Introduction
This European Association of Hospital Pharmacists (EAHP) guidance document for the handling of gene therapy medicinal products (GTMPs) outlines practical considerations for the hospital pharmacy handling of GTMPs. The term ‘handling’ encompasses receipt and storage, dispensing and reconstitution, transportation, administration, waste disposal, spills and accidental exposure. Practical experience from a committee of experts and published evidence were used in the development of these guidelines. All committee members provided feedback on these recommendations, which were then subsequently validated and approved by external experts.
These guidelines cover both European Commission (EC)-approved GTMPs and products under investigation in clinical trials. However, it is important to note that these guidelines are of a general nature and intended as a framework to aid the development of institutional standard operating procedures (SOPs), especially when policy-making committees may have difficulty accessing credible guidance on which to base their recommendations (1). As such, national regulations and the summary of product characteristics (SmPC)/relevant clinical trial documents should always be consulted for country- and product-specific requirements.
This document is an update on the EAHP guidance on the handling of gene medicines, published in 2007 (2) and takes into account the substantial advances in GTMP technology and marketing approval being granted for a number of GMTPs in Europe.
Overview of the guidance development process
This guidance document was developed by a Special Interest Group convened by the EAHP to update the EAHP’s Guidance on the Pharmacy Handling of Gene Medicines (2) and gain insight into the preparedness of hospital pharmacy departments for the delivery of in vivo GTMPs (3). The original guidance was developed by a steering committee of representatives from the EAHP in 2007, representing Austria, Czechia, Finland, Germany, The Netherlands, Spain, Sweden and the United Kingdom (UK) (2). The Special Interest Group for this update comprised a committee of representatives from the EAHP, representing Belgium, Denmark, France, Germany, Ireland, Italy, Portugal, Spain, Sweden, and the UK. All members of the Special Interest Group were recognised as experts in gene medicine pharmacy.
Areas for update were identified by a literature review of existing guidance and through the expert opinions of the Special Interest Group members. A series of draft documents were produced, with each incorporating comments and input from the members of the Special Interest Group. An initial kick-off meeting was held after development of the outline to discuss updates and additional content.
The Appraisal of Guidelines for Research (AGREE) II document was followed while preparing these guidelines (4). AGREE II consists of two global rating items and 23 further items within six domains (scope and purpose, stakeholder involvement, rigour of development, clarity of presentation, applicability and editorial independence), which allow the quality of a guidance document to be assessed (4).
Aims, scope and target of the guidance
This guidance document aims to provide practical recommendations for the handling of GTMPs in Europe, throughout the entire workflow of receiving a shipped product from the manufacturer, through to medicine reconstitution, transport, administration to the patient, advising patients/caregivers, waste disposal and procedures for accidental spills or exposures. However, we reiterate that this guidance should always be considered in the context of local procedures and regulations.
Definitions of key terms used throughout this guidance have been provided in Appendix 1, and a list of EC-approved GTMPs (as of January 2023) is provided in Appendix 2.
According to the European Medicines Agency (EMA), a GTMP must fulfil two conditions: 1) the product must be a biological medicinal product and contain recombinant nucleic acid(s) and 2) the recombinant nucleic acid(s) should be directly involved in the mechanism of action, and hence therapeutic action, of the product (5). In these guidelines, the term ‘GTMP’ is used in keeping with the EMA classification. GTMPs are a subset of advanced therapy medicinal products (ATMPs), which also include somatic cell therapy medicinal products and tissue engineered products (5).
This document provides guidance on the handling of in vivo GTMPs, wherein the genetic material is introduced (e.g., by injection or inhalation) directly into the body within a vector (viral or non-viral) or via physical delivery of naked DNA, to be taken up by individual cells (6, 7). At the time of writing, all EC-approved in vivo GTMPs were delivered via viral vectors (Appendix 2) and by injection. The handling of ex vivo GTMPs, wherein a GTMP is administered to a sample of the patient’s cells that has been removed from the body before being incubated, and the modified cells returned to the patient, is beyond the scope of this document. We refer the interested reader to the UK’s Specialist Pharmacy Service guidelines for handling of ex vivo GTMPs (adapting as required to comply with local regulations) (6).
These guidelines relate to the handling of EC-approved GTMPs and products under investigation in clinical trials. At the time of writing, all EC-approved GTMPs were required to be handled in accordance with biosafety level 1 or 2 procedures due to their status as genetically modified organisms (GMOs) (Appendix 2; please refer to local regulations for country-specific definitions of GMOs). According to the World Health Organization, organisms in risk group 1 (and requiring containment under biosafety level 1) pose “no or low individual and community risk” and are unlikely to cause human or animal disease, while risk group 2 (biosafety level 2) organisms pose “moderate individual risk, low community risk” and can cause human or animal disease, but are unlikely to be a serious hazard (8). Nebulised (inhaled) gene therapy poses additional safe handling considerations compared with injected gene therapy due to the increased risk of aerosol formation; in this case, guidance should be sought from the manufacturer. The EMA Summary of Product Characteristics or clinical trial documents for individual products should always be consulted for product-specific guidance, regardless of the mode of administration. In addition, local classifications, regulations, legislation, and biosafety requirements should always be consulted and adhered to when handling GTMPs. These may vary between jurisdictions. We recommend individuals familiarise themselves with their local regulations. This guidance is intended to be used as a framework for developing local policies, including how to respond to accidents, spills and accidental exposure.
This guidance document is relevant for any person handling a GTMP, although this will most often be a hospital pharmacist or pharmacy technician. Other healthcare professionals, such as physicians, theatre and ward nursing staff, biosafety/infection control officers, occupational health representatives, and cleaning and waste disposal staff may also consult this guidance, as is relevant to their role. General information for each step of handling a GTMP is presented in a series of charts and tables, with additional considerations and information provided in the text. As mentioned above, local legislation and regulations must always be followed, and these will always take priority over this guidance.
Need for updated guidance on the handling of GTMPs
The previous EAHP guidance on the hospital pharmacy handling of gene medicines was published in 2007 (2). At that time, a number of GTMPs were under investigation but none had been granted marketing approval for routine clinical use, with alipogene tiparvovec receiving the first approval in 2012 (9). Now, in 2023, a number of GTMPs have been approved for use in Europe (9), and additional novel GTMPs are expected to be granted marketing approval in Europe in the future.
As a result, the use of GTMPs in routine clinical practice, outside of clinical trials, is becoming more commonplace. Hospital pharmacists and other skilled personnel will be required to reconstitute and administer these agents. However, there is evidence to suggest that hospitals and healthcare professionals may not be adequately prepared to implement GTMPs (10). A recent survey by the Special Interest Group identified a wide range of experience across centres in Europe, with some centres having relatively extensive experience while other centres reported having very little experience (3).
Considering this lack of experience, many institutions are expected to need to procure additional equipment and/or develop new facilities, such as ultra-low temperature freezers and biosafety devices, to provide infrastructure for the safe handling of GTMPs (1). Different set-ups can be implemented depending on individual institution’s requirements and in accordance with local regulations. For example, in Denmark, an innovative approach using a mobile, single-use isolator was developed, allowing GTMPs to be reconstituted at the administration site by hospital pharmacists and in cooperation with the surgical or clinical team (11). The French Society of Oncological Pharmacy guidelines highlight the possibility of using a hybrid isolator, which has features that can be adjusted to the level of containment required (such as a removable glove panel) (12).
A number of resources providing guidance on the use of GTMPs in healthcare in the UK have been produced, including the Advanced Therapy Treatment Centres (ATCC) website (13), the GTMP Governance and Preparation Requirements published by the Specialist Pharmacy Service (SPS) (6), eLearning programs (14) and the Scientific Advisory Committee on Genetic Modification (SACGM) Compendium of Guidance (15-17). However, the degree of national-level guidance on the use of GTMPs in Europe varies between countries.
As such, there is a need for practical guidance to help hospital pharmacies create safe and effective workflows, and to support a degree of standardisation of procedures across Europe. Handling GTMPs may be perceived as novel and challenging, but many of the procedures will be familiar to healthcare professionals, often reflecting those already applied when handling hazardous drugs, such as cytotoxic agents. Appropriate training should help personnel feel more at ease with handling GTMPs.
Review of existing guidance
The SPS of the UK’s National Health Service (NHS) has produced specific guidelines for the handling of GTMPs, which were last updated in 2019 (6). This includes practical guidance across the entire handling procedure, as well as consideration of the facilities and personnel required for pharmacies to safely handle GTMPs (6). Guidance on differential handling of in vivo and ex vivo products is also included (6). Handling of in vivo versus ex vivo GTMPs is beyond the scope of this document as the processes are similar (although handling ex vivo GTMPs may require some additional training, particularly in aseptic technique, as well as specific facilities/equipment); we refer the interested reader to the above-mentioned guidelines (6). As mentioned in the previous section, several additional UK-specific resources are also available (13-17). Furthermore, other national guidelines exist, such as the French HCB Handbook for the Contained Use of GMOs (18). This handbook contains sections specific to the administration of GMOs for therapeutic purposes, as well as guidelines on the handling of GMO waste, viral vectors, and containment requirements for gene therapy (18).
Experts from the USA and Australia have also produced opinion and review articles providing guidance on the handling of GTMPs in a hospital pharmacy setting (1, 19-24). Common recommendations across documents include limiting access to GTMPs to trained personnel, using appropriate biosafety containment equipment and personal protective equipment (PPE), using appropriate containers for transport, developing institutional procedures and policies, and ensuring appropriate decontamination and waste management (19-24). Additional recommendations include adding GTMPs to the institutional hazardous drugs list, following contact precautions with patient material post-administration, instating a clinical biosafety committee, caregiver training, providing appropriate personnel training/education and storage of the GTMPs in a dedicated area (20-25).
Further resources can be found on The Gene Therapy Network website (26). The website includes practical advice, protocols and downloadable resources on the handling of GTMPs, from PPE, storage, transportation, decontamination and disposal to accidental exposure (26). The Gene Therapy Network is guided by a multidisciplinary steering committee comprised of internationally renowned experts, and aims to “build knowledge of the evolving gene therapy landscape among healthcare professionals by providing engaging, evidence-based educational content while fostering peer-to-peer interaction” (26).
In general, available guidelines and recommendations agree that GTMPs should only be handled by trained personnel in a manner appropriate for the biosafety level of the agent, following established procedures (6, 19-22, 26).
General operating procedures for handling GTMPs in hospital pharmacy
For ease of use, we have compiled suggested operating procedures into tables and charts. These tables and charts should be viewed only as a framework of guidelines to aid in the development of institutional SOPs. Thorough risk assessment should be conducted before the use of any new GTMP. The development of institutional SOPs should also consider local regulations, policies, and procedures – for example, in Denmark, each facility must go through a formal application process before using any GTMP, to ensure safety for personnel, patients and the environment. However, it has been noted that, as of 2022, few national and/or industry-wide guidelines relating to GTMPs had been developed because of the novelty of these therapies (1). All the information required for each step is included in the appropriate chart or table (or their accompanying footnotes). Additional considerations are highlighted in the text.
Handling of GTMPs and patient specimens
General requirements for the handling of GTMPs and associated patient specimens are outlined in
Table 1. Specific requirements may vary between countries, and as such adhering to local regulations should always be prioritised. The GMTP’s Summary of Product Characteristics or clinical trial documentation should also always be consulted.
A thorough risk assessment should always be performed for any new GTMP being introduced into a facility, preferably by a dedicated genetic modification safety committee, even if this is not required by national regulations (6, 19). Any risk assessment should consider risks to human health and the environment (including the risk group of the viral vector, replication competency, and the risk group of the transgene, including oncogenicity and genome integration ability) (6, 21). Additional considerations may include the ability to form toxins, cause allergic reactions, or produce biologically active substances, and antibiotic resistance (27). Recommendations regarding the risk assessment of virus-derived vectors used in gene therapy have been previously published (28).
