Submitted:
12 December 2023
Posted:
12 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Principles and Methods
2.1. Principle of CO2 Absorption by NaOH Solution
2.2. CO2 Absorption Efficiency and Liquid-to-Gas Ratio
2.3. Ship CO2 Absorption Cycle System
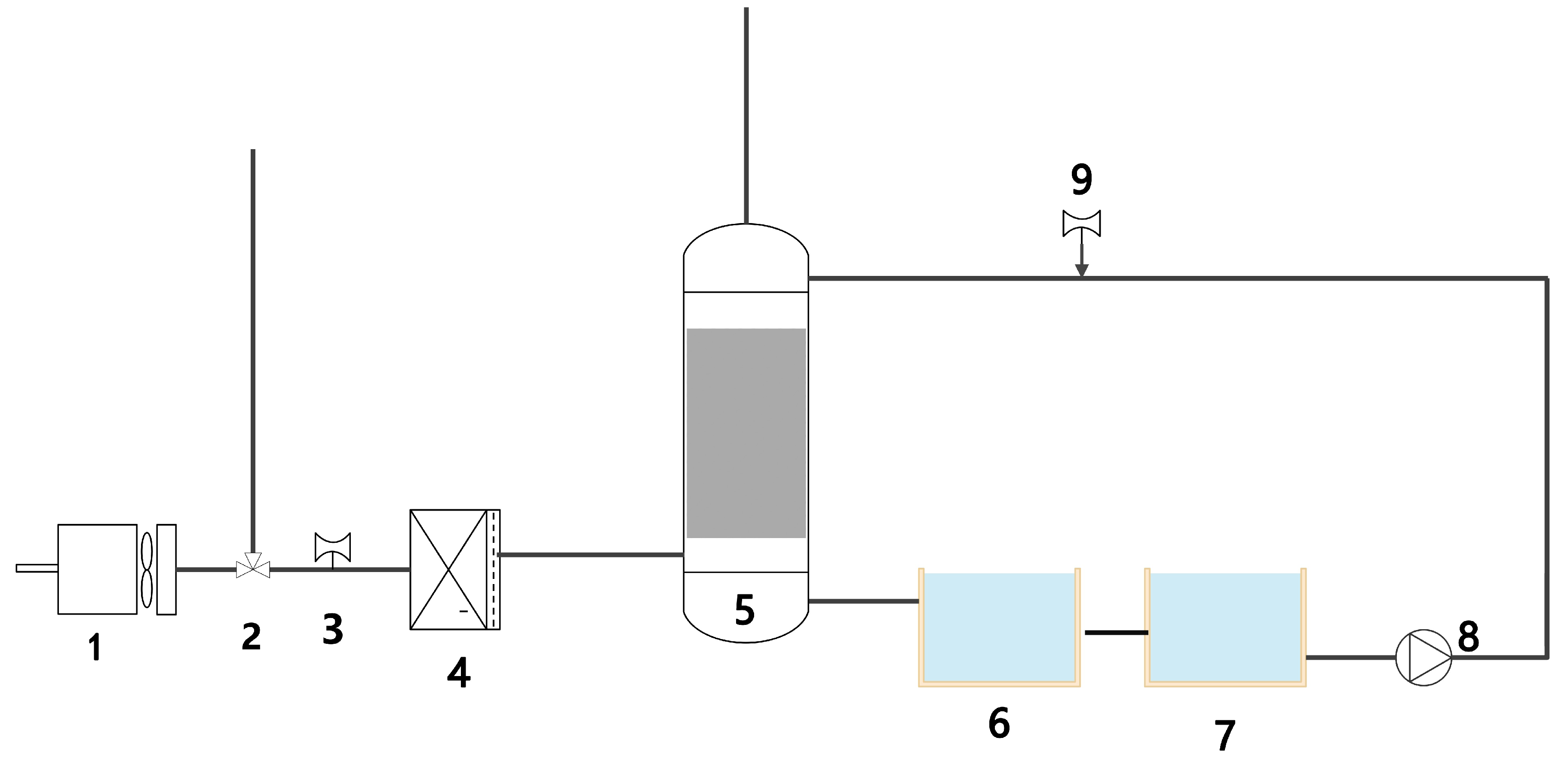
3. Experimental Equipment
3.1. Marine Diesel Engine

| Name | Value | Name | Value |
|---|---|---|---|
| Bore | 135mm | Lubricating Oil Temperature | 95℃ |
| Stroke | 140mm | Cooling Water Temperature | 60/95℃ |
| Compression Ratio | 16 | Exhaust Pipe Flange | 80/83mm |
| Piston Displacement | 12.9L | Exhaust temperature | <580℃ |
| Continuous Power/Speed | 146kw/ 1500r·min-1 | Temperature at 25% Rated Speed | 327℃ |
| 12-hour Power Fuel Consumption Rate | 225.8 g·(kw·h)-1 | Temperature at 50% Rated Speed | 390℃ |
| 12-hour Power Oil Consumption Rate | 1.65 g·(kw·h)-1 | Temperature at 75% Rated Speed | 450℃ |
