Submitted:
05 December 2023
Posted:
07 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
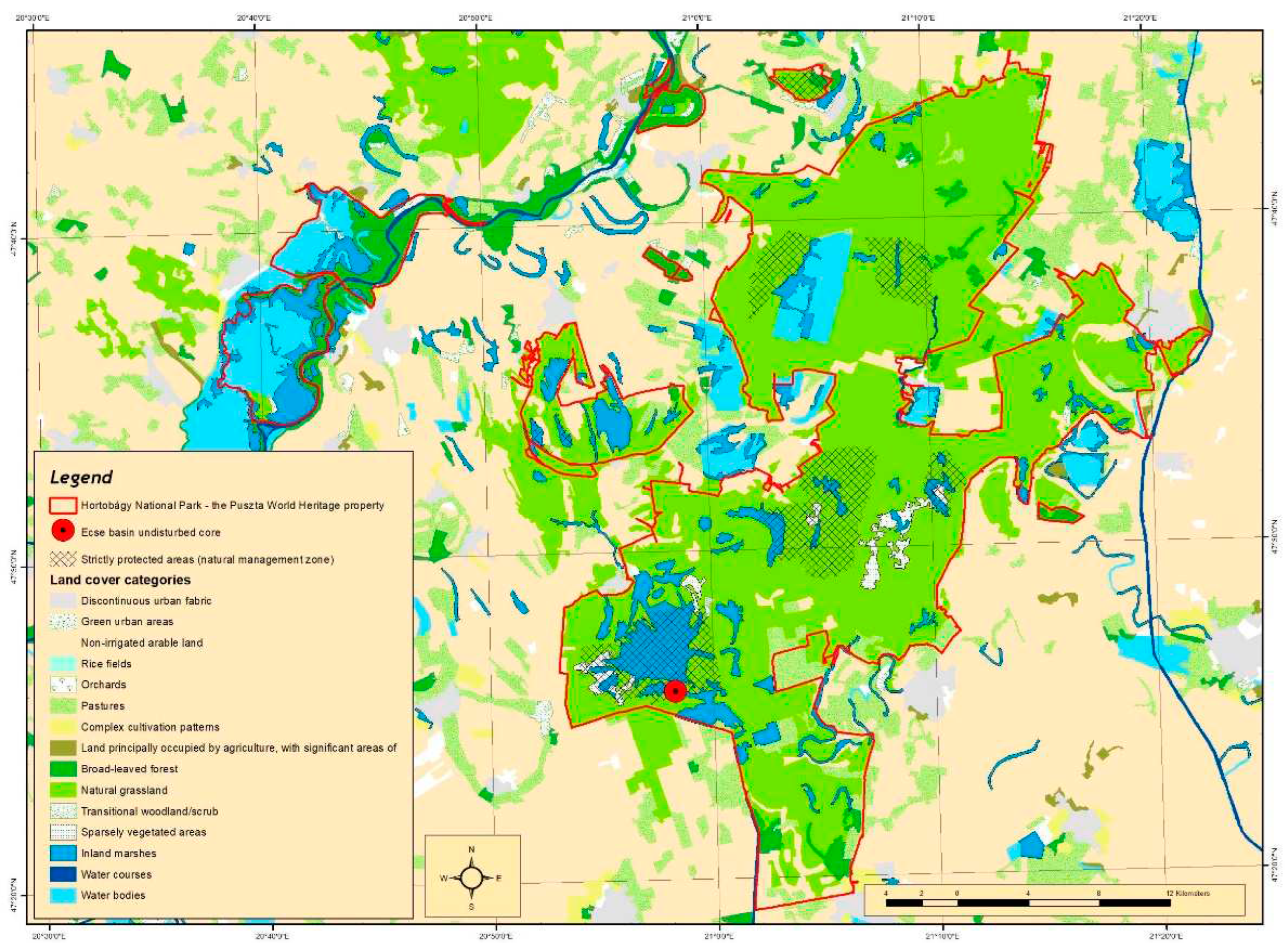
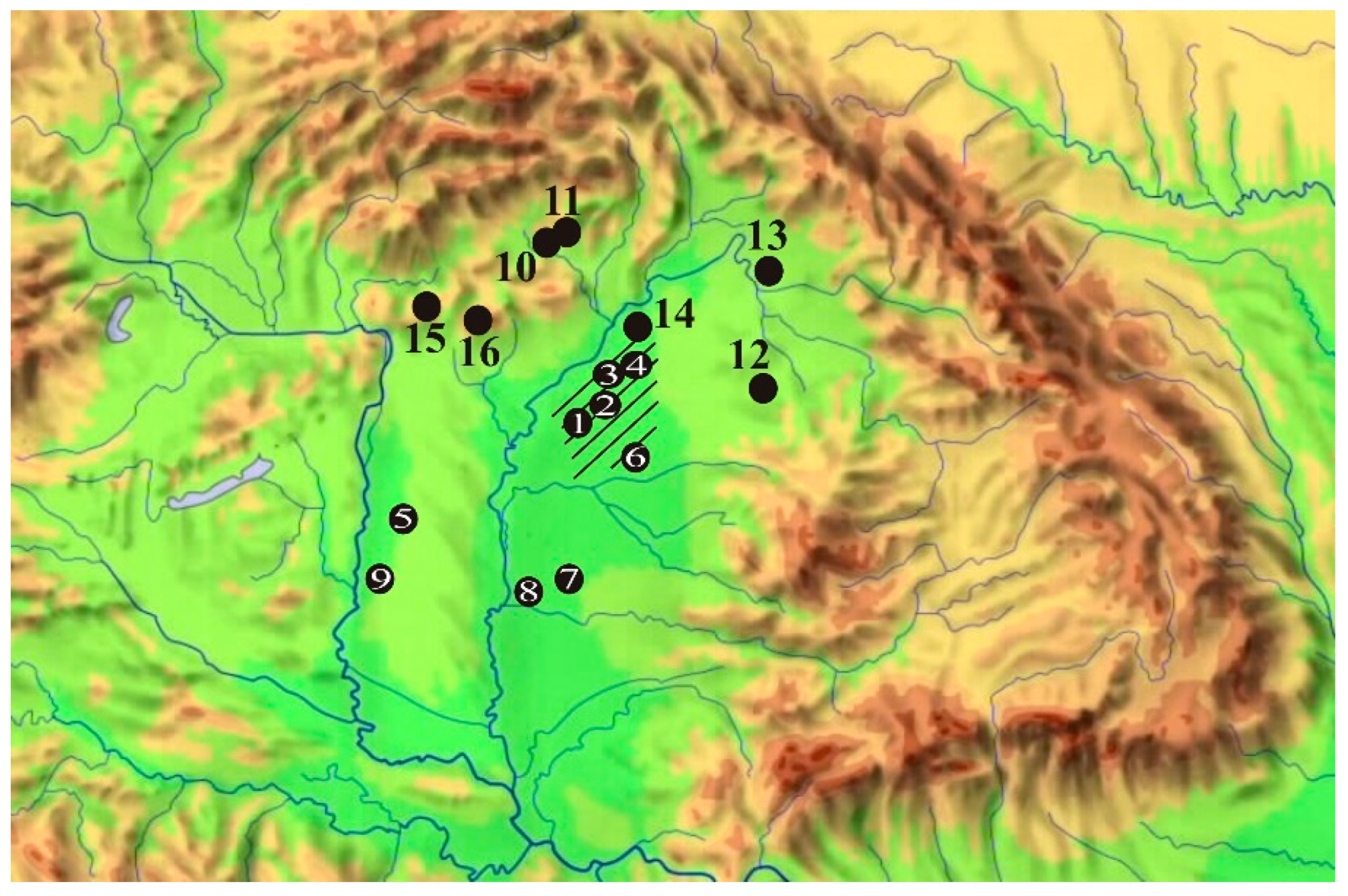
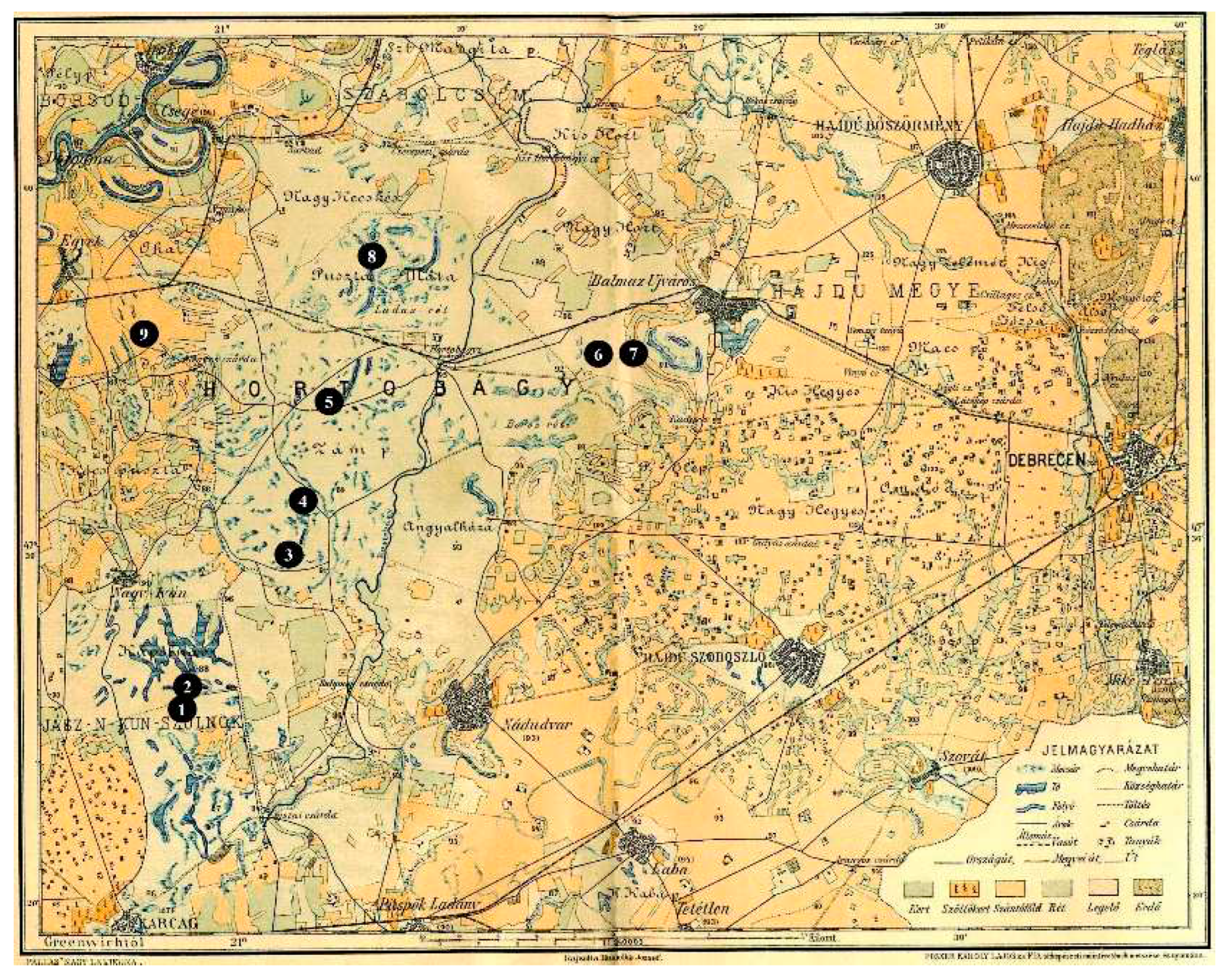
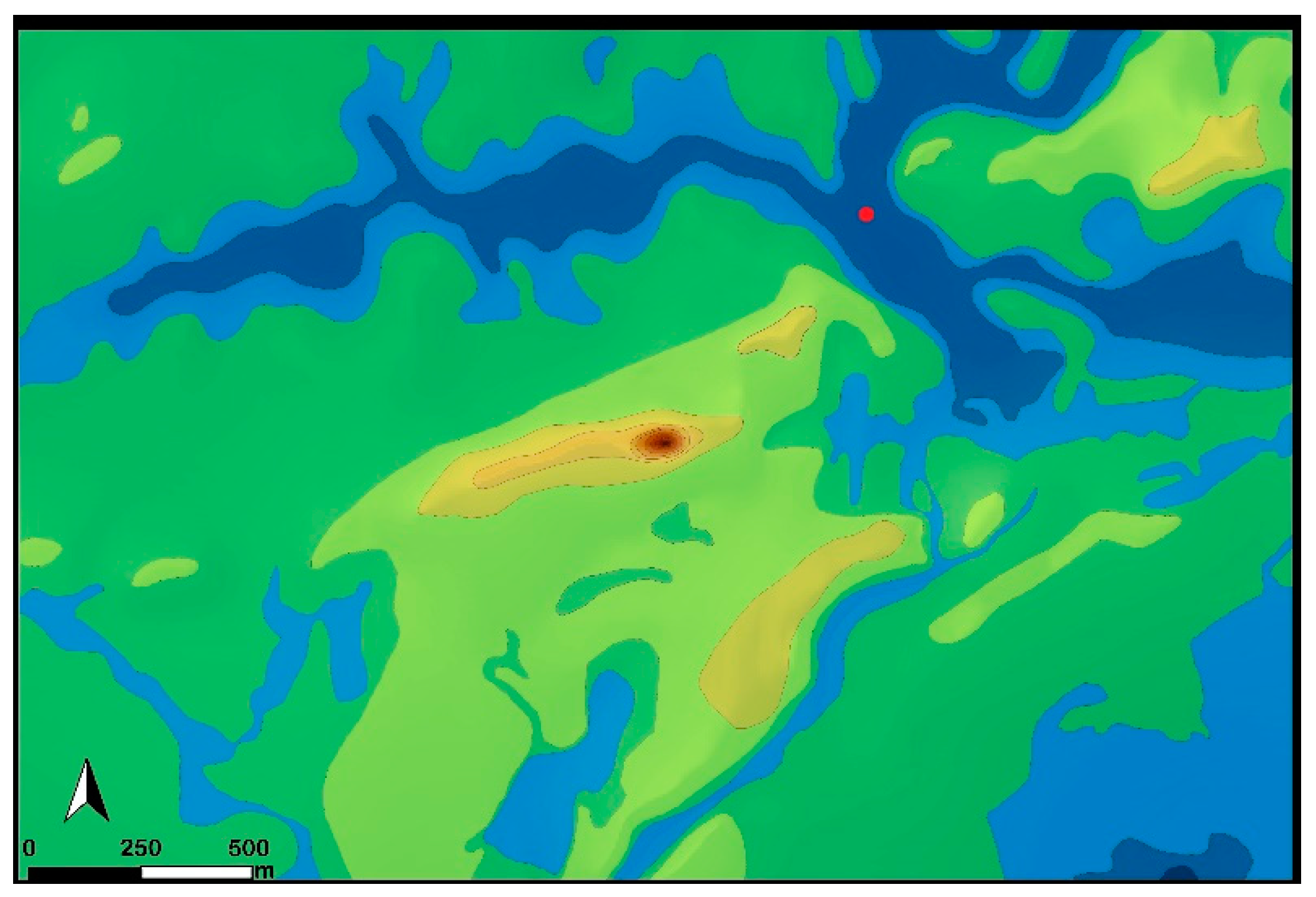
2. Materials and Methods
3. Results
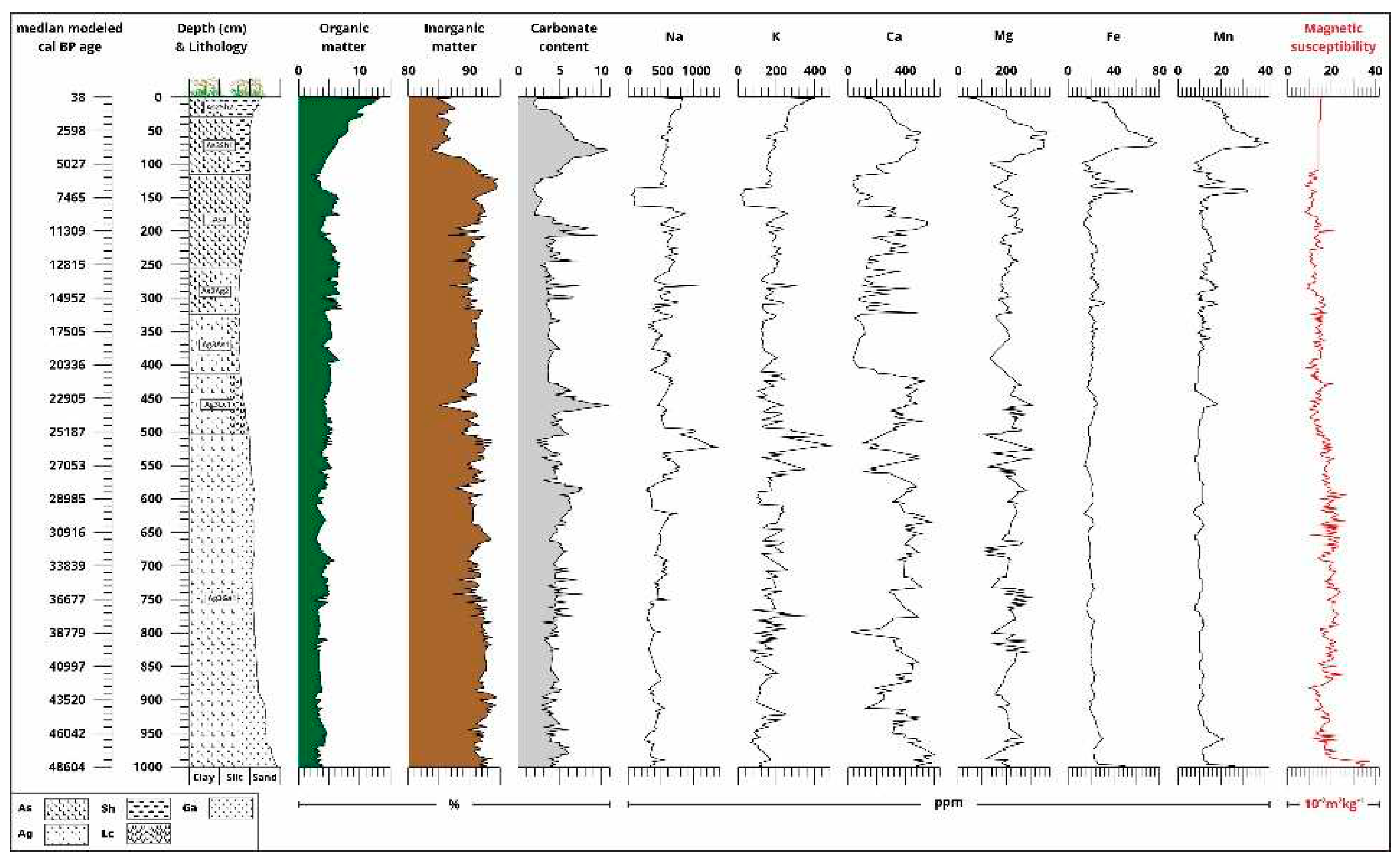
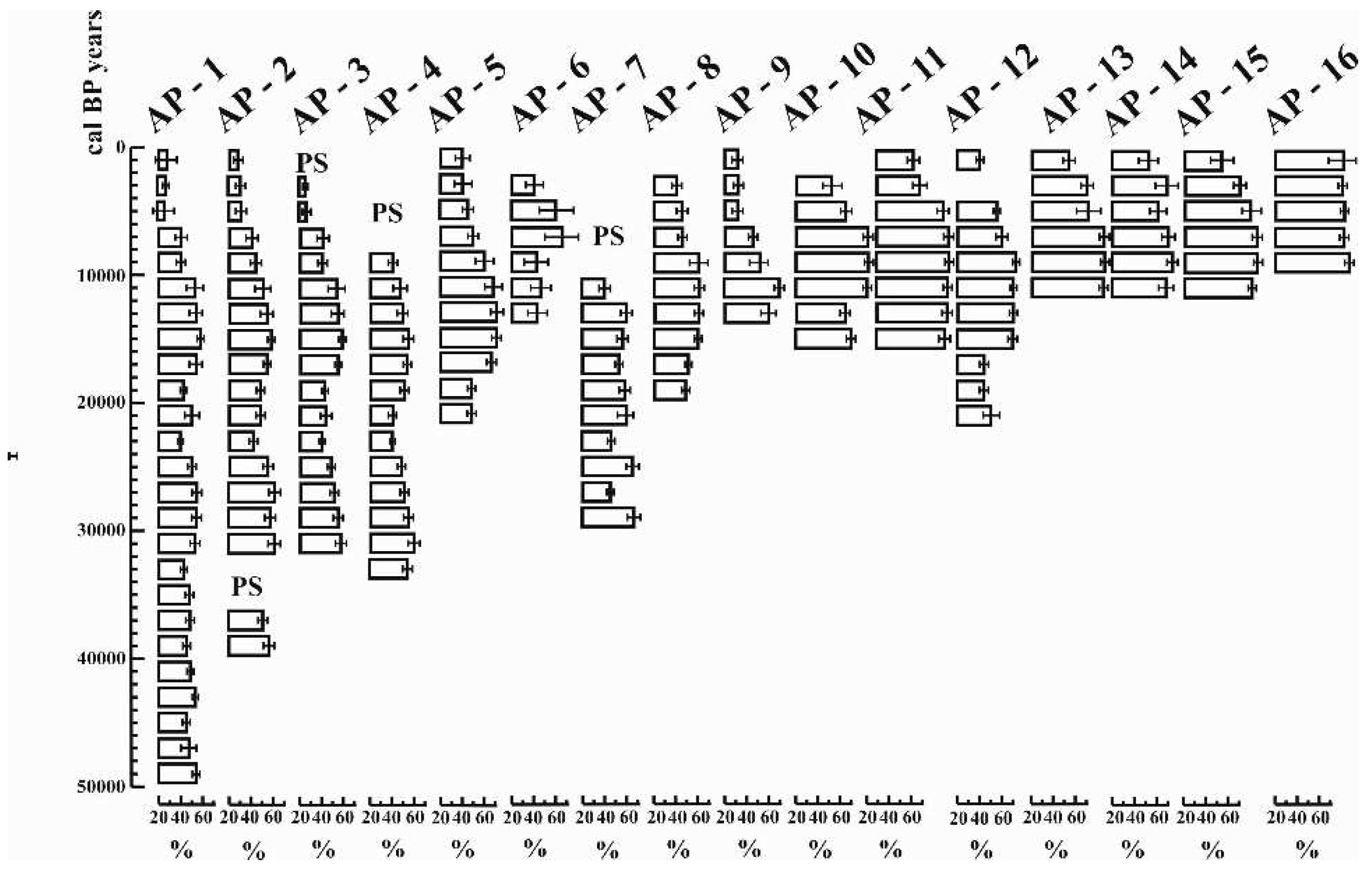
3.1. Geochronological Results
3.2. Sedimentological Results
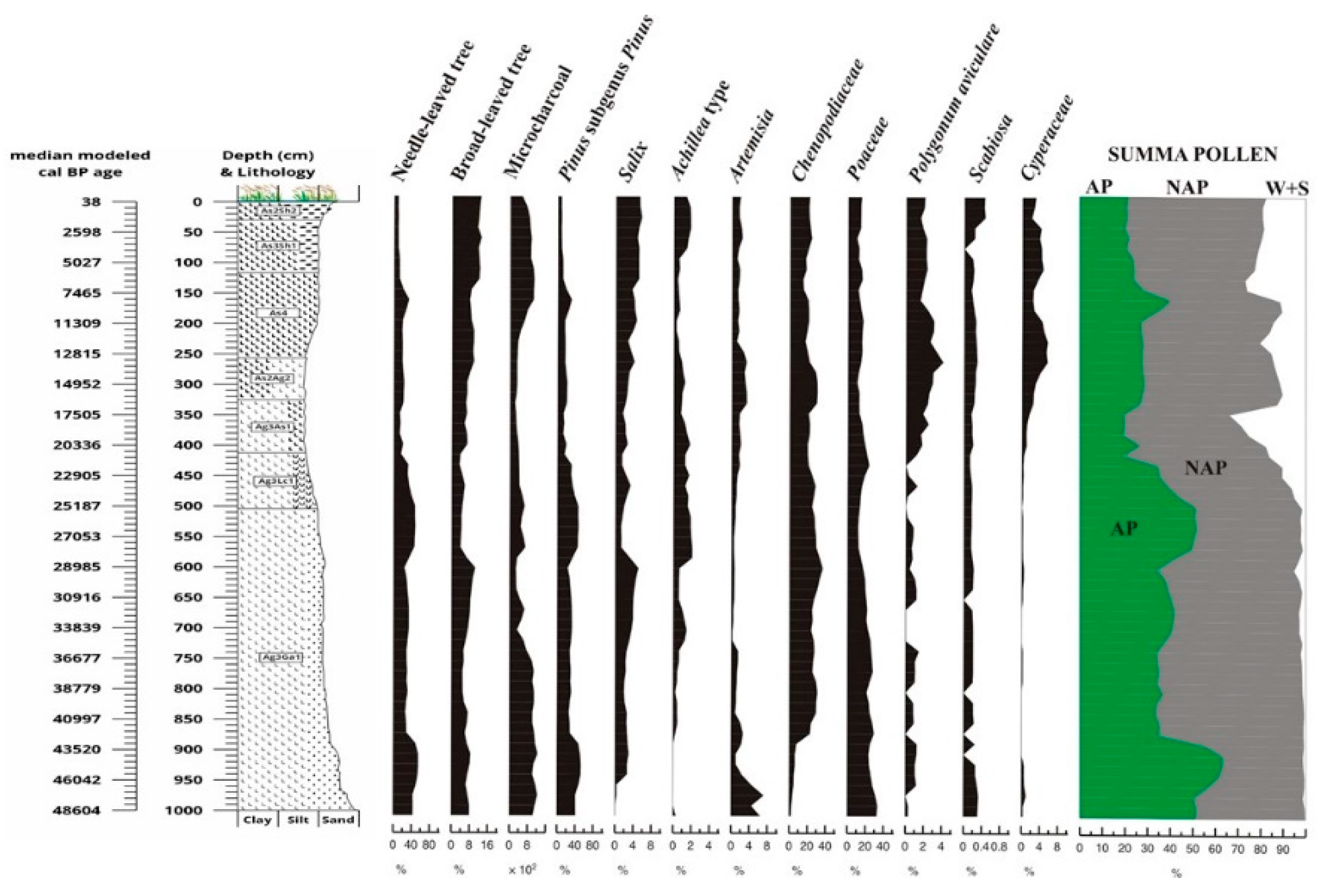
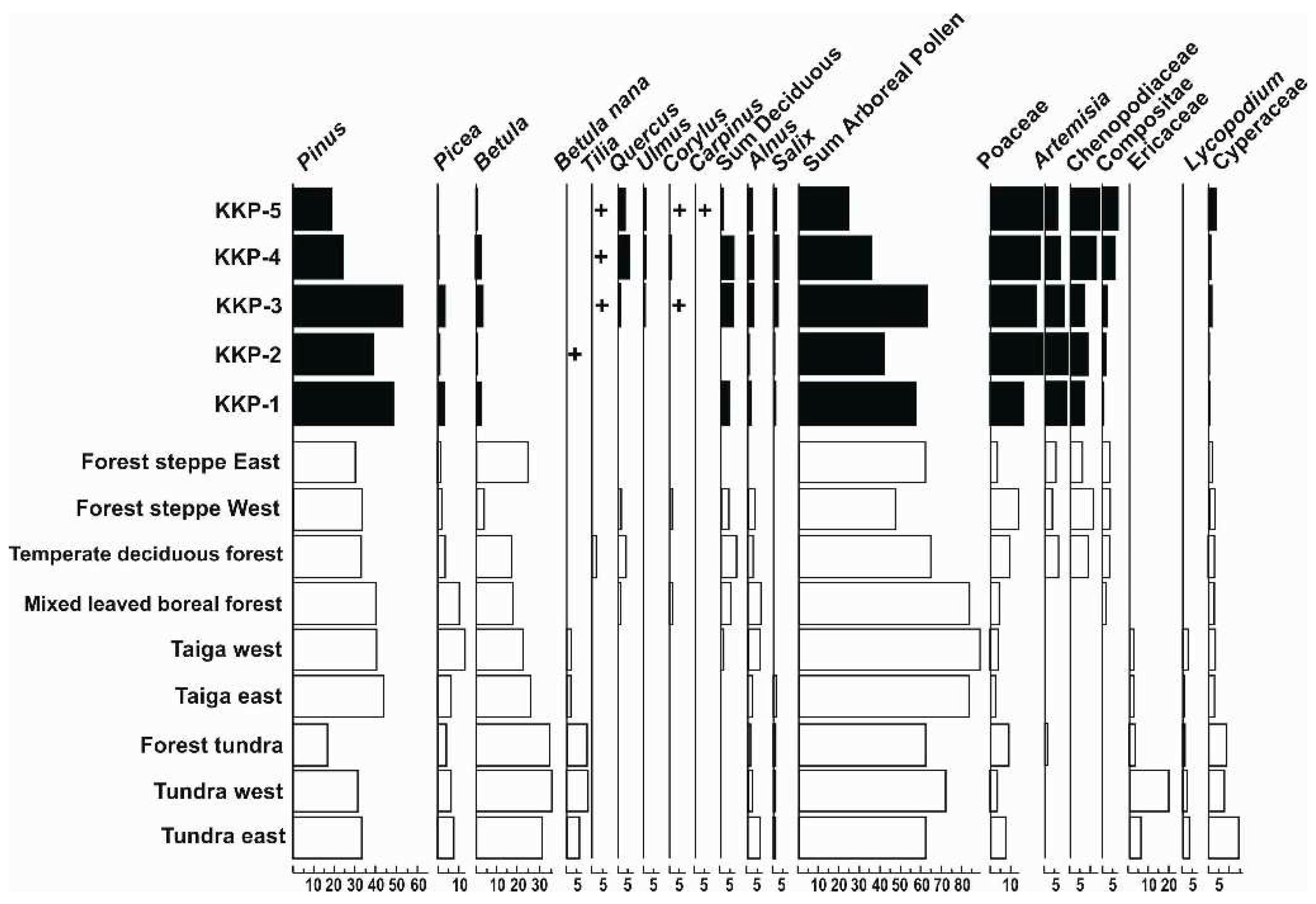
3.3. Results of the Pollen Analysis
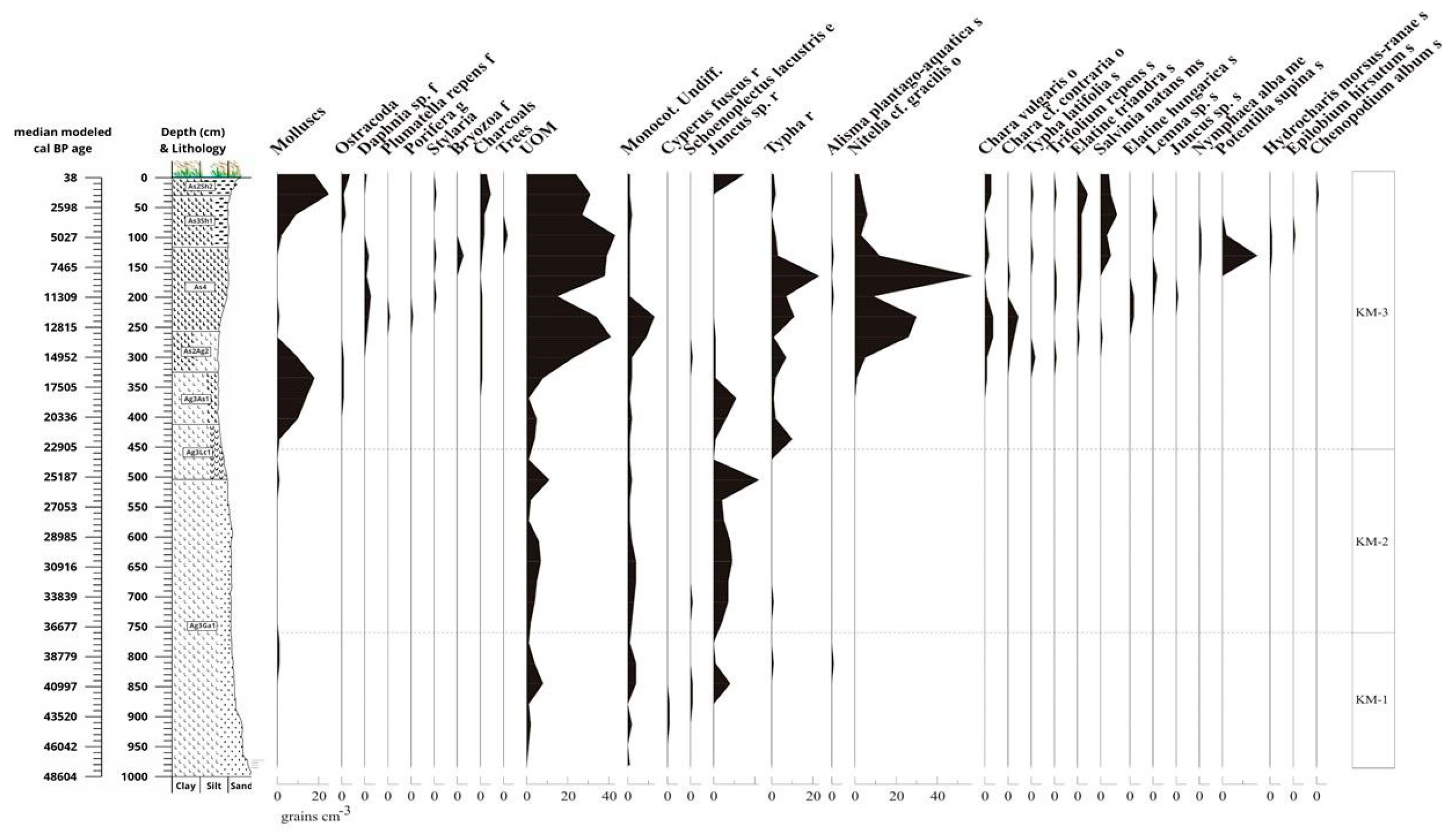
3.4. Macrobotanical Results
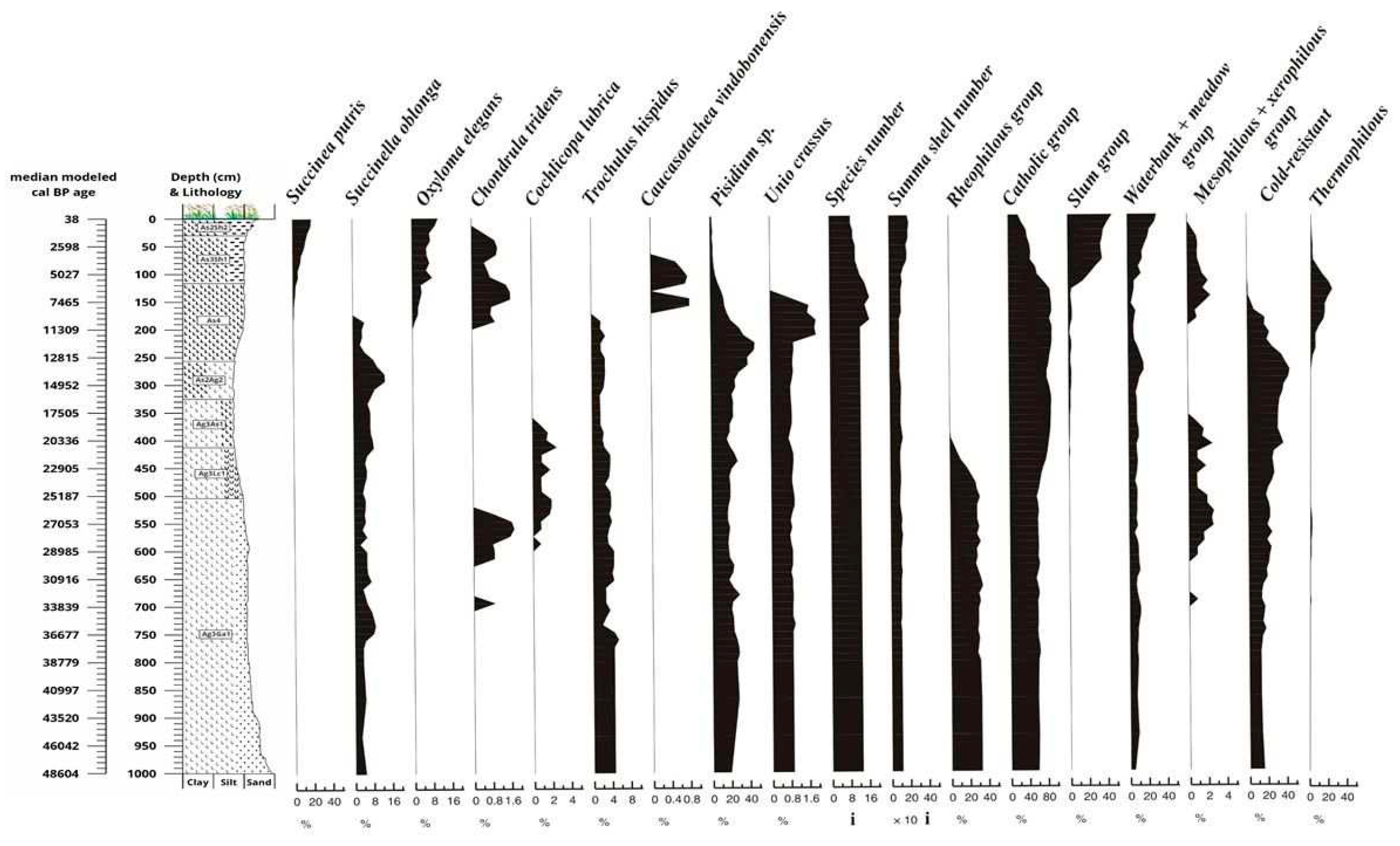
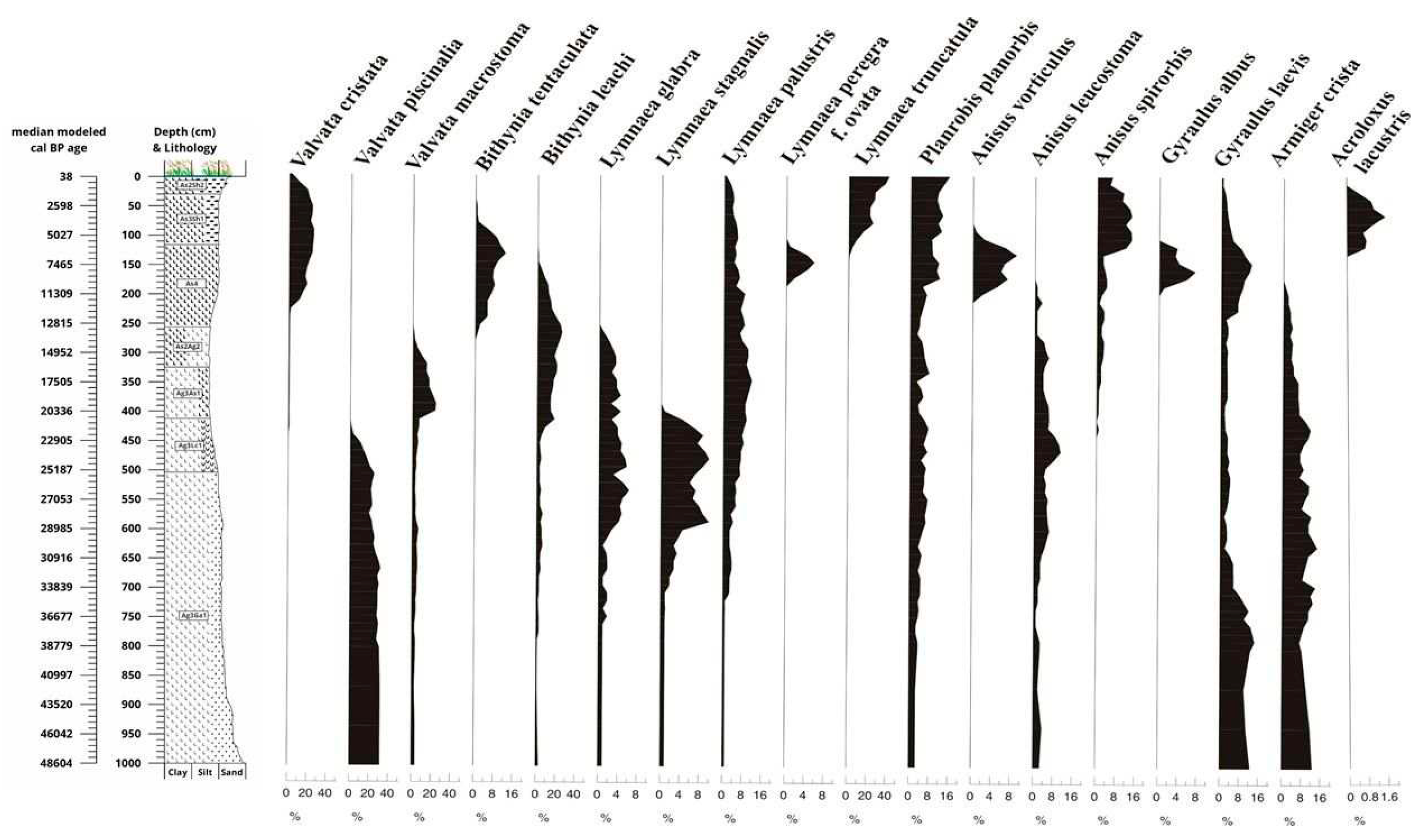
3.5. Malacological Results
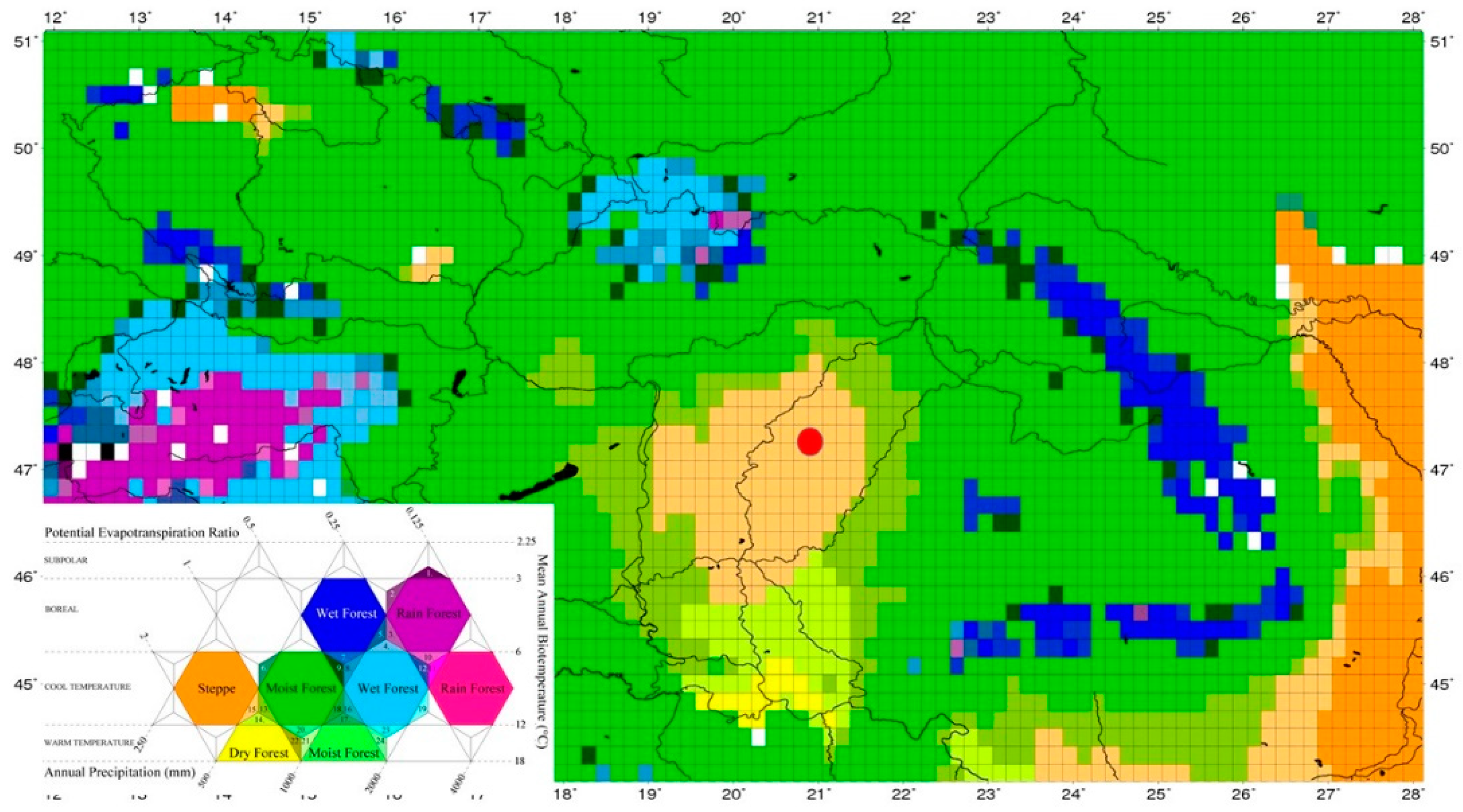
4. Discussion
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Béres, A.; Bodó I.; Jakuts, P. Hortobágy - A nomád Pusztától a Nemzeti Parkig; Kovács, G., Salamon, F.; Ed.; Natura: Budapest, 1976; p. 351.
