1. Introduction
Uterine myomas are benign monoclonal tumors in more than 70% of women of reproductive age. Clinical symptoms affect 20-50% of these cases and include excessive uterine bleeding, pelvic pressure, and pain, impaired fertility, and complications during pregnancy and delivery [
1,
2,
3].
The development and growth of myomas depend on many factors, including steroid hormones (estrogen and progesterone), numerous cytokines and growth factors, extracellular matrix - ECM (extracellular matrix), microRNA, genetic factors, stem cells, and vitamin D deficiency [
2,
4,
5,
6].
The α and β receptors of estrogens - those produced in the ovary as well as those formed by conversion from ovarian and adrenal androgens - affect the production of numerous cytokines and growth factors. This makes the myoma susceptible to the effects of progesterone, which is involved in regulating genes responsible for proliferation and apoptosis. In addition, both estrogen and progesterone are involved in the production and accumulation of ECM by increasing the expression of fibronectin and collagen [
2,
4]. A small non-coding microRNA has also been found to be involved in myoma development, also mainly affecting ECM formation. Overexpression of miR-21 has been shown to play a role in the pathomechanism of uterine myoma development. Through the TGF-β3 pathway, myoma cells showed increased expression of metalloproteinases (MMP2, MMP9, MMP11) and Serpin1 [
5].
A study by Firdaus et al. [
6] showed that 40-50% of tumors present chromosomal aberrations in exon
2MED12.
According to Islam et al. [
2] and Santamaria et al. [
7], stem cells located in the myometrial layer are involved in the development of myomas. Their activity occurs through the Hippo pathway associated with proliferation and apoptosis and the canonical WNT/β catenin pathway.
The results of numerous studies also indicate the role of vitamin D deficiency in the development of myomas [
8,
9].
Although the molecular mechanisms of the development and growth of myomas are only partially understood, they still represent an interesting model for research related to the conservative treatment of myomas. Numerous attempts at pharmacological management have so far proven unsatisfactory [
3,
10,
11]. Therefore, a search for the activity of new molecules - which, as in malignant tumors, may be involved in inhibiting the development of myomas is ongoing. These may include factors affecting angiogenesis, cell adhesion, or affecting ECM [
12,
13,
14].
Angiogenesis is a complex process involving stimulatory compounds, ECM components, and various cell types [
15,
16,
17]. Angiogenesis-stimulating factors include angiopoietins.
Angiopoietin 1 (ANG1) is one of the three best-known angiopoietins: it is encoded by ANGPT1, a gene located on chromosome 8q22.3q23. It is a ligand of the receptor with tyrosine kinase 2 activity. It causes the remodeling of immature vessels and stabilizes mature vessels. It is expressed on smooth muscle cells, fibroblasts, and pericytes and induces adhesion, migration, and survival of endothelial cells. ANG1 synthesis is regulated by the hypoxia-inducible factor HIF-1α and is associated with calcium ions. Angiopoietin 1 exerts a local effect in the ECM. Cell signal transduction is mediated by protein kinases, including MAPK and FAK [
2,
17,
18,
19].
Angiogenic functions of ANG1 and ANG2 have been shown to be modulated by Ca
2+-dependent calcium signaling pathways [
16,
19].
Endothelium-derived Ca
2+ enhances angiogenesis through multiple factors, including the afore-mentioned ANG1 and fibroblast growth factor [
19]. One mechanism promoting angiogenesis may be the influx of Ca
2+ ions into the mitochondria, resulting in hypoxia associated with the activation of proangiogenic factors [
16]. By contrast, a study by Yang et al. [
20] showed that under hypoxic conditions, Ca
2+ concentrations were higher in myoma than in normal uterine muscle cells, and this was responsible for the susceptibility of myomas to apoptosis. Changes in Ca
2+ concentrations in myoma were also dependent on estrogen concentrations [
21].
The final stage of myoma degeneration is the calcification of its cells, which is observed as a therapeutic effect of embolization [
22]. Calcium hemostasis is regulated by CaSR, the expression of which has been found mainly in the parathyroid glands, kidneys, and brain. It is involved in many biological processes of the body. It was recently discovered that CaSR regulates the fate of many cells in the body; its increased or decreased expression has been described in various types of cancer [
23]. Its presence in uterine myomas has not yet been described.
Focal Adhesion Kinase (FAK) is a non-receptor tyrosine kinase, the product of the 8q24-localized PTK2 gene. It is found on cells that form connections with ECM or other cells - these are foci of adhesion. The primary function of FAK is to transmit signals from the external environment through receptors to the inside of the cell, but also in the opposite direction. Its receptors can be integrins or growth factors. In addition to its involvement in endothelial cell migration and survival, FAK has been also known to be closely involved in controlling angiogenesis [
24,
25]. Its action is mainly through the ERK1/AKT and JNK signaling pathways [
26,
27]. According to Tavora et al. [
28], FAK is a possible target for anti-angiogenic targeted therapies.
2. Materials and Methods
Study Design
The study group consisted of tissue material from 70 patients with uterine myomas diagnosed on histopathological examination by two independent pathologists. The selected women ranged in age from 24 to 82, with a mean age of 50. The myomas ranged in diameter from 1 cm to 10 cm, with an average of 4.3 cm in largest dimension.
The control group included tissue sections, morphologically unchanged, from 12 women without known myomas after histopathological examination. These women had undergone surgery for endometrial hypertrophy or genital prolapse. They were between 56 and 69 years old, with a mean age of 61 years.
The patients included in our study - both those with myomas and those in the control group - had no chronic disease. They were selected for the myoma study group and the control group for abnormal uterine bleeding (endometrial hyperplasia) and genital prolapse (genital prolapsus). They were qualified for surgery by an internist and anesthesiologist. No diseases considered to be risk factors were identified in the participants – such as in the case of endometrial cancer (obesity). Therefore, a table with clinical data was not included so no correlation was made with the expression of the proteins analyzed. Thus, only the age of the patients was taken into account.
