Submitted:
04 December 2023
Posted:
05 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
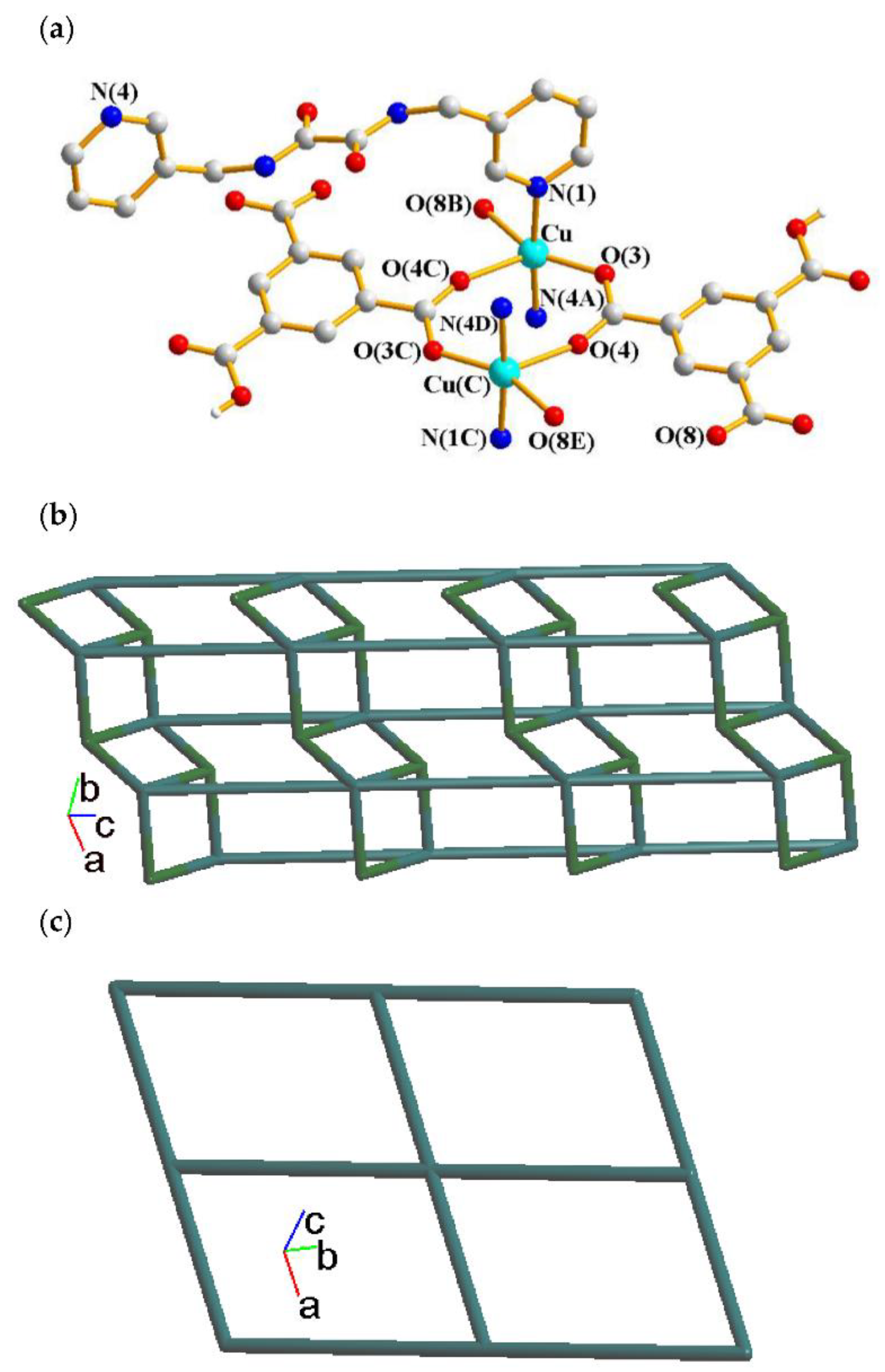
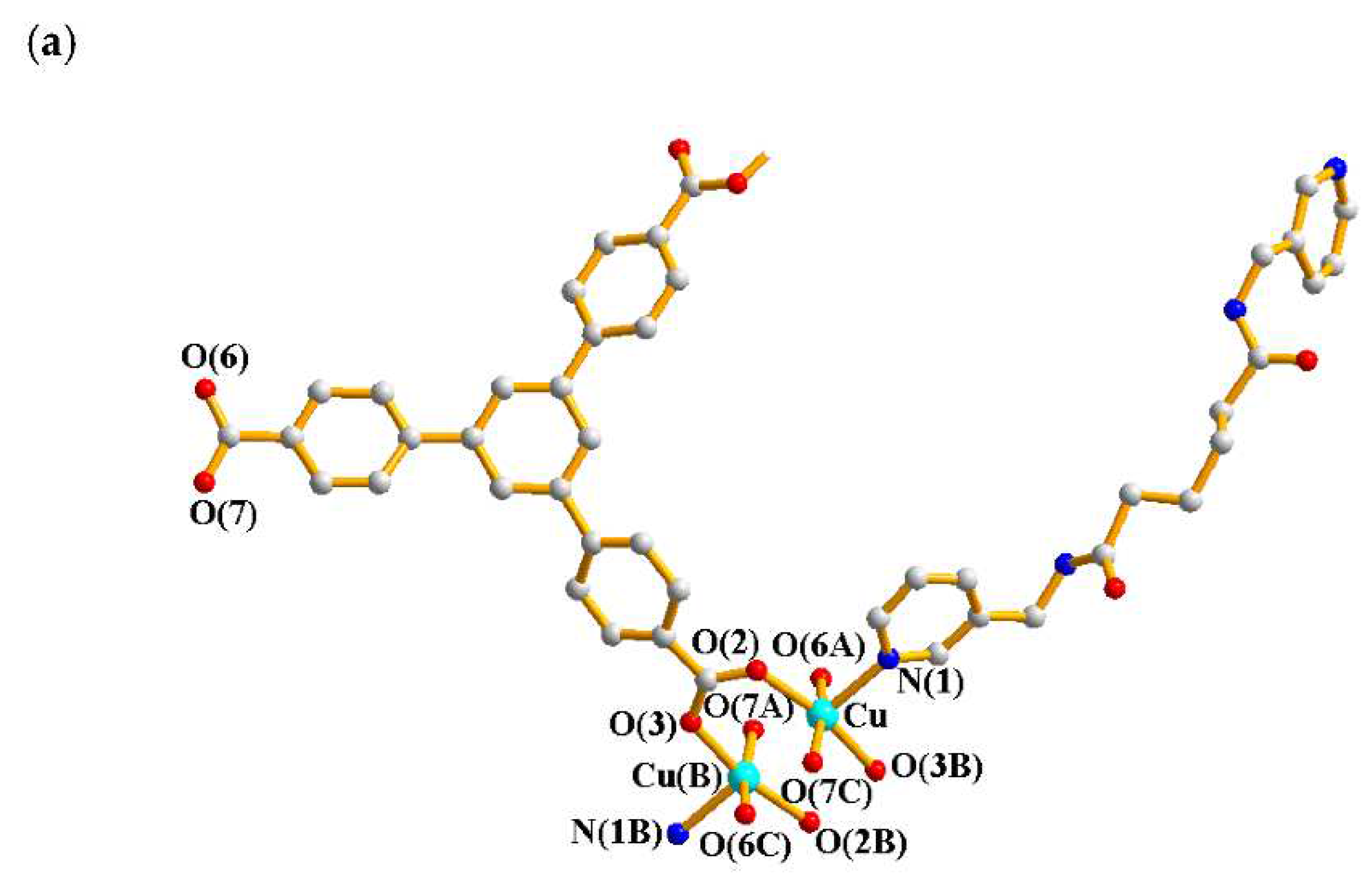
2.1. Structure of 1
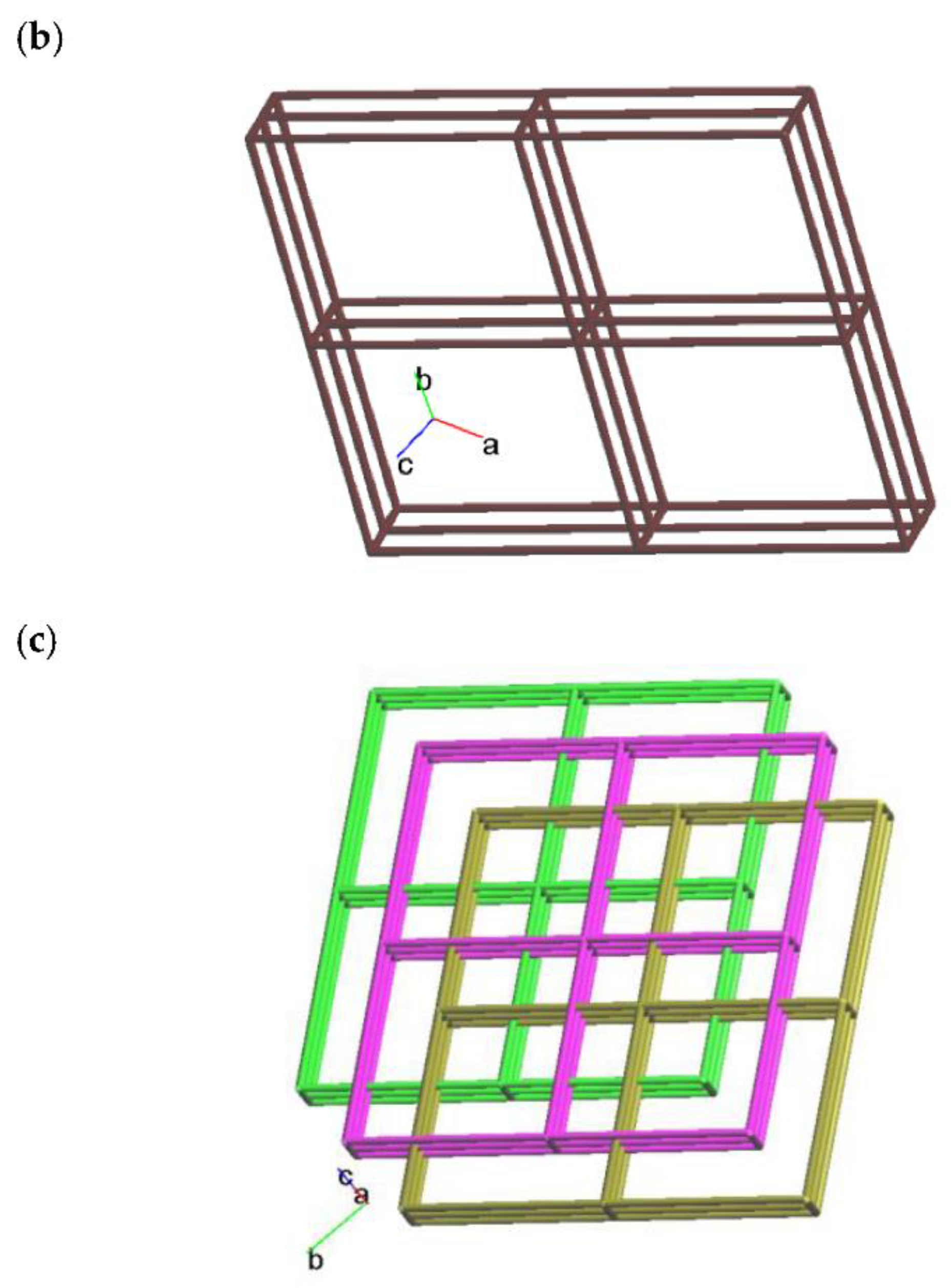
2.2. Structure of 2
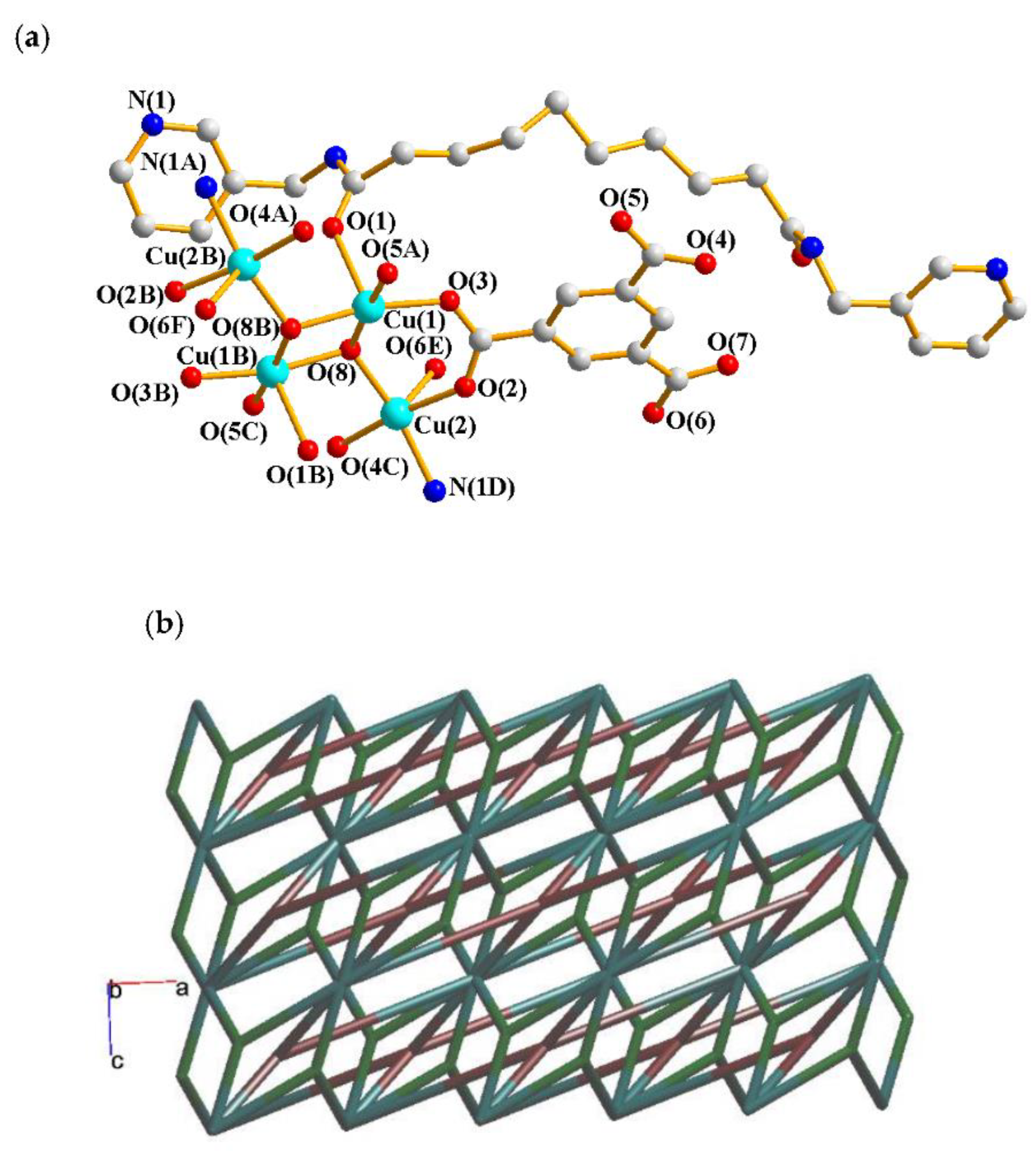
2.3. Structure of 3
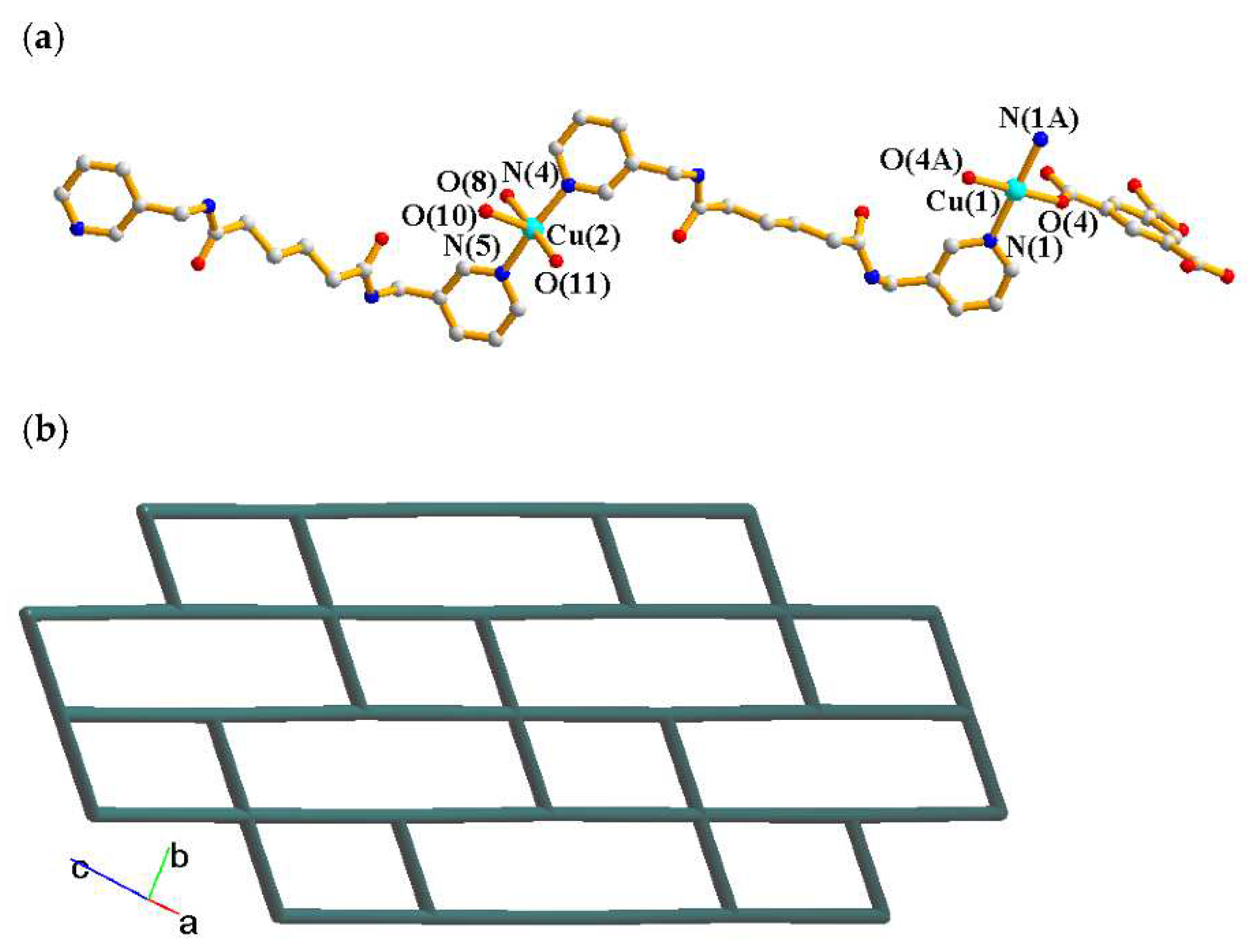
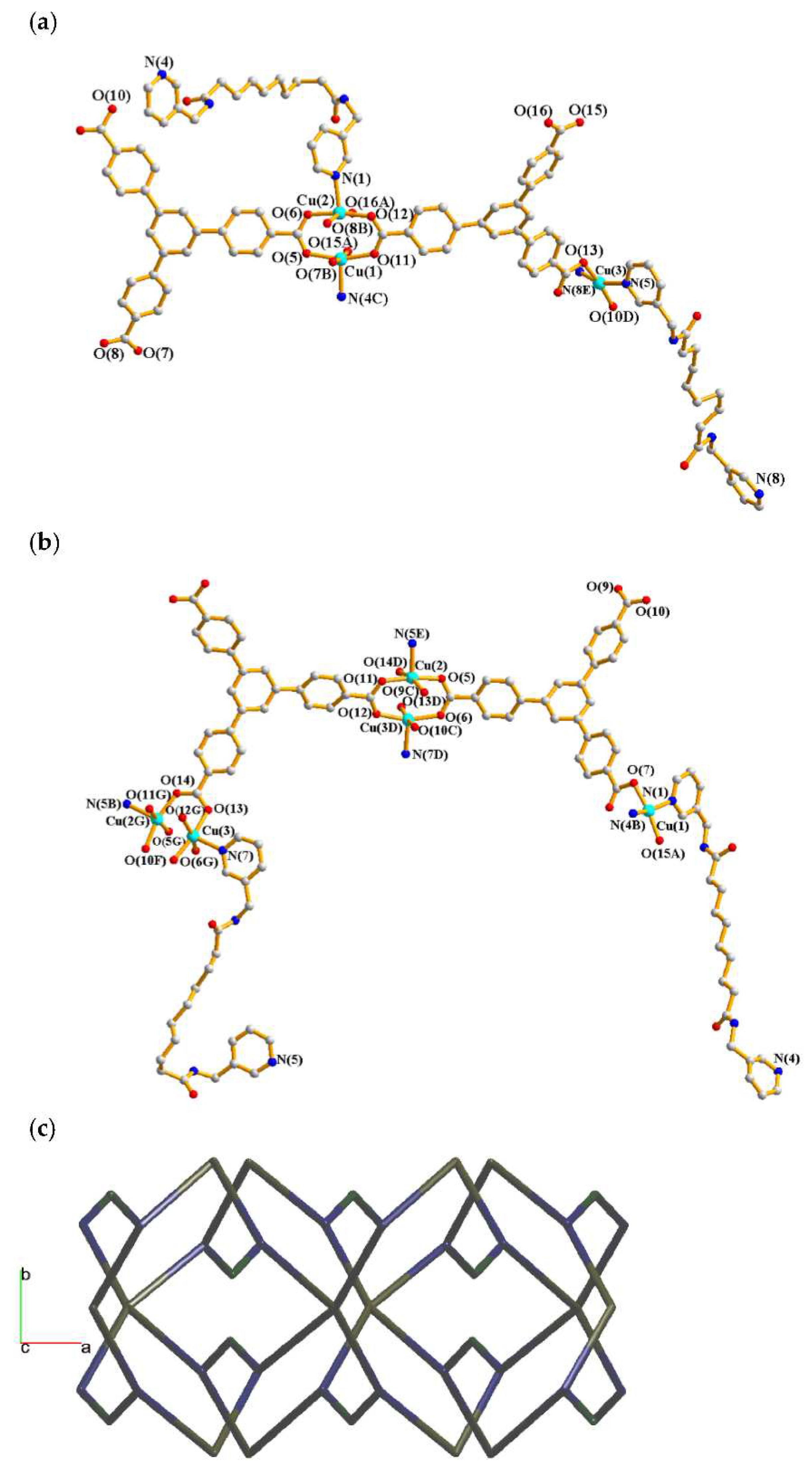
2.4. Structure of 4
2.5. Structure of 5 and 6
2.6. Ligand Conformations and Coordination Modes
2.7. Powder X-ray Analysis
2.8. Thermal Properties
2.9. Iodine Adsorption
2.10. Energy Dispersive X-ray (EDX) Analysis
2.11. Gas Adsorption
3. Materials and Methods
3.1. Materials
3.2. Preparations
3.3.1.{[. Cu(L1)(1,3,5-HBTC)]·H2O}n, 1
3.2.2.{[. Cu1.5(L2)1.5(1,3,5-BTC)(H2O)2]·6.5H2O}n, 2
3.3.3.[. Cu(L2)0.5(1,3,5-HBTB)]n, 3
3.3.4.[. Cu4(L3)(OH)2(1,3,5-BTC)2]n, 4
3.3.5.{[. Cu3(L3)2(1,3,5-BTB)2]·2.5MeOH·2H2O}n, 5, and {[Cu3(L3)2(1,3,5-BTB)2 ]·DMF·2H2O}n, 6
3.5. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiekink, E.R.; Vittal, J.J. Frontiers in Crystal Engineering. 2006.
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers Design, Analysis and Application; The Royal Society of Chemistry: London, 2009.
