1. Introduction
There are over 900,000 cases of head and neck cancer diagnosed worldwide each year [
1]. Approximately 75% of all head and neck cancer patients are treated with radiotherapy (RT) primarily or combined with surgery and/or chemotherapy [
2]. The oral cavity submucosa contains minor salivary glands which provide lubrication and moisture for the oral cavity [
3]. The oral cavity mucosa contains taste buds which are necessary for the sensation of taste [
4]. Radiotherapy to the oral cavity is associated with acute adverse events including painful oral mucositis, dysgeusia, sticky salivary secretions, dysphagia, and odynophagia [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14]. These adverse events result in secondary use of opioid pain medications, dehydration, weight loss, malnutrition, use of intravenous fluids, feeding tubes, medications to thin salivary secretions, and hospitalization [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14]. The acute toxicity can be severe or worse in 1-81 % of patients depending on the outcome measured and intensity of the treatment [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14]. Late adverse events include opioid pain medication dependency, mucosal infections, dysphagia, dysgeusia, altered diet with poor nutrition, food poisoning, sticky secretions, xerostomia, dental caries and extractions, osteoradionecrosis, stress, anxiety, anorexia, and depression [
5,
6,
9,
11,
14,
15,
16]. Late toxicity can be severe or worse in 1-54% of patients depending on the outcome measured and intensity of the treatment [
5,
6,
9,
11,
14,
15,
16]. These adverse events are detrimental to the patient’s quality of life and function and are costly to manage [
5,
6,
9,
11,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23].
A variety of oral cavity organ at risk (OAR) avoidance structures have been defined to aid in RT treatment planning (
Table S1), [
24,
25,
26,
27,
28,
29]. The treatment goal is to limit the volume of the oral cavity OAR avoidance structure exposed to RT dose to reduce the incidence and severity of acute and late adverse events. Currently there is no consensus on how to define the oral cavity OAR avoidance structure (
Table S1). Likewise, there is limited data available describing the association between RT dose, volume of oral cavity exposed to RT and the incidence and severity of acute and late adverse events [
24,
27,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54]. The goal of this study was to define an oral cavity OAR avoidance structure and to determine if there is a dose and volume relationship for the oral cavity OAR avoidance structure as defined which would be predictive of acute and late adverse events, and which would be useful for RT treatment planning. We found that the mean dose to the defined oral cavity OAR avoidance structure was significantly associated with opioid use and hospitalization during and within 30 days of completing RT.
2. Materials and Methods
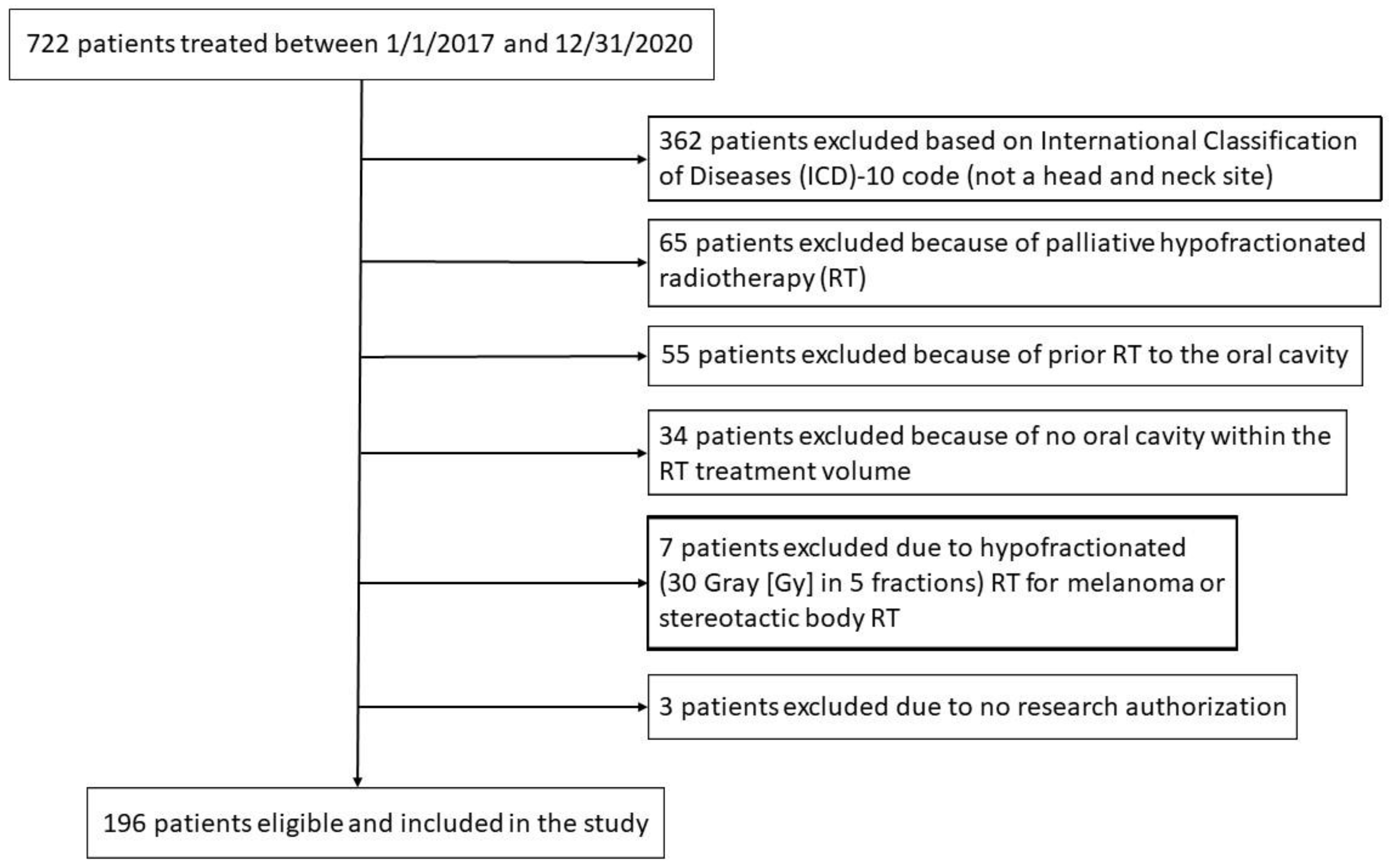
This retrospective review was approved by the Mayo Clinic Institutional Review Board (IRB #20-004915, June 17, 2020). Inclusion criteria included all patients with benign or malignant tumors within the head and neck region receiving RT to the oral cavity by one of the authors (RLF) between 1/1/2017 and 12/31/2020. The Mayo Clinic Rochester Department of Radiation Oncology Patient Outcomes Database (IRB #15-00136, March 15, 2015) was searched electronically by provider (RLF), date (1/1/2017 to 12/31/2020) and International Classification of Diseases (ICD)-10 codes to identify eligible patients.
Table S2 provides the ICD-10 codes included in the search. Exclusion criteria included a palliative course of hypofractionated RT, prior RT to the oral cavity, no oral cavity OAR within the RT treatment volume (no dose to the oral cavity), hypofractionated RT for melanoma, hypofractionated stereotactic body RT, and no consent provided to use medical records for research purposes as provided either by Department of Radiation Oncology Registry (IRB #15-000136, March 15, 2015) or by Minnesota state statute.
Figure 1 is a Consolidated Standards of Reporting Trials (CONSORT) diagram for patient inclusion and exclusion.
Presence of diabetes mellitus, hypertension, and leukoplakia prior to RT, use of concurrent chemotherapy, smoking status at the time of treatment, use of percutaneous endoscopic gastrostomy (PEG) tube, and weights were collected using a combination of chart review and data extraction from Mayo Clinic’s Unified Data Platform (UDP) which is a data warehouse for current and historical medical records. For diabetes mellitus, hypertension, and leukoplakia, diagnosis codes were retrieved from medical records. If the diagnosis date was before the RT end date, then the patient was considered to have had a co-morbidity during RT. Whether a patient received concurrent chemotherapy was collected from treatment summary notes. The medication administration database for specific drugs was searched to identify if the chemotherapy was cytotoxic therapy, hormonal therapy or immunotherapy, and to identify patients prescribed opioid pain medications 30 days prior to the start of RT, during RT, and up to 30 days after the completion of RT. Smoking status closest to the RT start date was extracted, and patients were placed into one of two groups: never smokers and former or current smokers. PEG tube insertion procedure codes were searched to identify patients who utilized a PEG tube after the onset of RT (2 patients with PEG tubes were excluded because the PEG tube placement was unrelated to the malignancy or the treatment). Patient weights were also collected from the UDP and evaluated at baseline prior to RT, post RT, and 3 months, 6 months, 12 months, and 24 months following the completion of RT. Glossectomy status was determined by ICD-10 code (C01 or C02) and treatment (postoperative RT). Hospitalization during and up to 30 days after the completion of RT was recorded from the Department of Radiation Oncology Hospitalization Dashboard.
