Submitted:
06 December 2023
Posted:
06 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Autosomal Dominant Hereditary Ataxias
2.1. SCA1
2.2. SCA2
2.3. SCA3 (Machado–Joseph Disease)
2.4. SCA6
2.5. SCA7
2.6. SCA17
2.7. SCA27B (GAA-FGF14 Ataxia)
3. Autosomal Recessive Cerebellar Ataxias
3.1. Friedreich Ataxia
3.2. CANVAS
3.3. ARSACS
3.4. Ataxia-Telangiectasia
3.5. ARCA2
4. Episodic Ataxias
5. X-Linked Degenerative Ataxias
6. Congenital Ataxias
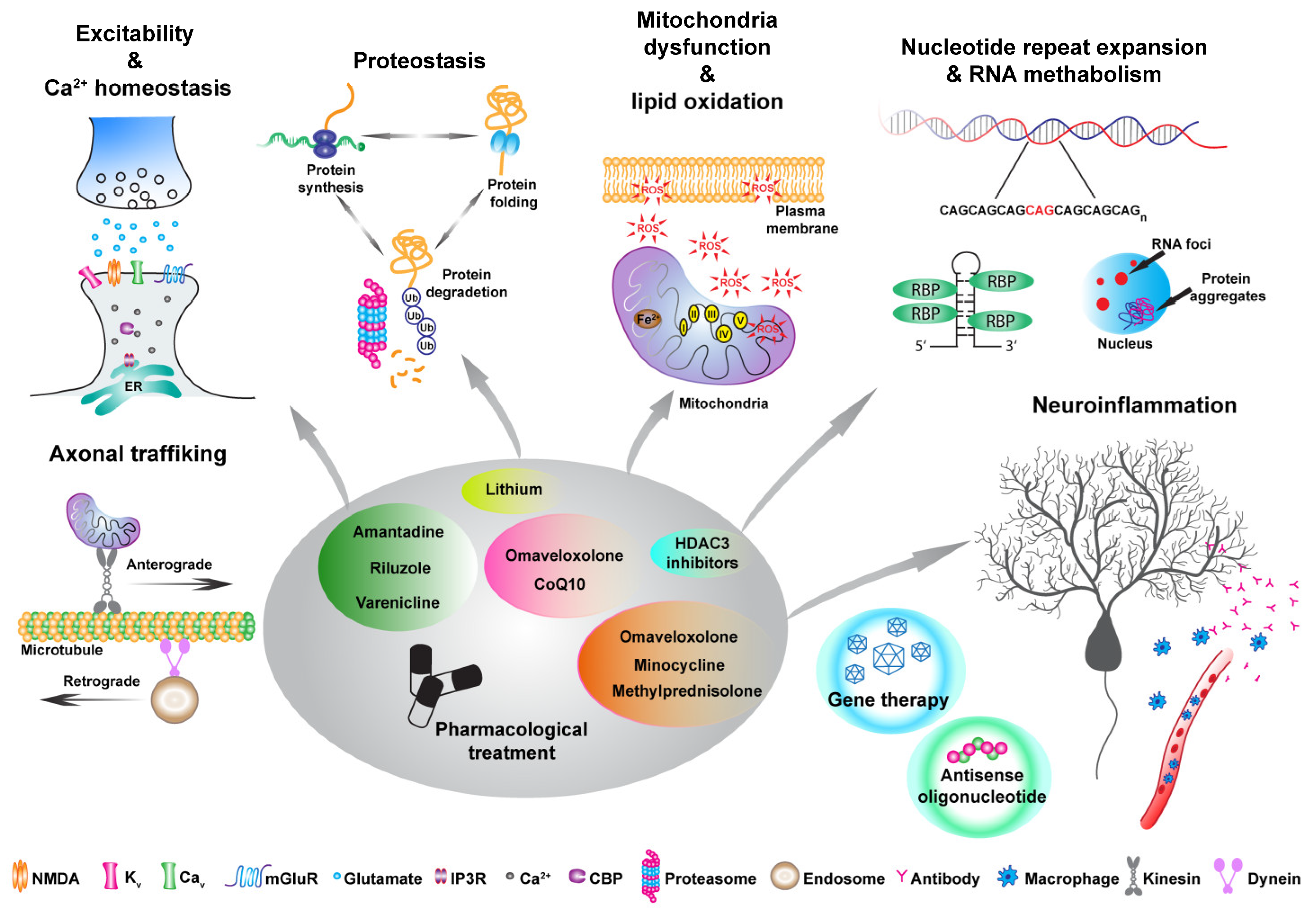
7. Different Approaches to Ameliorate Disease Outcome
- Genome editing strategies to correct the pathological mutation;
- Antisense oligonucleotides (ASO) or small RNA structures to interfere with repeat expansion translation or R-loop formation;
- Gene therapy approaches to rescue the levels of disease-mutated genes or key pathway regulators;
- Disease protein homeostasis to restore physiological protein levels;
- Pharmacological treatments, either to target specific pathophysiological mechanisms, to reduce toxic metabolites, or to supplement crucial compounds.
7.1. Genome Editing Strategies to Correct Pathological Mutation
7.2. Antisense Oligonucleotides (ASO) or Small RNA Structures to Interfere with Repeat Expansion Translation or R-Loop Formation
7.3. Gene Therapy Approaches to Rescue the Levels of Disease-Mutated Genes or Key Pathway Regulators
7.4. Disease Protein Homeostasis to Restore Physiological Protein Levels
7.5. Pharmacological Treatments to Target Specific Pathomechanism, Reduce Toxic Metabolites and Supplement Crucial Compounds
8. Ataxia FDA Approved Drugs and Treatments
9. Main Challenges and Limiting Factors
Author Contributions
Funding
Conflicts of Interest
References
- Pandolfo, M.; Manto, M. Cerebellar and Afferent Ataxias. Contin. Minneap. Minn. 2013, 19, 1312–1343. [CrossRef]
- Pilotto, F.; Saxena, S. Epidemiology of Inherited Cerebellar Ataxias and Challenges in Clinical Research. Clin. Transl. Neurosci. 2018, 2, 2514183X1878525. [CrossRef]
- Zoghbi, H.Y.; Orr, H.T. Glutamine Repeats and Neurodegeneration. Annu. Rev. Neurosci. 2000, 23, 217–247. [CrossRef]
- Palau, F.; Espinós, C. Autosomal Recessive Cerebellar Ataxias. Orphanet J. Rare Dis. 2006, 1, 47. [CrossRef]
- Koeppen, A.H. Friedreich’s Ataxia: Pathology, Pathogenesis, and Molecular Genetics. J. Neurol. Sci. 2011, 303, 1–12. [CrossRef]
- Coarelli, G.; Wirth, T.; Tranchant, C.; Koenig, M.; Durr, A.; Anheim, M. The Inherited Cerebellar Ataxias: An Update. J. Neurol. 2023, 270, 208–222. [CrossRef]
- Leehey, M.A. Fragile X-Associated Tremor/Ataxia Syndrome: Clinical Phenotype, Diagnosis, and Treatment. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2009, 57, 830–836. [CrossRef]
- Jen, J.C.; Graves, T.D.; Hess, E.J.; Hanna, M.G.; Griggs, R.C.; Baloh, R.W.; CINCH investigators Primary Episodic Ataxias: Diagnosis, Pathogenesis and Treatment. Brain J. Neurol. 2007, 130, 2484–2493. [CrossRef]
- Choi, K.-D.; Choi, J.-H. Episodic Ataxias: Clinical and Genetic Features. J. Mov. Disord. 2016, 9, 129–135. [CrossRef]
- Bushart, D.D.; Shakkottai, V.G. Ion Channel Dysfunction in Cerebellar Ataxia. Neurosci. Lett. 2019, 688, 41–48. [CrossRef]
- Pilotto, F.; Douthwaite, C.; Diab, R.; Ye, X.; Al Qassab, Z.; Tietje, C.; Mounassir, M.; Odriozola, A.; Thapa, A.; Buijsen, R.A.M.; et al. Early Molecular Layer Interneuron Hyperactivity Triggers Purkinje Neuron Degeneration in SCA1. Neuron 2023. [CrossRef]
- Ruegsegger, C.; Stucki, D.M.; Steiner, S.; Angliker, N.; Radecke, J.; Keller, E.; Zuber, B.; Rüegg, M.A.; Saxena, S. Impaired MTORC1-Dependent Expression of Homer-3 Influences SCA1 Pathophysiology. Neuron 2016, 89, 129–146. [CrossRef]
- Ronnebaum, S.M.; Patterson, C.; Schisler, J.C. Emerging Evidence of Coding Mutations in the Ubiquitin–Proteasome System Associated with Cerebellar Ataxias. Hum. Genome Var. 2014, 1, 14018. [CrossRef]
- Luo, H.; Todi, S.V.; Paulson, H.L.; Costa, M. do C. Regional and Age-Dependent Changes in Ubiquitination in Cellular and Mouse Models of Spinocerebellar Ataxia Type 3. Front. Mol. Neurosci. 2023, 16, 1154203. [CrossRef]
- Girard, M.; Larivière, R.; Parfitt, D.A.; Deane, E.C.; Gaudet, R.; Nossova, N.; Blondeau, F.; Prenosil, G.; Vermeulen, E.G.M.; Duchen, M.R.; et al. Mitochondrial Dysfunction and Purkinje Cell Loss in Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay (ARSACS). Proc. Natl. Acad. Sci. USA 2012, 109, 1661–1666. [CrossRef]
- Lynch, D.R.; Farmer, G. Mitochondrial and Metabolic Dysfunction in Friedreich Ataxia: Update on Pathophysiological Relevance and Clinical Interventions. Neuronal Signal. 2021, 5, NS20200093. [CrossRef]
- Manolaras, I.; Del Bondio, A.; Griso, O.; Reutenauer, L.; Eisenmann, A.; Habermann, B.H.; Puccio, H. Mitochondrial Dysfunction and Calcium Dysregulation in COQ8A-Ataxia Purkinje Neurons Are Rescued by CoQ10 Treatment. Brain J. Neurol. 2023, awad099. [CrossRef]
- Harmuth, T.; Weber, J.J.; Zimmer, A.J.; Sowa, A.S.; Schmidt, J.; Fitzgerald, J.C.; Schöls, L.; Riess, O.; Hübener-Schmid, J. Mitochondrial Dysfunction in Spinocerebellar Ataxia Type 3 Is Linked to VDAC1 Deubiquitination. Int. J. Mol. Sci. 2022, 23, 5933. [CrossRef]
- Stucki, D.M.; Ruegsegger, C.; Steiner, S.; Radecke, J.; Murphy, M.P.; Zuber, B.; Saxena, S. Mitochondrial Impairments Contribute to Spinocerebellar Ataxia Type 1 Progression and Can Be Ameliorated by the Mitochondria-Targeted Antioxidant MitoQ. Free Radic. Biol. Med. 2016, 97, 427–440. [CrossRef]
- Ward, J.M.; Stoyas, C.A.; Switonski, P.M.; Ichou, F.; Fan, W.; Collins, B.; Wall, C.E.; Adanyeguh, I.; Niu, C.; Sopher, B.L.; et al. Metabolic and Organelle Morphology Defects in Mice and Human Patients Define Spinocerebellar Ataxia Type 7 as a Mitochondrial Disease. Cell Rep. 2019, 26, 1189–1202.e6. [CrossRef]
- Li, P.P.; Moulick, R.; Feng, H.; Sun, X.; Arbez, N.; Jin, J.; Marque, L.O.; Hedglen, E.; Chan, H.Y.E.; Ross, C.A.; et al. RNA Toxicity and Perturbation of RRNA Processing in Spinocerebellar Ataxia Type 2. Mov. Disord. Off. J. Mov. Disord. Soc. 2021, 36, 2519–2529. [CrossRef]
- Zhang, N.; Ashizawa, T. RNA Toxicity and Foci Formation in Microsatellite Expansion Diseases. Curr. Opin. Genet. Dev. 2017, 44, 17–29. [CrossRef]
- Fiszer, A.; Krzyzosiak, W.J. RNA Toxicity in Polyglutamine Disorders: Concepts, Models, and Progress of Research. J. Mol. Med. 2013, 91, 683–691. [CrossRef]
- Matsuzono, K.; Imamura, K.; Murakami, N.; Tsukita, K.; Yamamoto, T.; Izumi, Y.; Kaji, R.; Ohta, Y.; Yamashita, T.; Abe, K.; et al. Antisense Oligonucleotides Reduce RNA Foci in Spinocerebellar Ataxia 36 Patient IPSCs. Mol. Ther. Nucleic Acids 2017, 8, 211–219. [CrossRef]
- Durr, A. Autosomal Dominant Cerebellar Ataxias: Polyglutamine Expansions and Beyond. Lancet Neurol. 2010, 9, 885–894. [CrossRef]
- Fujioka, S.; Sundal, C.; Wszolek, Z.K. Autosomal Dominant Cerebellar Ataxia Type III: A Review of the Phenotypic and Genotypic Characteristics. Orphanet J. Rare Dis. 2013, 8, 14. [CrossRef]
- Duenas, A.M. Molecular Pathogenesis of Spinocerebellar Ataxias. Brain 2006, 129, 1357–1370. [CrossRef]
- Schöls, L.; Bauer, P.; Schmidt, T.; Schulte, T.; Riess, O. Autosomal Dominant Cerebellar Ataxias: Clinical Features, Genetics, and Pathogenesis. Lancet Neurol. 2004, 3, 291–304. [CrossRef]
- Ruano, L.; Melo, C.; Silva, M.C.; Coutinho, P. The Global Epidemiology of Hereditary Ataxia and Spastic Paraplegia: A Systematic Review of Prevalence Studies. Neuroepidemiology 2014, 42, 174–183. [CrossRef]
- Buijsen, R.A.M.; Toonen, L.J.A.; Gardiner, S.L.; van Roon-Mom, W.M.C. Genetics, Mechanisms, and Therapeutic Progress in Polyglutamine Spinocerebellar Ataxias. Neurotherapeutics 2019, 16, 263–286. [CrossRef]
- Paulson, H.L.; Shakkottai, V.G.; Clark, H.B.; Orr, H.T. Polyglutamine Spinocerebellar Ataxias — from Genes to Potential Treatments. Nat. Rev. Neurosci. 2017, 18, 613–626. [CrossRef]
- Honti, V.; Vecsei, L. Genetic and Molecular Aspects of Spinocerebellar Ataxias. Neuropsychiatr. Dis. Treat. 2005, 1, 125–133. [CrossRef]
- Maltecca, F.; Filla, A.; Castaldo, I.; Coppola, G.; Fragassi, N.A.; Carella, M.; Bruni, A.; Cocozza, S.; Casari, G.; Servadio, A.; et al. Intergenerational Instability and Marked Anticipation in SCA-17. Neurology 2003, 61, 1441–1443. [CrossRef]
- Nethisinghe, S.; Pigazzini, M.L.; Pemble, S.; Sweeney, M.G.; Labrum, R.; Manso, K.; Moore, D.; Warner, J.; Davis, M.B.; Giunti, P. PolyQ Tract Toxicity in SCA1 Is Length Dependent in the Absence of CAG Repeat Interruption. Front. Cell. Neurosci. 2018, 12, 200. [CrossRef]
- Filla, A.; Mariotti, C.; Caruso, G.; Coppola, G.; Cocozza, S.; Castaldo, I.; Calabrese, O.; Salvatore, E.; De Michele, G.; Riggio, M.C.; et al. Relative Frequencies of CAG Expansions in Spinocerebellar Ataxia and Dentatorubropallidoluysian Atrophy in 116 Italian Families. Eur. Neurol. 2000, 44, 31–36. [CrossRef]
- Donato, S.D.; Mariotti, C.; Taroni, F. Spinocerebellar Ataxia Type 1. In Handbook of Clinical Neurology; Elsevier, 2012; Volume 103, pp. 399–421. ISBN 978-0-444-51892-7.
