1. Introduction
Glia cells or neuroglia are non-neuronal cells in the central nervous system (CNS) and peripheral nervous system (PNS) that provide physical and chemical support and protection for neurons. In addition to myelin, various nutrients, neurotrophic factors, and packaged vesicles are secreted and conveyed intercellularly for maintaining physiological and functional homeostasis of neural tissue [
1,
2,
3]. Following neurotoxic insult, neural trauma or degeneration, glial cells repair by removing dead cells and damaged tissue debris such as extracellular matrix, through phagocytosis. Additionally, secreted factors such as proteoglycans/glycosaminoglycans and diverse microchannel inducing proteases promote glial and neural cell or axon adhesion, guidance, and migration and scar (gliosis) formation. [
4,
5].
Microchanneling allows for reperfusion and flow of neurotrophic factors through vascular like mimicry in addition to permitting damaged neurons or severed axons to form cellular extensions, migrate and reform new cellular connections and interactions [
6,
7]. Scar formation is a neuroprotective process that represses necrosis or programmed cell death by secluding damaging proinflammatory or stress inducing factors and free radicals.
Secretion of soluble enzymes promotes extracellular matrix (ECM) digestion and consequently deposition of new proteoglycan and glycosaminoglycan fibrous like scaffolds that allow segregation of injured tissue and cell adhesion and migration necessary for tissue remodeling and repair [
8,
9]. The content of vesicles and composition of secreted factors that support and protect homeostasis and neural tissue repair following injury are still largely unknown and of critical importance for medical research and treatment in neuro -regeneration, -aging and -degeneration [
10,
11].
Recent technologies such as single cell and ultra-low input of mRNA sequencing, and ultra-sensitive immunoassays and mass spectrometry have identified new subtypes and functions of patient tissue cells through the unprecedented ability to characterize secreted factors and contents of vesicles [
12,
13,
14]. Regardless of the sensitivity of state-of-the-art technologies, determining normal and disease secreted human glial specific soluble factors, scaffolds and vesicles is greatly impeded due to “contamination” of animal products used for culturing 2D glial cell or and 3D ex situ tissue models. For instance, contaminating vesicles and factors are introduced with use of animal derived serum (such as, fetal bovine) and 3D scaffolds (non-fully defined Matrigel) used in 2D and 3D cultures of patient derived cells or cell lines.
CNS glial cell type lineages generated early in development include astrocytes, oligodendrocytes, microglia, ependymal cells and precursor cells NG-2 and radial glia. PNS glial cells are mostly classified according to their anatomical location and include Schwann, satellite, olfactory nerve and enteric glia [
15]. Glial cells, in contrast to neurons retain to some extent the ability to divide and therefore have the potential to detrimentally or beneficially remodel and therefore impair or promote neural tissue repair [
16,
17,
18]. CNS and PNS glial cells respond differently in disease and injury. Why this is so is of great clinical interest, and we propose it is due in part to differences in the composition of glial cell secreted factors, vesicles and scaffolds that invoke extracellular tissue remodeling and signaling. CNS glial cells have well established roles in neurodegeneration (e.g., Parkinson’s disease) [
19], or neural pathologies such as tumor formation and hyperactive vascularization (e.g., glioblastoma) [
20,
21,
22,
23]. In contrast, PNS glial cells are known to have pro-regenerative properties such as peripheral axon regeneration capacity [
24].
Previously, we studied the role of novel evolutionarily conserved transmembrane proteins, and in particular TMEM230, a likely Golgi complex membrane protein that regulate protein, vesicle and scaffold trafficking and secretion in glial cells [
23,
25,
26]. Using non-biogenic scaffolds and serum replacement conditions free of animal components allowed us to unambiguously demonstrate that conditioned media generated from human CNS glial cells contain secreted factors and vesicles can promote revascularization and microchannel formation, conditions necessary for neural tissue repair [
23].
In this study, we developed a novel 2D and 3D tissue culture method that allows for recapitulating the functional properties (such as microchanneling) and the isolation of secreted components of different types of glial cells, free of animal factors and contaminants normally associated with animal-based serum culture conditions.