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.X.; Zhang, W.H.; Abrahams, B.F.; Braunstein, P.; Lang, J.P. Fabrication of Photoactuators: Macroscopic Photomechanical Responses of Metal-Organic Frameworks to Irradiation by UV Light. Angew. Chem. Int. Ed. 2019, 58, 9453–9458. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.X.; Chen, H.H.; Zhang, W.H.; Day, G.S.; Lang, J.P.; Zhou, H.C. Photoinduced nonlinear contraction behavior in metalorganic frameworks. Chem. Eur. J. 2019, 25, 8543–8549. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lang, J.-P.; Abrahams, B.F. Highly Efficient Separation of a Solid Mixture of Naphthalene and Anthracene by a Reusable Porous Metal−Organic Framework through a Single-Crystal-to-Single Crystal Transformation. J. Am. Chem. Soc. 2011, 133, 11042. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Chen, X.-R.; Chen, H.-X.; Niu, Z.; Hirao, H.; Braunstein, P.; Lang, J.-P. Ultrafast Luminescent Light-Up Guest Detection Based on the Lock of the Host Molecular Vibration. J. Am. Chem. Soc. 2020, 142, 6690–6697. [Google Scholar] [CrossRef]
- Kedar, B.T.; Chen, J.-D. Crystal engineering of coordination polymers containing flexible bis-pyridyl-bis-amide ligands. CrystEngComm. 2015, 17, 4611–4626. [Google Scholar]
- Mondal, S.; Dastidar, P. Mixed Ligand Coordination Polymers for Metallogelation and Iodine Adsorption. Cryst. Growth Des. 2019, 19, 470–478. [Google Scholar] [CrossRef]