- Orengo, J.P.; van der Heijden, M.E.; Hao, S.; Tang, J.; Orr, H.T.; Zoghbi, H.Y. Motor Neuron Degeneration Correlates with Respiratory Dysfunction in SCA1. Dis. Model. Mech. 2018, dmm.032623. [CrossRef]
- Robitaille, Y.; Schut, L.; Kish, S.J. Structural and Immunocytochemical Features of Olivopontocerebellar Atrophy Caused by the Spinocerebellar Ataxia Type 1 (SCA-1) Mutation Define a Unique Phenotype. Acta Neuropathol. 1995, 90, 572–581. [CrossRef]
- Seidel, K.; Siswanto, S.; Brunt, E.R.P.; den Dunnen, W.; Korf, H.-W.; Rüb, U. Brain Pathology of Spinocerebellar Ataxias. Acta Neuropathol. 2012, 124, 1–21. [CrossRef]
- Orr, H.T.; Chung, M.; Banfi, S.; Kwiatkowski, T.J.; Servadio, A.; Beaudet, A.L.; McCall, A.E.; Duvick, L.A.; Ranum, L.P.W.; Zoghbi, H.Y. Expansion of an Unstable Trinucleotide CAG Repeat in Spinocerebellar Ataxia Type 1. Nat. Genet. 1993, 4, 221–226. [CrossRef]
- Irwin, S.; Vandelft, M.; Pinchev, D.; Howell, J.L.; Graczyk, J.; Orr, H.T.; Truant, R. RNA Association and Nucleocytoplasmic Shuttling by Ataxin-1. J. Cell Sci. 2005, 118, 233–242. [CrossRef]
- Klement, I.A.; Skinner, P.J.; Kaytor, M.D.; Yi, H.; Hersch, S.M.; Clark, H.B.; Zoghbi, H.Y.; Orr, H.T. Ataxin-1 Nuclear Localization and Aggregation. Cell 1998, 95, 41–53. [CrossRef]
- Lam, Y.C.; Bowman, A.B.; Jafar-Nejad, P.; Lim, J.; Richman, R.; Fryer, J.D.; Hyun, E.D.; Duvick, L.A.; Orr, H.T.; Botas, J.; et al. ATAXIN-1 Interacts with the Repressor Capicua in Its Native Complex to Cause SCA1 Neuropathology. Cell 2006, 127, 1335–1347. [CrossRef]
- Rousseaux, M.W.C.; Tschumperlin, T.; Lu, H.-C.; Lackey, E.P.; Bondar, V.V.; Wan, Y.-W.; Tan, Q.; Adamski, C.J.; Friedrich, J.; Twaroski, K.; et al. ATXN1-CIC Complex Is the Primary Driver of Cerebellar Pathology in Spinocerebellar Ataxia Type 1 through a Gain-of-Function Mechanism. Neuron 2018, 97, 1235–1243.e5. [CrossRef]
- Emamian, E.S.; Kaytor, M.D.; Duvick, L.A.; Zu, T.; Tousey, S.K.; Zoghbi, H.Y.; Clark, H.B.; Orr, H.T. Serine 776 of Ataxin-1 Is Critical for Polyglutamine-Induced Disease in SCA1 Transgenic Mice. Neuron 2003, 38, 375–387. [CrossRef]
- Huttlin, E.L.; Jedrychowski, M.P.; Elias, J.E.; Goswami, T.; Rad, R.; Beausoleil, S.A.; Villén, J.; Haas, W.; Sowa, M.E.; Gygi, S.P. A Tissue-Specific Atlas of Mouse Protein Phosphorylation and Expression. Cell 2010, 143, 1174–1189. [CrossRef]
- Serra, H.G.; Duvick, L.; Zu, T.; Carlson, K.; Stevens, S.; Jorgensen, N.; Lysholm, A.; Burright, E.; Zoghbi, H.Y.; Clark, H.B.; et al. RORα-Mediated Purkinje Cell Development Determines Disease Severity in Adult SCA1 Mice. Cell 2006, 127, 697–708. [CrossRef]
- de Chiara, C.; Menon, R.P.; Strom, M.; Gibson, T.J.; Pastore, A. Phosphorylation of S776 and 14-3-3 Binding Modulate Ataxin-1 Interaction with Splicing Factors. PloS ONE 2009, 4, e8372. [CrossRef]
- Antenora, A.; Rinaldi, C.; Roca, A.; Pane, C.; Lieto, M.; Saccà, F.; Peluso, S.; De Michele, G.; Filla, A. The Multiple Faces of Spinocerebellar Ataxia Type 2. Ann. Clin. Transl. Neurol. 2017, 4, 687–695. [CrossRef]
- Rüb, U.; Schöls, L.; Paulson, H.; Auburger, G.; Kermer, P.; Jen, J.C.; Seidel, K.; Korf, H.-W.; Deller, T. Clinical Features, Neurogenetics and Neuropathology of the Polyglutamine Spinocerebellar Ataxias Type 1, 2, 3, 6 and 7. Prog. Neurobiol. 2013, 104, 38–66. [CrossRef]
- Bürk, K. Cognition in Hereditary Ataxia. Cerebellum Lond. Engl. 2007, 6, 280–286. [CrossRef]
- Mutesa, L.; Pierquin, G.; Segers, K.; Vanbellinghen, J.F.; Gahimbare, L.; Bours, V. Spinocerebellar Ataxia Type 2 (SCA2): Clinical Features and Genetic Analysis. J. Trop. Pediatr. 2008, 54, 350–352. [CrossRef]
- Estrada, R.; Galarraga, J.; Orozco, G.; Nodarse, A.; Auburger, G. Spinocerebellar Ataxia 2 (SCA2): Morphometric Analyses in 11 Autopsies. Acta Neuropathol. 1999, 97, 306–310. [CrossRef]
- Vishwakarma, P.; Muthuswamy, S.; Agarwal, S. Current Molecular Insight to Reveal the Dynamics of CAG Repeating Units in Spinocerebellar Ataxia. Intractable Rare Dis. Res. 2018, 7, 79–86. [CrossRef]
- Elden, A.C.; Kim, H.-J.; Hart, M.P.; Chen-Plotkin, A.S.; Johnson, B.S.; Fang, X.; Armakola, M.; Geser, F.; Greene, R.; Lu, M.M.; et al. Ataxin-2 Intermediate-Length Polyglutamine Expansions Are Associated with Increased Risk for ALS. Nature 2010, 466, 1069–1075. [CrossRef]
- Van Damme, P.; Veldink, J.H.; van Blitterswijk, M.; Corveleyn, A.; van Vught, P.W.J.; Thijs, V.; Dubois, B.; Matthijs, G.; van den Berg, L.H.; Robberecht, W. Expanded ATXN2 CAG Repeat Size in ALS Identifies Genetic Overlap between ALS and SCA2. Neurology 2011, 76, 2066–2072. [CrossRef]
- Glass, J.D.; Dewan, R.; Ding, J.; Gibbs, J.R.; Dalgard, C.; Keagle, P.J.; Shankaracharya; García-Redondo, A.; Traynor, B.J.; Chia, R.; et al. ATXN2 Intermediate Expansions in Amyotrophic Lateral Sclerosis. Brain J. Neurol. 2022, 145, 2671–2676. [CrossRef]
- Kozlov, G.; Trempe, J.F.; Khaleghpour, K.; Kahvejian, A.; Ekiel, I.; Gehring, K. Structure and Function of the C-Terminal PABC Domain of Human Poly(A)-Binding Protein. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 4409–4413. [CrossRef]
- Shibata, H.; Huynh, D.P.; Pulst, S.M. A Novel Protein with RNA-Binding Motifs Interacts with Ataxin-2. Hum. Mol. Genet. 2000, 9, 1303–1313. [CrossRef]
- Watanabe, R.; Higashi, S.; Nonaka, T.; Kawakami, I.; Oshima, K.; Niizato, K.; Akiyama, H.; Yoshida, M.; Hasegawa, M.; Arai, T. Intracellular Dynamics of Ataxin-2 in the Human Brains with Normal and Frontotemporal Lobar Degeneration with TDP-43 Inclusions. Acta Neuropathol. Commun. 2020, 8, 176. [CrossRef]
- Juvonen, V.; Hietala, M.; Kairisto, V.; Savontaus, M.-L. The Occurrence of Dominant Spinocerebellar Ataxias among 251 Finnish Ataxia Patients and the Role of Predisposing Large Normal Alleles in a Genetically Isolated Population. Acta Neurol. Scand. 2005, 111, 154–162. [CrossRef]
- Vale, J.; Bugalho, P.; Silveira, I.; Sequeiros, J.; Guimarães, J.; Coutinho, P. Autosomal Dominant Cerebellar Ataxia: Frequency Analysis and Clinical Characterization of 45 Families from Portugal. Eur. J. Neurol. 2010, 17, 124–128. [CrossRef]
- Rosenberg, R.N. Machado-Joseph Disease: An Autosomal Dominant Motor System Degeneration. Mov. Disord. Off. J. Mov. Disord. Soc. 1992, 7, 193–203. [CrossRef]
- Bettencourt, C.; Santos, C.; Coutinho, P.; Rizzu, P.; Vasconcelos, J.; Kay, T.; Cymbron, T.; Raposo, M.; Heutink, P.; Lima, M. Parkinsonian Phenotype in Machado-Joseph Disease (MJD/SCA3): A Two-Case Report. BMC Neurol. 2011, 11, 131. [CrossRef]
- Riess, O.; Rüb, U.; Pastore, A.; Bauer, P.; Schöls, L. SCA3: Neurological Features, Pathogenesis and Animal Models. Cerebellum Lond. Engl. 2008, 7, 125–137. [CrossRef]
- Rüb, U.; Brunt, E.R.; Deller, T. New Insights into the Pathoanatomy of Spinocerebellar Ataxia Type 3 (Machado-Joseph Disease). Curr. Opin. Neurol. 2008, 21, 111–116. [CrossRef]
- Stefanescu, M.R.; Dohnalek, M.; Maderwald, S.; Thürling, M.; Minnerop, M.; Beck, A.; Schlamann, M.; Diedrichsen, J.; Ladd, M.E.; Timmann, D. Structural and Functional MRI Abnormalities of Cerebellar Cortex and Nuclei in SCA3, SCA6 and Friedreich’s Ataxia. Brain J. Neurol. 2015, 138, 1182–1197. [CrossRef]
- Wan, N.; Chen, Z.; Wan, L.; Tang, B.; Jiang, H. MR Imaging of SCA3/MJD. Front. Neurosci. 2020, 14, 749. [CrossRef]
- Ma, J.; Wu, C.; Lei, J.; Zhang, X. Cognitive Impairments in Patients with Spinocerebellar Ataxia Types 1, 2 and 3 Are Positively Correlated to the Clinical Severity of Ataxia Symptoms. Int. J. Clin. Exp. Med. 2014, 7, 5765–5771. [CrossRef]
- Doss-Pepe, E.W.; Stenroos, E.S.; Johnson, W.G.; Madura, K. Ataxin-3 Interactions with Rad23 and Valosin-Containing Protein and Its Associations with Ubiquitin Chains and the Proteasome Are Consistent with a Role in Ubiquitin-Mediated Proteolysis. Mol. Cell. Biol. 2003, 23, 6469–6483. [CrossRef]
- Liu, H.; Li, X.; Ning, G.; Zhu, S.; Ma, X.; Liu, X.; Liu, C.; Huang, M.; Schmitt, I.; Wüllner, U.; et al. The Machado-Joseph Disease Deubiquitinase Ataxin-3 Regulates the Stability and Apoptotic Function of P53. PLoS Biol. 2016, 14, e2000733. [CrossRef]
- Zeng, C.; Zhao, C.; Ge, F.; Li, Y.; Cao, J.; Ying, M.; Lu, J.; He, Q.; Yang, B.; Dai, X.; et al. Machado-Joseph Deubiquitinases: From Cellular Functions to Potential Therapy Targets. Front. Pharmacol. 2020, 11, 1311. [CrossRef]
- Bichelmeier, U.; Schmidt, T.; Hübener, J.; Boy, J.; Rüttiger, L.; Häbig, K.; Poths, S.; Bonin, M.; Knipper, M.; Schmidt, W.J.; et al. Nuclear Localization of Ataxin-3 Is Required for the Manifestation of Symptoms in SCA3: In Vivo Evidence. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 7418–7428. [CrossRef]
- Sasaki, H.; Kojima, H.; Yabe, I.; Tashiro, K.; Hamada, T.; Sawa, H.; Hiraga, H.; Nagashima, K. Neuropathological and Molecular Studies of Spinocerebellar Ataxia Type 6 (SCA6). Acta Neuropathol. 1998, 95, 199–204. [CrossRef]
- Casey, H.L.; Gomez, C.M. Spinocerebellar Ataxia Type 6. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington, Seattle: Seattle, WA, USA, 1993.
