Submitted:
05 December 2023
Posted:
06 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
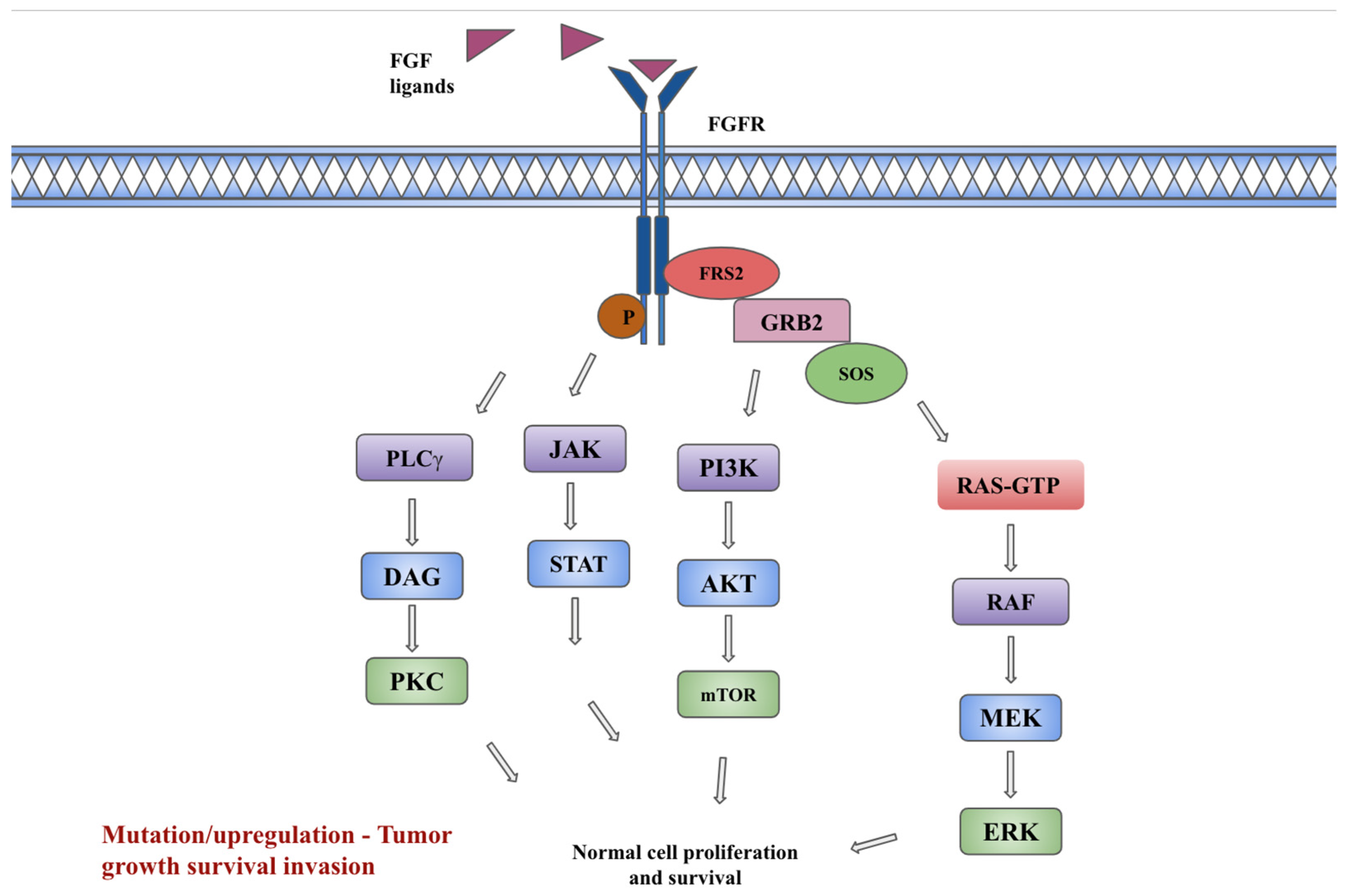
2. FGF/FGFR signaling pathway
3. FGFR Signaling diversity in cancer
3.1. Gynecologic cancers (Cervical, ovarian, and endometrial)
3.2. Gastrointestinal cancers
3.2.1. Cholangiocarcinoma
3.2.2. Gastric and gastroesophageal junction cancers
3.3. Urothelial cancers
3.4. Non-small cell lung cancer
3.5. Breast cancers
3.6. Glioblastoma
3.7. Gastrointestinal stromal tumors and other soft tissue sarcomas
3.7.1. Gastrointestinal stromal tumors
3.7.2. Rhabdomyosarcoma
3.8. Head and Neck Cancers
4. Generations of FGFR inhibitors
FDA approved FGFR inhibitors
| Drug | Trial | Phase | Study population, number (n) | ORR (%) | mDOR months | mOS months | mPFS months | FDA approval | Adverse effects |
|---|---|---|---|---|---|---|---|---|---|
|
Pemigatinib FGFR 1-3 inhibitor |
Abou-Alfa et al. FIGHT-202 trial [76,77] |
II |
Locally advanced, unresectable or metastatic CCA with FGFR2 gene fusion or rearrangements, progressed on at least one prior line of therapy. n = 146 |
35.5 | 9.1m | 17.5 |
- | April 17, 2020 as second line | Hyperphosphatemia, alopecia, dysgeusia, diarrhea, fatigue, stomatitis, dry mouth, arthralgia, hyponatremia, abdominal pain, fatigue, pyrexia, cholangitis, and pleural effusion |
| Gotlib et al. FIGHT-203 trial [80] |
II | MLNs with FGFR1 rearrangement regardless of prior lines of treatment. n = 34 | 64.7 (CR) |
Not reached | - | - | August 26, 2022 as second line | Hyperphosphatemia, alopecia, diarrhea, stomatitis, anemia, and pain in extremity | |
|
Infigratinib FGFR 1-3 inhibitor |
Javle et al. CBGJ398X2204 trial [84] |
II | Locally advanced, or metastatic CCA with FGFR2 fusions or rearrangements, progressed on at least one prior line. n = 108 | 23.1 | 5 | - | 7.3 | May 28, 2021 as second line | Hyperphosphatemia, eye disorders, hyponatremia, stomatitis, and fatigue |
|
Erdafitinib FGFR 1-4 inhibitors |
Siefker-Radtke et al. BLC2001 trial [88] |
II | Locally advanced, unresectable, or metastatic urothelial cancers with FGFR alterations, progressed on at least prior line or within 12 months after neoadjuvant or adjuvant chemotherapy. n = 99 | 40 | 5.6 | 13.8 | 5.5 | April 12, 2019 as second line | Hyperphosphatemia, stomatitis, diarrhea, and dry mouth, hyponatremia, and asthenia |
|
Futibatinib FGFR 1-4 inhibitor |
Goyal et al. FOENIX-CCA2 trial [93] |
II | Locally advanced, unresectable, or metastatic iCCA with FGFR2 fusions or rearrangements who progressed on at least one prior line. n=109 | 42 | 9.7 | 21.7 | 9.0 | September 30, 2022 as second line | Hyperphosphatemia, alopecia, dry mouth, diarrhea, dry skin, fatigue, increased aspartate aminotransferase level, and stomatitis |
5. Other FGFR inhibitors
5.1. Selective FGFR inhibitors
5.2. Multitarget tyrosine kinase inhibitors including FGFR
5.3. FGFR ligand trap
5.4. FGFR monoclonal antibody
5.5. Antibody drug conjugates
6. Resistance mechanisms to FGF/FGFR pathway inhibitors
7. Future Therapeutic Combinations with FGF/FGFR Inhibitors
8. Mechanism and management of the most relevant toxicity
9. Landscape of ongoing investigations, challenging issues, and future directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babina, I.S.; Turner, N.C. Advances and challenges in targeting FGFR signalling in cancer. Nature Reviews Cancer 2017, 17, 318-332. [CrossRef]
- Uehara, Y.; Ikeda, S.; Kim, K.H.; Lim, H.J.; Adashek, J.J.; Persha, H.E.; Okamura, R.; Lee, S.; Sicklick, J.K.; Kato, S.; et al. Targeting the FGF/FGFR axis and its co-alteration allies. ESMO Open 2022, 7, 100647. [CrossRef]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clinical Cancer Research 2016, 22, 259-267. [CrossRef]
- Gordon, A.; Johnston, E.; Lau, D.K.; Starling, N. Targeting FGFR2 Positive Gastroesophageal Cancer: Current and Clinical Developments. OncoTargets and Therapy 2022, Volume 15, 1183-1196. [CrossRef]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol 2015, 4, 215-266. [CrossRef]
- Beenken, A.; Mohammadi, M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 2009, 8, 235-253. [CrossRef]
- Chen, L.; Zhang, Y.; Yin, L.; Cai, B.; Huang, P.; Li, X.; Liang, G. Fibroblast growth factor receptor fusions in cancer: opportunities and challenges. Journal of Experimental & Clinical Cancer Research 2021, 40. [CrossRef]
- Teven, C.M.; Farina, E.M.; Rivas, J.; Reid, R.R. Fibroblast growth factor (FGF) signaling in development and skeletal diseases. Genes Dis 2014, 1, 199-213. [CrossRef]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduction and Targeted Therapy 2020, 5, 181. [CrossRef]
- Birrer, M.J.; Johnson, M.E.; Hao, K.; Wong, K.K.; Park, D.C.; Bell, A.; Welch, W.R.; Berkowitz, R.S.; Mok, S.C. Whole genome oligonucleotide-based array comparative genomic hybridization analysis identified fibroblast growth factor 1 as a prognostic marker for advanced-stage serous ovarian adenocarcinomas. J Clin Oncol 2007, 25, 2281-2287. [CrossRef]