- Chen, C.-J.; Chen, C.-L.; Lee, W.-T.; Hu, J.-H.; Chhetri, P.M.; Chen, J.-D. , Coordination polymers with a semi-rigid spacer ligand in a mixed system: Roles of benzene-1, 3, 5-tricarboxylate anions. J. Mol. Struct. 2021, 1244, 131252. [Google Scholar] [CrossRef]
- Hu, J.H.; Liu, Y.C.; Chen, J.-D. Cobalt (II) coordination polymers constructed from bis (N-pyrid-3-ylmethyl) adipoamide and polycarboxylic acids: Reversible structural transformation upon proton delivery and removal. CrystEngComm 2021, 23, 7471–7478. [Google Scholar] [CrossRef]
- Hu, J.H.; Liu, Y.C.; Liu, Y.H.; Chen, J.-D. Structural transformations in cobalt (ii) coordination polymers constructed from flexible N, N′-bis (3-pyridylmethyl) sebacoamide and benzene-1, 3, 5-tricarboxylic acid. CrystEngComm 2022, 24, 4120–4127. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Huang, W.-C.; Chen, W.-H.; Chen, C.-L.; Liao, T.-T.; Chen, Y.-W.; Chen, J.-D. Formation of entangled Co(II) coordination polymers based on bis-pyridyl-bis-amide and angular dicarboxylate ligands: A structural comparison. CrystEngComm 2023, 25, 5575–5587. [Google Scholar] [CrossRef]
- Safarifard, V.; Morsali, A. Influence of an amine group on the highly efficient reversible adsorption of iodine in two novel isoreticular interpenetrated pillared-layer microporous metal–organic frameworks. CrystEngComm. 2014, 16, 8660–8663. [Google Scholar] [CrossRef]
