Submitted:
05 December 2023
Posted:
06 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Ethnomedical study
2.2. Phytochemical composition of dry extracts and essential oil
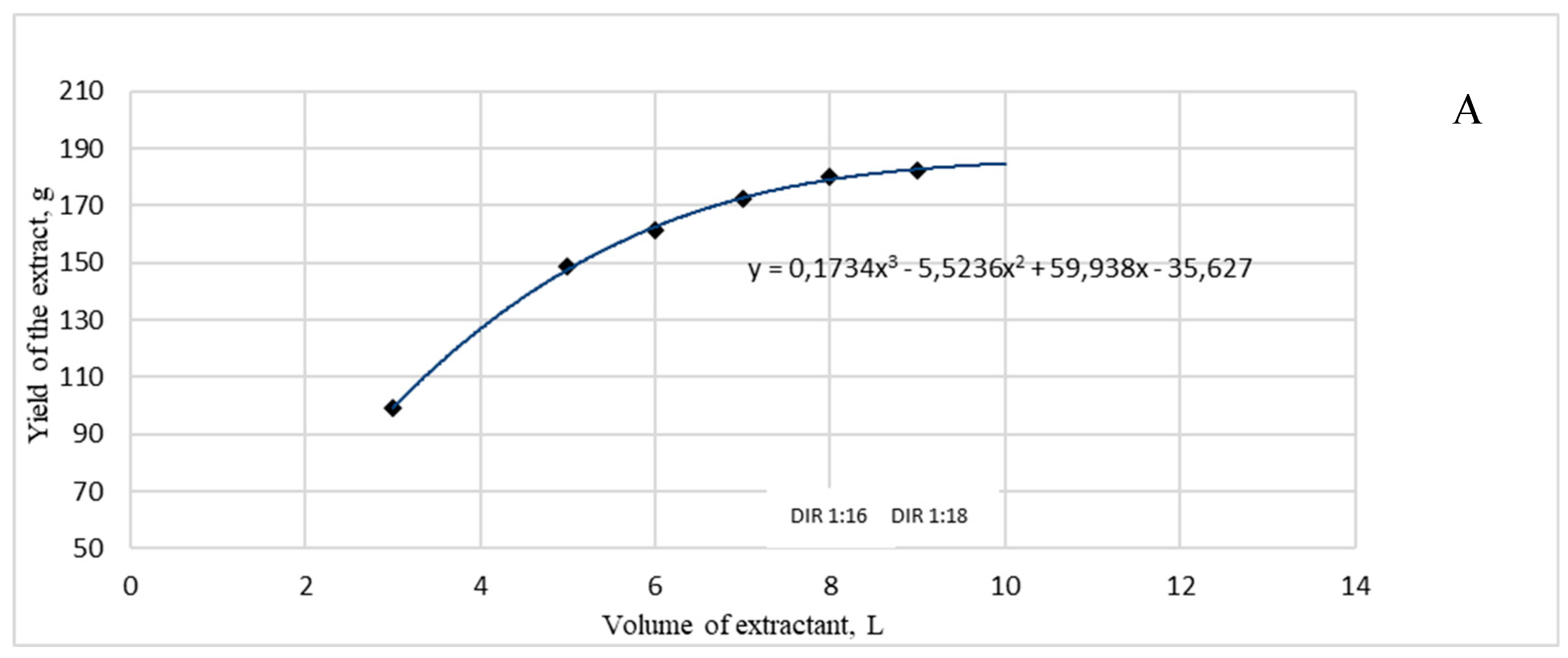
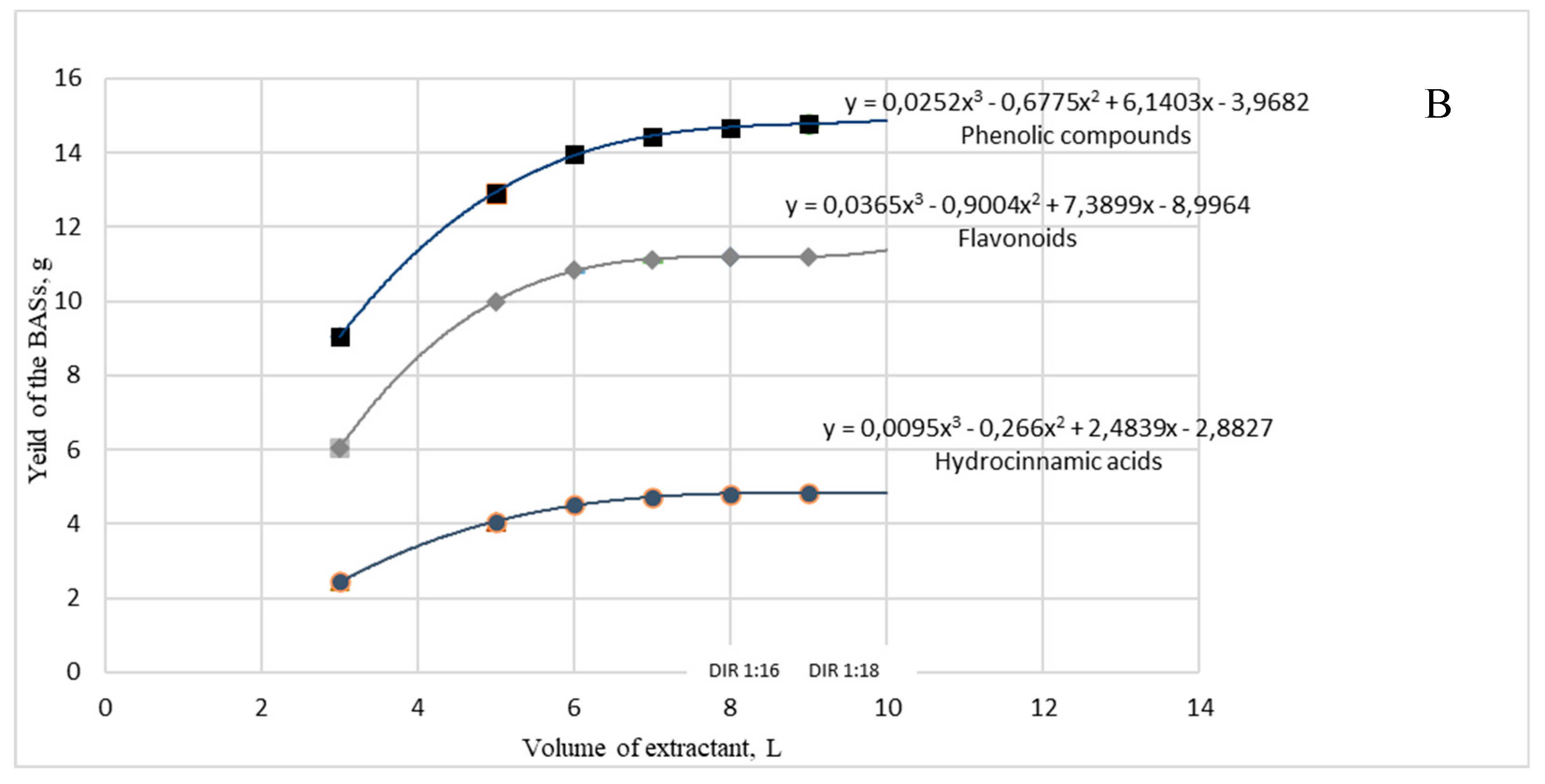
2.3. Optimization of a dry extract G2 preparation
2.4. Pharmacological study on analgesic and soporific activity
2.4.1. Analgesic activity
2.4.2. Soporific activity
3. Discussion
4. Materials and Methods
4.1. Ethnomedical study
4.2. Plant material
4.3. Preparation of extracts
4.4. Phytochemical analysis
4.4.1. Assay of main phytochemicals
4.4.2. Gas-chromatographic analysis of essential oil
4.4.3. Identification of phenolic compounds by UPLC-MS/MS
4.5. Pharmacological study
4.5.1. Analgesic activity
4.5.2. Soporific activity
4.6. Statistical analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sah, A.; Naseef, P.P.; Kuruniyan, M.S.; Jain, G.K.; Zakir, F.; Aggarwal, G. A Comprehensive Study of Therapeutic Applications of Chamomile. Pharmaceuticals 2022, 15, 1284. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Gupta, V.; Mittal, P.; Khokra, S.L.; Kaushik, D. Pharmacological potential of Matricaria recutita—A review. Int. J. Pharm. Sci. Drug Res. 2010, 2, 12–16. [Google Scholar]
- Chamomile. Drugs and Lactation Database [LactMed); National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile [Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef]
- Parra, J.; García-Barrantes, P.M.; Rodríguez, G.; Badilla, B. Physicochemical and chromatographic method of characterization of Matricaria recutita tinctures. Journal of Pharmacy and Pharmacognosy Research 2016, 4, 18–24. [Google Scholar] [CrossRef]