- Jakucs, P. A Hortobágy növényvilága. In Hortobágy - a nomád Pusztától a Nemzeti Parkig, Kovács, G., Salamon, F., Ed.; Natura: Budapest, 1976; pp. 38-56.
- Molnár, Z. A Hortobágy hazánk egyik legősibb növényzetű tája. In Válogatás az MTA Ökológiai és Botanikai Kutatóintézet kutatási eredményeiből, ÖBKI Műhelyfüzetek 2, Török, K., Keve, K.T., Kertész, M., Ed.; MTA Ökológiai és Botanikai Kutatóintézet: Vácrátót, Hungary, 2009; pp. 143-148.
- Lőkös, L. Diaria Itinerum Pauli Kitaibelii III; Természettudományi Múzeum: Budapest, 2001.
- Townson, R. Travels in Hungary, with a short account of Vienna in the year 1793; G G & J Robinson: London, UK, 1797; p. 506.
- Pók, J. Szabolcs vármegye katonai leírása 1782-1785; Nyíregyháza, 1994; Volume II, p. 128.
- Sümegi, P.; Szilágyi, G.; Gulyás, S.; Jakab, G.; Molnár, A. The Late Quaternary Paleoecology and Environmental History of the Hortobágy, a unique Mosaic Alkaline Steppe from the Heart of the Carpathian Basin, Central Europe. In Steppe Ecosystems Biological Diversity, Management and Restoration, Prieto, M.B.M., Diaz, T.B., Ed.; Nova Publishers: New York, USA, 2013; pp. 165-194.
- Sümegi, P.; Molnár, A.; Szilágyi, G. Szikesedés a Hortobágyon. Természet Világa 2000, 131, 213-216.
- Dapsy, L. Tiszaszabályozás befolyása a magyar talajra. Természettudományi Közlöny 1869, 1, 97-108.
- Boros, E.; Bíró, Cs. A Duna-Tisza-közi szikes tavak ökológiai állapot változásai a XVIII-XX. századokban (Ecological changes in natron lakes of the Danube-Tisza Interfluve between the 18th and 20th centuries). Acta Biologica Debrecina Oecologica Hungarica 1999, 9, 81-105.
- Molnár, Z. A Pitvarosi-puszták és környékük vegetáció- és tájtörténete a Középkortól napjainkig. Natura Bekesiensis 1996, 2, 65-97.
- Molnár, Z. Vegetation history of the Kardoskút area (S.E. Hungary) II: The lake Fehér-tó in the last 200 years. Tiscia 1997, 30, 27-34.
- Molnár, Z. Tájtörténeti adatok a hazai szikesek növényzetének ismeretéhez. In Ohattól Farkas-szigetig. Ökológiai kultúra – ökológiai nevelés, Tóth, A., Ed.; Alföldkutatásért Alapítvány: Budapes-Kisújszállás, 2003; pp. 71-95.
- Molnár, Z. Történeti tájökológiai kutatások az Alföldön. University of Pécs, Pécs, Hungary, 2007.
- Molnár, Z. A Duna – Tisza köze és a Tiszántúl fontosabb vegetáció típusainak holocén kori története: irodalmi értékelés egy vegetációkutató szemszögéből. Kanitzia 2008, 16, 93-118.
- Molnár, Z.; Bíró, M. Vegetation history of the Kardoskút area (SE Hungary) I: History of the steppes from the Middle Ages to the present. Tiscia 1997, 30, 15-25.