| Average Piston Speed at Rated Speed | 7.5m·s-1 | Temperature at 100% Rated Speed | 535℃ |
| Firing Order | 1-5-3-6-2-4 | Starting Method | Electric Start |
| Cooling Method r | Water-cooled |
3.2. Testing Equipment

4. Design of Decarbonization Tower


5. Experimental Results and Analysis
| Gas flow rate/Nm³·h-1 | Liquid flow rate/ m³·h-1 | Liquid-to-gas ratio | absorption rate/% |
|---|---|---|---|
| 500 | 0.5 | 1 | 55.84 |
| 500 | 1 | 2 | 73.99 |
| 500 | 2 | 4 | 81.59 |
| 750 | 0.5 | 0.67 | 35.63 |
| 750 | 1 | 1.33 | 45.27 |
| 750 | 2 | 2.67 | 61.51 |
| 1000 | 0.5 | 0.5 | 25.61 |
| 1000 | 1 | 1 | 35.07 |
| 1000 | 2 | 2 | 53.85 |
5.1. Influence of Liquid-to-Gas Ratio on Decarbonization Efficiency
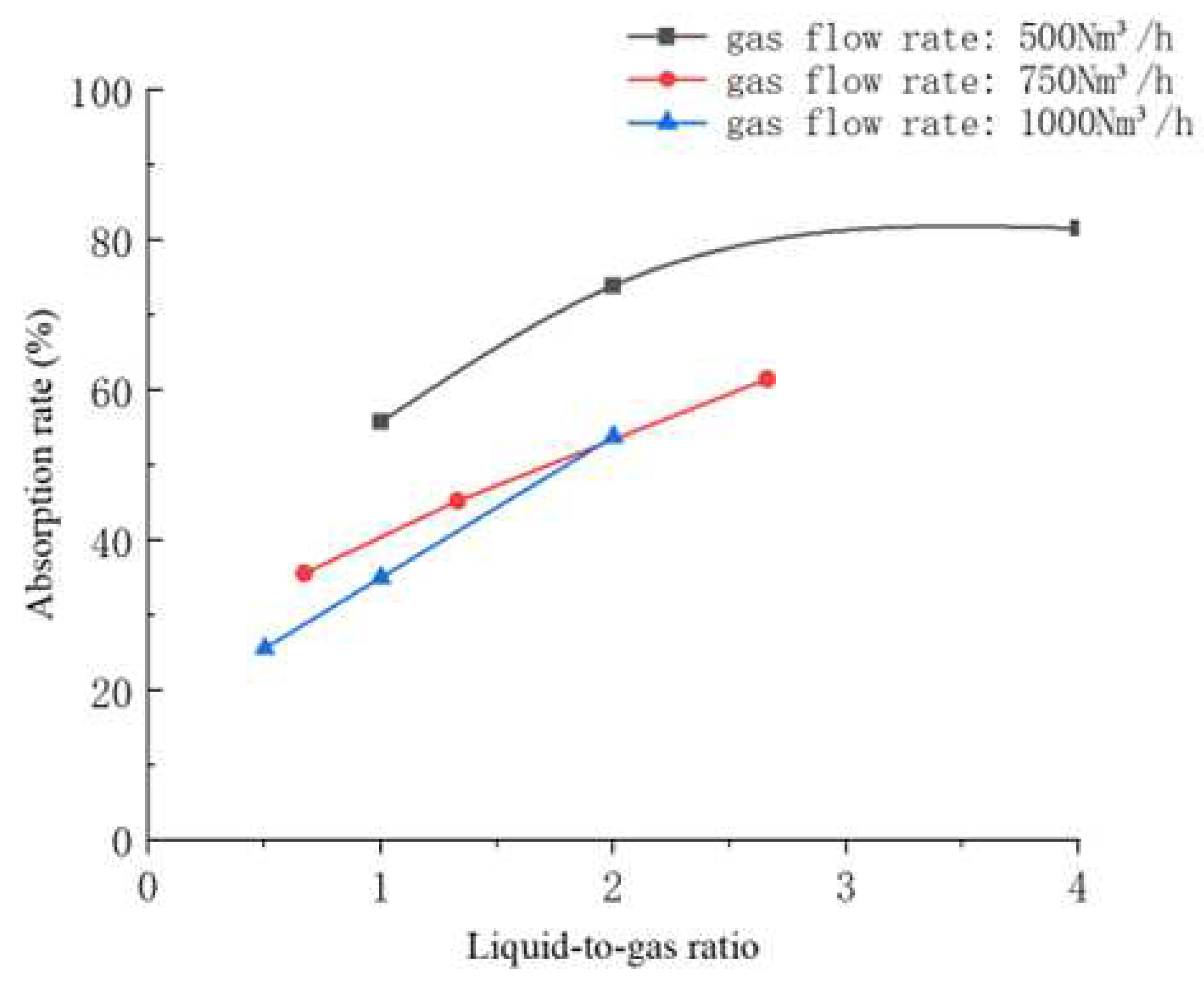
5.2. Influence of NaOH Concentration on Decarbonization Efficiency
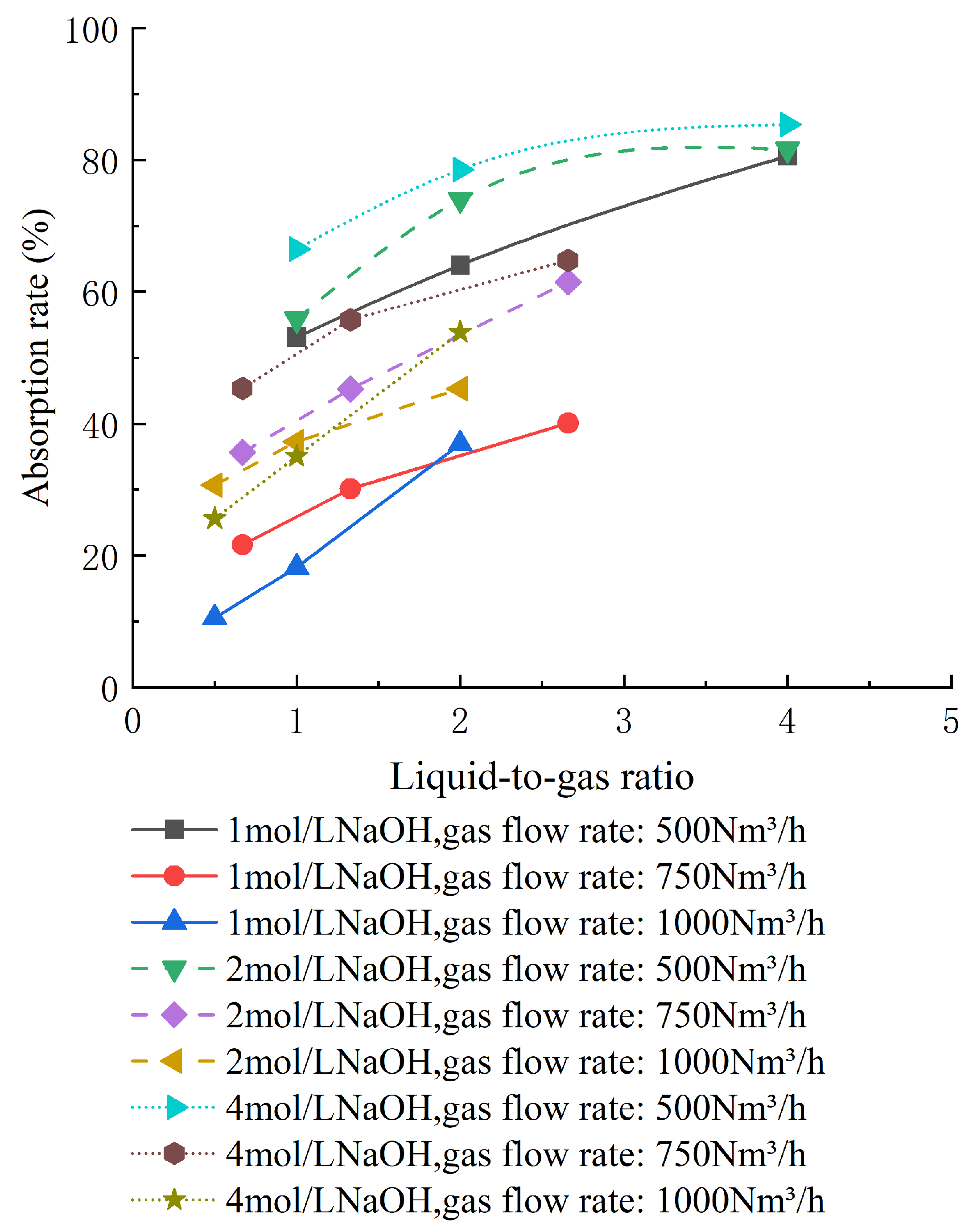