2. Materials and Methods
Animal
Newborn Sprague–Dawley rats (Charles River, Calco, Italy) were maintained in accordance with current European rules concerning the care and use of animals (Council Directive 2010/63/EU of the European Parliament and the Council of 22 September 2010 on the protection of animal used for scientific purposes) and 3R’s guidelines. The Ethical Committee of the University of Milan approved the use of animal obtained cells for research.
Schwann cell primary cultures
Schwann cells were obtained as previously described [
27,
28,
29]. Rat sciatic nerves were digested with 1% collagenase and 0.25% trypsin (Merk Life Science), then mechanically dissociated, filtered through a 100-μm filter (BD Biosciences), and centrifuged for 5 min at 900 rpm. Dissociated cells were suspended in Dulbecco’s modified Eagle’s medium (DMEM, Serotec, Oxford) plus 10% fetal calf serum (FCS; Thermo Fisher Scientific) and plated on 35mm Petri dishes. After 24 h, the medium was supplemented with 10 μM Ara-C (Merk Life Science). The medium was then changed with DMEM-FCS 10% plus 10 μM forskolin (Merk Life Science) and 200 μg/ml bovine pituitary extract (BPE; Thermo Fisher Scientific). Cells became confluent in 10 days. Immunopanning for final purification was carried out by incubating the cells for 30 min with mouse anti-rat Thy1.1 antibody (Bio-Rad Laboratories), followed by 500 μl of baby rabbit complement (Cedarlane). Cell suspension (6 × 104 cells) was seeded on 35 mm Petri dishes, in the presence of 2 μM forskolin.
At the third in vitro passage, Schwann cells were treated for 48 h with 4 μM forskolin and then used for research. Schwann cell purity (more than 98%) was tested with a specific antibody against glycoprotein P0 [
28].
Lentiviral construct generation
We used the lentiviral vector pCDH-CMV-MCS-EF1-copGFP (SBI, CD511b-1) expressing green fluorescent protein (GFP) for monitoring the transduction efficiency and cell visualization during culture.
Lentiviral particle production
Lentiviral particles were produced in HEK293T cells by transfecting the pCDH-GFP vector together with the plasmids psPAX2 and pMD2.G (Addgene, #12260 and #12259, gift from Didier Trono,) as helper vectors for 2nd generation viral packaging (with a ratio 4:3:1, respectively), with the Lipofectamine™ 2000 Transfection Reagent (ThermoFisher, 11668027) following manufacturer’s instructions.
Cell culture supernatants containing the lentiviral particles were harvested after 48 and 72 h, lentiviral particles were concentrated by ultracentrifugation at 120,000 rcf for 3 h and stored at -80° C for later use.
Adherent cell cultures
The human brain glioblastoma U87-MG cell line was obtained from the American Type Culture Collection (ATTC, Manassas, VA, USA) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, Euroclone, ECB7501L) supplemented with 10% fetal bovine serum (FBS, Sigma, F7524), 1% glutamine (Cambrex, BE17-605E) and 1% penicillin/streptomycin (P/S, Life Technology, 15140-122) in a humidified atmosphere of 5% CO2 at 37°C.
The human brain astrocytoma 1321-N1 cell line and primary rat Schwann cells were kindly provided by Prof. Valerio Magnaghi’s Lab at University of Milan, Italy. Both cells were maintained in DMEM supplemented with 10% FBS, 1% glutamine and 1% P/S. A dose of 2 µM of Forskolin (FSK, Sigma, F6886) was needed for growing of Schwann cells [
30], while an incremental dose was required for differentiating them [
31]. All cell types were cultured to 80% level of confluence in a humidified atmosphere of 5% CO
2 at 37°C.
