Submitted:
01 December 2023
Posted:
07 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. L. (L.) Infantum Cultures and Production of Crude Leishmania Antigen (CLA) and Leish-EVs
2.3. Ultrastructural Analyses of Promastigotes Releasing Leish-EVs by Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM)
2.4. THP-1 Cell Line and THP-1 Released EVs (THP-1-EVs) Production
2.5. Concentration Determination of Leish-EVs and THP-1-EVs by Nanoparticle Tracking Analysis (NTA)
2.6. Immunological Investigations
2.7. Cytokine and miRNA Analysis by Gene Expression in Quantitative Real Time PCR (qPCR)
2.8. Data Analysis
3. Results
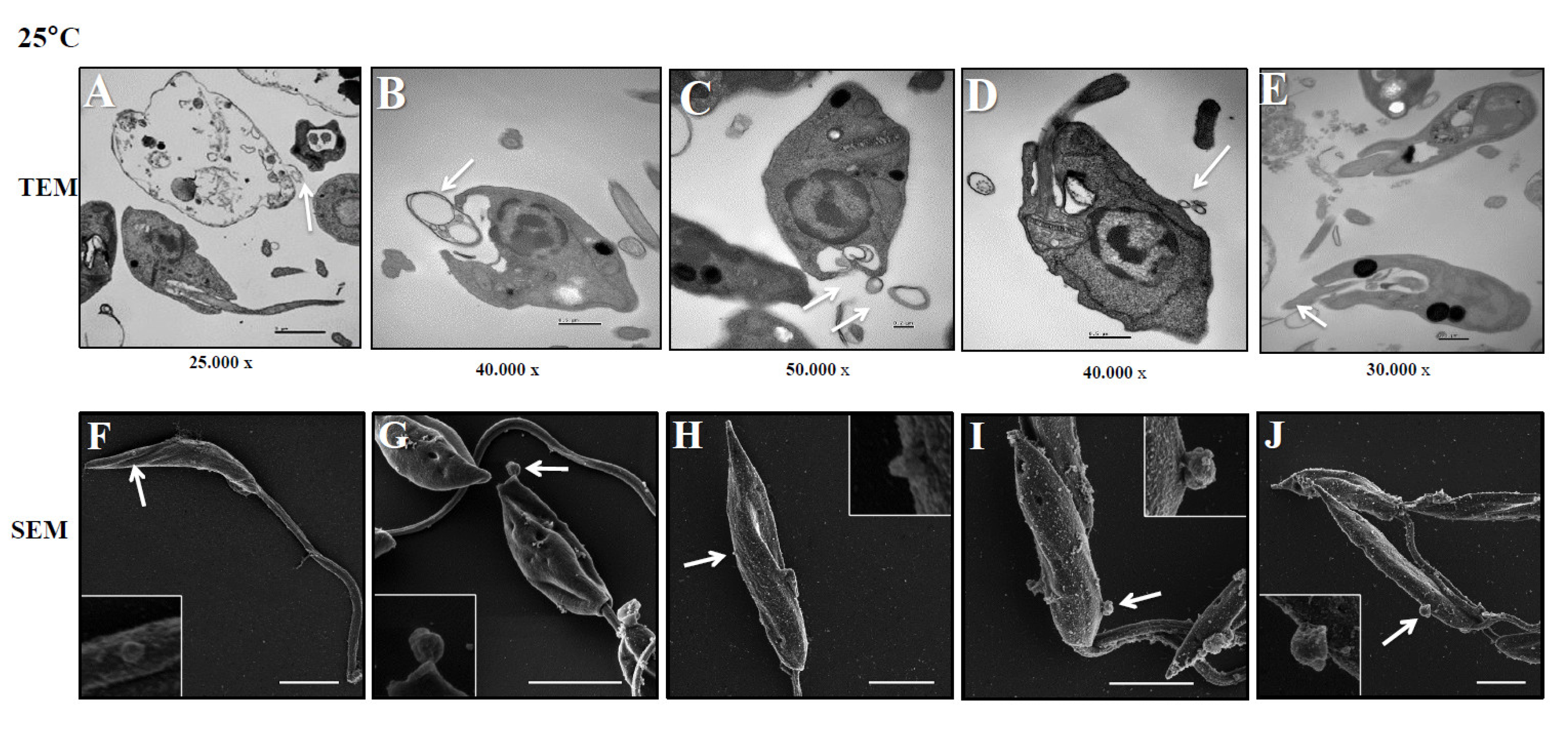
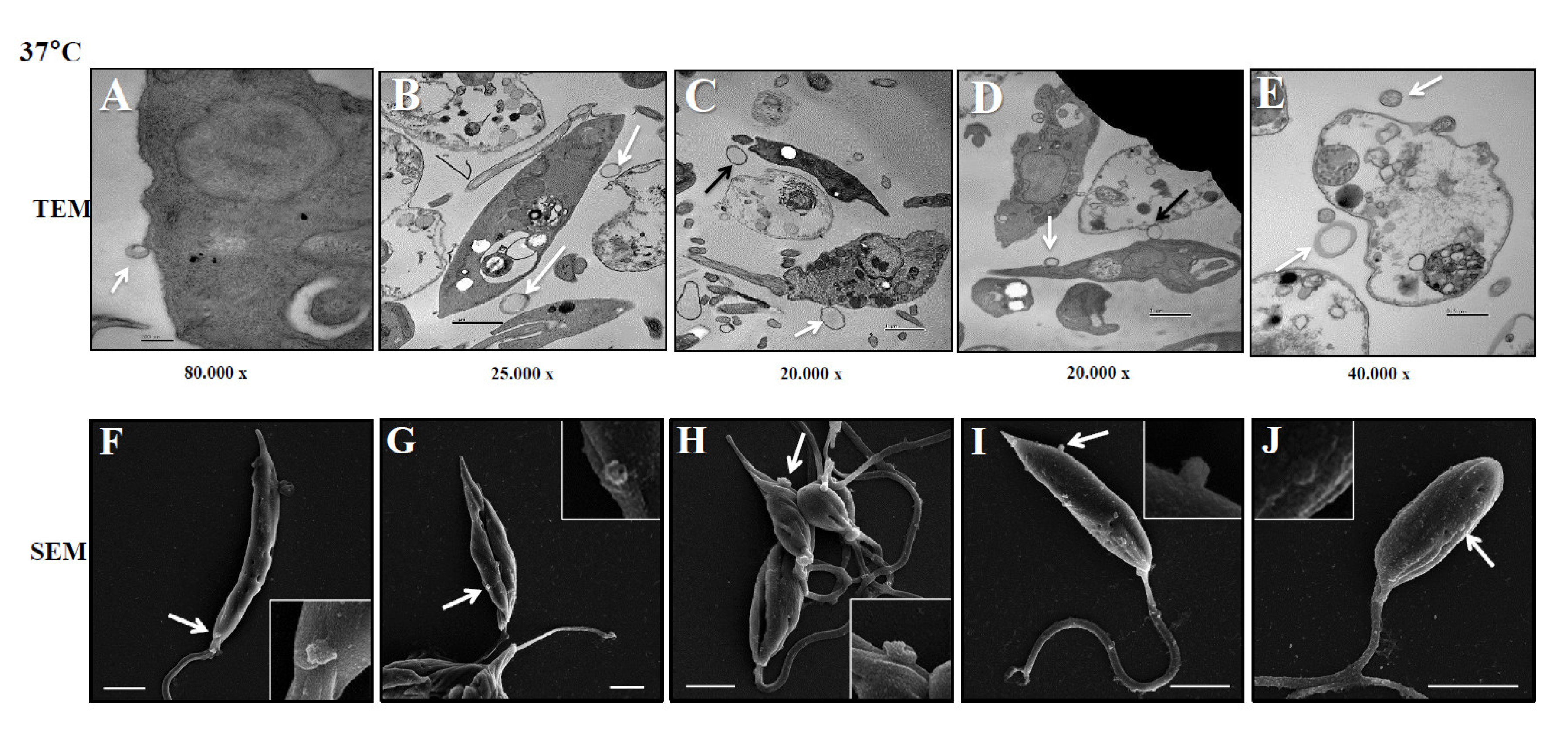
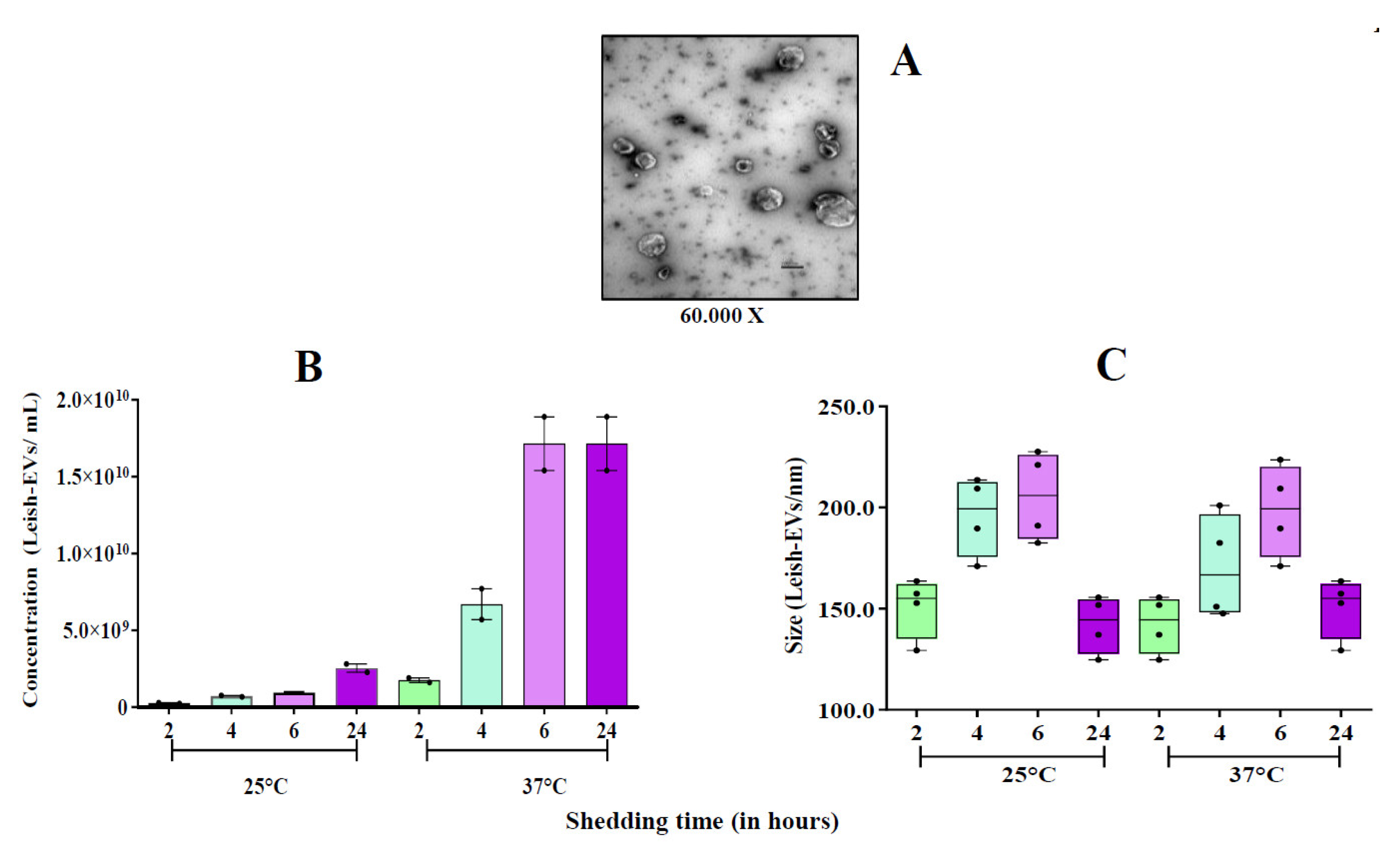
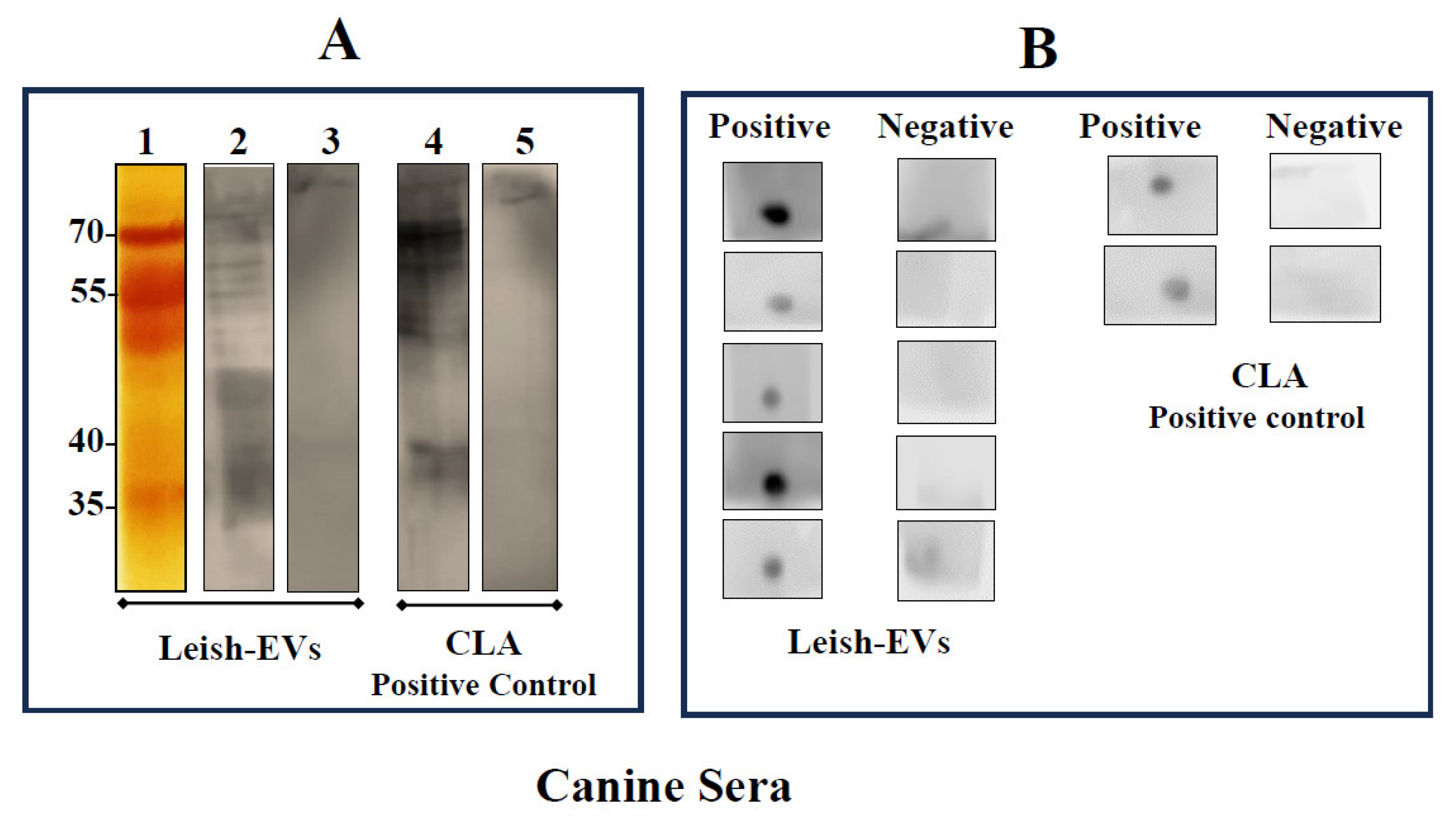