Protein detection was carried out using the following antibodies against CASR – Calcium Sensing Receptor Antibody - Affinity Biosciences #AF6296; ANGPT1 –Angiopoietin 1 Antibody - Affinity Biosciences #AF5184; PTK2 – FAK antibody - Affinity Biosciences #AF6397.
The study was conducted on tissue arranged in tissue microarray blocks - TMA prepared according to the procedure described above (Markowska et al., 2022)[
9] from 70 uterine myomas and 70 uterine tissues (tumor margin) identified by a pathologist. Each fragment consisted of elongated smooth muscle cells without atypia, with a very low mitotic rate, i.e., less than one mitosis/10 HPF (high power field). On microscopic examination, the tissue sections consisted of smooth muscle cells that revealed no atypia and showed no significant morphological changes among normal myometrium, tumor peripheral tissue, and tumor cells. There was no necrosis or other regressive changes. The only microscopic change was found in the distorted architecture of the tumor tissue. TMAs were assembled using the UNITMA Quick-Ray
® Manual Tissue Microarrayer. Each TMA contained 14 patient tissue sections and two control sections. The tissue cores were 5.0 mm in diameter. The sections intended for histopathological diagnosis preceding the described study were stained with hematoxylin and eosin. Each microarray also contained a fragment of normal uterine tissue from the control group.
An immunohistochemical examination was performed according to the procedure given by the manufacturer Vector Laboratories. Tissue microarrays were deparaffinized and then rehydrated with xylene and a series of alcohols. Antigens were exposed at 96°C for 20 minutes in citrate-based Vector Antigen® unmasking solution, pH 6.0 (H-3300). Endogenous peroxidase activity was quenched in BLOXALL blocking solution for 10 minutes.
Non-specific binding was blocked in 2.5% normal horse serum from the ImmPRESS® Horse Anti-Rabbit IgG PLUS Polymer Kit Peroxidase reagent kit (Vector Laboratories, CA, USA, MP-7801) for 20 minutes. The excess serum was then removed from the sections. They were next incubated for 30 minutes at 37ºC with antibodies against the proteins analyzed. The slides were then washed in PBS buffer for 5 minutes and incubated for 30 minutes with ImmPRESS reagent. The scrapings were then washed twice for 5 minutes in PBS buffer. After the buffer was removed from the scrapings, they were incubated in ImmPACT DAB EqV working solution until the desired color was achieved. Again, the slides were washed twice for 5 minutes in a PBS buffer. The slides were stained with hematoxylin. Finally, the sections were dehydrated in a series of alcohols and xylene and covered with coverslips.
For immunohistochemical reactions, tissue material from sections of the normal uterus was used as a positive control. The negative control for immunohistochemical reactions on the same material was subjected to the same procedure without using the original antibody during staining. In addition, the use of a TMA containing diverse biological material and control tissues allowed the observation of a variety of immunohistochemical images within each set of tissues. Thus, they provide a control system in relation to each other to evaluate the correctness of the immunohistochemical reactions.
Evaluation of the distinction between the myoma, both the center and periphery of the tumor, from the surrounding normal uterine tissue was performed by an experienced pathologist. In the opinion of the same specialist, no features indicative of necrosis were observed in the analyzed fragments.
Semi-quantitative evaluation of CASR, ANGPT1, and PTK2 protein expression.
For each patient, ten images of the visual field were taken at a total magnification of 400x. For this purpose, an Olympus Grundium Ocus 40 microscope scanner (Olympus, Tokyo, Japan) was used. Based on the above-mentioned photographic documentation, a semi-quantitative evaluation of immunohistochemical reactions was carried out in the Olympus commercial cellSens dimensional program. In the program, phase analysis of the stained preparation was performed by automatically detecting objects due to their color (brown chromogen DAB-3.3). Threshold values were entered, according to which the software automatically classified the data. At the preparation stage, the number of cells and the area of immunohistochemical reactions were evaluated. The obtained surface area values were expressed in mm2. The measurement results were then automatically exported to MS Excel sheets for further statistical analysis [
9,
29].
Statistical analysis
Average level of protein expression in each sample was calculated as median from 10 pictures captured. Normality of distribution by subgroups (tumor center, tumor periphery, controls) was verified using the Shapiro-Wilk test as well as skewness and kurtosis values. Subgroup comparison was carried out with ANOVA (Tukey post-hoc test) or with Kruskal-Wallis test (Dunn post-hoc test with Bonferroni correction). A significance level of 0.05 was assumed.
