1. Introduction
In chronic myeloid leukemia (CML), age as a prognostic factor has been significantly recognized and has been included into the Sokal and EURO scores [
1,
2]. However, the advent of tyrosine kinase inhibitors (TKIs) has revolutionized the clinical strategy for the disease, substantially enhancing outcomes, even among older patients [
3]. In the pre-TKIs era, the treatment approach for elderly CML patients primarily focused on minimizing the disease burden, considering therapy-related toxicities and the frailty of the patients [
4].
Significantly, starting from 2018, the average life expectancy for male CML patients aged over 65 is approximately 16 years, while for those aged over 75, it is around 9 years [
5]. However, despite these encouraging statistics, age continues to be considered in the latest ELTS score, albeit with reduced influence on outcomes [
6]. Managing elderly CML patients continues to pose a challenge, highlighting the need for tailored approaches in this population.
Comorbidity, geriatric syndromes, and polypharmacy are frequently heightened in elderly CML patients [
7]. Furthermore, age-related variations in pharmacokinetic and pharmacodynamic parameters can alter drug tolerance and efficacy [
8]. Consequently, older patients are often excluded from participation in clinical trials, and available data primarily stem from real-world experiences [
9]. Notably, Rohrbacher et al. demonstrated that CML patients enrolled in clinical trials are, on average, ten years younger than those who are not included [
10]. While imatinib (IMA) has demonstrated excellent safety and efficacy, even in very elderly patients (i.e., aged ≥ 75 years) [
11], there is limited knowledge regarding the use of next-generation TKIs in this population. Although age does not hinder the achievement of molecular responses in older patients treated with second (2G) or third-generation (3G) TKIs, the presence of comorbidities may limit their prescription [
12]. Consequently, a careful evaluation is necessary to mitigate the risk of life-threatening adverse events (AEs).
Dose-optimization strategies have shown favorable outcomes in real-world in minimizing toxicity while ensuring the attainment and sustenance of molecular responses [
13,
14,
15,
16]. This approach can be particularly advantageous for older or frail patients at diagnosis or during follow-up, helping to mitigate persistent or low-grade AEs such as fatigue or edema associated with IMA and diarrhea linked to bosutinib (BOS) [
17]. Furthermore, the goal of limiting cardiovascular and pulmonary toxicities associated with nilotinib (NIL), dasatinib (DAS), and ponatinib (PON) can be accomplished through dose reduction in patients achieving optimal response [
12].
Limited research has specifically focused on the efficacy and safety of TKIs in very elderly CML patients. Furthermore, data on the feasibility of using PON and asciminib (ASC) in very elderly patients who have shown resistance to prior treatment lines are still limited. Therefore, the aim of this retrospective multicentric study was to evaluate the safety and efficacy of TKIs in a cohort of 123 newly diagnosed very elderly CML patients, providing valuable real-world data.
2. Materials and Methods
We performed a retrospective analysis on 123 newly diagnosed CML patients aged ≥ 75 years, from January 2003 to January 2023. Four Italian centers collaborated and provided required data after obtaining informed consent from all patients. Diagnostic and response criteria were based on current European LeukemiaNet (ELN) recommendations [
18]. Information extracted from patients' records included data about to age, medical history, medication history, disease stage, and risk of progression based on Sokal and ELTS scores at the time of diagnosis [
1,
6]. Comorbidities burden have been evaluated with the Charlson Comorbidity Index (CCI) [
19]. Data regarding the treatment duration for each TKI in the first, second, third, and fourth lines of therapy were collected, including information on the initial doses and any dose reductions during follow-up. Hematological and non-hematological toxicities were assessed and graded based on the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [
20].
Bosutinib was administered as first-line treatment in a patient enrolled in the BFORE registration trial (NCT02130557), which compared bosutinib with imatinib as first-line therapy [
21].
Cytogenetic responses were evaluated using standard G-banded karyotype on bone marrow (BM) aspirates, or by fluorescent in situ hybridization (FISH) on BM interphasic cells. Transcription levels of
BCR::ABL1 were evaluated by RQ-PCR in certified laboratories and molecular responses (MR) defined according to standardized criteria as Major Molecular Response (MMR) (
BCR::ABL1IS ≤ 0.1%) and Deep Molecular Response (DMR) (MR4.0,
BCR::ABL1IS ≤0.01%; MR4.5,
BCR::ABL1IS ≤ 0.0032%; MR5.0,
BCR::ABL1IS ≤ 0.001%) [
18].