- Martin, N.; Madrid-López, C.; Villalba-Méndez, G.; Talens-Peiró, L. New Techniques for assessing critical raw material aspects in energy and other technologies. Environment. Sci. Technol. 2022, 56, 17236–17245. [Google Scholar] [CrossRef]
- Koshovyi, O.N.; Vovk, G.V.; Akhmedov, E.Yu.; Komissarenko, A.N. The study of the chemical composition and pharmacological activity of Salvia officinalis leaves extracts getting by complex processing. Azerbaijan Pharmaceutical and Pharmacotherapy Journal 2015, 15, 30–34. [Google Scholar]
- Shanaida, M.; Hudz, N.; Jasicka-Misiak, I.; Wieczorek, PP. Polyphenols and pharmacological screening of a Monarda fistulosa L. dry extract based on a hydrodistilled residue by-product. Front Pharmacol. 2021, 12, 563436. [Google Scholar] [CrossRef]
- Raal, A.; Arak, E.; Orav, A.; Ivask., K. Comparision of the essential oil from Matricaria recutita L. of different origins. Ars Pharmaceutica 2003, 44, 159–165. [Google Scholar]
- Orav, A.; Raal, A.; Arak, E. Content and composition of the essential oil of Chamomilla recutita [L.) Rauschert from some European countries. Nat. Prod. Res. 2010, 24, pp–48. [Google Scholar] [CrossRef] [PubMed]
- Raal, A.; Orav, A.; Püssa, T.; Valner, K.; Malmiste, B.; Arak, E. Content of essential oil, terpenoids and polyphenols in commercial chamomile [Chamomilla recutita L. Rauschert) teas from different countries. Food Chem. 2012, 131, 632–638. [Google Scholar] [CrossRef]
- Koshovyi, O.M.; Kukhtenko, O.S.; Kovalova, A.M.; Komissarenko, A.M.; Vinnyk, O.V.; Sholom, Yu.G. Optimization of the biologically active substances extraction process from eucalypt leaves: multiplicity of extraction. Current issues of pharmaceutical and medical science and practice 2010, XIII, 47–49. [Google Scholar]