3. Results
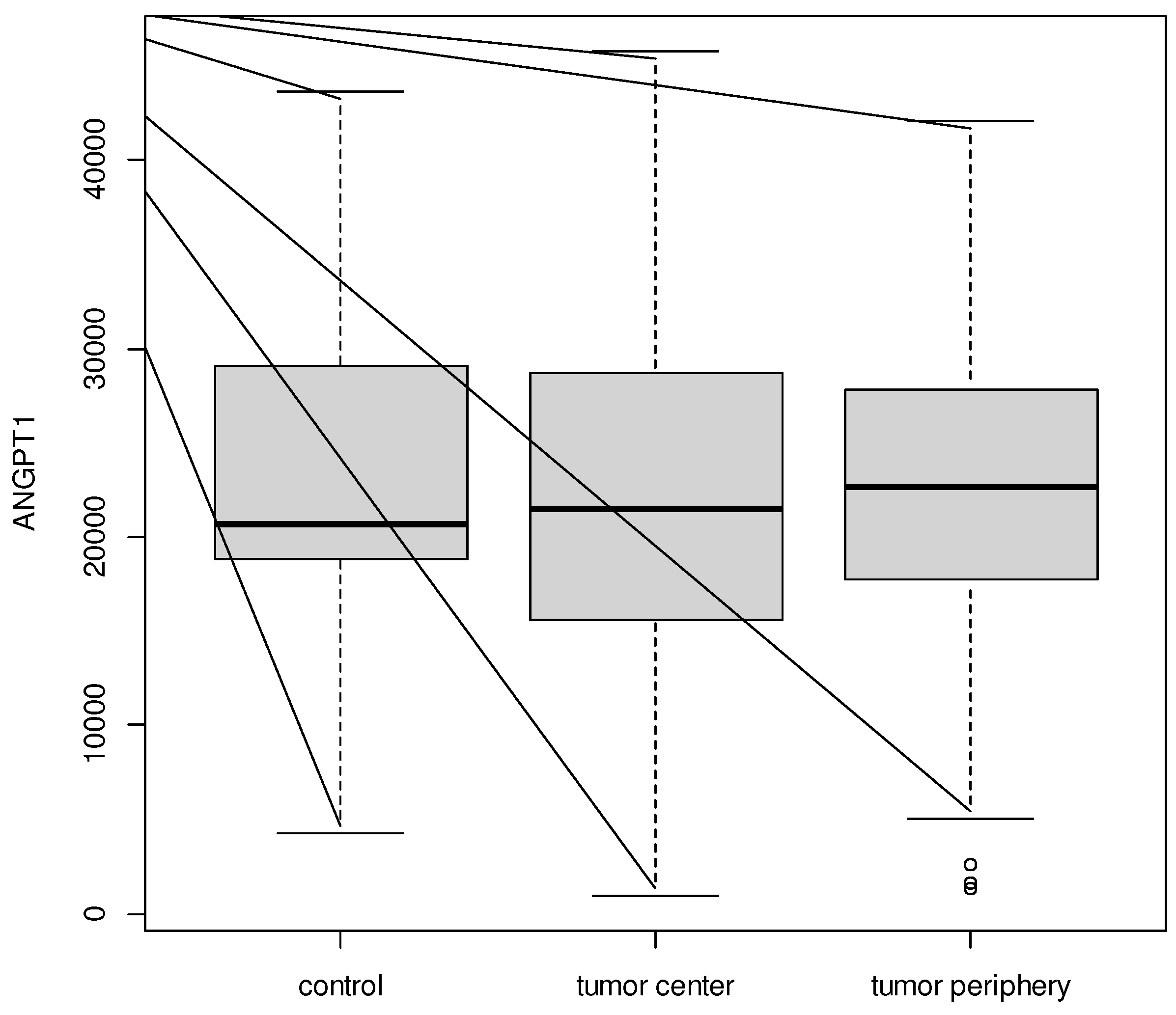
No significant difference between tumor center, tumor periphery and controls for ANGPT1 (p = 0.983) was recorded. Average level of ANGPT1 was from 22 355.37 ± 9 961.68 for the tumor center to 22 900.08 ± 10 762.30 for controls.
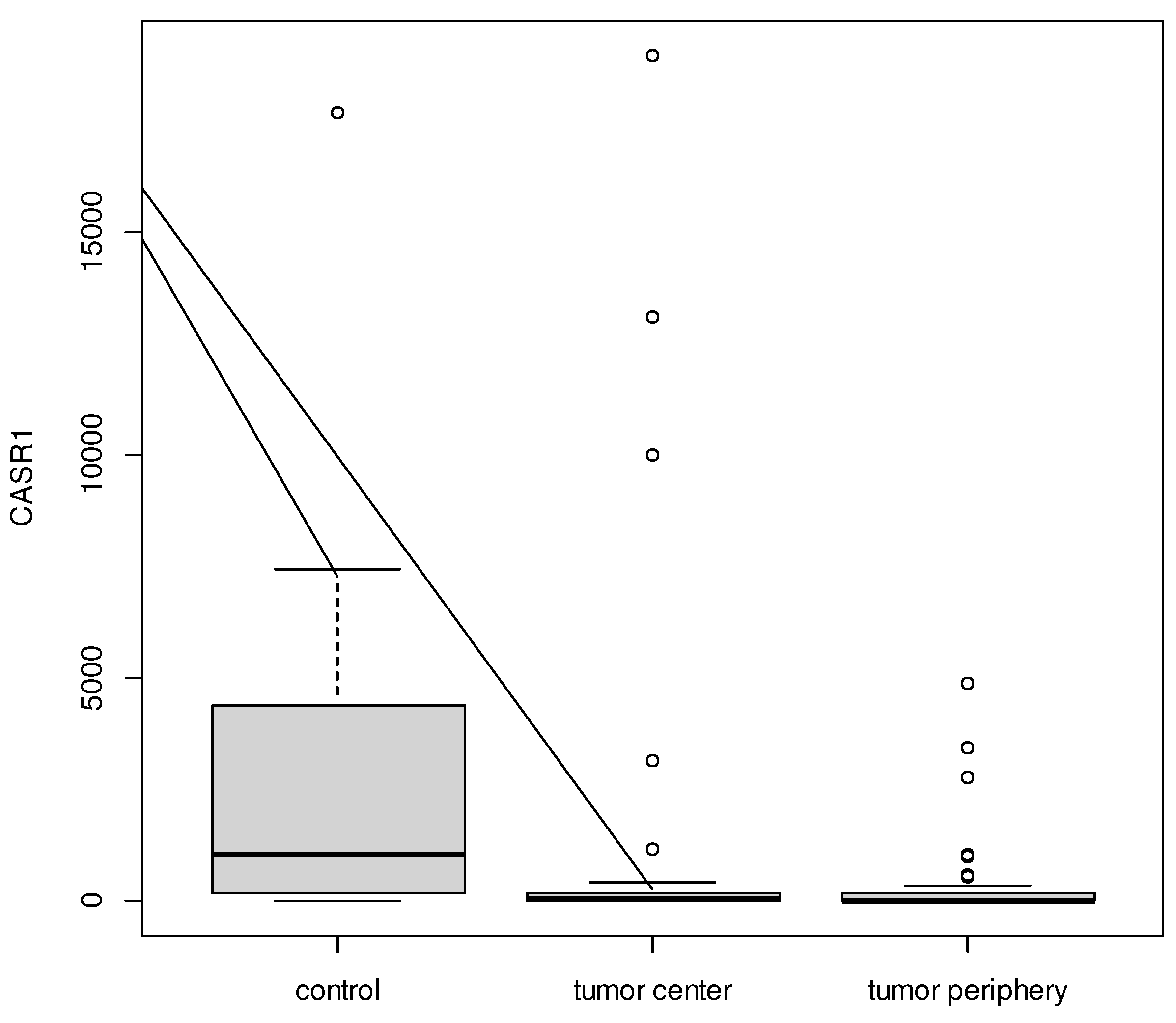
CASR1 expression was significantly different between the samples (p = 0.001). Post-hoc analysis confirmed, that it was lower in the tumor center (median = 34.31) and tumor periphery (median = 14.00) vs. controls (median = 1 056.15).