- Miháltz, I. A Duna-Tisza csatorna geológiai viszonyainak tanulmányozása. In A Duna-Tisza csatorna, Lampl, H., Hallósy F., Ed.; Egyetemi Nyomda: Budapest, Hungary, 1947.
- Sümegi, P. Hajdúság felső-pleisztocén fejlődéstörténete finomrétegtani (üledékföldtani, őslénytani, geokémiai) vizsgálatok alapján. Debreceni Egyetem, Debrecen, 1989.
- Sümegi, P. A negyedidőszak földtanának és őskörnyezettanának alapjai; JATEPress: Szeged, Hungary, 2001; p. 262.
- Sümegi, P. Régészeti geológia és történeti ökológia alapjai; JATEPress: Szeged, Hungary, 2003; p. 224.
- Szöőr, G.; Sümegi, P.; Balázs, É. Sedimentological and geochemical facies analysis Upper Pleistocene fossil soil zones discovered in the Hajdúság region, NE Hungary. In Quaternary environment in Hungary, Pécsi, M., Schweitzer, F., Ed.; Studies in geography in Hungary; Akadémiai Kiadó: Budapest, Hungary, 1991.
- Szöőr, G.; Sümegi, P.; Balázs, É. A Hajdúság területén feltárt felső pleisztocén fosszilis talajok szedimentológiai és geokémiai fácieselemzése. In Fáciesanalitikai, paleobiogeokémiai és paleoökológiai kutatások, Szöőr, G., Ed.; MTA Debreceni Bizottsága: Debrecen, Hungary, 1992; pp. 81-91.
- Sümegi, P. The Process of Sodification on Hortobágy in Space and Time according to geopedological investigation. In Hydro-Petro-Geology and Hungary. A field trip across the country. Excursion guide.; Magyarhoni Földtani Társulat: Budapest, Hungary, 1997; pp. 237-242.
- Sümegi, P.; Szilágyi, G. A quarter-malacological inventory of Hungarian kurgans. In Kurgan Studies: An environmental and archaeological multiproxy study of burial mounds in the Eurasian steppe zone, Pető, Á., Barczi, A., Ed.; Archeopress, British Archaeological Reports 2238: Oxford, UK, 2011.
- Sümegi, P.; Bodor, E.; Törőcsik, T. The origins of sodification in the Hortobágy region in the light of the palaeoenvironmental studies at Zám–Halasfenék. In Environmental Archaeology in North-Eastern Hungary, Gál, E., Juhász, I., Sümegi, P., Ed.; Varia Archaeologica Hungarica; MTA Régészeti Intézet: Budapest, Hungary, 2005.
- Sümegi, P.; Bodor, E.; Törőcsik, T. A hortobágyi szikesedés eredete. In Táj, környezet és társadalom. Ünnepi tanulmányok Keveiné Bárány Ilona professzor asszony tiszteletére, Kiss, A., Mezősi, G., Sümeghy, Z., Ed.; Szegedi Tudományegyetem: Szeged, Hungary, 2006; pp. 633-641.
- Sümegi, P.; Molnár, M.; Jakab, G.; Persaits, G.; Majkut, P.; Páll, D.G.; Gulyás, S.; Jull, A.J.T.; Törőcsik, T. Radiocarbon-dated paleoenvironmental changes on a lake and peat sediment sequence from the central part of the Great Hungarian Plain (Central Europe) during the last 25.000 years. Radiocarbon 2011, 52, 85-97. [CrossRef]
- Willis, K.J. Impact of the early Neolithic Körös culture on the landscape: evidence from palaeoecological investigations of Kiri-tó. In The Early Neolithic on the Great Hungarian Plain: investigations of the Körös culture site of Ecsegfalva 23, Co. Békés, Whittle, A., Ed.; Varia Archaeologica Hungarica; Budapest, Hungary, 2007; pp. 83-99.
- Sümegi, P.; Magyari, E.; Daniel, P.; Hertelendi, E.; Rudner, E. A kardoskúti Fehér-tó negyedidőszaki fejlődéstörténetének rekonstrukciója. Földtani Közlöny 1999, 129, 479-519.
- Sümegi, P.; Persaits, G.; Gulyás, S. Woodland-Grassland Ecotonal Shifts in Environmental Mosaics: Lessons Learnt from the Environmental History of the Carpathian Basin (Central Europe) During the Holocene and the Last Ice Age Based on Investigation of Paleobotanical and Mollusk Remains. In Ecotones Between Forest and Grassland, Myster, R.W., Ed.; Springer Press: New York, USA, 2012; pp. 17-57. [CrossRef]
- Jakab, G.; Sümegi, P.; Magyari, E. New Quantative Method for the Paleobotanical Description of Late Quaternary Organic Sediments (Mire-Development Pathway and Paleoclimatic Records from Southern Hungary). Acta Geologica Hungarica 2004, 47, 373-409. [CrossRef]
- Willis, K.J.; Braun, M.; Sümegi, P.; Tóth, A. Does soil change cause vegetation change or vice-versa? Ecology 1997, 78, 740-750. [CrossRef]
- Magyari, E.; Chapman, J.C.; Passmore, D. G.; Allen, J. R. M.; Huntley, J.P.; Huntley, B. Holocene persistence of wooded steppe in the Great Hungarian Plain. Journal of Biogeography 2010, 37, 915-935. [CrossRef]
- Willis, K.J.; Sümegi, P.; Braun, M.; Tóth A. . The Late Quaternary environmental history of Bátorliget, N.E. Hungary. Palaeogeography, Palaeoclimatology, Palaeoecology 1995, 118, 25-47. [CrossRef]
- Sümegi, P.; Törőcsik, T. Magyarország növényzetének története. Rosalia 2010, 1, 11-13.
- Gardner, A.R. The impact of Neolithic agriculture on the environments of south-east Europe. University of Cambridge, Cambridge, 1999.
- Borhidi, A. Magyarország növénytársulásai; Akadémiai Kiadó: Budapest, Hungary, 2007.
- Ábrányi, K.; Bokor, J.; Timon, Á.; Radó, A.; Gaál, J.; Wargha, S. A Pallas Nagy Lexikona; Pallas Irodalmi és Nyomdai Részvénytársaság - Révai Testvérek: Budapest, 1895.
- Sümegi, P. Loess and Upper Paleolithic environment in Hungary; Aurea Kiadó: Nagykovácsi, Hungary, 2005; p. 312.
- Troels-Smith, J. Karakterisering af lose jordater (Characterisation of Unconsolidated Sediments). Danmarks Geologiske Undersogelse 1955, 3, 39-73. [CrossRef]
- Vári, T.Z.; Molnár, D.; Sümegi, P.; Sümegi, B.P.; Törőcsik, T.; Szakál, E.; Benyó-Korcsmáros, R. Holocene environmental history of the Bottomless Lake (Tăul fără fund) sphagnum peat bog in Bǎgǎu, Romania. Studia Quaternaria 2020, 37(2), 69-77.
- Vári, T.Z.; Gulyás, S.; Sümegi, P. Reconstructing the Paleoenvironmental Evolution of Lake Kolon (Hungary) through Integrated Geochemical and Sedimentological Analyses of Quaternary Sediments. Quaternary 2023, 6(3), 39. [CrossRef]
- Dean, W.E. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition; comparison with other methods. Journal of the Sedimentary Research 1974, 44, 242-248. [CrossRef]
- Oldfield, F.; Thompson, R.; Barber, K.E. Changing atmospheric fallout of magnetic particles recorded in recent ombrotrophic peat sections. Science 1978, 199, 679-680. [CrossRef]
- Dearing, J. Environmental Magnetic Susceptibility: Using the Bartington MS2 System; Chi Publishing: Keniloworth, UK, 1994.
- Xu, X.W.; Qiang, X.K.; Fu, C.F.; Zhao, H.; Chen, T.; Sun, Y.F. Characteristics of frequency-dependent magnetic susceptibility in Bartington MS2 and Kappabridge MFK1-FA, and its application in loess-paleosol, red clay and lacustrine sediments. Chinese Journal of Geophysics 2012, 55(1), 197-206.
- Njalsson, T.; Novosselov, I. Design and optimization of a compact low-cost optical particle sizer. Journal of Aerosol Science 2018, 119, 1-12. [CrossRef]
- Hertelendi, E.; Csongor, É.; Záborszky, L.; Molnár, I.; Gál, I.; Győrffy, M.; Nagy, S. Counting system for high precision C-14 dating. Radiocarbon 1989, 32, 399-408. [CrossRef]
- Hertelendi, E.; Sümegi, P.; Szöőr, Gy. Geochronologic and paleoclimatic characterization of Quaternary sediments in the Great Hungarian Plain. Radiocarbon 1992, 34, 833-839. [CrossRef]
- Molnár, M.; Janovics, R.; Major, I.; Orsovszki, J.; Gönczi, R.; Veres, M.; Leonard, A.G.; Castle, S.M.; Lange, T.E.; Wacker, L.; Hajdas, I.; Jull, A.J.T. Status Report of the New AMS 14C Sample Preparation Lab of the Hertelendi Laboratory of Environmental Studies (Debrecen; Hungary). Radiocarbon 2013, 55, 665-676. [CrossRef]
- Reimer, P.; Austin, W.; Bard, E.; Bayliss, A.; Blackwell, P.G.; Bronk Ramsey, C.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; Grootes, P.M.; Guilderson, T.P.; Hajdas, I.; Heaton, T..J.; Hogg, A.G.; Hughen, K.A.; Kromer, B.; Manning, S.W.; Muscheler, R.; Palmer, J.G.; Pearson, C.; van der Plicht, J.; Reimer, R.W.; Richards, D.A.; Scott, E.M.; Southon, J.R.; Turney, C.S.M.; Wacker, L.; Adolphi, F.; Büntgen, U.; Capano, M.; Fahrni, S.; Fogtmann-Schulz, A.; Friedrich, R.; Köhler, P.; Kudsk, S.; Miyake, F.; Olsen, J.; Reinig, F.; Sakamoto, M.; Sookdeo, A.; Talamo, S. The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 2020, 62, 725-757. [CrossRef]
- Stuiver, M.; Reimer, P.J.; Braziunas, T.F. High-precision radiocarbon age calibration for terrestrial and marine samples. Radiocarbon 1998, 40, 1127-1151. [CrossRef]
- RBacon. Age-Depth Modelling using Bayesian Statistics. Available online: https://cran.r-project.org/web/packages/rbacon/index.html (accessed on 29 August 2023).
- RStudio Desktop—Posit. Available online: (accessed on 29 August 2023).
- Blaauw, M.; Christen, J.A. Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian Analysis 2011, 6(3), 457-474. [CrossRef]
- Berglund, B.E.; Ralska-Jasiewiczowa, M. Pollen analysis and pollen diagrams. In Handbook of Holocene Palaeoecology and Palaeohydrology, Berglund, B.E., Ed.; J. Wiley and Sons Ltd.: Chichester-Toronto, 1986.
- Zólyomi, B. Magyarország növénytakarójának fejlődéstörténete az utolsó jégkorszaktól. MTA Biológiai Osztályának Közleményei 1952, 1, 491-544.
- Magyari, E.K. Climatic versus human modification of the Late Quaternary vegetation in Eastern Hungary. Debrecen University, Debrecen, 2002.
- Stockmarr, J. Tablets with spores used in absolute pollen analysis. Pollen et Spores 1971, 13, 614-621.
- Maher, L.J.J. Nomograms for computing 0.95 confidence limits of pollen data. Review of Palaeobotany and Palynology 1972, 13, 85-93. [CrossRef]
- Clark, R.L. Point count estimation of charcoal in pollen preparations and thin sections of sediments. Pollen et Spores 1982, 24, 523-535.
- Moore, P.D.; Webb, J.A.; Collinson, M.E. Pollen Analysis; Blackwell Scientific Publications: Oxford, UK, 1991.
- Beug, H.J. Leitfaden der Pollenbestimmung für Mitteleuropa und angrenzende Gebiete; Pfeil: München, Germany, 2004.
- Kozáková, R.; Pokorný, P. Dynamics of the biotopes at the edge of a medieval town: pollen analysis of Vltava river sediments in Prague, Czech Republic. Preslia 2007, 79, 259-281.
- Reille, M. Pollen et spores d'europe et d'afrique du nord; Laboratoire de Botanique historique et Palynologie: Marseille, 1992; p. 543.
- Reille, M. Pollen et Spores d'Europe et d'Afrique du Nord, Supplément 1; Laboratoire de Botanique historique et Palynologie: Marseille, 1995; p. 331.
