1. Introduction
In the 21st century, inflammatory bowel disease (IBD) has become a global disease, and although the incidence in Western countries tends to stabilize, the incidence in newly developed industrialized countries, whose society is becoming more and more westernized, including Lithuania, is still increasing [
1,
2,
3]. IBD can affect not only the digestive tract, but also other organs outside the intestines. These lesions are called extraintestinal manifestations (EIMs) of IBD. In both Crohn's disease (CD) and Ulcerative colitis (UC), EIMs most commonly involve the musculoskeletal system, skin, hepatobiliary tract, and eyes. The frequency of different EIMs in IBD patients ranges from <1% up to 50% [
4,
5].
According to different authors, skin and mucosal lesions occur in 6-53% of patients with IBD [
5,
6,
7,
8]. The most common lesions associated with IBD are erythema nodosum, pyoderma gangrenosum, and oral mucosal lesions [
9]. Based on pathogenesis and relationship to the underlying intestinal disease, mucocutaneous lesions can be divided into five groups: specific manifestations, disorders associated with IBD, reactive manifestations, conditions secondary to the treatment and conditions caused nutritional malabsorption [
10].
The pathogenesis of mucocutaneous conditions associated with IBD is related to chronic inflammation and special human leukocyte antigen (HLA) genes (HLA-DR2 and HLAB27) [
11,
12]. It includes erythema nodosum, aphthous stomatitis, psoriasis, and epidermolysis bullosa acquisita. Aphthous stomatitis is the most common oral mucosal lesion in IBD patients, with a prevalence ranging from 0.7 to 20%, and is more common in CD than UC. This condition is characterized by painful round ulcers with erythematous edges and a fibrinous exudate in the center [
13]. Lesions are usually localized in the lips and inner mucosa of the cheeks [
9]. This lesion occurs more frequently in active bowel disease [
14]. In more than 25% of cases, aphthous stomatitis may occur before the diagnosis of IBD is established [
5]. Erythema nodosum is one of the most common skin lesions in IBD. This skin condition is more common in CD (4-15%) than in UC (3-10%) and is more frequent in women [
15]. Erythema nodosa is characterized by symmetrical, red or purple, painful, subcutaneous nodules 1-5 cm in diameter. Lesions are usually localized on the extensor surfaces of the lower limbs [
16]. Almost 15% of skin lesions may appear before the diagnosis of IBD [
5]. The prevalence of psoriasis among IBD patients is 2.7-11%, and this skin disease is more common in CD than in UC [
6,
17]. No relationship was found between the severity of psoriasis and the activity of IBD [
17]. Patients with psoriasis have a higher risk of developing IBD than the general population [
18,
19]. In IBD patients most commonly appears plaque psoriasis form characterized by well-bounded erythematous plaques covered with silver scales. Psoriasis usually occurs on the hairy scalp and extensor surfaces of the limbs. It has been observed that patients with IBD treated with tumor necrosis factor alpha (TNF-α) inhibitors (infliximab, adalimumab, etc.) may develop paradoxical psoriatic lesions, however it is not specific only for IBD [
20].
There is increasing evidence of secondary mucocutaneous diseases induced by IBD treatment with TNF-α antagonists [
21,
22]. These lesions include local and systemic adverse reactions, paradoxical skin lesions (typically anti-TNF agents are approved for the treatment of these disorders, for example psoriasis), and infectious complications [
10,
22]. The increased incidence of skin cancer with anti-TNF use is still controversial [
23,
24]. In a meta-analysis performed by Nigam et al. [
22], the overall incidence of any dermatological reaction in patients with IBD treated with anti-TNF therapy was 19.4%, while the most common event (in 39 studies) was psoriasis/psoriatic rash with an incidence of 5.6%. A retrospective study conducted by Weizman et al. [
25] found that the median time to onset of psoriasis was 19 months after initiation of anti-TNF, with an earlier onset of psoriasis in patients treated with adalimumab versus infliximab (15 vs. 26 months).
We would like to emphasize, that the data on the prevalence of mucocutaneus manifestation in IBD patients are limited, different numbers on the prevalence of these lesions are usually derived from small series and may be possibly biased. We could find only a few well-designed clinical trials [
5,
6,
7,
8,
14,
26]. Therefore, the aim of our study was to assess the prevalence of skin and mucosal lesions in IBD patients at University hospital and to determine the relationship of lesions with demographic factors, clinical features, and systemic treatment.
2. Materials and Methods
2.1. Study Design and Participants
The prospective study was conducted in 2022 from January to October. Out-patients with IBD who were managed in the hospital of Lithuanian University of Health Sciences Kaunas Clinics were included in this study. A questionnaire survey of the subjects was directly applied during the consultation of the gastroenterologist. A total of 162 IBD patients agreed to answer to the survey questions.