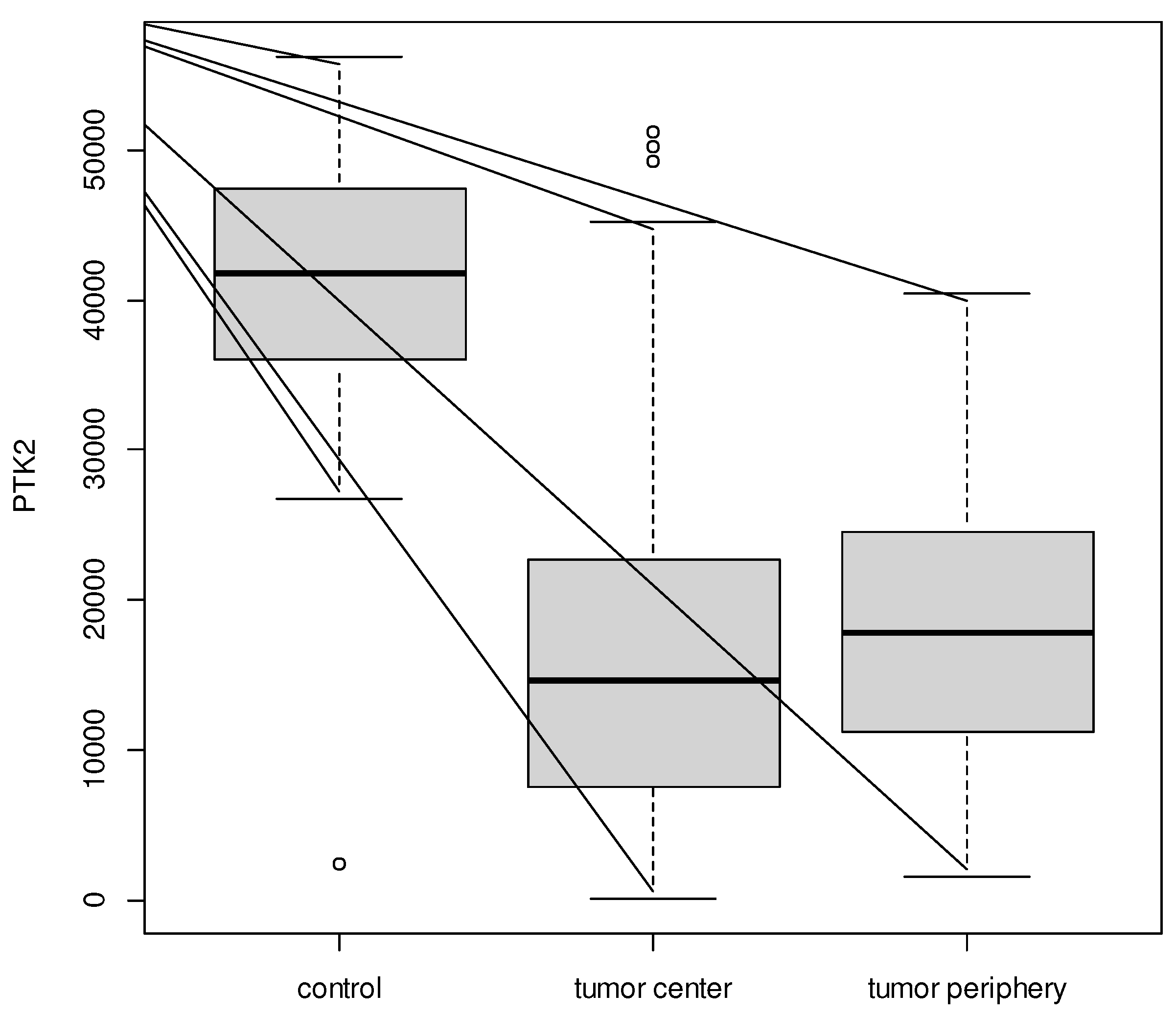
A similar result was confirmed for PTK2: expression was significantly different between the samples (p < 0.001) and post-hoc analysis confirmed that expression was lower in the tumor center (16 037.69 ± 11 342.30) and tumor periphery (18 338.54 ± 8 824.04) vs. controls (39 665.24 ± 14 231.92).
Table 1.
Comparison of tumor center, tumor periphery vs. controls for ANGPT1, CASR, PKT2.
Table 1.