Human umbilical vein endothelial cells (HUVECs) were grown in standard EGM2 medium with Ham’s F12/DMEM-Glutamax (Life Technologies, 21765-029/31966-021) at a ratio of 1:1 supplemented with additional factors: heparin (CC-4396A), hydrocortisone (CC-4112A), epidermal growth factor (EGF, CC-4317A), human basic fibroblast growth factor (bFGF, CC-4113A), vascular endothelial growth factor (VEGF, CC-4114A), ascorbic acid (CC-4116A), FBS (CC-4101A), gentamicin (CC-4381A) and R3 Insulin-like growth factor (R3IGF-like, CC-4115A). HUVECs were cultured in a humidified atmosphere of 5% CO2 at 37°C to 80% level of confluence and medium was replaced twice a week.
Transduction was performed on adherent glial cells at a concentration of 10,000 cells/cm
2 (U87-MG and 1321-N1) and 4,500 cells/cm
2 (Schwann) using green fluorescent protein (GFP) lentiviral vector. Cells were monitored using a fluorescence microscope with UV excitation filter. Cells were allowed to recover for two passages in adherent culture. While cells were maintained or passaged with FBS, the ability to form microchannels in 2D and 3D conditions assayed with high (10%), low (2%) and no FBS (serum replacement, SR) for comparative analysis of microchannel formation capacity with different concentrations of FBS, see
Table 1.
Microchannel assay
20,000 HUVECs were plated in growth factor reduced and defined Matrigel (BD Biosciences, 356231) in 48-well plates (Greiner, Twin-Helix, 677180) for 24 h in EGM2 medium. Also 20,000 GFP expressing U87-MG glioblastoma, 1321-N1 astrocytoma