The inclusion criteria were age ≥18 years, history of UC or CD, voluntary participation, ability to answer the questionnaire independently.
According to the time of appearance, skin lesions were divided into two groups: 1) those appeared before the diagnosis of IBD; 2) appearing together with IBD or during the course of the disease. Our results and analysis were made using all cases of skin and mucosal lesions. The relationship of skin lesions with systemic drugs for the treatment of IBD was analyzed by evaluating skin lesions that appeared during the course of IBD, i.e., in treatment. We must note that the assessment of the relationship of used medications with skin or mucous lesions and some other parameters was based on the opinion of the patients.
2.2. Ethical Consideration
This study was conducted in accordance with the Declaration of Helsinki and approved by the Lithuanian University of Health Sciences Bioethics Center, study No. BEC-MF-168, notification of determination received on 12 Jan 2022. Additionally, informed consent was obtained from all subjects involved in this study. The confidentiality and anonymity of the participants’ responses were guaranteed.
2.3. Statistical Analysis
Data were tabulated in Microsoft Excel, and statistical analyses were performed using IBM SPSS Statistics for Windows v25.0. Continuous variables were expressed as mean ± standard deviation for those conforming to a normal distribution. Categorical variables were expressed as frequency (%). The Chi-square test and Fisher's exact test were used to compare the frequency of skin and mucosal lesions between UC and CD patient groups (subgroups) and between men and women. The Mann–Whitney U test was used to compare the duration of IBD between subjects with and without skin lesions. A p-value < 0.05 was deemed statistically significant.
3. Results
3.1. Characteristics of Patients
A total of 162 patients were included in the study, with a mean age (MA) of all patients of 42.5 ±14.2 years, with no significant difference between sexes. The youngest patient was 19 years old, and the oldest was 75 years old. Ulcerative colitis was diagnosed in 117 (72.2%) patients: 17 (14.5%) had a proctitis, 30 (25.6%) – left-sided colitis, and 70 (59.8%) had a pancolitis. A total of 45 (27.8%) patients had Crohn's disease – including 17 (37.8%) with ileitis, 11 (24.4%) patients with colitis, and 17 (37.8%) with ileocolitis. Additional demographic and IBD information is presented in
Table 1.
3.2. Skin and Mucosal Lesions
Skin lesions were reported by 66 (40.7%) patients: 37 (39.8%) men and 29 (42.0%) women, p > 0.05. Skin lesions occurred in 51 (43.6%) UC group and in 15 (33.3%) CD group patients, p > 0.05.
The subjects indicated the following skin lesions: psoriasis (8.0%), erythema nodosum (5.6%), pyoderma gangrenosum and acne (3.7% each), atopic dermatitis (2.5%), vitiligo and seborrheic dermatitis (1.9% each), epidermolysis bullosa acquisita and vasculitis (0.6% each), unspecified dermatitis (6.8%) and others (10.5%). The most common skin lesions among patients with UC were psoriasis (9.4%), pyoderma gangrenosum and acne (4.3% each), while the most common lesions among CD patients were erythema nodosum (11.1%) and psoriasis (4.4%). The detailed distribution of skin lesions among IBD patients is presented in
Table 2.
Oral mucosal lesions were aphthous stomatitis or isolated aphthae. It has been reported by 27 (16.7%) subjects: the prevalence was slightly higher in women than men, respectively 15 (21.7%) versus 12 (12.9%). These disorders occurred in 17 (14.5%) UC and 10 (22.2%) CD patients. The frequency of oral mucosal lesions was not found to be significantly different between sexes or between UC and CD patients.
After evaluating the total number of patients with both skin and mucosal lesions, it was determined that 79 (48.8%) of the patients participating in this study reported mucocutaneous lesions.