- Reille, M. Pollen et spores d'Europe et d'Afrique du Nord: Supplément 2; Laboratoire de botanique historique et palynologie: Marseille, 1998; p. 521.
- Bennett, K.D. Psimpoll manual. 2005.
- Birks, H.J.B.; Gordon, A.D. Numerical methods in Quaternary pollen analysis; Academic Press: London, UK, 1985.
- Barber, K.E.; Chambers, F. M.; Maddy, D.; Brew, J. A sensitive high resolution record of the Holocene climatic change from a raised bog in northern England. The Holocene 1994, 4, 198-205. [CrossRef]
- Sugita, S. Pollen representation of vegetation in Quaternary sediments: theory and method in patchy vegetation. Journal of Ecology 1994, 82, 881-897. [CrossRef]
- Ložek, V. Quartärmollusken der Tschechoslowakei. Rozpravy Ústredniho ústavu geologického 1964, 31, 1-374.
- Sparks, B.W. Non-marine Mollusca and Quaternary ecology. The Journal of Animal Ecology 1964, 33, 87-98. [CrossRef]
- Alexandrowicz, W.P. Molluscan assemblages of Late Glacial and Holocene calcareous tufa in Southern Poland. Folia Quaternaria 2004, 75, 3-309.
- Alexandrowicz, W.P. Malacological sequence of Weichselian (MIS 5-2) loess series from a profile in Grodzisko Dolne (southern Poland) and its palaeogeographic significance. Quaternary International 2014, 319, 109-118. [CrossRef]
- Krolopp, E. Mollusc fauna of the sedimentary formations of the Quaternary period, Hungary. Acta Geologica Hungarica 1965, 9, 153-160.
- Krolopp, E. Quaternary malacology in Hungary. Földrajzi Közlemények 1973, 21, 161-171.
- Krolopp, E. Biostratigraphic division of Hungarian Pleistocene Formations according to their Mollusc fauna. Acta Geologica Hungarica 1983, 26, 62-89.
- Welter-Schultes, F. European non-marine mollusc, a guide for species identification; Planet Poster Edition: Göttingen, 2012; p. 760.
- Birks, H.J.B.; Birks, H.H. Quaternary paleoecology; Edward Arnold: London, 1980; p. 289.
- Fall, P. Pollen taphonomy in a canyon stream. Quaternary Research 1987, 28(3), 393-406. [CrossRef]
- Hall, S.A. Pollen analysis and paleoecology of alluvium. 31(3) 1989, 435-438. [CrossRef]
- Prentice, I.C.; Guiot, J.; Huntley, B.; Jolly, D.; Cheddadi, R. Reconstructing biomes from palaeoecological data: a general method and its application to European pollen data at 0 and 6 ka. Climate Dynamics 1996, 12, 185-194. [CrossRef]
- Magyari, E.K. Late quaternary vegetation history in the Hortobágy steppe and Middle Tisza floodplain, NE Hungary. Studia Botanica Hungarica 2011, 42, 185-203.
- Sümegi, P.; Szilágyi, G. A Hortobágy új felszínfejlődési modellje és a szikesedés eredete. Acta Biologica Debrecina 2010, 22, 37-36.
- Timár, G.; Sümegi, P.; Horváth, F. Late Quaternary dynamics of Tisza River: Evidence of climatic and tectonic controls. Tectonophysics 2005, 410, 97-110. [CrossRef]
- Sümegi, P.; Gulyás, S.; Molnár, D.; Sümegi, B.P.; Almond, P.C.; Vandenberghe, J.; Liping Zhou; Pál-Molnár; E.; Törőcsik, T.; Hao, Q.; Smalley, I.; Molnár, M.; Marsi, I. New chronology of the best developed loess/paleosol sequence of Hungary capturing the past 1.1 ma: Implications for correlation and proposed pan Eurasian stratigraphic schemes. Quaternary Sciences Reviews 2018, 191, 144-166. [CrossRef]
- Sümegi, P.; Molnár, D.; Náfrádi, K.; Makó, L.; Cseh, P.; Törőcsik, T.; Molnár, M.; Zhou, L. Vegetation and land snail-based reconstruction of the palaeocological changes in the forest steppe eco-region of the Carpathian Basin during last glacial warming. Global Ecology and Conservation 2022, 33, e01976. [CrossRef]
- Willis, K.J.; Sümegi, P.; Braun, M.; Bennett, K.D.; Tóth, A. Prehistoric land degradation in Hungary: who, how and why? Antiquity 1998, 72, 101-113. [CrossRef]
- Sümegi, P.; Náfrádi, K.; Molnár, D.; Sávai, S. Results of paleoecological studies in the loess region of Szeged-Öthalom (SE Hungary). Quaternary International 2015, 372, 66-78. [CrossRef]
- Dániel, P. Methods of the five-step extraction-digestion method. In The geohistory of Bátorliget Marshland, Sümegi, P., Gulyás, S., Ed.; Archaeolingua Press: Budapest, Hungary, 2004.
- Dániel, P.; Kovács, B.; Győri, Z.; Sümegi, P. A Combined Sequential Extraction Method for Analysis of Ions Bounded to Mineral Component. In Proceedings of the Workshop of the 4th Soil and Sediment Contaminant Analysis, Lausanne, Switzerland, 1996.
- Rónai, A. A síkvidékek földtani kutatásának jelentősége. Földtani Intézet Évi Jelentése 1961-ről 1964, 5-17.
- Rónai, A. Negyedkori üledékképződés és éghajlattörténet az Alföld Medencéjében: Quartärsedimentation und Klimageschichte im Becken der ungarischen Tiefebene (Alföld). Magyar Állami Földtani Intézet évkönyve 56. köt. 1. füz.; Műszaki Könyvkiadó: Budapest, Hungary, 1972; p. 421.
- Rónai, A. Negyedidőszaki kéregmozgások a Magyar medencében. Földtani Közlöny 1977, 107, 431-436.
- Rónai, A. Komplex síkvidéki földtani kutatások és agrogeológiai kapcsolataik. MTA X. Osztály Közleményei 1982, 15, 183-188.
- Rónai, A. Az Alföld földtana. Acta Geologica Hungarica 1985, 21, 1-445.
- Lehmkuhl, F.; Bösken, J.; Hošek, J.; Sprafke, T.; Marković, S.B.; Obreht, I.; Hambach, U.; Sümegi, P.; Lindner, H. Loess distribution and related Quaternary sediments in the Carpathian Basin. Journal of Maps 2018, 14, 673-682. [CrossRef]
- Lehmkuhl, F.; Bösken, J.; Pötter, S.; Sprafke, T.; Schulte, P.; Jary, Z.; Antoine, P.; Wacha, L.; Wolf, D.; Zerboni, A.; Hošek, J.; Marković, S.B.; Obreht, I.; Sümegi, P.; Veres, D.; Boemke, B.; Schaubert, V.; Viehweger, J.; Hambach, U. Loess landscapes of Europe – mapping, geomorphology and zonal differentiation. Earth-Science Reviews 2021, 211, 1-82. [CrossRef]
- Sümegi, P.; Gulyás, S. (eds.). The geohistory of Bátorliget Marshland; Archaeolingua Press: Budapest, 2004; p. 353.
- Willis, K.J.; Rudner, E.; Sümegi, P. The full-glacial forests of central and southeastern Europe: Evidence from Hungarian palaeoecological records. Quaternary Research 2000, 53, 203-213. [CrossRef]
- Jacobson, G.L.; Bradshaw, R.H.W. The selection of sites for palaeovegetational studies. Quaternary Research 1981, 16, 80-96. [CrossRef]
- Prentice, I.C. Pollen representation, source area, and basin size: toward a unified theory of pollen analysis. Quaternary Research 1985, 23, 76-86. [CrossRef]
- Prentice, I.C.; Webb III, T. BIOME 6000: reconstructing global mid-Holocene vegetation patterns from palaeoecological records. Journal of Biogeography 1988, 25, 997-1005. [CrossRef]
- Prentice, I.C.; Cramer W., Harrison; S.P.; Leemans, R.; Monserud, R.A.; Solomon, A.M. A global biome model based on plant physiology and dominance, soil properties and climate. Journal of Biogeography 1992, 19, 1177-1134. [CrossRef]
- Magyari, E.K.; Kuneš, P.; Jakab, G.; Sümegi, P.; Pelánková, B.; Schäbitz, F.; Braun, M.; Chytrý, M. Late Pleniglacial vegetation in eastern-central Europe: are there modern analogues in Siberia? Quaternary Science Reviews 2014, 95, 60-79. [CrossRef]
- Buiron, D.; Stenni, B.; Chappellaz, J.; Landais, A.; Baumgartner, M.; Bonazza, M.; Capronc, E.; Frezzotti, M.; Kageyama, M.; Lemieux-Dudon, B.; Masson-Delmotte, B.; Parrenin, F.; Schilt, A.; Selmo, E.; Severi, M.; Swingedouwc, D.; Udisti, R. Regional imprints of millennial variability during the MIS 3 period around Antarctica. Quaternary Science Reviews 2012, 48, 99-112. [CrossRef]
- Bond, G.C.; Heinrich, H.; Broecker, W.; Labeyrie, L.; McManus, J.; Andrews, J.; Huon, S.; Jantschik, R.; Clasen, S.; Simet, C.; Tedesco, K.; Klas, M.; Bonani, G.; Ivy, S. Evidence for massive discharges of icebergs into the North Atlantic ocean during the last glacial. Nature 1992, 360, 245-249. [CrossRef]
- Genty, D.; Blamart, D.; Ouahdi, R.; Gilmour, M.; Baker, A.; Jouzel, J.; Van Exter, S. Precise dating of Dansgaard-Oeschger climate oscillations in Western Europe from stalagmite data. Nature 2003, 421, 833-837. [CrossRef]
- Grimm, E.C.; Jacobson, G.L.; Watts, W.A.; Hansen, B.C.S.; Maasch, K.A. A 50,000-Year Record of Climate Oscillations from Florida and Its Temporal Correlation with the Heinrich Events. Science 1993, 261, 198-200. [CrossRef]
- Grimm, E.C.; Watts, W.A.; Jacobson, G.L.; Hansen, B.C.S.; Almquist, H.R.; Dieffenbacher-Krall, A.C. Evidence for warm wet Heinrich events in Florida. Quaternary Science Reviews 2006, 25, 2197–2211. [CrossRef]
- Grootes, P.M.; Stuiver, M.; White, J.W.C.; Johnsen, S.J.; Jouzel, J. Comparison of oxygen isotope records from the GISP2 and GRIP Greenland ice cores. Nature 1993, 366, 552-554. [CrossRef]
- Svensson, A.; Andersen, K.K.; Bigler, M.; Clausen, H.B.; Dahl-Jensen, D.; Davies, S.M.; Johnsen, S.J.; Muscheler, R.; Parrenin, F.; Rasmussen, S.O. A 60 000 year Greenland stratigraphic ice core chronology. Climate of the Past 2008, 4, 47-57. [CrossRef]
- Timmermann, A.; Menviel, L.; Okumura, Y.; Schilla, A.; Merkel, U.; Timm, O.; Hu, A.; Otto-Bliesner, B.; Schulz, M. Towards a quantitative understanding ofmillennial-scale Antarctic warming events. Quaternary Science Reviews 2010, 29, 74-85. [CrossRef]
- Yiou, P.; Jouzel, J.; Johnsen, S.; Rögnvaldsson, Ö.E. Rapid oscillations in Vostok and GRIP Ice Cores. Geophyical Research Letters 1995, 22, 2179–2182. [CrossRef]
- Björck, S.; Walker, M.J.C.; Cwynar, L.C.; Johnsen, S.; Knudsen, K.L.; Lowe, J.J.; Wohlfarth, B. and INTIMATE Members. An event stratigraphy for the Last Termination in the North Atlantic region based on the Greenland ice-core record: A proposal by the INTIMATE group. Journal of Quaternary Science 1998, 13, 282-292. [CrossRef]
- Walker, M.J.C.; Björck, S.; Lowe, J.J.; Cwynar, L.C.; Johnsen, S.; Knudsen, K.L.; Wohlfarth, B. and INTIMATE GROUP. Isotopic “events” in the GRIP ice core: A stratotype for the Late Pleistocene. Quaternary Science Reviews 1999, 18, 1143–1150. [CrossRef]
- Kremenetski, K.V. Steppe and forest-steppe belt of Eurasia: Holocene environmental history. In Prehistoric steppe adaptation and the horse, Levine, M., Renfrew, C., Boyle, K., Ed.; McDonald Institute for Archaeological Research, University of Cambridge: Cambridge, 2003; pp. 11-27.