3.1. Biological Characteristics of Leish-EVs Released by L. (L.) Infantum
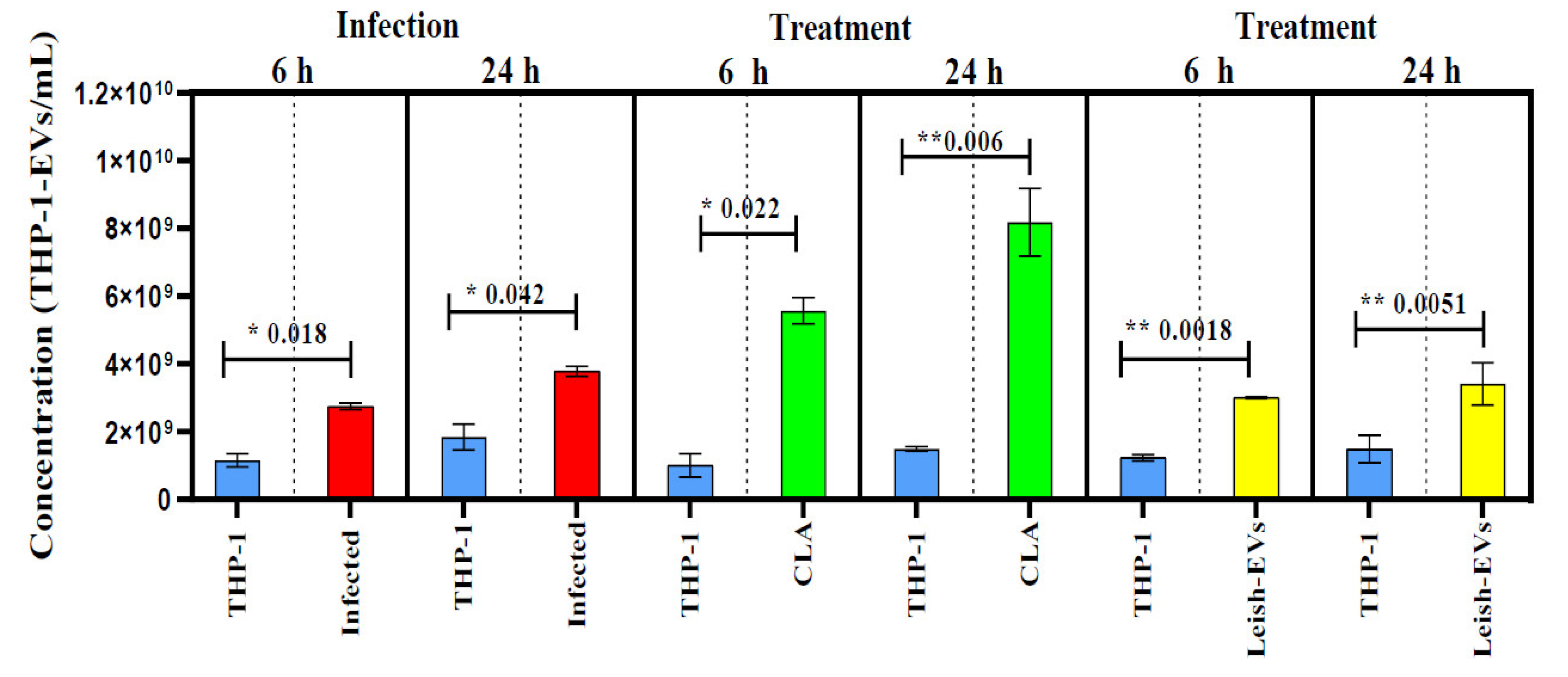
3.2. THP-1-EVs Releasing Was Stimulated by L. (L.) Infantum Antigens and Leish-EVs
3.3. Immunological Experiments
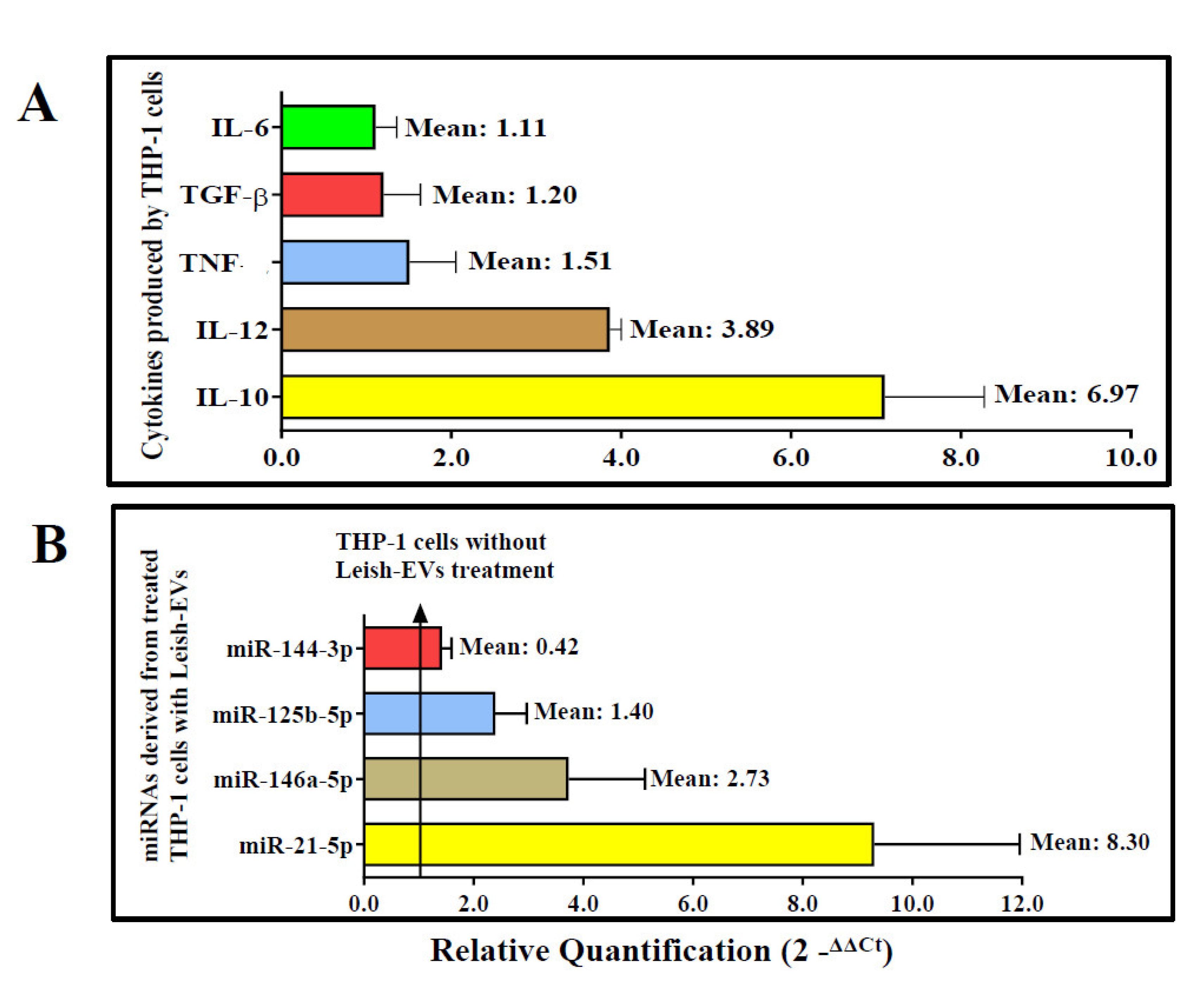
3.4. Leish-EVs Stimulated the THP-1 Cells to Produce Cytokines and miRNAs
4. Discussion
Author Contributions
Funding
Acknowledgments
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Leishmaniasis, 2022. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab (accessed on 2 October 2023).