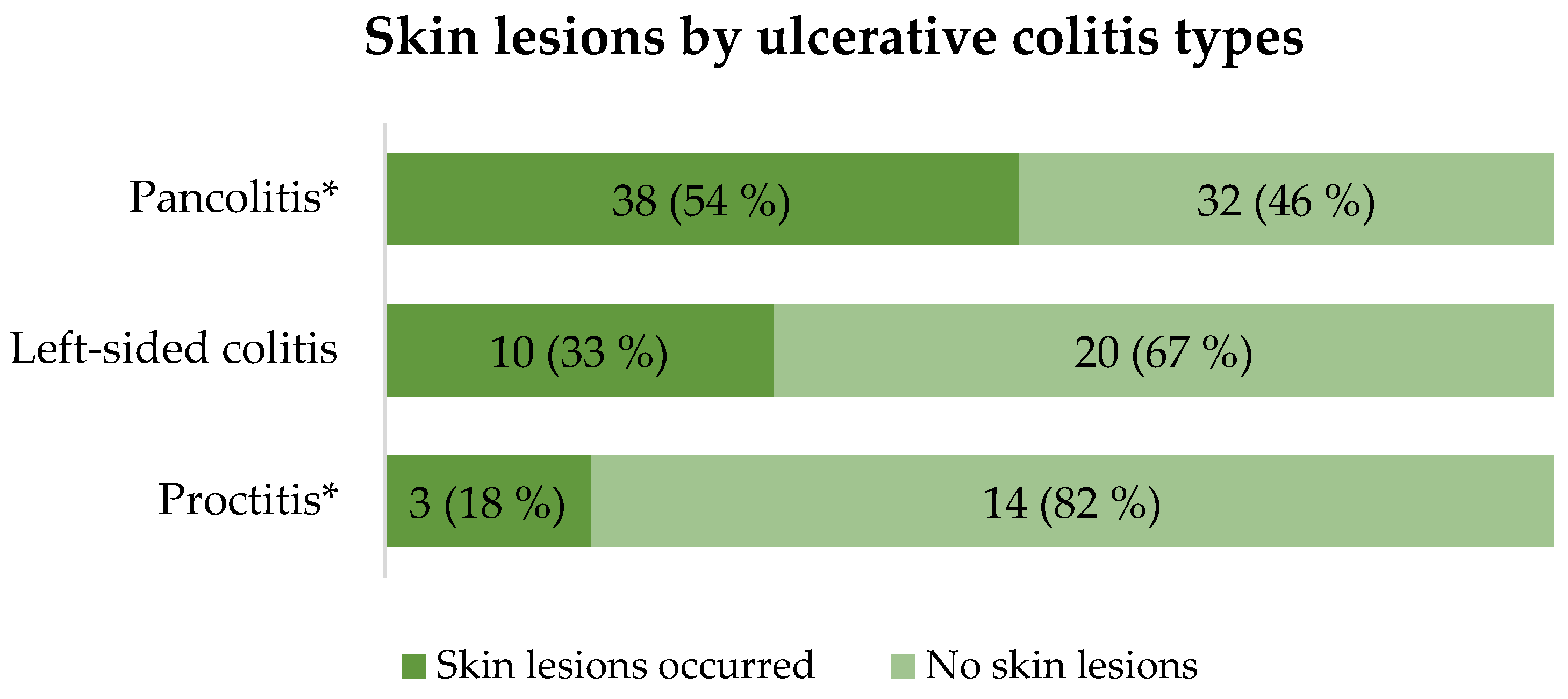
3.3. The Relationship Between Skin and Mucosal Lesions with Extension of IBD
Depending on the extension of UC, it was found that skin lesions more frequently occur in pancolitis patients compared to proctitis group (p = 0.01). The detailed distribution of skin lesions between UC groups is presented in
Figure 1.
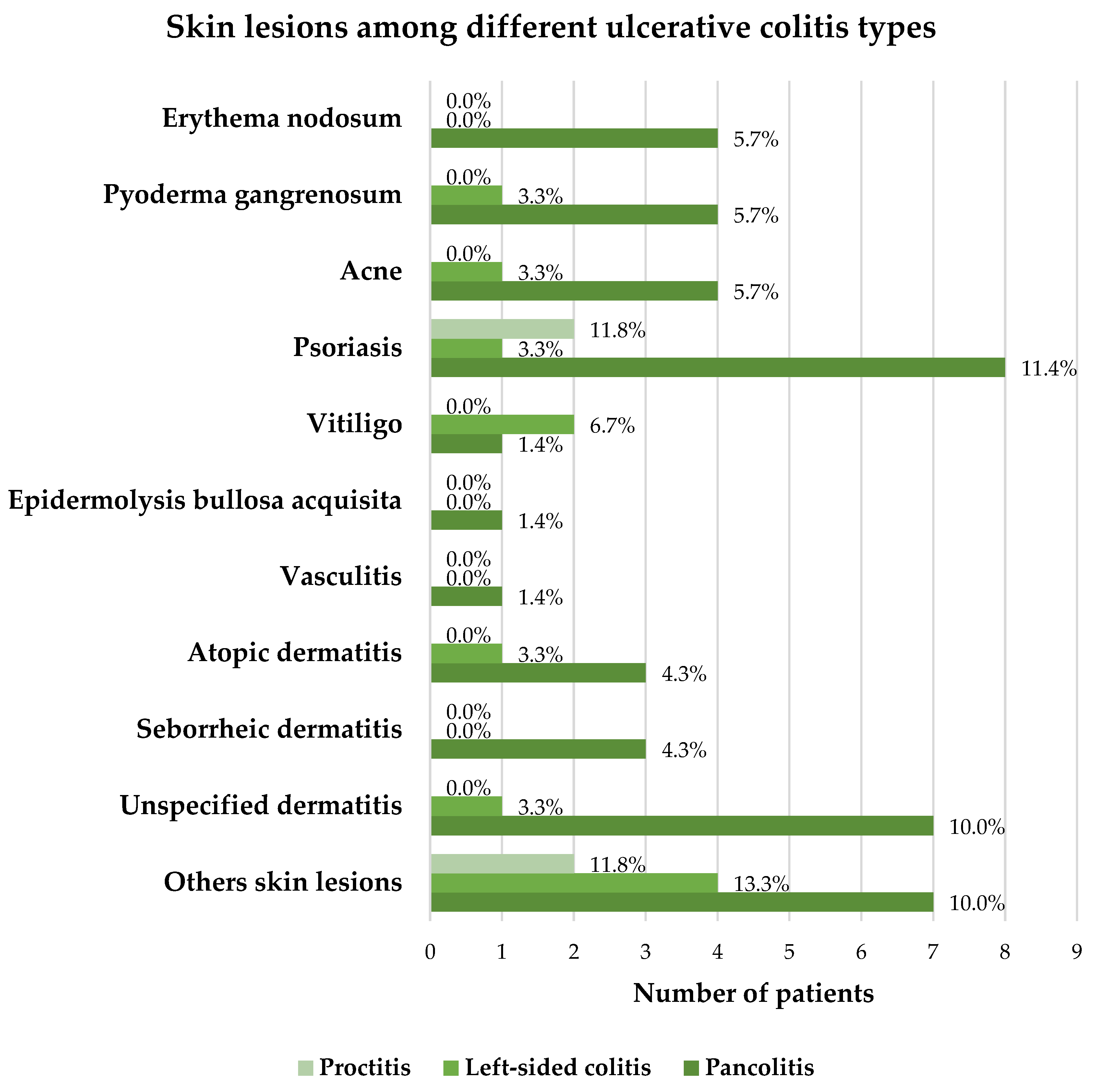
Lesions of the oral mucosa were reported by 3 of 17 (17.6%) proctitis patients, 6 of 30 (20.0%) patients with left-sided colitis and 8 of 70 (11.4%) patients suffering from pancolitis. The frequency of mucosal lesions was not statistically significantly different between different types of UC. It is important to mention that some patients reported more than 1 type of mucocutaneous lesions. The detailed distribution of skin lesions among different types of UC is presented in
Figure 2.
When comparing different types of CD, skin lesions were reported by 3 of 17 (17.6%) patients with ileitis, 5 of 11 (45.5%) patients with colitis and 7 of 17 (41.2%) patients with ileocolitis. The oral mucosal lesions occurred in 4 of 17 (23.5%) patients with ileitis, 3 of 11 (27.3%) colitis and 3 of 17 (17.6%) patients with ileocolitis. In contrast to UC, the incidence of skin and mucosal lesions was not statistically significantly different between different types of CD. The detailed distribution of skin lesions depending on the type of CD is presented in
Table 3.
3.4. The Relationship Between Skin and Mucosal Lesions with duration of IBD
The average duration of IBD in subjects who reported skin lesions was similar to those without lesions (9.3 ±6.7 vs. 9.4 ±6.7 years, respectively), p > 0.05. The same trend is observed with lesions of the oral mucosa: the average duration of IBD in lesions group was 10.2 ±8.9 years, comparing with those without lesions 9.2 ±6.1 years, p > 0.05. Also, there were no significant differences when comparing groups of UC and CD patients separately. The frequency of mucocutaneous lesions does not depend on the duration of the IBD, and a longer duration of illness is not a predictive factor for the appearance of mucocutaneous lesions.