- Guo, B.; Li, F.; Wang, C.; Zhang, L.; Sun, D. A rare (3, 12)-connected zirconium metal–organic framework with efficient iodine adsorption capacity and pH sensing. J. Mater. Chem. A. 2019, 7, 13173–13179. [Google Scholar] [CrossRef]
- Arici, M.; Arici, T.A.; Demiral, H. , Taş, M.; Yeşilel, O.Z. A porous Zn (II)-coordination polymer based on a tetracarboxylic acid exhibiting selective CO 2 adsorption and iodine uptake. Dalton Trans. 2020, 49, 10824–10831. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.T.; Liao, T.T.; Chen, J.-D. Nickel (II) Coordination Polymers Supported by Bis-pyridyl-bis-amide and Angular Dicarboxylate Ligands: Role of Ligand Flexibility in Iodine Adsorption. Int. J. Mol. Sci. 2022, 23, 3603. [Google Scholar] [CrossRef] [PubMed]
- Mohanambe, L.; Vasudevan, S. Insertion of iodine in a functionalized inorganic layered solid. Inorg. Chem. 2004, 43, 6421–6425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, L.X.; Bai, F.Y.; Xing, Y.H. Thorium–organic framework constructed with a semirigid triazine hexacarboxylic acid ligand: Unique structure with thorium oxide wheel clusters and iodine adsorption behavior. Inorg. Chem. 2020, 59, 3964–3973. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Qi, Y.J.; Zhao, D.; Li, H.H.; Zheng, S.T. Heterometallic organic frameworks built from trinuclear indium and cuprous halide clusters: Ligand-oriented assemblies and iodine adsorption behavior. Inorg. Chem. 2018, 58, 516–523. [Google Scholar] [CrossRef]
- Spek, A.L. checkCIF validation ALERTS: What they mean and how to respond. Acta Crystallogr. 2020, E76, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.F.; Hu, J.H.; Lee, W.T.; Yang, X.K.; Chen, J.-D. Structural transformations of cobalt (II) coordination polymers constructed from N, N′-di-3-pyridyladipoamide and tetracarboxylic acids: Disentanglement upon water coordination. Cryst. Growth Des. 2020, 20, 7211–7218. [Google Scholar] [CrossRef]
- Liao, T.-T.; Lin, S.-Y.; Chen, J.-D. Co (ii) coordination polymers supported by a benzenetetracarboxylate and bis-pyridyl-bis-amides with different flexibilities. CrystEngComm 2023, 25, 1723–1730. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Liao, T.-T.; Lin, S.-Y.; Zhong, S.-Y.; Chen, T.-R.; Chen, J.-D. Cd (II) and Zn (II) coordination polymers constructed from bis-pyridyl-bis-amide and dicarboxylic or tetracarboxylic acid: Synthesis, structures and luminescent properties. Inorg. Chim. Acta. 2023, 556, 121641. [Google Scholar] [CrossRef]
- Bruker AXS. APEX2, V2008.6, SADABS V2008/1, SAINT V7.60A, SHELXTL V6.14; Bruker AXS Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