- Zhuchenko, O.; Bailey, J.; Bonnen, P.; Ashizawa, T.; Stockton, D.W.; Amos, C.; Dobyns, W.B.; Subramony, S.H.; Zoghbi, H.Y.; Lee, C.C. Autosomal Dominant Cerebellar Ataxia (SCA6) Associated with Small Polyglutamine Expansions in the Alpha 1A-Voltage-Dependent Calcium Channel. Nat. Genet. 1997, 15, 62–69. [CrossRef]
- Ikeuchi, T.; Takano, H.; Koide, R.; Horikawa, Y.; Honma, Y.; Onishi, Y.; Igarashi, S.; Tanaka, H.; Nakao, N.; Sahashi, K.; et al. Spinocerebellar Ataxia Type 6: CAG Repeat Expansion in Alpha1A Voltage-Dependent Calcium Channel Gene and Clinical Variations in Japanese Population. Ann. Neurol. 1997, 42, 879–884. [CrossRef]
- Riess, O.; Schöls, L.; Bottger, H.; Nolte, D.; Vieira-Saecker, A.M.; Schimming, C.; Kreuz, F.; Macek, M.; Krebsová, A.; Macek, M. Sen; et al. SCA6 Is Caused by Moderate CAG Expansion in the Alpha1A-Voltage-Dependent Calcium Channel Gene. Hum. Mol. Genet. 1997, 6, 1289–1293. [CrossRef]
- Shizuka, M.; Watanabe, M.; Ikeda, Y.; Mizushima, K.; Okamoto, K.; Shoji, M. Molecular Analysis of a de Novo Mutation for Spinocerebellar Ataxia Type 6 and (CAG)n Repeat Units in Normal Elder Controls. J. Neurol. Sci. 1998, 161, 85–87. [CrossRef]
- Ishikawa, K.; Tanaka, H.; Saito, M.; Ohkoshi, N.; Fujita, T.; Yoshizawa, K.; Ikeuchi, T.; Watanabe, M.; Hayashi, A.; Takiyama, Y.; et al. Japanese Families with Autosomal Dominant Pure Cerebellar Ataxia Map to Chromosome 19p13.1-P13.2 and Are Strongly Associated with Mild CAG Expansions in the Spinocerebellar Ataxia Type 6 Gene in Chromosome 19p13.1. Am. J. Hum. Genet. 1997, 61, 336–346. [CrossRef]
- Du, X.; Wang, J.; Zhu, H.; Rinaldo, L.; Lamar, K.-M.; Palmenberg, A.C.; Hansel, C.; Gomez, C.M. Second Cistron in CACNA1A Gene Encodes a Transcription Factor Mediating Cerebellar Development and SCA6. Cell 2013, 154, 118–133. [CrossRef]
- La Spada, A.R. Spinocerebellar Ataxia Type 7. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington, Seattle: Seattle (WA), 1993.
- Aleman, T.S.; Cideciyan, A.V.; Volpe, N.J.; Stevanin, G.; Brice, A.; Jacobson, S.G. Spinocerebellar Ataxia Type 7 (SCA7) Shows a Cone-Rod Dystrophy Phenotype. Exp. Eye Res. 2002, 74, 737–745. [CrossRef]
- Benton, C.S.; de Silva, R.; Rutledge, S.L.; Bohlega, S.; Ashizawa, T.; Zoghbi, H.Y. Molecular and Clinical Studies in SCA-7 Define a Broad Clinical Spectrum and the Infantile Phenotype. Neurology 1998, 51, 1081–1086. [CrossRef]
- Turk, K.W.; Flanagan, M.E.; Josephson, S.; Keene, C.D.; Jayadev, S.; Bird, T.D. Psychosis in Spinocerebellar Ataxias: A Case Series and Study of Tyrosine Hydroxylase in Substantia Nigra. Cerebellum Lond. Engl. 2018, 17, 143–151. [CrossRef]
- David, G.; Abbas, N.; Stevanin, G.; Dürr, A.; Yvert, G.; Cancel, G.; Weber, C.; Imbert, G.; Saudou, F.; Antoniou, E.; et al. Cloning of the SCA7 Gene Reveals a Highly Unstable CAG Repeat Expansion. Nat. Genet. 1997, 17, 65–70. [CrossRef]
- Ansorge, O.; Giunti, P.; Michalik, A.; Van Broeckhoven, C.; Harding, B.; Wood, N.; Scaravilli, F. Ataxin-7 Aggregation and Ubiquitination in Infantile SCA7 with 180 CAG Repeats. Ann. Neurol. 2004, 56, 448–452. [CrossRef]
- Cheon, Y.; Kim, H.; Park, K.; Kim, M.; Lee, D. Dynamic Modules of the Coactivator SAGA in Eukaryotic Transcription. Exp. Mol. Med. 2020, 52, 991–1003. [CrossRef]
- Palhan, V.B.; Chen, S.; Peng, G.-H.; Tjernberg, A.; Gamper, A.M.; Fan, Y.; Chait, B.T.; La Spada, A.R.; Roeder, R.G. Polyglutamine-Expanded Ataxin-7 Inhibits STAGA Histone Acetyltransferase Activity to Produce Retinal Degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 8472–8477. [CrossRef]
- Nakamura, Y.; Tagawa, K.; Oka, T.; Sasabe, T.; Ito, H.; Shiwaku, H.; La Spada, A.R.; Okazawa, H. Ataxin-7 Associates with Microtubules and Stabilizes the Cytoskeletal Network. Hum. Mol. Genet. 2012, 21, 1099–1110. [CrossRef]
- Monin, M.-L.; Tezenas du Montcel, S.; Marelli, C.; Cazeneuve, C.; Charles, P.; Tallaksen, C.; Forlani, S.; Stevanin, G.; Brice, A.; Durr, A. Survival and Severity in Dominant Cerebellar Ataxias. Ann. Clin. Transl. Neurol. 2015, 2, 202–207. [CrossRef]
- Toyoshima, Y.; Yamada, M.; Onodera, O.; Shimohata, M.; Inenaga, C.; Fujita, N.; Morita, M.; Tsuji, S.; Takahashi, H. SCA17 Homozygote Showing Huntington’s Disease-like Phenotype. Ann. Neurol. 2004, 55, 281–286. [CrossRef]
- Stevanin, G.; Brice, A. Spinocerebellar Ataxia 17 (SCA17) and Huntington’s Disease-like 4 (HDL4). Cerebellum Lond. Engl. 2008, 7, 170–178. [CrossRef]
- Nolte, D.; Sobanski, E.; Wissen, A.; Regula, J.U.; Lichy, C.; Müller, U. Spinocerebellar Ataxia Type 17 Associated with an Expansion of 42 Glutamine Residues in TATA-Box Binding Protein Gene. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1396–1399. [CrossRef]
- Yang, S.; Li, X.-J.; Li, S. Molecular Mechanisms Underlying Spinocerebellar Ataxia 17 (SCA17) Pathogenesis. Rare Dis. Austin Tex 2016, 4, e1223580. [CrossRef]
- Rafehi, H.; Read, J.; Szmulewicz, D.J.; Davies, K.C.; Snell, P.; Fearnley, L.G.; Scott, L.; Thomsen, M.; Gillies, G.; Pope, K.; et al. An Intronic GAA Repeat Expansion in FGF14 Causes the Autosomal-Dominant Adult-Onset Ataxia SCA27B/ATX-FGF14. Am. J. Hum. Genet. 2023, 110, 1018. [CrossRef]
- Bonnet, C.; Pellerin, D.; Roth, V.; Clément, G.; Wandzel, M.; Lambert, L.; Frismand, S.; Douarinou, M.; Grosset, A.; Bekkour, I.; et al. Optimized Testing Strategy for the Diagnosis of GAA-FGF14 Ataxia/Spinocerebellar Ataxia 27B. Sci. Rep. 2023, 13, 9737. [CrossRef]
- Wang, Q.; Bardgett, M.E.; Wong, M.; Wozniak, D.F.; Lou, J.; McNeil, B.D.; Chen, C.; Nardi, A.; Reid, D.C.; Yamada, K.; et al. Ataxia and Paroxysmal Dyskinesia in Mice Lacking Axonally Transported FGF14. Neuron 2002, 35, 25–38. [CrossRef]
- Bosch, M.K.; Carrasquillo, Y.; Ransdell, J.L.; Kanakamedala, A.; Ornitz, D.M.; Nerbonne, J.M. Intracellular FGF14 (IFGF14) Is Required for Spontaneous and Evoked Firing in Cerebellar Purkinje Neurons and for Motor Coordination and Balance. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 6752–6769. [CrossRef]
- Xiao, M.; Bosch, M.K.; Nerbonne, J.M.; Ornitz, D.M. FGF14 Localization and Organization of the Axon Initial Segment. Mol. Cell. Neurosci. 2013, 56, 393–403. [CrossRef]
- Traschütz, A.; Reich, S.; Adarmes, A.D.; Anheim, M.; Ashrafi, M.R.; Baets, J.; Basak, A.N.; Bertini, E.; Brais, B.; Gagnon, C.; et al. The ARCA Registry: A Collaborative Global Platform for Advancing Trial Readiness in Autosomal Recessive Cerebellar Ataxias. Front. Neurol. 2021, 12, 677551. [CrossRef]
- Anheim, M.; Tranchant, C.; Koenig, M. The Autosomal Recessive Cerebellar Ataxias. N. Engl. J. Med. 2012, 366, 636–646. [CrossRef]
- Synofzik, M.; Németh, A.H. Recessive Ataxias. Handb. Clin. Neurol. 2018, 155, 73–89. [CrossRef]
- Rossi, M.; Anheim, M.; Durr, A.; Klein, C.; Koenig, M.; Synofzik, M.; Marras, C.; van de Warrenburg, B.P.; International Parkinson and Movement Disorder Society Task Force on Classification and Nomenclature of Genetic Movement Disorders the Genetic Nomenclature of Recessive Cerebellar Ataxias. Mov. Disord. Off. J. Mov. Disord. Soc. 2018, 33, 1056–1076. [CrossRef]
- Pierson, T.M.; Adams, D.; Bonn, F.; Martinelli, P.; Cherukuri, P.F.; Teer, J.K.; Hansen, N.F.; Cruz, P.; Mullikin for the Nisc Comparative Sequencing Program, J.C.; Blakesley, R.W.; et al. Whole-Exome Sequencing Identifies Homozygous AFG3L2 Mutations in a Spastic Ataxia-Neuropathy Syndrome Linked to Mitochondrial m-AAA Proteases. PLoS Genet. 2011, 7, e1002325. [CrossRef]
- Lise, S.; Clarkson, Y.; Perkins, E.; Kwasniewska, A.; Sadighi Akha, E.; Schnekenberg, R.P.; Suminaite, D.; Hope, J.; Baker, I.; Gregory, L.; et al. Recessive Mutations in SPTBN2 Implicate β-III Spectrin in Both Cognitive and Motor Development. PLoS Genet. 2012, 8, e1003074. [CrossRef]
- Gerber, S.; Alzayady, K.J.; Burglen, L.; Brémond-Gignac, D.; Marchesin, V.; Roche, O.; Rio, M.; Funalot, B.; Calmon, R.; Durr, A.; et al. Recessive and Dominant De Novo ITPR1 Mutations Cause Gillespie Syndrome. Am. J. Hum. Genet. 2016, 98, 971–980. [CrossRef]
- Nasca, A.; Rizza, T.; Doimo, M.; Legati, A.; Ciolfi, A.; Diodato, D.; Calderan, C.; Carrara, G.; Lamantea, E.; Aiello, C.; et al. Not Only Dominant, Not Only Optic Atrophy: Expanding the Clinical Spectrum Associated with OPA1 Mutations. Orphanet J. Rare Dis. 2017, 12, 89. [CrossRef]
- Othman, B.A.; Ong, J.E.; Dumitrescu, A.V. Biallelic Optic Atrophy 1 (OPA1) Related Disorder-Case Report and Literature Review. Genes 2022, 13, 1005. [CrossRef]
- Harding, A.E. Classification of the Hereditary Ataxias and Paraplegias. Lancet Lond. Engl. 1983, 1, 1151–1155. [CrossRef]
- Schulz, J.B.; Boesch, S.; Bürk, K.; Dürr, A.; Giunti, P.; Mariotti, C.; Pousset, F.; Schöls, L.; Vankan, P.; Pandolfo, M. Diagnosis and Treatment of Friedreich Ataxia: A European Perspective. Nat. Rev. Neurol. 2009, 5, 222–234. [CrossRef]
- Ruano, L.; Melo, C.; Silva, M.C.; Coutinho, P. The Global Epidemiology of Hereditary Ataxia and Spastic Paraplegia: A Systematic Review of Prevalence Studies. Neuroepidemiology 2014, 42, 174–183. [CrossRef]