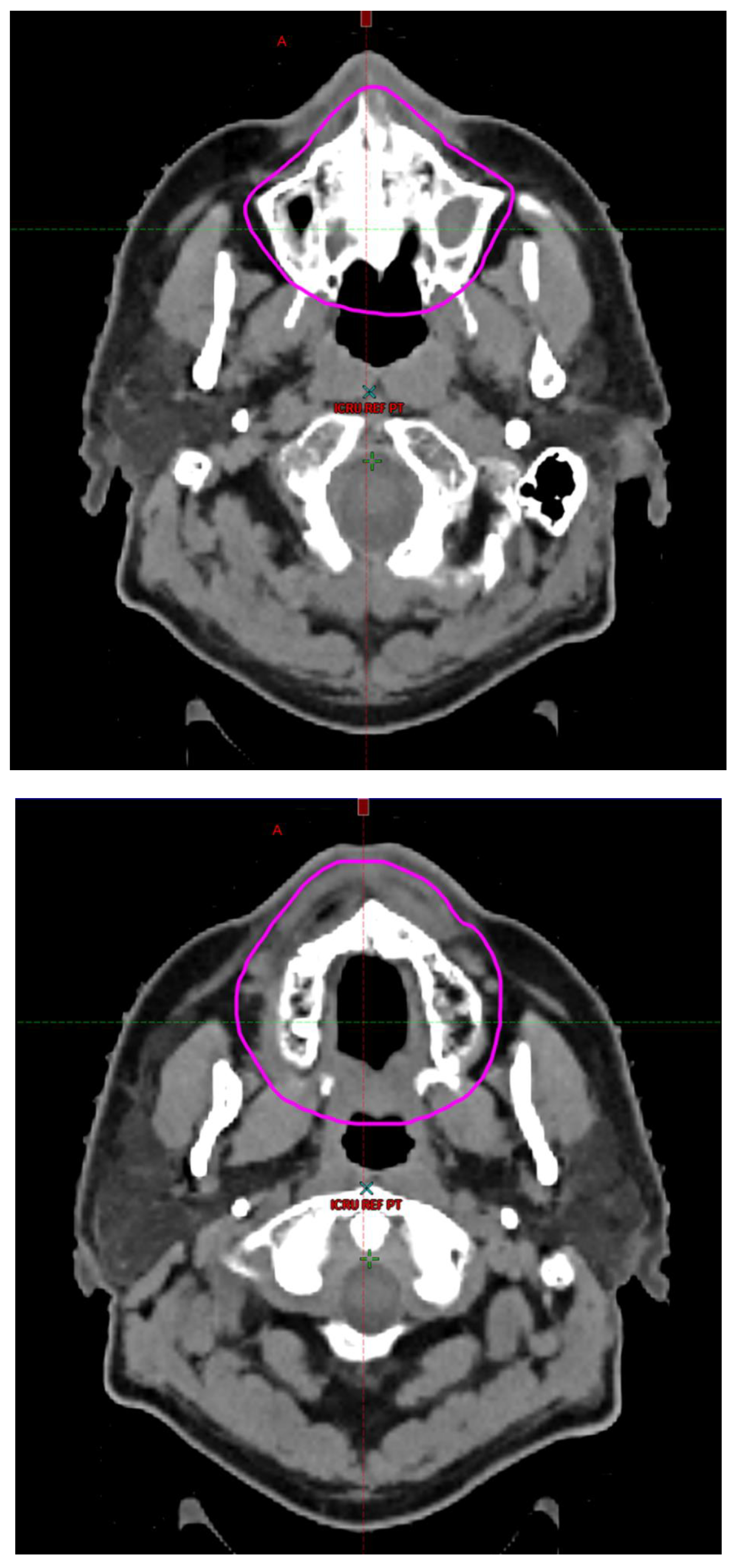
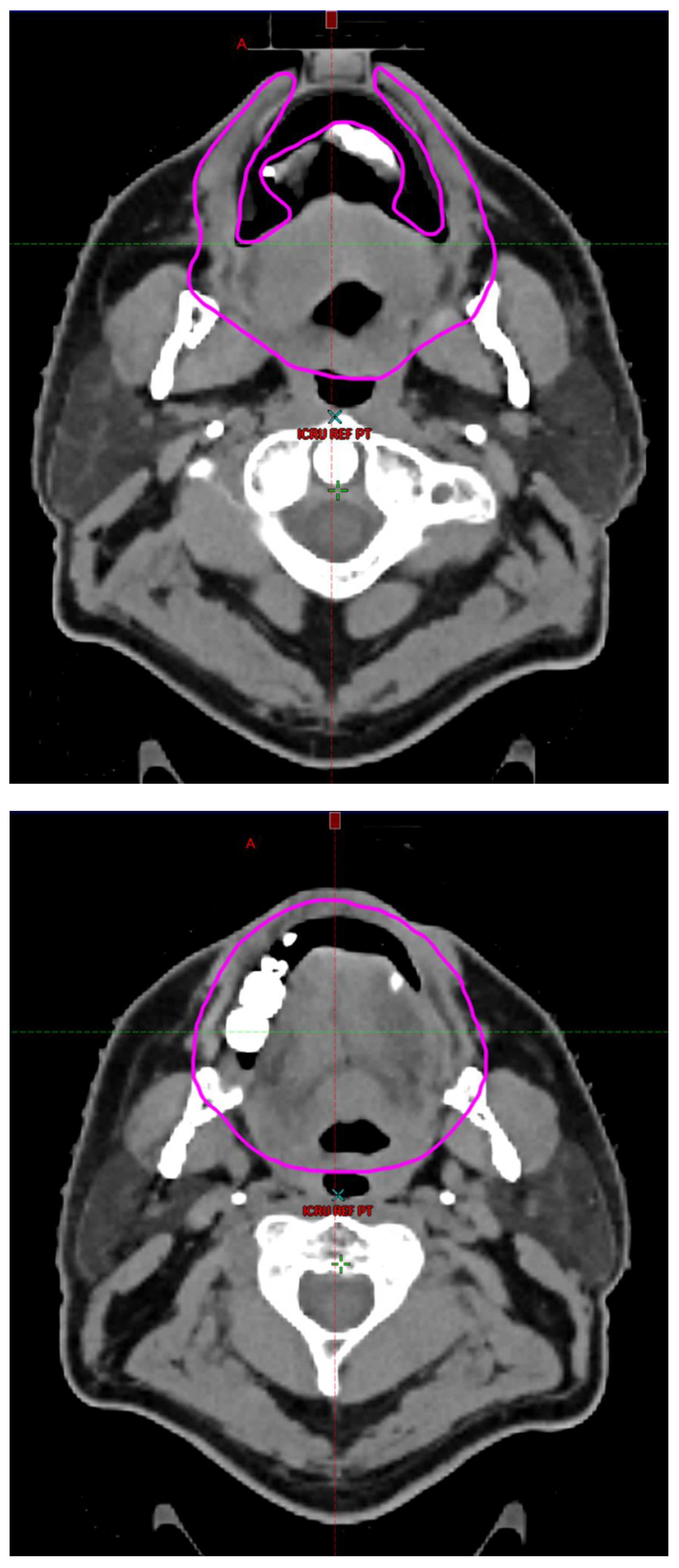
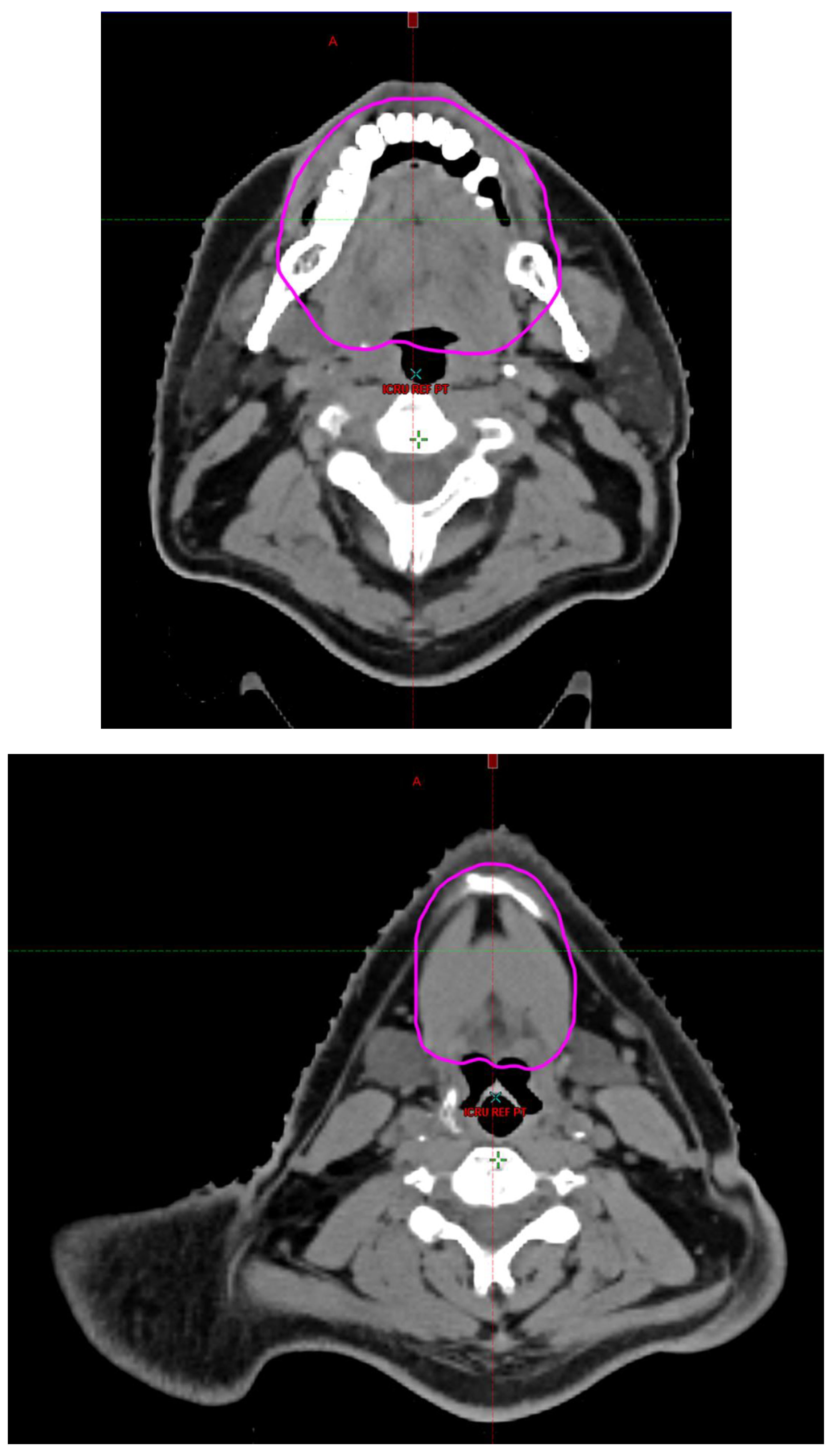
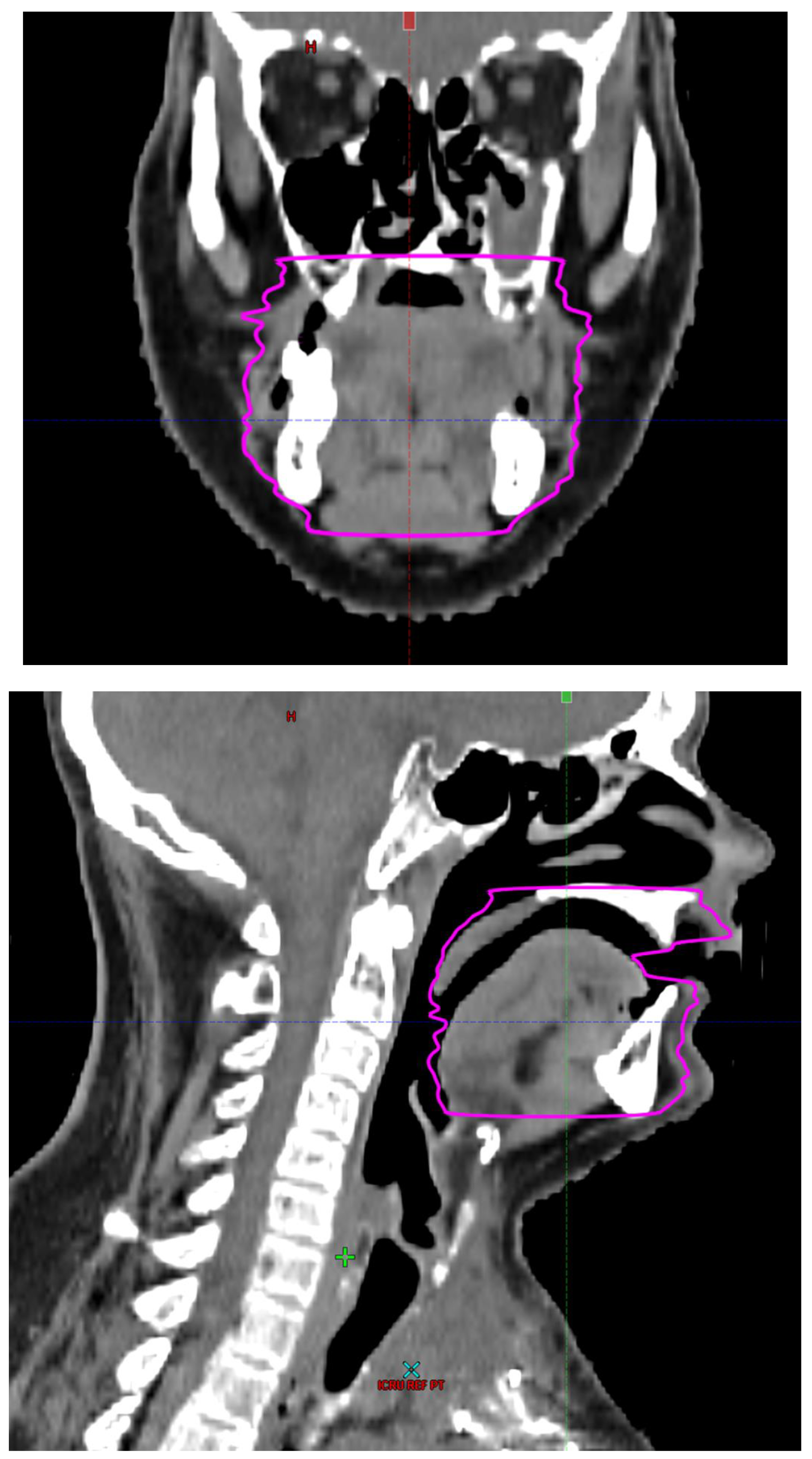
Radiotherapy treatment plans were created, and dose volume histograms (DVH) were generated using the Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA). The standard photon treatment plans consisted of three volumetric modulated arc therapy fields. The standard proton treatment plans consisted of three to four fields with the pencil beam scanning technique. For proton therapy plans, the RT dose was scaled by 1.1 to account for the difference in relative biological effect when compared to conventional photon treatments. A definition for oral cavity OAR avoidance structure evolved at our institution between 2003 and 2016 when it was formalized and accepted as the consensus standard for treatment planning (
Figure 2). The DVH statistics for the oral cavity OAR, and the mean dose to the left and right submandibular glands, left and right parotid glands and total parotid glands were calculated and extracted. Pharyngeal constrictor, intrinsic and extrinsic muscles of the tongue, and laryngeal DVH statistics were not included since swallowing function is not a primary endpoint for this study. There were four patients who did not complete the planned course of treatment. In these cases, the prescribed dose was scaled to the delivered dose.
The superior extent of the oral cavity OAR avoidance structure includes all the mucosa of the hard palate. The contouring of the OAR begins superiorly at the first sign of mucosa on the alveolar ridge of the maxilla (medial and lateral) and hard palate. It then continues inferiorly to include the mucosa of the upper and lower lip, mucosa of the hard and soft palate including the uvula, the buccal mucosa including the buccinator muscles, the mucosa of the retromolar trigone, the entire tongue (anterior two-thirds, dorsal surface and tongue base), floor of mouth, sublingual glands, gingival mucosa of the mandible (lingual and buccal surfaces), and ending at the level of the cranial edge of the hyoid bone and caudal edge of the mandible. It also includes the maxillary and mandibular teeth if present. The posterior extent includes the soft palate, uvula, and tongue base. The anterior extent includes the mucosal surface of the posterior one-half of the lips and the gingival mucosa of the maxillary and mandibular alveolar ridges and retromolar trigone. The lateral extent includes the buccal mucosa and buccinator muscles. The oral cavity OAR contains most of the taste buds which are located within the mucosa of the anterior two-thirds of the tongue, the floor of mouth, the buccal mucosa, the lips, the pharynx (including the soft palate, uvula, and base of tongue), the larynx (epiglottis) and upper third of the esophagus [
4]. The oral cavity OAR also contains the minor salivary glands located within buccal, labial, lingual, soft palate, lateral parts of the hard palate, and floor of the mouth submucosa and in the trough circling the circumvallate papillae on the dorsal surface of the tongue near the terminal sulcus [