Appropriate protective clothing should always be worn whenever a GTMP is handled; however, the quality of the protective clothing may differ depending on the activity. For example, receipt of the GTMP upon delivery and reconstitution of the GTMP are associated with differing exposure risks, and as such, will require different levels of protective clothing. For biosafety class 1 GTMPs, universal precautions should be used unless a higher level of risk has been determined following a risk assessment; for biosafety class 2 GTMPs protective clothing should always be worn. The quality and type of protective clothing should be appropriate for the grade of the working area and process being performed. During reconstitution of a GTMP, protective clothing must be worn in such a way as to protect the GTMP from microbial contamination, as well as protecting the person. For further detail, see Appendix 3.
We recommend that dispensing and reconstitution of GTMPs be performed in either a (minimum) class 2, type B biological safety cabinet or pharmaceutical grade isolator (compliant with European standard EN12469:2000) that removes air to the outside. A mobile or disposable isolator unit may be an alternative to simplify logistics or clean-up, particularly if local regulations only allow GTMP reconstitution in specific areas or if regulations discourage transport of the reconstituted GTMP. In addition, if only small numbers of patients are being treated, it may not be cost-effective to build a dedicated clean-room and in this situation a mobile unit may be preferred (11). Alternatively, a hybrid isolator may be used, which is a unidirectional flow isolator allowing modifications to be made to the isolator depending on the level of containment required (12).
Precautions for the decontamination of needles and sharps, as well as work surfaces, should be in place before the decision to initiate treatment with a GTMP is made.
Contact procedures with the patient following administration will be dependent on the biosafety level of the GTMP; for example, specimens from a patient treated with a biosafety level 1 agent often do not need to be biohazard labelled (subject to local regulations). However, patient laundry should be decontaminated/cleaned according to the procedures for blood- or body fluid-soiled laundry, unless evidence-based regulatory guidance indicates that the patient is unlikely to be shedding viral vectors and this procedure is not necessary. Viral vector shedding in stools may occur for some GTMPs, but there are no overarching recommendations regarding necessary precautions for patients eliminating stools and urine. The Summary of Product Characteristics and local regulations should be considered for each individual GMTP. Patients administered a GTMP may return home shortly after treatment, so hospital pharmacists should ensure that the risks associated with viral shedding, and their management, should be considered and discussed with the patient (and their carer[s]), as necessary.
Samples from patients who have received a GTMP should be handled in a similar manner as samples from patients with infectious diseases. Handling processes should be agreed upon following consultation with a multidisciplinary team and/or biosafety committee. Patient specimens should always be transported and stored in a double-enclosed, labelled, leak-proof container. Waste disposal should ensure decontamination of any remaining GTMP, by autoclaving, heat inactivation or incineration.
A spill kit should always be readily available whenever transporting, storing, reconstituting, dispensing, administering, or disposing of a GTMP or patient specimens.
We strongly recommend that institutional policies and SOPs be put in place for handling GTMPs, and all GTMPs be added to the hazardous drugs list, if applicable, to ensure adequate protection of personnel (22).
All parties involved in the handling of GTMPs must clearly understand their roles and responsibilities along the process. A suggested working model is provided in Appendix 4; this uses the RASCI (responsible, accountable, support, consulted, informed) model (29). Each institution will need to follow local regulations. Every individual involved in the process of handling a GTMP within an institution is ultimately accountable to that institution’s management team. We recommend forming a biosafety committee comprising at least a hospital pharmacist, physician, nurse, biosafety or environmental safety officer, occupational health officer, waste services personnel and laboratory scientist with relevant experience working with GTMPs (26). The advice of an independent biosafety committee may also be sought (26).
Responsibility for all aspects of GTMP handling that occur in a hospital pharmacy ultimately lies with the hospital pharmacy’s chief pharmacist but may be delegated to other members of the hospital pharmacy team, as appropriate (Appendix 4). The broader hospital pharmacy team may also support the chief pharmacist with GMTP handling, as required. The treating physician and nursing staff should usually be informed when a GTMP is received from the manufacturer.
When the decision to initiate treatment is made, the treating physician is responsible for ensuring that the required conditions for handling and administering GTMPs are established. Treatment must not begin until all necessary processes are in place. Consultation and collaboration between the treating physician, chief pharmacist, hospital pharmacy staff, nursing staff, biosafety/infection control officer, occupational health and waste disposal services is required to establish the local workflow in advance of treatment.
The chief pharmacist or delegated responsible pharmacist, treating physician and biosafety/infection control officer should conduct a detailed assessment of the training needs of the staff and the ability of the facility to handle GTMPs. Correct use of the equipment, safe handling of GTMPs and adherence to local requirements and regulations should be included as part of mandatory training modules for personnel who handle GMTPs to generate confidence in handling GTMPs. The chief pharmacist is responsible for all hospital pharmacy-related procedures, including ensuring that appropriate equipment is available, and that hospital pharmacy staff are appropriately trained for their roles. The chief pharmacist may delegate responsibility to one of their team of pharmacists. The treating physician is responsible for all clinic-related procedures, including ensuring that the required facilities are available and medical/surgical personnel are appropriately trained. The biosafety/infection control officer is responsible for all other areas of assessment.
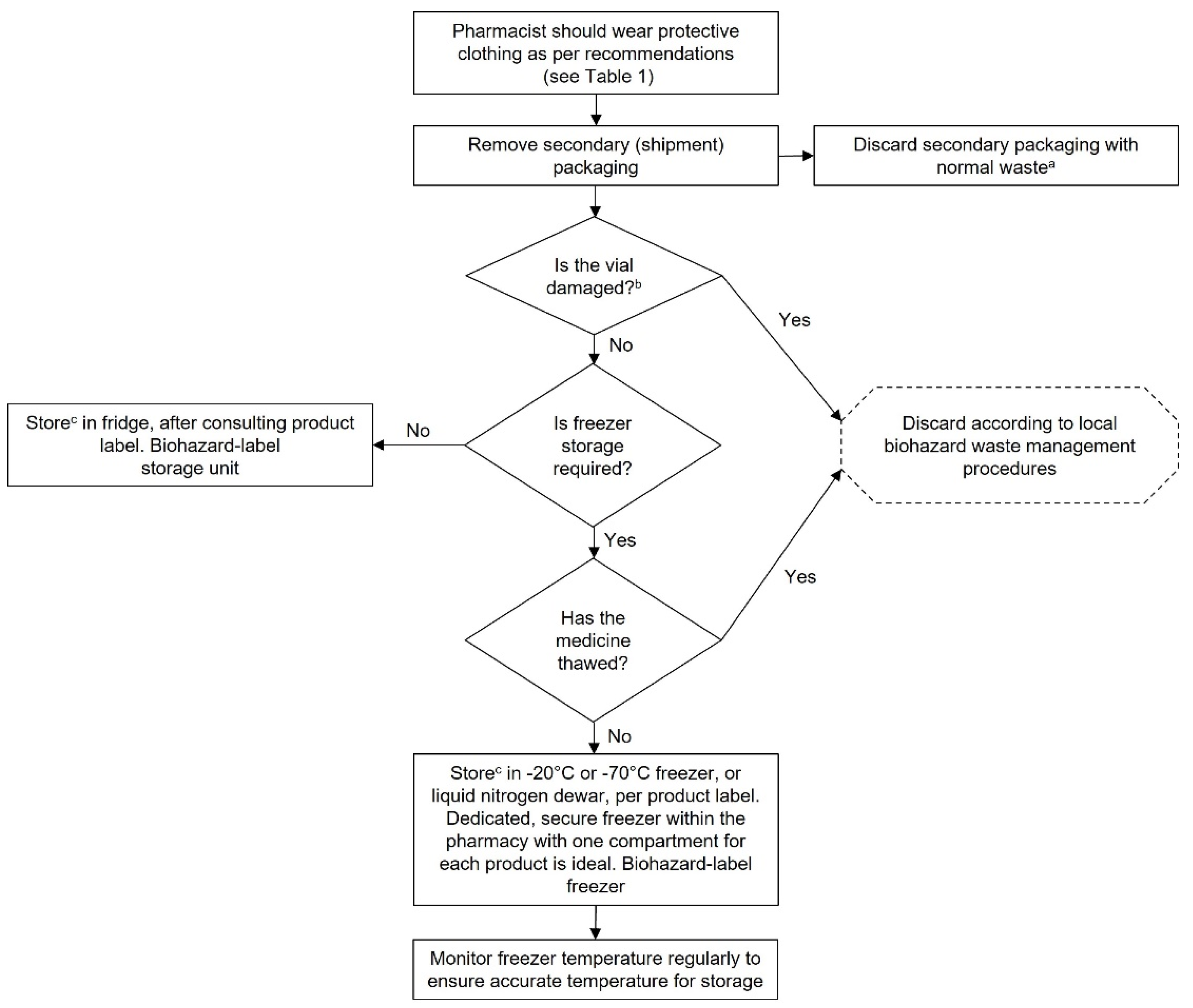
Receipt and Storage of GTMPs
Recommended procedures for the receipt and storage of GTMPs are outlined in
Chart 1. These procedures should be considered in the context of local procedures and regulations, which will always take priority. As such, all charts in this document should be viewed as a framework for the development of local SOPs, which may be edited as required to comply with local regulations.
Couriers must be licenced and use approved procedures and methods for transporting GTMPs. Given that GTMPs are both extremely costly and temperature-sensitive, cold chain transportation with temperature logging is required. If any temperature excursions occur during transport, the GTMP should be stored safely in temporary quarantine and the supplier contacted to determine the next steps.
The secondary packaging for GTMPs is guaranteed as being uncontaminated; however, PPE appropriate to the biosafety level of the GTMP should always be worn when unpacking the GTMP upon delivery in case it has been damaged during transit. If the vial has been damaged, it should be disposed of immediately in line with defined waste management procedures, in accordance with local procedures.
Storage temperature requirements will vary between GTMPs; consult the Summary of Product Characteristics for the storage temperature required each GTMP. Some GTMPs may require storage in liquid nitrogen.
GTMPs should be stored in separate fridges/freezers where possible, and labelled appropriately with a biohazard label, if required by local regulations. If it is not possible to use a dedicated fridge/freezer for GTMPs in isolation, a separate shelf within a fridge or freezer may be sufficient, but this depends on local regulations. Some countries in Europe require GTMPs to be stored separately. Furthermore, individual GTMPs should be stored in isolation from each other, for example, by using clearly labelled, separate shelves within a storage facility for each GTMP.
Freezers should be temperature-monitored with an alarm set to trigger if the temperature rises above a threshold. Care should be taken to limit the duration of freezer door opening when accessing GTMPs to prevent warming of the contents of the freezer. Once thawed, GTMPs should generally not be refrozen; see the Summary of Product Characteristics or clinical trial documents for storage requirements for thawed vials.
Institutions should prepare for instances of storage equipment dysfunction. For example, an alternate freezer for storing GTMPs could be identified for use in the event of a freezer failure (17). A responsible person should be assigned and notified in the event of a freezer failure and a preformulated plan for moving GTMPs to alternate storage facilities actioned.
Similarly, procedures for validation of storage facilities should be put in place because these facilities may be used infrequently. A negative pressure room may be required for storage; consult local regulations. If storage within the hospital pharmacy is not possible due to a lack of appropriate storage facilities, an outside location may be used if local regulations allow. This location should be known to other staff members such as the physician(s) and nurse(s). The alternative location should only be accessible by authorised personnel and should not carry a risk of undue exposure to a GTMP for people or the environment.