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- STRECK C,KEENLYSIDE P, VONUNGER M. The paris agreement: A new beginning [J]. J Eur Environ Plan L, 2016,13(1): 3-29. [CrossRef]
- International Energy Agency. CO2 emissions in 2022[R]. Paris: IEA, 2023.
- HASAN M F, FIRST E L, BOUKOUVALA F, et al. A multiscale framework for CO2 capture, utilization, and sequestration: CCUS and CCU [J]. Comput Chem Eng, 2015, 81: 2-21. [CrossRef]
- Marine Environment Protection Committee (MEPC),80th session,2023. Initial IMO Strategy on Reduction of GHG Emissions from Ships[S].
- Jingliang Zhang, Sulin Zhao, Rongxiang Zhao et al. Research on modern carbon dioxide absorption process[J]. Contemporary Chemical Industry,2011,40(01):88-91.
- Chung,S.J.et al. "Dual-Phase Metal Carbonate Membrane for High-Temperature Carbon Dioxide Separation." Industrial & Engineering Chemistry Research 44 (2005): 7999-8006. [CrossRef]
- Dinda S .Development of solid adsorbent for carbon dioxide capture from flue gas[J].Separation and Purification Technology,2013,10964-71. [CrossRef]
- TONG Siqi, JIAN Weiwei, HAI Qiuyan, XIE Weixin, SUN Yi. Progress of CO2 adsorption on porous solid materials[J]. Journal of Liaoning University of Petrochemical Technology, 2022, 42(2): 30-37.
- Lei Ting, Yu Shunan, Zhou Changan et al. Research progress on molding technology of solid amine adsorbent for carbon capture by adsorption[J].Chemical Prgress 2022,41(12):62136225.DOI:10.16085/j.issn.1000-6613.2021-2238. [CrossRef]
- YU Hang,MENG Hong,YANG Xiangfu et al. Research progress of carbon-based carbon dioxide adsorbent materials[J]. Clean Coal Technology,2023,29(11):35-48.DOI:10.13226/j.issn.1006-6772.22081602. [CrossRef]
- Bae T ,Hudson R M ,Mason A J , et al.Evaluation of cation-exchanged zeolite adsorbents for post-combustion carbon dioxide capture[J].Energy environmental science: EES,2013,6(1):128-138.
