B.; Giugliano, M.; Christophe Verbist, C. et al. Large and fast human pyramidal neurons associated with intelligence. Elife. 2018, 18(7), e41714. [CrossRef]
- Shipton, O. A., El-Gaby, M., Apergis-Schoute, J., Deisseroth, K., Bannerman, D. M., Paulsen, O., & Kohl, M. M. Left–right dissociation of hippocampal memory processes in mice. PNAS 2014, 111(42), 15238–15243. [CrossRef]
- Sá, M.J.; Ruela, C. and Madeira, M.D. Dendritic right/left asymmetries in the neurons of the human hippocampal formation: a quantitative Golgi study. Arq Neuropsiquiatr 2007, 65(4B), 1105-13. [CrossRef]
- Shinohara, Y.; Hirase, H.; Watanabe, M.; +2, and Ryuichi Shigemoto, R. Left-right asymmetry of the hippocampal synapses with differential subunit allocation of glutamate receptors. PNAS 2008, 105 (49) 19498-19503. [CrossRef]
- Goto, K.; Kurashima R.; Gokan, H.; Inoue, N.; Ito, I. and Watanabe, S. Left−Right Asymmetry Defect in the Hippocampal Circuitry Impairs Spatial Learning and Working Memory in iv Mice. PLOS ONE 2010, 5(11), e15468. [CrossRef]
- Ukai, H.; Kawahara, A.; Hirayama, K.; Show all 17 Ito Isao, I. ItPirB regulates asymmetries in hippocampal circuitry. PLOS ONE 2017, 12(6). [CrossRef]
- Luengo-Sanchez, S.; Bielza, C.; Benavides-Piccione, R.; Fernaud-Espinosa, I.;DeFelipe, J. and Larrañaga, P. A univocal definition of the neuronal soma morphology using Gaussian mixture models. Front. Neuroanat., 2015, 9. [CrossRef]
- Rockland, K.S. Pyramidal Neurons: Looking for the origins of axons. Evolutionary Biology. Neuroscience 2022. [CrossRef]
- Abrous, D.N., Koehl, M. & Lemoine, M. A. Baldwin interpretation of adult hippocampal neurogenesis: from functional relevance to physiopathology. Mol Psychiatry 2022. 27, 383–402. [CrossRef]
- Leguey, I.; Benavides-Piccione, R.; Rojo, C.; Larrañaga, P.; Bielza, C. and DeFelipe, J. Patterns of Dendritic Basal Field Orientation of Pyramidal Neurons in the Rat Somatosensory Cortex. eNeuro 2018, 5 (6). [CrossRef]
- Weiler, S.; Nilo, D.G.; Bonhoeffer, T.; Hübener, M.; Rose, T. and Scheuss, V. Orientation and direction tuning align with dendritic morphology and spatial connectivity in mouse visual cortex. Current Biology 2020, 32(8), 1743-1753.e7. [CrossRef]
- Wahle, P.; Sobierajski, E.; Gasterstädt, I.; Lehmann,N.; Weber, S.; Lübke, H.R.; Engelhardt, M.; Distler, C. and Meyer, G. Neocortical pyramidal neurons with axons emerging from dendrites are frequent in non-primates, but rare in monkey and human. eLife. 2022, 11, e76101. [CrossRef]
- Musall, S., Sun, X.R., Mohan, H. et al. Pyramidal cell types drive functionally distinct cortical activity patterns during decision-making. Nat Neurosci 2023. [CrossRef]
- Park, J.; Papoutsi, A.; Ash, R.T. et al. Contribution of apical and basal dendrites to orientation encoding in mouse V1 L2/3 pyramidal neurons. Nat Commun 10, 5372 (2019). [CrossRef]
- Elaine M. Pinheiro, E.M.; Xie, Z.; Norovich, A.L.; Vidaki, M.; Tsai, L-H. and Gertler, F.B. Lpd depletion reveals that SRF specifies radial versus tangential migration of pyramidal neurons. Nat Cell Biol. 2011, 13(8), 989–995. [CrossRef]