- Vankan, P. Prevalence Gradients of Friedreich’s Ataxia and R1b Haplotype in Europe Co-Localize, Suggesting a Common Palaeolithic Origin in the Franco-Cantabrian Ice Age Refuge. J. Neurochem. 2013, 126 (Suppl 1), 11–20. [CrossRef]
- Fogel, B.L. Autosomal-Recessive Cerebellar Ataxias. Handb. Clin. Neurol. 2018, 147, 187–209. [CrossRef]
- Harding, A.E. Friedreich’s Ataxia: A Clinical and Genetic Study of 90 Families with an Analysis of Early Diagnostic Criteria and Intrafamilial Clustering of Clinical Features. Brain J. Neurol. 1981, 104, 589–620. [CrossRef]
- Pandolfo, M. Friedreich Ataxia: The Clinical Picture. J. Neurol. 2009, 256 (Suppl 1), 3–8. [CrossRef]
- Collins, A. Clinical Neurogenetics: Friedreich Ataxia. Neurol. Clin. 2013, 31, 1095–1120. [CrossRef]
- Koeppen, A.H.; Ramirez, R.L.; Becker, A.B.; Mazurkiewicz, J.E. Dorsal Root Ganglia in Friedreich Ataxia: Satellite Cell Proliferation and Inflammation. Acta Neuropathol. Commun. 2016, 4, 46. [CrossRef]
- Kemp, K.C.; Cook, A.J.; Redondo, J.; Kurian, K.M.; Scolding, N.J.; Wilkins, A. Purkinje Cell Injury, Structural Plasticity and Fusion in Patients with Friedreich’s Ataxia. Acta Neuropathol. Commun. 2016, 4, 53. [CrossRef]
- Selvadurai, L.P.; Harding, I.H.; Corben, L.A.; Georgiou-Karistianis, N. Cerebral Abnormalities in Friedreich Ataxia: A Review. Neurosci. Biobehav. Rev. 2018, 84, 394–406. [CrossRef]
- Campuzano, V.; Montermini, L.; Moltò, M.D.; Pianese, L.; Cossée, M.; Cavalcanti, F.; Monros, E.; Rodius, F.; Duclos, F.; Monticelli, A.; et al. Friedreich’s Ataxia: Autosomal Recessive Disease Caused by an Intronic GAA Triplet Repeat Expansion. Science 1996, 271, 1423–1427. [CrossRef]
- Groh, M.; Lufino, M.M.P.; Wade-Martins, R.; Gromak, N. R-Loops Associated with Triplet Repeat Expansions Promote Gene Silencing in Friedreich Ataxia and Fragile X Syndrome. PLoS Genet. 2014, 10, e1004318. [CrossRef]
- Colin, F.; Martelli, A.; Clémancey, M.; Latour, J.-M.; Gambarelli, S.; Zeppieri, L.; Birck, C.; Page, A.; Puccio, H.; Ollagnier de Choudens, S. Mammalian Frataxin Controls Sulfur Production and Iron Entry during de Novo Fe4S4 Cluster Assembly. J. Am. Chem. Soc. 2013, 135, 733–740. [CrossRef]
- Martelli, A.; Puccio, H. Dysregulation of Cellular Iron Metabolism in Friedreich Ataxia: From Primary Iron-Sulfur Cluster Deficit to Mitochondrial Iron Accumulation. Front. Pharmacol. 2014, 5, 130. [CrossRef]
- Synofzik, M.; Puccio, H.; Mochel, F.; Schöls, L. Autosomal Recessive Cerebellar Ataxias: Paving the Way toward Targeted Molecular Therapies. Neuron 2019, 101, 560–583. [CrossRef]
- Cortese, A.; Tozza, S.; Yau, W.Y.; Rossi, S.; Beecroft, S.J.; Jaunmuktane, Z.; Dyer, Z.; Ravenscroft, G.; Lamont, P.J.; Mossman, S.; et al. Cerebellar Ataxia, Neuropathy, Vestibular Areflexia Syndrome Due to RFC1 Repeat Expansion. Brain J. Neurol. 2020, 143, 480–490. [CrossRef]
- Szmulewicz, D.J.; Waterston, J.A.; MacDougall, H.G.; Mossman, S.; Chancellor, A.M.; McLean, C.A.; Merchant, S.; Patrikios, P.; Halmagyi, G.M.; Storey, E. Cerebellar Ataxia, Neuropathy, Vestibular Areflexia Syndrome (CANVAS): A Review of the Clinical Features and Video-Oculographic Diagnosis. Ann. N. Y. Acad. Sci. 2011, 1233, 139–147. [CrossRef]
- Cortese, A.; Simone, R.; Sullivan, R.; Vandrovcova, J.; Tariq, H.; Yau, W.Y.; Humphrey, J.; Jaunmuktane, Z.; Sivakumar, P.; Polke, J.; et al. Biallelic Expansion of an Intronic Repeat in RFC1 Is a Common Cause of Late-Onset Ataxia. Nat. Genet. 2019, 51, 649–658. [CrossRef]
- Dominik, N.; Magri, S.; Currò, R.; Abati, E.; Facchini, S.; Corbetta, M.; MacPherson, H.; Di Bella, D.; Sarto, E.; Stevanovski, I.; et al. Normal and Pathogenic Variation of RFC1 Repeat Expansions: Implications for Clinical Diagnosis. Brain J. Neurol. 2023, awad240. [CrossRef]
- Ronco, R.; Perini, C.; Currò, R.; Dominik, N.; Facchini, S.; Gennari, A.; Simone, R.; Stuart, S.; Nagy, S.; Vegezzi, E.; et al. Truncating Variants in RFC1 in Cerebellar Ataxia, Neuropathy, and Vestibular Areflexia Syndrome. Neurology 2023, 100, e543–e554. [CrossRef]
- Abdi, M.H.; Zamiri, B.; Pazuki, G.; Sardari, S.; Pearson, C.E. Pathogenic CANVAS-Causing but Not Nonpathogenic RFC1 DNA/RNA Repeat Motifs Form Quadruplex or Triplex Structures. J. Biol. Chem. 2023, 299, 105202. [CrossRef]
- Bouchard, J.P.; Barbeau, A.; Bouchard, R.; Bouchard, R.W. Electromyography and Nerve Conduction Studies in Friedreich’s Ataxia and Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay (ARSACS). Can. J. Neurol. Sci. J. Can. Sci. Neurol. 1979, 6, 185–189. [CrossRef]
- Bouhlal, Y.; Amouri, R.; El Euch-Fayeche, G.; Hentati, F. Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay: An Overview. Parkinsonism Relat. Disord. 2011, 17, 418–422. [CrossRef]
- Engert, J.C.; Bérubé, P.; Mercier, J.; Doré, C.; Lepage, P.; Ge, B.; Bouchard, J.P.; Mathieu, J.; Melançon, S.B.; Schalling, M.; et al. ARSACS, a Spastic Ataxia Common in Northeastern Québec, Is Caused by Mutations in a New Gene Encoding an 11.5-Kb ORF. Nat. Genet. 2000, 24, 120–125. [CrossRef]
- Synofzik, M.; Soehn, A.S.; Gburek-Augustat, J.; Schicks, J.; Karle, K.N.; Schüle, R.; Haack, T.B.; Schöning, M.; Biskup, S.; Rudnik-Schöneborn, S.; et al. Autosomal Recessive Spastic Ataxia of Charlevoix Saguenay (ARSACS): Expanding the Genetic, Clinical and Imaging Spectrum. Orphanet J. Rare Dis. 2013, 8, 41. [CrossRef]
- Longo, F.; De Ritis, D.; Miluzio, A.; Fraticelli, D.; Baets, J.; Scarlato, M.; Santorelli, F.M.; Biffo, S.; Maltecca, F. Assessment of Sacsin Turnover in Patients With ARSACS: Implications for Molecular Diagnosis and Pathogenesis. Neurology 2021, 97, e2315–e2327. [CrossRef]
- Xiromerisiou, G.; Dadouli, K.; Marogianni, C.; Provatas, A.; Ntellas, P.; Rikos, D.; Stathis, P.; Georgouli, D.; Loules, G.; Zamanakou, M.; et al. A Novel Homozygous SACS Mutation Identified by Whole Exome Sequencing-Genotype Phenotype Correlations of All Published Cases. J. Mol. Neurosci. MN 2020, 70, 131–141. [CrossRef]
- Parkinson, M.H.; Bartmann, A.P.; Clayton, L.M.S.; Nethisinghe, S.; Pfundt, R.; Chapple, J.P.; Reilly, M.M.; Manji, H.; Wood, N.J.; Bremner, F.; et al. Optical Coherence Tomography in Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay. Brain J. Neurol. 2018, 141, 989–999. [CrossRef]
- Ricca, I.; Morani, F.; Bacci, G.M.; Nesti, C.; Caputo, R.; Tessa, A.; Santorelli, F.M. Clinical and Molecular Studies in Two New Cases of ARSACS. Neurogenetics 2019, 20, 45–49. [CrossRef]
- Parfitt, D.A.; Michael, G.J.; Vermeulen, E.G.M.; Prodromou, N.V.; Webb, T.R.; Gallo, J.-M.; Cheetham, M.E.; Nicoll, W.S.; Blatch, G.L.; Chapple, J.P. The Ataxia Protein Sacsin Is a Functional Co-Chaperone That Protects against Polyglutamine-Expanded Ataxin-1. Hum. Mol. Genet. 2009, 18, 1556–1565. [CrossRef]
- Anderson, J.F.; Siller, E.; Barral, J.M. The Sacsin Repeating Region (SRR): A Novel Hsp90-Related Supra-Domain Associated with Neurodegeneration. J. Mol. Biol. 2010, 400, 665–674. [CrossRef]
- Duncan, E.J.; Larivière, R.; Bradshaw, T.Y.; Longo, F.; Sgarioto, N.; Hayes, M.J.; Romano, L.E.L.; Nethisinghe, S.; Giunti, P.; Bruntraeger, M.B.; et al. Altered Organization of the Intermediate Filament Cytoskeleton and Relocalization of Proteostasis Modulators in Cells Lacking the Ataxia Protein Sacsin. Hum. Mol. Genet. 2017, 26, 3130–3143. [CrossRef]
- Gentil, B.J.; Lai, G.-T.; Menade, M.; Larivière, R.; Minotti, S.; Gehring, K.; Chapple, J.-P.; Brais, B.; Durham, H.D. Sacsin, Mutated in the Ataxia ARSACS, Regulates Intermediate Filament Assembly and Dynamics. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 2982–2994. [CrossRef]
- Del Bondio, A.; Longo, F.; De Ritis, D.; Spirito, E.; Podini, P.; Brais, B.; Bachi, A.; Quattrini, A.; Maltecca, F. Restoring Calcium Homeostasis in Purkinje Cells Arrests Neurodegeneration and Neuroinflammation in the ARSACS Mouse Model. JCI Insight 2023, 8, e163576. [CrossRef]
- Bradshaw, T.Y.; Romano, L.E.L.; Duncan, E.J.; Nethisinghe, S.; Abeti, R.; Michael, G.J.; Giunti, P.; Vermeer, S.; Chapple, J.P. A Reduction in Drp1-Mediated Fission Compromises Mitochondrial Health in Autosomal Recessive Spastic Ataxia of Charlevoix Saguenay. Hum. Mol. Genet. 2016, 25, 3232–3244. [CrossRef]
- Chun, H.H.; Gatti, R.A. Ataxia-Telangiectasia, an Evolving Phenotype. DNA Repair 2004, 3, 1187–1196. [CrossRef]
- Perlman, S.L.; Boder Deceased, E.; Sedgewick, R.P.; Gatti, R.A. Ataxia-Telangiectasia. Handb. Clin. Neurol. 2012, 103, 307–332. [CrossRef]
- Shiloh, Y. ATM and Related Protein Kinases: Safeguarding Genome Integrity. Nat. Rev. Cancer 2003, 3, 155–168. [CrossRef]
- Fernandez-Capetillo, O.; Lee, A.; Nussenzweig, M.; Nussenzweig, A. H2AX: The Histone Guardian of the Genome. DNA Repair 2004, 3, 959–967. [CrossRef]
- Chang, J.R.; Ghafouri, M.; Mukerjee, R.; Bagashev, A.; Chabrashvili, T.; Sawaya, B.E. Role of P53 in Neurodegenerative Diseases. Neurodegener. Dis. 2012, 9, 68–80. [CrossRef]
- Lagier-Tourenne, C.; Tazir, M.; López, L.C.; Quinzii, C.M.; Assoum, M.; Drouot, N.; Busso, C.; Makri, S.; Ali-Pacha, L.; Benhassine, T.; et al. ADCK3, an Ancestral Kinase, Is Mutated in a Form of Recessive Ataxia Associated with Coenzyme Q10 Deficiency. Am. J. Hum. Genet. 2008, 82, 661–672. [CrossRef]