- Tímár, G.; Gábris, G. Estimation of water conductivity of the natural flood channels on the Tisza flood-plain, the Great Hungarian Plain. Geomorphology 2008, 98(3-4), 250-261. [CrossRef]
- Allen, J.R.M.; Watts, W.A.; Huntley, B. Weichselian palynostratigraphy, palaeovegetation and palaeoenvironment: the record from Lago Grande di Monticchio, southern Italy. Quaternary International 2000, 73-74, 91–110. [CrossRef]
- Tarasov, P.E.; Webb, T.III; Andreev, A.A.; Afanaseva, N.B.; Berezina, N.A.; Bezusko, L.G.; Blyakharchuk, T.A.; Bolikhovskaya, N.S.; Cheddadi, R.; Chernavskaya, M.M.; Chernova, G.M.; Dorofeyuk, N.I.; Dirksen, V.G.; Elina, G.A.; Filimonova, L.V.; Glebov, F.Z.; Guiot, J.; Gunova, V.S.; Harrison, S.P.; Jolly, D.; Khomutova, V.I.; Kvavadze, E.V.; Osipova, I.M.; Panova, N.K.; Prentice, I.C.; Saarse, L.; Sevastyanov, D.V.; Volkova, V.S.; Zernitskaya, V.P. Present-day and mid-Holocene biomes reconstructed from pollen and plant macrofossil data from the Former Soviet Union and Mongolia. Journal of Biogeography 1998, 25, 1029-1053. [CrossRef]
- Tarasov, P.E.; Volkova, V.S.; Webb, T.III; Guiot, J.; Andreev, A.A.; Bezusko, L.G.; Bezusko, T.V.; Bykova, G.V.; Dorofeyuk, N.I.; Kvavadze, E.V.; Osipova, I.M.; Panova, N.K.; Sevastyanov, D.V. Last glacial maximum biomes reconstructed from pollen and plant macrofossil data from northern Eurasia. Journal of Biogeography 2000, 27, 609-620. [CrossRef]
- Szelepcsényi, Z.; Breuer, H.; Kis, A.; Pongrácz, R.; Sümegi, P. Assessment of projected climate change in the Carpathian Region using the Holdridge life zone system. Theoretical and Applied Climatology 2018, 131, 593-610. [CrossRef]
- Pełechata, A.; Pełechaty, M.; Pukacz, A. Factors influencing cyanobacteria community structure in Chara-lakes. Ecological Indicators 2016, 71, 477-490. [CrossRef]
- Vári, T.Z.; Pál-Molnár, R.; Sümegi P. Reconstructing the paleoenvironmental Evolution of Lake Kolon (Hungary) through Malacological, Macrobotanical and Pollen Analyses. Diversity 2023, This volume. [CrossRef]
- Várallyay, G. Soil water problems in Hungary. Agrokémia és Talajtan 1989, 38(3-4), 577-595.
- Bába, K. History of the investigation of the terrestrial snails of the Great Hungarian Plain and its present situation - II. Tiscia 1983, 18, 83-95.
- Molnár, B. Pliocén és pleisztocén lehordási területek az Alföldön. Földtani Közlöny 1960, 89, 403-413.
- Molnár, B. A magyarországi folyók homoküledékeinek nehézásvány-összetétel vizsgálata. Hidrológiai Közlöny 1964, 44, 347-355.
- Molnár, B. Changes in Area and Directions of Stream Erosion in the Eastern Part of the Hungarian Basin (Great Plain) during the Pliocene and Pleistocene. Acta Minerologica et Petrographica 1965, 17, 39-52.
- Molnár, B. A Dél-Alföld feltöltődésének ritmusai és vízföldtani jelentőségük. Hidrológiai Közlöny 1967, 47, 537-552.
- Molnár, B. Az Alföld harmadidőszak-végi és negyedkori feltöltődési ciklusai. Földtani Közlöny 1973, 103, 294-310.
- Magyari, E.; Jakab, G.; Rudner, E.; Sümegi, P. Palynological and plant macrofossil data on Late Pleistocen short term climatic osscilations in Northeast Hungary. Acta Palaeobotanica Supplement 1999, 2, 491-502.
- Sümegi, P. Reconstruction of flora, soil and landscape evolution, and human impact on the Bereg Plain from late, glacial up to the present, based on palaeoecological analysis. In The Upper Tisa Valley. Tiscia Monograph Series, Hamar, J., Sárkány-Kiss, A., Ed.; Szeged, 1999; pp. 173-204.
- Peterson, G.M. Recent pollen spectra and zonal vegetation in the western USSR. Quaternary Science Reviews 1983, 2(4), 281-321. [CrossRef]