Continuous variables were reported as medians and ranges, while categorical variables were presented as frequencies and percentages. Overall survival (OS) was calculated from the date of diagnosis until the time of death or last follow-up, while event-free survival (EFS) was calculated from the initiation of each TKI treatment until treatment failure, discontinuation for any reason, progression to accelerated phase (AP), or blast phase (BP). Progression-free survival (PFS) was calculated from the start date until progression to AP or BP. Group comparisons were conducted using appropriate statistical tests such as unpaired t-tests, chi-square tests, and Fisher's exact test. A significance level of p<0.05 was considered statistically significant. Survival analysis was performed using Kaplan-Meier curves, and differences were assessed using log-rank tests. Univariate and multivariate analyses were carried out using Cox regression analysis to determine hazard ratios (HR) and 95% confidence intervals (95% CI) for factors associated with survival. The analysis was conducted using R (R Core Team, 2020) and RStudio (RStudio Team, 2020).
3. Results
3.1. Patients
A total of 123 very elderly newly diagnosed CML patients were included in the study. The patient characteristics are displayed in
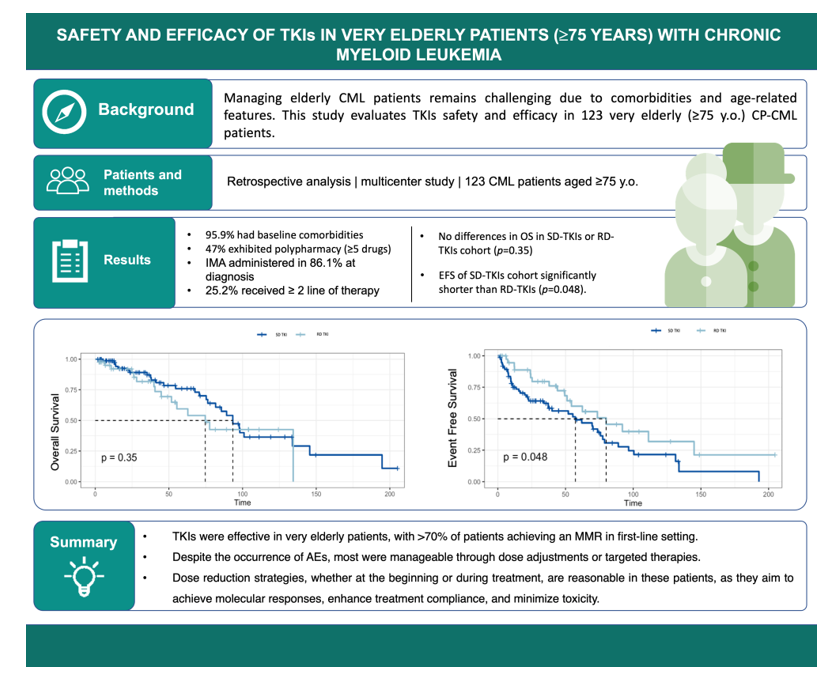
Table 1. The median age at diagnosis was 80 years (range: 75-96). Among the patients, 48% were aged 85 years or older. All patients were in chronic phase (CP) of CML. Based on the Sokal score, 0.8% of patients had a low risk, 71.5% had an intermediate risk, and 27.6% had a high risk. According to the ELTS score, 3.2% of patients were classified as low risk, 61.7% as intermediate risk, and 34.9% as high risk. Baseline comorbidities were documented in 118 out of 123 patients (95.9%).
Collectively, 103 patients (83.7%) had cardiovascular (CV) risk factors at baseline, including hypertension, dyslipidemia, type 2 diabetes, and smoking habit, while 52 (42.2%) had a history of cardiovascular disease (CVD). Among these patients, 7 (5.6%) had chronic heart failure, and 13 (10.4%) had atrial fibrillation. Moreover, 10 patients (8.1%) had a history of myocardial infarction and 13 (10.4%) of stroke or transient ischemic attack (TIA). The CCI was 0 in 55 patients, 1 in 28 patients and ≥ 2 in 40 patients. In total, 116 patients (94.3%) assumed concomitant medications for any causes, with an average of 4.5 drugs per person (range: 0-13). Polypharmacy, defined as the use of ≥ 5 drugs, was found in 47% of the patients.
3.2. Response rate and survival
In frontline therapy, patients were treated with IMA (n=101; 86.1%), DAS (n=9; 7.1%), NIL (n=7; 5.6%) and BOS (n=1; 0.81%). Median doses and duration for each line of therapy are shown in Table 2.
Dose-reduced TKIs were administered in 45 patients (36.5%) in first line; patients treated with reduced doses of TKIs (RD-TKIs) were older compared to those treated with standard doses (SD-TKIs) (median age: 79 years vs. 82 years, p=0.0003), with no significant differences in the number of comorbidities between the two groups (p=0.11).