Access to GTMPs should be restricted to authorised personnel who have been trained in their handling.
Cost may also be a significant barrier to storage that should be considered when establishing facilities for the handling and storage of GTMPs. Here, a mobile isolator unit may be a more cost-effective option when establishing procedures for GTMP handling.
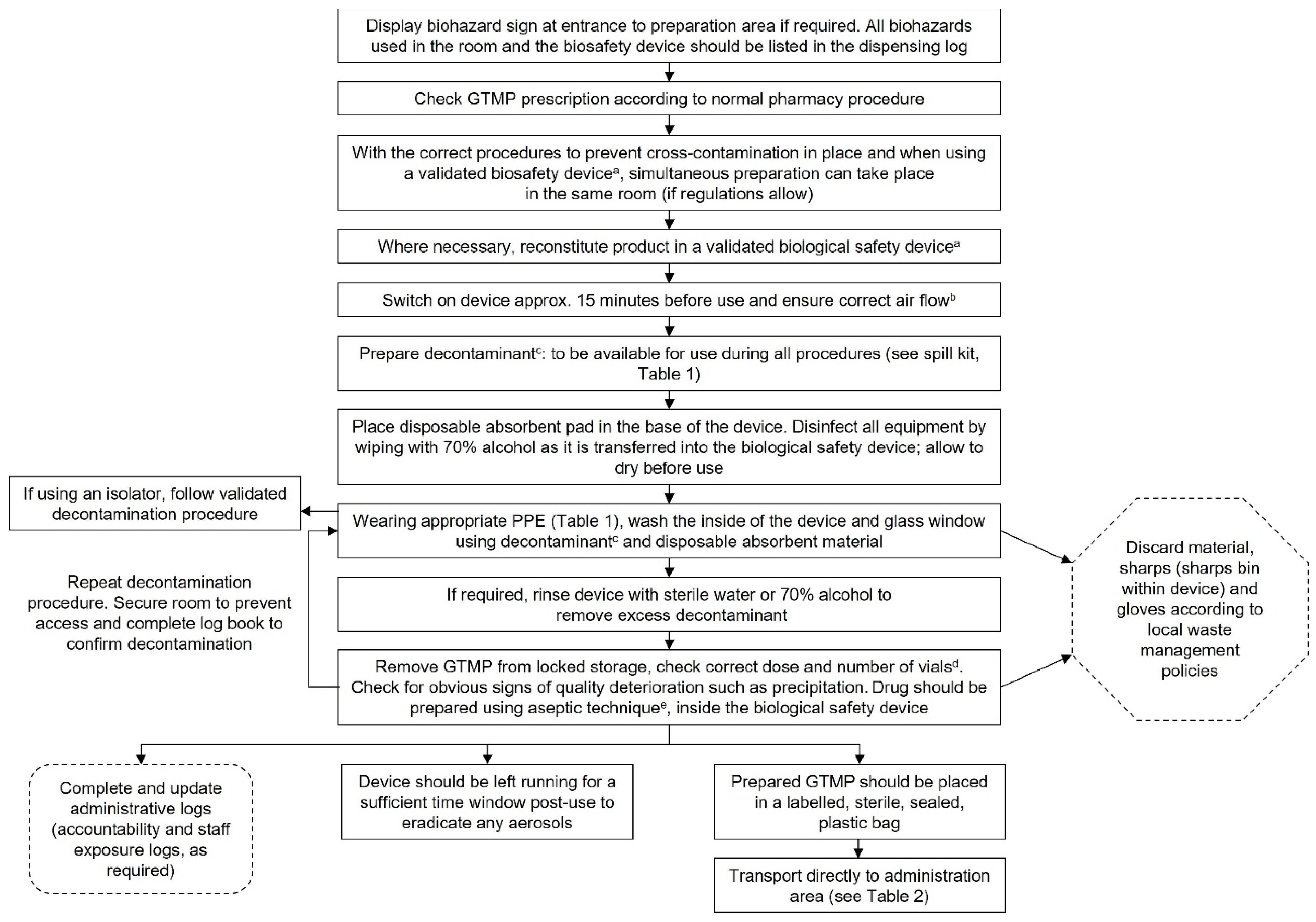
Reconstitution and dispensing of GTMPs
GTMPs should be reconstituted using aseptic technique. The procedure for aseptic reconstitution and dispensing of GTMPs is outlined in
Chart 2. As the vast majority of GTMPs will need to be administered rapidly following reconstitution, the procedure for reconstitution and dispensing should be intertwined into one clear process when developing local SOPs. Appropriate PPE should always be worn when reconstituting and dispensing GTMPs.
All GTMP prescriptions should be checked according to usual hospital pharmacy procedures. Any queries should be directed to the treating physician. If necessary, theatre or ward nurses may also be consulted. Assuming that all procedures for the handling of GTMPs have been implemented, the biosafety/infection control officer, hospital hygiene services and occupational health should not need to be informed of every instance when a GTMP prescription is issued. It should only be necessary to notify appropriate personnel when a GTMP is issued within an institution for the first time. Local regulations should be consulted for country-specific requirements.
SOPs for the entire reconstitution and dispensing process should be developed, including for the transfer process between aseptic and non-sterile facilities/devices (including between institutions on occasion), reconstitution, consumables required, waste disposal, form of the final packaging, labelling of the transport container, stability of the GTMP and spill procedures (6).
Where possible, the GTMP should be reconstituted within the hospital pharmacy to reduce the risks of contamination and medication errors (6). However, this may not be possible in some countries due to legal requirements, e.g., Denmark. In these cases, local regulations must always be followed. In the case of Denmark, a solution has been identified whereby GTMPs are reconstituted in a mobile, single-use isolator at the administration site, which enables aseptic preparation of the GTMP, prompt administration, and close cooperation between hospital pharmacy and clinical personnel (11). However, in other countries (such as France), GTMPs must be reconstituted within the hospital pharmacy, in alignment with regulatory requirements. As such, it is essential to check and adhere to national requirements when developing institutional SOPs.
Similar guidance applies to reconstituting agents in the clinical area. While this may be allowed in some countries for Class 1 agents or Class 2 agents within a closed system transfer device, other countries may forbid it. Some hospitals may have dedicated clean rooms or aseptic facilities for the reconstitution of GTMPs.
At the time of writing, all GTMPs approved by the EC could be handled under biosafety level 1 or 2 conditions (see Appendix 2 for a list of EC-approved GTMPs). If an agent with a higher biosafety level is to be handled, e.g., as part of a clinical trial, a thorough biosafety risk assessment should be performed to determine appropriate handling procedures. An overview of considerations for the biosafety risk assessment of genetically modified organisms has been previously published and may be a useful resource (28).
A biological safety device that complies with EN12469:2000, such as a (minimum) class 2, type B biological safety cabinet or pharmaceutical-grade isolator, should always be used for the reconstitution of GTMPs to protect both the handler and the product. The safety device should have an exhaust to the external environment and not recirculate air into the room. Negative pressure may be required, depending on the risk level of the agent. As always, consult local regulations for specific requirements and conduct a thorough risk assessment. Generally, biosafety class 2 GTMPs and above should be reconstituted in a negative pressure isolator (6).
A biohazard sign should be posted on the outside of the door to the reconstitution room whenever a biosafety level 2 GTMP is being handled. Consult local requirements as to whether a biohazard sign is required for biosafety level 1 agents.
Steps to prevent cross-contamination should be taken, including disinfection of the device before and after use, disinfection of all equipment entering the device, and leaving the device running for a period (in general, 15 minutes to 1 hour is sufficient) following reconstitution, to allow for the elimination of aerosols. Other drugs, doses and supplies should also be cleared from the hood to reduce the risk of cross contamination (1). Decontamination of the device must occur after this time window, immediately before the device is shut down.
If possible, we recommend that a dedicated biosafety device be used for the reconstitution of GTMPs. However, if this is not possible, a common device may be used provided that strict decontamination methods before and after each use are adhered to, if this is allowed by local regulations (19). It is recommended that replication-competent GTMP vectors are reconstituted in a separate device because of the higher risk of cross-contamination (19).
Before beginning to reconstitute a GTMP, always ensure that sufficient consumables (needles, labels, plasticware, etc.) are available and ensure that the GTMP has not passed its expiry date. Consult the Summary of Product Characteristics or clinical trial documents for information regarding the shelf life of the reconstituted product; some agents have a very short shelf-life at room temperature, so reconstitution may need to be performed quickly and in coordination with the administration schedule.
Appropriate viricidal agents should be readily available in the event of a spill, and for decontamination of the safety cabinet before and after use. These may include 1000 ppm chlorine/10% bleach, 1–2% Virkon or 6% hydrogen peroxide. Always ensure that the contact time for the decontamination agent is adhered to. Different viral vectors may have different susceptibilities to decontamination agents; if in doubt, consult the manufacturer of the GTMP for appropriate decontamination practices (26). However, note that some decontaminants can damage stainless steel surfaces on biosafety cabinets; this should be monitored (26).
We recommend that a log book be kept for each biological safety cabinet that is used to reconstitute GTMPs, to inform other members of staff when the device was last used and to confirm that decontamination has been performed. Accountability or exposure logs may also be prudent, particularly for institutions that are new to handling GTMPs. Health surveillance requirements should be checked according to local regulations. An exposure log, if used, should be held centrally (e.g., the occupational health department) and retained for an appropriate timeframe, according to local regulations. Some individuals may be at higher risk of exposure, such as pregnant or immunocompromised staff, or staff with persistent infections (such as herpes simplex) that may be related to the vector used to generate the GTMP. These individuals should be provided with sufficient information to self-assess the risk of handling the GTMP, as well as the opportunity to speak with an occupational health professional. However, local regulations should also be consulted, as some countries may prohibit these individuals from handling GTMPs.