- Hobert, O. Development of left/right asymmetry in the Caenorhabditis elegans nervous system: From zygote to postmitotic neuron. Genesis 2014, 52(6), 528-43. [CrossRef]
- Tee, Y., Shemesh, T., Thiagarajan, V. et al. Cellular chirality arising from the self-organization of the actin cytoskeleton. Nature Cell Biolohy 2015, 17, 445 – 457. [CrossRef]
- Falk,J.; Boubakar, L. and Valérie Castellani, V. Septin functions during neuro-development, a yeast perspective Current Opinion in Neurobiology 2019. 57, 102-109. [CrossRef]
- Inaki, M.; Sasamura. T. and Kenji Matsuno, K. Review. Cell Chirality Drives Left-Right Asymmetric Morphogenesis. Front. Cell Dev. Biol. 2018, 6. [CrossRef]
- Konietzny,A.; Bär, J. and Mikhaylova, M. Review. Dendritic Actin Cytoskeleton: Structure, Functions, and Regulations. Front. Cell. Neurosci. Sec. Cellular Neurophysiology 2017, 11. [CrossRef]
- Satir, P. Chirality of the cytoskeleton in the origins of cellular asymmetry Philos Trans R Soc Lond B Biol Sci. 2016, 371(1710), 20150408. [CrossRef]
- Parato, J. and Bartolini, F.The microtubule cytoskeleton at the synapse Neurosci Lett. 2021, 14;753,135850. [CrossRef]
- Lamprecht, R. The actin cytoskeleton in memory formation. Progress in Neurobiology 2014, 117, 1-19. [CrossRef]
- Sánchez-Ponce, D.; Blázquez-Llorca, L.; DeFelipe, J.; Garrido, J.J. and Muñoz, A. Colocalization of α-actinin and Synaptopodin in the Pyramidal Cell Axon Initial Segment. Cerebral Cortex 2012, 22(7), 1648–1661. [CrossRef]
- Radler, M.R.; Liu, X.; Peng, M.; Doyle, B. and Toyo-Oka, K. Pyramidal neuron morphogenesis requires a septin network that stabilizes filopodia and suppresses lamellipodia during neurite initiation. Curr Biol. 2023 Feb 6;33(3):434-448.e8. [CrossRef]
- Bocquet, A.; Berges, R.; Frank, R.; Robert, P.; Peterson, A.C. and Eyer, J. Neurofilaments Bind Tubulin and Modulate Its Polymerization. J Neurosci. 2009, 29(35), 11043–11054. [CrossRef]
- Uylings, H.B.; Jacobsen, A.M.; Zilles, K. and Amunts, K. Left-right asymmetry in volume and number of neurons in adult Broca’s area. Cortex. 2006, 42(4), 652-8. [CrossRef]
- El-Gaby, M.; Reeve, H.M.; Lopes-dos-Santos,V.; Campo-Urriza, N.; Perestenko, P.V. Morley, A.; Strickland, L.A.M.; Lukács, I.P.; Paulsen, O. and Dupret, D. An Emergent Neural Coactivity Code for Dynamic Memory. Nat Neurosci. 2021, 24(5): 694–704. [CrossRef]
- Cullen, T.J.; Walker, M.A.; Eastwood, S.L.; Esiri, M.M.; Harrison, P.J. and Crow, T.J. Anomalies of asymmetry of pyramidal cell density and structure in dorsolateral prefrontal cortex in schizophrenia Br J Psychiatry 2006, 188:26-31. [CrossRef]
- Souta, R. An Introductory Perspective on the Emerging Application of qEEG. In the book Introduction to Quantitative EEG and Neurofeedback. Advsanced Theory and Applications. By Budzynski, T.H.; Washington, P.; Budzynski, H.K.; Washington, P.; Evans, J.R. and Abarbanel, A. Sec Ed. Acad. Press. 2009.