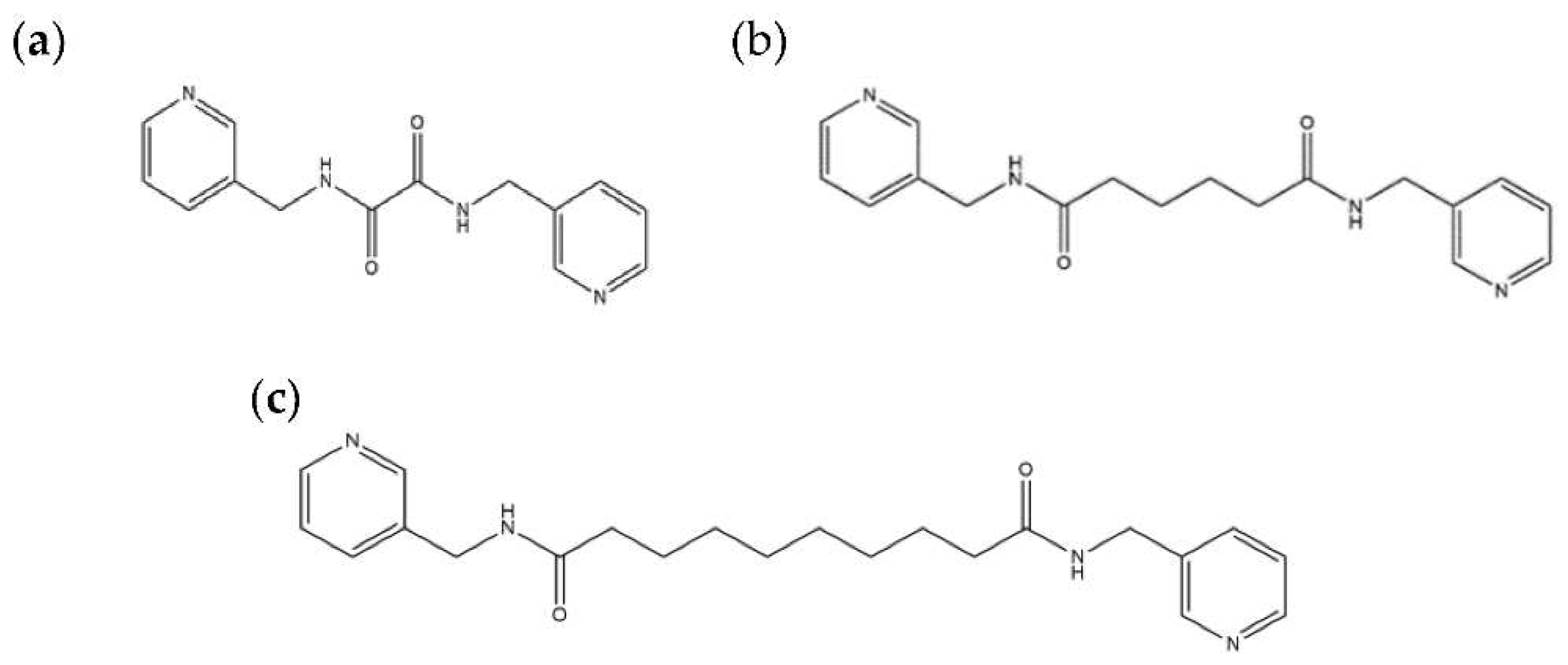
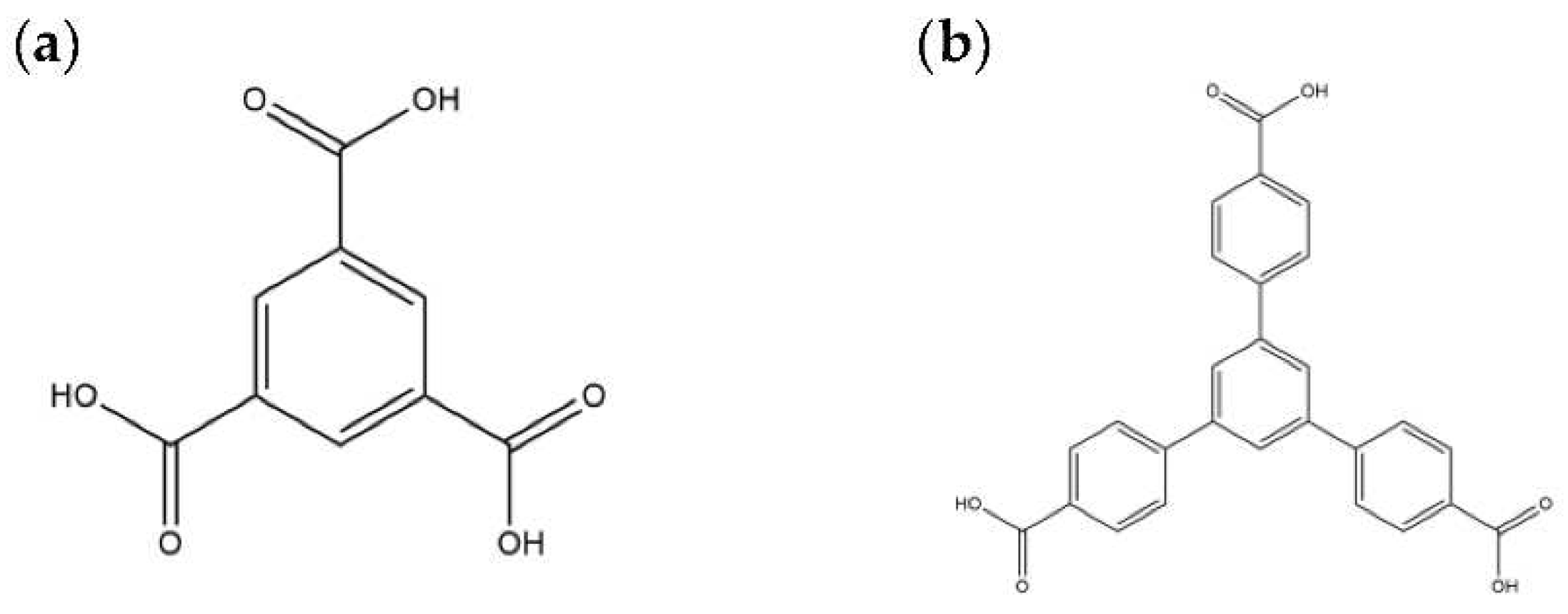
| Ligand conformation | Coordination mode | |
| 1 |
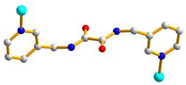 trans syn-syn |
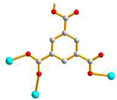 μ3-κO: κO’: κO’’ |
| 2 |
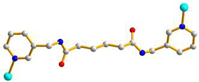 AAA trans syn-syn |
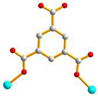 μ2-κO:κO’ |
| 3 |
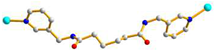 AGA cis anti-anti |
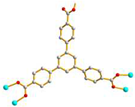 μ4-κO: κO’: κO’’: κO’’’ |
| 4 |
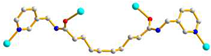 AAAAAAA cis anti-anti |
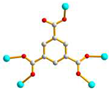 μ5-κO:κO’:O’’:O’’’:O’’’’ |
| 5 |
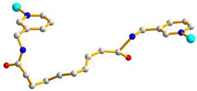 GGAAAAA cis syn-syn |
 μ5-κO:κO’:O’’:O’’’:O’’’’ |
| 6 |
 AAAAAAA trans anti-anti |
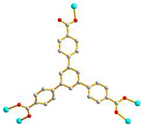 μ5-κO:κO’:O’’:O’’’:O’’’’ |
| Complex | Weight loss of solvent oC (calc/found), % |
Weight loss of ligand oC (calc/found), % |
| 1 | H2O 30 - 250 (3.21/4.38) |
L1 + 1,3,5-HBTC2- 250 - 800 (85.14/85.15) |
| 2 | 6.5 H2O 30 - 120 (16.19/14.38) |
1.5 (L2) + 1,3,5-BTC3- 120 - 900 (73.64/70.97) |
| 3 | 0.5 DMA + 3 H2O 30 - 250 (12.83/7.86) |
0.5 (L2) + 1,3,5-HBTB2- 250 - 800 (78.75/81.97) |
| 4 | - |
L3 + 2 (1,3,5-BTC3-) + 2 (OH-) 270 - 800 (76.50/80.31) |
| 5 | 2.5 MeOH + 2 H2O 30 - 270 (5.97/4.28) |