Overall, the 3-month cytogenetic response was assessed in 75 patients, with 29.3% achieving Partial Cytogenetic Response (PCyR) and 45.3% achieving Complete Cytogenetic Response (CCyR). Among patients evaluable for MR, 71.9% (64/89) achieved an early molecular response (EMR, i.e. 3-month BCR::ABL1IS <10%). At 6, 12, and 24 months of treatment, MR3 was achieved by 35.7% (30/84), 55.7% (39/70), and 75.0% (42/56) of patients, respectively. Additionally, at these timepoints, 16.6%, 35.7%, and 51.7% of patients achieved a DMR. Overall, 29.9% of patients did not achieve a MR in the first-line treatment.
The cumulative incidence of MMR at 6, 12, and 24 months in evaluable patients was 37% (95% CI: 26-48), 56% (95% CI: 43-67), and 81% (95% CI: 67-89) for patients treated with SD-TKIs. For those treated with RD-TKI, the cumulative incidence was 18% (95% CI: 6.9-32), 52% (95% CI: 32-68), and 74% (95% CI: 51-87). No statistically significant differences were observed between the two patient groups (p=0.3).
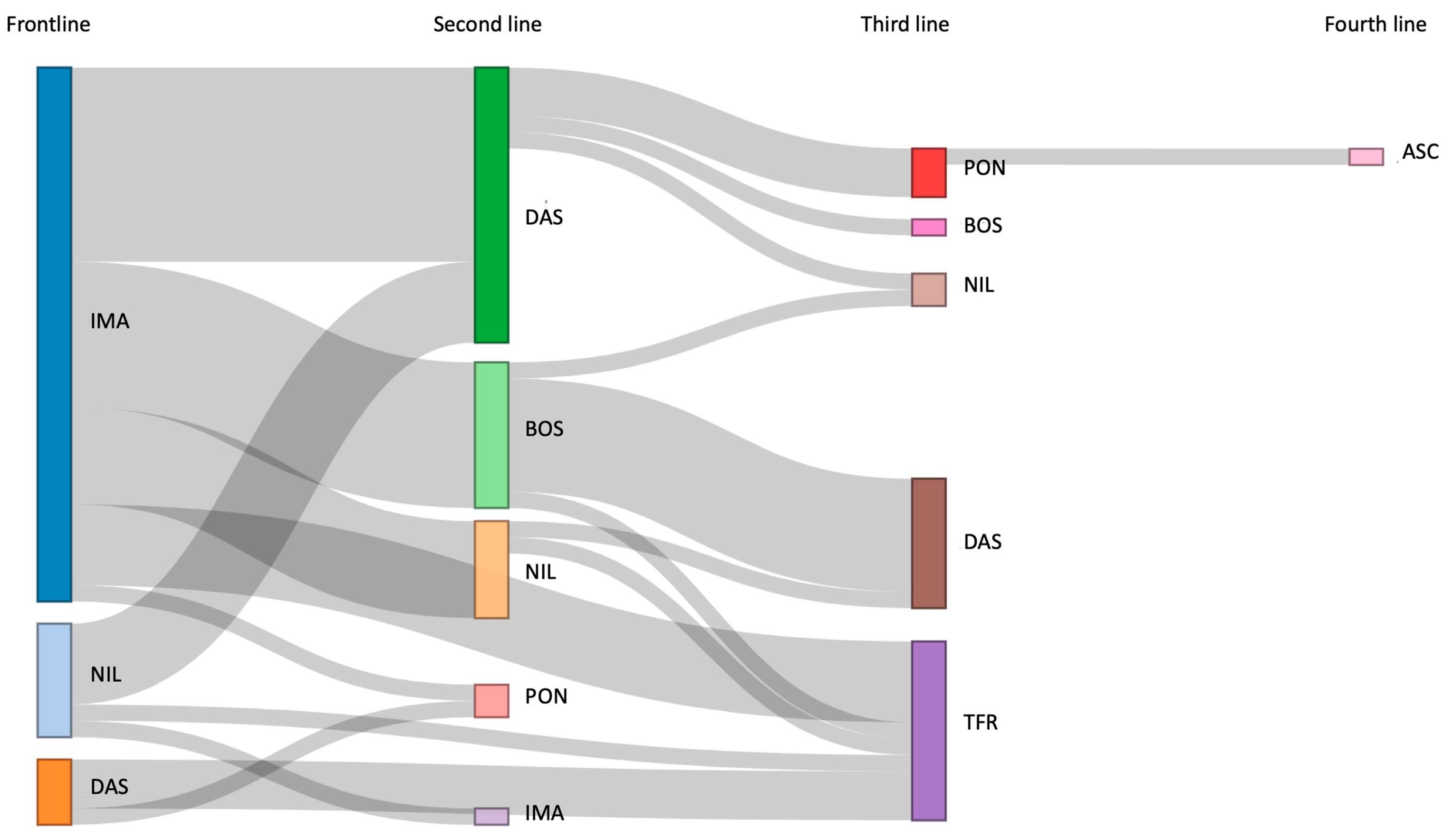
Thirty-one patients (25.2%) switched to a second line of treatment (
Figure 1), mainly for resistance to treatment (64.5%): thirteen patients switched to DAS (41.9%), nine to BOS (29.0%), six to NIL (19.3%), two to PON (6.4%) and one to IMA (3.2%). In the second line, 19 patients (61.2%) were treated with RD-TKIs. Over the 24 months of follow-up, 48.3% of patients achieved either MR3 or DMR, with only one patient being unevaluable for MR. In the second line as well, the cumulative incidence of MMR at 6, 12, and 24 months did not differ between those administered with SD-TKIs or RD-TKIs (p=0.2).