- Beniaguev, D.; Segev, I. and London, M. Single cortical neurons as deep artificial neural networks. Neuron 2021. 109(17), 2727-2739.e3. [CrossRef]
- Toxvaerd, S. Scilit. The emergence of the bilateral symmetry in animals: A review and a new hypothesis. Symmetry, 2021, 13(2), 261. [CrossRef]
- Matsuo, K.; Tamura, R.; Hotta, K.; Okada,M.; Takeuchi, A.; Yanlin Wu, Y. et al. Bilaterally Asymmetric Helical Myofibrils in Ascidian Tadpole Larvae. Front. Cell Dev. Biol. Sec. Morphogenesis and Patterning 2021, 9 – 2021. [CrossRef]
- Hoffmann, L.A, and Giomi, L. Theory of cellular homochirality and trait evolution in flocking systems. 2923, arXiv:2307.14360 [q-bio.CB]. [CrossRef]
- Zhu, DY., Cao, TT., Fan, HW. et al. The increased in vivo firing of pyramidal cells but not interneurons in the anterior cingulate cortex after neuropathic pain. Mol Brain. 2022, 15, 12. [CrossRef]
- Koga, K. Early evolution of membrane lipids: how did the lipid divide occur? Journal of Molecular Evolution, 2011, 72(3), 274-82. [CrossRef]
- Qi, H-X.; Jain, N.; Preuss, T.M. and Kaas, J.K. Inverted pyramidal neurons in chimpanzee sensorimotor cortex are revealed by immunostaining with monoclonal antibody SMI-32. Somatosensory & Motor Research 1999; 16(1), 49-56. [CrossRef]
- Steger, R.; Ramos, R.L.; Cao, R.; Yang, Q,; Chen, Ch-Ch.; Dominici, J. and Brumberg, J.C. Physiology and morphology of inverted pyramidal neurons in the rodent neocortex. Neuroscience. 2013, 17; 0: 165–179. [CrossRef]
- Martin, H.S.; Podolsky, K.A. and Devaraj, N.K. Probing the role of chirality in phospholipid membranes. ChemBioChem 2021, 22(22). 3148-315. [CrossRef]
- Kong, X-Z.; Postema, M.C.; Guadalupe, T.; de Kovel, C.; Boedhoe, P.C.W.; Hoogman, M.; Mathias, S.R.; van Rooij, D.; Schijven, D.; Glahn, D.C.; Medland, S.E. et al., Mapping brain asymmety in health and disease through the ENIGMA consortium. Hum Brain Mapp. 2022, 43(1), 167–181. [CrossRef]
- Kay, J.W. and Phillips, W.A. Contextual Modulation in Mammalian Neocortex is Asymmetric. Symmetry 2020, 12(5), 815. [CrossRef]
- Tripathi, S.; Dey,A.; Shanmugam, M.; Narayanan, R.S.and Chandrsekhar, V. Cobalt (II) Complexes as Single-Ion Magnets. Topics in Organometallic Chem 2019, 64. [CrossRef]
- Kagamiyama, H. and Hayashi, H. Crystallographic Structures. Branched-Chain Amino Acids, Part B. In the book Methods in Enzymoligy. Elsevier 2000.
- Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. ISBN 978-0-13-039913-7.
- Gradišar, H.; Božič, S.; Doles, T.; Vengust, D.; Hafner-Bratkovič, I.; Mertelj, A.; Webb, B.; Šali, A.; Klavžar, S. and Roman Jerala, R. Design of a single-chain polypeptide tetrahedron assembled from coiled-coil segments. Nat Chem Biol. 2013, 9(6), 362–366. [CrossRef]
- Le Bel, J.A. Bull. Soc. Chim. Fr., 1874, 22, 337.
- Mun, J.; Kim, M.; Yang, Y.; Badloe, T.; Ni, J.; Chen, Y.; Qiu C., and Rho, J, Electromagnetic chirality: From fundamentals to nontraditional chiroptical phenomena. Light. Sci. Appl. 2020, 9,139. [CrossRef]
- Wheeler RJ. Use of chiral cell shape to ensure highly directional swimming in trypanosomes. PLoS Comput Biol 2017, 13(1), e1005353. [CrossRef]
- Jacobus Henricus Van‘t Hoff, J.H. Arch. Neérl. Sci. Exactes. Nat., 1874, 9(1), 445-454, https://www.chemteam.info/Chem-History/Van’t-Hoff-1874.html.