- Kafarov, V.V. Methods of cybernetics in chemistry and chemical technology; M.: Chemistry, USSR, 1976. [Google Scholar]
- Marzullo, L.; Ochkur, O.; Renai, L.; Gotti, R.; Koshovyi, O.; Furlanetto, S.; Orlandini, S.; Del Bubba, M. Quality by design in optimizing the extraction of (poly)phenolic compounds from Vaccinium myrtillus berries. J. Chromatograpgy A 2022, 1677, 463329. [Google Scholar] [CrossRef] [PubMed]
- Sak, K.; Jürisoo, K.; Raal, A. Estonian folk traditional experiences on natural anticancer remedies: From past to the future. Pharm. Biol. 2014, 52, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Raal, A.; Nguyen, T.H.; Ho, V.D.; Do, TT. Selective cytotoxic action of Scots pine [Pinus sylvestris L.) needles extract in human cancer cell lines. Pharmacogn. Mag. 2015, 11, S290–S295. [Google Scholar] [CrossRef] [PubMed]
- Raal, A.; Jaama, M.; Utt, M.; Püssa, T.; Žvikas, V.; Jakštas, V.; Koshovyi, O.; Nguyen, K. V.; Nguyen, T. H. The phytochemical profile and anticancer activity of Anthemis tinctoria and Angelica sylvestris used in Estonian ethnomedicine. Plants 2022, 11, 994. [Google Scholar] [CrossRef]
- Raal, A.; Sõukand, R. Classification of remedies and medicinal plants of Estonian ethnopharmacology. Trames 2005, 9, 259–267. [Google Scholar] [CrossRef]
- Sõukand, R.; Raal, A. Data on medicinal plants in Estonian folk medicine: collection, formation and overview of previous researches. Folklore 2005, 30, 171–198. [Google Scholar]
- Herba: Historical Estonian Folk Medicine Botanical Database. Available online: https://herba.folklore.ee/ (accessed on 11 November 2023). (In Estonian).