- Smith, G.; Ng, M.T.; Shepherd, L.; Herrington, C.S.; Gourley, C.; Ferguson, M.J.; Wolf, C.R. Individuality in FGF1 expression significantly influences platinum resistance and progression-free survival in ovarian cancer. Br J Cancer 2012, 107, 1327-1336. [CrossRef]
- Steele, I.A.; Edmondson, R.J.; Bulmer, J.N.; Bolger, B.S.; Leung, H.Y.; Davies, B.R. Induction of FGF receptor 2-IIIb expression and response to its ligands in epithelial ovarian cancer. Oncogene 2001, 20, 5878-5887. [CrossRef]
- Taniguchi, F.; Itamochi, H.; Harada, T.; Terakawa, N. Fibroblast growth factor receptor 2 expression may be involved in transformation of ovarian endometrioma to clear cell carcinoma of the ovary. Int J Gynecol Cancer 2013, 23, 791-796. [CrossRef]
- Zaid, T.M.; Yeung, T.L.; Thompson, M.S.; Leung, C.S.; Harding, T.; Co, N.N.; Schmandt, R.S.; Kwan, S.Y.; Rodriguez-Aguay, C.; Lopez-Berestein, G.; et al. Identification of FGFR4 as a potential therapeutic target for advanced-stage, high-grade serous ovarian cancer. Clin Cancer Res 2013, 19, 809-820. [CrossRef]
- Choi, C.H.; Chung, J.Y.; Kim, J.H.; Kim, B.G.; Hewitt, S.M. Expression of fibroblast growth factor receptor family members is associated with prognosis in early stage cervical cancer patients. J Transl Med 2016, 14, 124. [CrossRef]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin Cancer Res 2016, 22, 259-267. [CrossRef]
- Wu, Y.M.; Su, F.; Kalyana-Sundaram, S.; Khazanov, N.; Ateeq, B.; Cao, X.; Lonigro, R.J.; Vats, P.; Wang, R.; Lin, S.F.; et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov 2013, 3, 636-647. [CrossRef]
- Arai, Y.; Totoki, Y.; Hosoda, F.; Shirota, T.; Hama, N.; Nakamura, H.; Ojima, H.; Furuta, K.; Shimada, K.; Okusaka, T.; et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 2014, 59, 1427-1434. [CrossRef]
- Jain, A.; Borad, M.J.; Kelley, R.K.; Wang, Y.; Abdel-Wahab, R.; Meric-Bernstam, F.; Baggerly, K.A.; Kaseb, A.O.; Al-Shamsi, H.O.; Ahn, D.H.; et al. Cholangiocarcinoma With FGFR Genetic Aberrations: A Unique Clinical Phenotype. JCO Precis Oncol 2018, 2, 1-12. [CrossRef]
- Kongpetch, S.; Jusakul, A.; Lim, J.Q.; Ng, C.C.Y.; Chan, J.Y.; Rajasegaran, V.; Lim, T.H.; Lim, K.H.; Choo, S.P.; Dima, S.; et al. Lack of Targetable FGFR2 Fusions in Endemic Fluke-Associated Cholangiocarcinoma. JCO Glob Oncol 2020, 6, 628-638. [CrossRef]
- Helsten, T.; Schwaederle, M.; Kurzrock, R. Fibroblast growth factor receptor signaling in hereditary and neoplastic disease: biologic and clinical implications. Cancer Metastasis Rev 2015, 34, 479-496. [CrossRef]
- Gu, W.; Yang, J.; Wang, Y.; Xu, J.; Wang, X.; Du, F.; Hu, X.; Guo, H.; Song, C.; Tao, R.; et al. Comprehensive identification of FGFR1-4 alterations in 5 557 Chinese patients with solid tumors by next-generation sequencing. Am J Cancer Res 2021, 11, 3893-3906.
- Costa, R.; Carneiro, B.A.; Taxter, T.; Tavora, F.A.; Kalyan, A.; Pai, S.A.; Chae, Y.K.; Giles, F.J. FGFR3-TACC3 fusion in solid tumors: mini review. Oncotarget 2016, 7, 55924-55938. [CrossRef]
- Singh, D.; Chan, J.M.; Zoppoli, P.; Niola, F.; Sullivan, R.; Castano, A.; Liu, E.M.; Reichel, J.; Porrati, P.; Pellegatta, S.; et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 2012, 337, 1231-1235. [CrossRef]
- Su, X.; Zhan, P.; Gavine, P.R.; Morgan, S.; Womack, C.; Ni, X.; Shen, D.; Bang, Y.J.; Im, S.A.; Ho Kim, W.; et al. FGFR2 amplification has prognostic significance in gastric cancer: results from a large international multicentre study. Br J Cancer 2014, 110, 967-975. [CrossRef]
- Matsumoto, K.; Arao, T.; Hamaguchi, T.; Shimada, Y.; Kato, K.; Oda, I.; Taniguchi, H.; Koizumi, F.; Yanagihara, K.; Sasaki, H.; et al. FGFR2 gene amplification and clinicopathological features in gastric cancer. Br J Cancer 2012, 106, 727-732. [CrossRef]
- Jung, E.J.; Jung, E.J.; Min, S.Y.; Kim, M.A.; Kim, W.H. Fibroblast growth factor receptor 2 gene amplification status and its clinicopathologic significance in gastric carcinoma. Hum Pathol 2012, 43, 1559-1566. [CrossRef]
- Tomlinson, D.C.; Hurst, C.D.; Knowles, M.A. Knockdown by shRNA identifies S249C mutant FGFR3 as a potential therapeutic target in bladder cancer. Oncogene 2007, 26, 5889-5899. [CrossRef]
- AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov 2017, 7, 818-831. [CrossRef]
- Sfakianos, J.P.; Cha, E.K.; Iyer, G.; Scott, S.N.; Zabor, E.C.; Shah, R.H.; Ren, Q.; Bagrodia, A.; Kim, P.H.; Hakimi, A.A.; et al. Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur Urol 2015, 68, 970-977. [CrossRef]