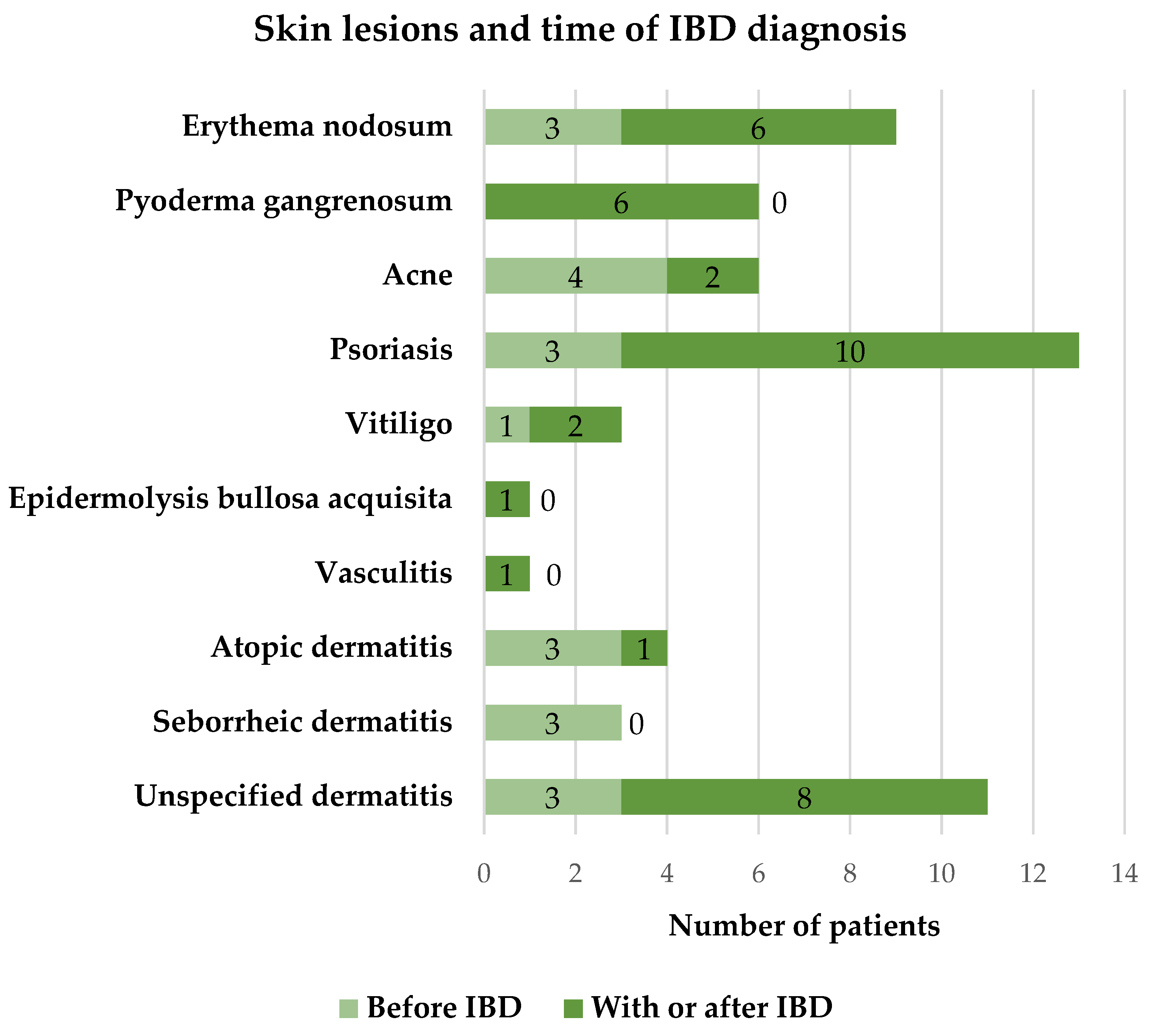
3.5. The Relationship Between Skin Lesions with the Time of IBD Diagnosis
According to the time of appearance, skin lesions can be divided into two groups – those that appeared before the diagnosis of IBD and those that were diagnosed together with IBD or during its course. Analyzing 66 patients who reported about skin lesions, 47 (71.2%) of them indicated that skin lesions appeared together with the onset of IBD or during the course of the disease, while 19 (28.8%) of subjects had skin lesions before the diagnosis of IBD. The detailed distribution of skin lesions according to the time of onset is presented in
Figure 3.
3.6. The Relationship of Skin Lesions and Drugs Used for the Treatment of IBD
Skin lesions in total were reported by 34 of 82 (41.5%) patients treated with biological therapy compared with 13 of 80 (16.3%) in the non-biological treatment group, a statistically significant difference was found between these two groups, p=0.003 (however, data on the occurrence of skin lesions before or after the onset of biological treatment were not collected in this study). The most common skin lesions among patients using biological therapy were unspecified dermatitis, pyoderma gangrenosum, psoriasis and erythema nodosum.
According to the patients’ opinion, 16 of 66 (24.2%) subjects observed an association between exacerbation of skin lesions and drugs used to treat IBD. The exacerbation of skin lesions, possibly caused by biological therapy, was indicated by 10 of 66 (15.2%) patients, caused by azathioprine – 6 of 66 (9.1%), prednisolone – 4 of 66 (6.1%) patients. Comparing UC and CD patients, worsening of skin symptoms was slightly more often reported by patients in the CD group, although there was no statistically significant difference (33,3% vs. 21,6%), p > 0.05.
4. Discussion
In this study, we found that the frequency of skin lesions among IBD patients in our series is 40.7%, and the overall frequency of skin-mucosal lesions is 48.8%. According to the data of previous studies, the frequency of skin and mucosal lesions varies from 5.8% to 53.3% [
6,
7,
8]. The differences between the results can be explained by the fact that different skin lesions were evaluated. In this study, we analyzed all skin lesions reported by patients with IBD. It is also important to note that we analyzed patients treated at the tertiary care center. Possibly, these patients are more resistant to treatment, with more severe forms of the disease and hypothetically more frequent extraintestinal, including mucocutaneous, manifestations. We would like to stress, that there are very limited data and we hardly could find well designed prospective epidemiological studies regarding the skin lesions in IBD patients.