2 (L3) + 2 (1,3,5-BTB3-) 270 - 800 (84.12/85.60) |
| 6 | DMF + H2O 30 - 250 (4.71/6.03) |
2 (L3) + 2 (1,3,5-BTB3-) 250 - 800 (84.42/84.54) |
| Complex | 1 | 2 | 3 |
|---|---|---|---|
| Formula | C23H20CuN4O9 | C36H53Cu1.5N6O17.50 | C36H27CuN2O7 |
| Formula weight | 599.97 | 945.15 | 663.13 |
| Crystal system | Triclinic | Triclinic | Monoclinic |
| Space group | Pī | Pī | C2/c |
| a, Å | 10.1482(9) | 8.9062(2) | 18.8531(6) |
| b, Å | 11.1855(10) | 11.2565(3) | 25.8032(8) |
| c, Å | 11.7055(11) | 22.7418(5) | 17.4503(6) |
| α, ° | 111.285(3) | 99.7718(14) | 90 |
| β, ° | 97.429(3) | 94.3827(15) | 99.4362(19) |
| γ,° | 108.425(3) | 105.8728(14) | 90 |
| V, Å3 | 1128.97(18) | 2142.95(9) | 8374.2(5) |
| Z | 2 | 2 | 8 |
| Dcalc, Mg/m3 | 1.647 | 1.465 | 1.052 |
| F(000) | 574 | 989 | 2736 |
| µ(Mo Kα), mm-1 | 1.032 | 0.831 | 0.561 |
| Range(2θ) for data collection, deg | 3.88 ≤ 2θ ≤ 51.99 | 3.66 ≤ 2θ ≤ 56.62 | 3.34 ≤ 2θ ≤ 56.62 |
| Independent reflection | 4415 [R(Int) = 0.0643] |
10315 [R(Int) = 0.0539] |
10067 [R(Int) = 0.0745] |
| Data / restraint / parameter | 4415 / 0 / 338 | 10315 / 0 / 556 | 10067 / 0 / 432 |
| quality-of-fit indicatorc | 1.054 | 1.015 | 0.990 |
| Final R indices [I > 2σ(I)] a,b |
R1 = 0.0555, wR2 = 0.1419 |
R1 = 0.0550, wR2 = 0.1228 |
R1 = 0.0536, wR2 = 0.1164 |
| R indices (all data) | R1 = 0.0755, wR2 = 0.1668 |
