1. Introduction
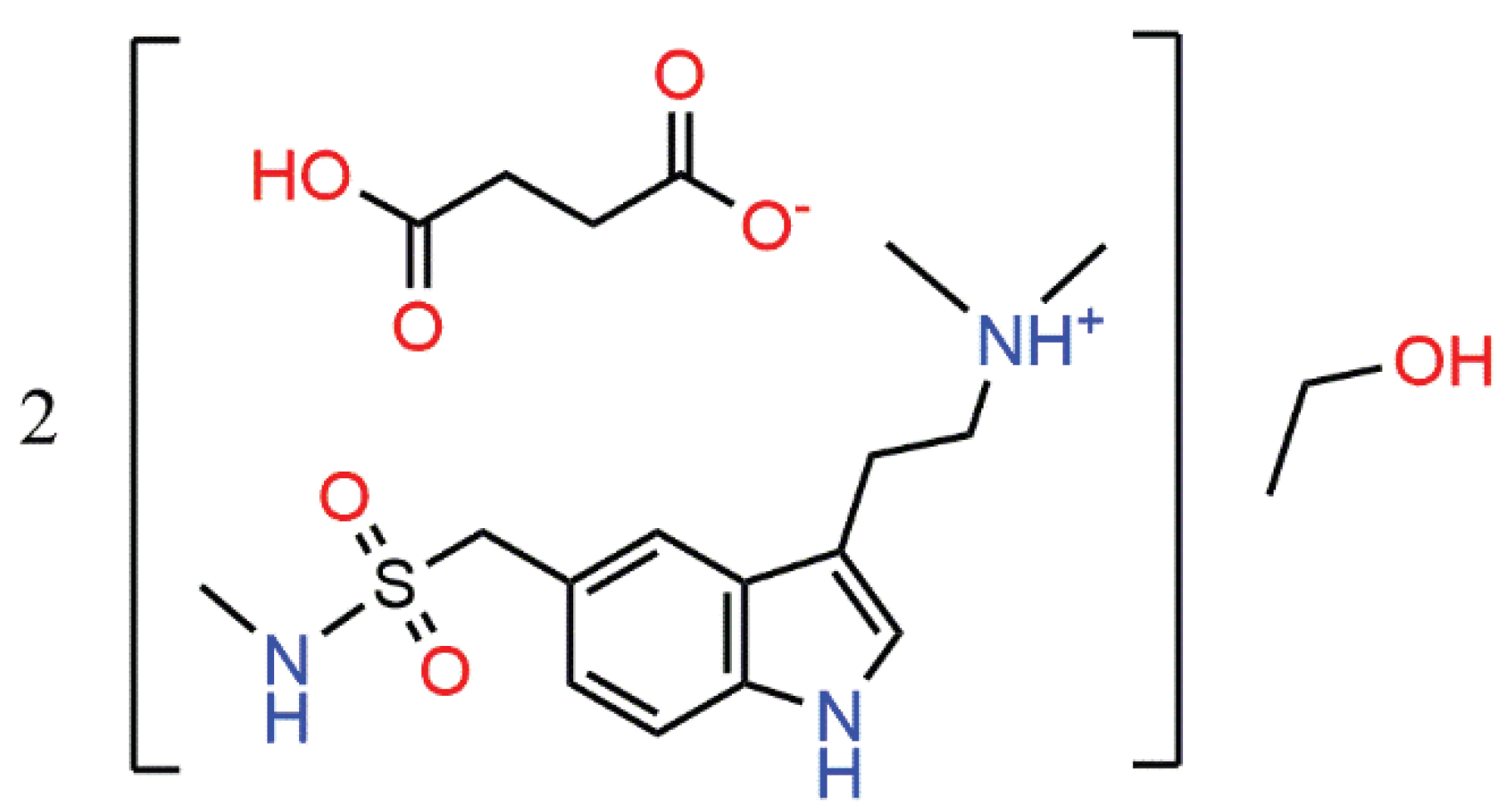
Sumatriptan succinate (HSum
+·HSucc
–) is the pharmaceutical active ingredient of commercially available drugs
Imitrex, Treximet and others used to treat migraine headaches and cluster headaches. In addition, its anti-inflammatory properties were also reported [
1]. The corresponding free base Sumatriptan (Sum) like all triptanes acts as serotonin 5-HT1B/5-HT1D receptor agonist [
2,
3]. During its metabolism Sum transform to glucuronide of indol-3-yl-acetic acid derivative via several steps [
4]. The crystal structures of both Sum and HSum
+·HSucc
– were published before [
5,
6].