- Mollet, J.; Delahodde, A.; Serre, V.; Chretien, D.; Schlemmer, D.; Lombes, A.; Boddaert, N.; Desguerre, I.; de Lonlay, P.; de Baulny, H.O.; et al. CABC1 Gene Mutations Cause Ubiquinone Deficiency with Cerebellar Ataxia and Seizures. Am. J. Hum. Genet. 2008, 82, 623–630. [CrossRef]
- Eto, M.; Watanabe, K.; Ishii, K. Apolipoprotein E Alleles and Hyperlipoproteinemia in Japan. Clin. Genet. 1988, 34, 246–251. [CrossRef]
- Traschütz, A.; Schirinzi, T.; Laugwitz, L.; Murray, N.H.; Bingman, C.A.; Reich, S.; Kern, J.; Heinzmann, A.; Vasco, G.; Bertini, E.; et al. Clinico-Genetic, Imaging and Molecular Delineation of COQ8A-Ataxia: A Multicenter Study of 59 Patients. Ann. Neurol. 2020, 88, 251–263. [CrossRef]
- Floyd, B.J.; Wilkerson, E.M.; Veling, M.T.; Minogue, C.E.; Xia, C.; Beebe, E.T.; Wrobel, R.L.; Cho, H.; Kremer, L.S.; Alston, C.L.; et al. Mitochondrial Protein Interaction Mapping Identifies Regulators of Respiratory Chain Function. Mol. Cell 2016, 63, 621–632. [CrossRef]
- Stefely, J.A.; Reidenbach, A.G.; Ulbrich, A.; Oruganty, K.; Floyd, B.J.; Jochem, A.; Saunders, J.M.; Johnson, I.E.; Minogue, C.E.; Wrobel, R.L.; et al. Mitochondrial ADCK3 Employs an Atypical Protein Kinase-like Fold to Enable Coenzyme Q Biosynthesis. Mol. Cell 2015, 57, 83–94. [CrossRef]
- Stefely, J.A.; Licitra, F.; Laredj, L.; Reidenbach, A.G.; Kemmerer, Z.A.; Grangeray, A.; Jaeg-Ehret, T.; Minogue, C.E.; Ulbrich, A.; Hutchins, P.D.; et al. Cerebellar Ataxia and Coenzyme Q Deficiency through Loss of Unorthodox Kinase Activity. Mol. Cell 2016, 63, 608–620. [CrossRef]
- Cullen, J.K.; Abdul Murad, N.; Yeo, A.; McKenzie, M.; Ward, M.; Chong, K.L.; Schieber, N.L.; Parton, R.G.; Lim, Y.C.; Wolvetang, E.; et al. AarF Domain Containing Kinase 3 (ADCK3) Mutant Cells Display Signs of Oxidative Stress, Defects in Mitochondrial Homeostasis and Lysosomal Accumulation. PloS ONE 2016, 11, e0148213. [CrossRef]
- Hassan, A. Episodic Ataxias: Primary and Secondary Etiologies, Treatment, and Classification Approaches. Tremor Hyperkinetic Mov. N. Y. N 2023, 13, 9. [CrossRef]
- Jen, J.C.; Wan, J. Episodic Ataxias. Handb. Clin. Neurol. 2018, 148, 521–529. [CrossRef]
- Martínez-Monseny, A.F.; Edo, A.; Casas-Alba, D.; Izquierdo-Serra, M.; Bolasell, M.; Conejo, D.; Martorell, L.; Muchart, J.; Carrera, L.; Ortez, C.I.; et al. CACNA1A Mutations Causing Early Onset Ataxia: Profiling Clinical, Dysmorphic and Structural-Functional Findings. Int. J. Mol. Sci. 2021, 22, 5180. [CrossRef]
- Brussino, A.; Gellera, C.; Saluto, A.; Mariotti, C.; Arduino, C.; Castellotti, B.; Camerlingo, M.; de Angelis, V.; Orsi, L.; Tosca, P.; et al. FMR1 Gene Premutation Is a Frequent Genetic Cause of Late-Onset Sporadic Cerebellar Ataxia. Neurology 2005, 64, 145–147. [CrossRef]
- Cabal-Herrera, A.M.; Tassanakijpanich, N.; Salcedo-Arellano, M.J.; Hagerman, R.J. Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS): Pathophysiology and Clinical Implications. Int. J. Mol. Sci. 2020, 21, 4391. [CrossRef]
- Tassone, F.; Adams, J.; Berry-Kravis, E.M.; Cohen, S.S.; Brusco, A.; Leehey, M.A.; Li, L.; Hagerman, R.J.; Hagerman, P.J. CGG Repeat Length Correlates with Age of Onset of Motor Signs of the Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS). Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2007, 144B, 566–569. [CrossRef]
- Jacquemont, S.; Hagerman, R.J.; Leehey, M.; Grigsby, J.; Zhang, L.; Brunberg, J.A.; Greco, C.; Des Portes, V.; Jardini, T.; Levine, R.; et al. Fragile X Premutation Tremor/Ataxia Syndrome: Molecular, Clinical, and Neuroimaging Correlates. Am. J. Hum. Genet. 2003, 72, 869–878. [CrossRef]
- Cronister, A.; Schreiner, R.; Wittenberger, M.; Amiri, K.; Harris, K.; Hagerman, R.J. Heterozygous Fragile X Female: Historical, Physical, Cognitive, and Cytogenetic Features. Am. J. Med. Genet. 1991, 38, 269–274. [CrossRef]
- Tassone, F.; Hagerman, R.J.; Taylor, A.K.; Gane, L.W.; Godfrey, T.E.; Hagerman, P.J. Elevated Levels of FMR1 MRNA in Carrier Males: A New Mechanism of Involvement in the Fragile-X Syndrome. Am. J. Hum. Genet. 2000, 66, 6–15. [CrossRef]
- Bertini, E.; Zanni, G.; Boltshauser, E. Nonprogressive Congenital Ataxias. Handb. Clin. Neurol. 2018, 155, 91–103. [CrossRef]
- Steinlin, M. Non-Progressive Congenital Ataxias. Brain Dev. 1998, 20, 199–208. [CrossRef]
- Romani, M.; Micalizzi, A.; Valente, E.M. Joubert Syndrome: Congenital Cerebellar Ataxia with the Molar Tooth. Lancet Neurol. 2013, 12, 894–905. [CrossRef]
- Brooker, S.M.; Edamakanti, C.R.; Akasha, S.M.; Kuo, S.; Opal, P. Spinocerebellar Ataxia Clinical Trials: Opportunities and Challenges. Ann. Clin. Transl. Neurol. 2021, 8, 1543–1556. [CrossRef]
- Duan, W.; Urani, E.; Mattson, M.P. The Potential of Gene Editing for Huntington’s Disease. Trends Neurosci. 2023, 46, 365–376. [CrossRef]
- Gaj, T.; Ojala, D.S.; Ekman, F.K.; Byrne, L.C.; Limsirichai, P.; Schaffer, D.V. In Vivo Genome Editing Improves Motor Function and Extends Survival in a Mouse Model of ALS. Sci. Adv. 2017, 3, eaar3952. [CrossRef]
- Yang, S.; Chang, R.; Yang, H.; Zhao, T.; Hong, Y.; Kong, H.E.; Sun, X.; Qin, Z.; Jin, P.; Li, S.; et al. CRISPR/Cas9-Mediated Gene Editing Ameliorates Neurotoxicity in Mouse Model of Huntington’s Disease. J. Clin. Invest. 2017, 127, 2719–2724. [CrossRef]
- Duan, Y.; Ye, T.; Qu, Z.; Chen, Y.; Miranda, A.; Zhou, X.; Lok, K.-C.; Chen, Y.; Fu, A.K.Y.; Gradinaru, V.; et al. Brain-Wide Cas9-Mediated Cleavage of a Gene Causing Familial Alzheimer’s Disease Alleviates Amyloid-Related Pathologies in Mice. Nat. Biomed. Eng. 2022, 6, 168–180. [CrossRef]
- Yoon, H.H.; Ye, S.; Lim, S.; Jo, A.; Lee, H.; Hong, F.; Lee, S.E.; Oh, S.-J.; Kim, N.-R.; Kim, K.; et al. CRISPR-Cas9 Gene Editing Protects from the A53T-SNCA Overexpression-Induced Pathology of Parkinson’s Disease In Vivo. CRISPR J. 2022, 5, 95–108. [CrossRef]
- Lee, B.; Lee, K.; Panda, S.; Gonzales-Rojas, R.; Chong, A.; Bugay, V.; Park, H.M.; Brenner, R.; Murthy, N.; Lee, H.Y. Nanoparticle Delivery of CRISPR into the Brain Rescues a Mouse Model of Fragile X Syndrome from Exaggerated Repetitive Behaviours. Nat. Biomed. Eng. 2018, 2, 497–507. [CrossRef]
- Ouyang, S.; Xie, Y.; Xiong, Z.; Yang, Y.; Xian, Y.; Ou, Z.; Song, B.; Chen, Y.; Xie, Y.; Li, H.; et al. CRISPR/Cas9-Targeted Deletion of Polyglutamine in Spinocerebellar Ataxia Type 3-Derived Induced Pluripotent Stem Cells. Stem Cells Dev. 2018, 27, 756–770. [CrossRef]
- Mazzara, P.G.; Muggeo, S.; Luoni, M.; Massimino, L.; Zaghi, M.; Valverde, P.T.-T.; Brusco, S.; Marzi, M.J.; Palma, C.; Colasante, G.; et al. Frataxin Gene Editing Rescues Friedreich’s Ataxia Pathology in Dorsal Root Ganglia Organoid-Derived Sensory Neurons. Nat. Commun. 2020, 11, 4178. [CrossRef]
- Li, Y.; Li, J.; Wang, J.; Zhang, S.; Giles, K.; Prakash, T.P.; Rigo, F.; Napierala, J.S.; Napierala, M. Premature Transcription Termination at the Expanded GAA Repeats and Aberrant Alternative Polyadenylation Contributes to the Frataxin Transcriptional Deficit in Friedreich’s Ataxia. Hum. Mol. Genet. 2022, 31, 3539–3557. [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-Replace Genome Editing without Double-Strand Breaks or Donor DNA. Nature 2019, 576, 149–157. [CrossRef]
- Simpson, B.P.; Yrigollen, C.M.; Izda, A.; Davidson, B.L. Targeted Long-Read Sequencing Captures CRISPR Editing and AAV Integration Outcomes in Brain. Mol. Ther. J. Am. Soc. Gene Ther. 2023, 31, 760–773. [CrossRef]
- Li, Y.; Polak, U.; Bhalla, A.D.; Rozwadowska, N.; Butler, J.S.; Lynch, D.R.; Dent, S.Y.R.; Napierala, M. Excision of Expanded GAA Repeats Alleviates the Molecular Phenotype of Friedreich’s Ataxia. Mol. Ther. J. Am. Soc. Gene Ther. 2015, 23, 1055–1065. [CrossRef]
- Xia, H.; Mao, Q.; Eliason, S.L.; Harper, S.Q.; Martins, I.H.; Orr, H.T.; Paulson, H.L.; Yang, L.; Kotin, R.M.; Davidson, B.L. RNAi Suppresses Polyglutamine-Induced Neurodegeneration in a Model of Spinocerebellar Ataxia. Nat. Med. 2004, 10, 816–820. [CrossRef]
- Keiser, M.S.; Geoghegan, J.C.; Boudreau, R.L.; Lennox, K.A.; Davidson, B.L. RNAi or Overexpression: Alternative Therapies for Spinocerebellar Ataxia Type 1. Neurobiol. Dis. 2013, 56, 6–13. [CrossRef]
- Keiser, M.S.; Boudreau, R.L.; Davidson, B.L. Broad Therapeutic Benefit after RNAi Expression Vector Delivery to Deep Cerebellar Nuclei: Implications for Spinocerebellar Ataxia Type 1 Therapy. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 588–595. [CrossRef]
- Keiser, M.S.; Kordower, J.H.; Gonzalez-Alegre, P.; Davidson, B.L. Broad Distribution of Ataxin 1 Silencing in Rhesus Cerebella for Spinocerebellar Ataxia Type 1 Therapy. Brain J. Neurol. 2015, 138, 3555–3566. [CrossRef]
- Alves, S.; Nascimento-Ferreira, I.; Auregan, G.; Hassig, R.; Dufour, N.; Brouillet, E.; Pedroso de Lima, M.C.; Hantraye, P.; Pereira de Almeida, L.; Déglon, N. Allele-Specific RNA Silencing of Mutant Ataxin-3 Mediates Neuroprotection in a Rat Model of Machado-Joseph Disease. PloS ONE 2008, 3, e3341. [CrossRef]