Comparison of tumor center, tumor periphery vs. controls for ANGPT1, CASR, PKT2.
| |
n |
Mean±SD
/ Median (Q1;Q3) |
Range |
p |
post-hoc |
| ANGPT1 |
|
|
|
|
|
| Tumor center |
70 |
22355.37±9961.68 |
(940.08;45829.46) |
0.983 |
|
| Tumor periphery |
70 |
22370.11±8540.59 |
(1313.09;42077.36) |
| Control |
12 |
22900.08±10762.30 |
(4256.68;43689.11) |
| CASR |
|
|
|
|
|
| Tumor center |
70 |
34.31 (7.64;179.37) |
(0.00;18997.74) |
0.001 |
Center < control
Periphery < control |
| Tumor periphery |
70 |
14.00 (2.08;145.02) |
(0.00;4906.41) |
| Control |
12 |
1056.15 (171.81;4343.91) |
(1.38;17712.65) |
| PTK2 |
|
|
|
|
|
| Tumor center |
70 |
16037.69±11342.30 |
(66.05;51188.30) |
<0.001 |
Center < controlPeriphery < control |
| Tumor periphery |
70 |
18338.54±8824.04 |
(1601.30;40503.59) |
| Control |
12 |
39665.24±14231.92 |
(2358.86;56274.69) |
Figure 1.
Boxplot for ANGPT1 between tumor center, tumor periphery, and controls.
Figure 1.
Boxplot for ANGPT1 between tumor center, tumor periphery, and controls.
Figure 2.
Boxplot for CASR1 between tumor center, tumor periphery, and controls.
Figure 2.
Boxplot for CASR1 between tumor center, tumor periphery, and controls.
Figure 3.
Boxplot for PTK2 between tumor center, tumor periphery, and controls.
Figure 3.
Boxplot for PTK2 between tumor center, tumor periphery, and controls.
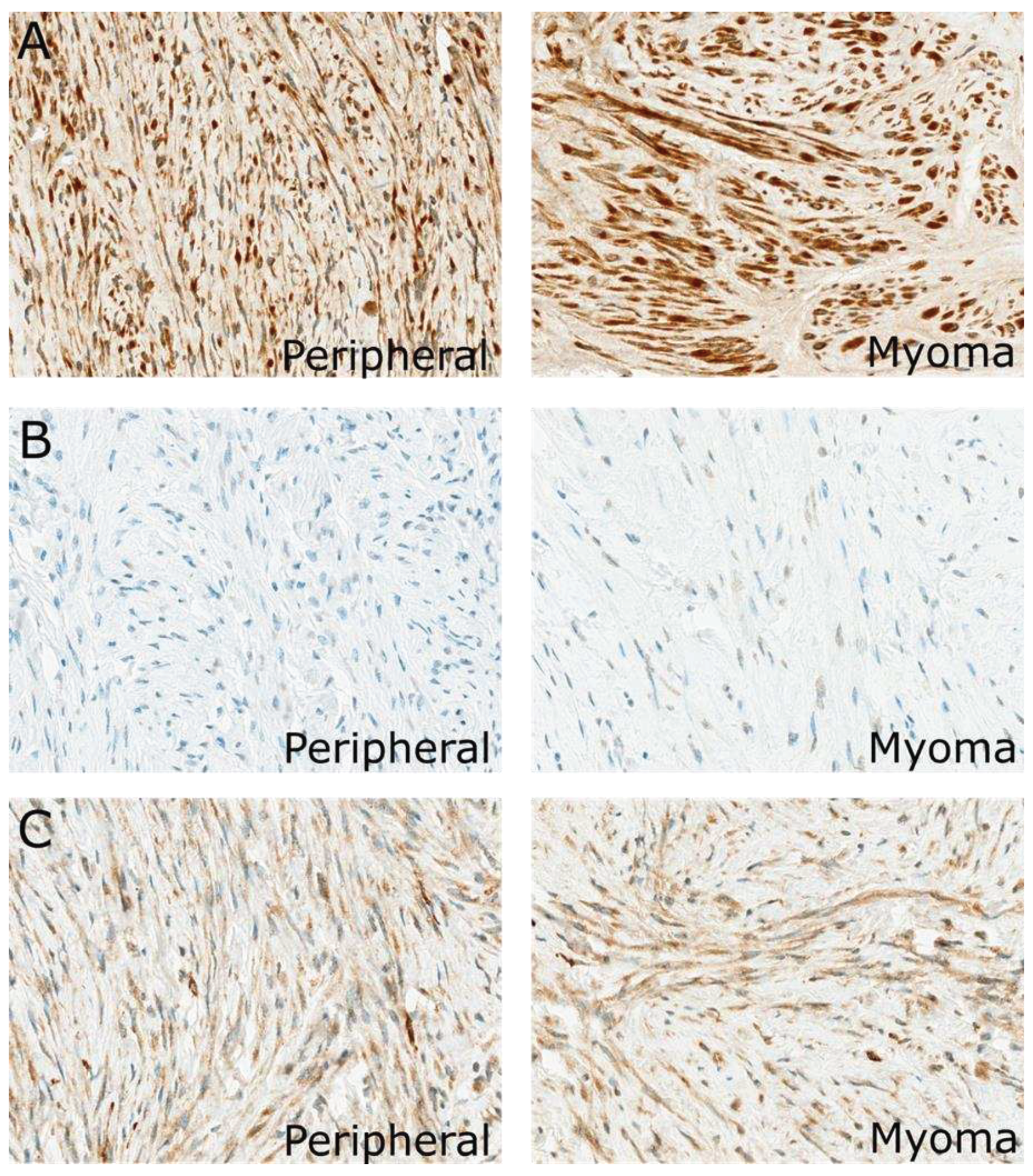
Figure 4.
Immunohistochemical expression of the analyzed protein in the uterus of the same patients: (A) ANGPT1, (B) CASR, (C) PTK2. Magnification for all pictures is 400X.
Figure 4.
Immunohistochemical expression of the analyzed protein in the uterus of the same patients: (A) ANGPT1, (B) CASR, (C) PTK2. Magnification for all pictures is 400X.
4. Discussion
Uterine myomas are heterogeneous tumors by number, size, location, and vascularization. Their numerous associated symptoms - such as bleeding, anemia, pressure on the bladder, and fertility disorders - seriously affect women’s health [
4,
30,
31]. Numerous factors and molecular signaling pathways have been studied for many years, suggesting therapeutic alternatives to surgery [
3,
5,
8,
32].