and Schwann cells were plated on top of Matrigel bed in 48-well plates for the microchannel assay. U87-MG, 1321-N1 and Schwann cells were monitored over time for 24, 48, and 72 24 h, respectively using a fluorescence microscope (Olympus IX51). Three different culture conditions were used for each type of glial cells, as described in
Table 1. These were: 1) glial growth medium; 2) EGM2 medium (HUVEC medium) [
23] and 3) SR medium, consisting of DMEM, 1% glutamine, with 10% Serum Replacement (SR, Life technologies, 10828-028) instead of 10% FBS [
23].
Nuclear staining
Cell cultures on Matrigel were stained with 4’,6’-diamidino-2-phenylindole (DAPI, Sigma, D9542) after 24 (Schwann cells), 48 (U87-MG cells) and 72 h (1321-N1 cells), respectively. After incubation for 5 min at room temperature cells were observed with fluorescence microscope using DAPI filter.
3. Results
Analyses were performed on CNS glial cells U87-MG and 1321-N1 and PNS tissue derived Schwann cells.
3.1. Glial cells cultured in conventional FBS-based culture conditions.
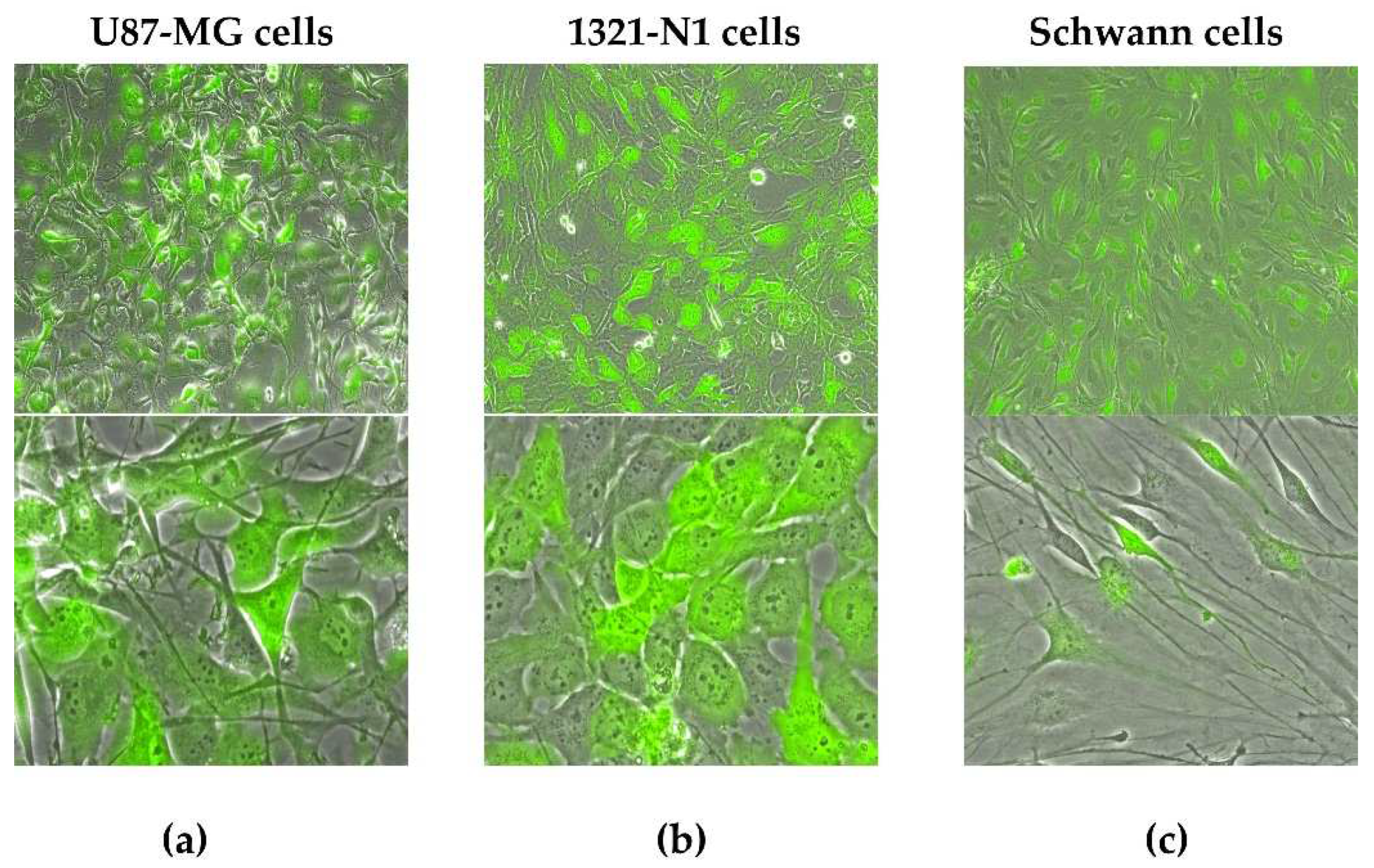
Glial U87-MG, 1321-N1 and Schwann adherent cells were transduced using GFP reporter lentiviral vector (
Figure 1) and monitored with fluorescence microscopy. U87-MG and 1321-N1 showed a higher transduction efficiency than primary Schwann cells. In fetal bovine sera (FBS) media, U87-MG and 1321-N1 cells reached confluence in two or three days (
Figure 1a,b), while Schwann as primary cells required 1 week (see
Figure 1c, Schwann cells are shown at passage 2).