Taking into account strong effect of solvent molecules on properties of solids, such as solubility, tabletability, stability and others, pharmaceutical industry is highly interested in crystal structures of all solid forms of active pharmaceutical ingredients which can occur upon drug production. This information is required for phase identification and purity control. In our study of novel solid forms of known active pharmaceutical ingredients [
7,
8,
9,
10], the ability of sumatriptan succinate to form various solvates was examined. Recrystallization from ethanol afforded hemisolvate, HSum
+·HSucc
–·0.5EtOH (
1). Herein we report on the molecular and crystal structures of
1,
Scheme 1.
2. Results and Discussion
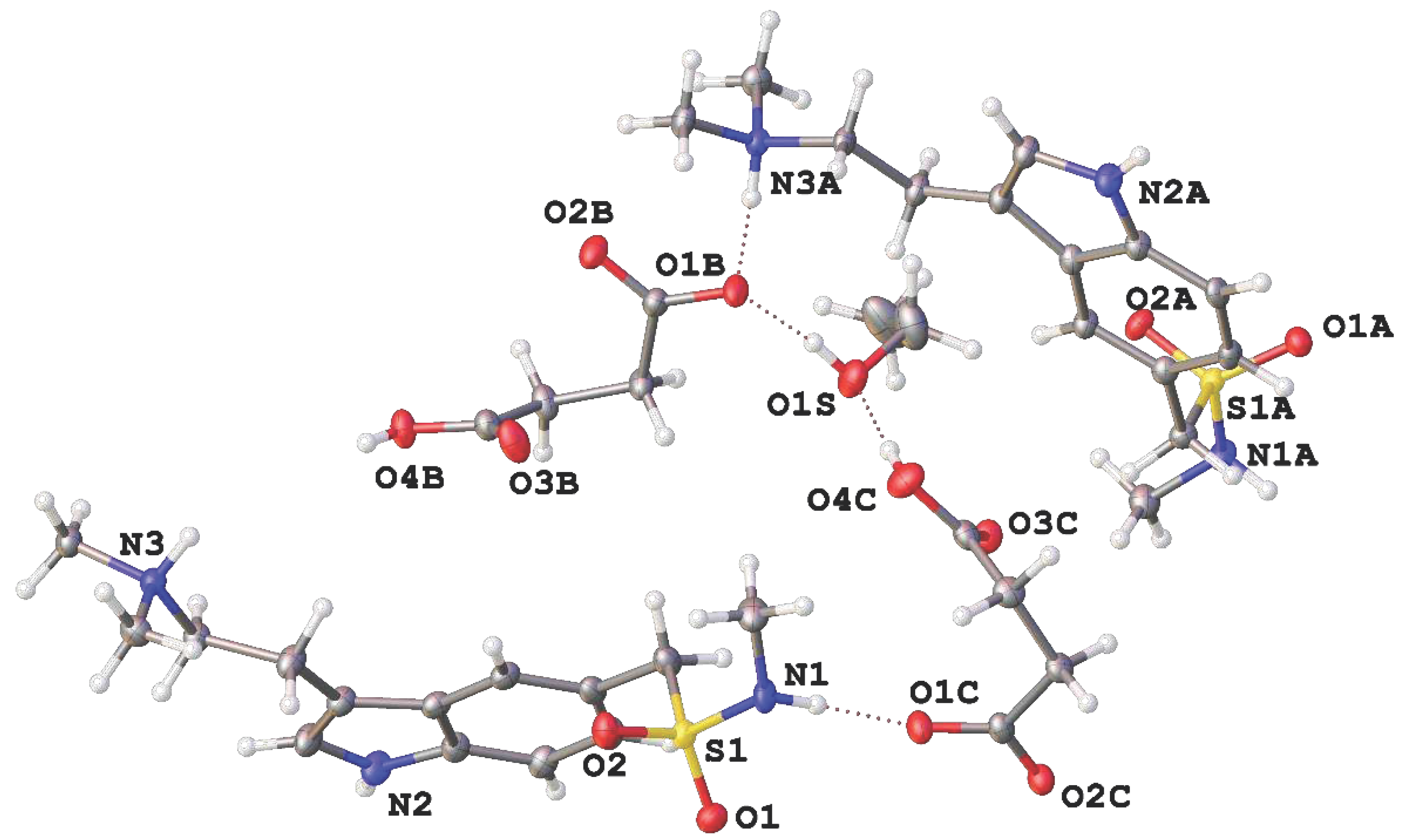
Sumatriptane succinate purchased from Sigma Aldrich was dissolved at ethanol without purification. After several days of standing on air at r.t. orange prismatic crystals precipitated. The precipitate was filtered off and studied using single-crystal X-ray diffraction. The asymmetric unit of
1 is represented on