- Matsumoto, P.S.S; Taniguchi, H.H.; Pereira, V.B.R.; Hiramoto, R.M.; Seviero Rampazzi, K.L.; de Raeffray Barbosa, J.E.; Puci Neto, R.A.; Camprigher, V.M.; de Barros Cortez, L.R.P.; Rahaman, K.R.; Novak, M.; Tolezano, J.E. Efficacies of insecticide dog collars against visceral leishmaniasis in low and high-income areas and the effects for non-collared neighbor dogs. Acta Trop. 2022, 235, 106626. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J. ; den Boer M; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. 2012, 7: e35671. PLoS ONE. [CrossRef]
- Alvar, J.; Yactayo, S.; Bern, C. Leishmaniasis and poverty. Trends Parasitol. 2006, 22, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet. Parasitol. 2007, 149, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Otranto, D. Best practices for preventing vector-borne diseases in dogs and humans. Trends Parasitol. 2016, 32, 43–55. [Google Scholar] [CrossRef] [PubMed]
- PAHO–Pan American Health Organization, Visceral leishmaniasis, 2022. Available online: https://www.paho.org/en/topics/leishmaniasis/visceral-leishmaniasis and https://www.paho.org/en/topics/leishmaniasis (accessed on 10 October 2023).
- Grimaldi, G. Jr.; Tesh, R. B. Leishmaniases of the New World: Current concepts and implications for future research. Clin. Microbiol. Rev. 1993, 6, 230–250. [Google Scholar] [CrossRef] [PubMed]
- Degrave, W.; Fernandes, O.; Campbell, D.; Bozza, M.; Lopes, U. Use of molecular probes and PCR for detection and typing of Leishmania-a mini-review. Mem. Inst. Oswaldo Cruz. 1994, 89, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Lainson, R. ; Shaw, J, J. New world leishmaniasis. The neotropical Leishmania species. In: Collier L, Balows A, Sussman M (Eds) Microbiology and Microbial Infectious Diseases, 1998. Volume 5 Topley & Wilson’s 9th, London.
- Lainson, R.; Rangel, E.F. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil: A review. Mem. Inst. Oswaldo Cruz. 2005, 100, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracellular Vesicles. 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Torrecilhas, A.C.; Soares, R.P.; Schenkman, S.; Fernandez-Prada, C.; Olivier, M. Extracellular vesicles in Trypanosomatids: Host cell communication. Front. Cell. Infect. Microbiol. 2020, 10, 602502. [Google Scholar] [CrossRef] [PubMed]
- Marti, M.; Johnson, P.J. Emerging roles for extracellular vesicles in parasitic infections. Curr. Opin. Microbiol. 2016, 32, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Sampey, G.; Chung, M.C.; Bailey, C.; van Hoek, M.L.; Kashanchi, F.; Hakami, R.M. The carrying pigeons of the cell: Exosomes and their role in infectious diseases caused by human pathogens. Pathog. Dis. 2014, 71, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, K.; Obi, P.O.; Saleem, A. Review of exosomal isolation methods: Is size exclusion chromatography the best option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef] [PubMed]
- Szempruch, A.J.; Sykes, S.E.; Kieft Dennison, L.; Becker, A.C; Gartrell, A.; Martin, W.J; Nakayasu, E.S.; Almeida, I.C.; Hajduk, S.L.; Harrington, J.M. Extracellular vesicles from Trypanosoma brucei mediate virulence factor transfer and cause host anemia. Cell 2016, 14 164, 246–257. [Google Scholar] [CrossRef]