- Ross, J.S.; Wang, K.; Khaira, D.; Ali, S.M.; Fisher, H.A.; Mian, B.; Nazeer, T.; Elvin, J.A.; Palma, N.; Yelensky, R.; et al. Comprehensive genomic profiling of 295 cases of clinically advanced urothelial carcinoma of the urinary bladder reveals a high frequency of clinically relevant genomic alterations. Cancer 2016, 122, 702-711. [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540-556.e525. [CrossRef]
- di Martino, E.; Tomlinson, D.C.; Williams, S.V.; Knowles, M.A. A place for precision medicine in bladder cancer: targeting the FGFRs. Future Oncol 2016, 12, 2243-2263. [CrossRef]
- Tomlinson, D.C.; Baldo, O.; Harnden, P.; Knowles, M.A. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol 2007, 213, 91-98. [CrossRef]
- Zhou, Z.; Liu, Z.; Ou, Q.; Wu, X.; Wang, X.; Shao, Y.; Liu, H.; Yang, Y. Targeting FGFR in non-small cell lung cancer: implications from the landscape of clinically actionable aberrations of FGFR kinases. Cancer Biol Med 2021, 18, 490-501. [CrossRef]
- Ou, S.I.; Horn, L.; Cruz, M.; Vafai, D.; Lovly, C.M.; Spradlin, A.; Williamson, M.J.; Dagogo-Jack, I.; Johnson, A.; Miller, V.A.; et al. Emergence of FGFR3-TACC3 fusions as a potential by-pass resistance mechanism to EGFR tyrosine kinase inhibitors in EGFR mutated NSCLC patients. Lung Cancer 2017, 111, 61-64. [CrossRef]
- Reis-Filho, J.S.; Simpson, P.T.; Turner, N.C.; Lambros, M.B.; Jones, C.; Mackay, A.; Grigoriadis, A.; Sarrio, D.; Savage, K.; Dexter, T.; et al. FGFR1 emerges as a potential therapeutic target for lobular breast carcinomas. Clin Cancer Res 2006, 12, 6652-6662. [CrossRef]
- Santolla, M.F.; Vivacqua, A.; Lappano, R.; Rigiracciolo, D.C.; Cirillo, F.; Galli, G.R.; Talia, M.; Brunetti, G.; Miglietta, A.M.; Belfiore, A.; et al. GPER Mediates a Feedforward FGF2/FGFR1 Paracrine Activation Coupling CAFs to Cancer Cells toward Breast Tumor Progression. Cells 2019, 8. [CrossRef]
- Turner, N.; Pearson, A.; Sharpe, R.; Lambros, M.; Geyer, F.; Lopez-Garcia, M.A.; Natrajan, R.; Marchio, C.; Iorns, E.; Mackay, A.; et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res 2010, 70, 2085-2094. [CrossRef]
- Formisano, L.; Lu, Y.; Servetto, A.; Hanker, A.B.; Jansen, V.M.; Bauer, J.A.; Sudhan, D.R.; Guerrero-Zotano, A.L.; Croessmann, S.; Guo, Y.; et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun 2019, 10, 1373. [CrossRef]
- Xie, N.; Tian, C.; Wu, H.; Yang, X.; Liu, L.; Li, J.; Xiao, H.; Gao, J.; Lu, J.; Hu, X.; et al. FGFR aberrations increase the risk of brain metastases and predict poor prognosis in metastatic breast cancer patients. Ther Adv Med Oncol 2020, 12, 1758835920915305. [CrossRef]
- Chew, N.J.; Nguyen, E.V.; Su, S.P.; Novy, K.; Chan, H.C.; Nguyen, L.K.; Luu, J.; Simpson, K.J.; Lee, R.S.; Daly, R.J. FGFR3 signaling and function in triple negative breast cancer. Cell Commun Signal 2020, 18, 13. [CrossRef]
- Tomlinson, D.C.; Knowles, M.A.; Speirs, V. Mechanisms of FGFR3 actions in endocrine resistant breast cancer. Int J Cancer 2012, 130, 2857-2866. [CrossRef]
- Yamaguchi, F.; Saya, H.; Bruner, J.M.; Morrison, R.S. Differential expression of two fibroblast growth factor-receptor genes is associated with malignant progression in human astrocytomas. Proc Natl Acad Sci U S A 1994, 91, 484-488. [CrossRef]
- Morrison, R.S.; Yamaguchi, F.; Saya, H.; Bruner, J.M.; Yahanda, A.M.; Donehower, L.A.; Berger, M. Basic fibroblast growth factor and fibroblast growth factor receptor I are implicated in the growth of human astrocytomas. J Neurooncol 1994, 18, 207-216. [CrossRef]
- Wang, F.; Kan, M.; Yan, G.; Xu, J.; McKeehan, W.L. Alternately spliced NH2-terminal immunoglobulin-like Loop I in the ectodomain of the fibroblast growth factor (FGF) receptor 1 lowers affinity for both heparin and FGF-1. J Biol Chem 1995, 270, 10231-10235. [CrossRef]
- Fukai, J.; Yokote, H.; Yamanaka, R.; Arao, T.; Nishio, K.; Itakura, T. EphA4 promotes cell proliferation and migration through a novel EphA4-FGFR1 signaling pathway in the human glioma U251 cell line. Mol Cancer Ther 2008, 7, 2768-2778. [CrossRef]
- Gouazé-Andersson, V.; Delmas, C.; Taurand, M.; Martinez-Gala, J.; Evrard, S.; Mazoyer, S.; Toulas, C.; Cohen-Jonathan-Moyal, E. FGFR1 Induces Glioblastoma Radioresistance through the PLCγ/Hif1α Pathway. Cancer Res 2016, 76, 3036-3044. [CrossRef]
- Miyake, A.; Hattori, Y.; Ohta, M.; Itoh, N. Rat oligodendrocytes and astrocytes preferentially express fibroblast growth factor receptor-2 and -3 mRNAs. J Neurosci Res 1996, 45, 534-541. [CrossRef]
- Ohashi, R.; Matsuda, Y.; Ishiwata, T.; Naito, Z. Downregulation of fibroblast growth factor receptor 2 and its isoforms correlates with a high proliferation rate and poor prognosis in high-grade glioma. Oncol Rep 2014, 32, 1163-1169. [CrossRef]
- Daido, S.; Takao, S.; Tamiya, T.; Ono, Y.; Terada, K.; Ito, S.; Ouchida, M.; Date, I.; Ohmoto, T.; Shimizu, K. Loss of heterozygosity on chromosome 10q associated with malignancy and prognosis in astrocytic tumors, and discovery of novel loss regions. Oncol Rep 2004, 12, 789-795.