Figure 1. It contains two cations, two anions, and one ethanol molecule. The positions of H(C), H(N) and H(O) can be easily revealed from difference Fourier maps. Thus, protonation of dimethylamine moiety of Sum and deprotonation of only one of two carboxylic groups of Succ was observed for all symmetrically independent species. Our conclusion about positions of hydrogen atoms is supported by interatomic and intermolecular distances. Particularly, C–O distances for deprotonated carboxylic groups vary from 1.238(4) to 1.274(4) Å. These values are intermediate between C=O and C–O(H) bond lengths for protonated groups in
1 equal to, respectively, 1.207(4) – 1.210(4) and 1.313(4) – 1.315(4) Å.
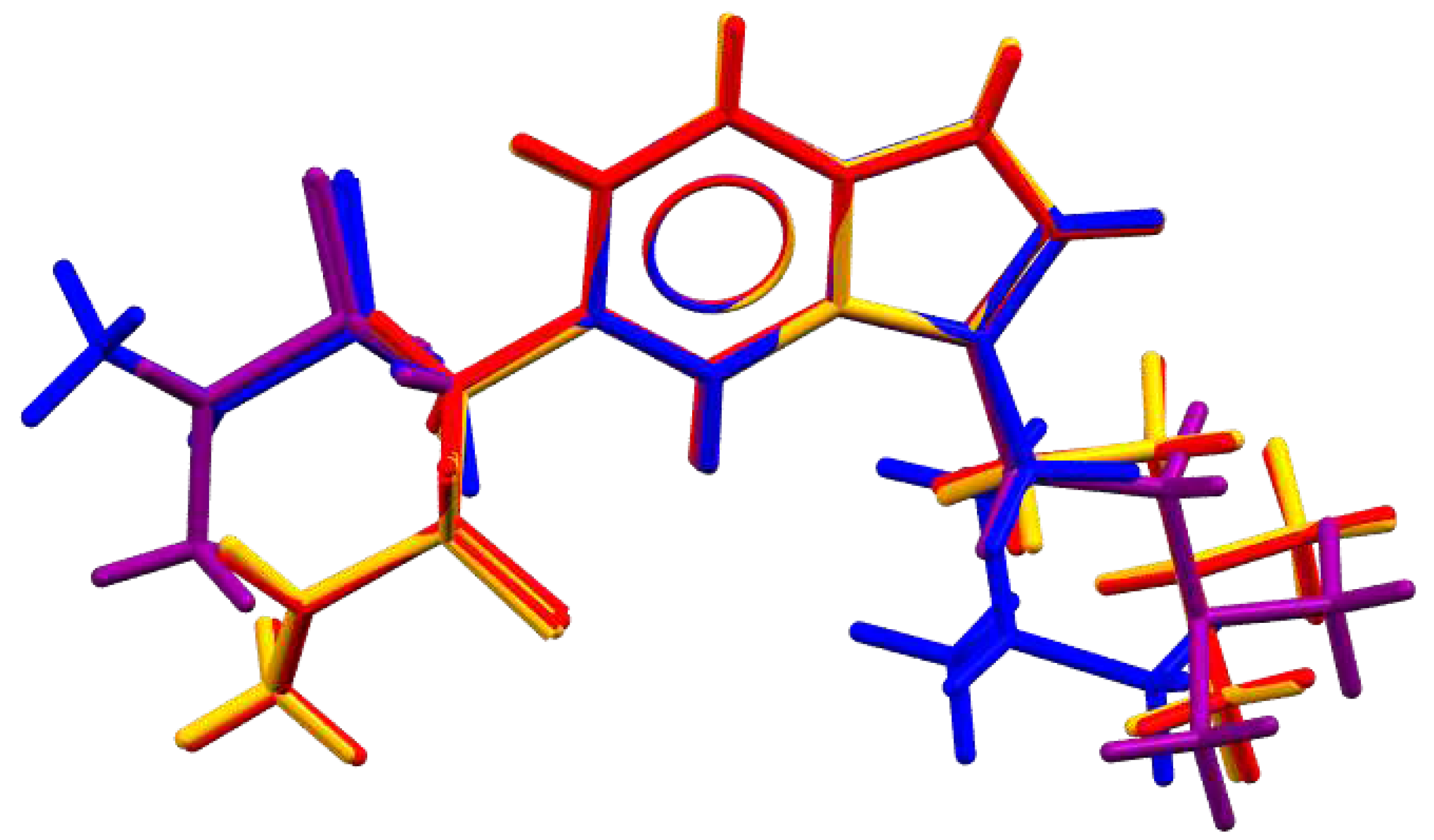
Molecular conformations of cations in
1 are nearly identical with average R.M.S.D for non-hydrogen atoms equal to 0.069 Å. On
Figure 2 Sum conformations in different solid forms are compared by superimposing of non-hydrogen atoms of the bicycle. It is clearly seen that rotation along single C–C, S–N and C–N groups is possible so that disposition of dimethylammonioethyl (dimethylaminoethyl) and N-methylmethanesulfonamide groups in all solids is different. Staggered conformation of succinate anions in two solvatomorphs is nearly equal: maximal deviation of non-hydrogen atoms is 0.639 Å only; the C–C–C–C torsion angle is c.a. 60°.
Different cation conformation should be associated with different H-bonded motifs. Both in Sum and in HSum
+·HSucc
– salts the number of H-bond donors and acceptors is inequivalent, thus, different functional groups compete each other to form the most stable H-bonding pattern. What is more, presence of a solvent molecule in this case is expected only if propensity of H-bond formation with this solvent is comparable or higher that propensity of H-bond formation between functional groups of main components [
11]. Propensities of H-bond formation for functional groups present in HSum
+·HSucc
– salts with and without ethanol were estimated using H-bond Propensities tool of Mercury package [
12] as described in Refs. [
13,
14]. The data obtained are listed in
Table 1.