- Xie, N.; Liu, S.; Yang, X.; Xiaoxiao He.; Huang, J. and Kemin Wang, K. DNA tetrahedron nanostructures for biological applications: biosensors and drug delivery. Analyst 2017, 18(142), 3322-3332. [CrossRef]
- Mathur, D.; Rogers, K.E.; Díaz, S.A.; Muroski, M.E.; Klein, W.P.; Nag, O.K.; Lee, K.; Field, L.D.; Delehanty, J.B. and Medin, I.L. Determining the Cytosolic Stability of Small DNA Nanostructures in Cellula. Nano Lett. 2022 22(12): 5037–5045. [CrossRef]
- Aumiller, Jr., W.A.; Masaki Uchida, and Trevor DouglasProtein cage assembly across multiple length scales. Chem Soc Rev. 2018 May 21; 47(10): 3433–3469. [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E. and Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 11, 16982–17015. [CrossRef]
- Hong, S.; Jiang, W.; Ding, Q.; Lin, K.; Zhao, C. and Wang, X. The Current Progress of Tetrahedral DNA Nanostructure for Antibacterial Application and Bone Tissue Regeneration. Int J Nanomedicine. 2023; 18: 3761–3780. [CrossRef]
- Zheng, Y.; Mao, K.; Chen,S. and Zhu, H. Chirality Effects in Peptide Assembly Structures. Front in Bioengineering and Biothechnology. 2021, 9, 703004. [CrossRef]
- Hassabis, D.; Kumaran, D.; Summerfield, C. and Botvinick, M. Neuroscience-Inspired Artificial Intelligence. Neuron 2017, 95(2), P245-258. [CrossRef]
- Zhao, J.; Wu, M.; Zhou, L.; Wang, X. and Jia, J. REVIEW. The Application of Artificial Intelligence in Brain-Computer Interface and Neural System Rehabilitation, Front. Neurosci., Sec. Neuroprosthetics 2022, 16. Research-Topic Cognitive psychology-based artificial intelligence review. [CrossRef]
- Surianarayanan, C.; Lawrence, J.J.; Chelliah, P.R.; Prakash, E. and Hewage, C. Gwanggil Jeon, Academic Editor. Convergence of Artificial Intelligence and Neuroscience towards the Diagnosis of Neurological Disorders—A Scoping Review. Sensors (Basel). 2023, 23(6): 3062. [CrossRef]
- Strychalski, W. 3D Computational Modeling of Bleb Initiation Dynamics. Front. Phys., Sec. Biophysics 2021, 9. [CrossRef]
- Johnson, E.; Cascio, D.; Sawaya, M.R.; Gingery, M. and Schröder, I. Crystal Structures of a Tetrahedral Open Pore Ferritin from the Hyperthermophilic Archaeon Archaeoglobus fulgidus. Structure. 2005 IF = 13(4), 637-648. [CrossRef]
- Zhang, K., Ezemaduka, A., Wang, Z. et al. A Novel Mechanism for Small Heat Shock Proteins to Function as Molecular Chaperones. Sci Rep 2015, 5, 8811 (2015). [CrossRef]
- Yoo, S.H. and Lee, H-S. Foldectures: 3D Molecular Architectures from Self-Assembly of Peptide Foldamers. Acc. Chem. Res. 2017, 50, 4, 832–841. [CrossRef]
- Sasaki, E., Böhringer, D., van de Waterbeemd, M. et al. Structure and assembly of scalable porous protein cages. Nat Commun 2017, 8, 14663. [CrossRef]
- Quaye, I.K. Extracellular hemoglobin: the case of a friend turned foe. Front Physiol. 2015; 6, 96. [CrossRef]
- Eaton, A.W. Retrospective on statistical mechanical models for hemoglobin allostery editors-pick The Journal of Chemical Physics 2022, 157, 184104. [CrossRef]
- Richter, F.; Meurers, B.H.; Zhu, C.; Medvedeva, V.P. and Chesselet, M-F. Neurons Express Hemoglobin α- and β-Chains in Rat and Human Brains. J Comp Neurol. 2009, 15(5): 538–547. [CrossRef]
- Zhang, D.; Ronson, T.K.; Mosquera, J.; Martinez, A.; Guy, L. and Nitschke, J.R. Anion Binding in Water Drives Structural Adaptation in an Azaphosphatrane-Functionalized FeII4L4 Tetrahedron. J. Am. Chem. Soc. 2017, 139, 19, 6574–6577. [CrossRef]
- 205. Shao X, Li Q, Mogilner A, Bershadsky AD, and Shivashankar GV. Mechanical stimulation induces formin-dependent assembly of a perinuclear actin rim. Proc. Natl. Acad. Sci. U.S.A. 2015; 112(20):E2595-601. [CrossRef]