All cases of skin and mucosal lesions were analyzed in this study, but it is theoretically possible to assume that the real skin lesions associated with IBD are those lesions that were diagnosed together with IBD onset or during the course of IBD: in this case skin lesions frequency is 29%. However, considering that some skin lesions may occur before the diagnosis of UC or CD, we preferred a detailed analysis of all skin lesions.
In our study, the most common skin lesion among IBD patients was psoriasis (8%). This condition was slightly more common among UC (9.4%) than CD (4.4%), but not statistically significant. In a systematic review and meta-analysis conducted by Alinaghi et al. [
18] the frequency of psoriasis among IBD patients was found to be half as low (4.2%) and, in contrast to our study, psoriasis was slightly more frequent among CD patients (3.6%) than UC (2.8%) patients. Two studies conducted in Turkey and Greece found psoriasis to be more common in UC than in CD patients, but a statistically significant difference was also not reached [
6,
7]. Psoriasis or psoriatic rash may occur as a paradoxical reaction in patients treated with TNF-α inhibitors [
25,
27,
28,
29]. Therefore, a slightly higher frequency of psoriasis in our study could possibly be related to the fact that a large proportion of patients (50.6%) were treated with biological therapy, while 56.1% of these patients were treated specifically with TNF-α inhibitors (although in this study data about previous treatment with biological drugs was not collected. In our center, TNF-α inhibitors remain one of the first-line biological agents for IBD treatment. Therefore, it cannot be ruled out that the rest of this group patients probably were previously treated with TNF -α inhibitors). Psoriasis caused by these medications occurs in 1.1-7.3% of IBD patients [
29]. In our study, psoriasis occurred in 8.7% of patients who were treated with TNF-α antagonists.
We found that the frequency of erythema nodosum among IBD patients is 5.6% and this condition is more common in CD (11.1%) than in UC (3.4%), p>0.05. In the Greek study [
6], the incidence of erythema nodosum was almost the same as in our study (5.3%), and also more frequently affected CD group patients. In the study performed by Ampuero et al. [
26], the incidence of erythema nodosum (7.1%) was slightly higher than our results.
In our study, the incidence of pyoderma gangrenosum among IBD patients was 3.7%. This skin condition was reported more often by UC than CD patients (4.3% vs. 2.2%; p > 0.05). According to other authors, the frequency of pyoderma gangrenosum is slightly lower and reaches 0.8-2.3%. [
5,
6]. In a Turkish study, similarly to our study, pyoderma gangrenosum was more common among patients with UC [
7].
According to our data, lesions of the oral mucosa affected 16.7% of patients. These lesions were more frequent among CD (22.2%) than UC (14.5%) patients, p > 0.05. We did not collect data about separate oral mucosal lesions. In a Turkish study, aphthous stomatitis (40.2%) was the most common of all mucocutaneous lesions and occur more frequently in UC (44.6%) than CD (33.3%) patients [
7]. While in the study conducted in Greece, the incidence of aphthous stomatitis was only 6.1% and it affected more often CD (6.9%) than UC (5.2%) patients [
6].
In this study, we found that the frequency of mucocutaneous lesions does not depend on the duration of the IBD, and a longer duration of illness does not lead to a higher frequency of lesions. In UC patients, skin lesions are more common in the more extended form of the disease, it was found that skin lesions statistically significantly more frequently occur in pancolitis patients compared to proctitis group. This might be explained that the larger extent of mucosal inflammation is related with more extensive inflammatory response in different parties of the human body and the skin inclusively. In CD the frequency of mucocutaneous lesions does not differ significantly between different forms, though it has some trends to be more frequent in CD involving colon. A Turkish study also found no association between the frequency of skin and mucosal lesions and duration of IBD or the different forms of CD. However, in contrast to our study, no associations were found between the frequency of skin lesions and the extension of UC [
7]. The study by Ampuero and co-authors [
26] also found no association with extension of IBD.
We have to recognize that our study has some disadvantages as it was based on patients completed data and the sample size is not very large. Nevertheless, the prospective design of our research must be considered as an advantage and data can be considered as confident. We think that our data could add the significant information on this topic as there are too little high-quality data. It is clear, that there is an urgent need to investigate this issue in large scale prospective well-designed studies.
5. Conclusions
In conclusion, it was found that overall mucocutaneous lesions were reported in 48.8% of inflammatory bowel disease patients in Lithuanian tertiary care University center. Skin lesions were reported by 40.7% of IBD patients, and oral mucosal lesions appear in 16.7%. The most common skin lesion in ulcerative colitis was psoriasis, and in Crohn’s disease it was erythema nodosum. The frequency of mucocutaneous lesions does not differ significantly between ulcerative colitis and Crohn’s disease, and a longer duration of illness is not a predictive factor for the appearance of mucocutaneous lesions. The larger extension of ulcerative colitis leads to a higher frequency of skin lesions, while in Crohn’s disease the frequency of skin lesions did not depend on the type of the disease.