3.2. Evaluation of glial cell microchannel forming capacity in 3D culture conditions based on the FBS concentration.
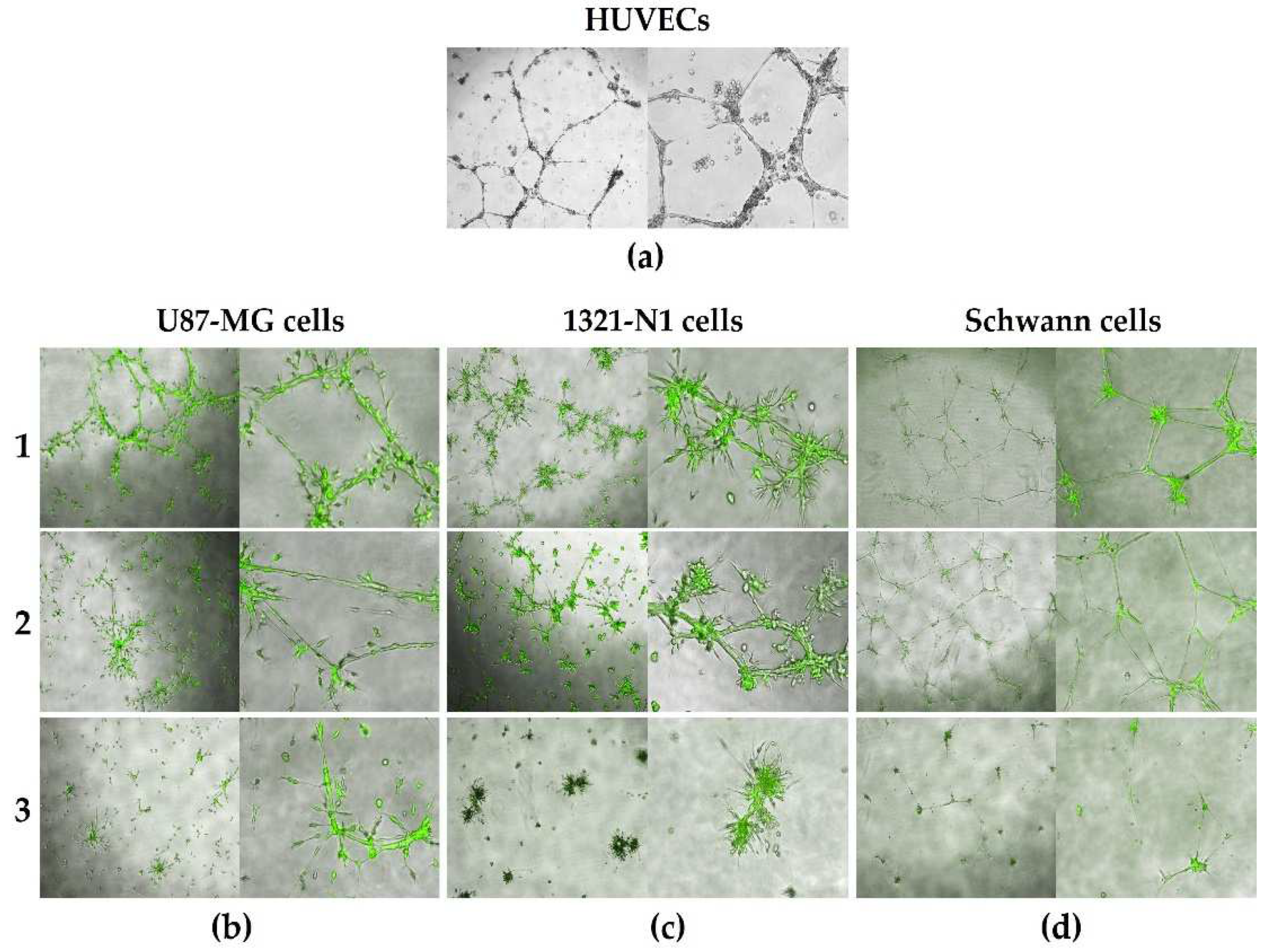
To evaluate the capacity of glial cells to form microchannels, we compared 3 FBS concentrations for culture of these cells in 3D Matrigel (
Figure 2). As described in
Table 1, Glial Growth Medium (1) containing 10% FBS, EGM2 Medium (2) containing 2% FBS and SR Medium (3) containing no FBS were used. Moreover, to evaluate the microchannel forming capacity of glial cells, the ability to form microchannels was compared with HUVECs (
Figure 2a). HUVEC microchannel formation was inducible in low FBS culture conditions (EGM2 medium), therefore, microchannel formation was evaluated in glial cells also using this condition.
We observed that microchanneling was induced in all glial cell lines and the primary Schwann cells (
Figure 2b–d). Using high, low and no FBS concentrations, all cell types showed similar 3D structural morphologies, and ability to generate microchanneling in Matrigel. As expected, primary Schwann cells with increasing passaging in culture showed limited cell proliferation and lumen structure formation capacity compared to established immortalized glial cell lines (U87-MG and 1321-N1 astrocyte). Moreover, the primary glial cells had less pronounced microchanneling capacity in culture conditions with no FBS due to the limited ability to passage these cells (
Figure 2d, condition 3).