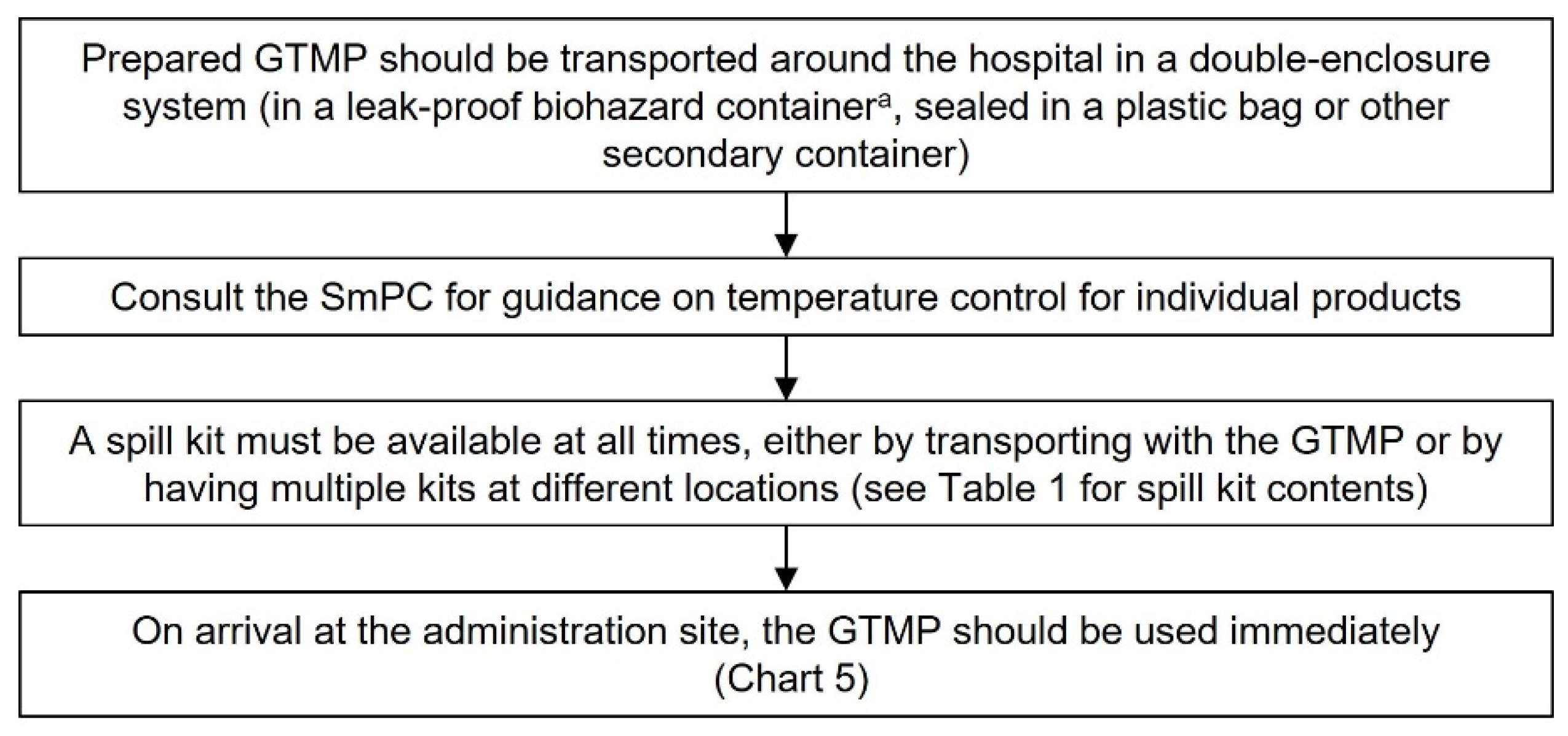
Transportation of GTMPs
The use of the term transportation in this guidance refers to transport within the hospital or on a public road using hospital transport. In both cases, the same recommendations apply, as described below (using a double-enclosed container, appropriate temperature control, availability of a spill kit and using a delivery log). As for all steps along the handling pathway, a risk analysis should always be conducted to ensure safe transport of GTMPs within the hospital system. Courier transport (e.g., from the supplier to the hospital/reconstitution site) is addressed in ‘Receipt and Storage of GTMPs’. Some countries may not allow transportation of the reconstituted GTMP around the hospital; in this case, a mobile isolator unit may be used to aseptically reconstitute the GTMP at the site of administration.
Once reconstituted, the GTMP should be transported in a double-enclosed system, in a biohazard-labelled, leak-proof container (
Chart 3).
Consult the Summary of Product Characteristics or clinical trial documents for temperature requirements after reconstitution and select an appropriate container for transport.
A spill kit should be on hand at every GTMP handling step, including transportation. Ideally, a spill kit should be carried with the GTMP, but an alternative would be to place spill kits along the delivery route. This may depend on local regulations and practicality.
Preferably, the reconstituted GTMP will be brought directly to the administration site and administered immediately, rather than being stored at the administration site.
We suggest having a delivery log as a record that each drug has been delivered, and handover should occur directly to the staff member responsible for administering the agent (26).
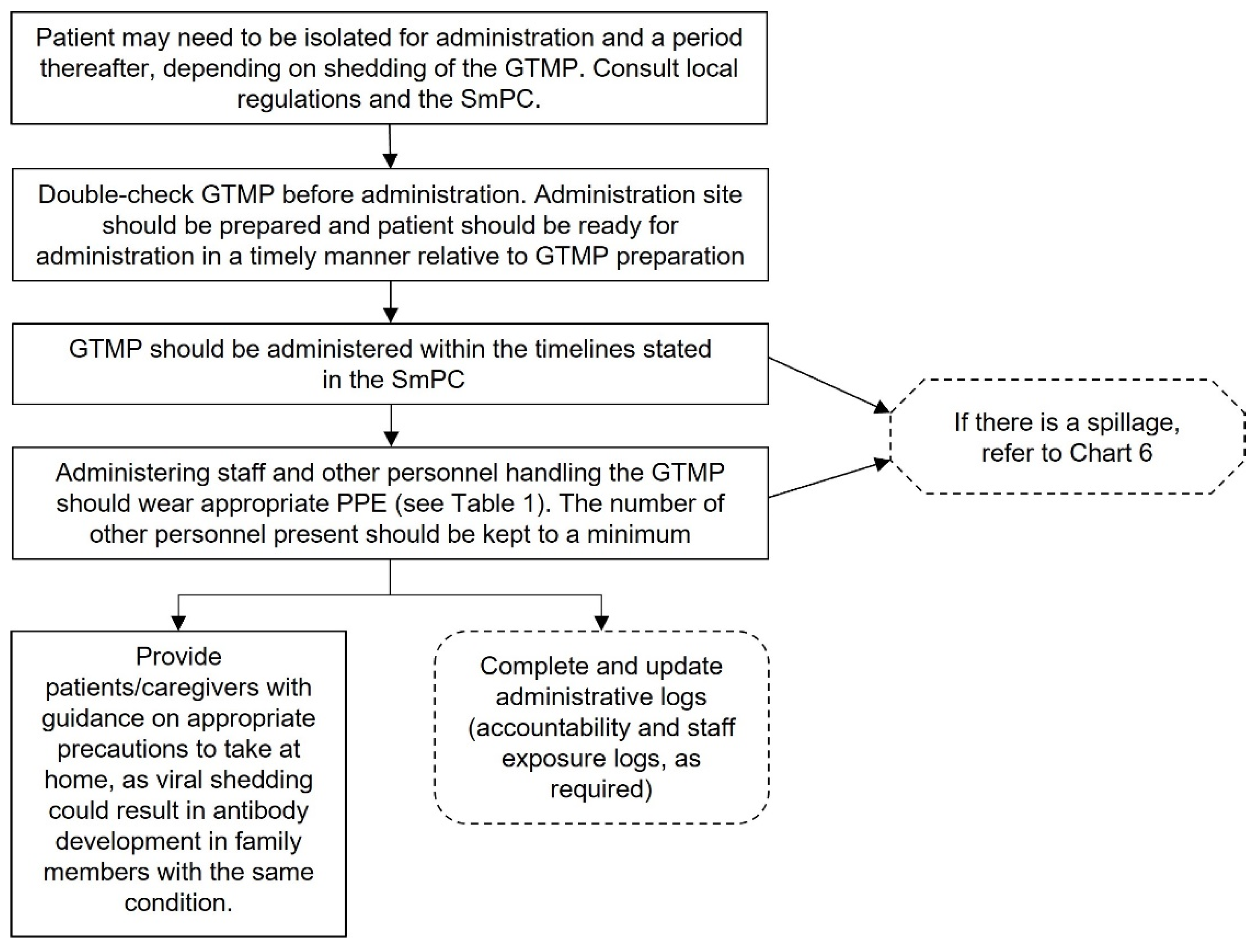
Administering GTMPs
Administration of the GTMP is usually the responsibility of the treating physician, with support from nursing staff, but appropriately trained nurses may also be able to administer GTMPs, in accordance with local regulations (Appendix 4).
Coordination between clinical and hospital pharmacy staff is essential to ensure that the GTMP is reconstituted and then administered in a timely manner. Ideally, the dose should be administered immediately rather than being stored at a second location (1).
Appropriate PPE should be worn when administering the GTMP, dependent on local regulations, product-specific requirements, and institutional risk assessment. Before beginning the administration procedures, ensure that the spill kit, waste disposal containers and appropriate dressings are available. Place a biohazard sign on the door if required by the biosafety class of the agent and local regulations.
A disposable mat should be placed under the body area of the patient, where the GTMP is to be administered. Standard precautions, aseptic technique and product-specific instructions should be adhered to. After administration, cover the site with a dressing and apply pressure to stop bleeding, as required. Dispose of all waste in accordance with local procedures.
It is possible viral shedding may occur from the patient following administration (17). Consult the Summary of Product Characteristics or clinical trial documents for individual GTMPs to determine whether this is likely to occur. If viable viruses (i.e., replication competent viruses) are likely to be shed, appropriate steps should be taken to protect people and the environment. This could include keeping the patient in isolation until shedding ceases. In addition, patients and caregivers should be advised on how to protect themselves, others, and the environment upon returning home. As with every GTMP handling step, local regulations and procedures should be consulted.
Broad guidance for the administration procedure is outlined in
Chart 4; this can be used by clinical staff to help develop SOPs for the administration of GTMPs.
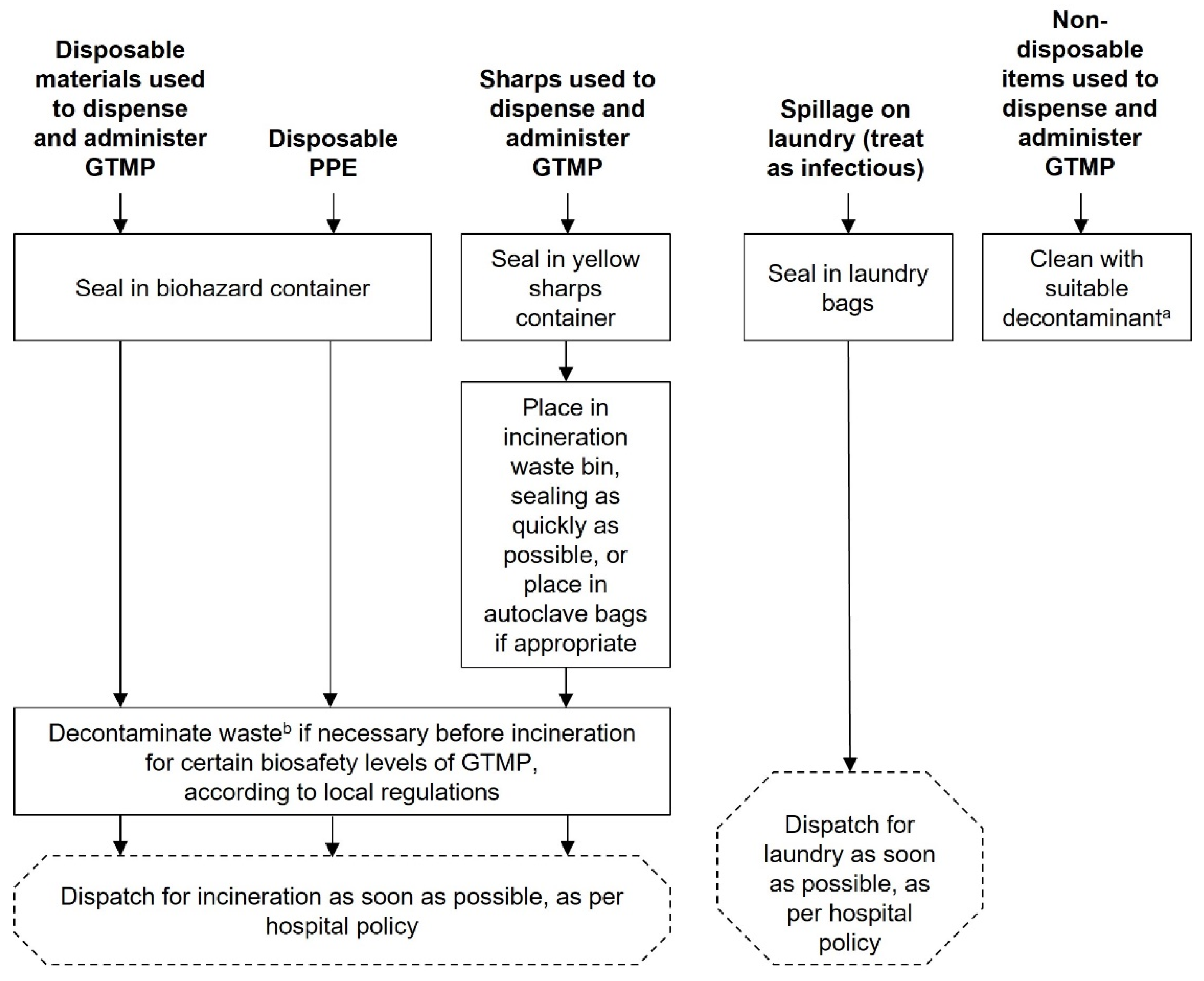
GTMP waste disposal
Disposal and cleaning of waste products should occur as soon as possible using the procedures outlined in
Chart 5 and in accordance with local waste management procedures.