- Shi, E.; Chmielecki, J.; Tang, C.M.; Wang, K.; Heinrich, M.C.; Kang, G.; Corless, C.L.; Hong, D.; Fero, K.E.; Murphy, J.D.; et al. FGFR1 and NTRK3 actionable alterations in "Wild-Type" gastrointestinal stromal tumors. J Transl Med 2016, 14, 339. [CrossRef]
- Pantaleo, M.A.; Urbini, M.; Indio, V.; Ravegnini, G.; Nannini, M.; De Luca, M.; Tarantino, G.; Angelini, S.; Gronchi, A.; Vincenzi, B.; et al. Genome-Wide Analysis Identifies MEN1 and MAX Mutations and a Neuroendocrine-Like Molecular Heterogeneity in Quadruple WT GIST. Mol Cancer Res 2017, 15, 553-562. [CrossRef]
- Javidi-Sharifi, N.; Traer, E.; Martinez, J.; Gupta, A.; Taguchi, T.; Dunlap, J.; Heinrich, M.C.; Corless, C.L.; Rubin, B.P.; Druker, B.J.; et al. Crosstalk between KIT and FGFR3 Promotes Gastrointestinal Stromal Tumor Cell Growth and Drug Resistance. Cancer Res 2015, 75, 880-891. [CrossRef]
- Li, F.; Huynh, H.; Li, X.; Ruddy, D.A.; Wang, Y.; Ong, R.; Chow, P.; Qiu, S.; Tam, A.; Rakiec, D.P.; et al. FGFR-Mediated Reactivation of MAPK Signaling Attenuates Antitumor Effects of Imatinib in Gastrointestinal Stromal Tumors. Cancer Discov 2015, 5, 438-451. [CrossRef]
- Boichuk, S.; Galembikova, A.; Dunaev, P.; Valeeva, E.; Shagimardanova, E.; Gusev, O.; Khaiboullina, S. A Novel Receptor Tyrosine Kinase Switch Promotes Gastrointestinal Stromal Tumor Drug Resistance. Molecules 2017, 22. [CrossRef]
- Taylor, J.G.t.; Cheuk, A.T.; Tsang, P.S.; Chung, J.Y.; Song, Y.K.; Desai, K.; Yu, Y.; Chen, Q.R.; Shah, K.; Youngblood, V.; et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J Clin Invest 2009, 119, 3395-3407. [CrossRef]
- Wachtel, M.; Rakic, J.; Okoniewski, M.; Bode, P.; Niggli, F.; Schäfer, B.W. FGFR4 signaling couples to Bim and not Bmf to discriminate subsets of alveolar rhabdomyosarcoma cells. Int J Cancer 2014, 135, 1543-1552. [CrossRef]
- Chudasama, P.; Renner, M.; Straub, M.; Mughal, S.S.; Hutter, B.; Kosaloglu, Z.; Schweßinger, R.; Scheffler, M.; Alldinger, I.; Schimmack, S.; et al. Targeting Fibroblast Growth Factor Receptor 1 for Treatment of Soft-Tissue Sarcoma. Clin Cancer Res 2017, 23, 962-973. [CrossRef]
- Agelopoulos, K.; Richter, G.H.; Schmidt, E.; Dirksen, U.; von Heyking, K.; Moser, B.; Klein, H.U.; Kontny, U.; Dugas, M.; Poos, K.; et al. Deep Sequencing in Conjunction with Expression and Functional Analyses Reveals Activation of FGFR1 in Ewing Sarcoma. Clin Cancer Res 2015, 21, 4935-4946. [CrossRef]
- Bao, Y.; Gabrielpillai, J.; Dietrich, J.; Zarbl, R.; Strieth, S.; Schröck, F.; Dietrich, D. Fibroblast growth factor (FGF), FGF receptor (FGFR), and cyclin D1 (CCND1) DNA methylation in head and neck squamous cell carcinomas is associated with transcriptional activity, gene amplification, human papillomavirus (HPV) status, and sensitivity to tyrosine kinase inhibitors. Clin Epigenetics 2021, 13, 228. [CrossRef]
- Tillman, B.N.; Yanik, M.; Birkeland, A.C.; Liu, C.J.; Hovelson, D.H.; Cani, A.K.; Palanisamy, N.; Carskadon, S.; Carey, T.E.; Bradford, C.R.; et al. Fibroblast growth factor family aberrations as a putative driver of head and neck squamous cell carcinoma in an epidemiologically low-risk patient as defined by targeted sequencing. Head Neck 2016, 38 Suppl 1, E1646-1652. [CrossRef]
- Brands, R.C.; Knierim, L.M.; De Donno, F.; Steinacker, V.; Hartmann, S.; Seher, A.; Kübler, A.C.; Müller-Richter, U.D.A. Targeting VEGFR and FGFR in head and neck squamous cell carcinoma in vitro. Oncol Rep 2017, 38, 1877-1885. [CrossRef]
- Nayak, S.; Goel, M.M.; Makker, A.; Bhatia, V.; Chandra, S.; Kumar, S.; Agarwal, S.P. Fibroblast Growth Factor (FGF-2) and Its Receptors FGFR-2 and FGFR-3 May Be Putative Biomarkers of Malignant Transformation of Potentially Malignant Oral Lesions into Oral Squamous Cell Carcinoma. PLoS One 2015, 10, e0138801. [CrossRef]
- Marshall, M.E.; Hinz, T.K.; Kono, S.A.; Singleton, K.R.; Bichon, B.; Ware, K.E.; Marek, L.; Frederick, B.A.; Raben, D.; Heasley, L.E. Fibroblast growth factor receptors are components of autocrine signaling networks in head and neck squamous cell carcinoma cells. Clin Cancer Res 2011, 17, 5016-5025. [CrossRef]
- Kim, E.K.; Cho, Y.A.; Koh, Y.W.; Shin, H.A.; Cho, B.C.; Yoon, S.O. Prognostic implications of Fibroblast growth factor receptor 1 (FGFR1) gene amplification and protein overexpression in hypopharyngeal and laryngeal squamous cell carcinoma. BMC Cancer 2020, 20, 348. [CrossRef]
- Seiwert, T.Y.; Zuo, Z.; Keck, M.K.; Khattri, A.; Pedamallu, C.S.; Stricker, T.; Brown, C.; Pugh, T.J.; Stojanov, P.; Cho, J.; et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res 2015, 21, 632-641. [CrossRef]
- Yuan, L.; Liu, Z.H.; Lin, Z.R.; Xu, L.H.; Zhong, Q.; Zeng, M.S. Recurrent FGFR3-TACC3 fusion gene in nasopharyngeal carcinoma. Cancer Biol Ther 2014, 15, 1613-1621. [CrossRef]
- Koole, K.; Clausen, M.J.; van Es, R.J.; van Kempen, P.M.; Melchers, L.J.; Koole, R.; Langendijk, J.A.; van Diest, P.J.; Roodenburg, J.L.; Schuuring, E.; et al. FGFR Family Members Protein Expression as Prognostic Markers in Oral Cavity and Oropharyngeal Squamous Cell Carcinoma. Mol Diagn Ther 2016, 20, 363-374. [CrossRef]
- Shi, S.; Li, X.; You, B.; Shan, Y.; Cao, X.; You, Y. High Expression of FGFR4 Enhances Tumor Growth and Metastasis in Nasopharyngeal Carcinoma. J Cancer 2015, 6, 1245-1254. [CrossRef]
- Yue, S.; Li, Y.; Chen, X.; Wang, J.; Li, M.; Chen, Y.; Wu, D. FGFR-TKI resistance in cancer: current status and perspectives. Journal of Hematology & Oncology 2021, 14, 23. [CrossRef]
- Krook, M.A.; Lenyo, A.; Wilberding, M.; Barker, H.; Dantuono, M.; Bailey, K.M.; Chen, H.-Z.; Reeser, J.W.; Wing, M.R.; Miya, J.; et al. Efficacy of FGFR Inhibitors and Combination Therapies for Acquired Resistance in FGFR2-Fusion Cholangiocarcinoma. Molecular Cancer Therapeutics 2020, 19, 847-857. [CrossRef]
- Mahapatra, S.; Jonniya, N.A.; Koirala, S.; Ursal, K.D.; Kar, P. The FGF/FGFR signalling mediated anti-cancer drug resistance and therapeutic intervention. J Biomol Struct Dyn 2023, 1-25. [CrossRef]
- Helsinn to Withdraw NDA for Cholangiocarcinoma. Available online: https://www.fdanews.com/articles/209797-helsinn-to-withdraw-nda-for-cholangiocarcinoma (accessed on November 6, 2023).
- FDA, P. FDA grants accelerated approval to pemigatinib for cholangiocarcinoma with an FGFR2 rearrangement or fusion. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pemigatinib-cholangiocarcinoma-fgfr2-rearrangement-or-fusion (accessed on 08/27/2023).
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. The lancet oncology 2020, 21, 671-684. [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.M.; Melisi, D.; Al-Rajabi, R.M.d.T.; Paulson, A.S.; Borad, M.J.; Gallinson, D.H.; Murphy, A.G.; et al. Pemigatinib for previously treated locally advanced/metastatic cholangiocarcinoma (CCA): Update of FIGHT-202. Journal of Clinical Oncology 2021, 39, 4086-4086. [CrossRef]
- Bekaii-Saab, T.S.; Valle, J.W.; Cutsem, E.V.; Rimassa, L.; Furuse, J.; Ioka, T.; Melisi, D.; Macarulla, T.; Bridgewater, J.A.; Wasan, H.S.; et al. FIGHT-302: Phase III study of first-line (1L) pemigatinib (PEM) versus gemcitabine (GEM) plus cisplatin (CIS) for cholangiocarcinoma (CCA) with FGFR2 fusions or rearrangements. Journal of Clinical Oncology 2020, 38, TPS592-TPS592. [CrossRef]
- Bekaii-Saab, T.S.; Valle, J.W.; Cutsem, E.V.; Rimassa, L.; Furuse, J.; Ioka, T.; Melisi, D.; Macarulla, T.; Bridgewater, J.; Wasan, H.; et al. FIGHT-302: first-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncology 2020, 16, 2385-2399. [CrossRef]
- Gotlib, J.; Kiladjian, J.-J.; Vannucchi, A.; Rambaldi, A.; Reiter, A.; Shomali, W.; George, T.I.; Patel, J.L.; Colucci, P.; Walker, C.; et al. A Phase 2 Study of Pemigatinib (FIGHT-203; INCB054828) in Patients with Myeloid/Lymphoid Neoplasms (MLNs) with Fibroblast Growth Factor Receptor 1 (FGFR1) Rearrangement (MLN FGFR1). Blood 2021, 138, 385. [CrossRef]
- FDA, P. FDA approves pemigatinib for relapsed or refractory myeloid/lymphoid neoplasms with FGFR1 rearrangement. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pemigatinib-relapsed-or-refractory-myeloidlymphoid-neoplasms-fgfr1-rearrangement#:~:text=On%20August%2026%2C%202022%2C%20the,receptor%201%20(FGFR1)%20rearrangement. (accessed on 08/27/2023).