- KONG Lingcong,SUN Yarong,XIE Yu et al. Progress and application of carbon dioxide capture technology by metal-organic framework materials[J]. Xinjiang Oil and Gas,2022,18(02):78-83. [CrossRef]
- Song T ,Zhao H ,Hu Y , et al.Facile assembly of mesoporous silica nanoparticles with hierarchical pore structure for CO2 capture[J].Chinese Chemical Letters,2019,30(12):2347-2350. [CrossRef]
- Vijaya T ,Viswateja K ,Spandana G , et al.Technoeconomic Investigation of Amine-Grafted Zeolites and Their Kinetics for COsub2/sub Capture.[J].ACS omega,2021,6(9):6153-6162. [CrossRef]
- WU Qiang, GAO Ming, ZHANG Gang et al. Preparation of biomass-based carbon materials with different morphologies and their application properties by hydrothermal/soft template method J]. Nanotechnology, 2019,30(18): 185702. [CrossRef]
- Zhang, Xueshi, Liu, Xinmin. Preparation and Performance of Modified Molecular Sieve for Carbon Dioxide Capture [J]. Techniques and Equipment for Environmental Pollution Control, 2015(10): 4995-4999. (in Chinese). [CrossRef]
- Zhang, Xueshi. Preparation of Amine Functional Porous Materials and Adsorption Properties for CO2 [D].Qingdao: Qingdao University of Science & Technology, 2015: 51-55. (in Chinese).
- Irani M, Gasem KA, Dutcher B, et al. CO2 captureusing nanoporous TiO(OH)2 /tetraethylenepentamine[J].Fuel,2016(183):601-608. [CrossRef]
- Cecilia J,Vilarrasa-García E,García-Sancho C, et al. Functionalization of hollow silica microspheres by impregnation or grafted of amine groups for the CO2 capture[J]. International Journal of Greenhouse Gas Control,2016,52. [CrossRef]
- Xu N ,Li X ,Franks A M , et al.Silver-molten carbonate composite as a new high-flux membrane for electrochemical separation of CO2 from flue gas[J].Journal of Membrane Science,2012,401-402190-194. [CrossRef]
- Ghezel-Ayagh H ,Jolly S ,Patel D , et al.Electrochemical Membrane Technology for Carbon Dioxide Capture from Flue Gas[J].Energy Procedia,2017,1082-9. [CrossRef]
- Electrochemical Membrane Technology for Carbon Dioxide Capturefrom Flue Gas[J].ECS Meeting Abstracts,2016. [CrossRef]
- Zhang N ,Pan Z ,Zhang Z , et al.CO2 capture from coalbed methane using membranes: a review[J].Environmental Chemistry Letters,2020,18(8):79-96. [CrossRef]
- Li X.C. Research on flue gas decarbonization technology of coal-fired power plant [J]. Clean Coal Technology,2009,15(03):6266. [CrossRef]
- Wang Hongbo. Research on carbon dioxide absorption by organic amine method[D]. Shanghai Normal University,2012.
- Park S W,Song K,Jo H. Laboratory-Scale Experiment on a Novel Mineralization-Based Method of CO2 Capture Using Alkaline Solution[J].Energy,2017,124:589598. [CrossRef]
- Chiang C Y,Lee D W,Liu H S. Carbon Dioxide Capture by Sodium Hydroxide-Glycerol Aqueous Solution in a Rotating Packed Bed[J]. Journal of the Taiwan Institute of Chemical Engineers,2017,72:29-36. [CrossRef]
- SHEN Heming,WU Canbin,LI Zhihua et al. Functional study on carbon sequestration of calcium hydroxide-the effect of CO2 concentration and carbonization time[J]. Functional Materials,2020,51(01):1115-1119. [CrossRef]
- Wang Z C, Liu X Y, Zhou P L, et al..Impacts of CaO Solid Particles in Carbon Dioxide Absorption Process from Ship Emission with NaOH Solution[J].Journal of Shanghai Jiaotong University(Science),2018,23(02):320-326. [CrossRef]
- Johny N,Murali T,Mathew M P, et al. Experiment on carbon dioxide removal from flue gas[J]. Materials Today: Proceedings,2019,11(Pt 3). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