3]. Therefore, the oral cavity OAR for radiotherapy (RT) treatment planning purposes is defined as including the anterior two-thirds of the tongue, floor of mouth, buccal mucosa, mucosal surface of the lips, soft palate, uvula, base of tongue, hard palate and circumvallate papillae on the dorsal surface of the tongue. The gingival mucosa of the alveolar ridges of the mandible and maxilla and the mucosa of the retromolar trigone are also included in the definition to further reduce the incidence and severity of painful oral mucositis. Finally, the sublingual glands are included in the oral cavity OAR structure. The larynx (epiglottis) and upper third of the esophagus are not included in this OAR volume because they are included in their own OAR avoidance structure (larynx, cricopharyngeal inlet, cervical esophagus).
Provider reported adverse events using Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03 (Published: June 14, 2010. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute), and patient reported outcomes using the European Organisation For Research and Treatment Of Cancer Quality of Life Questionnaire Head and Neck (EORTC QLQ-H&N35) and the Patient-Reported Outcomes Measurement Information System (PROMIS) Global-10 were collected prospectively at baseline prior to RT, at completion of RT, and at 3 months, 6 months, 12 months, and annually thereafter following completion of RT [
55,
56]. Hospitalization and the start of opioid pain medications during and up to 30 days after the completion of RT was also recorded. Patients prescribed opioid pain medications within 30 days of starting RT were presumed to be taking opioid pain medications at the start of RT and were excluded from the analysis of this endpoint. Patients were censored at last follow-up if alive, at death, or when a second course of RT was administered to the oral cavity. The primary endpoints of interest were the incidence of CTCAE v4.03
>grade 2 oral pain, salivary duct inflammation, dysgeusia, dehydration and dry mouth; EORTC QLQ-H&N35 subscales for senses, saliva, speech, social eating with
>10 point change, and scores for pain, pain medication, feeding tube use, and weight loss; PROMIS Global-10 physical and mental health subscale T-scores with
>10 point change, measured weight loss
>10% at end of treatment, 3 months, 6 months, 12 months, and 24 months following completion of RT; start of opioid pain medication use during and up to 30 days after the completion of RT, and hospitalization during and up to 30 days after the completion of RT. Secondary endpoints of interest were CTCAE v4.03
>grade 2 dysphagia and EORTC QLQ-H&N35 subscales for swallow with
>10-point change.
Patients presenting with baseline pre-RT CTCAE v4.03 adverse events secondary to surgery, malignancy, chemotherapy, medications, or co-morbid illnesses were excluded from the specific adverse event analysis. This included 2 patients with baseline CTCAE v4.03 >grade 1 dehydration, 3 patients with >grade 2 dry mouth, 5 patients with >grade 2 dysgeusia, 11 patients with >grade 2 dysphagia, 5 patients with >grade 2 oral pain and 4 patients with >grade 2 salivary inflammation.