- Szelepcsényi, Z.; Breuer, H.; Ács, F.; Kozma, I. Biofizikai klímaklasszifikációk (1. rész: a módszerek bemutatása). Légkör 2009a, 54, 21-26.
- Szelepcsényi, Z.; Breuer, H.; Ács, F.; Kozma, I. Biofizikai klímaklasszifikációk (2. rész: magyarországi alkalmazások). Légkör 2009b, 54, 21-26.
- Szelepcsényi, Z.; Breuer, H.; Sümegi, P. The climate of Carpathian Region in the 20th century based on the original and modified Holdridge life zone system. Central European Journal of Geosciences 2014, 6, 293-307. [CrossRef]
- Szilágyi, G.; Náfrádi, K.; Sümegi, P. A preliminary chronological study to understand the construction phases of a Late Copper–Early Bronze Age kurgan (kunhalom). Central European Geology 2019, 62(3), 1-29. [CrossRef]
- Németh, A.; Bárány, A.; Csorba, G.; Magyari, E.; Pazonyi, P.; Pálfy, J. Holocene mammal extinctions in the Carpathian Basin: A review. Mammal Review 2017, 47(1), 38-52. [CrossRef]


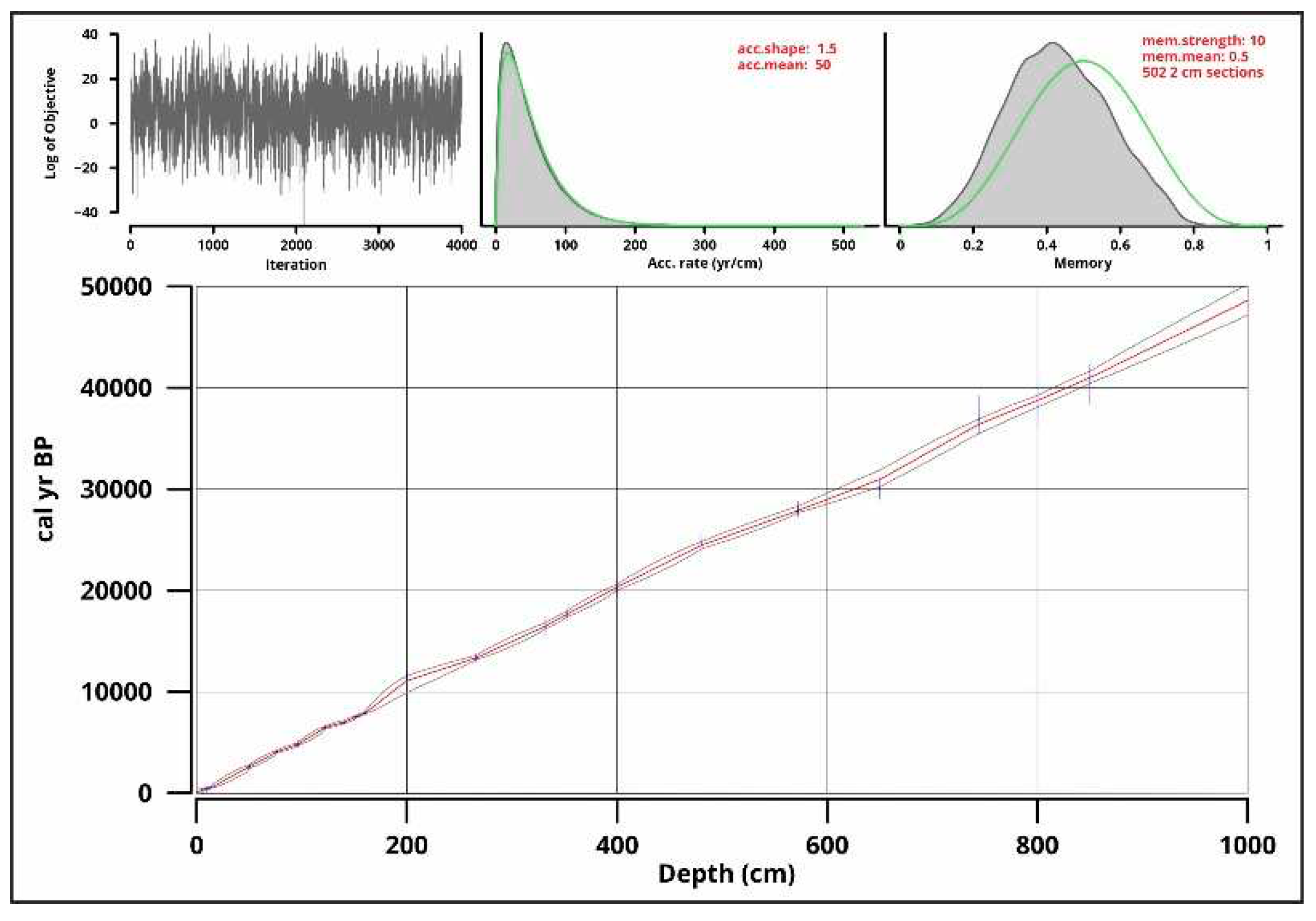
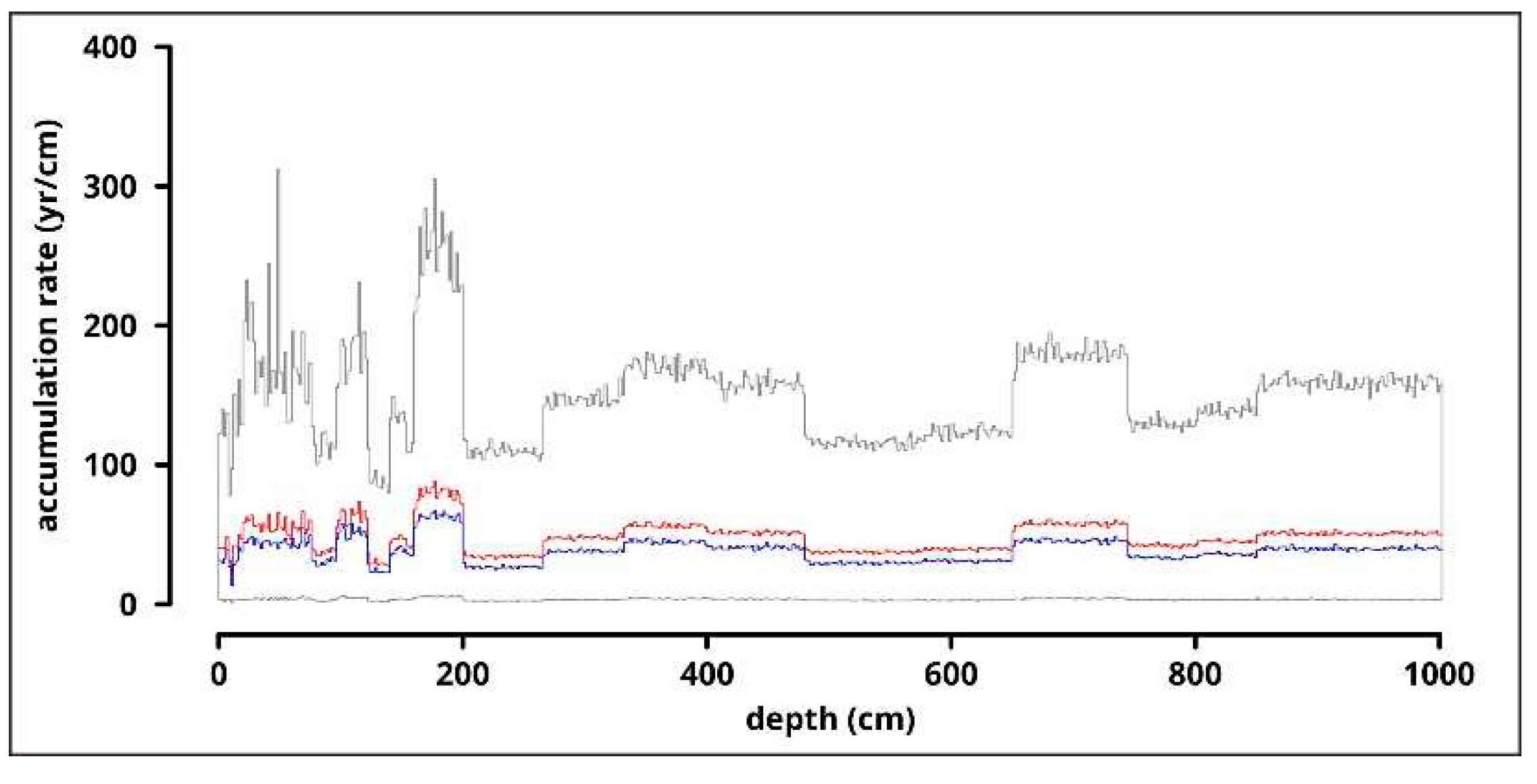
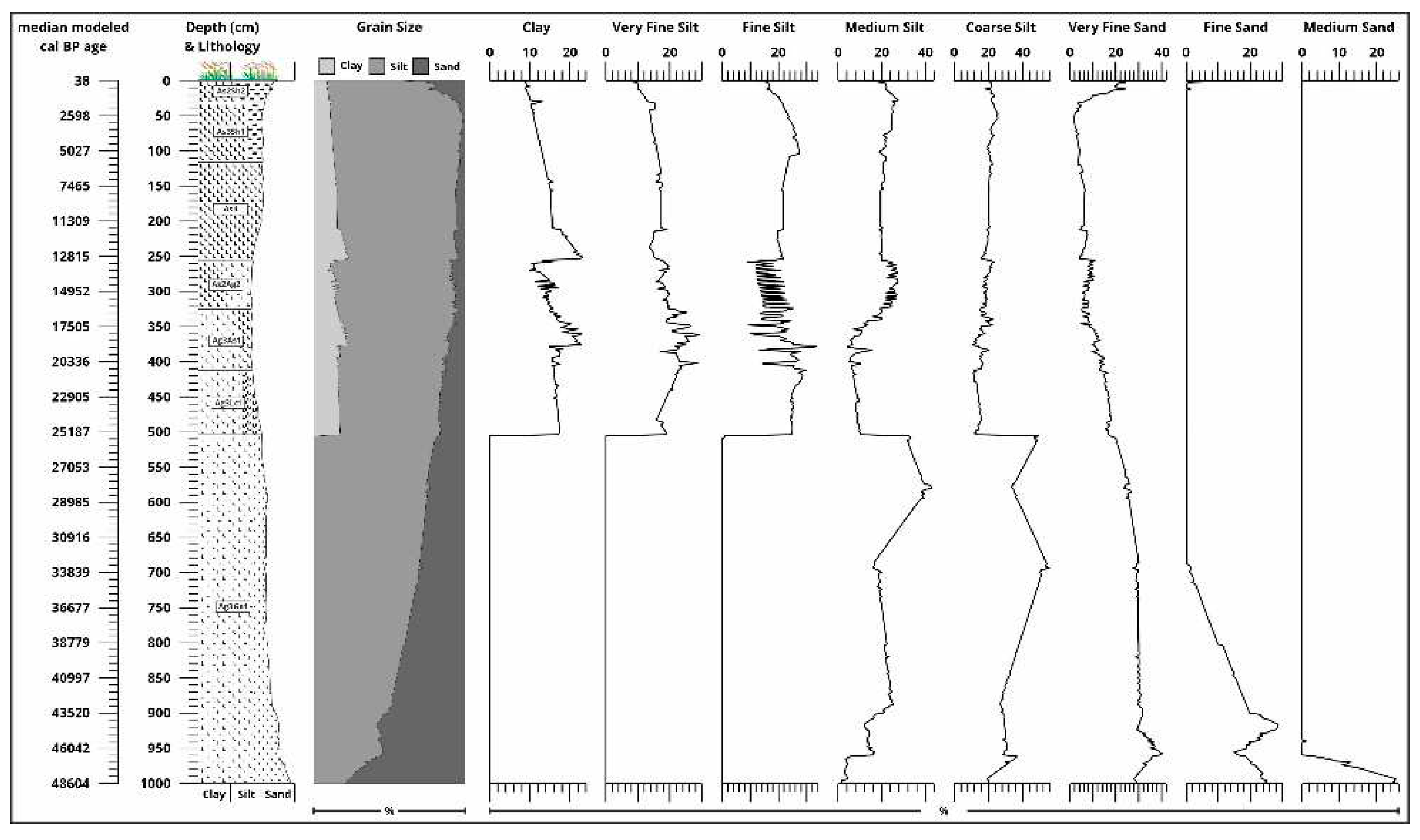
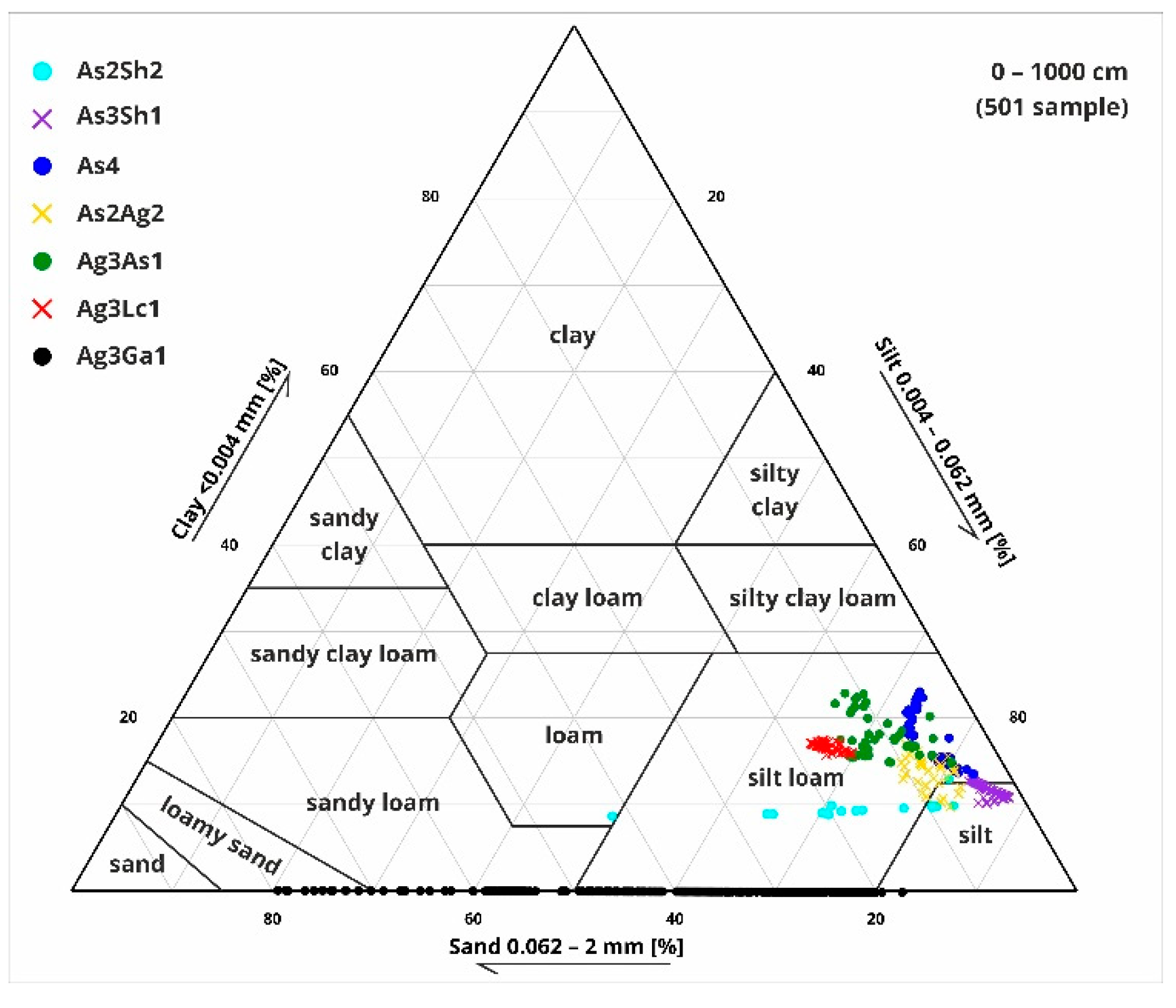
| cm | type of organic material | uncal BP | +/- | cal BP | +/- | cal BP interval | cal BC/AD | +/- | cal BC/AD interval | Code |
|---|---|---|---|---|---|---|---|---|---|---|
| (from 1950) | ||||||||||
| 10 | Planorbis shell | 403 | 17 | 422 | 84 | 339-506 | 1527 AD | 84 | 1444-1611 AD | DeA-130891 |
| 12 | Planorbis shell | 444 | 21 | 493 | 32 | 461-524 | 1448 AD | 22 | 1426-1469 AD | D-AMS21141 |
| 50 | Planorbis shell | 2524 | 23 | 2616 | 120 | 2496-2736 | 767 BC | 120 | 547 - 787 BC | D-AMS21152 |
| 76 | Planorbis shell | 3732 | 20 | 4169 | 82 | 3987-4151 | 2120 BC | 82 | 2038-2202 BC | DeA-130902 |
| 96 | Lymnaea shell | 4232 | 29 | 4753 | 104 | 4649-4857 | 2804 BC | 104 | 2700-2908 BC | D-AMS2113 |
| 122 | Unio shell | 5696 | 22 | 6479 | 73 | 6406-6552 | 4530 BC | 73 | 4457-4603 BC | DeA-130913 |
| 140 | Unio shell | 6065 | 26 | 6973 | 174 | 6799-7146 | 5023 BC | 174 | 4850-5197 BC | D-AMS21112 |
| 152 | Unio shell | 6698 | 30 | 7549 | 76 | 7504-7655 | 5628 BC | 73 | 5555-5701 BC | D-AMS21123 |
| 160 | Bithynia shell | 7067 | 29 | 7900 | 65 | 7836-7965 | 5949 BC | 67 | 5887-6016 BC | D-AMS21104 |
| 200 | Unio shell | 10,055 | 33 | 11,580 | 223 | 11,357-11,803 | 9629 BC | 225 | 9404-9854 BC | DeA-130914 |
| 266 | Pisidium shell | 11,417 | 52 | 13,292 | 118 | 13,174-13,411 | 11,343 BC | 118 | 11,225-11,462 BC | D-AMS21095 |
| 332 | Pisidium shell | 13,598 | 70 | 16,431 | 234 | 16,197-16,654 | 14,457 BC | 249 | 14,208-14,705 BC | D-AMS21086 |
| 352 | Cochlicopa shell | 14,474 | 58 | 17,645 | 233 | 17,412-17,878 | 15,696 BC | 233 | 15,463-15,929 BC | D-AMS21077 |
| 400 | Succinella shell | 16,847 | 78 | 20,342 | 191 | 20,151-20,535 | 18,262 BC | 159 | 18,203-18,521 BC | D-AMS21068 |
| 480 | Trochulus hispidus | 20,529 | 72 | 24,676 | 310 | 24,366-24,986 | 22,727 BC | 310 | 22,417-23,037 BC | DeA-130965 |
| 572 | Succinella shell | 23,725 | 85 | 27,851 | 157 | 27,694-28,008 | 25,902 BC | 157 | 25,745-26,059 BC | D-AMS21049 |
| 650 | Pinus microharcoal | 25,661 | 121 | 29,935 | 254 | 29,681-30,189 | 27,986 BC | 254 | 27,732-28,240 BC | DeA-131026 |
| 744 | Helicopsis shell | 32,535 | 175 | 36,845 | 481 | 36,364-37,325 | 34,896 BC | 481 | 34,415-35,376 BC | D-AMS210510 |
| 800 | Succinella shell | 33,433 | 232 | 38,308 | 853 | 37,455-39,161 | 36,359 BC | 853 | 35,506-37,212 BC | DeA-130977 |
| 850 | Trochulus shell | 35,696 | 297 | 40,740 | 583 | 40,158-41,323 | 38,791 BC | 583 | 38,209-39,374 BC | DeA-130988 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