- Dong, G.; Filho, A.L.; Olivier, M. Modulation of Host-Pathogen Communication by Extracellular Vesicles (EVs) of the Protozoan Parasite Leishmania. Front. Cell Infect. Microbiol. 2019, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Wagner, V.; Minguez-Menendez, A.; Fernandez-Prada, C.; Olivier, M. Extracellular vesicles and leishmaniasis: Current knowledge and promising avenues for future development. Mol. Immunol. 2021, 135, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Clos, J.; de’Oliveira, C.C.; Shirvani, O.; Fang, Y.; Wang, C.; Foster, L.J.; Reiner, N.E. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J. Cell. Sci. 2010, 123, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, Á.M.; Galué-Parra, A.; Pereira, W.L.A.; Pedersen, K.W.; da Silva, E.O. Leishmania 360°: Guidelines for Exosomal Research. Microorganisms. 2021, 2, 2081. [Google Scholar] [CrossRef]
- Hassani, K.; Antoniak, E.; Jardim, A.; Olivier, M. Temperature-induced protein secretion by Leishmania mexicana modulates macrophage signaling and function. 2011, 3: e18724. PLoS ONE. [CrossRef]
- Cruz, A.B.; Carneiro, F.M.; Maia, M.M.; Pereira, I.S.; Taniwaki, N.N.; Namiyama, G.M.; Gava, R.; Hiramoto, R.M.; Pereira-Chioccola, VL. Dogs with canine visceral leishmaniasis have a boost of extracellular vesicles and miR-21-5p up-expression. Parasite Immunol. 2023, 45, e13004. [Google Scholar] [CrossRef]
- Colombo, F.A.; Odorizzi, R.M.; Laurenti, M.D.; Galati, E.A.; Canavez, F.; Pereira-Chioccola, VL. Detection of Leishmania (Leishmania) infantum RNA in fleas and ticks collected from naturally infected dogs. Parasitol Res. [CrossRef]
- Hippólito, D.D.C.; Gomes, A.H.S.; Maia, M.M.; Meira-Strejevitch, C.D.S.; Kanamura, C.T.; Lauletta Lindoso, J.A.; Pereira-Chioccola, V.L. Gene expression profile of cytokines produced in biopsies from patients with American cutaneous leishmaniasis. Acta Trop. 2019, 189, 69–75. [Google Scholar] [CrossRef]
- Cruz, A.B.; Maia, M.M.; Pereira, I.S.; Taniwaki, N.N.; Namiyama, G.M.; Telles, J.P.M.; Vidal, J.E.; Spegiorin, L.C.J.F.; Brandão de Mattos, C.C.; Mattos, L.C.; Meira-Strejevitch, C.D.S.; Pereira-Chioccola, V.L. Human extracellular vesicles and correlation with two clinical forms of toxoplasmosis. PLoS ONE 2020, 15, e0229602. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods. 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Nogueira, P.M.; Ribeiro, K.; Silveira, A.C.O; Campos, J.H.; Martins-Filho, A.O.; Bela, S.R.; Campos, M.A.; Pessoa, N.L.; Colli, W.; Alves, M.J.M.; Soares, R.P.; Torrecilhas, A.C. Vesicles from differential innate and chronic imune responses. J. Extracell. Vesicles. 2015, 26, 28734. [Google Scholar] [CrossRef]
- Lambertz, U.; Silverman, J.M.; Nandan, D.; McMaster, W.R.; Clos, J.; Foster, L.J.; Reiner, N.E. Secreted virulence factors and immune evasion in visceral leishmaniasis. J. Leukoc. Biol. 2012, 91, 887–899. [Google Scholar] [CrossRef]
- Hassani, K.; Shio, M.T.; Martel, C.; Faubert, D.; Olivier, M. Absence of metalloprotease GP63 alters the protein content of Leishmania exosomes. PLoS ONE. 2014, 15, e95007. [Google Scholar] [CrossRef]
- Barbosa, F.M.C.; Dupin, T.V.; Toledo, M.D.S.; Reis, N.F.D.C.; Ribeiro, K.; Cronemberger- Andrade, A.; Rugani, J.N.; De Lorenzo, B.H.P.; Novaes, E.; Brito, R.R.; Soares, R.P.; Torrecilhas, A.C.; Xander, P. Extracellular vesicles released by Leishmania (Leishmania) amazonensis promote disease progression and induce the production of different cytokines in macrophages and B-1 cells. Front. Microbiol. 2018, 9, 3056. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Reiner, N.E. Leishmania exosomes deliver preemptive strikes to create an environment permissive for early infection. Front. Cell Infect. Microbiol. 2012, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Tomiotto-Pellissier, F.; Bortoleti, B.T.S.; Assolini, J.P.; Gonçalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage Polarization in Leishmaniasis: Broadening Horizons. Front Immunol. 2018, 9, 2529. [Google Scholar] [CrossRef]
- Barbiéri, C.L. Immunology of canine leishmaniasis. Parasite Immunol. 2006, 28, 329–337. [Google Scholar] [CrossRef]