Nine patients switched to a third line of treatment, primarily due to resistance in seven patients (77.7%), and intolerance in two patients (22.2%). Mutation analysis identified the T315I mutation in one patient and G442E mutation in another. PON was administered in five patients, while two patients switched to NIL, and two to DAS and BOS each. Four patients achieved an MR3 or DMR during the 24 months of follow-up, other four patients never achieved any MR, while one patient was not evaluable for MR.
Only one patient switched to a fourth line due to the T315I mutation and initiated ASC at the dose of 200 mg BID. Basal BCR::ABL1IS was 35%; after 3 months of treatment the patient achieved the MMR and maintained it until the last follow-up. After 58 days of treatment, the patient experienced a grade 3 increase of transaminases, leading to a temporary discontinuation of ASC. The TKI was reintroduced at a reduced dosage once toxicity resolved, and no new episodes were reported thereafter.
Overall, median follow-up was 46.62 months (range: 1.8-206.2). Out of the total cohort, 43 patients (34.9%) died, mainly from causes unrelated to CML (93%), while three patients died due to progression to BP.
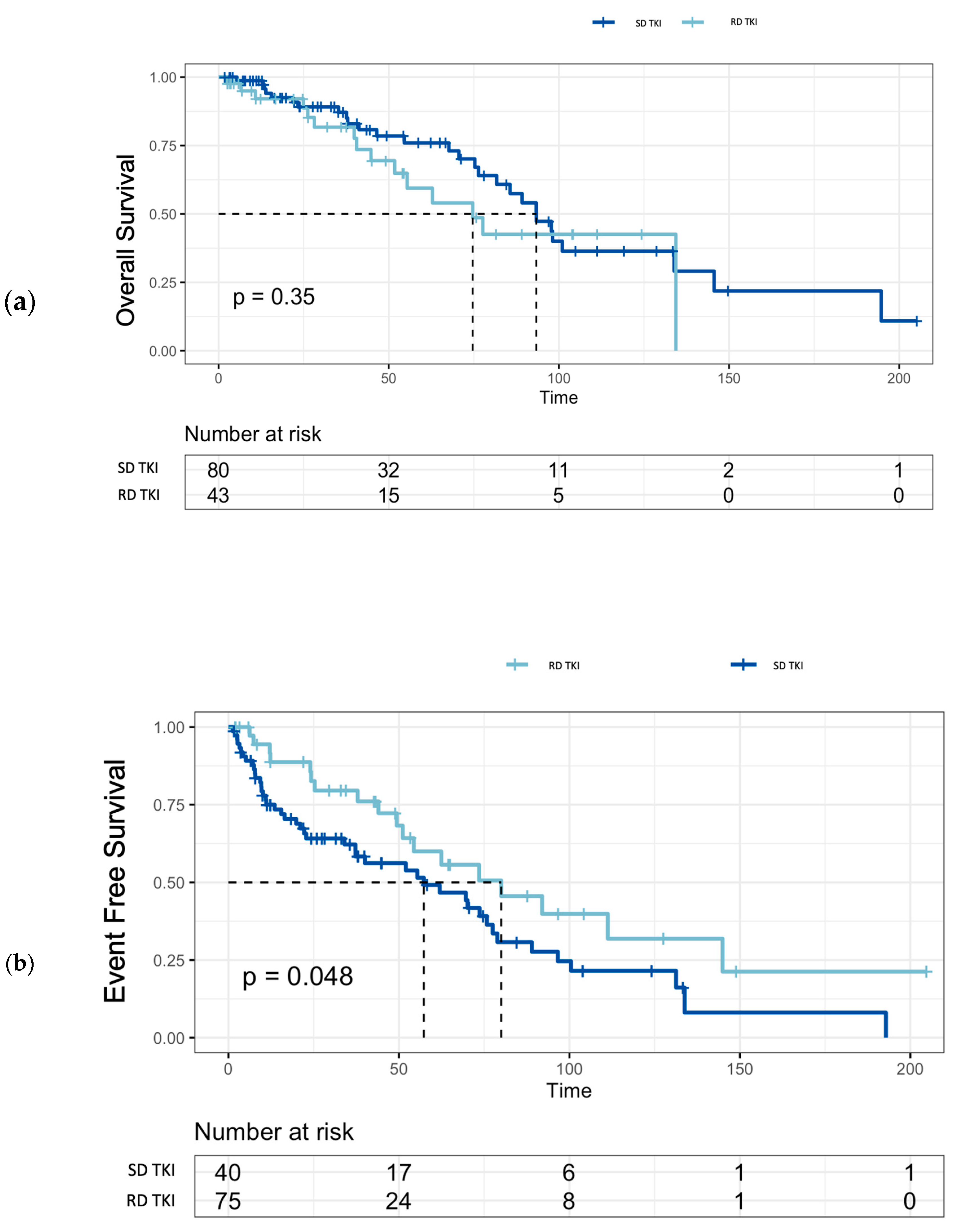
The OS rates for the entire cohort were 96.6% (95% CI: 0.93-0.99) at 1 year, 71.9% (95% CI: 0.63-0.81) at 5 years and 37.7% (95% CI: 0.27-0.52) at 10 years of follow-up. There was no significant difference in OS between patients treated with SD-TKIs or RD-TKIs (p=0.35) (
Figure 2). In evaluable patients (n=115), EFS was 62 months (95% CI: 44-78) in the first-line treatment, while it was 57 months (95% CI: 20-90) in the second-line treatment. Additionally, median EFS of patients treated with SD-TKIs was significantly shorter compared to RD-TKIs (80 months vs. 57 months, p=0.048) (