CTCAE v4.03 adverse events at specified time points with insufficient
>grade 2 events to run logistical models predicting
>grade 2 toxicity were excluded from univariate logistic modeling. A decrease in the PROMIS Global-10 physical health T-score or mental health T-score of
>10 points is considered clinically significant [
57]. A decrease in the EORTC QLQ-H&N35 subscale score
>10 points is considered clinically significant [
58]. Variables included in the univariate analysis included age, sex, ever smoker, diabetes mellitus, hypertension, cytotoxic chemotherapy, oral cavity OAR D
max, mean dose, and V10Gy-V70Gy in 10 Gy increments, mean right, left and total parotid gland dose, mean right and left submandibular gland dose, RT modality, and glossectomy. For the multivariable logistic models, 14 outcomes having at least 30 events (or at least 30 non-events if the number of non-events is fewer than events) and having a univariate p-value of <0.05 were included in full and parsimonious multivariable modeling.
The data analysis plan was to evaluate DVH statistics for the oral cavity OAR avoidance structure (Dmax [maximum dose to 0.01 cc], mean dose, and V10Gy to V70Gy in 10 Gy increments where VxGy is the volume of oral cavity OAR receiving xGy or more) to determine if there is a statistically significant association with provider reported adverse events (CTCAE v4.03), patient reported outcomes (EORTC QLQ-H&N35 subscales and PROMIS Global-10 physical and mental health T-scores), start of opioid pain medications during or within 30 days of completing RT, and/or with hospitalization during or within 30 days of completing RT at specified times.
Logistic regression models were used to identify predictive clinical parameters for the endpoints of this study. Endpoints included provider reported adverse events using CTCAE v4.03 >grade 2 oral pain, salivary duct inflammation, dysgeusia, dehydration, dysphagia, and dry mouth; patient reported outcomes using EORTC QLQ-H&N35 subscales for swallow, senses, speech, social eating, saliva with >10 point change, and scores for pain, pain medication, feeding tube use, and weight loss; PROMIS Global-10 T-scores for physical and mental health with >10 point change; measured weight loss, at end of treatment, 3 months, 6 months, 12 months and 24 months following completion of RT, starting opioid pain medication during and up to 30 days after the completion of RT, and hospitalization during and up to 30 days after completion of RT. A multivariable logistic model was identified for each outcome including those predictors deemed clinically relevant. A parsimonious model was identified retaining those variables with statistical significance of <0.05. The alpha level was set at 0.05 for statistical significance. All analyses were completed using SAS version 9.4. Pearson correlations were used to evaluate the correlation between oral cavity OAR DVH statistics. DVH statistics were compared between RT modalities using a two-sample T test. For DVH parameters, the total oral cavity OAR avoidance structure volume was evaluated for correlation with provider reported adverse events (CTCAE v4.03), patient reported outcomes (EORTC QLQ-H&N35 subscales, PROMIS Global-10 subscales), opioid pain medication use, and hospitalization. Sensitivity and specificity estimates from a receiver operating characteristic curve were used to identify cut-points to achieve at least 90%, 80%, and 70% sensitivity and maximizing the subsequent specificity.
3. Results
Table 1 summarizes the patient, tumor, and treatment characteristics for the 196 patients included. The study group included 142 males (72.4%). The median age was 63.0 years (IQR 55.1, 72.5).
3.1. Univariable analysis
The univariable analysis revealed the oral cavity OAR DVH statistics were associated with the following CTCAE v4.03 adverse event endpoints: dehydration >grade 1 at end of treatment; dry mouth >grade 1 at end of treatment, 3 months, 6 months, 12 months and 24 months; dysgeusia >grade 1 at end of treatment and 3 months; dysphagia >grade 1 at end of treatment, 3 months, 6 months, 12 months and 24 months; oral pain >grade 1 at end of treatment; salivary duct inflammation >grade 1 at end of treatment and 3 months; and weight loss
>10% at end of treatment, 3 months, 6 months, 12 months and 24 months (see
Table S3 for odds ratios and 95% Confidence Intervals).
The univariable analysis also revealed the oral cavity OAR DVH statistics were associated with a clinically significant decrease in the Promis-10 Physical T-score of
>10 points at 3 months and a decrease in the Promis-10 Mental T-score of
>10 points at 12 months (
Table S3).
The oral cavity OAR DVH statistics were also found to be associated with the following changes in EORTC QLQ-H&N35 subscales: swallow
>10 points at 3 months, 6 months and 24 months; saliva
>10 points at end of treatment; senses
>10 points at end of treatment and 6 months; speech
>10 points at 24 months; social eating
>10 points at end of treatment, 3 months, 6 months and 12 months; and feeding tube use at end of treatment (
Table S3).
Finally, the oral cavity OAR DVH statistics were found to be associated with the start of opioid pain medications during and within 30 days of completing RT. The oral cavity OAR DVH statistics were also found to be associated with hospitalization during and within 30 days of completing RT (
Table S3).
Table 2 demonstrates the high correlation between mean oral cavity OAR dose and V10Gy-V70Gy. Therefore, only the mean oral cavity OAR dose was used in the multivariable analysis.
3.2. Multivariable modeling
Table 3 summarizes the multivariable modeling. The mean oral cavity OAR dose was associated with the start of opioid pain medication during and within 30 days of completing RT (odds ratio [OR] 2, [95% Confidence Interval {CI} 1-2), p=0.008), and with hospitalization during and within 30 days of completing RT (OR 1, 95% CI 1-2; p=0.036). The model was driven largely by the dose to the major salivary glands. The use of cytotoxic chemotherapy was associated with a higher risk of developing CTCAE v4.03 dehydration >grade 1 at the end of treatment (OR 5, 95% CI 3-12; p<0.001), the start of opioid pain medication during and withing 30 days of completing RT (OR 4, 95% CI 2-11; p=0.002), and with hospitalization during and within 30 days of completing RT (OR 2, 95% CI 1-5; p=0.022). The use of protons was associated with a lower risk of developing CTCAE v4.03 dysphagia >grade 1 at end of treatment and at 3 months (OR 0.3, 95% CI 0.1-0.6; p=0.003 and OR 0.4, 95% CI 0.1-0.9; p=0.029, respectively). The use of protons was also associated with a lower risk of developing CTCAE v4.03 salivary duct inflammation >grade 1 at end of treatment (OR 0.4, 95% CI 0.2-1; p=0.047). The C-statistic suggests the models are good to strong.
Table 4 compares RT modality and oral cavity OAR DVH statistics. The dose to the oral cavity OAR was significantly lower when protons were used compared to photons.
The data was assessed to identify a mean oral cavity OAR dose cut point which could be used to guide RT treatment planning to minimize the risk of opioid pain medication use and hospitalization during and within 30 days of completing RT.
Table 5 and 6 provide the mean oral cavity OAR dose cut points to achieve at least 90%, 80% and 70% sensitivity for correctly predicting the need for opioid pain medications or hospitalization during or within 30 days of completing RT while maximizing specificity.
4. Discussion
A systematic review of acute taste impairment following RT suggested approximately 96% of patients will experience objective taste impairment, and approximately 79% will report subjective taste impairment [
34]. In a prospective longitudinal study of taste impairment in patients with stage III and IV oropharyngeal carcinoma treated with intensity modulated radiotherapy (IMRT), severe taste impairment was reported by 50% of patients 1 month after completing treatment, 40% at 3 months, 22% at 6 months, and 23% at 12 months [
24]. The University of Michigan definition of oral cavity OAR was used in the study. Taste impairment was measured using the Head-and-Neck QOL (HNQOL) questionnaire and University of Washington Head and Neck-Related QOL (UWQOL) questionnaire. Patient reported severe taste impairment was significantly associated with RT dose to the oral cavity and tongue. Normal tissue complication probability (NCTP) revealed the mean oral cavity dose at which 50% of patients develop severe taste impairment is 53 Gy and 57 Gy, according to the HNQOL and UWQOL instrument results, respectively. The NCTP mean oral cavity dose at which 25% of patients develop severe taste impairment is 39 and 42 Gy, respectively. The present study did not reveal an association between oral cavity OAR dose and taste impairment on multivariable analysis using the CTCAE v4.03 and EORTC QLQ-H&N35 instruments.
Chen et al prospectively evaluated patient-reported taste impairment using the EORTC QLQ-H&N35 taste related question (question 44, 0=no taste impairment, >=33.3=taste impairment) in patients undergoing curative primary or postoperative IMRT with or without concurrent chemotherapy [
41]. They used the University of Michigan definition of oral cavity OAR [
36]. They excluded patients treated with chemotherapy or RT for recurrent cancer or second primary tumors, patients using medications that could impair taste, and/or patients with occupational exposures that could cause impairment of taste. In multivariable analyses, partial or total glossectomy was significantly associated with long-term taste impairment. When the authors excluded patients treated with surgery from the analyses, the mean RT dose to the oral cavity was not associated with taste impairment. When the mean RT dose was <5000 cGy, 14.3% of patients experienced taste impairment. When the mean RT dose was ≥5000 cGy, 28.3% of patients experienced taste impairment. The authors concluded that glossectomy is a major cause of long-term taste impairment in head and neck cancer patients receiving IMRT. The study was limited by incomplete baseline data and a small sample size. In the present study, the multivariable analysis did not suggest that glossectomy had an impact on taste impairment.
Fried et al pooled patients with human papillomavirus or p16 related squamous cell carcinoma of the oropharynx (favorable risk) from 3 multiinstitutional phase II studies evaluating deintensified treatments [
31]. Exclusion criteria included less than 6 months of follow-up and patient without RT plans available for analysis. The oral cavity OAR was defined as the oral tongue, tongue base, floor of mouth, hard palate, soft palate, buccal mucosa, and lip mucosa (upper and lower). PRO-CTCAE related to dry mouth and taste impairment at 6 and 12 months were used as the outcomes for this study. Multivariable analyses revealed dry mouth severity at 6 months was significantly associated with the mean RT dose to the contralateral parotid gland, oral cavity OAR, and patient reported dry mouth at baseline. Dry mouth severity at 12 months was significantly associated with baseline dry mouth and the mean RT dose to the contralateral submandibular gland. Taste impairment at 12 months was significantly associated with the mean RT dose to the oral cavity OAR. Evaluation of substructures within the oral cavity OAR revealed that dry mouth severity at 6 months was related to the mean dose to the floor of mouth. Taste impairment at 12 months was associated with mean dose to the oral tongue. The authors concluded the floor of mouth and oral tongue should be prioritized during RT treatment planning over the rest of the oral cavity OAR structures. The study had two limitations, 1) biases associated with pooled analyses, and 2) missing patient reported quality of life metrics data.