We previously showed that protein and glycoconjugate degradation of ECM is critical for lumen and microchannel formation, endothelial cell migration and sprouting, and that these processes were driven by secreted vesicles, factors, or scaffolds [
8,
9,
23].
The ability of the Glial Medium (1) and EGM2 Medium (2) to induce enhanced microchanneling and vascular mimicry [
23] was due to FBS supplementing components such as bovine pro-angiogenic factors to the media [
32]. Since the media with no FBS (SR Medium, condition 3) also induced microchanneling in both CNS and PNS glial cells, this medium is ideal for glial cell secreted molecule and vesicle isolation and for glial characterization, free from animal contaminants in future assays and experiments.
3.3. Evaluation of microchannel 3D structures by nuclear staining.
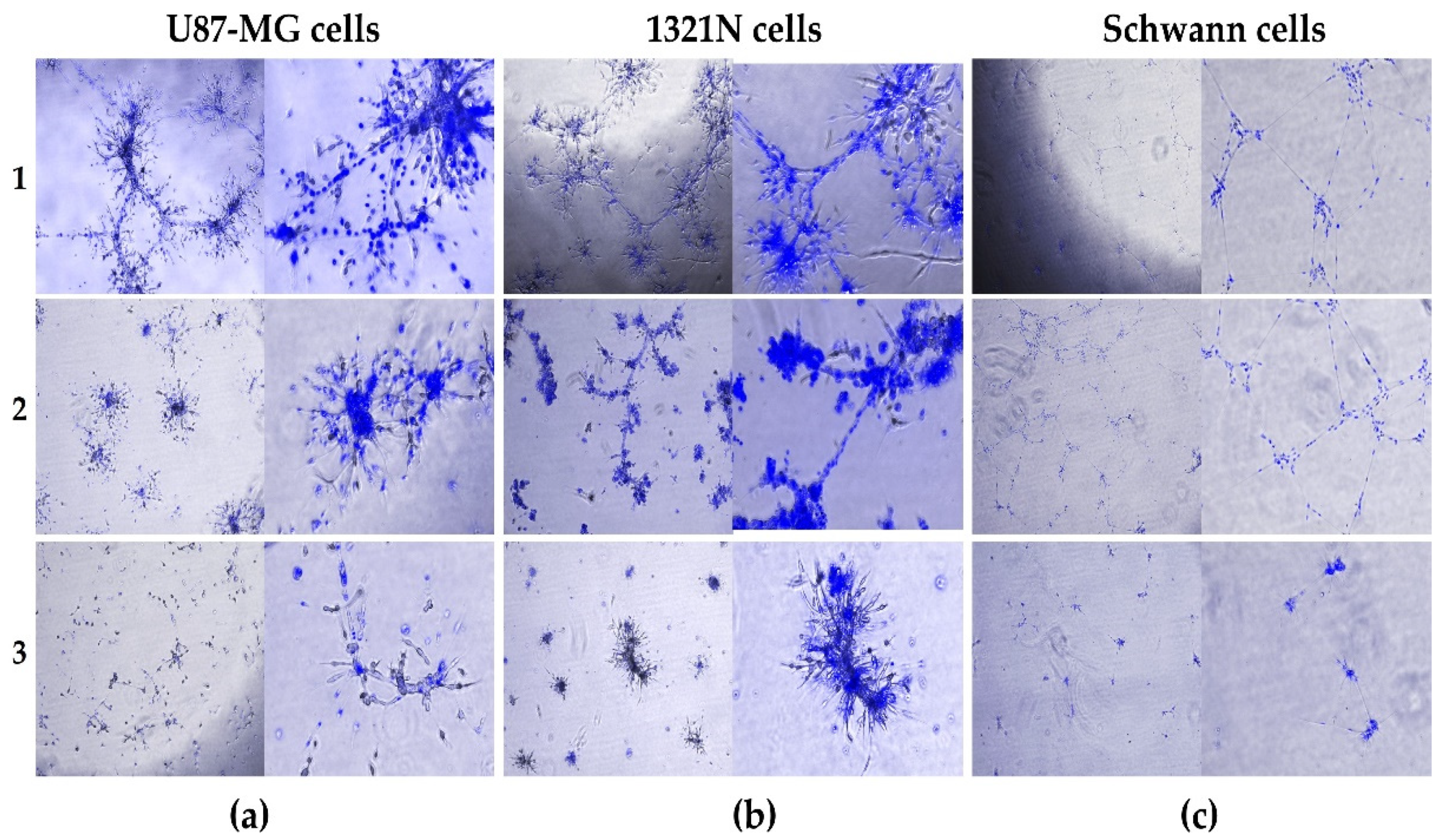
We performed DAPI staining to identify the positions of nuclei and the physical extent and boundaries of microchannel structures of each glial cell type in 3D Matrigel (
Figure 3). As DAPI is a dye for nuclei detection, it was observable that the microchannels contained few cells, suggesting that the 3D lumen structures were generated from cell migration and likely by the long cytoplasmic projections of the glial cells secreting proteases and scaffold degrading enzymes. For U87-MG cells (
Figure 3a) grown in conditions with high FBS (1) or low FBS (2), the DAPI staining clearly showed these structures contained lumen and were hollow. Also, for 1321-N1 cells, the DAPI staining allowed to observe that the structures generated were assembled in 3D (
Figure 3b).
Regarding the Schwann cells, as stated above (
Figure 2d), the nuclei in the structures were positioned throughout the microchannels (
Figure 3c, conditions 1 and 2) and showed that Schwann inferred projections were similar to that seen with HUVECs (
Figure 3c, condition 1 and 2;
Figure 2a). Collectively, these results suggest that process of glial cell microchannel formation is similar to that of HUVECs.
4. Discussion
In both physiological and pathological conditions, glial cells secrete factors, vesicles and scaffolds that promote ECM digestion, resulting in microchannel formation [
23,
33,
34]. These microchannels have various functions depending on the anatomical location of the glial cells. In the CNS glial cells, microchanneling may enhance new blood vessel formation through extracellular signals inducing sprouting or recapitulating vascular mimicry [