Figure 2).
Overall, the PFS was 95.3% (95% CI: 0.90-1). Univariate and multivariate analyses were performed to assess the predictive role of various baseline characteristics (age at diagnosis, sex, Sokal score, ELTS score, CCI, comorbidities, and polypharmacy) as well as treatment-related factors (number of treatment lines, grade ≥ 3 AEs) on survival outcomes in the entire patient cohort. Among these factors, only age at diagnosis ≥ 80 years demonstrated a negative prognostic impact in both the univariate analysis (p=0.00017) and the multivariate analysis (p=0.00079), indicating that very older age at diagnosis was associated with poor survival outcome.
3.3. Adverse events
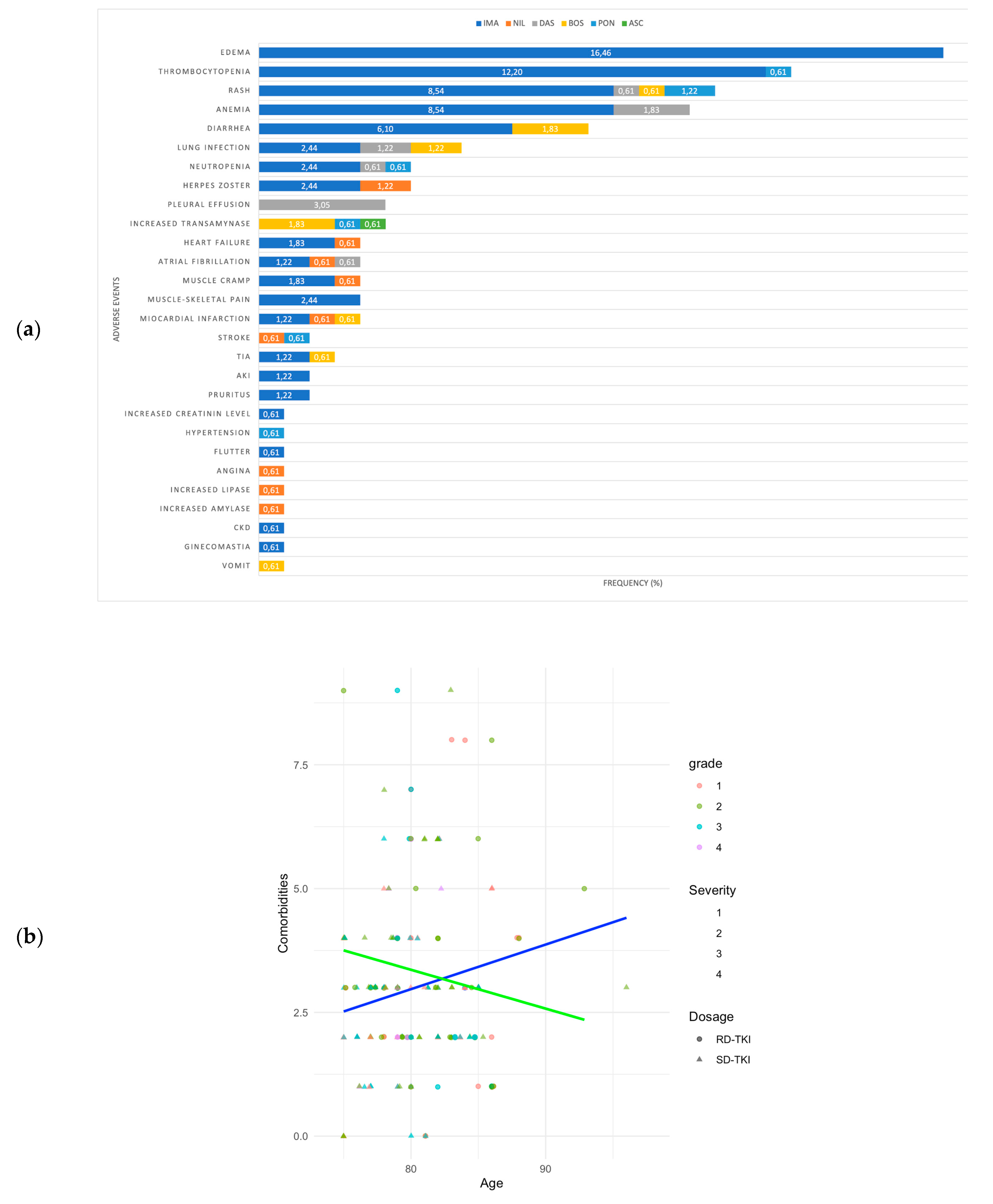
During the follow-up period, AEs were observed in 97 patients (78.8%), with the majority classified as non-hematological (72.4%) and graded as 1-2 according to the CTCAE criteria.
Figure 3 (a) provides a summary of the most commonly reported AEs. The most frequently observed AEs included edema, diarrhea, and cutaneous rash, predominantly of grade 1 (range: 1-3).
Pleural effusion was detected in patients treated with DAS both in frontline (n=1, 80 mg/day; n=2, 100 mg/day) and second-line setting (n=2, 100 mg/day). The median age of these patients was 79.3 years (range: 76-83), and the median time of onset was 34.6 months (range: 4.9-86.7) in the frontline setting and 11 months (range: 5-17) in the second-line setting. Permanent discontinuation of treatment was required for two patients, one with grade 3 and another with recurrent grade 2 pleural effusion, while others were managed through dose reduction and/or specific therapy.