- FDA, I. FDA grants accelerated approval to infigratinib for metastatic cholangiocarcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-infigratinib-metastatic-cholangiocarcinoma (accessed on.
- Javle, M.M.; Kelley, R.K.; Springfeld, C.; Abou-Alfa, G.K.; Macarulla, T.; Tanasanvimon, S.; Goyal, L.; Borbath, I.; Bitzer, M.; Yong, W.-P.; et al. A phase II study of infigratinib in previously treated advanced/metastatic cholangiocarcinoma with FGFR gene fusions/alterations. Journal of Clinical Oncology 2021, 39, TPS356-TPS356. [CrossRef]
- Javle, M.M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.B.; Yong, W.-P.; et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. Journal of Clinical Oncology 2021, 39, 265-265. [CrossRef]
- discontinuation, I. Helsinn Therapeutics – Discontinuation of Truseltiq® (infigratinib). Available online: https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-withdrawls/drugwithdrawal_truseltiq_2022-1117.pdf (accessed on 08/27/2023).
- FDA, E. FDA grants accelerated approval to erdafitinib for metastatic urothelial carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-erdafitinib-metastatic-urothelial-carcinoma (accessed on.
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. New England Journal of Medicine 2019, 381, 338-348. [CrossRef]
- Siefker-Radtke, A.O.; Necchi, A.; Park, S.H.; García-Donas, J.; Huddart, R.A.; Burgess, E.F.; Fleming, M.T.; Rezazadeh Kalebasty, A.; Mellado, B.; Varlamov, S.; et al. Efficacy and safety of erdafitinib in patients with locally advanced or metastatic urothelial carcinoma: long-term follow-up of a phase 2 study. Lancet Oncol 2022, 23, 248-258. [CrossRef]
- Janssen Submits Supplemental New Drug Application to the U.S. Food and Drug Administration Seeking Full Approval of BALVERSA® (erdafitinib) for the Treatment of Patients with Locally Advanced or Metastatic Urothelial Carcinoma and Selected Fibroblast Growth Factor Receptor Gene Alterations. Available online: https://www.jnj.com/janssen-submits-supplemental-new-drug-application-to-the-u-s-food-and-drug-administration-seeking-full-approval-of-balversa-erdafitinib-for-the-treatment-of-patients-with-locally-advanced-or-metastatic-urothelial-carcinoma-and-selected-fibroblast-growth-factor-receptor-gene-alterations (accessed on 11/6/2023).
- Loriot, Y.; Matsubara, N.; Park, S.H.; Huddart, R.A.; Burgess, E.F.; Houede, N.; Banek, S.; Laguerre, B.; Guadalupi, V.; Ku, J.H.; et al. Phase 3 THOR study: Results of erdafitinib (erda) versus chemotherapy (chemo) in patients (pts) with advanced or metastatic urothelial cancer (mUC) with select fibroblast growth factor receptor alterations (<i>FGFRalt</i>). Journal of Clinical Oncology 2023, 41, LBA4619-LBA4619. [CrossRef]
- Loriot, Y.; Matsubara, N.; Park, S.H.; Huddart, R.A.; Burgess, E.F.; Houede, N.; Banek, S.; Guadalupi, V.; Ku, J.H.; Valderrama, B.P.; et al. Erdafitinib or Chemotherapy in Advanced or Metastatic Urothelial Carcinoma. New England Journal of Medicine 2023. [CrossRef]
- Romero, D. THOR provides new data on the efficacy of erdafitinib. Nature Reviews Clinical Oncology 2023. [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N Engl J Med 2023, 388, 228-239. [CrossRef]
- FDA, F. FDA grants accelerated approval to futibatinib for cholangiocarcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-futibatinib-cholangiocarcinoma (accessed on 08/27/2023).
- Borad, M.J.; Bridgewater, J.A.; Morizane, C.; Shroff, R.T.; Oh, D.-Y.; Moehler, M.H.; Furuse, J.; Benhadji, K.A.; He, H.; Valle, J.W. A phase III study of futibatinib (TAS-120) versus gemcitabine-cisplatin (gem-cis) chemotherapy as first-line (1L) treatment for patients (pts) with advanced (adv) cholangiocarcinoma (CCA) harboring fibroblast growth factor receptor 2 (FGFR2) gene rearrangements (FOENIX-CCA3). Journal of Clinical Oncology 2020, 38, TPS600-TPS600. [CrossRef]
- Schuler, M.; Cho, B.C.; Sayehli, C.M.; Navarro, A.; Soo, R.A.; Richly, H.; Cassier, P.A.; Tai, D.; Penel, N.; Nogova, L.; et al. Rogaratinib in patients with advanced cancers selected by FGFR mRNA expression: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol 2019, 20, 1454-1466. [CrossRef]
- Sternberg, C.N.; Petrylak, D.P.; Bellmunt, J.; Nishiyama, H.; Necchi, A.; Gurney, H.; Lee, J.L.; van der Heijden, M.S.; Rosenbaum, E.; Penel, N.; et al. FORT-1: Phase II/III Study of Rogaratinib Versus Chemotherapy in Patients With Locally Advanced or Metastatic Urothelial Carcinoma Selected Based on FGFR1/3 mRNA Expression. J Clin Oncol 2023, 41, 629-639. [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Droz dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. British Journal of Cancer 2019, 120, 165-171. [CrossRef]
- Javle, M.M.; Abou-Alfa, G.K.; Macarulla, T.; Personeni, N.; Adeva, J.; Bergamo, F.; Malka, D.; Vogel, A.; Knox, J.J.; Evans, T.R.J.; et al. Efficacy of derazantinib in intrahepatic cholangiocarcinoma patients with FGFR2 mutations or amplifications: Interim results from the phase 2 study FIDES-01. Journal of Clinical Oncology 2022, 40, 427-427. [CrossRef]
- Necchi, A.; Todenhöfer, T.; Deville, J.-L.; Häckl, M.; Marszewska, M.; McKernan, P.; Saulay, M.; Engelhardt, M.; Santis, M.D. Efficacy and safety of derazantinib (DZB) in patients with metastatic urothelial carcinoma (mUC) with activating FGFR1/2/3 genetic aberrations (GA): Results from the phase 1b/2 FIDES-02 study. Journal of Clinical Oncology 2023, 41, 501-501. [CrossRef]
- Available online: clinicaltrials.gov. (accessed on 11/18/2023).