21]. Microchannels also favor glial cell migration and glial cell cytoplasm extensions to form and secrete fibrous like scaffolds. The scars that form from the secreted scaffolds limit tissue damage or degeneration. Cytoplasmic extensions also allow neural cells and or their axons to “migrate” and form new connections during neural regeneration [
24,
29,
33,
34].
We previously demonstrated that both hypervascularization and vascular mimicry generated in high-grade CNS glioma tumors were induced by secretion of glial cell vesicles, factors, and scaffolds [
23]. Here, beside the CNS oligodendrocytes previously studied, we tested the microchannel forming capacity of other human CNS tumor astrocytes and rat normal PNS primary Schwann cells.
We generated GFP expressing CNS and PNS glial cells to observe their morphologies and extracellular structure forming capacities through fluorescence microscopy. The diverse morphologies, size and length of cellular projections shown in
Figure 1 are likely due to the type glial cells (CNS for oligodendrocytes and astrocytes, and PNS for Schwann cells) used in the assays and likely to their tissue functions.
We tested the ability of different glial cells to form microchannels in 3D Matrigel (
Figure 2) and observed that in addition to our previous results using oligodendrocytes (
Figure 2b), astrocytes (
Figure 2c) and Schwann cells (
Figure 2d) showed the capacity to generate 3D microchannels.
The DAPI staining (
Figure 3) allowed to visualize simultaneously 3D structures and the number of cells in the lumen of microchannels. Our results have important clinical implications. Glial cell induced microchanneling may allow neural cell migration and formation of new axon connections in neural regeneration or promote nutrient and oxygen exchange following injury [
35,
36].
The method presented here will allow for isolating and characterizing vesicles free of animal (for instance, bovine) factors, normally associated with conventional serum-based culture conditions and identify glial specific factors and vesicle contents using state-the-art RNA sequencing, and glycoconjugate and protein characterization technologies. We propose our culture protocols can be utilized in future studies to understand how glial cells promote or rescue neural degeneration or injury and underly the clinical potential of using enriched human glial cell secreted vesicles free of animal products for promoting re-normalization of human pathological conditions.