Overall, there were 22 cardiovascular adverse events (CVAEs) in 17 patients (13.8%), including 15 patients with CV risk or a CVD at baseline. Among these, 9 patients were < 80 years old, while 6 patients were 80 years old or older. Specifically, in the first-line setting, 3 patients treated with IMA experienced acute heart failure, 3 had a myocardial infarction, 3 had a TIA, 3 had atrial fibrillation, and 1 had atrial flutter. The average dose of IMA administered was 400 mg/day. Among patients treated with next-generation TKIs as first-line therapy, one patient receiving NIL at a daily dose of 600 mg experienced episodes of angina. In the second-line treatment, one patient receiving NIL at 600 mg/day exhibited acute heart failure and atrial fibrillation, while another patient suffered a stroke. A patient treated with DAS at 50 mg/day developed atrial fibrillation. A patients treated with BOS 500 mg/day had a TIA and a newly diagnosed hypertension was observed in a patient treated with PON at a daily dose of 45 mg. In the third-line setting, a patient receiving PON at 15 mg/day experienced a stroke and a patient treated with BOS 500 mg/day had a myocardial infarction. No statistically significant difference was found between SD-TKIs and RD-TKIs (p=1).
Hematological AEs were observed across all treatment lines, typically manifesting with a median onset time of 3.53 months and mostly of grade 3 (range: 1-4). Among these, grade 3 thrombocytopenia (range: 1-4) was the most commonly observed hematological AE. Permanent discontinuation was necessary in three patients due to grade 4 hematological AEs, while most of the grade 1-3 AEs were managed through dose reductions or temporary drug withdrawal.