- Joumaa, M.M.El.; Borjac, J.M. Matricaria chamomilla: A valuable insight into recent advances in medicinal uses and pharmacological activities. Phytochem. Rev. 2022, 21, 1913–1940. [Google Scholar] [CrossRef]
- Catani, M.V.; Rinaldi, F.; Tullio, V.; Gasperi, V.; Savini, I. Comparative analysis of phenolic composition of six commercially available chamomile [Matricaria chamomilla L.) extracts: Potential biological implications. Int. J. Mol. Sci. 2021, 22, 10601. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Romani, A.; Pinelli, P.; Vinvieri, F.F.; Prucher, D. Characterisation of M. recutita L. flower extract by HPLCMS and HPLC-DAD analysis. Chromatographia 2000, 51, 301–307. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, Z.; Chang, X.; Xue, H.; Yahefu, W.; Zhang, X. 4-Hydroxyphenylacetic acid prevents acute APAP-induced liver injury by increasing phase II and antioxidant enzymes in mice. Front. Pharmacol 2018, 9, 653. [Google Scholar] [CrossRef]
- European Pharmacopoeia, Supplement 11.2 ed; Strasbourg: Council of Europe, 2023.
- Orav, A.; Sepp, J.; Kailas, T.; Müürisepp, M.; Arak, E.; Raal, A. Composition of essential oil of aerial parts of Chamomilla suaveolens from Estonia. Nat. Prod. Comm. 2010, 5, 133–136. [Google Scholar] [CrossRef]
- Pino, J.A.; Bayat, F.; Marbot, R.; Aguero, J. Essential oil of chamomile Chamomilla recutita [L.) Rausch. From Iran. J. Essent. Oil Res. 2002, 14, 407–408. [Google Scholar] [CrossRef]
- Matos, F.J.A.; Machado, M.I.L.; Alencar, J.W.; Craveiro, A.A. Constituents of brazilian chamomile oil. J. Essent. Oil Res. 2011, 5, 337–339. [Google Scholar] [CrossRef]
- Ramadan, M.; Goeters, S.; Watzer, B.; Krause, E.; Lohmann, K.; Bauer, R.; Hempel, B.; Imming, P. Chamazulene carboxylic acid and matricin: A natural profen and its natural prodrug, identified through similarity to synthetic drug substances. J. Nat. Prod. 2006, 69, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Chaves, P.; Hocayen, P.; Dallazen, J.L.; de Paula Werner, M.F.; Iacomini, M.; Andreatini, R.; Cordeiro, L. Chamomile tea: Source of a glucuronoxylan with antinociceptive, sedative and anxiolytic-like effects. Int. J. Biol. Macromol. 2020, 164, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Heo, Y.; Kim, Y.C. Effect of German chamomile oil application on alleviating atopic dermatitis-like immune alterations in mice. J. Vet. Sci. 2010, 11, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, N.; Shukla, S.; Srivastava, J.K.; Gupta, S. Chamomile: An anti-inflammatory agent inhibits inducible nitric oxide synthase expression by blocking RelA/p65 activity. Int. J. Mol. Med. 2010, 26, 935–940. [Google Scholar]
- Fan, X.; Du, K.; Li, N.; Zheng, Z.; Qin, Y.; Liu, J.; Sun, R.; Su, Y. Evaluation of anti-nociceptive and anti-inflammatory effect of Luteolin in mice. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Flemming, M.; Kraus, B.; Rascle, A.; Jürgenliemk, G.; Fuchs, S.; Fürst, R.; Heilmann, J. Revisited anti-inflammatory activity of matricine in vitro: Comparison with chamazulene. Fitoterapia 2015, 106, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Singh, M.; Dubey, V.; Srivastava, S.; Luqman, S.; Bawankule, D.U. -[-)-bisabolol reduces pro-inflammatory cytokine production and ameliorates skin inflammation. Curr. Pharm. Biotechnol. 2014, 15, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Tomi´c, M.; Popovi´c, V.; Petrovi´c, S.; Stepanovi´c-Petrovi´c, R.; Micov, A.; Pavlovi´c-Drobac, M.; Couladis, M. Antihyperalgesic and antiedematous activities of bisabolol-oxides-rich matricaria oil in a rat model of inflammation. Phytother. Res. 2014, 28, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Shanaida, M.; Hudz, N.; Korzeniowska, K.; Wieczorek, P. Antioxidant activity of essential oils obtained from aerial part of some Lamiaceae species. Internat. J. Green Pharm. 2018, 12, 200–204. [Google Scholar]