- Dayakar, A.; Chandrasekaran, S.; Kuchipudi, S.V.; Kalangi, S.K. Cytokines: Key Determinants of Resistance or Disease Progression in Visceral Leishmaniasis: Opportunities for Novel Diagnostics and Immunotherapy. Front Immunol. 2019, 5, 10:670. [Google Scholar] [CrossRef] [PubMed]
- Stäger, S.; Maroof, A.; Zubairi, S.; Sanos, S.L.; Kopf, M.; Kaye, P.M. Distinct roles for IL-6 and IL-12p40 in mediating protection against Leishmania donovani and the expansion of IL-10+ CD4+ T cells. Eur. J. Immunol. [CrossRef]
- Bragato, J.P.; Rebech, G.T.; Freitas, J.H.; Santos, M.O.D; Costa, S.F.; Eugênio, F.R.; Santos, P.S.P.D; de Lima, V.M.F. miRNA-21 regulates CD69 and IL-10 expression in canine leishmaniasis. PLoS ONE. 2022, 24, e0265192. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004, 23, 281–297. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Shinagawa, K.; Castellino, F.J.; Schorey, J.S. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007, 110, 3234–3244. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Pandey, R.K.; Prajapati, P.; Prajapati, V.K. Circulating MicroRNAs: Potential and Emerging Biomarkers for Diagnosis of Human Infectious Diseases. Front. Microbiol. 2016, 15, 1274. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Stewart, T.; Sheng, L.; Li, N.; Bullock, K.; Song, N.; Shi, M.; Banks, W.A.; Zhang, J. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: Another mechanism for initiation and progression of Parkinson's disease? Acta Neuropathol. Commun. 2017, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Lin, X.; Zhou, F.; Li, C.; Wang, X.; Yu, H.; Pan, Y.; Fei, H.; Ma, L.; Zhang, S. A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation. Acta Biomater. 2020, 113, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Varikuti, S.; Verma, C.; Holcomb, E.; Jha, B.K.; Viana, A.; Maryala, R.; Lamenza, F.; McElwain, BK. , Doni, N.Y.; Papenfuss, T., Oghumu, S.; Gannavaram, S.; Nakhasi, H.L.; Satoskar, A.R. MicroRNA-21 Deficiency Promotes the Early Th1 Immune Response and Resistance toward Visceral Leishmaniasis. J. Immunol. 2021, 1, 1322–1332. [Google Scholar] [CrossRef]
- Ganguly, S.; Ghoshal, B.; Banerji, I.; Bhattacharjee, S.; Chakraborty, S.; Goswami, A.; Mukherjee, K.; Bhattacharyya, S.N. Leishmania survives by exporting miR-146a from infected to resident cells to subjugate inflammation. Life Sci. Alliance 2022, 24, e202101229. [Google Scholar] [CrossRef]
| Gene symbol | Gene name | Biological function | Assay ID1 | AmpliconLength | Chromosome location |
|---|---|---|---|---|---|
| IFN-γ | Interferon gamma | Protein coding | Hs00989291_m1 | 73 | 12 |
| IL10 | Interleukin 10 | Protein coding | Hs00961622_m1 | 74 | 1 |
| IL12 | Interleukin 12 | Protein coding | Hs01011518_m1 | 72 | 5 |
| IL6 | Interleukin 6 | Protein coding | Hs00985639_m1 | 66 | 7 |
| TNF | Tumor necrosis factor | Protein coding | Hs01113624_g1 | 143 | 6 |
| TGF-β | Transforming growth factor beta 1 | Protein coding | Hs00998133_m1 | 57 | 19 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Protein coding | Hs02758991_g1 | 93 | 12 |
| Assay name 1 | Gene family | Assay ID | Chromosome location | Mature miRNA sequence |
|---|---|---|---|---|
| 1: miR-21-5p | MI0000077 | 477975_miR | 17 | UAGCUUAUCAGACUGAUGUUGA |
| 2: miR-146a-5p | MI0000477 | 478399_miR | 5 | UGAGAACUGAAUUCCAUGGGUU |
| 3: miR-125b-5p | MIMAT0000423 | 477885_miR | 11 | UCC CUG AGA CCC UAA CUU GUGA |
| 4: miR-144-3p | MI0000460 | MC11051 | 17 | UACAGUAUAGAUGAUGUACU |
| 5: cel-miR-39-3p 2 | MIMAT0000010 | 478293_miR | ND | UCACCGGGUGUAAAUCAGCUUG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