Studies have shown the critical effect of vascularization on the development of myomas [
17,
18,
30]. Angiopoietin 1 (ANGPT1) expression on smooth muscle and perivascular cells plays an essential role in vascular maturation and mediates cell migration, adhesion, and survival [
17,
19,
33].
In the study presented here, ANGPT1 showed expression in both myoma tissue and surrounding myoma tissue, as well as in the unaltered uterine myoma of women in the control group. However, in the 70 women in the study (both myoma and peripheral tissue) as well as the 12 women in the control group (healthy uterine muscle), the values were not significantly different. In the study by Nakayama et al. [
34], ANGPT1 expression in myoma tissue was examined in 17 women, 13 (76.5%) were found to be positive. However, ANGPT1 values were not determined in the surrounding tissue or in the control group. We are the first to determine ANG1 expression of myomas, and in the surrounding tissue and in the unaltered uterine muscle.
The important role of angiopoietin 1 is evidenced by papers on its effect on endometrial bleeding and its beneficial effect in reducing the risk of early cervical cancer metastasizing to lymph nodes [
35,
36].
Angiopoietins (including ANGPT1) regulate angiogenesis and vasculogenesis through a variety of pro-angiogenic, anti-angiogenic, and growth factors - but also through calcium (Ca
2+)-related signaling [
16,
19].
Serum Ca
2+ levels in 149 women with uterine myomas in Nigeria have been described. These patients were found to have low Ca
2+ levels, and dietary supplementation with this element was suggested [
37]. Controversial data come from studies on Chinese women. In a case-control study, Ca
2+ concentrations were not statistically different (267 and 267 women, respectively) between them [
38]. Kopf et al. [
39] described a case of a large myoma in a pregnant woman (weighing 2834 g) that caused hypercalcemia with significant morbid symptoms that resolved after tumor removal.
The above-described controversial results of serum Ca2+ determinations were the reason for the calcium receptor (CASR) determination in the myometrial tumor, its periphery, and normal muscle of control women. Such determinations have not been encountered in the literature available. In 70 women with uterine myomas, CASR was determined in the tumor and its periphery. In both the myoma tumor and its periphery, CASR concentrations were lower than in the myometrium of healthy women. This result would support the suggestion by researchers at the University Hospital of Nigeria for calcium supplementation to prevent the development of large myomas in women with small myomas.
The relationship between angiogenesis, PTK2 (FAK1) expression, and Ca2+ concentration has been described [
19,
40]. Ang-1 can induce PTK2 phosphorylation, but at the same time, Ang-1 is associated with calcium activity [
19]. This was the rationale for studies on the mentioned molecules. There are no studies on PTK2 in uterine myomas in the literature.
In the pathology of many tumors, especially malignant ones, PTK2 is involved in the transmission of signals to and from the cell, migration, cell survival, and tumor invasion participating in its progression. Clinical trials with FAK inhibitors are currently underway [
40,
41,
42].
5. Conclusions
In our study, we detected that FAK (PTK2) expression is significantly lower in uterine myomas and their periphery compared to healthy uterine muscle tissue. This fact indicates that FAK is not involved in growth in benign cancerous tumors, and therefore, any clinical trials of FAK inhibitors are out of the question. It may be yet another histological biomarker that distinguishes benign from malignant tumors.
Author Contributions
Conceptualization, A.M.; methodology, J.Ż.; validation, P.K.; formal analysis, J.M.; investigation, M.K., M.deM.; resources, A.G., W.B., M.G; data curation, J.Ż.; writing—original draft preparation, A.M., M.deM., J.M.; writing—review and editing, M.deM.; visualization, M.deM.; supervision, J.Ż.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Bioethics Committee of the Karol Marcinkowski University of Medical Sciences in Poznan. According to the Bioethics Committee statement of January 16, 2020, the study does not have the characteristics of a medical experiment and, following Polish law and Good Clinical Practice (GCP), is not subject to evaluation by the Bioethics Committee. Informed consent was obtained from all participants taking part in the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pavone, D.; Clemenza, S.; Sorbi, F.; Fambrini, M.; Petraglia, F. Epidemiology and Risk Factors of Uterine Fibroids. Best Practice & Research Clinical Obstetrics & Gynaecology 2018, 46, 3–11. [Google Scholar] [CrossRef]