- Hossain, M. F.; Talukder, B.; Rana, M.N.; Tasnim, R.; Nipun, T.S.; Uddin, S. M.; Hossen, S. M. In vivo sedative activity of methanolic extract of Stericulia villosa Roxb. leaves. BMC Complement. Alternat. Med. 2016, 16, 398. [Google Scholar] [CrossRef] [PubMed]
- Staufenbiel, S.M.; Penninx, B.W.; Spijker, A.T.; Elzinga, B.M.; van Rossum, E.F. Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinol. 2013, 38, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, L.L.; Carvalho, J.P.; Tyrka, A.R.; Wier, L.M.; Mello, A.F.; Mello, M.F.; Anderson, G.M.; Wilkinson, C.W.; Price, L.H. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatry 2007, 62, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Nakamura, K.; Furukawa, K.; Konishi, Y.; Ogino, T.; Higashiura, K.; Yago, H.; Okamoto, K.; Otsuka, M. A new nonpeptide tachykinin NK1 receptor antagonist isolated from the plants of Compositae. Chem. Pharm. Bull. 2002, 50, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Miura, T.; Mimaki, Y.; Sashida, Y. Effect of inhalation of chamomile oil vapour on plasma ACTH level in ovariectomized-rat under restriction stress. Biol. Pharm. Bull. 1996, 19, 1244–1246. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; Li, Q.S.; Xie, S.X.; Mao, J.J. Putative antidepressant effect of chamomile [Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J. Altern. Complement. Med. 2020, 26, 813–819. [Google Scholar] [CrossRef]
- Hashemi, P.; FahanikBabaei, J.; Vazifekhah, S.; Nikbakht, F. Evaluation of the neuroprotective, anticonvulsant, and cognitionimprovement effects of apigenin in temporal lobe epilepsy: Involvement of the mitochondrial apoptotic pathway. Iran. J. Basic Med. Sci. 2019, 22, 752–758. [Google Scholar]
- Dobrochaeva, D.N.; Kotov, M.I.; Prokudin, Y.N.; Barbarich, A.I. Key to Higher Plants of Ukraine; Naukova Dumka: Kyiv, Ukraine, 1999. [Google Scholar]
- Koshovyi, O.M.; Zagayko, A.L.; Kolychev, I.O.; Akhmedov, E.Yu.; Komissarenko, A.N. Phytochemical study of the dry extract from bilberry leaves. Azerbaijan Pharmaceutical and Pharmacotherapy Journal 2016, 16, 18–23. [Google Scholar]
- Koshovyi, O.; Granica, S.; Piwowarski, J.P.; Stremoukhov, O.; Kostenko, Y.; Kravchenko, G.; Krasilnikova, O.; Zagayko, A. Highbush blueberry [Vaccinium corymbosum L.) leaves extract and its modified arginine preparation for the management of metabolic syndrome – chemical analysis and bioactivity in rat model. Nutrients 2021, 13, 2870. [Google Scholar] [CrossRef] [PubMed]
- Huzio, N.; Grytsyk, A.; Raal, A.; Grytsyk, L.; Koshovyi, O. Phytochemical and pharmacological research in Agrimonia eupatoria L. herb extract with anti-Inflammatory and hepatoprotective properties. Plants 2022, 11, 2371. [Google Scholar] [CrossRef] [PubMed]
- Ilina, T.; Skowronska, W.; Kashpur, N.; et al. Immunomodulatory activity and phytochemical profile of infusions from cleavers herb. Molecules 2020, 25, 3721. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudone, L.; Petrikaite, V. Phenolic fractions from Vaccinium vitis-idaea L. and their antioxidant and anticancer activities assessment. Antioxidants 2020, 9, 1261. [Google Scholar] [CrossRef]
- European Convention for the protection of vertebrate animals used for experimental and other scientific purposes. 21999A0824(01). Official Journal L 222, 24/08/1999 P. 0031 – 0037, Strasbourg, 1986.