- Subbiah, V.; Sahai, V.; Maglic, D.; Bruderek, K.; Touré, B.B.; Zhao, S.; Valverde, R.; O'Hearn, P.J.; Moustakas, D.T.; Schönherr, H.; et al. RLY-4008, the First Highly Selective FGFR2 Inhibitor with Activity across FGFR2 Alterations and Resistance Mutations. Cancer Discovery 2023, 13, 2012-2031. [CrossRef]
- Voss, M.H.; Hierro, C.; Heist, R.S.; Cleary, J.M.; Meric-Bernstam, F.; Tabernero, J.; Janku, F.; Gandhi, L.; Iafrate, A.J.; Borger, D.R.; et al. A Phase I, Open-Label, Multicenter, Dose-escalation Study of the Oral Selective FGFR Inhibitor Debio 1347 in Patients with Advanced Solid Tumors Harboring FGFR Gene Alterations. Clin Cancer Res 2019, 25, 2699-2707. [CrossRef]
- Kim, R.D.; Sarker, D.; Meyer, T.; Yau, T.; Macarulla, T.; Park, J.-W.; Choo, S.P.; Hollebecque, A.; Sung, M.W.; Lim, H.-Y.; et al. First-in-Human Phase I Study of Fisogatinib (BLU-554) Validates Aberrant FGF19 Signaling as a Driver Event in Hepatocellular Carcinoma. Cancer Discovery 2019, 9, 1696-1707. [CrossRef]
- Tjulandin, S.; Statsenko, G.; Artamonova, E.; Vladimirova, L.Y.; Besova, N.; Mochalova, A.; Rykov, I.; Moiseyenko, V.; Utyashev, I.A.; Iugai, S.; et al. A first-in-human phase 1b study of a novel allosteric extracellular FGFR2 inhibitor alofanib in patients with refractory metastatic gastric cancer. Journal of Clinical Oncology 2022, 40, 304-304. [CrossRef]
- Tyulyandina, A.; Harrison, D.; Yin, W.; Stepanova, E.; Kochenkov, D.; Solomko, E.; Peretolchina, N.; Daeyaert, F.; Joos, J.B.; Van Aken, K.; et al. Alofanib, an allosteric FGFR2 inhibitor, has potent effects on ovarian cancer growth in preclinical studies. Invest New Drugs 2017, 35, 127-133. [CrossRef]
- Chan, S.L.; Schuler, M.; Kang, Y.K.; Yen, C.J.; Edeline, J.; Choo, S.P.; Lin, C.C.; Okusaka, T.; Weiss, K.H.; Macarulla, T.; et al. A first-in-human phase 1/2 study of FGF401 and combination of FGF401 with spartalizumab in patients with hepatocellular carcinoma or biomarker-selected solid tumors. J Exp Clin Cancer Res 2022, 41, 189. [CrossRef]
- Aggarwal, C.; Redman, M.W.; Lara, P.N., Jr.; Borghaei, H.; Hoffman, P.; Bradley, J.D.; Newman, A.J., 3rd; Feldman, M.J.; Minichiello, K.; Miao, J.; et al. SWOG S1400D (NCT02965378), a Phase II Study of the Fibroblast Growth Factor Receptor Inhibitor AZD4547 in Previously Treated Patients With Fibroblast Growth Factor Pathway-Activated Stage IV Squamous Cell Lung Cancer (Lung-MAP Substudy). J Thorac Oncol 2019, 14, 1847-1852. [CrossRef]
- Chae, Y.K.; Hong, F.; Vaklavas, C.; Cheng, H.H.; Hammerman, P.; Mitchell, E.P.; Zwiebel, J.A.; Ivy, S.P.; Gray, R.J.; Li, S.; et al. Phase II Study of AZD4547 in Patients With Tumors Harboring Aberrations in the FGFR Pathway: Results From the NCI-MATCH Trial (EAY131) Subprotocol W. Journal of Clinical Oncology 2020, 38, 2407-2417. [CrossRef]
- Coombes, R.C.; Badman, P.D.; Lozano-Kuehne, J.P.; Liu, X.; Macpherson, I.R.; Zubairi, I.; Baird, R.D.; Rosenfeld, N.; Garcia-Corbacho, J.; Cresti, N.; et al. Results of the phase IIa RADICAL trial of the FGFR inhibitor AZD4547 in endocrine resistant breast cancer. Nature Communications 2022, 13, 3246. [CrossRef]
- Huynh, H.; Chow, P.K.; Tai, W.M.; Choo, S.P.; Chung, A.Y.; Ong, H.S.; Soo, K.C.; Ong, R.; Linnartz, R.; Shi, M.M. Dovitinib demonstrates antitumor and antimetastatic activities in xenograft models of hepatocellular carcinoma. J Hepatol 2012, 56, 595-601. [CrossRef]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. New England Journal of Medicine 2015, 372, 621-630. [CrossRef]
- Hui, R.; Pearson, A.; Cortes, J.; Campbell, C.; Poirot, C.; Azim, H.A., Jr.; Fumagalli, D.; Lambertini, M.; Daly, F.; Arahmani, A.; et al. Lucitanib for the Treatment of HR(+)/HER2(-) Metastatic Breast Cancer: Results from the Multicohort Phase II FINESSE Study. Clin Cancer Res 2020, 26, 354-363. [CrossRef]
- Hayashi, H.; Makiyama, A.; Okumura, N.; Yasufuku, I.; Saigo, C.; Takeuchi, T.; Miyazaki, T.; Tanaka, Y.; Matsuhashi, N.; Murase, K.; et al. Gastric carcinosarcoma with FGFR2 amplification under long-term control with pazopanib: a case report and literature review. BMC Gastroenterology 2022, 22, 360. [CrossRef]
- Tan, F.H.; Putoczki, T.L.; Stylli, S.S.; Luwor, R.B. Ponatinib: a novel multi-tyrosine kinase inhibitor against human malignancies. Onco Targets Ther 2019, 12, 635-645. [CrossRef]
- Harding, T.C.; Long, L.; Palencia, S.; Zhang, H.; Sadra, A.; Hestir, K.; Patil, N.; Levin, A.; Hsu, A.W.; Charych, D.; et al. Blockade of Nonhormonal Fibroblast Growth Factors by FP-1039 Inhibits Growth of Multiple Types of Cancer. Science Translational Medicine 2013, 5, 178ra139-178ra139, doi:doi:10.1126/scitranslmed.3005414.
- Kollmannsberger, C.; Britten, C.D.; Olszanski, A.J.; Walker, J.A.; Zang, W.; Willard, M.D.; Radtke, D.B.; Farrington, D.L.; Bell-Mcguinn, K.M.; Patnaik, A. A phase 1 study of LY3076226, a fibroblast growth factor receptor 3 (FGFR3) antibody–drug conjugate, in patients with advanced or metastatic cancer. Investigational New Drugs 2021, 39, 1613-1623. [CrossRef]
- Blackwell, C.; Sherk, C.; Fricko, M.; Ganji, G.; Barnette, M.; Hoang, B.; Tunstead, J.; Skedzielewski, T.; Alsaid, H.; Jucker, B.M.; et al. Inhibition of FGF/FGFR autocrine signaling in mesothelioma with the FGF ligand trap, FP-1039/GSK3052230. Oncotarget 2016, 7, 39861-39871. [CrossRef]
- Van Brummelen, E.M.J.; Levchenko, E.; Dómine, M.; Fennell, D.A.; Kindler, H.L.; Viteri, S.; Gadgeel, S.; López, P.G.; Kostorov, V.; Morgensztern, D.; et al. A phase Ib study of GSK3052230, an FGF ligand trap in combination with pemetrexed and cisplatin in patients with malignant pleural mesothelioma. Investigational New Drugs 2020, 38, 457-467. [CrossRef]
- Necchi, A.; Castellano, D.E.; Mellado, B.; Pang, S.; Urun, Y.; Park, S.H.; Vaishampayan, U.N.; Currie, G.; Abella-Dominicis, E.; Pal, S.K. Fierce-21: Phase II study of vofatmab (B-701), a selective inhibitor of FGFR3, as salvage therapy in metastatic urothelial carcinoma (mUC). Journal of Clinical Oncology 2019, 37, 409-409. [CrossRef]
- Trudel, S.; Bergsagel, P.L.; Singhal, S.; Niesvizky, R.; Comenzo, R.L.; Bensinger, W.I.; Lebovic, D.; Choi, Y.; Lu, D.; French, D.; et al. A Phase I Study of the Safety and Pharmacokinetics of Escalating Doses of MFGR1877S, a Fibroblast Growth Factor Receptor 3 (FGFR3) Antibody, in Patients with Relapsed or Refractory t(4;14)-Positive Multiple Myeloma. Blood 2012, 120, 4029. [CrossRef]
- ODonnell, P.; Goldman, J.; Gordon, M.; Shih, K.; Choi, Y.; Lu, D.; Kabbarah, O.; Ho, W.; Rooney, I.; Lam, E. 621 a phase I dose-escalation study of MFGR1877S, a human monoclonal anti-fibroblast growth factor receptor 3 (FGFR3) antibody, in patients (pts) with advanced solid tumors. European Journal of Cancer 2012, 191-192.