The responsibility for waste disposal, decontamination of spills and accidental exposures lie with the person who created these (Appendix 4). Cleaners, porters, and waste services will be supportive in waste disposal and can help advise in the creation of SOPs.
Following the correct waste disposal procedures is important to limit contact of the GTMP with people and the environment.
Some country regulations may require autoclaving waste prior to incineration; these regulations should be frequently checked for updates. Regulations should also be consulted as to whether contractors handling waste need a special licence.
All waste must be contained and labelled as outlined in Chart 5 and following the guidelines for transport.
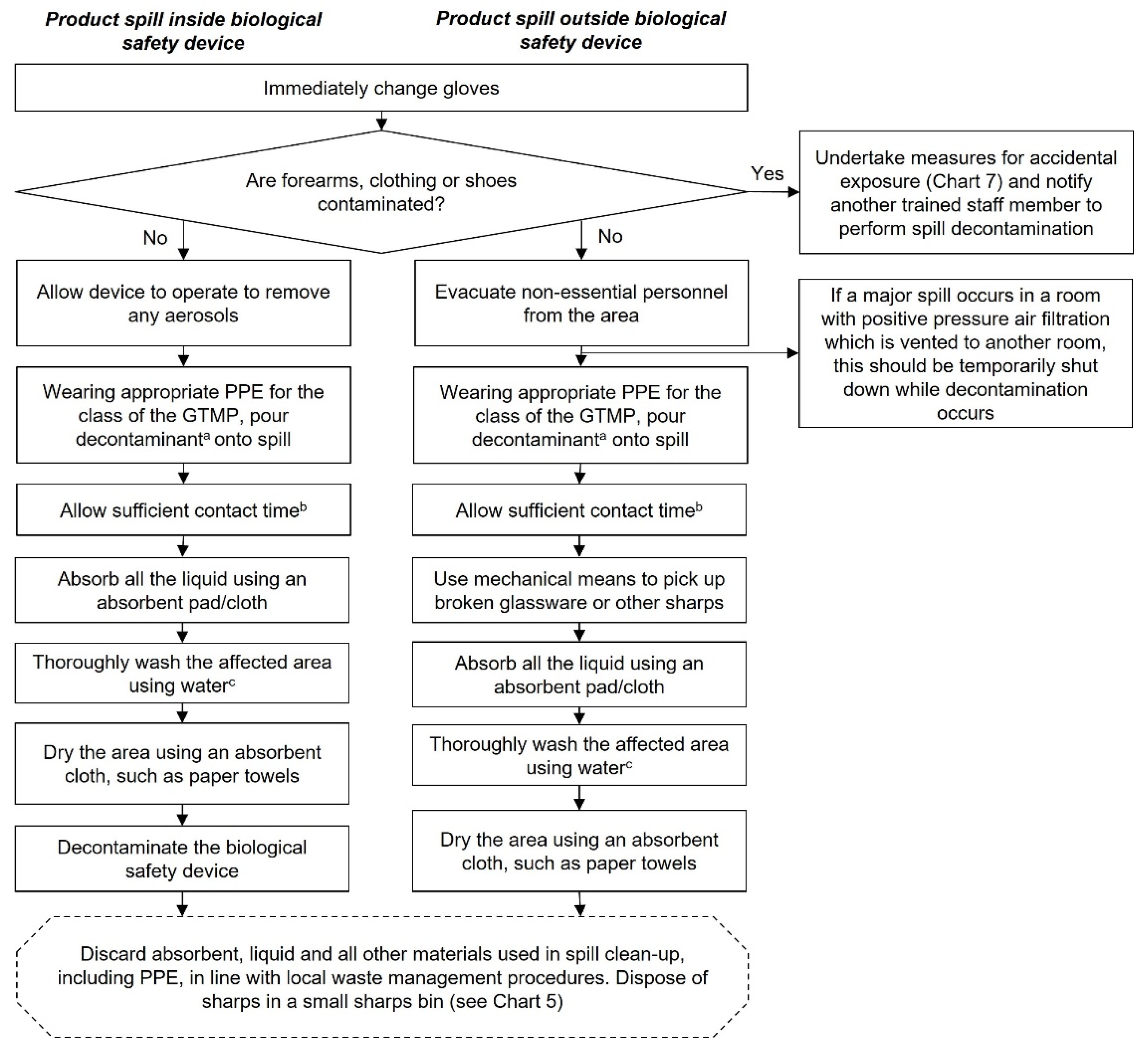
Decontamination of GTMP spills
Decontamination procedures are outlined in
Chart 6 and the recommended contents of a spill kit can be found in
Table 1. Always consult local regulations when developing an SOP for the decontamination of spills.
The responsibility for waste disposal, decontamination of spills and accidental exposures lies with the person who created these (Appendix 4). In the case of a spill or accidental exposure, an occupational health and the biosafety/infection control officer must always be informed and consulted as required. For spills, hospital hygiene services and cleaners should also be informed. Consult local regulations for any reporting requirements. The pharmacist/pharmacy staff are ultimately responsible for decontamination of GTMP spills within the hospital pharmacy, although the biosafety/infection control officer is responsible for ensuring that the correct procedures are in place to deal with spills.
A spill kit should always be readily to hand, either carried with the GTMP, or by placing multiple kits at all locations associated with reconstitution, transport, and administration of GMTPs.
Decontamination procedures vary depending on whether the spill occurs inside or outside a biosafety device.
All staff handling GTMPs should be trained in decontaminating spills so that immediate action can be taken, if required.
All spills, including minor splashes, must be decontaminated, but minor spills do not require evacuation of the area. Major spills (see Appendix 1 for a glossary of terms) include spillage of a whole vial and are a potentially serious incident, even if there is no obvious accidental exposure. Gloves of the staff member affected by the spill should be immediately changed and the forearms/clothing/shoes checked for contamination. Affected items should be removed immediately and measures for accidental exposure undertaken. In this situation, a separate staff member should decontaminate the area.
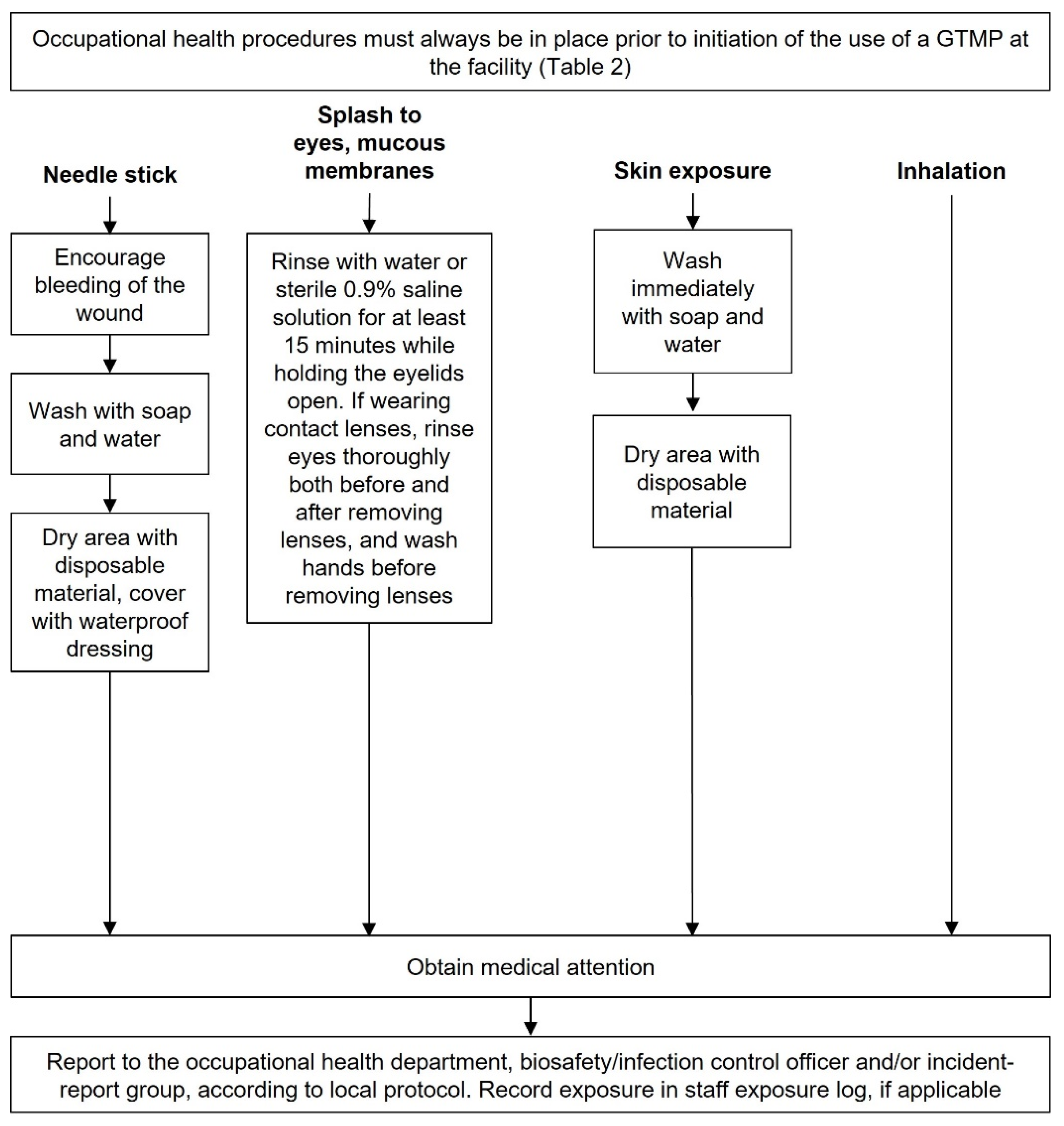
Accidental exposure to GTMPs
Medical attention should usually be sought after accidental exposure and first aid should occur according to
Chart 7. Consult local regulations for reporting requirements.
The responsibility for waste disposal, decontamination of spills and accidental exposures lie with the person who created these (Appendix 4). In the case of a spill or accidental exposure, occupational health and the biosafety/infection control officer must always be informed and consulted, as required. For accidental exposures, physicians, nurses, and hospital hygiene services may also need to be informed, where appropriate. Local regulations and procedures should always be followed when dealing with an accidental exposure.
Product-specific procedures for accidental exposure should be included in the safety data sheet, which should always be to hand when handling GTMPs.
The appropriate hospital staff should be notified in the event of an accidental exposure, including occupational health, the biological safety officer and incident report group. These people should be aware that GTMPs are being used in the hospital and of their potential risks and side effects.