- Rodríguez-Lebrón, E.; Costa, M. do C.; Luna-Cancalon, K.; Peron, T.M.; Fischer, S.; Boudreau, R.L.; Davidson, B.L.; Paulson, H.L. Silencing Mutant ATXN3 Expression Resolves Molecular Phenotypes in SCA3 Transgenic Mice. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 1909–1918. [CrossRef]
- Costa, M. do C.; Luna-Cancalon, K.; Fischer, S.; Ashraf, N.S.; Ouyang, M.; Dharia, R.M.; Martin-Fishman, L.; Yang, Y.; Shakkottai, V.G.; Davidson, B.L.; et al. Toward RNAi Therapy for the Polyglutamine Disease Machado-Joseph Disease. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 1898–1908. [CrossRef]
- Bushart, D.D.; Zalon, A.J.; Zhang, H.; Morrison, L.M.; Guan, Y.; Paulson, H.L.; Shakkottai, V.G.; McLoughlin, H.S. Antisense Oligonucleotide Therapy Targeted Against ATXN3 Improves Potassium Channel-Mediated Purkinje Neuron Dysfunction in Spinocerebellar Ataxia Type 3. Cerebellum Lond. Engl. 2021, 20, 41–53. [CrossRef]
- McLoughlin, H.S.; Gundry, K.; Rainwater, O.; Schuster, K.H.; Wellik, I.G.; Zalon, A.J.; Benneyworth, M.A.; Eberly, L.E.; Öz, G. Antisense Oligonucleotide Silencing Reverses Abnormal Neurochemistry in Spinocerebellar Ataxia 3 Mice. Ann. Neurol. 2023. [CrossRef]
- Miyazaki, Y.; Du, X.; Muramatsu, S.-I.; Gomez, C.M. An MiRNA-Mediated Therapy for SCA6 Blocks IRES-Driven Translation of the CACNA1A Second Cistron. Sci. Transl. Med. 2016, 8, 347ra94. [CrossRef]
- Pastor, P.D.H.; Du, X.; Fazal, S.; Davies, A.N.; Gomez, C.M. Targeting the CACNA1A IRES as a Treatment for Spinocerebellar Ataxia Type 6. Cerebellum Lond. Engl. 2018, 17, 72–77. [CrossRef]
- Ramachandran, P.S.; Bhattarai, S.; Singh, P.; Boudreau, R.L.; Thompson, S.; Laspada, A.R.; Drack, A.V.; Davidson, B.L. RNA Interference-Based Therapy for Spinocerebellar Ataxia Type 7 Retinal Degeneration. PloS One 2014, 9, e95362. [CrossRef]
- Ramachandran, P.S.; Boudreau, R.L.; Schaefer, K.A.; La Spada, A.R.; Davidson, B.L. Nonallele Specific Silencing of Ataxin-7 Improves Disease Phenotypes in a Mouse Model of SCA7. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 1635–1642. [CrossRef]
- Keiser, M.S.; Monteys, A.M.; Corbau, R.; Gonzalez-Alegre, P.; Davidson, B.L. RNAi Prevents and Reverses Phenotypes Induced by Mutant Human Ataxin-1. Ann. Neurol. 2016, 80, 754–765. [CrossRef]
- Rinaldi, C.; Wood, M.J.A. Antisense Oligonucleotides: The next Frontier for Treatment of Neurological Disorders. Nat. Rev. Neurol. 2018, 14, 9–21. [CrossRef]
- Smith, R.A.; Miller, T.M.; Yamanaka, K.; Monia, B.P.; Condon, T.P.; Hung, G.; Lobsiger, C.S.; Ward, C.M.; McAlonis-Downes, M.; Wei, H.; et al. Antisense Oligonucleotide Therapy for Neurodegenerative Disease. J. Clin. Invest. 2006, 116, 2290–2296. [CrossRef]
- Miller, T.M.; Pestronk, A.; David, W.; Rothstein, J.; Simpson, E.; Appel, S.H.; Andres, P.L.; Mahoney, K.; Allred, P.; Alexander, K.; et al. An Antisense Oligonucleotide against SOD1 Delivered Intrathecally for Patients with SOD1 Familial Amyotrophic Lateral Sclerosis: A Phase 1, Randomised, First-in-Man Study. Lancet Neurol. 2013, 12, 435–442. [CrossRef]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [CrossRef]
- van Roon-Mom, W.M.C.; Roos, R.A.C.; de Bot, S.T. Dose-Dependent Lowering of Mutant Huntingtin Using Antisense Oligonucleotides in Huntington Disease Patients. Nucleic Acid Ther. 2018, 28, 59–62. [CrossRef]
- Rook, M.E.; Southwell, A.L. Antisense Oligonucleotide Therapy: From Design to the Huntington Disease Clinic. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2022, 36, 105–119. [CrossRef]
- Scoles, D.R.; Meera, P.; Schneider, M.D.; Paul, S.; Dansithong, W.; Figueroa, K.P.; Hung, G.; Rigo, F.; Bennett, C.F.; Otis, T.S.; et al. Antisense Oligonucleotide Therapy for Spinocerebellar Ataxia Type 2. Nature 2017, 544, 362–366. [CrossRef]
- Shen, X.; Wong, J.; Prakash, T.P.; Rigo, F.; Li, Y.; Napierala, M.; Corey, D.R. Progress towards Drug Discovery for Friedreich’s Ataxia: Identifying Synthetic Oligonucleotides That More Potently Activate Expression of Human Frataxin Protein. Bioorg. Med. Chem. 2020, 28, 115472. [CrossRef]
- Li, L.; Shen, X.; Liu, Z.; Norrbom, M.; Prakash, T.P.; O’Reilly, D.; Sharma, V.K.; Damha, M.J.; Watts, J.K.; Rigo, F.; et al. Activation of Frataxin Protein Expression by Antisense Oligonucleotides Targeting the Mutant Expanded Repeat. Nucleic Acid Ther. 2018, 28, 23–33. [CrossRef]
- Park, J.; Al-Ramahi, I.; Tan, Q.; Mollema, N.; Diaz-Garcia, J.R.; Gallego-Flores, T.; Lu, H.-C.; Lagalwar, S.; Duvick, L.; Kang, H.; et al. RAS-MAPK-MSK1 Pathway Modulates Ataxin 1 Protein Levels and Toxicity in SCA1. Nature 2013, 498, 325–331. [CrossRef]
- Perdomini, M.; Belbellaa, B.; Monassier, L.; Reutenauer, L.; Messaddeq, N.; Cartier, N.; Crystal, R.G.; Aubourg, P.; Puccio, H. Prevention and Reversal of Severe Mitochondrial Cardiomyopathy by Gene Therapy in a Mouse Model of Friedreich’s Ataxia. Nat. Med. 2014, 20, 542–547. [CrossRef]
- Belbellaa, B.; Reutenauer, L.; Monassier, L.; Puccio, H. Correction of Half the Cardiomyocytes Fully Rescue Friedreich Ataxia Mitochondrial Cardiomyopathy through Cell-Autonomous Mechanisms. Hum. Mol. Genet. 2019, 28, 1274–1285. [CrossRef]
- Munoz-Zuluaga, C.; Gertz, M.; Yost-Bido, M.; Greco, A.; Gorman, N.; Chen, A.; Kooner, V.; Rosenberg, J.B.; De, B.P.; Kaminsky, S.M.; et al. Identification of Safe and Effective Intravenous Dose of AAVrh.10hFXN to Treat the Cardiac Manifestations of Friedreich’s Ataxia. Hum. Gene Ther. 2023, 34, 605–615. [CrossRef]
- Piguet, F.; de Montigny, C.; Vaucamps, N.; Reutenauer, L.; Eisenmann, A.; Puccio, H. Rapid and Complete Reversal of Sensory Ataxia by Gene Therapy in a Novel Model of Friedreich Ataxia. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 1940–1952. [CrossRef]
- Britti, E.; Delaspre, F.; Feldman, A.; Osborne, M.; Greif, H.; Tamarit, J.; Ros, J. Frataxin-Deficient Neurons and Mice Models of Friedreich Ataxia Are Improved by TAT-MTScs-FXN Treatment. J. Cell. Mol. Med. 2018, 22, 834–848. [CrossRef]
- Erwin, G.S.; Grieshop, M.P.; Ali, A.; Qi, J.; Lawlor, M.; Kumar, D.; Ahmad, I.; McNally, A.; Teider, N.; Worringer, K.; et al. Synthetic Transcription Elongation Factors License Transcription across Repressive Chromatin. Science 2017, 358, 1617–1622. [CrossRef]
- Bondarev, A.D.; Attwood, M.M.; Jonsson, J.; Chubarev, V.N.; Tarasov, V.V.; Schiöth, H.B. Recent Developments of HDAC Inhibitors: Emerging Indications and Novel Molecules. Br. J. Clin. Pharmacol. 2021, 87, 4577–4597. [CrossRef]
- Soragni, E.; Gottesfeld, J.M. Translating HDAC Inhibitors in Friedreich’s Ataxia. Expert Opin. Orphan Drugs 2016, 4, 961–970. [CrossRef]
- Chan, P.K.; Torres, R.; Yandim, C.; Law, P.P.; Khadayate, S.; Mauri, M.; Grosan, C.; Chapman-Rothe, N.; Giunti, P.; Pook, M.; et al. Heterochromatinization Induced by GAA-Repeat Hyperexpansion in Friedreich’s Ataxia Can Be Reduced upon HDAC Inhibition by Vitamin B3. Hum. Mol. Genet. 2013, 22, 2662–2675. [CrossRef]
- Lei, L.-F.; Yang, G.-P.; Wang, J.-L.; Chuang, D.-M.; Song, W.-H.; Tang, B.-S.; Jiang, H. Safety and Efficacy of Valproic Acid Treatment in SCA3/MJD Patients. Parkinsonism Relat. Disord. 2016, 26, 55–61. [CrossRef]
- Soragni, E.; Miao, W.; Iudicello, M.; Jacoby, D.; De Mercanti, S.; Clerico, M.; Longo, F.; Piga, A.; Ku, S.; Campau, E.; et al. Epigenetic Therapy for Friedreich Ataxia. Ann. Neurol. 2014, 76, 489–508. [CrossRef]
- Ding, Y.; Adachi, H.; Katsuno, M.; Sahashi, K.; Kondo, N.; Iida, M.; Tohnai, G.; Nakatsuji, H.; Sobue, G. BIIB021, a Synthetic Hsp90 Inhibitor, Induces Mutant Ataxin-1 Degradation through the Activation of Heat Shock Factor 1. Neuroscience 2016, 327, 20–31. [CrossRef]
- Williams, A.J.; Knutson, T.M.; Colomer Gould, V.F.; Paulson, H.L. In Vivo Suppression of Polyglutamine Neurotoxicity by C-Terminus of Hsp70-Interacting Protein (CHIP) Supports an Aggregation Model of Pathogenesis. Neurobiol. Dis. 2009, 33, 342–353. [CrossRef]
- Tsou, W.-L.; Hosking, R.R.; Burr, A.A.; Sutton, J.R.; Ouyang, M.; Du, X.; Gomez, C.M.; Todi, S.V. DnaJ-1 and Karyopherin A3 Suppress Degeneration in a New Drosophila Model of Spinocerebellar Ataxia Type 6. Hum. Mol. Genet. 2015, 24, 4385–4396. [CrossRef]
- Alves, S.; Cormier-Dequaire, F.; Marinello, M.; Marais, T.; Muriel, M.-P.; Beaumatin, F.; Charbonnier-Beaupel, F.; Tahiri, K.; Seilhean, D.; El Hachimi, K.; et al. The Autophagy/Lysosome Pathway Is Impaired in SCA7 Patients and SCA7 Knock-in Mice. Acta Neuropathol. 2014, 128, 705–722. [CrossRef]
- Parkinson, M.H.; Schulz, J.B.; Giunti, P. Co-Enzyme Q10 and Idebenone Use in Friedreich’s Ataxia. J. Neurochem. 2013, 126 (Suppl 1), 125–141. [CrossRef]
- Wu, Y.-L.; Chang, J.-C.; Sun, H.-L.; Cheng, W.-L.; Yen, Y.-P.; Lin, Y.-S.; Chao, Y.-C.; Liu, K.-H.; Huang, C.-S.; Liu, K.-L.; et al. Coenzyme Q10 Supplementation Increases Removal of the ATXN3 Polyglutamine Repeat, Reducing Cerebellar Degeneration and Improving Motor Dysfunction in Murine Spinocerebellar Ataxia Type 3. Nutrients 2022, 14, 3593. [CrossRef]