The propensities evaluated indicate that in pure HSum
+·HSucc
– all donors take part in H-bonding with the most likely acceptors. Presence of ethanol molecule becames possible because it is as likely donor of H-bond as COOH and R
3NH groups. In HSum
+·HSucc
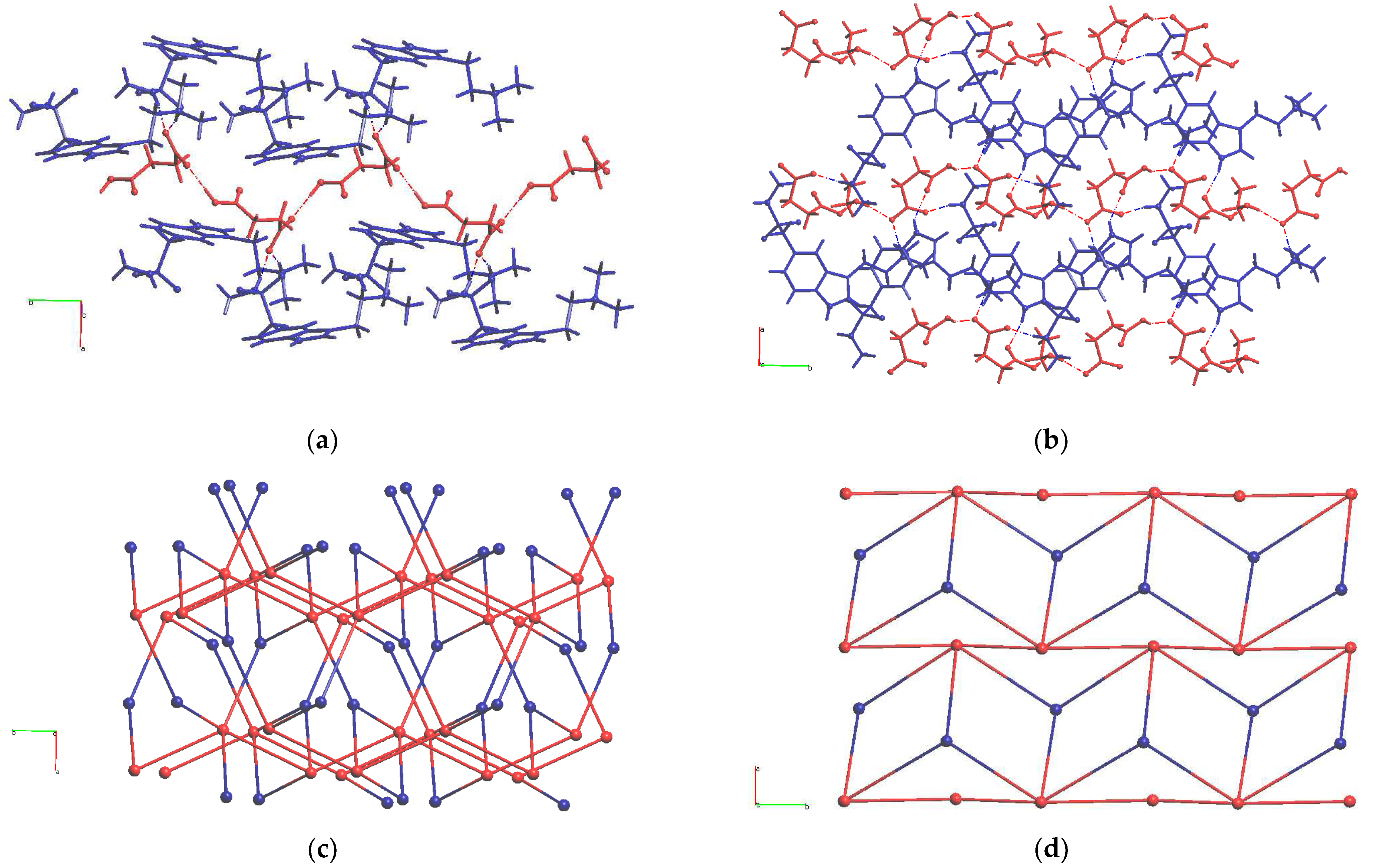
–·0.5EtOH two unlikely H-bonds are present, thus more stable polymorphs of this salt can exist. Fragments of experimentally obtained H-bonded networks in these two salts are compared in
Figure 3. Parameters of H-bonds in solid HSum
+·HSucc
–·0.5EtOH are listed in
Table 2.