- Kruppa, A.J.; Kishi-Itakura, C.; Masters, T.A.; Rorbach, J.E.; Grice, G.L.; Kendrick-Jones, J.; James A. Nathan, J.A. et al. Myosin VI-Dependent Actin Cages Encapsulate Parkin-Positive Damaged Mitochondria. Dev Cell. 2018. 26; 44(4): 484–499.e6. [CrossRef]
- Heid H, Rickelt S, Zimbelmann R, Winter S, Schumacher H, Dörflinger Y, Kuhn C, and Franke WW. On the formation of lipid droplets in human adipocytes: the organization of the perilipin-vimentin cortex. PLoS ONE 2014; 9(2), e90386. [PMID: 24587346]. [CrossRef]
- Tessarz, P., Schwarz, M., Mogk, A. & Bukau, B. The yeast AAA+ chaperone Hsp104 is part of a network that links the actin cytoskeleton with the inheritance of damaged proteins. Mol. Cell Biol. 2009, 29, 3738–3745. [CrossRef]
- Franke WW, Hergt M, and Grund C. Rearrangement of the vimentin cytoskeleton during adipose conversion: formation of an intermediate filament cage around lipid globules. Cell 1987; 49(1):131-41. [PMID: 3548999]. [CrossRef]
- Mostowy S, Bonazzi M, Hamon MA, Tham TN, Mallet A, Lelek M, Gouin E, Demangel C, Brosch R, Zimmer C, Sartori A, Kinoshita M, Lecuit M, and Cossart P. Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microbe 2010; 8(5):433-44. [CrossRef]
- Biliński, S.M. and Jaglarz, M.K. Organization and possible functions of microtubule cytoskeleton in hymenopteran nurse cells. Cell Motil Cytoskeleton. 1999, 43(3):213-20. [CrossRef]
- Xia, Z.; Wang, P.; Liu, X.; Liu, T.; Yan, Y.; Juan Yan, J. et al. Tumor-Penetrating Peptide-Modified DNA Tetrahedron for Targeting Drug Delivery. Biochemistry 2016, 55, 9, 1326–1331. [CrossRef]
- Chen, Gf., Xu, Th., Yan, Y. et al. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin 2017, 38, 1205–1235. [CrossRef]
- Bao, Y.; Wu, S.; Chu, L.T.; Kwong, H.K.; Hartanto, H.; Huang, Y. et al. Early Committed Clockwise Cell Chirality Upregulates Adipogenic Differentiation of Mesenchymal Stem Cells. Adv Biosyst. 2020, 10, e2000161. [CrossRef]
- Yao, X.; Hu, Y.; Cao, B.; Peng, R. and Ding, J. Effects of surface molecular chirality on adhesion and differentiation of stem cells. Biomaterials 2013, 34 9001-9009. [CrossRef]
- Zheng, H.; Yoshitomi, T. and Yoshimoto, K. Analysis of Chirality Effects on Stem Cell Fate Using Three-dimensional Fibrous Peptide Hydrogels ACS Appl. Bio Mater. 2018, 1, 3, 538–543. [CrossRef]
- Walser, M.; Svensson, J.; Karlsson, Motalleb, R.; Åberg, M.; Kuhn, H.G.; Isgaard, J. and Åberg, N.D. Growth Hormone and Neuronal Hemoglobin in the Brain—Roles in Neuroprotection and Neurodegenerative Diseases. Front. Endocrinol. Sec. Neuroendocrine Science 2021, 11. [CrossRef]
- Zheng, R.; Yan, Y.; Pu, J. and Zhang, B. Physiological and Pathological Functions of Neuronal Hemoglobin: A Key Underappreciated Protein in Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23(16), 9088. [CrossRef]
- Flynn, K.S. The cytoskeleton and neurite initiation. Bioarchitecture. 2013, 3(4): 86–109. [CrossRef]
- Giuliana Indelicato, Newton Wahome, Philippe Ringler. Shirley A. Müller, Mu-Ping Nieh, Peter Burkhard, Reidun Twarock. Principles Governing the Self-Assembly of Coiled-Coil Protein Nanoparticles. Biophysical Journal 2016, 110(3), P646-660. [CrossRef]
- Heberle, F.A. and Feigenson, G.W. Phase Separation in Lipid Membranes. Cold Spring Harb Perspect Biol. 2011, 3(4), a004630. [CrossRef]
- Wu, X.; Cai, Q.;Feng, Z. and Mingjie Zhang, M. Review. Liquid-Liquid Phase Separation in Neuronal Development and Synaptic Signaling. Developmental Cell 2020, 55(1), 18-29. [CrossRef]
- Wonka, P. and Gervautz. M. Ray Tracing of Nonlinear Fractals. Journal of WSCG. 1998, 6(1-3), 424-431, http://wscg.zcu.cz/wscg1998/wscg98.htm.