Discussion
This document updates the EAHP guidance on handling GTMPs, which was first published in 2007 (2). In general, the basic processes and precautions for handling GTMPs remain the same – GTMPs should be treated as infectious agents, which means that appropriate PPE must always be worn, and GTMPs should always be reconstituted in a minimum class 2, type B biosafety device. However, most GTMPs present a small biosafety risk, being classified as either biosafety level 1 or 2 (Appendix 2). Institutions should conduct a thorough risk assessment for all new agents entering their facility and establish institutional SOPs and policies accordingly. Local regulations and legislation should also always be adhered to and regularly checked for updates. The Summary of Product Characteristics or clinical trial documents (depending on whether the agent is approved for medicinal use or investigational) should always be consulted for GTMP-specific requirements.
Hospital pharmacists and pharmacies will need to be equipped to handle GTMPs, with increasing numbers of GTMPs gaining regulatory approval. However, there may still be issues with a lack of facilities and staff training (10). A European survey of 216 hospital pharmacies conducted by the EAHP in 2022 found a wide range of preparedness for GTMPs (3). Overall, 40% of survey respondents indicated that their pharmacy had no readiness for GTMPs, while only 9% indicated high readiness, and 37% were currently providing gene therapies (3). Amongst hospital pharmacies providing GTMPs, a number of weaknesses were identified: 53% did not have a biosafety committee, 43% did not have a GTMP-specific spill kit, and 37% were not provided with specific training on the handling of GTMPs (3). Webinars and eLearning were the preferred method of training delivery for most survey respondents (3).
Similarly, a 2018 article highlighted that UK hospital pharmacy departments are not ready to handle GTMPs, based on results from a survey (10). Only seven hospitals had the GTMP talimogene laherparepvec in their formulary, and only five had aseptic facilities for the reconstitution of GTMPs (10). In addition, a low response rate (73/~500 hospitals) may be indicative of a lack of familiarity with GTMPs (10).
The guidance in this document should help institutions prepare for GTMPs. The main barriers are likely to be procurement of appropriate biosafety devices and temperature-controlled storage if these are not already present in the facility, as well as a lack of experience. We recommend that less experienced centres link with more experienced centres and invest in facilities to support the future delivery of GTMPs and other ATMPs. Dedicated personnel will be required to manage GTMPs and provide training, and appropriate governance for the handling of GTMPs must be implemented where it does not already exist. Always consult local regulations; these will always supersede any advice given in this document.
This document does not cover background information on GTMPs, including mechanism of action, underlying biology, and challenges in development. For example, personnel should understand viral vectors and replication-competent viruses, their key characteristics, and how this influences the biosafety risk level of different GTMPs. We recommend that adequate background information be provided as part of staff training, to aid understanding of the reasoning behind the safety processes for handling GTMPs, and to improve confidence with these products. The EAHP will aim to provide training opportunities in the future; however, in the meantime, we refer the interested reader to resources that cover this information (26, 30). In the UK, eLearning is available through the NHS (14).
This document covers the handling of in vivo GTMPs but does not cover other classes of ATMPs (ex vivo GTMPs, somatic cell therapies and tissue engineered products). Future guidance will need to be developed on the handling of these agents.
This update of the EAHP guidance on handling GTMPs provides practical suggestions for hospital pharmacists handling GTMPs in Europe. Adherence to this guidance, within the framework of any local regulations, will ensure consistency of practice across Europe. This will allow safe and effective use of approved GTMPs, and the ability to take on new GTMPs with confidence when they are approved for clinical use.
Acknowledgements and funding support
Financial support for developing this manuscript, including funding for medical writing support, was provided by unrestricted grants from Biomarin, CSL Behring and Pfizer. Medical writing support for the preparation of this manuscript was provided by Linda Buss PhD and Blair Hesp PhD CMPP (Kainic Medical Communications, Dunedin, New Zealand). All authors contributed to all drafts and editorial control remained with the authors at all times.
Competing interest statement
S.A. has received consulting fees from Pfizer, Roche and Biomarin, and travel support from Pfizer. N.C. has provided consultancy to Pfizer and Roche. H.E., H.M., M.P and V.P. have no disclosures. J.V.G. is member of the Spanish Society of Hospital Pharmacy ATMP working group and has been co-author of the EAHP 2007 guidance on handling gene medicines. L.H. has received consulting fees from Roche Belgium, and speaker honoraria from conference organisers for attending the MFC symposium UZ Gent 2021 and 2023, Besedim Congress 2023, LOK: medical cannabis 2021 and PUO Clinical Pharmacist 2021. B.P. has received consulting fees from Pfizer, Novartis and Biomarin, speaker honoraria from Pfizer and Novartis, and travel support from Pfizer. B.P. is also co-ordinator of the ATMP guidelines group for the French Society for Oncology Pharmacy (SFPO). N.S. is member of the Pan UK ATMP pharmacy working group and has been a co-author on the SPS ATMP UK guidance and the EAHP 2007 guidance on handling gene medicines, has received grants from the NIHR and Astellas, receives royalties as editor of the Oxford Handbook of Clinical Pharmacy, has received consulting fees from Astellas and speaker honoraria from Astellas and Pfizer, has participated on advisory boards for Novartis, Eisai, Roche, Sanofi and CSL Behring, is Chair of the Royal Pharmaceutical Society Consultant Pharmacist Group, and is a Visiting Professor at the University of Reading (unpaid).
Copyright statement
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors a full copyright assignment to permit this article (if accepted) to be published in European Journal of Hospital Pharmacy, as set out in in the copyright assignment
http://journals.bmj.com/site/authors/editorial-policies.xhtml#copyright. The Corresponding Author accepts and understands that any supply made under these terms is made by BMJPGL to the Corresponding Author. Where the Corresponding Author wishes to make the article available on an open access basis (and intends to pay the relevant open access fee), the terms of such open access shall be governed by a Creative Commons licence – details of these licences and which Creative Commons licence will apply to this article are set out in our licence referred to above.
Appendices
Appendix 1. Glossary of Terms.
| Term |
Definition |
| Accidental exposure |
Accidental release of the GTMP from containment, resulting in unintended exposure of staff or the public to the agent. |
| Administration |
The process of treating the patient with the GTMP, from receipt of the GTMP by clinical staff up to the point where the patient leaves the hospital and all waste has been disposed of safely. |
| Advanced therapy medicinal product (ATMP) |
A GTMP, somatic cell therapy medicinal product, or a tissue engineered product (5). |
| Biosafety |
The safe working practices required for the handling of biological materials, especially infectious agents. |
| Biosafety level 1 |
The containment level required for a microorganism that is unlikely to cause human or animal disease (no or low individual and community risk; risk group 1). Often no biosafety device is required for this level; check local regulations (8). |
| Biosafety level 2 |
The containment level required for a pathogen that can cause human or animal disease, but is unlikely to be a serious hazard. Laboratory exposure could cause serious infection, but effective treatment and prevention measures are available and the risk of spread is limited (moderate individual risk, low community risk; risk group 2). Biosafety device, PPE and biohazard sign usually required; check local regulations (8). |
| Biosafety level 3 |
The containment level required for a pathogen that causes serious human or animal disease but does not usually spread between individuals. Effective treatment and preventative measures are available (high individual risk, low community risk; risk group 3). Biosafety device, PPE, biohazard sign, controlled access and directional airflow usually required; check local regulations (8). |
| Biosafety level 4 |
The containment level required for a pathogen that usually causes serious human or animal disease and is easily transmissible. Effective treatment and preventative measures are not usually available (high individual and community risk; risk group 4). Highly restricted access with airlock entry, shower exit and specialist waste disposal usually required. Specialised PPE and/or class 3 biosafety devices may be required, as well as additional safety measures; check local requirements (8). |
| Containment |
The culturing, storage, transport, destruction, disposal, or any other use of a genetically modified organism within the bounds of physical, chemical and/or biological barriers to limit their contact with people and the environment (17) . |
| Decontamination |
Any process for removing/killing microorganisms (8). |
| Disinfection |
A physical or chemical means of killing microorganisms, but not necessarily spores (8). |
| Genetically modified organism (GMO) |
An organism that has undergone genetic modification. |
| Genetic modification |
Occurs when the genetic material of an organism has been altered in a way that does not occur naturally (either by mating or natural recombination), and uses recombinant nucleic acid techniques to form new combinations of genetic material (17). |
| Gene therapy |
Treatment or prophylaxis of disease by the deliberate introduction of genetic material into the isolated cells of a patient (ex vivo) or directly into the patient (in vivo) (6). |
| Gene therapy medicinal product (GTMP) |
A GTMP must fulfil two conditions: 1) the product must be a biological medicinal product and contain recombinant nucleic acid(s) and 2) the recombinant nucleic acid(s) should be directly involved in the mechanism of action and hence therapeutic action of the product (5). |
| Handling |
Includes storage, dispensing and reconstitution, transportation, administration, waste disposal, spills and accidental exposure, and any other process where the GTMP is in use. |
| Naked DNA |
DNA that is free in solution, not packaged in a vector. |
| Spill, majora
|
More than 5 mL or 5 g of a substance. |
| Spill, minora
|
Less than 5 mL or 5 g of a substance. |
| Storage |
Containment of a GTMP when it is not in use. |
| Reconstitution |
The process of making the GTMP ready-to-administer. Also referred to as preparation (6). |
| Transportation |
Movement of the GTMP around and/or between hospitals using hospital transport. NOT courier transport. |
aSpill definitions are based on the definitions for major and minor spills of cytotoxic agents (31).
Appendix 2. List of EC-approved GTMPs, as of January 2023 (9).
| GTMP |
Manufacturer |
Indication |
in vivo/
ex vivo
|
Viral vector |
Biosafety level of vectorb (28) |
| Glybera (alipogene tiparvovec)a (32) |
uniQure biopharma |
Familial lipoprotein lipase deficiency |
in vivo |
AAV-1 |
1 |
| Imlygic (talimogene laherparepvec) (33) |
Amgen/IDT Biologika |
Unresectable, metastatic melanoma |
in vivo |
HSV-1 |
1/2d
|
| Strimvelisc (34) |
AGC Biologics |
ADA-SCID |
ex vivo |
Retroviral |
2 |
| Yescarta (axicabtagene ciloleucel)c (35) |
Kite Pharma |
DLBCL, HGBL, PMBCL, FL |
ex vivo |
Retroviral |
2 |
| Kymriah (tisagenlecleucel)c (36) |
Novartis |
ALL, DLBCL, FL |
ex vivo |
Lentiviral |
2 |
| Luxturna (voretigene neparvovec) (37) |
Spark Therapeutics/Novartis |
Inherited retinal dystrophy |
in vivo |
AAV-2 |
1 |
| Zynteglo (betibeglogene autotemcel)a,c (38) |
Minaris Regenerative Medicine |
TDT |
ex vivo |
Lentiviral |
2 |
| Zolgensma (onasemnogene abeparvovec) (39) |
Novartis/Almac Pharma Services |
5q SMA |
in vivo |
AAV-9 |
1 |
| Libmeldy (atidarsagene autotemcel)c (40) |
AGC Biologics |
ARSA-mutant MLD |
ex vivo |
Lentiviral |
2 |
| Tecartus (brexucabtagene autoleucel)c (41) |
Kite Pharma |
MCL, ALL |
ex vivo |
Retroviral |
2 |
| Skysona (elivaldogene autotemcel)a,c (42) |
Minaris Regenerative Medicine |
ABCD1-mutant early cerebral adrenoleukodystrophy |
ex vivo |
Lentiviral |
2 |
| Abecma (idecabtagene vicleucel)c (43) |
Celgene |
Multiple myeloma |
ex vivo |
Lentiviral |
2 |
| Breyanzi (lisocabtagene maraleucel)c (44)
|
Juno Therapeutics/Celgene |
DLBCL, PMBCL, FL |
ex vivo |
Lentiviral |
2 |
| Carvykti (ciltacaptagene autoleucel)c (45) |
Janssen |
Multiple myeloma |
ex vivo |
Lentiviral |
2 |
| Upstaza (eladocagene exuparvovec) (46) |
MassBiologics South Coast/Almac Pharma Services |
AADC deficiency |
in vivo |
AAV-2 |
1 |
| Roctavian (valoctocogene roxaparvovec) (47) |
BioMarin |
Haemophilia A |
in vivo |
AAV-5 |
1 |
| Hemgenix (etranacogene dezaparvovec) (48) |
CSL Behring |
Haemophilia B |
in vivo |
AAV-5 |
1 |
Please note this table is intended as a RESOURCE ONLY and DOES NOT REPLACE institutional risk assessment. The SmPC for individual products should always be consulted and local regulations should always be checked. aMarketing authorisation not renewed. bBiosafety level based on the Belgian classification (28); local classifications should be used where possible. Classification does not take into account the risk level of the transgene. cCellular product; may require additional training for appropriate handling (6). dThe Belgian classification classes HSV-1 as risk group 1 when used as a vector (28); however, the NIH guidelines for research involving recombinant or synthetic nucleic acid molecules classes all herpesviruses as risk group 2 (49). Consult local regulations for guidance. Abbreviations: AADC, aromatic l-amino acid decarboxylase; AAV, adeno-associated virus; ADA-SCID, adenosine deaminase-severe combined immunodeficiency; ALL, acute lymphoblastic leukaemia; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma; HGBL, high-grade B cell lymphoma; HSV, herpes simplex virus; MCL, mantle cell lymphoma; MLD, metachromatic leukodystrophy; NIH, National Institutes of Health; PMBCL, primary mediastinal large B cell lymphoma; SMA, spinal muscular atrophy; TDT, transfusion-dependent beta-thalassaemia.