- Council Directive 2010/63/EU of 22 September 2010 on the protection of animals used for scientific purposes, 2010. Official Journal of the European Communities. L 276. P. 33–79.
- The Law of Ukraine “On the Protection of Animals from Cruel Treatment” dated 12/15/2009. Available online: https://zakon.rada.gov.ua/laws/show/3447-15#Text (accessed on 8 September 2021).
- The Order of the Ministry of Health of Ukraine, No. 944 dated 14.12.2009 “On approval of the Procedure for Preclinical Study of Medicinal Products and Examination of Materials of Preclinical Study of Medicinal Products”. Available online: https://zakon.rada.gov.ua/laws/show/z0972-01#Text (accessed on 1 February 2010).
- Council Directive of 18 December 1986 on the Lows, Regulating the Application of Principles of Good Laboratory Practice and the Verification of Their Applications for Tests on Chemical Substances [87/18/EEC)/The Rules Governing Medicinal Products in the European Community; Commission of the European Communities: Brussels, Belgium, 1991; Volume 1, pp. 145–146. 18 December.
- Ihnatova, T.; Kaplaushenko, A.; Frolova, Yu.; Pryhlo, E. Synthesis and antioxidant properties of some new 5-phenethyl-3-thio-1,2,4-triazoles. Pharmacia 2021, 68, 129–133. [Google Scholar] [CrossRef]
- State Pharmacopoeia of Ukraine, 2nd ed.; Ukrainian Scientific Pharmacopoeial Center of Drugs Quality: Kharkiv, Ukraine, 2015.
| Category | Number of records | Percentage of total, % |
|---|---|---|
| Unspecified | 45 | 30.0 |
| Respiratory tract diseases | 45 | 30.0 |
| Ocular diseases | 23 | 15.3 |
| Inflammation | 9 | 6.0 |
| Trauma | 8 | 5.3 |
| Pain | 5 | 3.3 |
| Infection diseases | 5 | 3.3 |
| Other | 5 | 3.3 |
| Sedative | 3 | 2.0 |
| Spasms | 2 | 1.3 |
| Substance | Content in the extract | ||
|---|---|---|---|
| G1 | G2 | G3 | |
| UPLC-MS/MS, µg/g of dry extract | |||
| Neochlorogenic acid | 1672.30 ± 85.39 | 444.94 ± 20.16 | 441.14 ± 13.32 |
| Luteolin | 83.99 ± 12.65 | 310.93 ± 22.73 | 74.87 ± 3.871 |
| Isoquercitrin | 477.46 ± 68.82 | 921.16 ± 85.20 | 42.15 ± 14.12 |
| Cryptochlorogenic acid | 16.51 ± 2.42 | 80.74 ± 13.48 | 0 |
| Luteolin-4-O-glucoside | 16.98 ± 2.86 | 45.11 ± 3.67 | 0 |
| Chlorogenic acid | 3930.89 ± 224.37 | 11742.31 ± 376.34 | 1280.86 ± 98.96 |
| Quercetin | 18.87 ± 1.20 | 172.15 ± 12.01 | 9.37 ±1.03 |
| Isorhamnetin-3-O-rutinoside | 9.34 ± 0.56 | 15.40 ± 1.60 | 0 |