In the present study we did not include patients with baseline xerostomia >grade 1. On multivariable analysis mean dose to the parotid glands or oral cavity OAR was not associated with xerostomia using provider reported CTCAE v4.03. Xerostomia at end of treatment was associated with mean right submandibular gland dose. A clinically significant decrease in the EORTC QLQ-N&N35 saliva subscale of >10 points was also associated with the mean right submandibular gland dose. In addition, dysgeusia at 12 months using provider reported CTCAE v4.03 was not found to be related to mean dose to the oral cavity OAR. However, dysgeusia at end of treatment was related to mean dose to the right submandibular gland.
In the study by Chen et al, the whole-mouth solution method for 4 tastes (salt, sweet, sour, and bitter) was used to measure taste function in patients with head and neck cancer treated IMRT or volumetric modulated arc therapy (VMAT) [
32]. Patients were excluded if they had received previous RT to head and neck regions and/or had abnormal taste function prior to RT. Additional endpoints included subjective provider evaluations using CTCAE v4.03 and the Subjective Total Taste Acuity scale. Patient self-reported quality of life was evaluated using EORTC QLQ-H&N35. The oral cavity OAR as defined by the University of Michigan was used [
36]. The authors reported a positive correlation between the subjective perception of impaired taste and the objectively measured impairment of the 4 taste qualities. An oral cavity mean dose
>4000 cGy was associated with taste impairment 3 months after completing RT. With a mean oral cavity RT dose <4000 cGy, 15.6% of patients developed taste impairment. With a mean oral cavity dose
>4000 cGy, 44.9% of patients developed taste impairment. The mean oral cavity NTCP doses at 3 months were 25 Gy (15%), 38 Gy (25%), and 60 Gy (50%). The mean oral cavity NTCP doses at 6 months were 57 Gy (15%), 60 Gy (25%), and 64 Gy (50%). The authors concluded that a high mean oral cavity dose is associated with taste impairment in patients with head and neck cancer receiving IMRT. Reducing oral cavity dose may promote early recovery of taste function after IMRT. Limitations of the study include data from a single institution with limited sample size. The present study, on multivariable analysis, did not reveal an association between taste impairment and mean dose to the oral cavity OAR as defined.
The univariable analysis reported in the present study revealed the oral cavity OAR DVH statistics were associated with the CTCAE v4.03 adverse event endpoints studied, a clinically significant decrease in the Promis-10 Physical T-score and Mental T-score, and with the EORTC QLQ-H&N35 subscales studied (p<0.05, Supplemental
Table 3). However, on multivariable analysis, they were not.
The multivariable models revealed the CTCAE v4.03 adverse event endpoints of dehydration, dry mouth, dysgeusia, dysphagia, salivary duct inflammation, weight loss >10%, and EORTC QLQ-H&N35 salivary scale decrease >10 points were significantly associated with the use of cytotoxic chemotherapy, proton therapy and mean dose to the parotid and submandibular glands. This highlights the impact of radiation dose to salivary glands on the important functions of saliva production, taste, and swallowing with subsequent weight loss.
The mean dose to the oral cavity OAR, as defined at our institution, was found to be significantly associated with the use of opioid pain medication and hospitalization during and within 3 months of completing RT. This would suggest the definition of the oral cavity OAR as used would be helpful in RT treatment planning to reduce the likelihood of these adverse events. The limitations of this study include the retrospective analysis from a single institution with incomplete collection of PROMIS-10 and EORTC QLQ-H&N35 data. The strengths include uniform oral cavity OAR avoidance structure contouring, prospective collection of CTCAE v4.03 adverse events, and completeness of the CTCAE v4.03, opioid use and hospitalization data.
In RT treatment planning for head and neck cancer the principle of “as low as reasonably achievable” should be followed when planning for dose to organs at risk such as the parotid glands, oral cavity, submandibular glands, floor of mouth, and oral tongue in order to minimize acute and late dysgeusia, dysphagia, xerostomia, weight loss, use of opioid pain medications and hospitalization using current guidelines until there is additional information available regarding dose constraints for the oral cavity OAR avoidance structure [
59].
5. Conclusions
The oral cavity OAR avoidance structure as defined in this study predicts for patients requiring opioid pain medication and hospitalization during and within 30 days of completing a course of RT for tumors of the head and neck region.
NRG-HN001: no definition, mean dose < 40 Gy to OC-PTV
NRG-HN002, Oral Cavity: The oral cavity will be defined as a composite structure posterior to lips consisting of the anterior ½ to 2/3 of the oral tongue/floor of mouth, buccal mucosa, and superiorly the palate, and inferiorly to the plane containing the tip of the mandible. Oral Cavity: Reduce the dose as much as possible. The mean dose should be < 32 Gy for the oral cavity. Efforts should also be made to avoid hot spots (> 60 Gy) within the non-involved oral cavity. Mean dose <=32 Gy excluding PTVs.
NRG-HN003, Cavity_Oral: For non-oral cavity cancers, the oral cavity will be defined as a composite structure consisting of the anterior 1/2 to 2/3 of the oral tongue/floor of mouth, buccal mucosa, and palate. For oral cavity cancers, the oral cavity will be defined as the subset of this composite structure that does not overlap with the PTVs. Mean dose (Dmean) <= 30 Gy. Avoid hot spots (> 60 Gy). For non-oral cavity cancers.
NRG-HN004, Lips and Oral Cavity: These should be contoured as 2 separate structures as the goal is to keep the lip dose much lower than the oral cavity dose. The definition of lips is self-explanatory. The oral cavity will be defined as a composite structure consisting of the anterior one half to two thirds of the oral tongue/floor of mouth, buccal mucosa, and palate. Mean dose < 30 Gy. D0.03cc < 60 Gy. To non-involved oral cavity.
NRG-HN005, Cavity_Oral: The oral cavity will be defined as a composite structure posterior to lips consisting of the anterior 1/2 to 2/3 of the oral tongue/floor of mouth, buccal mucosa, and superiorly the palate, and inferiorly to the plane containing the tip of the mandible (external to PTVs). Mean dose < 35 Gy to uninvolved oral cavity.
NRG-HN006, Cavity_Oral: The oral cavity will be defined as a composite structure posterior to lips consisting of the anterior 1/2 to 2/3 of the oral tongue/floor of mouth, buccal mucosa, and superiorly the palate, and inferiorly to the plane containing the tip of the mandible (external to PTVs). Mean dose <=35 Gy. Avoid hot spots > 60 Gy. To uninvolved oral cavity.
NRG-HN008, Cavity_Oral: The oral cavity will be defined as a composite structure posterior to lips consisting of the anterior 1/2 to 2/3 of the oral tongue/floor of mouth, buccal mucosa, and superiorly the palate, and inferiorly to the plane containing the tip of the mandible (external to PTVs). Mean dose <= 35 Gy to uninvolved oral cavity.
NRG-HN009, Cavity_Oral: The oral cavity will be defined as a composite structure posterior to lips consisting of the anterior 1/2 to 2/3 of the oral tongue/floor of mouth, buccal mucosa, and superiorly the palate, and inferiorly to the plane containing the tip of the mandible (external to PTVs). Mean dose < 30 Gy to uninvolved oral cavity.
RTOG-1216, Cavity_Oral: For non-oral cavity cancers, the oral cavity will be defined as a composite structure consisting of the anterior 1/2 to 2/3 of the oral tongue/floor of mouth, buccal mucosa, and palate. For oral cavity cancers, the oral cavity will be defined as the subset of this composite structure that does not overlap with the PTVs. Mean dose <= 30 Gy, D0.03cc <= 60 Gy.
University of Michigan: The surfaces of the inner lips, buccal mucosa, tongue, base of tongue, floor of mouth, and palate were outlined on each patient’s CT data set composing a distinct organ, “oral cavity” (extending to include the surface of the base of tongue). Significant associations were found between patient-reported severe dysgeusia and radiation dose to the oral cavity (
P=.005) and tongue (
P=.019); normal tissue complication probability for severe dysgeusia at 3 months showed mean oral cavity D
50 doses 53 Gy and 57 Gy in the HNQOL and WUQOL questionnaires, respectively [
36].
University of Toronto: Obviously, there is no single anatomic structure that would encompass all minor salivary glands. Therefore, we propose the definition of a surrogate structure, called the “Minor Oral Including Sublingual Salivary Tissue (MOIST) Target” that is intended to contain the majority of the minor salivary glands located within the mucosa covering the linings of the oral cavity and anterior oropharynx. The MOIST Target is defined to include the minor glands of the floor of mouth, tongue, base of tongue, hard palate, soft palate, uvula, buccal mucosa, inner lips, retromolar trigone, lateral alveolar margin, anterior faucial pillar, and the sublingual glands. The sublingual glands are included as they are difficult to identify separately on cross-sectional imaging. The anterior border of the oral cavity includes the gingival reflection and the inner side of the upper and lower lips. The posterior border includes the soft palate, uvula, retromolar trigone mucosa, and more inferiorly the base of tongue. The lateral border will encompass the mandible bone and the maxilla to include the overlying gingival mucosal surfaces bilaterally and the buccal mucosa. No attempt is made to include the whole volume of the buccinator muscle. Superiorly, the “MOIST Target” includes the hard palate mucosa and mucosal reflections near the maxilla. The most superior volume of gingival mucosa is delineated up to the level of the bottom of the maxillary sinus. The inferior border is defined to include the base of tongue mucosa posteriorly and the mucosal reflection of the alveolar ridges on the mandible laterally and anteriorly. Centrally, the inferior border includes the mylohyoid and the geniohyoid muscle to include the sublingual glands. Given the differences in head position, especially when the head is in hyperextension position during the planning CT scan, the inferior border may vary slightly and include a larger part of the posterior base of tongue [
25].