Appendix 3. HSE 2007 guidance on containment measures required for GTMPs in a clinical research
setting.
| Containment measure |
Level 1 |
Level 2 |
| Autoclave |
Required on site |
Required in the building |
| Access restricted to authorised personnel |
Not required |
Required |
| Measures to control aerosol dissemination |
Not required |
Required |
| PPE |
Required |
Required |
| Specified disinfection procedures |
Required |
Required |
| Safe storage of GTMP |
Required |
Required |
| Inactivation of GTMP in contaminated material and waste |
Required by validated means |
Required by validated means |
‘Level’ refers to the containment level and not the biosafety level of the agents themselves. However, the risk group of the agent does correlate directly with the containment level, e.g., risk group 2 agents require containment level 2. This table details the requirements from a containment perspective; additional precautions may be required to protect the product. Abbreviations: GMTP, gene therapy medicinal products; UK Health and Safety Executive.
Appendix 4. Model for the roles and responsibilities for handling GTMPs.
| Handling stage |
Chief pharmacist |
Hospital pharmacy staff |
Physician |
Theatre/ward nurse |
Biosafety officera/hygiene services/infection control |
Occupational health |
Cleaners |
Porters |
Waste services |
| Initiation of GTMP treatment and setting up conditions, including environmental considerations |
CI |
CI |
R |
CI |
CI |
CI |
CI |
I |
CI |
| Assessment: ability to handle, staff trainingb
|
R |
S |
RA |
|
RS |
S |
|
|
|
| Screening GTMP prescriptions (patient basis) |
R |
RS |
AC |
CI |
Ic
|
Ic
|
|
|
|
| Receipt of GTMP from the manufacturer and inspection |
R |
S |
CI |
CI |
Ic
|
Ic
|
|
|
|
| Transportation |
RA |
S |
CI |
CI |
SC |
C |
|
I |
|
| Storage |
R |
S |
|
|
SC |
1 |
|
|
|
| Preparation and decontamination of biological safety device |
R |
SI |
|
|
SC |
|
|
|
|
| Dispensing |
RA |
S |
|
|
|
|
|
|
|
| Administration (product-dependent) |
|
|
R |
RS |
|
|
|
|
|
| Waste disposal |
R |
S |
R |
R |
SC |
|
S |
S |
S |
| Decontamination of GTMP spills |
Rd
|
RdS |
Rd
|
Rd
|
ACI |
CI |
SI |
|
|
| Accidental exposure |
Rd
|
Rd
|
RdI |
RdI |
ACI |
CI |
|
|
|
R, responsible person; A, person to whom ‘R’ is accountable; S, can be supportive; C, should be consulted; I, should be informed. aIn organisations where there is no appointed biosafety/infection control officer, the responsibilities should be taken by a member of the infection control body. bTraining will be limited to pharmacy training for pharmacists. cShould be informed once at the start of the process when conditions for the use of GTMPs are being established. dThe person who spilled the GTMP, or was accidentally exposed, should be responsible for initiating the decontamination. Ultimate responsibility for ensuring the spill is appropriately decontaminated remains with the pharmacist. Abbreviations: GMTP, gene therapy medicinal products.
References
- Hernandez, JM. Biosafety considerations for viral vector gene therapy: An explanation and guide for the average everyday-hero pharmacist. J Pharm Pract. 2022:8971900221104250.
- Vulto AG, Stoner N, Balásová H, Cercos A-C, Hoppe-Tichy T, Genestar JLV, et al. European Association of Hospital Pharmacists (EAHP) Guidance on the Pharmacy Handling of Gene Medicines. European Journal of Hospital Pharmacy. 2007, 13:29-39.
- European Association of Hospital Pharmacists. Special Interest Group Report: Hospital pharmacist’s preparedness for in-vivo gene therapy medicinal products 2023 [Available from: https://www.eahp.eu/sites/default/files/eahp_sig_gene_therapy_report.pdf.
- Appraisal of Guidelines for Research and Evaluation II 2017 [Available from: https://www.agreetrust.org/wp-content/uploads/2017/12/AGREE-II-Users-Manual-and-23-item-Instrument-2009-Update-2017.pdf.
- European Medicines Agency. Reflection paper on classification of advanced therapy medicinal products 2015 [updated 21 May 2015. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-classification-advanced-therapy-medicinal-products_en-0.pdf.
- Pan UK Pharmacy Working Group for ATMPs. Gene therapy medicinal products: Governance and preparation requirements 2019 [updated October 2019. Available from: https://www.sps.nhs.uk/wp-content/uploads/2019/09/PAN-UK-PWG-for-ATMPs-Gene-Therapy-Guidance-issue-2.pdf.
- Sung YK, Kim SW. Recent advances in the development of gene delivery systems. Biomater Res. 2019, 23:8.
- World Health Organization. Laboratory biosafety manual. 3 ed 2004.
- European Medicines Agency. CAT quarterly highlights and approved ATMPs 2023 [updated 31 January
2023. Available from: https://www.ema.europa.eu/en/documents/report/cat-quarterly-highlights-approved-atmps-january-2023_en.pdf.
- Stoner, N. Are UK hospital pharmacy departments ready for the rise of gene therapy medicinal products? Expert Opin Biol Ther. 2018, 18, 837–40. [Google Scholar] [CrossRef] [PubMed]
- McNulty HBO, Duckert LR. Good Practice Initiative: Development of method for mobile aseptic preparation of advanced therapy medicinal products. 26th Congress of the European Association of Hospital Pharmacists; 23-25 March 2022, Vienna, Austria2022. p. PC11229.
- Société Française de Pharmacie Oncologique. Recommandations S.F.P.O. sur le circuit hospitalier des Médicaments de Thérapie Innovante (MTI) 2023 [updated August 2023. Available from: https://sfpo.com/wp-content/uploads/2023/08/Recommandations-MTI-V2-Aout-2023.pdf.
- Advanced Therapy Treatment Centres. Advanced therapies NHS readiness toolkit 2023 [Available from: https://www.theattcnetwork.co.uk/advanced-therapies-nhs-readiness-toolkit.
- National Health Service. Advanced Therapy Medicinal Products programme 2023 [Available from: https://www.e-lfh.org.uk/programmes/advanced-therapy-medicinal-products/.
- Health and Safety Executive. Containment and control of activities involving genetically modified microorganisms 2014 [Available from: https://www.hse.gov.uk/biosafety/gmo/acgm/acgmcomp/part3.pdf.
- Health and Safety Executive. Risk assessment of genetically modified microorganisms (other than those associated with plants) 2014 [Available from: https://www.hse.gov.uk/biosafety/gmo/acgm/acgmcomp/part2.pdf.
- Health and Safety Executive. Guidance on the use of genetically modified microorganisms in a clinical setting 2014 [updated 2014. Available from: https://www.hse.gov.uk/biosafety/gmo/acgm/acgmcomp/part6.pdf.
- Haut Conseil des Biotechnologies. Manuel du HCB pour l’utilisation confinée d’organismes génétiquement modifiés 2019 [updated 4 July 2019. Available from: https://www.ecologie.gouv.fr/sites/default/files/manuelduconfine2019.pdf.
- Ellison SL, Hunt DL. Perceived versus real risks of handling gene transfer agents in the pharmacy environment. Am J Health Syst Pharm. 2010, 67, 838–48.
- Webb TL, Hong E. GMO medicines and hospital pharmacy practice: a review. Journal of Pharmacy Practice and Research. 2021, 51, 203–10.
- Petrich J, Marchese D, Jenkins C, Storey M, Blind J. Gene replacement therapy: A primer for the health-system pharmacist. J Pharm Pract. 2020, 33, 846–55. [CrossRef] [PubMed]
- Blind JE, McLeod EN, Brown A, Patel H, Ghosh S. Biosafety practices for in vivo viral-mediated gene therapy in the health care setting. Appl Biosaf. 2020, 25, 194–200. [CrossRef] [PubMed]
- Blind JE, McLeod EN, Campbell KJ. Viral-mediated gene therapy and genetically modified therapeutics: A primer on biosafety handling for the health-system pharmacist. American Journal of Health-System Pharmacy. 2019, 76, 795–802.
- Armitstead JA, Zilich AJ, Williams KL, Sitzlar SC, Wermeling D. Hospital and pharmacy departmental policies and procedures for gene therapy at a teaching institution. Hosp Pharm. 2001, 36:56-66.
- Myers, C.J. Preparing pharmacists to manage gene therapies. J Am Pharm Assoc (2003). 2021, 61, e78–e82. [Google Scholar] [CrossRef] [PubMed]
- Gene Therapy Network 2020 [Available from: https://genetherapynetwork.com/emea.
- Danish Arbejdstilsynet. Risikovurdering af genteknologiske forskningsprojekter mv. 2001.
- Baldo A, van den Akker E, Bergmans HE, Lim F, Pauwels K. General considerations on the biosafety of virus-derived vectors used in gene therapy and vaccination. Current Gene Therapy. 2013, 13:385-94.
- What is RASCI/RACI: A comprehensive guide on how and when to use them 2023 [Available from: https://www.interfacing.com/what-is-rasci-raci.
- Sinclair A, Islam S, Jones S. Gene therapy: An overview of approved and pipeline technologies. Canadian Agency for Drugs and Technologies in Health. 2018(171).
- University of British Columbia. Cytotoxic spill clean up 2021 [updated January 2021. Available from: https://riskmanagement.sites.olt.ubc.ca/files/2021/01/CHEM-SWP-004-Cytotoxic-Spill-Clean-Up.pdf.
- GLYBERA Summary of Product Characteristics 2017 [updated 10 July 2017. Available from: https://www.ema.europa.eu/en/documents/product-information/glybera-epar-product-information_en.pdf.
- IMLYGIC Summary of Product Characteristics 2022 [updated 22 November 2022. Available from: https://www.ema.europa.eu/en/documents/product-information/imlygic-epar-product-information_en.pdf.