| Isorhamnetin-3-glucoside | 257.7 ± 27.04 | 410.75 ± 52.07 | 46.43 ± 4.38 |
| Luteolin-3,7-diglucoside | 18.11 ± 4.59 | 20.72 ± 1.88 | 0 |
| Vanilic acid | 175.96 ± 13.28 | 86.58 ± 5.54 | 71.59 ± 7.22 |
| Caffeic acid | 42.98 ± 3.82 | 43.04 ± 3.22 | 33.46 ± 2.95 |
| 3,4-Dihydroxyphenylacetic acid | 376.15 ± 27.,47 | 184.05 ± 13.38 | 159.71 ± 12.16 |
| Isorhamnetin | 17.99 ± 1.89 | 125,32 ± 12.71 | 15.51 ± 2.27 |
| Apigenin | 84.93 ± 4.88 | 578.65 ± 63.91 | 12.98 ± 2 |
| Kaempherol-3-O-glucoside | 34.79 ± 1.46 | 50.76 ± 2.10 | 0 |
| Rutin | 45.34 ± 5.55 | 126.49 ± 5.73 | 0 |
| Hyperoside | 224.42 ± 21.56 | 366.82 ± 21.21 | 65.15 ± 2.36 |
| Luteolin-7-O-glucoside | 616.65 ± 63.46 | 1061.82 ± 83.68 | 123.64 ± 31.82 |
| 4.5-Dicaffeoylquinic acid | 3565.27 ± 266.90 | 4912.17 ± 416.85 | 541.70 ± 26.44 |
| 3.5-Dicaffeoylquinic acid | 1823.72 ± 136.53 | 2512.69 ± 213.23 | 277.09 ± 13.52 |
| 3.4-Dicaffeoylquinic acid | 3739.46 ± 279.94 | 5152.17 ± 437.22 | 568.17 ± 27.73 |
| Spectrophotometry, % | |||
| Phenolic compounds | 6.19 ± 0.29 | 9.70 ± 0.52 | 2.27 ± 0.11 |
| Hydrocinnamic acids | 1.57 ± 0.09 | 3.47 ± 0.15 | 0.21 ± 0.01 |
| Flavonoids | 3.63 ± 0.11 | 9.92 ± 0.32 | 0.45± 0.01 |
| RI (DB-5) | Compound | Content in the oil, % | References [9,10,11] |
|---|---|---|---|
| 1455 | (E)-ß-Farnesene | 4.30 | 1450-1456 [9,10,11] |
| 1570 | Spathulenol | 2.24 | 1568-1570 [9,10,11] |
| 1649 | α-Bisabolol oxide B | 22.00 | 1648-1649 [9,10,11] |
| 1656 | β-Bisabolol | 1.21 | 1640-1656 [9,10,11] |
| 1673 | α-Bisabolone oxide A | 15.92 | 1670-1674 [9,10,11] |
| 1740 | α-Bisabolol oxide A | 44.01 | 1734-1741 [9,10,11] |
| 1876 | cis-Enyne-bicycloether | 4.13 | 1867-1876 [9,10,11] |
| 1978 | Hexadecanoic acid | 1.70 | 1977 [11] |
| 2143 | cis-Linoleic acid | 1.79 | 2143 [11] |
| In total | 97.30 | ||
| Extraction stage | Dry residue, % | Content (%) in the dry residue | ||
|---|---|---|---|---|
| Phenolic compounds | Hydrocinnamic acids | Flavonoids | ||
| 1 | 5.00 ± 0.28 | 9.13 ± 0.34 | 2.46 ± 0.12 | 6.12 ± 0.19 |
| 2 | 2.57 ± 0.13 | 7.80 ± 0.10 | 3.24 ± 0.17 | 7.89 ± 0.04 |
| 3 | 1.4 ± 0.06 | 8.29 ± 0.27 | 3.79 ± 0.23 | 6.89 ± 0.26 |
| 4 | 1.1 ± 0.08 | 4.39 ± 0.24 | 1.91 ± 0.11 | 2.53 ± 0.04 |
| 5 | 0.8 | 2.85 ± 0.13 | 0.72 ± 0.04 | 0.58 ± 0.03 |
| 6 | 0.2 | 1.35± 0.05 | 0.99 ± 0.03 | 0.28 ± 0.01 |