Affiliated Quanzhou First Hospital of Fujian Medical University, Xinyu People’s Hospital, Zhejiang Cancer Hospital: Two methods, Oral Cavity Contour method- the oral mucosa was limited as follows: above to hard palate, underneath to floor of mouth, anterior to the buccal mucosa around the teeth, and posterior to tongue surface and uvula,
>grade 3 toxicity V30 Gy; Mucosa Surface Contour method- the oral mucosa was defined as a 3-mm thick wall of tissue and included the following surface: buccal mucosa, buccal gingiva, gingiva proper, lingual gingiva, lingual frenulum, alveolar mucosa, labial mucosa, labial gingiva, labial frenulum, mucosal surface of the floor of the mouth, the mucosal surface of the tongue anterior to the terminal sulcus, the mucosal surface of the hard palate, and the inferior mucosal surface of the soft palate,
>grade 3 toxicity V50 Gy [
27].
Sacro Cuore Don Calabria Hospital, University of Palermo: The oral mucosa limits were defined as follows: hard palate superiorly, cricoid cartilage inferiorly, the buccal mucosa around the teeth anteriorly, and the posterior pharyngeal wall posteriorly. Mucositis
>G2 was found to be statistically related to chemotherapy, weight loss, dysphagia
>G2, total oral mucosa D
mean >50 Gy and D
max >65 Gy, V
45 Gy >40%, V
50 Gy >30%, and V
55 Gy >20% of the oral mucosa minus target PTVs. A ratio between total oral mucosa and oral mucosa minus target PTVs >2.5 is related to G3 mucositis (p =.03) [
54].
The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust: The mucosal surface contours were defined as a 3 mm thick wall of tissue. The outlined mucosal surface contours included the following surfaces: buccal mucosa, buccal gingiva, gingiva proper, lingual gingiva, lingual frenulum, alveolar mucosa, labial mucosa, labial gingiva, labial frenulum, mucosal surface of the floor of mouth, mucosal surface of the tongue anterior to the terminal sulcus, and the mucosal surface of the hard palate. The superior extent was defined to be the superior border of the labial mucosa of the upper lip anteriorly, the roof of the palate posteriorly and the superior extent of the buccal mucosa laterally. The inferior extent was formed by the inferior border of the labial mucosa of the lower lip anteriorly, the surface of the tongue posteriorly and the inferior extents of the floor of mouth mucosa and buccal mucosa laterally. The lateral extents of the buccal mucosa formed the lateral borders. The anterior border followed the alveolar mucosa, and the posterior extent of the hard palate formed the posterior border. The mucosal surface contours were initially delineated as a single line and once complete, expanded to a 3 mm annulus [
29].
International Consensus OAR Contouring Guidelines: The delineation of the extended oral cavity was based partly on Hoebers et al. For the sake of simplicity and consistency, the extended oral cavity structure was defined posterior to the internal arch of the mandible and maxilla. The mucosa anterior to the mandible and maxilla is included in the contour of the lips, and the mucosa lateral to the mandible and maxilla is included in the buccal mucosa. Anatomic boundaries: Cranial-Hard palate mucosa and mucosal reflections near the maxilla, Caudal-The base of tongue, mucosa and hyoid, posteriorly, and the mylohyoid muscle and anterior belly of the digastric muscle, anteriorly, Anterior-Inner surface of the mandible and maxilla, Posterior-Posterior borders of soft palate, uvula, and more inferiorly the base of tongue, Lateral-Inner surface of the mandible and maxilla. Posterior to mandible and maxilla, no inner surface of the lips.
For research purposes, the extended oral cavity can be subdivided into oral tongue and anterior oropharynx, by drawing a vertical line from the posterior hard palate to the hyoid (circumvallate line) [
26].
Chang Gung Memorial Hospital: The surfaces of the inner lips, buccal mucosa, tongue, base of the tongue, floor of the mouth, and palate were outlined on each computed tomography (CT) data set to form a distinct organ, the “oral cavity” (extended to include the surface of the base of the tongue). Positive correlation occurred between objectively measured taste loss for the 4 taste qualities and subjective perception of taste loss. Only oral cavity mean dose 4000 cGy or greater predicted taste dysfunction 3 months after RT. The mean oral cavity doses to the predicted 15%(D
15), 25%(D
25), and 50% (D
50) probabilities were 25, 38, and 60 Gy at 3 months and 57, 60, and 64 Gy at 6 months, respectively [
32].
University of North Carolina: The OARs were generally segmented as described in a publication by Brouwer et al. (International Consensus OAR Contouring Guidelines outlined above). The anterior two-thirds of the tongue were defined as the oral tongue, and the posterior third as the base of tongue. The region inferior and anterior to the tongue was delineated as the floor of mouth. Nineteen total structures were contoured including the left and right parotid glands, left and right submandibular glands, left and right sublingual glands, oral tongue, base of tongue, floor of mouth, soft and hard palates, buccal mucosa, upper and lower lips. The only significant factor related to dysgeusia at 12 months was mean dose to the oral cavity (
P = .009). On examining substructures, the mean dose to the floor of mouth was implicated for the dose relationship to 6-month xerostomia (
P = .04), and the oral tongue was found to be implicated for the relationship for 12-month dysgeusia (
P = .04) [
31].
The University of Hong Kong-Shenzhen Hospital, National Cancer Center Singapore, Memorial Sloan-Kettering Cancer Center, State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center: Tongue/Oral Cavity, Cranial-Post. edge of the hard palate or soft palate, Caudal- Disappearance of anterior belly of digastric muscle, Anterior-Post. edge of mandible or is free, Posterior- Palate, oropharynx, the palatine tonsil, hyoid bone, Lateral- Med. edge of the mandible or inferior alveoli socket [
28].
Supplementary Materials
Table S1. Oral cavity OAR avoidance structure definitions and dose constraints. Table S2. International Classification of Diseases (ICD)-10 codes used in the database search. Table S3. Univariable Analysis-Variables Associated with Toxicity.
Author Contributions
Conceptualization, RLF, WSH, OMM, and SS.; Methodology, RLF, WSH, OMM, SCL, SHP and SS; Formal Analysis, RLF, WSH, OMM, and SS; Investigation, RLF, WSH, OMM, and SS; Resources, RLF, WSH, ACA, EJM, KMVA, LXY, OMM and SS; Data Curation, RLF, WSH, ACA, EJM, KMVA, LXY, OMM and SS; Writing – Original Draft Preparation, RLF, WSH, and SS; Writing – Review & Editing, RLF, WSH, ACA, ABC, MEG, YIG, SCL, DJM, LAM, EJM, MANW, SHP, DMR, J-CMR, KMVA, LXY, OMM, and SS.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Mayo Clinic (IRB #20-004915, June 17, 2020; and IRB #15-00136, March 15, 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
The authors acknowledge the assistance of Brady Himle for manuscript preparation and submission.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hyuna Sung, Jacques Ferlay, Rebecca L. Siegel, Mathieu Laversanne, Isabelle Soerjomataram, Ahmedin Jemal, Freddie Bray. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71:209-249. [CrossRef]
- Ratko TA, Douglas GW, de Souza JA, et al. Radiotherapy Treatments for Head and Neck Cancer Update [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014 Dec. (Comparative Effectiveness Review, No. 144.) Introduction. Available from: https://www.ncbi.nlm.nih.gov/books/NBK269010/.