- Siefker-Radtke, A.O.; Currie, G.; Abella, E.; Vaena, D.A.; Kalebasty, A.R.; Curigliano, G.; Tupikowski, K.; Andric, Z.G.; Lugowska, I.; Kelly, W.K. FIERCE-22: Clinical activity of vofatamab (V) a FGFR3 selective inhibitor in combination with pembrolizumab (P) in WT metastatic urothelial carcinoma, preliminary analysis. Journal of Clinical Oncology 2019, 37, 4511-4511. [CrossRef]
- Wainberg, Z.A.; Enzinger, P.C.; Kang, Y.-K.; Qin, S.; Yamaguchi, K.; Kim, I.-H.; Saeed, A.; Oh, S.C.; Li, J.; Turk, H.M.; et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): a randomised, double-blind, placebo-controlled, phase 2 study. The lancet oncology 2022, 23, 1430-1440. [CrossRef]
- Catenacci, D.V.T.; Kang, Y.-K.; Saeed, A.; Yamaguchi, K.; Qin, S.; Lee, K.-W.; Kim, I.-H.; Oh, S.C.; Li, J.; Turk, H.M.; et al. FIGHT: A randomized, double-blind, placebo-controlled, phase II study of bemarituzumab (bema) combined with modified FOLFOX6 in 1L FGFR2b+ advanced gastric/gastroesophageal junction adenocarcinoma (GC). Journal of Clinical Oncology 2021, 39, 4010-4010. [CrossRef]
- Smyth, E.C.; Chao, J.; Muro, K.; Yen, P.; Yanes, R.E.; Zahlten-Kumeli, A.; Rha, S.Y. Trial in progress: Phase 3 study of bemarituzumab + mFOLFOX6 versus placebo + mFOLFOX6 in previously untreated advanced gastric or gastroesophageal junction (GEJ) cancer with FGFR2b overexpression (FORTITUDE-101). Journal of Clinical Oncology 2022, 40, TPS4164-TPS4164. [CrossRef]
- Kim, S.-B.; Meric-Bernstam, F.; Kalyan, A.; Babich, A.; Liu, R.; Tanigawa, T.; Sommer, A.; Osada, M.; Reetz, F.; Laurent, D.; et al. First-in-Human Phase I Study of Aprutumab Ixadotin, a Fibroblast Growth Factor Receptor 2 Antibody–Drug Conjugate (BAY 1187982) in Patients with Advanced Cancer. Targeted Oncology 2019, 14, 591-601. [CrossRef]
- Poźniak, M.; Porębska, N.; Krzyścik, M.A.; Sokołowska-Wędzina, A.; Jastrzębski, K.; Sochacka, M.; Szymczyk, J.; Zakrzewska, M.; Otlewski, J.; Opaliński, Ł. The cytotoxic conjugate of highly internalizing tetravalent antibody for targeting FGFR1-overproducing cancer cells. Molecular Medicine 2021, 27. [CrossRef]
- Kas, S.M.; de Ruiter, J.R.; Schipper, K.; Schut, E.; Bombardelli, L.; Wientjens, E.; Drenth, A.P.; de Korte-Grimmerink, R.; Mahakena, S.; Phillips, C.; et al. Transcriptomics and Transposon Mutagenesis Identify Multiple Mechanisms of Resistance to the FGFR Inhibitor AZD4547. Cancer Res 2018, 78, 5668-5679. [CrossRef]
- Zhou, Y.; Wu, C.; Lu, G.; Hu, Z.; Chen, Q.; Du, X. FGF/FGFR signaling pathway involved resistance in various cancer types. J Cancer 2020, 11, 2000-2007. [CrossRef]
- Wang, L.; Šuštić, T.; Leite de Oliveira, R.; Lieftink, C.; Halonen, P.; van de Ven, M.; Beijersbergen, R.L.; van den Heuvel, M.M.; Bernards, R.; van der Heijden, M.S. A Functional Genetic Screen Identifies the Phosphoinositide 3-kinase Pathway as a Determinant of Resistance to Fibroblast Growth Factor Receptor Inhibitors in FGFR Mutant Urothelial Cell Carcinoma. Eur Urol 2017, 71, 858-862. [CrossRef]
- Grygielewicz, P.; Dymek, B.; Bujak, A.; Gunerka, P.; Stanczak, A.; Lamparska-Przybysz, M.; Wieczorek, M.; Dzwonek, K.; Zdzalik, D. Epithelial-mesenchymal transition confers resistance to selective FGFR inhibitors in SNU-16 gastric cancer cells. Gastric Cancer 2016, 19, 53-62. [CrossRef]
- Kim, S.Y.; Ahn, T.; Bang, H.; Ham, J.S.; Kim, J.; Kim, S.T.; Jang, J.; Shim, M.; Kang, S.Y.; Park, S.H.; et al. Acquired resistance to LY2874455 in FGFR2-amplified gastric cancer through an emergence of novel FGFR2-ACSL5 fusion. Oncotarget 2017, 8, 15014-15022. [CrossRef]
- Chae, Y.K.; Ranganath, K.; Hammerman, P.S.; Vaklavas, C.; Mohindra, N.; Kalyan, A.; Matsangou, M.; Costa, R.; Carneiro, B.; Villaflor, V.M.; et al. Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget 2017, 8, 16052-16074. [CrossRef]
- Musolino, A.; Campone, M.; Neven, P.; Denduluri, N.; Barrios, C.H.; Cortes, J.; Blackwell, K.; Soliman, H.; Kahan, Z.; Bonnefoi, H.; et al. Phase II, randomized, placebo-controlled study of dovitinib in combination with fulvestrant in postmenopausal patients with HR+, HER2− breast cancer that had progressed during or after prior endocrine therapy. Breast Cancer Research 2017, 19. [CrossRef]
- Katoh, M. Fibroblast growth factor receptors as treatment targets in clinical oncology. Nature Reviews Clinical Oncology 2019, 16, 105-122. [CrossRef]
- Singleton, K.R.; Hinz, T.K.; Kleczko, E.K.; Marek, L.A.; Kwak, J.; Harp, T.; Kim, J.; Tan, A.C.; Heasley, L.E. Kinome RNAi Screens Reveal Synergistic Targeting of MTOR and FGFR1 Pathways for Treatment of Lung Cancer and HNSCC. Cancer Research 2015, 75, 4398-4406. [CrossRef]
- Scheller, T.; Hellerbrand, C.; Moser, C.; Schmidt, K.; Kroemer, A.; Brunner, S.M.; Schlitt, H.J.; Geissler, E.K.; Lang, S.A. mTOR inhibition improves fibroblast growth factor receptor targeting in hepatocellular carcinoma. British Journal of Cancer 2015, 112, 841-850. [CrossRef]
- Formisano, L.; Lu, Y.; Servetto, A.; Hanker, A.B.; Jansen, V.M.; Bauer, J.A.; Sudhan, D.R.; Guerrero-Zotano, A.L.; Croessmann, S.; Guo, Y.; et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nature Communications 2019, 10. [CrossRef]
- Sobhani, N.; Fassl, A.; Mondani, G.; Generali, D.; Otto, T. Targeting Aberrant FGFR Signaling to Overcome CDK4/6 Inhibitor Resistance in Breast Cancer. Cells 2021, 10, 293. [CrossRef]
- Palakurthi, S.; Kuraguchi, M.; Zacharek, S.J.; Zudaire, E.; Huang, W.; Bonal, D.M.; Liu, J.; Dhaneshwar, A.; DePeaux, K.; Gowaski, M.R.; et al. The Combined Effect of FGFR Inhibition and PD-1 Blockade Promotes Tumor-Intrinsic Induction of Antitumor Immunity. Cancer Immunology Research 2019, 7, 1457-1471. [CrossRef]
- Sweis, R.F.; Spranger, S.; Bao, R.; Paner, G.P.; Stadler, W.M.; Steinberg, G.; Gajewski, T.F. Molecular Drivers of the Non-T-cell-Inflamed Tumor Microenvironment in Urothelial Bladder Cancer. Cancer Immunol Res 2016, 4, 563-568. [CrossRef]
- Abdul-Karim, R.M.; Chaudhry, A.; Patrikidou, A.; Gonzalez, A.F.; Racca, F.; Loriot, Y.; Pouessel, D.; Deville, J.-L.; Lee, H.J.; Cantero, F.; et al. Derazantinib (DZB) in combination with atezolizumab (AZB) in patients with solid tumors: Results from the dose-finding phase Ib substudy of FIDES-02. Journal of Clinical Oncology 2021, 39, 437-437. [CrossRef]
- Touat, M.; Ileana, E.; Postel-Vinay, S.; André, F.; Soria, J.C. Targeting FGFR Signaling in Cancer. Clin Cancer Res 2015, 21, 2684-2694. [CrossRef]
- Kommalapati, A.; Tella, S.H.; Borad, M.; Javle, M.; Mahipal, A. FGFR Inhibitors in Oncology: Insight on the Management of Toxicities in Clinical Practice. Cancers (Basel) 2021, 13. [CrossRef]