Symmetry codes: (i) 1+x,y,z; (ii) x,-1+y,z; (iii) -1+x,y,z.
HSucc
– anions form infinite chains in HSum
+·HSucc
– [
6] in accord with the most likely H-bonds (red chains in
Figure 3a). In HSum
+·HSucc
–·0.5EtOH ethanol molecules act as linkers within similar chains (red chains in
Figure 3b). HSum
+ cations connect these chains into infinite frameworks and layers, respectively. In both solids, the cation acts as a three-connected node of H-bonded network, and the anion is a five-connected node. The resulting topologies of underlying 3,5-c binodal H-bonded nets in these compounds evaluated with the ToposPro package [
15] are, respectively, seh-3,5-P2
1/c and 3,5L24 (for notation of nets see Ref. [
16]).
To sum up, by recrystallization from ethanol we obtained a novel solid form of sumatriptan succinate used to treat migraine and cluster headaches. Co-crystallization with ethanol is in accord with the most likely H-bonds in a three-component mixture as estimated using H-bond propensity tool, because the propensities of OH…O2C and COOH…O2C bonds were found to be similar. In both solids the cations and anions act as three- and five-connected nodes, and ethanol molecules – as simple linkers between two anions. Nevertheless, presence of solvent molecules strongly affects overall H-bonding network. In pure HSum+·HSucc– 3D H-bonded framework is observed, while in HSum+·HSucc–·0.5EtOH 2D layers are found.
3. Materials and Methods
Fine powder of sumatriptane succinate (0.012 g, 0.0046 mmol) was dissolved in 3 ml of water-ethanol mixture. Single crystals were grown by slow evaporation. NMR spectra were obtained for 1H at 400 MHz, for 13C at 100 MHz and for 15N at 40 MHz, using Bruker AVANCE III WB 400 spectrometer (Bruker, Billerica, MA, USA). FTIR spectrum was recorded on an IR spectrometer with a Fourier transformer Shimadzu IRTracer100 (Kyoto, Japan) in the range of 4000–600 cm−1 at a resolution of 1 cm−1 (Nujol mull, KBr pellets).
3.1. X-Ray diffraction
The intensities of reflections were collected at Centre for Molecular Studies of INEOS RAS with Bruker D8 QUEST diffractometer at 100K (MoKα = 0.71072 Å, φ and ω-scans). The structure was solved by the dual-space algorithm [
17] and refined by full-matrix least squares against F
2 as two component inversion twin (SHELXL program [
18]) using OLEX2 package [
19], scale factors for two components are equal to 0.32(7) and 0.68(7), respectively. Non-hydrogen atoms were refined in an anisotropic approximation. Hydrogen atoms at carbon ones were calculated and included in the refinement with U
iso(H) = 1.2U
eq(C). Hydrogen atoms of N-H and O-H groups were located in difference Fourier maps and refined with unconstrained U
iso and fixed bond distances (0.88 and 0.85Å, respectively).
Crystal Data for C38H60N6O13S2 (M = 873.04 g/mol): monoclinic, space group Pc (no. 7), a = 9.834(9), b = 12.609(10), c = 16.946(16) Å, α = 90, β = 90.94(3), γ = 90°, V = 2101(3) Å3, Z = 2, µ = 0.198 mm–1, Dcalc = 1.380 g cm-3, F(000) = 932, 22256 reflections measured (4.0° ≤ 2Θ ≤ 61.8°), 10388 unique (Rint = 0.0602, Rsigma = 0.0763) which were used in all calculations. The final R1 was 0.0467 (I > 2σ(I)) and wR2 was 0.1188 (all data).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. crystallographic data in Crystallographic Information File (CIF) format..
Author Contributions
Conceptualization, A.A.K.; methodology, P.A.B.; investigation, A.V.V. and P.V.D.; writing—A.A.K. and A.V.V.; funding acquisition, A.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 20-13-00241.
Data Availability Statement
The X-ray data are available at CCDC under ref. code CCDC 2306805.