- Arthur R. Hand, Dharmini Pathmanathan and Ruth B. Field. Morphological features of the minor salivary glands. Archives of Oral Biology 44 (1999) S3-SlO. [CrossRef]
- Susan S. Schiffman. Taste and Smell in Disease. NEJM 308 (21): 1275-1279, 1983. [CrossRef]
- T. S. Deshpande, P. Blanchard, L. Wang, R. L. Foote, X. Zhang, S. J. Frank. Radiation-Related Alterations of Taste Function in Patients With Head and Neck Cancer: a Systematic Review. Curr. Treat. Options in Oncol. 19:72, 2018. [CrossRef]
- Herve Y. Sroussi, Joel B. Epstein, Rene-Jean Bensadoun, Deborah P. Saunders, Rajesh V. Lalla, Cesar A. Migliorati, Natalie Heaivilin, Zachary S. Zumsteg. Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis Cancer Medicine 2017; 6(12):2918–2931. [CrossRef]
- Naomi Kiyota, Makoto Tahara, Junki Mizusawa, Takeshi Kodaira, Hirofumi Fujii, Tomoko Yamazaki, Hiroki Mitani, Shigemichi Iwae, Yasushi Fujimoto, Yusuke Onozawa , Nobuhiro Hanai, Takenori Ogawa, Hiroki Hara, Nobuya Monden, Eiji Shimura, Shujiro Minami, Takashi Fujii, Kaoru Tanaka, Akihiro Homma, Seiichi Yoshimoto, Nobuhiko Oridate, Koichi Omori, Tsutomu Ueda, Kenji Okami, Ichiro Ota, Kiyoto Shiga, Masashi Sugasawa, Takahiro Asakage, Yuki Saito, Shigeyuki Murono, Yasumasa Nishimura, Kenichi Nakamura, Ryuichi Hayashi, Head and Neck Cancer Study Group of the Japan Clinical Oncology Group (JCOG-HNCSG). Weekly Cisplatin Plus Radiation for Postoperative Head and Neck Cancer (JCOG1008): A Multicenter, Noninferiority, Phase II/III Randomized Controlled Trial. J Clin Oncol. 2022 Mar 1; JCO2101293. Online ahead of print. [CrossRef]
- Robert L Ferris, Yael Flamand, Gregory S Weinstein, Shuli Li, Harry Quon, Ranee Mehra, Joaquin J Garcia, Christine H Chung, Maura L Gillison, Umamaheswar Duvvuri, Bert W O'Malley Jr, Enver Ozer, Giovana R Thomas, Wayne M Koch, Neil D Gross, R Bryan Bell, Nabil F Saba, Miriam Lango, Eduardo Méndez, Barbara Burtness. Phase II Randomized Trial of Transoral Surgery and Low-Dose Intensity Modulated Radiation Therapy in Resectable p16+ Locally Advanced Oropharynx Cancer: An ECOG-ACRIN Cancer Research Group Trial (E3311). J Clin Oncol. 2022 Jan 10;40(2):138-149. [CrossRef]
- Sue S. Yom, Pedro Torres-Saavedra, Jimmy J. Caudell, John N. Waldron, Maura L. Gillison, Ping Xia, Minh T. Truong, Christina Kong, Richard Jordan, Rathan M. Subramaniam, Min Yao, Christine H. Chung, Jessica L. Geiger, Jason W. Chan, Brian O’Sullivan, Dukagjin M. Blakaj, Loren K. Mell, Wade L. Thorstad, Christopher U. Jones, Robyn N. Banerjee, Christopher Lominska, and Quynh-Thu Le. Reduced-Dose Radiation Therapy for HPV-Associated Oropharyngeal Carcinoma (NRG Oncology HN002). J Clin Oncol 39:956-965, 2021. [CrossRef]
- Devendra Chaukar, Kumar Prabash, Pawan Rane, Vijay Maruti Patil, Shivakumar Thiagarajan, Sarbani Ghosh-Laskar, Shilpi Sharma, Prathamesh S. Pai, Pankaj Chaturvedi, Gouri Pantvaidya, Anuja Deshmukh, Deepa Nair, Sudhir Nair, Richa Vaish, Vanita Noronha, Asawari Patil, Supreeta Arya, and Anil D’Cruz. Prospective Phase II Open-Label Randomized Controlled Trial to Compare Mandibular Preservation in Upfront Surgery With Neoadjuvant Chemotherapy Followed by Surgery in Operable Oral Cavity Cancer. J Clin Oncol 40:272-281, 2021. [CrossRef]
- Bhishamjit S. Chera, Robert J. Amdur, Rebecca Green, Colette Shen, Gaorav Gupta, Xianming Tan, Mary Knowles, David Fried, Neil Hayes, Jared Weiss, Juneko Grilley-Olson, Shetal Patel, Adam Zanation, Trevor Hackman, Jose Zevallos, Jeffrey Blumberg, Samip Patel, Mohit Kasibhatla, Nathan Sheets, Mark Weissler, Wendell Yarbrough, and William Mendenhall. Phase II Trial of De-Intensified Chemoradiotherapy for Human Papillomavirus–Associated Oropharyngeal Squamous Cell Carcinoma. Clin Oncol 37:2661-2669, 2019. [CrossRef]
- Anthony C. Nichols, Julie Theurer, Eitan Prisman, Nancy Read, Eric Berthelet, Eric Tran, Kevin Fung, John R. de Almeida, Andrew Bayley, David P. Goldstein, Michael Hier, Khalil Sultanem, Keith Richardson, Alex Mlynarek, Suren Krishnan, Hien Le, John Yoo, S, Danielle MacNeil, Eric Winquist, J. Alex Hammond, Varagur Venkatesan, Sara Kuruvilla, Andrew Warner, Sylvia Mitchell, Jeff Chen, Martin Corsten, Stephanie Johnson-Obaseki, Libni Eapen, Michael Odell, Christina Parker, Bret Wehrli, Keith Kwan, David A. Palma. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomised trial. Lancet Oncol 2019; 20: 1349–59. [CrossRef]
- Y. Zhang, L. Chen, G. Q. Hu, N. Zhang, X. D. Zhu, K. Y. Yang, F. Jin, M. Shi, Y. P. Chen, W. H. Hu, Z. B. Cheng, S. Y. Wang, Y. Tian, X. C. Wang, Yan Sun, J. G. Li, W. F. Li, Y. H. Li, L. L. Tang, Y. P. Mao, G. Q. Zhou, R. Sun, X. Liu, R. Guo, G. X. Long, S. Q. Liang, L. Li, J. Huang, J. H. Long, J. Zang, Q. D. Liu, L. Zou, Q. F. Su, B. M. Zheng, Y. Xiao, Y. Guo, F. Han, H. Y. Mo, J. W. Lv, X. J. Du, C. Xu, N. Liu, Y. Q. Li, M.L.K. Chua, F. Y. Xie, Ying Sun, and J. Ma. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N Engl J Med 2019; 381:1124-35. [CrossRef]
- Maura L. Gillison, Andy M. Trotti, Jonathan Harris, Avraham Eisbruch, Paul M. Harari, David J. Adelstein, Erich M. Sturgis, Barbara Burtness, John A. Ridge, Jolie Ringash, James Galvin, Min Yao, Shlomo A. Koyfman, Dukagjin M. Blakaj, Mohammed A. Razaq, A. Dimitrios Colevas, Jonathan J. Beitler, Christopher U. Jones, Neal E. Dunlap, Samantha A. Seaward, Sharon Spencer, Thomas J. Galloway, Jack Phan, James J. Dignam, Quynh Thu Le. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 2019; 393: 40–50. [CrossRef]
- Ali Hosni, Kevin Chiu, Shao Hui Huang, Wei Xu, Jingyue Huang, Andrew Bayley, Scott V. Bratman, John Cho, Meredith Giuliani, John Kim, Brian O’Sullivan, Jolie Ringash, John Waldron, Anna Spreafico, John R. de Almeida, Eric Monteiro, Ian Witterick, Douglas B. Chepeha, R.W. Gilbert, Jonathan C. Irish, David P. Goldstein, Andrew Hope. Non-operative management for oral cavity carcinoma: Definitive radiation therapy as a potential alternative treatment approach. Radiotherapy and Oncology 154 (2021) 70–75. [CrossRef]
- Mutlay Sayan, Richard J. Cassidy, Jeffrey M. Switchenko, Oluwatosin A. Kayode, Nabil F. Saba, Conor E. Steuer, Dong M. Shin, J. Trad Wadsworth, Mark El-Deiry, Mihir Patel, Jonathan J. Beitler and Kristin A. Higgins. Development of late Toxicities in Patients with Oral Tongue cancer Treated with surgical resection and adjuvant radiation Therapy. Front. Oncol. 6:272. [CrossRef]
- James J. Sciubba, David Goldenberg. Oral complications of radiotherapy. Lancet Oncol 2006; 7: 175–83. [CrossRef]
- Sean T. Massa, Nosayaba Osazuwa-Peters, Eric Adjei Boakye, Ronald J. Walker, Gregory M. Ward. Comparison of the Financial Burden of Survivors of Head and Neck Cancer With Other Cancer Survivors. JAMA Otolaryngol Head Neck Surg. 2019;145(3):239-249. [CrossRef]
- Kathleen Lang, Matthew Sussman, Mark Friedman, Jun Su, Hong J. Kan, David Mauro, Eskinder Tafesse, Joseph Menzin. Incidence and Costs of Treatment-Related Complications Among Patients With Advanced Squamous Cell Carcinoma of the Head and Neck. Arch Otolaryngol Head Neck Surg. 2009;135(6):582-588. [CrossRef]
- Linda S. Elting, Yu-Chia Chang. Costs of Oral Complications of Cancer Therapies: Estimates and a Blueprint for Future Study. J Natl Cancer Inst Monogr (2019) 2019(53): lgz010. [CrossRef]
- Jed J. Jacobson, Joel B. Epstein, Frederick C. Eichmiller, Teresa B. Gibson, Ginger S. Carls, Emily Vogtmann, Shaohung Wang and Barbara Murphy. The cost burden of oral, oral pharyngeal, and salivary gland cancers in three groups: commercial insurance, medicare, and medicaid. Head & Neck Oncology 2012, 4:15. [CrossRef]
- LINDA S. ELTING, CATHERINE D. COOKSLEY, MARK S. CHAMBERS, ADAM S. GARDEN. Risk, Outcomes, and Costs of Radiation-Induced Oral Mucositis among Patients with Head-and-Neck Malignancies. Int. J. Radiation Oncology Biol. Phys., Vol. 68, No. 4, pp. 1110-1120, 2007. [CrossRef]
- David I. Rosenthal. Consequences of mucositis-induced treatment breaks and dose reductions on head and neck cancer treatment outcomes. J Support Oncol. 2007 Oct;5(9 Suppl 4):23-31.