- Bensinger, W.; Schubert, M.; Ang, K.-K.; Brizel, D.; Brown, E.; Eilers, J.G.; Elting, L.; Mittal, B.B.; Schattner, M.A.; Spielberger, R.; et al. NCCN Task Force Report: Prevention and Management of Mucositis in Cancer Care. Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw 2008, 6, S-1-S-21. [CrossRef]
- Prensky, C.; Marlow, E.; Gupta, M.; Sales, C.; Kiss, S.; D’Amico, D.J. Reversible Macular Lesions in the Setting of Oral Pan-Fibroblast Growth Factor Inhibitor for the Treatment of Bladder Cancer. Journal of VitreoRetinal Diseases 2018, 2, 111-114. [CrossRef]
- Jogo, T.; Nakamura, Y.; Shitara, K.; Bando, H.; Yasui, H.; Esaki, T.; Terazawa, T.; Satoh, T.; Shinozaki, E.; Nishina, T.; et al. Circulating Tumor DNA Analysis Detects FGFR2 Amplification and Concurrent Genomic Alterations Associated with FGFR Inhibitor Efficacy in Advanced Gastric Cancer. Clinical Cancer Research 2021, 27, 5619-5627. [CrossRef]
- Akbulut, O.; Lengerli, D.; Saatci, O.; Duman, E.; Seker, U.O.S.; Isik, A.; Akyol, A.; Caliskan, B.; Banoglu, E.; Sahin, O. A Highly Potent TACC3 Inhibitor as a Novel Anticancer Drug Candidate. Mol Cancer Ther 2020, 19, 1243-1254. [CrossRef]
| Multi-TKIs | Targets |
|---|---|
| Dovitinib (TKI258) | FGFR, VEGFR, PDGFR inhibitor [111] |
| Lenvatinib (E7080) | VEGFR 1-3, FGFR 1-4, PDGFR α, RET, KIT [112] |
| Lucatinib (E-3810) | FGFR1-2, VEGFR1-3, and PDGFRα-β [113] |
| Pazopanib (GW786034) | FGFR 1-2, VEGFR 1-3, PDGFRα-β, C-kit (stem cell factor receptor) [114] |
| Ponatinib (AP24534) | FGFR 1-4, VEGFR2, PDGFRα, c-SRC, c-Kit, FLT3, RET [115] |
| Agent | NCT | Status | Conditions | Phase |
|---|---|---|---|---|
| Pemigatinib | NCT03914794 | R | Non-muscle invasive bladder cancer (NMIBC) with recurrent low or intermediate risk tumors (as neoadjuvant) | II |
| Pemigatinib | NCT03011372 | A, NR | Previously treated myeloid/lymphoid neoplasms with FGFR1 rearrangement (FIGHT-203) | II |
| Pemigatinib vs Gemcitabine +cisplatin |
NCT03656536 | R | Untreated unresectable or metastatic cholangiocarcinoma with FGFR2 rearrangement (FIGHT-302) | III |
| Pemigatinib | NCT05565794 | R | Intrahepatic cholangiocarcinoma with FGFR2 gene mutation, rearrangement, or translocation after curative local therapy | II |
| Pemigatinib | NCT05267106 |
R | Previously treated glioblastoma or other primary central nervous system tumors harboring activating FGFR1-3 alterations (FIGHT-209) | II |
| Pemigatinib | NCT05253807 | A, NR | Relapsed or refractory advanced non-small cell lung cancer with an FGFR alteration (FIGHT-210) | II |
| Pemigatinib | NCT04659616 | R | Acute myeloid leukemia after initial induction chemotherapy with adverse or intermediate risk cytogenetics | I |
| Futibatinib | NCT04189445 | A, NR | Previously treated advanced or metastatic solid tumors, gastric or gastroesophageal cancers, myeloid or lymphoid neoplasms with FGFR 1-4 rearrangements | II |
| Futibatinib +/- fulvestrant |
NCT04024436 | A, NR | Previously treated metastatic breast cancer with FGFR1 and FGFR2 amplification | II |
| Futibatinib vs gemcitabine + cisplatin |
NCT04093362 | A, NR | Previously untreated advanced cholangiocarcinoma harboring FGFR2 gene rearrangements (FOENIX-CCA3) | III |
| Futibatinib | NCT05727176 | R | Previously treated advanced cholangiocarcinoma with FGFR2 fusion or rearrangement (FOENIX-CCA4) | II |
| Futibatinib + pembrolizumab |
NCT04828486 | R | Advanced or metastatic hepatocellular carcinoma with FGF19 expression | II |
| Futibatinib + pembrolizumab |
NCT04601857 | R | Advanced or metastatic urothelial carcinoma who are not candidates to receive a platinum-based treatment regimen | II |
| Futibatinib + pembrolizumab |
NCT05036681 | R | Previously treated locally advanced or metastatic microsatellite stable endometrial carcinoma | II |
| Infigratinib | NCT04233567 | A, NR | Previously treated advanced or metastatic solid tumors, cholangiocarcinoma and refractory malignant solid neoplasm with FGFR gene mutations | II |
| Infigratinib | NCT04228042 | A, NR | Renal pelvis and upper tract urothelial cancer as neoadjuvant treatment | I/II |
| Erdafitinib | NCT04917809 | R | Recurrent non-Invasive Bladder Cancer with FGFR3 gene mutation after treatment with instillations of BCG or chemotherapy into the bladder | II |
| Erdafitinib | NCT04083976 |
A, NR | Advanced or metastatic solid tumors with FGFR alterations (mutations or gene fusions) after at least one prior line of systemic therapy (RAGNAR) | II |
| Erdafitinib | NCT04754425 | R | Castration-resistant prostate cancer after progressed on second generation androgen receptor targeting agents | II |
| Erdafitinib + fulvestrant + palbociclib | NCT03238196 | A, NR | Previously treated HR positive HER2 negative FGFR amplified metastatic breast cancer | I |
| Fisogatinib | NCT02508467 | A, NR | Hepatocellular carcinoma with FGF19 expression with or without prior tyrosine kinase inhibitors | I |
| Derazantinib + atezolizumab |
NCT05174650 | R | Previously treated advanced or metastatic intrahepatic cholangiocarcinoma with FGFR2 fusions/rearrangements | II |
| Lirafugratinib (RLY4008) |
NCT04526106 |
R | Previously treated unresectable/metastatic intrahepatic cholangiocarcinoma and other advanced tumors with FGFR2 alterations (REFOCUS) | I/II |
| Dovitinib + PARP inhibitor Stenoparib (2X-121) |
NCT05571969 |
R | Advanced Solid Tumors | I |
| Rogaratinib (BAY1163877) + atezolizumab |
NCT03473756 | A, NR | Metastatic or locally advanced cisplatin ineligible patients with urothelial carcinoma and FGFR 1 or 3 alterations (FORT-2) | Ib/II |
| Rogaratinib | NCT04595747 | A, NR | Previously treated advanced or metastatic sarcoma with FGFR 1-4 alterations and in patients with SDH-deficient Gastrointestinal Stromal Tumor (GIST) | II |
| Fexagratinib AZD4547 + tislelizumab |
NCT05775874 | R | Locally advanced or metastatic urothelial cancer with FGFR2/3 alterations in Chinese patient population | II |
| Bemarituzumab |
NCT05325866 | R | Refractory or relapsed advanced or metastatic solid tumors with FGFR2b overexpression after at least one prior line (FORTITUDE-301) | I |
| Bemarituzumab+ mFOLFOX6+ nivolumab vs mFOLFOX6+ nivolumab |
NCT05111626 | R | Untreated advanced or metastatic gastric and gastroesophageal junction (GEJ) cancer with FGFR2b overexpression (FORTITUDE-102) | III |
| Bemarituzumab+ + mFOLFOX vs mFOLFOX |
NCT05052801 | R | Previously treated advanced or metastatic Gastric or GEJ Cancers with FGFR2b overexpression (FORTITUDE-101) | III |
| Bemarituzumab +anti-cancer therapy |
NCT05267470 | A, NR | Advanced or metastatic squamous lung cancer with FGFR2b overexpression (FORTITUDE-201) | I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