Acknowledgments
Ministry of Science and Higher Education of the Russian Federation is acknowledged for providing access to scientific literature.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ala, M.; Ghasemi, M.; Mohammad Jafari, R.; Dehpour, A.R. Beyond Its Anti-Migraine Properties, Sumatriptan Is an Anti-Inflammatory Agent: A Systematic Review. Drug Dev. Res. 2021, 82, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Sumatriptan/Naproxen Sodium: A Review in Migraine. Drugs 2016, 76, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Tfelt-Hansen, P.; De Vries, P.; Saxena, P.R. Triptans in Migraine. Drugs 2000, 60, 1259–1287. [Google Scholar] [CrossRef]
- Pöstges, T.; Lehr, M. Metabolism of Sumatriptan Revisited. Pharm. Res. Persp. 2023, 11, e01051. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, K.; Sridhar, B.; Krishnan, H. Sumatriptan, an Anti migraine Drug. Acta Cryst., Sect. E 2006, 62, o1086–o1088. [Google Scholar] [CrossRef]
- Ravikumar, K.; Swamy, G.Y.S.K.; Krishnan, H. α-{3-[2-(Di methyl ammonio) ethyl]-1H-Indol-5-Yl}-N-Methyl methanesulfon amide Succinate (Sumatriptan Succinate). Acta Cryst., Sect. E 2004, 60, o618–o620. [Google Scholar] [CrossRef]
- Goloveshkin, A.S.; Korlyukov, A.A.; Vologzhanina, A.V. Novel Polymorph of Favipiravir—An Antiviral Medication. Pharmaceutics 2021, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Korlyukov, A.A.; Dorovatovskii, P.V.; Vologzhanina, A.V. N-(4-Methyl-3-((4-(Pyridin-3-Yl)Pyrimidin-2-Yl)Amino)Phenyl)-4-((4-Methylpiperazin-1-Yl)Methyl)Benzamide. Molbank 2022, 2022, M1461. [Google Scholar] [CrossRef]
- Korlyukov, A.A.; Buikin, P.A.; Dorovatovskii, P.V.; Vologzhanina, A.V. Synthesis, NoSpherA2 Refinement, and Noncovalent Bonding of Abiraterone Bromide Monohydrate. Struct. Chem. 2023, 34, 1927–1934. [Google Scholar] [CrossRef]
- Buikin, P.; Vologzhanina, A.; Novikov, R.; Dorovatovskii, P.; Korlyukov, A. Abiraterone Acetate Complexes with Biometals: Synthesis, Characterization in Solid and Solution, and the Nature of Chemical Bonding. Pharmaceutics 2023, 15, 2180. [Google Scholar] [CrossRef] [PubMed]
- Delori, A.; Galek, P.T.A.; Pidcock, E.; Jones, W. Quantifying Homo- and Heteromolecular Hydrogen Bonds as a Guide for Adduct Formation. Chem. – Eur. J. 2012, 18, 6835–6846. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Galek, P.T.A.; Allen, F.H.; Fábián, L.; Feeder, N. Knowledge-Based H-Bond Prediction to Aid Experimental Polymorph Screening. CrystEngComm 2009, 11, 2634–2639. [Google Scholar] [CrossRef]
- Vologzhanina, A.V. Intermolecular Interactions in Functional Crystalline Materials: From Data to Knowledge. Crystals 2019, 9, 478. [Google Scholar] [CrossRef]
- Shevchenko, A.P.; Blatov, V.A. Simplify to Understand: How to Elucidate Crystal Structures? Struct. Chem. 2021, 32, 507–519. [Google Scholar] [CrossRef]
- Blatov, V.; O’Keeffe, M.; M. Proserpio, D. Vertex-, Face-, Point-, Schläfli-, and Delaney-Symbols in Nets, Polyhedra and Tilings: Recommended Terminology. CrystEngComm 2010, 12, 44–48. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Cryst., Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst., Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J. a. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).