- Schirinzi, T.; Favetta, M.; Romano, A.; Sancesario, A.; Summa, S.; Minosse, S.; Zanni, G.; Castelli, E.; Bertini, E.; Petrarca, M.; et al. One-Year Outcome of Coenzyme Q10 Supplementation in ADCK3 Ataxia (ARCA2). Cerebellum Ataxias 2019, 6, 15. [CrossRef]
- Jauslin, M.L.; Meier, T.; Smith, R.A.J.; Murphy, M.P. Mitochondria-Targeted Antioxidants Protect Friedreich Ataxia Fibroblasts from Endogenous Oxidative Stress More Effectively than Untargeted Antioxidants. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 1972–1974. [CrossRef]
- Márquez, B.T.; Leung, T.C.S.; Hui, J.; Charron, F.; McKinney, R.A.; Watt, A.J. A Mitochondrial-Targeted Antioxidant (MitoQ) Improves Motor Coordination and Reduces Purkinje Cell Death in a Mouse Model of ARSACS. Neurobiol. Dis. 2023, 183, 106157. [CrossRef]
- Yang, B.; Dan, X.; Hou, Y.; Lee, J.-H.; Wechter, N.; Krishnamurthy, S.; Kimura, R.; Babbar, M.; Demarest, T.; McDevitt, R.; et al. NAD+ Supplementation Prevents STING-Induced Senescence in Ataxia Telangiectasia by Improving Mitophagy. Aging Cell 2021, 20, e13329. [CrossRef]
- Hourez, R.; Servais, L.; Orduz, D.; Gall, D.; Millard, I.; de Kerchove d’Exaerde, A.; Cheron, G.; Orr, H.T.; Pandolfo, M.; Schiffmann, S.N. Aminopyridines Correct Early Dysfunction and Delay Neurodegeneration in a Mouse Model of Spinocerebellar Ataxia Type 1. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 11795–11807. [CrossRef]
- Jayabal, S.; Chang, H.H.V.; Cullen, K.E.; Watt, A.J. 4-Aminopyridine Reverses Ataxia and Cerebellar Firing Deficiency in a Mouse Model of Spinocerebellar Ataxia Type 6. Sci. Rep. 2016, 6, 29489. [CrossRef]
- Egorova, P.A.; Zakharova, O.A.; Vlasova, O.L.; Bezprozvanny, I.B. In Vivo Analysis of Cerebellar Purkinje Cell Activity in SCA2 Transgenic Mouse Model. J. Neurophysiol. 2016, 115, 2840–2851. [CrossRef]
- Kasumu, A.W.; Hougaard, C.; Rode, F.; Jacobsen, T.A.; Sabatier, J.M.; Eriksen, B.L.; Strøbæk, D.; Liang, X.; Egorova, P.; Vorontsova, D.; et al. Selective Positive Modulator of Calcium-Activated Potassium Channels Exerts Beneficial Effects in a Mouse Model of Spinocerebellar Ataxia Type 2. Chem. Biol. 2012, 19, 1340–1353. [CrossRef]
- Maltecca, F.; Baseggio, E.; Consolato, F.; Mazza, D.; Podini, P.; Young, S.M.; Drago, I.; Bahr, B.A.; Puliti, A.; Codazzi, F.; et al. Purkinje Neuron Ca2+ Influx Reduction Rescues Ataxia in SCA28 Model. J. Clin. Invest. 2015, 125, 263–274. [CrossRef]
- Coarelli, G.; Heinzmann, A.; Ewenczyk, C.; Fischer, C.; Chupin, M.; Monin, M.-L.; Hurmic, H.; Calvas, F.; Calvas, P.; Goizet, C.; et al. Safety and Efficacy of Riluzole in Spinocerebellar Ataxia Type 2 in France (ATRIL): A Multicentre, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Neurol. 2022, 21, 225–233. [CrossRef]
- Ghanekar, S.D.; Kuo, S.-H.; Staffetti, J.S.; Zesiewicz, T.A. Current and Emerging Treatment Modalities for Spinocerebellar Ataxias. Expert Rev. Neurother. 2022, 22, 101–114. [CrossRef]
- Ristori, G.; Romano, S.; Visconti, A.; Cannoni, S.; Spadaro, M.; Frontali, M.; Pontieri, F.E.; Vanacore, N.; Salvetti, M. Riluzole in Cerebellar Ataxia: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Neurology 2010, 74, 839–845. [CrossRef]
- Perlman, S.L. Update on the Treatment of Ataxia: Medication and Emerging Therapies. Neurother. J. Am. Soc. Exp. Neurother. 2020, 17, 1660–1664. [CrossRef]
- Zesiewicz, T.A.; Greenstein, P.E.; Sullivan, K.L.; Wecker, L.; Miller, A.; Jahan, I.; Chen, R.; Perlman, S.L. A Randomized Trial of Varenicline (Chantix) for the Treatment of Spinocerebellar Ataxia Type 3. Neurology 2012, 78, 545–550. [CrossRef]
- Botez, M.I.; Botez-Marquard, T.; Elie, R.; Pedraza, O.L.; Goyette, K.; Lalonde, R. Amantadine Hydrochloride Treatment in Heredodegenerative Ataxias: A Double Blind Study. J. Neurol. Neurosurg. Psychiatry 1996, 61, 259–264. [CrossRef]
- Nissenkorn, A.; Hassin-Baer, S.; Lerman, S.F.; Levi, Y.B.; Tzadok, M.; Ben-Zeev, B. Movement Disorder in Ataxia-Telangiectasia: Treatment with Amantadine Sulfate. J. Child Neurol. 2013, 28, 155–160. [CrossRef]
- Cunha-Santos, J.; Duarte-Neves, J.; Carmona, V.; Guarente, L.; Pereira de Almeida, L.; Cavadas, C. Caloric Restriction Blocks Neuropathology and Motor Deficits in Machado-Joseph Disease Mouse Models through SIRT1 Pathway. Nat. Commun. 2016, 7, 11445. [CrossRef]
- Yiu, E.M.; Tai, G.; Peverill, R.E.; Lee, K.J.; Croft, K.D.; Mori, T.A.; Scheiber-Mojdehkar, B.; Sturm, B.; Praschberger, M.; Vogel, A.P.; et al. An Open-Label Trial in Friedreich Ataxia Suggests Clinical Benefit with High-Dose Resveratrol, without Effect on Frataxin Levels. J. Neurol. 2015, 262, 1344–1353. [CrossRef]
- Leuzzi, V.; Micheli, R.; D’Agnano, D.; Molinaro, A.; Venturi, T.; Plebani, A.; Soresina, A.; Marini, M.; Ferremi Leali, P.; Quinti, I.; et al. Positive Effect of Erythrocyte-Delivered Dexamethasone in Ataxia-Telangiectasia. Neurol. Neuroimmunol. Neuroinflammation 2015, 2, e98. [CrossRef]
- Saberi-Karimian, M.; Beyraghi-Tousi, M.; Jamialahmadi, T.; Sahebkar, A. The Positive Short-Term Effect of Dexamethasone on Ataxia Symptoms in a Patient with Ataxia-Telangiectasia: A Case Report. Clin. Case Rep. 2022, 10, e05895. [CrossRef]
- Sarkar, S.; Floto, R.A.; Berger, Z.; Imarisio, S.; Cordenier, A.; Pasco, M.; Cook, L.J.; Rubinsztein, D.C. Lithium Induces Autophagy by Inhibiting Inositol Monophosphatase. J. Cell Biol. 2005, 170, 1101–1111. [CrossRef]
- Watase, K.; Gatchel, J.R.; Sun, Y.; Emamian, E.; Atkinson, R.; Richman, R.; Mizusawa, H.; Orr, H.T.; Shaw, C.; Zoghbi, H.Y. Lithium Therapy Improves Neurological Function and Hippocampal Dendritic Arborization in a Spinocerebellar Ataxia Type 1 Mouse Model. PLoS Med. 2007, 4, e182. [CrossRef]
- Kieling, C.; Rieder, C.R.M.; Silva, A.C.F.; Saute, J. a. M.; Cecchin, C.R.; Monte, T.L.; Jardim, L.B. A Neurological Examination Score for the Assessment of Spinocerebellar Ataxia 3 (SCA3). Eur. J. Neurol. 2008, 15, 371–376. [CrossRef]
- Saccà, F.; Puorro, G.; Brunetti, A.; Capasso, G.; Cervo, A.; Cocozza, S.; de Leva, M.; Marsili, A.; Pane, C.; Quarantelli, M.; et al. A Randomized Controlled Pilot Trial of Lithium in Spinocerebellar Ataxia Type 2. J. Neurol. 2015, 262, 149–153. [CrossRef]
- Liu, J.; Wang, L. Mitochondrial Enhancement for Neurodegenerative Movement Disorders: A Systematic Review of Trials Involving Creatine, Coenzyme Q10, Idebenone and Mitoquinone. CNS Drugs 2014, 28, 63–68. [CrossRef]
- Grimaldi, G.; Argyropoulos, G.P.; Boehringer, A.; Celnik, P.; Edwards, M.J.; Ferrucci, R.; Galea, J.M.; Groiss, S.J.; Hiraoka, K.; Kassavetis, P.; et al. Non-Invasive Cerebellar Stimulation--a Consensus Paper. Cerebellum Lond. Engl. 2014, 13, 121–138. [CrossRef]
- Hartley, H.; Cassidy, E.; Bunn, L.; Kumar, R.; Pizer, B.; Lane, S.; Carter, B. Exercise and Physical Therapy Interventions for Children with Ataxia: A Systematic Review. Cerebellum Lond. Engl. 2019, 18, 951–968. [CrossRef]
- Lee, A. Omaveloxolone: First Approval. Drugs 2023, 83, 725–729. [CrossRef]
- Lynch, D.R.; Chin, M.P.; Delatycki, M.B.; Subramony, S.H.; Corti, M.; Hoyle, J.C.; Boesch, S.; Nachbauer, W.; Mariotti, C.; Mathews, K.D.; et al. Safety and Efficacy of Omaveloxolone in Friedreich Ataxia ( MOXIE Study). Ann. Neurol. 2021, 89, 212–225. [CrossRef]
- Strawser, C.; Schadt, K.; Hauser, L.; McCormick, A.; Wells, M.; Larkindale, J.; Lin, H.; Lynch, D.R. Pharmacological Therapeutics in Friedreich Ataxia: The Present State. Expert Rev. Neurother. 2017, 17, 895–907. [CrossRef]
- Ashizawa, T.; Öz, G.; Paulson, H.L. Spinocerebellar Ataxias: Prospects and Challenges for Therapy Development. Nat. Rev. Neurol. 2018, 14, 590–605. [CrossRef]
- Shakkottai, V.; Paulson, H. Expanding the Genetic Basis of Ataxia. Nat. Genet. 2019, 51, 580–581. [CrossRef]
- Wong, M.M.K.; Watson, L.M.; Becker, E.B.E. Recent Advances in Modelling of Cerebellar Ataxia Using Induced Pluripotent Stem Cells. J. Neurol. Neuromedicine 2017, 2, 11–15. [CrossRef]
- Georges, P.; Boza-Moran, M.-G.; Gide, J.; Pêche, G.A.; Forêt, B.; Bayot, A.; Rustin, P.; Peschanski, M.; Martinat, C.; Aubry, L. Induced Pluripotent Stem Cells-Derived Neurons from Patients with Friedreich Ataxia Exhibit Differential Sensitivity to Resveratrol and Nicotinamide. Sci. Rep. 2019, 9, 14568. [CrossRef]
- Hansen, S.K.; Stummann, T.C.; Borland, H.; Hasholt, L.F.; Tümer, Z.; Nielsen, J.E.; Rasmussen, M.A.; Nielsen, T.T.; Daechsel, J.C.A.; Fog, K.; et al. Induced Pluripotent Stem Cell - Derived Neurons for the Study of Spinocerebellar Ataxia Type 3. Stem Cell Res. 2016, 17, 306–317. [CrossRef]
- Buijsen, R.A.M.; Hu, M.; Sáez-González, M.; Notopoulou, S.; Mina, E.; Koning, W.; Gardiner, S.L.; van der Graaf, L.M.; Daoutsali, E.; Pepers, B.A.; et al. Spinocerebellar Ataxia Type 1 Characteristics in Patient-Derived Fibroblast and IPSC-Derived Neuronal Cultures. Mov. Disord. Off. J. Mov. Disord. Soc. 2023. [CrossRef]
- He, L.; Wang, S.; Peng, L.; Zhao, H.; Li, S.; Han, X.; Habimana, J. de D.; Chen, Z.; Wang, C.; Peng, Y.; et al. CRISPR/Cas9 Mediated Gene Correction Ameliorates Abnormal Phenotypes in Spinocerebellar Ataxia Type 3 Patient-Derived Induced Pluripotent Stem Cells. Transl. Psychiatry 2021, 11, 479. [CrossRef]