| Agent | Group | Dose, mg/kg | The time of discomfort occurrence (seconds) / Analgesic activity (%) in relation to [control] and (reference drug) after administration in | ||||
|---|---|---|---|---|---|---|---|
| 30 min | 60 min | 120 min | 180 min | 240 min | |||
| Intact animals | 1 | 7.20±0.29 | 7.10±0.61 | 7.08±0.27 | 7.15±0.65 | 6.73±0.94 | |
| Extract G1 | 2 | 25 | 7.85±0.39 / [9%] (-25%) * |
8.48±0.39 / [19%] (-18%) * |
8.60±0.34 / [21%] # (-18%) * |
7.97±0.21 / [11%] (-16%) |
7.67±0.34 / [14%] (-8%) |
| 3 | 50 | 9.65±0.45 / [34] # (-8%) |
9.83±0.53 / [38%] # (-5%) |
9.63±0.50 / [36%] # (-9%) |
8.77±0.27 / [23%] (-7%) |
8.23±0.28 / [22%] (-1%) |
|
| 4 | 100 | 9.80±0.59 / [36%] # (-6%) |
10.13±0.61/ [43%] # (-2%) |
9.97±0.59 / [41%] # (-5%) |
9.32±0.57 / [30%] # (-1%) |
8.28±0.37 / [23%] (-1%) |
|
| Extract G2 | 5 | 25 | 9.63±0.54 / [34%] # (-8%) |
9.97±0.60 / [40%] # (-4%) |
8.65±0.48 / [22%] # (-18%) |
7.98±0.12 / [12%] (-16%) |
8.43±0.21 / [25%] (1%) |
| 6 | 50 | 11.43±0.85 / [59%] # (9%) |
11.83±0.77 / [67%] # (14%) |
11.72±0.73 / [65%] # (11%) |
11.13±0.73 / [56%] # (18%) |
8.67±0.31 / [29%] (4%) |
|
| 7 | 100 | 12.50±0.36 / [74%] # (20%) * |
12.52±0.31 / [76%] # (21%) * |
12.47±0.30 / [76%] # (18%) * |
9.63±0.50 / [35%] # (2%) |
9.02±0.39 / [34%] (8%) |
|
| Extract G3 | 8 | 25 | 8.32±0.39 / [16%] # (-20%) * |
8.88±0.31 / [25%] (-14%) |
8.30±0.17 / [17%] # (-21%) * |
8.13±0.30 / [14%] (-14%) |
7.72±0.35 / [15%] (-8%) |
| 9 | 50 | 7.80±0.48 / [8%] (-25%) * |
8.33±0.45 / [17%] (-20%) |
8.33±0.37 / [18%] (-21%) |
7.95±0.34 / [11%] (-16%) |
7.70±0.24 / [14%] (-8%) |
|
| 10 | 100 | 8.80±0.64 / [22%] (-16%) |
8.88±0.65 / [25%] (-14%) |
8.67±0.55 / [22%] (-18%) |
8.38±0.54 / [17%] (-11%) |
8.28±0.51 / [23%] (-1%) |
|
| Acetaminophen | 11 | 50 | 10.54±0.73 | 10.38±0.62 | 10.53±0.74 | 9.45±0.60 | 8.35±0.36 |
| Agent | Group | Dose, mg/kg | Average duration of a sleep, min | Soporific effect, % |
|---|---|---|---|---|
| Control group | 1 | 40 | 104.83±8.76 | 100% |
| Extract G1 | 2 | 25 | 140.33±6.52*# | 133.9% |
| 3 | 50 | 201.83±4.69* | 192.5% | |
| 4 | 100 | 148.83±3.88*# | 142.0% | |
| Extract G2 | 5 | 25 | 186.33±6.12* | 177.7% |
| 6 | 50 | 227.83±7.59* | 217.3% | |
| 7 | 100 | 190.00±6.97* | 181.2% | |
| Extract G3 | 8 | 25 | 177.83±4.00*# | 169.6% |
| 9 | 50 | 136.83±4.74*# | 130.5% | |
| 10 | 100 | 166.33±9.93*# | 158.7% | |
| Valerian extract | 11 | 2.15 | 204.17±8.39 | 194.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