Author Contributions
Conceptualization, V.K. and I.R.J.; methodology, I.R.J. and V.K.; software, I.R.J.; validation, L.V.J. and G.K.; formal analysis, I.R.J. and L.V.J.; investigation, I.R.J. and L.V.J.; resources, V.K. and I.R.J.; data curation, I.R.J.; writing—original draft preparation, V.K. and I.R.J.; writing—review and editing, L.V.J., G.K. and J.K.; visualization, V.K.; supervision, L.V.J., G.K. and J.K.; project administration, L.V.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by Ethics Committee of the Lithuanian University of Health Sciences Bioethics Center (study No. BEC-MF-168, date of approval 12 January 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. The Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Karpavičiūtė, V.; Kiudelis, G.; Kupčinskas, J.; Kupčinskas, L. Trends in the Prevalence of Inflammatory Bowel Disease in Lithuania during 2001-2020. J Crohn‘s Colitis 2023, 17, 965–966. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef] [PubMed]
- Vavricka, S.R.; Rogler, G.; Gantenbein, C.; Spoerri, M.; Vavricka, M.P.; Navarini, A.A.; French, L.E.; Safroneeva, E.; Fournier, N.; Straumann, A.; et al. Chronological Order of Appearance of Extraintestinal Manifestations Relative to the Time of IBD Diagnosis in the Swiss Inflammatory Bowel Disease Cohort. Inflamm Bowel Dis 2015, 21, 1794–800. [Google Scholar] [CrossRef] [PubMed]
- Karmiris, K.; Avgerinos, A.; Tavernaraki, A.; Zeglinas, C.; Karatzas, P.; Koukouratos, T.; Oikonomou, K.A.; Kostas, A.; Zampeli, E.; Papadopoulos, V.; et al. Prevalence and Characteristics of Extra-intestinal Manifestations in a Large Cohort of Greek Patients with Inflammatory Bowel Disease. J Crohn‘s Colitis 2016, 10, 429–436. [Google Scholar] [CrossRef]
- Demir, F.T.; Kocatürk, E.; Yorulmaz, E.; Adali, G.; Kavala, M. Mucocutaneous manifestations of inflammatory bowel disease in Turkey. J Cutan Med Surg 2014, 18, 397–404. [Google Scholar] [CrossRef]
- Zippi, M.; Corrado, C.; Pica, R.; Avallone, E.V.; Cassieri, C.; De Nitto, D. ; Paoluzi, P.; Vernia, P. Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients. World J Gastroenterol 2014, 20, 17463–17467. [Google Scholar] [CrossRef]
- Greuter, T.; Navarini, A.; Vavricka, S.R. Skin Manifestations of Inflammatory Bowel Disease. Clin Rev Allergy Immunol 2017, 53, 413–427. [Google Scholar] [CrossRef]
- Antonelli, E.; Bassotti, G.; Tramontana, M.; Hansel, K.; Stingeni, L.; Ardizzone, S.; Genovese, G.; Marzano, A.V.; Maconi, G. Dermatological Manifestations in Inflammatory Bowel Diseases. J Clin Med 2021, 10, 364. [Google Scholar] [CrossRef]
- Keyal, U.; Liu, Y.; Bhatta, A.K. Dermatologic manifestations of inflammatory bowel disease: A review. Discov Med 2018, 25, 225–233. [Google Scholar]
- Plumptre, I.; Knabel, D.; Tomecki, K. Pyoderma Gangrenosum: A Review for the Gastroenterologist. Inflamm Bowel Dis 2018, 24, 2510–2517. [Google Scholar] [CrossRef]
- Sbeit, W.; Kadah, A.; Mahamid, M.; Karayanni, H.; Mari, A.; Tali, S.; Srouji, S.; Khoury, T. Oral manifestations of inflammatory bowel disease: the neglected piece of the puzzle. Eur J Gastroenterol Hepatol 2020, 32, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Laranjeira, N.; Fonseca, J.; Meira, T.; Freitas, J.; Valido, S.; Leitão, J. Oral mucosa lesions and oral symptoms in inflammatory bowel disease patients. Arq Gastroenterol 2015, 52, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Harbord, M.; Annese, V.; Vavricka, S.R.; Allez, M.; Acosta, M.B.-D.; Boberg, K.M.; Burisch, J.; De Vos, M.; De Vries, A.-M.; Dick, A.D.; et al. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J Crohn’s Colitis 2016, 10, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Vavricka, S.R.; Schoepfer, A.; Scharl, M.; Lakatos, P.L.; Navarini, A.; Rogler, G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm Bowel Dis 2015, 21, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Marzano, A. V.; Borghi, A.; Stadnicki, A.; Crosti, C.; Cugno, M. Cutaneous Manifestations in Patients with Inflammatory Bowel Diseases: Pathophysiology, Clinical Features, and Therapy. Inflamm Bowel Dis 2014, 20, 213–227. [Google Scholar] [CrossRef]