- Taylor, L.LK; Riddell, I.A. and Smulders, M.M.J. Review. Self-Assembly of Functional Discrete Three-Dimensional Architectures in Water. Angewanter Chemie (Int Edition) 2019, 58(5), 1280-1307. [CrossRef]
- Chirikjian, G.S. Group theory and biomolecular conformation: I. Mathematical and computational models. J Phys Condens Matter. 2010, 22(32): 323103. [CrossRef]
- Dyakin V.V. and Posner, E.V. Molecular Chirality as an Integral Biomarker of Lactation, Nutrition and Cognitive Development Express-Review. Biomedical Science & Research. 2020, 8(5). [CrossRef]
- Liu, Y.; Wu, Z.; Armstrong, D.W.; Wolosker, H. and Zheng, Y. Detection and analysis of chiral molecules as disease biomarkers. Nat Rev Chem. 2023, 7(5): 355–373. [CrossRef]
- Ariga, K.; Richards, G.J.; Ishihara, S.; Izawa, H. and Jonathan P. Hill, J.P. Intelligent Chiral Sensing Based on Supramolecular and Interfacial Concepts Sensors (Basel). 2010; 10(7): 6796–6820. [CrossRef]
- Li, J., Li, J., Liu, R. et al. Autonomous discovery of optically active chiral inorganic perovskite nanocrystals through an intelligent cloud lab. Nat Commun 2020. 11, 2046. [CrossRef]
- Rainey, P.B. Major evolutionary transitions in individuality between humans and AI. Phil. Trans.of Royal Soc. B. 2023, 378 (1872). [CrossRef]
- Shinohara, Y.; Hirase, H.; Watanabe, M.; Itakura, M.; Takahashi, M. and Shigemoto, R. Left-right asymmetry of the hippocampal synapses with differential subunit allocation of glutamate receptors. Proc Natl Acad Sci U S A. 2008. 105(49):19498-503. [CrossRef]
- Goto K, Kurashima R, Gokan H, Inoue N, Ito I, Watanabe S. Left−Right Asymmetry Defect in the Hippocampal Circuitry Impairs Spatial Learning and Working Memory in iv Mice. PLoS ONE 2010, 5(11): e15468. [CrossRef]
- El-Gaby, M.; Shipton, O.A. Paulsen, O. Synaptic Plasticity and Memory: New Insights from Hippocampal Left–Right Asymmetries, The Sage Journal. The Neuroscientist 2014, 21(5).
- Shimbo, A.; Kosaki, Y.; Ito, I. and Watanabe, S. Mice lacking hippocampal left-right asymmetry show non-spatial learning deficits. Behavioural Brain Research 2018, 336(15) 156-165. [CrossRef]
- Shipton, O. A., El-Gaby, M., Apergis-Schoute, J., Deisseroth, K., Bannerman, D. M., Paulsen, O., & Kohl, M. M. (2014). Left–right dissociation of hippocampal memory processes in mice. PNAS 2014, 111(42), 15238–15243. [CrossRef]
- Sha, Z., Schijven, D., Carrion-Castillo, A. et al. The genetic architecture of structural left–right asymmetry of the human brain. Nat Hum Behav 2021, 5, 1226–1239. [CrossRef]
- Jianguo Wang, Sidi Ma, Peijie Yu, Xionglei He Author NotesEvolution of Human Brain Left–Right Asymmetry: Old Genes with New Functions. Molecular Biology and Evolution, 2023, 40(9), msad181. [CrossRef]
- Cathryn A. Cutia, Leanna K. Leverton and Catherine A. Christian-HinmanSex and Estrous Cycle Stage Shape Left-Right Asymmetry in Chronic Hippocampal Seizures in Mice. eNeuro 29 May 2023, 10 (6). [CrossRef]
- Shiptona, O.A.; Tanga, C.S.; Paulsena, O. and Vargas-Caballero, M. Differential vulnerability of hippocampal CA3-CA1 synapses to Ab. Acta Neuropathologica Communications 2022, 10, 45. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