- Islam, M.S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular Matrix in Uterine Leiomyoma Pathogenesis: A Potential Target for Future Therapeutics. Hum Reprod Update 2018, 24, 59–85. [Google Scholar] [CrossRef]
- Ciebiera, M.; Ali, M.; Prince, L.; Jackson-Bey, T.; Atabiekov, I.; Zgliczyński, S.; Al-Hendy, A. The Evolving Role of Natural Compounds in the Medical Treatment of Uterine Fibroids. J Clin Med 2020, 9, 1479. [Google Scholar] [CrossRef]
- Moravek, M.B.; Yin, P.; Ono, M.; Coon, J.S.; Dyson, M.T.; Navarro, A.; Marsh, E.E.; Chakravarti, D.; Kim, J.J.; Wei, J.-J.; et al. Ovarian Steroids, Stem Cells and Uterine Leiomyoma: Therapeutic Implications. Hum Reprod Update 2015, 21, 1–12. [Google Scholar] [CrossRef]
- Cardozo, E.R.; Foster, R.; Karmon, A.E.; Lee, A.E.; Gatune, L.W.; Rueda, B.R.; Styer, A.K. MicroRNA 21a-5p Overexpression Impacts Mediators of Extracellular Matrix Formation in Uterine Leiomyoma. Reprod Biol Endocrinol 2018, 16, 46. [Google Scholar] [CrossRef]
- Firdaus, R.; Agrawal, P.; Anagani, M.; Vijayalakshmi, K.; Hasan, Q. Multiple Mutations in Exon-2 of Med-12 Identified in Uterine Leiomyomata. J Reprod Infertil 2021, 22, 201–209. [Google Scholar] [CrossRef]
- Santamaria, X.; Mas, A.; Cervelló, I.; Taylor, H.; Simon, C. Uterine Stem Cells: From Basic Research to Advanced Cell Therapies. Hum Reprod Update 2018, 24, 673–693. [Google Scholar] [CrossRef]
- Ciebiera, M.; Włodarczyk, M.; Ciebiera, M.; Zaręba, K.; Łukaszuk, K.; Jakiel, G. Vitamin D and Uterine Fibroids-Review of the Literature and Novel Concepts. Int J Mol Sci 2018, 19, 2051. [Google Scholar] [CrossRef]
- Markowska, A.; Kurzawa, P.; Bednarek, W.; Gryboś, A.; Mardas, M.; Krzyżaniak, M.; Majewski, J.; Markowska, J.; Gryboś, M.; Żurawski, J. Immunohistochemical Expression of Vitamin D Receptor in Uterine Fibroids. Nutrients 2022, 14, 3371. [Google Scholar] [CrossRef]
- Lee, J.-W.; Choi, H.J.; Kim, E.-J.; Hwang, W.Y.; Jung, M.-H.; Kim, K.S. Fisetin Induces Apoptosis in Uterine Leiomyomas through Multiple Pathways. Sci Rep 2020, 10, 7993. [Google Scholar] [CrossRef]
- Afrin, S.; Islam, M.S.; Patzkowsky, K.; Malik, M.; Catherino, W.H.; Segars, J.H.; Borahay, M.A. Simvastatin Ameliorates Altered Mechanotransduction in Uterine Leiomyoma Cells. Am J Obstet Gynecol 2020, 223, 733.e1–733.e14. [Google Scholar] [CrossRef]
- Russo, T.A.; Banuth, A.M.M.; Nader, H.B.; Dreyfuss, J.L. Altered Shear Stress on Endothelial Cells Leads to Remodeling of Extracellular Matrix and Induction of Angiogenesis. PLoS One 2020, 15, e0241040. [Google Scholar] [CrossRef]
- Chauhan, A.; Khan, T. Focal Adhesion Kinase-An Emerging Viable Target in Cancer and Development of Focal Adhesion Kinase Inhibitors. Chem Biol Drug Des 2021, 97, 774–794. [Google Scholar] [CrossRef]
- Machado-Lopez, A.; Simón, C.; Mas, A. Molecular and Cellular Insights into the Development of Uterine Fibroids. Int J Mol Sci 2021, 22, 8483. [Google Scholar] [CrossRef]
- Vega, R.; Carretero, M.; Travasso, R.D.M.; Bonilla, L.L. Notch Signaling and Taxis Mechanisms Regulate Early Stage Angiogenesis: A Mathematical and Computational Model. PLoS Comput Biol 2020, 16, e1006919. [Google Scholar] [CrossRef]
- Moccia, F.; Negri, S.; Shekha, M.; Faris, P.; Guerra, G. Endothelial Ca2+ Signaling, Angiogenesis and Vasculogenesis: Just What It Takes to Make a Blood Vessel. Int J Mol Sci 2019, 20, 3962. [Google Scholar] [CrossRef]
- Hwang-Bo, J.; Park, J.-H.; Chung, I.S. 3-O-Acetyloleanolic Acid Inhibits Angiopoietin-1-Induced Angiogenesis and Lymphangiogenesis via Suppression of Angiopoietin-1/Tie-2 Signaling. Phytother Res 2020, 34, 359–367. [Google Scholar] [CrossRef]
- Harel, S.; Sanchez-Gonzalez, V.; Echavarria, R.; Mayaki, D.; Hussain, S.N. Roles of miR-640 and Zinc Finger Protein 91 (ZFP91) in Angiopoietin-1-Induced In Vitro Angiogenesis. Cells 2020, 9, 1602. [Google Scholar] [CrossRef]
- Pafumi, I.; Favia, A.; Gambara, G.; Papacci, F.; Ziparo, E.; Palombi, F.; Filippini, A. Regulation of Angiogenic Functions by Angiopoietins through Calcium-Dependent Signaling Pathways. Biomed Res Int 2015, 2015, 965271. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, Z.; Dai, H. Calcium Concentration Response to Uterine Ischemia: A Comparison of Uterine Fibroid Cells and Adjacent Normal Myometrial Cells. Eur J Obstet Gynecol Reprod Biol 2014, 174, 123–127. [Google Scholar] [CrossRef]
- Wang, S.; Duan, H.; Li, B. Rapid Effects of Oestrogen on Intracellular Ca2+ in the Uterine Junctional Myometrium of Patients With and Without Adenomyosis in Different Phases of the Menstrual Cycle. Reprod Sci 2020, 27, 1992–2001. [Google Scholar] [CrossRef]
- Nicholson, T.A.; Pelage, J.P.; Ettles, D.F. Fibroid Calcification after Uterine Artery Embolization: Ultrasonographic Appearance and Pathology. J Vasc Interv Radiol 2001, 12, 443–446. [Google Scholar] [CrossRef]