- Eli Sapir, Yebin Tao, Felix Feng, Stuart Samuels, Issam El Naqa, Carol A. Murdoch-Kinch, Mary Feng, Matthew Schipper, and Avraham Eisbruch. Predictors of Dysgeusia in Patients With Oropharyngeal Cancer Treated With Chemotherapy and Intensity Modulated Radiation Therapy. Int J Radiation Oncol Biol Phys, Vol. 96, No. 2, pp. 354e361, 2016. [CrossRef]
- Frank Hoebers, Eugene Yu,Avi Eisbruch, Wade Thorstad, Brian O’Sullivan, Laura A. Dawson, and Andrew Hope. A Pragmatic Contouring Guideline for Salivary Gland Structures in Head and Neck Radiation Oncology. The MOIST Target. Am J Clin Oncol 2013;36:70–76. [CrossRef]
- Charlotte L. Brouwer, Roel J.H.M. Steenbakkers, Jean Bourhis, Wilfried Budach, Cai Grau, Vincent Grégoire, Marcel van Herk, Anne Lee, Philippe Maingon, Chris Nutting, Brian O’Sullivan, Sandro V. Porceddu, David I. Rosenthal, Nanna M. Sijtsema, Johannes A. Langendijk. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiotherapy and Oncology 117 (2015) 83–90. [CrossRef]
- Kaixin Li, Ling Yang, Qiang-ying Hu, Xiao-zhong Chen, MingChen and Yuanyuan Chen. Oral Mucosa Dose Parameters Predicting Grade ≥3 Acute Toxicity in Locally Advanced Nasopharyngeal Carcinoma, Patients Treated With Concurrent Intensity-Modulated Radiation Therapy and Chemotherapy: An Independent Validation Study Comparing Oral Cavity versus Mucosal Surface Contouring Techniques. Translational Oncology (2017) 10, 752–759. [CrossRef]
- Sun Y, Yu XL, Luo W, Lee AW, Wee JT, Lee N, Lee N, Zhou GQ, Tang LL,and Tao CJ, et al (2014). Recommendation for a contouring method and atlas of organs at risk in nasopharyngeal carcinoma patients receiving intensity-modulated radiotherapy. Radiother Oncol 110, 390–397. [CrossRef]
- Dean JA, Welsh LC, Gulliford SL, Harrington KJ, and Nutting CM (2015). A novel method for delineation of oral mucosa for radiotherapy dose-response studies. Radiother Oncol 115, 63–66. Jamie A. Dean, Liam C. Welsh, Sarah L. Gulliford, Kevin J. Harrington, Christopher M. Nutting. A novel method for delineation of oral mucosa for radiotherapy dose–response studies. Radiotherapy and Oncology 115 (2015) 63–66. [CrossRef]
- J. K. Kaae, L. Johnsen, C. R. Hansen, M. H. Kristensen, C. Brink & J. G. Eriksen. Relationship between patient and physician-rated xerostomia and dose distribution to the oral cavity and salivary glands for head and neck cancer patients after radiotherapy. Acta Oncologica, 58:10, 1366-1372. [CrossRef]
- David V. Fried, Shiva K. Das, Colette Shen, Lawrence B. Marks, and Bhishamjit S. Chera. Impact of Oral Cavity Dosimetry on Patient Reported Xerostomia and Dysgeusia in the Setting of Deintensified Chemoradiotherapy. Advances in Radiation Oncology (2022) 7, 100952. doi.org/10.1016/j.adro.2022.100952. David V. Fried, Shiva K. Das, Colette Shen, Lawrence B. Marks, and Bhishamjit S. Chera. Impact of Oral Cavity Dosimetry on Patient Reported Xerostomia and Dysgeusia in the Setting of Deintensified Chemoradiotherapy. Advances in Radiation Oncology (2022) 7, 100952. UNC 2022. [CrossRef]
- Wen-Cheng Chen, Cheng-Ming Hsu, Yao-Te Tsai, Meng-Hung Lin, Ming-Shao Tsai, Geng-He Chang, Chia-Hsuan Lai, Fumin Fang, Miao-Fen Chen. Prospective Evaluation of Taste Function in Patients With Head and Neck Cancer Receiving Intensity-Modulated Radiotherapy. JAMA Otolaryngol Head Neck Surg. doi:10.1001/jamaoto.2022.0850. Wen-Cheng Chen, Cheng-Ming Hsu, Yao-Te Tsai, Meng-Hung Lin, Ming-Shao Tsai, Geng-He Chang, Chia-Hsuan Lai, Fumin Fang, Miao-Fen Chen. Prospective Evaluation of Taste Function in Patients With Head and Neck Cancer Receiving Intensity-Modulated Radiotherapy. JAMA Otolaryngol Head Neck Surg. [CrossRef]
- Wang X, Eisbruch A. IMRT for head and neck cancer: Reducing xerostomia and dysphagia. J Radiat Res. 2016;57:i69–i75. [CrossRef]
- Gunn L, Gilbert J, Nenclares P, et al. Taste dysfunction following radiotherapy to the head and neck: A systematic review. Radiother Oncol. 2021;157:130–140. Gunn L, Gilbert J, Nenclares P, et al. Taste dysfunction following radiotherapy to the head and neck: a systematic review. Radiother Oncol. 2021; 157:130-140. [CrossRef]
- Jellema AP, Doornaert P, Slotman BJ, Rene Leemans C, Langendijk JA. Does radiation dose to the salivary glands and oral cavity predict patient-rated xerostomia and sticky saliva in head and neck cancer patients treated with curative radiotherapy? Radiother Oncol. 2005;77:164–171. [CrossRef]
- Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:695–704. [CrossRef]
- Blanco AI, Chao KS, El Naqa I, et al. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1055–1069. [CrossRef]
- Riva G, Raimondo L, Ravera M, et al. Late sensorial alterations in different radiotherapy techniques for nasopharyngeal cancer. Chem Senses. 2015;40(4):285-292. [CrossRef]
- Maes A, Huygh I, Weltens C, et al. De Gustibus: time scale of loss and recovery of tastes caused by radiotherapy. Radiother Oncol. 2002;63(2):195-201. [CrossRef]
- Mirza N, Machtay M, Devine PA, Troxel A, Abboud SK, Doty RL. Gustatory impairment in patients undergoing head and neck irradiation. Laryngoscope. 2008;118(1):24-31. [CrossRef]
- Chen WC, Tsai MS, Tsai YT, Lai CH, Lee CP, Chen MF. Long-term taste impairment after intensity-modulated radiotherapy to treat head-and-neck cancer: correlations with glossectomy and the mean radiation dose to the oral cavity. Chem Senses. 2019;44(5):319-326. [CrossRef]
- Yamashita H, Nakagawa K, Nakamura N, et al. Relation between acute and late irradiation impairment of four basic tastes and irradiated tongue volume in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;66(5): 1422-1429. [CrossRef]
- Yamashita H, Nakagawa K, Tago M, et al. Taste dysfunction in patients receiving radiotherapy. Head Neck. 2006;28(6):508-516. [CrossRef]
- Baharvand M, ShoalehSaadi N, Barakian R, Moghaddam EJ. Taste alteration and impact on quality of life after head and neck radiotherapy. J Oral Pathol Med. 2013;42(1):106-112. [CrossRef]
- Negi P, Kingsley PA, Thomas M, Sachdeva J, Srivastava H, Kalra B. Pattern of gustatory impairment and its recovery after head and neck irradiation. Iran J Otorhinolaryngol. 2017;29(95): 319-327.
- Fernando IN, Patel T, Billingham L, et al. The effect of head and neck irradiation on taste dysfunction: a prospective study. Clin Oncol (R Coll Radiol). 1995;7(3):173-178. [CrossRef]
- Kamprad F, Ranft D, Weber A, Hildebrandt G. Functional changes of the gustatory organ caused by local radiation exposure during radiotherapy of the head-and-neck region. Strahlenther Onkol. 2008;184(3):157-162. [CrossRef]
- Sandow PL, Hejrat-Yazdi M, Heft MW. Taste loss and recovery following radiation therapy. J Dent Res. 2006;85(7):608-611. [CrossRef]
- Barbosa da Silva JL, Doty RL, Miyazaki JVMK, et al. Gustatory disturbances occur in patients with head and neck cancer who undergo radiotherapy not directed to the oral cavity. Oral Oncol. 2019;95: 115-119. [CrossRef]
- Martini S, Iorio GC, Arcadipane F, et al. Prospective assessment of taste impairment and nausea during radiotherapy for head and neck cancer. Med Oncol. 2019;36(5):44. [CrossRef]
- Mossman KL. Quantitative radiation dose-response relationships for normal tissues in man. I. gustatory tissue response during photon and neutron radiotherapy. Radiat Res. 1982;91(2): 265-274. [CrossRef]
- Narayan S, Lehmann J, Coleman MA, Vaughan A, Yang CC, Enepekides D, Farwell G, Purdy JA, Laredo G, and Nolan K, et al (2008). Prospective evaluation to establish a dose response for clinical oral muscositis in patients undergoing head-and-neck conformal radiotherpy. Int J Radiat Oncol Biol Phys 73, 756–762. [CrossRef]
- Wang ZH, Zhang SZ, Zhang ZY, Zhang CP, Hu HS, Tu WY, Kirwan J, and Mendenhall WM(2012). Protecting the oral mucosa in patients with oral tongue squamous cell carcinoma treated postoperatively with intensity-modulated radiotherapy: A randomized study. Laryngoscope 122, 291–298. [CrossRef]
- Mazzola R, Ricchetti F, Fersino S, Fiorentino A, Levra NG, Paola GD, Ruggieri R, and Alongi F (2016). Predictors of mucositis in oropharyngeal and oral cavity cancer in patients treated with volumetric modulated radiation treatment: A dose-volume analysis. Head Neck 38(Issue S1), E815-819. [CrossRef]
- Bjordal K, Ahlner-Elmqvist M, Tollesson E, Jensen AB, Razavi D, Maher EJ, Kaasa S (1994) Development of a European Organization for Research and Treatment of Cancer (EORTC) questionnaire module to be used in quality of life assessments in head and neck cancer patients. EORTC Quality of Life Study Group. Acta Oncol 33(8):879–885. [CrossRef]
- Cella, D., Riley, W., Stone, A. A., Rothrock, N., Reeve, B. B., Yount, S., Amtmann, D., D., B., Choi, S., Cook, K. F., et al. (2010). The Patient Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. Journal of Clinical Epidemiology 63, 1179-1194. [CrossRef]
- Atallah, E. et al. Design and rationale for the life after stopping tyrosine kinase inhibitors (LAST) study, a prospective, single-group longitudinal study in patients with chronic myeloid leukemia. BMC Cancer 18(1), 359 (2018). [CrossRef]
- King MT: The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res 5:555-567, 1996. [CrossRef]
- Brodin NP, Kabarriti R, Garg MK, Guha C, Tomé WA, A systematic review of normal-tissue complication models relevant to standard fractionation radiation therapy of the head and neck region published after the QUANTEC reports, International Journal of Radiation Oncology • Biology• Physics (2017). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).