- STRIMVELIS Summary of Product Characteristics 2022 [updated 08 July 2022. Available from: https://www.ema.europa.eu/en/documents/product-information/strimvelis-epar-product-information_en.pdf.
- YESCARTA Summary of Product Characteristics 2023 [updated 30 January 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/yescarta-epar-product-information_en.pdf.
- KYMRIAH Summary of Product Characteristics 2022 [updated 07 November 2022. Available from: https://www.ema.europa.eu/en/documents/product-information/kymriah-epar-product-information_en.pdf.
- LUXTURNA Summary of Product Characteristics 2023 [updated 04 May 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/luxturna-epar-product-information_en.pdf.
- ZYNTEGLO Summary of Product Characteristics 2022 [updated 20 January 2022. Available from: https://www.ema.europa.eu/en/documents/product-information/zynteglo-epar-product-information_en-1.pdf.
- ZOLGENSMA Summary of Product Characteristics 2023 [updated 13 March 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/zolgensma-epar-product-information_en.pdf.
- LIBMELDY Summary of Product Characteristics 2023 [updated 17 April 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/libmeldy-epar-product-information_en.pdf.
- TECARTUS Summary of Product Characteristics 2023 [updated 30 January 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/tecartus-epar-product-information_en.pdf.
- SKYSONA Summary of Product Characteristics 2022 [updated 04 April 2022. Available from: https://www.ema.europa.eu/en/documents/product-information/skysona-epar-product-information_en.pdf.
- ABECMA Summary of Product Characteristics 2022 [updated 28 October 2021. Available from: https://www.ema.europa.eu/en/documents/product-information/abecma-epar-product-information_en.pdf.
- BREYANZI Summary of Product Characteristics 2022 [updated 08 April 2022. Available from: https://www.ema.europa.eu/en/documents/product-information/breyanzi-epar-product-information_en.pdf.
- CARVYKTI Summary of Product Characteristics 2023 [updated 14 April 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/carvykti-epar-product-information_en.pdf.
- UPSTAZA Summary of Product Characteristics 2023 [updated 04 April 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/upstaza-epar-product-information_en-0.pdf.
- ROCTAVIAN Summary of Product Characteristics 2022 [updated 09 November 2022. Available from: https://www.ema.europa.eu/en/documents/product-information/roctavian-epar-product-information_en.pdf.
- HEMGENIX Summary of Product Characteristics 2023 [updated 20 June 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/hemgenix-epar-product-information_en.pdf.
- National Institutes of Health. NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines) 2019 [Available from: https://osp.od.nih.gov/wp-content/uploads/NIH_Guidelines.pdf.
Chart 1.
Storage of GTMPs. aWhen GTMPs are delivered, the secondary packaging is guaranteed as not contaminated. bIf vial is damaged, the primary packaging should be decontaminated. cLocation of storage must ensure that no unauthorised person can gain access and there is no undue risk of exposure of hospital staff to the product. A biosafety label should be included on the door of the storage room and the storage unit. This chart is intended as a framework to aid the development of institutional SOPs and should be amended as required to comply with local regulations. Abbreviations: GTMP, gene therapy medicinal product.
Chart 1.
Storage of GTMPs. aWhen GTMPs are delivered, the secondary packaging is guaranteed as not contaminated. bIf vial is damaged, the primary packaging should be decontaminated. cLocation of storage must ensure that no unauthorised person can gain access and there is no undue risk of exposure of hospital staff to the product. A biosafety label should be included on the door of the storage room and the storage unit. This chart is intended as a framework to aid the development of institutional SOPs and should be amended as required to comply with local regulations. Abbreviations: GTMP, gene therapy medicinal product.
Chart 2.
Aseptic reconstitution and dispensing of GTMPs. aMinimum class 2, type B, with background grading per local guidance/regulations. bThe running of the safety device should be according to local procedures for aseptic reconstitution. cDecontaminant with proved viricidal activity, e.g., an oxidising agent such as 1000 ppm chlorine, 1–2% Virkon or 6% hydrogen peroxide. The chosen decontaminant should be validated for each individual GTMP using data provided by the supplier/manufacturer, as detailed in the Summary of Product Characteristics. dThe delivery device should be suitably decontaminated after use. eAny spills should be decontaminated according to Chart 6. This chart is intended as a framework to aid the development of institutional SOPs and should be amended as required to comply with local regulations. Abbreviations: GTMP, gene therapy medicinal product; PPE, personal protective equipment.
Chart 2.
Aseptic reconstitution and dispensing of GTMPs. aMinimum class 2, type B, with background grading per local guidance/regulations. bThe running of the safety device should be according to local procedures for aseptic reconstitution. cDecontaminant with proved viricidal activity, e.g., an oxidising agent such as 1000 ppm chlorine, 1–2% Virkon or 6% hydrogen peroxide. The chosen decontaminant should be validated for each individual GTMP using data provided by the supplier/manufacturer, as detailed in the Summary of Product Characteristics. dThe delivery device should be suitably decontaminated after use. eAny spills should be decontaminated according to Chart 6. This chart is intended as a framework to aid the development of institutional SOPs and should be amended as required to comply with local regulations. Abbreviations: GTMP, gene therapy medicinal product; PPE, personal protective equipment.
Chart 3.
Transportation of GTMPs. aLeak-proof biohazard container can be reused following decontamination. This chart is intended as a framework to aid the development of institutional SOPs and should be amended as required to comply with local regulations. Abbreviations: GTMP, gene therapy medicinal product; SmPC, summary of product characteristics.
Chart 3.
Transportation of GTMPs. aLeak-proof biohazard container can be reused following decontamination. This chart is intended as a framework to aid the development of institutional SOPs and should be amended as required to comply with local regulations. Abbreviations: GTMP, gene therapy medicinal product; SmPC, summary of product characteristics.
Chart 4.
Guidance for clinical staff to develop SOPs for administering GTMPs. This chart is intended as a framework to aid the development of institutional SOPs and should be amended as required to comply with local regulations. Abbreviations: GTMP, gene therapy medicinal product; SmPC, summary of product characteristics; PPE, personal protective equipment.
Chart 4.
Guidance for clinical staff to develop SOPs for administering GTMPs. This chart is intended as a framework to aid the development of institutional SOPs and should be amended as required to comply with local regulations. Abbreviations: GTMP, gene therapy medicinal product; SmPC, summary of product characteristics; PPE, personal protective equipment.
Chart 5.
GTMP waste disposal. aDecontaminant with proven viricidal activity, e.g., an oxidising agent such as 1000 ppm chlorine, 1–2% Virkon or 6% hydrogen peroxide. The chosen decontaminant should be validated for each individual GTMP using data provided by the supplier/manufacturer, as detailed in the Summary of Product Characteristics. bInactivation should be conducted according to local regulations. This chart is intended as a framework to aid the development of institutional SOPs and should be amended as required to comply with local regulations. Abbreviations: GTMP, gene therapy medicinal product; PPE, personal protective equipment.
Chart 5.
GTMP waste disposal. aDecontaminant with proven viricidal activity, e.g., an oxidising agent such as 1000 ppm chlorine, 1–2% Virkon or 6% hydrogen peroxide. The chosen decontaminant should be validated for each individual GTMP using data provided by the supplier/manufacturer, as detailed in the Summary of Product Characteristics. bInactivation should be conducted according to local regulations. This chart is intended as a framework to aid the development of institutional SOPs and should be amended as required to comply with local regulations. Abbreviations: GTMP, gene therapy medicinal product; PPE, personal protective equipment.
Chart 6.
Decontamination of GTMP spills. aDecontaminant with proven viricidal activity, e.g., an oxidising agent such as 1000 ppm chlorine, 1–2% Virkon or 6% hydrogen peroxide. The chosen decontaminant should be validated for each individual GTMP using data provided by the supplier/manufacturer, as detailed in the Summary of Product Characteristics. bContact time is dependent on the disinfectant used. Exceeding the contact time will not improve decontamination but may damage surfaces. cUsing other disinfectants or alcohol to wash the area rather than water will likely cause frothing or smearing, which may be difficult to remove. This chart is intended as a framework to aid the development of institutional SOPs and should be amended as required to comply with local regulations. Abbreviations: GTMP, gene therapy medicinal product; PPE, personal protective equipment.
Chart 6.
Decontamination of GTMP spills. aDecontaminant with proven viricidal activity, e.g., an oxidising agent such as 1000 ppm chlorine, 1–2% Virkon or 6% hydrogen peroxide. The chosen decontaminant should be validated for each individual GTMP using data provided by the supplier/manufacturer, as detailed in the Summary of Product Characteristics. bContact time is dependent on the disinfectant used. Exceeding the contact time will not improve decontamination but may damage surfaces. cUsing other disinfectants or alcohol to wash the area rather than water will likely cause frothing or smearing, which may be difficult to remove. This chart is intended as a framework to aid the development of institutional SOPs and should be amended as required to comply with local regulations. Abbreviations: GTMP, gene therapy medicinal product; PPE, personal protective equipment.
Chart 7.
Accidental exposure to a GTMP. This broadly refers to agents that are biosafety level 2; those that are level 1 may require less stringent decontamination agents. See SmPC for specific information on decontaminants for particular agents. This chart is intended as a framework to aid the development of institutional SOPs and should be amended as required to comply with local regulations. Abbreviations: GTMP, gene therapy medicinal product; SmPC, Summary of Product Characteristics.
Chart 7.
Accidental exposure to a GTMP. This broadly refers to agents that are biosafety level 2; those that are level 1 may require less stringent decontamination agents. See SmPC for specific information on decontaminants for particular agents. This chart is intended as a framework to aid the development of institutional SOPs and should be amended as required to comply with local regulations. Abbreviations: GTMP, gene therapy medicinal product; SmPC, Summary of Product Characteristics.
Table 1.
General guidance regarding the handling of GTMPs and patient specimens.
Table 1.
General guidance regarding the handling of GTMPs and patient specimens.
| Precaution |
Details |
| Protective clothinga
|
Disposable apron, gown, or lab coatbSafety glasses or gogglesGlovesMucous membrane splash protector (face mask) (26) |
| Biological safety cabinet (minimum class 2, type B) or pharmaceutical grade isolator (compliant with European standard EN12469:2000) |
See Chart 2
|
| Care with use and disposal of needles and sharps |
See Chart 5
|
| Decontamination of work surface areas |
See Chart 2
|
| Decontaminate patient bedding according to procedures for blood- or body-fluid-soiled laundryb
|
See Chart 5
|
| No special precautions for patient elimination of stools or urine, unless specified in the Summary of Product Characteristics for product-specific information |
Patients may use normal bathroom facilities unless advised otherwise |
| All transport and storage of patient specimens, GTMPs and associated waste must be in a double-enclosed, biohazard-labelled, leak-proof container (20, 26) |
See Table 2 |
| Disposal of GTMPs, contaminated waste and patient specimens must follow institutional decontamination procedures |
See Chart 5
|
| A spill kit must always be on hand (6, 20, 26) |
Suggested spill kit contents:
2× disposable gowns or arm covers
4× gloves
2× masks
2× aprons
2× goggles
4× disposable shoe covers
2× disinfectant sachets (or other, pre-prepared, decontaminant)Absorbent paper towels
2× disposable forceps
2× biohazard incineration bags
Emergency contact numberCopy of spillage procedure |
| Institutional procedures and policies should be put in place for all steps along the GTMP handling pipeline (21) |
Steps include storage, transport, reconstitution, dispensing, administration, disposal and decontamination, accidental exposure and spills and personnel training (21) |
| Hazardous signage must be used (20, 21, 26) |
On all containers, storage cabinets/fridges/freezers and the door to the reconstitution room (20, 26) |
| All GTMPs should be added to the institutional hazardous drugs list (22) |
As required by local legislation |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).