- Gorvin, C.M. Calcium-Sensing Receptor Signaling - How Human Disease Informs Biology. Curr Opin Endocr Metab Res 2021, 16, 10–28. [Google Scholar] [CrossRef]
- Lechertier, T.; Reynolds, L.E.; Kim, H.; Pedrosa, A.R.; Gómez-Escudero, J.; Muñoz-Félix, J.M.; Batista, S.; Dukinfield, M.; Demircioglu, F.; Wong, P.P.; et al. Pericyte FAK Negatively Regulates Gas6/Axl Signalling to Suppress Tumour Angiogenesis and Tumour Growth. Nat Commun 2020, 11, 2810. [Google Scholar] [CrossRef]
- Pedrosa, A.-R.; Bodrug, N.; Gomez-Escudero, J.; Carter, E.P.; Reynolds, L.E.; Georgiou, P.N.; Fernandez, I.; Lees, D.M.; Kostourou, V.; Alexopoulou, A.N.; et al. Tumor Angiogenesis Is Differentially Regulated by Phosphorylation of Endothelial Cell Focal Adhesion Kinase Tyrosines-397 and -861. Cancer Res 2019, 79, 4371–4386. [Google Scholar] [CrossRef]
- Islam, M.S.; Castellucci, C.; Fiorini, R.; Greco, S.; Gagliardi, R.; Zannotti, A.; Giannubilo, S.R.; Ciavattini, A.; Frega, N.G.; Pacetti, D.; et al. Omega-3 Fatty Acids Modulate the Lipid Profile, Membrane Architecture, and Gene Expression of Leiomyoma Cells. J Cell Physiol 2018, 233, 7143–7156. [Google Scholar] [CrossRef]
- Katoh, K. FAK-Dependent Cell Motility and Cell Elongation. Cells 2020, 9, 192. [Google Scholar] [CrossRef]
- Tavora, B.; Reynolds, L.E.; Batista, S.; Demircioglu, F.; Fernandez, I.; Lechertier, T.; Lees, D.M.; Wong, P.-P.; Alexopoulou, A.; Elia, G.; et al. Endothelial-Cell FAK Targeting Sensitizes Tumours to DNA-Damaging Therapy. Nature 2014, 514, 112–116. [Google Scholar] [CrossRef]
- Chalcarz, M.; Żurawski, J. Injection of Aquafilling® for Breast Augmentation Causes Inflammatory Responses Independent of Visible Symptoms. Aesthetic Plast Surg 2021, 45, 481–490. [Google Scholar] [CrossRef]
- Ciarmela, P.; Delli Carpini, G.; Greco, S.; Zannotti, A.; Montik, N.; Giannella, L.; Giuliani, L.; Grelloni, C.; Panfoli, F.; Paolucci, M.; et al. Uterine Fibroid Vascularization: From Morphological Evidence to Clinical Implications. Reproductive BioMedicine Online 2022, 44, 281–294. [Google Scholar] [CrossRef]
- Don, E.E.; Middelkoop, M.-A.; Hehenkamp, W.J.K.; Mijatovic, V.; Griffioen, A.W.; Huirne, J.A.F. Endometrial Angiogenesis of Abnormal Uterine Bleeding and Infertility in Patients with Uterine Fibroids-A Systematic Review. Int J Mol Sci 2023, 24, 7011. [Google Scholar] [CrossRef]
- Grube, M.; Neis, F.; Brucker, S.Y.; Kommoss, S.; Andress, J.; Weiss, M.; Hoffmann, S.; Taran, F.-A.; Krämer, B. Uterine Fibroids - Current Trends and Strategies. Surg Technol Int 2019, 34, 257–263. [Google Scholar]
- Bowler, E.; Oltean, S. Alternative Splicing in Angiogenesis. Int J Mol Sci 2019, 20, 2067. [Google Scholar] [CrossRef]
- Nakayama, T.; Inaba, M.; Naito, S.; Mihara, Y.; Miura, S.; Taba, M.; Yoshizaki, A.; Wen, C.-Y.; Sekine, I. Expression of Angiopoietin-1, 2 and 4 and Tie-1 and 2 in Gastrointestinal Stromal Tumor, Leiomyoma and Schwannoma. World J Gastroenterol 2007, 13, 4473–4479. [Google Scholar] [CrossRef]
- Middelkoop, M.-A.; Don, E.E.; Hehenkamp, W.J.K.; Polman, N.J.; Griffioen, A.W.; Huirne, J.A.F. Angiogenesis in Abnormal Uterine Bleeding: A Narrative Review. Hum Reprod Update 2023, 29, 457–485. [Google Scholar] [CrossRef]
- Cai, E.; Yang, D.; Zhang, Y.; Cai, J.; Sun, S.; Yang, P.; Huang, Y.; Han, Q.; Xiong, Z.; Wang, S. Angiopoietin-1 Is Associated with a Decreased Risk of Lymph Node Metastasis in Early Stage Cervical Cancer. Histol Histopathol 2020, 35, 1029–1034. [Google Scholar] [CrossRef]
- Adeboje-Jimoh, F.; Okunade, K.S.; Olorunfemi, G.; Olamijulo, J.A. Serum Calcium and Magnesium Levels in Women with Uterine Fibroids at a University Teaching Hospital in Southwest Nigeria: A Comparative Cross-Sectional Study. Res Sq 2023, rs.3.rs-2877359. [Google Scholar] [CrossRef]
- Li, S.; Chen, B.; Sheng, B.; Wang, J.; Zhu, X. The Associations between Serum Vitamin D, Calcium and Uterine Fibroids in Chinese Women: A Case-Controlled Study. J Int Med Res 2020, 48, 300060520923492. [Google Scholar] [CrossRef]
- Kropf, J.; Vo, M.; Cheyney, S.; Kayaleh, O.; McWhorter, J.; Carlan, S.J. Hypercalcemia Resulting from Necrotizing Leiomyoma in a Pregnant Female. Am J Case Rep 2020, 21, e923412. [Google Scholar] [CrossRef]
- Brullo, C.; Tasso, B. New Insights on Fak and Fak Inhibitors. Curr Med Chem 2021, 28, 3318–3338. [Google Scholar] [CrossRef]
- Dawson, J.C.; Serrels, A.; Stupack, D.G.; Schlaepfer, D.D.; Frame, M.C. Targeting FAK in Anticancer Combination Therapies. Nat Rev Cancer 2021, 21, 313–324. [Google Scholar] [CrossRef]
- Quispe, P.A.; Lavecchia, M.J.; León, I.E. Focal Adhesion Kinase Inhibitors in the Treatment of Solid Tumors: Preclinical and Clinical Evidence. Drug Discov Today 2022, 27, 664–674. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).