- Alinaghi, F.; Tekin, H.G.; Burisch, J.; Wu, J.J.; Thyssen, J.P.; Egeberg, A. Global Prevalence and Bidirectional Association Between Psoriasis and Inflammatory Bowel Disease—A Systematic Review and Meta-analysis. J Crohn’s Colitis 2020, 14, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Blegvad, C.; Egeberg, A.; Tind Nielsen, T.E.; Gislason, G.H.; Zachariae, C.; Nybo Andersen, A.M.; Skov, L. Autoimmune Disease in Children and Adolescents with Psoriasis: A Cross-sectional Study in Denmark. Acta Derm Venereol 2017, 97, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Lolli, E.; Saraceno, R.; Calabrese, E.; Ascolani, M.; Scarozza, P.; Chiricozzi, A.; Onali, S.; Petruzziello, C.; Chimenti, S.; Pallone, F.; et al. Psoriasis Phenotype in Inflammatory Bowel Disease: A Case-Control Prospective Study. J Crohn’s Colitis 2015, 9, 699–707. [Google Scholar] [CrossRef]
- Garcovich, S.; De Simone, C.; Genovese, G.; Berti, E.; Cugno, M.; Marzano, A.V. Paradoxical skin reactions to biologics in patients with rheumatologic disorders. Front Pharmacol 2019, 10, 282. [Google Scholar] [CrossRef]
- Nigam, G.B.; Bhandare, A.P.; Antoniou, G.A.; Limdi, J.K. Systematic review and meta-analysis of dermatological reactions in patients with inflammatory bowel disease treated with anti-tumour necrosis factor therapy. Eur J Gastroenterol Hepatol 2021, 33, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.D.; Stovin, C.; Alveyn, E.; Adeyemi, O.; Chan, C.K.D.; Patel, V.; Adas, M.A.; Atzeni, F.; Ng, K.K.H.; Rutherford, A.I.; et al. JAK inhibitors and the risk of malignancy: a meta-analysis across disease indications. Ann Rheum Dis 2023, 82, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; Yamaoka, K.; Chen, Y.H.; Bhatt, D.L.; Gunay, L.M.; Sugiyama, N.; Connell, C.A.; Wang, C.; Wu, J.; Menon, S.; et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: results from the open-label, randomised controlled ORAL Surveillance trial. Ann Rheum Dis 2023, 82, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Weizman, A.V.; Sharma, R.; Afzal, N.M.; Xu, W.; Walsh, S.; Stempak, J.M.; Nguyen, G.C.; Croitoru, K.; Steinhart, A.H.; Silverberg, M.S. Stricturing and Fistulizing Crohn's Disease Is Associated with Anti-tumor Necrosis Factor-Induced Psoriasis in Patients with Inflammatory Bowel Disease. Dig Dis Sci 2018, 63, 2430–2438. [Google Scholar] [CrossRef] [PubMed]
- Ampuero, J.; Rojas-Feria, M.; Castro-Fernández, M.; Cano, C.; Romero-Gómez, M. Predictive factors for erythema nodosum and pyoderma gangrenosum in inflammatory bowel disease. J Gastroenterol Hepatol 2014, 29, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Denadai, R.; Teixeira, F.V.; Steinwurz, F.; Romiti, R.; Saad-Hossne, R. Induction or exacerbation of psoriatic lesions during anti-TNF-α therapy for inflammatory bowel disease: a systematic literature review based on 222 cases. J Crohn‘s Colitis 2013, 7, 517–524. [Google Scholar] [CrossRef]
- Cleynen, I.; Van Moerkercke, W.; Billiet, T.; Vandecandelaere, P.; Vande Casteele, N.; Breynaert, C.; Ballet, V.; Ferrante, M.; Noman, M.; Assche, G.V.; et al. Characteristics of Skin Lesions Associated with Anti-Tumor Necrosis Factor Therapy in Patients with Inflammatory Bowel Disease: A Cohort Study. Ann Intern Med. 2016, 164, 10–22. [Google Scholar] [CrossRef]
- Au, M.; Heddle, G.; Young, E.; Ryan, E.; Graf, S.; Tee, D.; Philpott, H. Anti-tumour necrosis factor-induced skin rashes in inflammatory bowel disease: a systematic review and evidence-based management algorithm. Intern Med J 2023, 53, 1854–1865. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).