- Chen, Y.; Bury, L.; Chen, F.; Aldinger, K.A.; Miranda, H.C.; Wynshaw-Boris, A. Generation of Advanced Cerebellar Organoids for Neurogenesis and Neuronal Network Development. Hum. Mol. Genet. 2023, ddad110. [CrossRef]
- Silva, T.P.; Fernandes, T.G.; Nogueira, D.E.S.; Rodrigues, C.A.V.; Bekman, E.P.; Hashimura, Y.; Jung, S.; Lee, B.; Carmo-Fonseca, M.; Cabral, J.M.S. Scalable Generation of Mature Cerebellar Organoids from Human Pluripotent Stem Cells and Characterization by Immunostaining. J. Vis. Exp. JoVE 2020. [CrossRef]
- Watson, L.M.; Wong, M.M.K.; Vowles, J.; Cowley, S.A.; Becker, E.B.E. A Simplified Method for Generating Purkinje Cells from Human-Induced Pluripotent Stem Cells. Cerebellum Lond. Engl. 2018, 17, 419–427. [CrossRef]
- Buchholz, D.E.; Carroll, T.S.; Kocabas, A.; Zhu, X.; Behesti, H.; Faust, P.L.; Stalbow, L.; Fang, Y.; Hatten, M.E. Novel Genetic Features of Human and Mouse Purkinje Cell Differentiation Defined by Comparative Transcriptomics. Proc. Natl. Acad. Sci. USA 2020, 117, 15085–15095. [CrossRef]
- Hommersom, M.P.; Buijsen, R.A.M.; van Roon-Mom, W.M.C.; van de Warrenburg, B.P.C.; van Bokhoven, H. Human Induced Pluripotent Stem Cell-Based Modelling of Spinocerebellar Ataxias. Stem Cell Rev. Rep. 2022, 18, 441–456. [CrossRef]
- Chow, S.-C.; Huang, Z. Innovative Design and Analysis for Rare Disease Drug Development. J. Biopharm. Stat. 2020, 30, 537–549. [CrossRef]
| Strategy | Stage | Model | Outcomes | Reference | |
|---|---|---|---|---|---|
| Spinocerebellar ataxia type-1 (SCA1) | |||||
| Small RNA structures | shRNA and miRNA | Pre-clinical | SCA1 mouse models | Improvement of motor coordination, restoration of cerebellar morphology and absence of ataxin-1 inclusions | [184,185,186] |
| Small RNA structures | miRNA | Pre-clinical | SCA1 non-human primate | Reduction of endogenous ATXN1 mRNA | |
| Proteostasis | HSP inhibitors | Pre-clinical | Human SCA1 cell lines | Reduction of ataxin-1 aggregates | [219] |
| Pharmacological treatment | MitoQ | Pre-clinical | SCA1 mouse models | Restoration of mitochondrial function, attenuation of PN degeneration and improvement of motor coordination | [19] |
| Pharmacological treatment | 4-animopyridine | Pre-clinical | SCA1 mouse model | Restoration of PN firing, improvement of motor coordination, and partial protection against cell atrophy | [229] |
| Pharmacological treatment | Lithium | Pre-clinical | SCA1 mouse model | Improvement in motor coordination | [246] |
| Spinocerebellar ataxia type-2 (SCA2) | |||||
| Genome editing | CRISPR | Pre-clinical | SCA2 mouse models | / | [182] |
| Small RNA structure | ASO | Pre-clinical | SCA2 mouse models | Improvement of motor performance and restoration of physiological properties and deregulated genes and proteins | [204] |
| Pharmacological treatment | Chlorzoxazone | Pre-clinical | SCA2 mouse model | Normalization of PN firing and alleviation of ataxic phenotype | [231] |
| Pharmacological treatment | NS13001 | Pre-clinical | SCA2 mouse model | Improvement of motor ability and reduced PN degeneration | [232] |
| Spinocerebellar ataxia type-3 (SCA3) | |||||
| Genome editing | CRISPR | Pre-clinical | Human iPSCs | Restoration of ataxin-3 functionality without the formation of toxic aggregates | [178] |
| Small RNA structure | shRNA | Pre-clinical | SCA3 rat model | Reduced of ataxin-3 inclusions and prevention of neurodegeneration | [188] |
| Small RNA structure | siRNA | Pre-clinical | SCA3 mouse model | ATXN3 downregulation and prevention of its aggregation | [189,190] |
| Small RNA structure | ASO | Pre-clinical | SCA3 mouse model | Improvement of motor ability, restoration of PN dysfunction and rescue of altered neurometabolites | [191] |
| Gene activation | HDAC inhibitor, valproic acid | Clinical phase 1/2 | SCA3 patients | Patients treated with valproic acid improved locomotor function (SARA scale) | [217] |
| Pharmacological treatment | Coenzyme Q10 | Pre-clinical | SCA3 mouse model | Recovery of motor coordination, reduced PN degeneration and muscle atrophy | [224] |
| Pharmacological treatment | Varenicline | Clinical | SCA3 patients | Improvement in SARA scale score | [238] |
| Pharmacological treatment | Resveratrol | Clinical | SCA3 mouse model | Reduced motor incoordination | [241] |
| Spinocerebellar ataxia type-6 (SCA6) | |||||
| Small RNA structure | miRNA | Pre-clinical | SCA6 mouse model | Reduced ataxic phenotype and PN degeneration | [193,194] |
| Pharmacological treatment | 4-aminopyridine | Pre-clinical | SCA6 mouse model | Improvement of motor ability and restoration of PN firing | [230] |
| Spinocerebellar ataxia type-7 (SCA7) | |||||
| Small RNA structure | miRNA | Pre-clinical | SCA7 mouse model | Improvement of motor deficit, increased PN survival and ATXN7 downregulation | [195,196] |
| Spinocerebellar ataxia type-28 (SCA28) | |||||
| Pharmacological treatment | Ceftriaxone | Pre-clinical | SCA28 mouse model | Reduced PN degeneration and improvement of motor performance | [144] |
| Friedreich Ataxia (FA) | |||||
| Genome editing | CRISPR | Pre-clinical | Human iPSCs | Deletion of the expanded CAG tract is not always sufficient to revert the phenotype | [179,180] |
| Genome editing | ZFN | Pre-clinical | Human iPSC-derived neurons and cardiomyocytes | Decreased aconitase activity and ATP levels in iPSC-derived neurons and corrected the cardiomyopathy in cardiomyocytes | [183] |
| Small RNA structure | ASO | Pre-clinical | Human FA cell lines | Activation of FXN expression and consequent restoration of frataxin levels | [206] |
| Small RNA structure | Gapmer | Pre-clinical | Human FA cell lines | Activation of FXN expression | [205] |
| Gene therapy | Pre-clinical | FA mouse model | Complete and rapid recovery of cardiac functionality | [208,209,210] | |
| Gene therapy | Pre-clinical | FA mouse model | Complete and rapid rescue of sensory neuropathy and ganglionopathy | [211] | |
| Gene therapy | Clinical phase 1/2 | FA patients | Ongoing sponsored by Lexeo Therapeutics (NCT05445323) | ||
| Protein replacement | TAT peptides | Pre-clinical | FA mouse model | Decreased neurite degeneration and apoptotic markers resulting in increased cell survival. And restoration of mitochondrial features | [212] |
| Protein replacement | TAT peptides | Clinical phase 2 | FA patients | Ongoing sponsored by Larimar Therapeutics (NCT05579691) | |
| Gene activation | Syn-TEF1 | Pre-clinical | Human iPSC-derived neurons and cardiomyocytes | Activation of FXN expression | [213] |
| Gene activation | Syn-TEF1 | Clinical phase 1a | FA patients | Ongoing sponsored by Design Therapeutics (NCT05285540) | |
| Gene activation | HDAC inhibitors, 2-aminobenzamide | Pre-clinical | Human cell lines and FA mouse models | Activation of FXN expression | [215] |
| Gene activation | HDAC inhibitors, nicotinamide | Pre-clinical | FA mouse model | FXN upregulation | [216] |
| Gene activation | HDAC inhibitors, RG2833 | Clinical phase 1 | FA patients | Increased FXN levels, but a toxic metabolites was detected | [218] |
| Pharmacological treatment | Omaveloxolone | Clinical phase 2 | FA patients | Approved: sponsored by Biogen (NCT02255435) | [253] |
| Pharmacological treatment | Vatiquinone | Clinical phase 2/3 | FA patients | Ongoing sponsored by PTC Therapeutics (NCT04577352) | |
| Pharmacological treatment | MitoQ | Pre-clinical | FA cell lines | Reduced cell death | [226] |
| Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay (ARSACS) | |||||
| Pharmacological treatment | MitoQ | Pre-clinical | ARSACS mouse model | Decreased PN degeneration, increased DCN innervation and prevention of motor decline | [227] |
| Pharmacological treatment | Ceftriaxone | Pre-clinical | ARSACS mouse model | Restoration of calcium homeostasis, reduced neuroinflammation and improvement of motor ability | [233] |
| Ataxia-telangiectasia (AT) | |||||
| Pharmacological treatment | Nicotinamide riboside | Pre-clinical | AT mouse model | Prevention of neuroinflammation, reduced mitochondrial dysfunction and PN death, and improvement in motor ability | [228] |
| Pharmacological treatment | Amantadine | Clinical phase 4 | AT patients | Improvement in ataxic phenotype, involuntary movements and parkinsonism symptoms | [240] |
| Pharmacological treatment | Dexamethasone | Clinical phase 3 | AT patients | Sponsored by Erydel (NCT02770807) | |
| Autosomal Recessive Ataxia tye-2 (ARCA2) | |||||
| Pharmacological treatment | Coenzyme Q10 | Clinical | ARCA2 patients | Mild improvement of motor features | [225] |
| Multi disease trials | |||||
| Pharmacological treatment | Riluzole | Clinical phase 2 | SCA1, SCA2, SCA17, SCA28 and FA patients | Improvement of ICARS scale score | [236] |
| Pharmacological treatment | Riluzole | Clinical phase 3 | SCA1, SCA2, SCA3, SCA6, SCA7, SCA8 and SCA10 patients | Ongoing sponsored by Biohaven Pharmaceutical, Inc. (NCT03701399) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