Collectively, grade ≥ 3 AEs occurred in 46 patients, with 35 treated with SD-TKIs and 11 with RD-TKIs (p=0.033). No statistically significant differences were found in OS between those who experienced grade ≥ 3 AEs and those who did not (p=0.24). Discontinuation of first-line treatment were required in 50 patients (40.7%), with 70% being temporary and 30% permanent. These interruptions were more frequent in patients aged ≥ 80 years (50%) compared to patients aged < 80 years (30.5%, p=0.042).
3.4. Treatment free remission
Overall, treatment-free remission (TFR) was successfully achieved in 11 patients after a median follow-up of 81.80 months (range: 12.6-103.3) (
Figure 1). Specifically, discontinuation was considered in seven patients based on the duration and depth of MR, while in four patients it was performed due to the emergence of severe AEs. The median age at suspension was 85 years (range: 82-93). Two patients had received more than one line of treatment, and five patients were treated with first-line RD-TKIs. After a median follow-up of 15.9 months (range: 1-46), 8 patients maintained DMR, while molecular recurrence (MMR or higher) was observed in three patients (27.2%) after a median time of 10.6 months (range: 1-27). Consequently, previous TKIs was restarted, and one out of the three patients had regained a DMR (MR4).
4. Discussion
The improvement in life expectancy of the general population has led to an increasing number of CML diagnoses in advanced age, with approximately 30% of patients falling into this category [
22]. Furthermore, the enhanced survival rates in CML, now comparable to those of the general population, have resulted in a greater number of patients aging during treatment, thereby complicating the decision-making process due to age-related changes, impaired functional status, comorbidities, or reduced social support [
3,
8]. Overall, these factors make it particularly important to assess the safety and efficacy of TKIs even for very elderly patients.
Consistent with literature trends, most very elderly patients in our cohort received IMA [
9]. However, there were no significant differences in age (
p=0.32) or comorbidity count (
p=0.73) between those treated with IMA vs. 2G TKIs. Additionally, CVR factors or a history of CVD did not significantly impact the initial treatment choice, with no statistically significant difference IMA and next-generation TKI-treated patients (
p=0.77).
In recent decades, the ageing population has seen a rise in multimorbidity (i.e., two or more chronic conditions) and polypharmacy [
23]. Despite this trend, evidence in literature supports the efficacy of TKIs in this context, including those aged ≥ 75 years [
11,
24,
25,
26]. Consistent with this, polypharmacy did not impact patient outcomes in our study. Furthermore, while comorbidities at diagnosis have been linked to poorer outcomes [
27], they did not significantly influence survival in our cohort.
In literature, experiences have reported symptoms ranging from moderate to severe in up to one-third of CML patients, while persistent mild symptoms were noted in up to 90% of cases [
29,
30]. Our cohort exhibited good tolerability to TKIs. The data from our study align with existing literature, with most AEs being of mild grade. Commonly reported events, such as edema, thrombocytopenia, and skin rash, were more prevalent in the cohort treated with IMA, reflecting the larger cohort size and the older age of these patients compared to those treated with next-generation TKIs. Importantly, the development of grade ≥ 3 AEs did not significantly impact on the patients' prognosis. Pleural effusion is a known event during treatment with DAS, with a frequency ranging from 20-30% [
26,
31,
32,
33]. In addition, the multivariate analysis of the DASISION and CA180-034 trial only identified age as a risk factor associated with the development of pleural effusion [
33]. We reported pleural effusion in 22.7% of patients treated with DAS in first and second line, predominantly of mild grade and managed with specific therapy and temporary withdrawal of TKI.
In addition, we observed CVAEs in 13.8% of the enrolled patients. Age is considered the primary driver for CV diseases, as their incidence increases with aging due to increased oxidative stress, inflammation, apoptosis, and myocardial dysfunction [
34,
35]. Importantly, our analysis has reported, for the first time, the actual incidence of CVAEs in a large cohort of very elderly patients treated with various TKIs in subsequent lines of treatment. Despite age being identified as a risk factor, we observed a higher number of CVAEs in patients under 80 years old. One possible explanation, as mentioned earlier, could be a higher tendency to use SD-TKIs in those aged ≤ 80 years, although without significative differences in CVAE incidence between SD and RD-TKIs.