R1 = 0.1158, wR2 = 0.1452 |
R1 = 0.1022, wR2 = 0.1345 |
| Formula | C40H38Cu4N4O16 | C100.50H104Cu3N8O20.50 | C101H101Cu3N9O19 |
| Formula weight | 1084.90 | 1942.53 | 1935.52 |
| Crystal system | Monoclinic | Orthorhombic | Orthorhombic |
| Space group | C2/c | Pna21 | Pna21 |
| a, Å | 16.5969(9) | 20.9682(10) | 22.0739(18) |
| b, Å | 13.9067(4) | 25.3489(11) | 24.3392(18) |
| c, Å | 17.6110(5) | 18.1699(8) | 17.9481(15) |
| α, ° | 90 | 90 | 90 |
| β, ° | 90.2248(9) | 90 | 90 |
| γ,° | 90 | 90 | 90 |
| V, Å3 | 4064.73(19) | 9657.7(8) | 9642.8(13) |
| Z | 4 | 4 | 4 |
| Dcalc, Mg/m3 | 1.773 | 1.336 | 1.333 |
| F(000) | 2200 | 4056 | 4036 |
| µ(Mo Kα), mm-1 | 2.145 | 0.728 | 0.728 |
| Range (2θ) for data collection,deg | 3.82 ≤ 2θ ≤ 56.59 | 2.75 ≤ 2θ ≤ 51.99 | 2.82 ≤ 2θ ≤ 56.63 |
| Independent reflection | 5049 [R(Int) = 0.0283] |
18994 [R(Int) = 0.0510] |
20288 [R(Int) = 0.0765] |
| Data / restraint / parameter | 5049 / 0 / 311 | 18994 / 2119 / 1157 | 20288 / 1 / 1181 |
| quality-of-fit indicatorc | 1.085 | 1.026 | 1.002 |
| Final R indices [I > 2σ(I)] a,b |
R1 = 0.0265, wR2 = 0.0690 |
R1 = 0.0526, wR2 = 0.1408 |
R1 = 0.0571, wR2 = 0.1019 |
| R indices (all data) | R1 = 0.0323, wR2 = 0.0747 |
R1 = 0.0664, wR2 = 0.1503 |
R1 = 0.1364, wR2 = 0.1244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