Recently, attention has been focused to dose optimization of TKIs to achieve a delicate balance between efficacy and safety. Emerging evidence consistently supports the safety and feasibility of dose adjustments at baseline and throughout the course of treatment, aiming to enhance treatment adherence, minimize drug interruptions, and ensure the maintenance of cytogenetic and molecular response [
17]. Moreover, safety and efficacy of RD-TKIs have been reported in elderly patients [
4,
11,
14,
26,
36,
37]. For instance, Seo et al. conducted a comprehensive analysis of TKIs dosing patterns in a cohort of 378 patients with a median age of 75 years, revealing that RD-TKIs were administered in 65.9% of patients at the latest follow-up, with no discernible impact on OS [
38]. In our cohort, 36.5% of patients started treatment with RD-TKIs, while the proportion of patients receiving RD-TKIs had increased to >60% in second line and in all patients in subsequent lines. Among them, 27 patients experienced dose reduction due to severe or persistent AEs. In any case, TKIs were well tolerated, and treatment resistance was the main reason for switching TKIs in each treatment lines. Importantly, we observed a significant decrease in grade ≥3 AEs in the first-line RD-TKIs cohort compared to SD-TKIs (
Figure 3 b). This reduction in AEs can be primarily attributed to the initial dose reduction strategy.
Furthermore, the comparison between patients treated with SD-TKIs or RD-TKIs did not show any differences in terms of OS (
Figure 2); on the contrary, EFS in the standard-dose group was shorter compared to the patients in the reduced-dose group, suggesting that in this patient population, a rational use of reduced doses may contribute to improving treatment adherence and maintaining the MMR without compromising OS.
To date, real-life data on the use of PON in the elderly population are lacking. The phase 2 PACE trial included individuals aged ≥65 years, demonstrating PON efficacy in a heavily pretreated population and satisfactory safety in selected patients. Adverse occlusive events (AOEs) were observed in 26% of CML patients at 5 years, with a higher relative risk (RR) in patients with ≥2 risk factors (RR: 2.2, 95% CI: 0.5-1.2) and those aged ≥65 years (RR: 1.7, 95% CI: 1.2-2.5) [
39]. Moreover, a discrete-time Markov model analysis conducted on dose-optimization strategy OPTIC trial showed that patients aged ≥ 65 years had a lower likelihood of achieving MR2 at any dose (45 mg, 30 mg or 15 mg/daily) compared to those aged < 65 years. Age was significantly associated with the development of AOEs, with an increased risk in older ages (HR: 1.2, 95% CI: 1.07-1.44) [
40,
41]. We reported outcomes of seven very elderly patients treated with PON at median age of 82 years (range: 79-84), including two in second line setting and five in the third-line setting. In total, the median follow-up was 34 months. All patients switched treatment due to resistance to previous lines, including two cases with the T315I mutation. Among them, only one patient, who received PON in the third-line setting at a dosage of 15 mg per day, achieved a MMR and experienced DMR within 6 months of treatment. In the remaining patients, treatment response was influenced by poor adherence to therapy, leading to frequent interruptions and modifications of the treatment schedule. Five patients experienced AEs, including a case of ischemic stroke in a patient with a history of hypertension and dyslipidemia, who was treated with PON 30 mg. Taken together, these data suggest the potential efficacy of PON even in this setting of elderly and heavily pretreated patients. However, caution is required when prescribing PON in very elderly patients, necessitating a careful evaluation of CVR factors and the development of a prevention plan once the medication is initiated. Nonetheless, dedicated studies are necessary to assess the feasibility and true safety of PON in patients aged ≥ 65 years.
We also present the case of a patient of 86 years treated with fourth-line ASC. To date, there are no specific studies available in this setting. Elderly patients were included in the pivotal trial ASCEMBL, which compared ASC 40 mg BID with BOS 500 mg QD in patients previously treated with ≥ 2 TKIs, including 29 patients aged ≥ 65 years and 4 patients aged ≥ 75 years. At the last follow-up at 96 weeks, the MMR rate was 37.6% in patients treated with ASC compared to 15.8% with BOS. ASC showed favorable MMR rates at 96 weeks in patients aged ≥65 years (4.6%, 95% CI: -25.1 to 34.3) and ≥75 years (50%, 95% CI: -19.3 to 100.0). Grade ≥3 AEs occurred in 56.4% of ASC-treated patients, with more CV events compared to the BOS arm [
42]. Our patient, resistant to previous therapies including PON, achieved MMR within 3 months with ASC (200 mg twice daily). Good tolerability was observed, except for a temporary grade 3 elevation in liver transaminases. Further studies are needed to assess efficacy and safety of ASC, especially in the elderly population.
Previously, the treatment goal for CML was long-term disease control. However, achieving TFR has now emerged as an important objective for many CML patients who obtain a stable response to treatment.
43 Pioneering studies with IMA, followed by those with 2G TKIs, have demonstrated the feasibility of treatment suspension, even in the elderly population [
36,
44]. The findings from our cohort are in line with the literature's reported data. Additionally, administering reduced doses to five patients did not hinder their ability to attain an optimal molecular response for TFR, while maintaining favorable outcomes.