5. Patents
Ileana Zucchi and Rolland Reinbold are recipient of EU Patent N. EP3576763B1 05/02/2018 and US Patent and US11566070B2 granted on 2023-01-31.
Author Contributions
Conceptualization, C.C. and R.R.; methodology, C.C.; T.M.; E.P.; P.P and E.A.; validation, C.C; resources, R.R.; I.Z. and V.M.; data curation, C.C.; E.M.; G.P.; G.D.P.; J.K. and R.R.; writing—original draft preparation, C.C.; writing—review and editing, R.R.; I.Z.; M.G.; supervision, R.R. and I.Z.; project administration, C.C. and R.R.; funding acquisition, R.R.; I.Z and V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Marie Skłodowska-Curie Actions (MSCA), call HORIZON-MSCA-2021-SE-01 project number 101086322; CNR (R.R.) call Nutrage 2022 (R.R.); CNR-MIUR Flagship Epigen and Interomics (I.Z.); FRRB LYRA_2015-00100 project (I.Z.); by institutional grants from the University of Milan and by PRIN 2017BJJ5EE from the Ministry of Italian Research (V.M.).
Institutional Review Board Statement
The animal study and use of rat Schwann cells was reviewed and approved by the Ethics Committee University of Milan, Italy.
Informed Consent Statement
Not applicable
Data Availability Statement
This study did not generate gene datasets. No publicly archived datasets analyzed or generated during the study.
Acknowledgments
The authors would like to thank Loredana Ansalone and Francesco Incardona from the ITB Unit for administrative support.
Conflicts of Interest
The authors declare Patent Application US16/482,455 may represent a potential conflict of interest.
References
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Rasband, M.N. Glial contributions to neural function and disease. Mol Cell Proteomics 2016, 15, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.D. Myelin formation and remodeling. Cell 2014, 156, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Nicaise, A.M.; D’Angelo, A.; Ionescu, R-B. ; Krzak, G.; Willis, C.M.; Pluchino, S.; The role of neural stem cells in regulating glial scar formation and repair. Cell Tissue Res 2022, 387, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Wei, Z.; Feng, S. Progression in translational research on spinal cord injury based on microenvironment imbalance. Bone Research 2022, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Akram, R.; Anwar, H.; Javed, M.S.; Rasul, A.; Imran, A.; Malik, S.A.; Raza, C.; Khan, I.U.; Sajid, F.; Iman, T.; Sun, T.; Han, H.S.; Hussain, G. Axonal regeneration: underlying molecular mechanisms and potential therapeutic targets. Biomedicines 2022, 10, 3186. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.; Janowitz, H.; Dougherty, S.E. Neuronal redevelopment and the regeneration of neuromodulatory axons in the adult mammalian central nervous system. Front Cell Neurosci, 2022, 16, 872501. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.C.O.B.; Kumar, A.V.; Katakam, S.K.; Cocola, C.; Pelucchi, P.; Graf, M.; Kiesel, l.; Reinbold, R.; Pavão, M.S.G.; Greve, B.; Götte, M. The heparan sulfate sulfotransferases HS2ST1 and HS3ST2 are novel regulators of breast cancer stem-cell properties. Front Cell Dev Biol 2020, 8, 559554. [Google Scholar] [CrossRef] [PubMed]
- Katakam, S.K.; Pelucchi, P.; Cocola, C.; Reinbold, R.; Vlodavsky, I.; Greve, B.; Götte, M. Syndecan-1-dependent regulation of heparanase affects invasiveness, stem cell properties, and therapeutic resistance of Caco2 colon cancer cells. Front Oncol 2020, 10, 774. [Google Scholar] [CrossRef]
- Tosato, M.; Zamboni, V.; Ferrini, A.; Cesari, M. The aging process and potential interventions to extend life expectancy. Clin Interv Aging 2007, 3, 401–412. [Google Scholar] [CrossRef]
- Fishman, J.R.; Binstock, R.H.; Lambrixc, M.A. Anti-aging science: the emergence, maintenance, and enhancement of a discipline. J Aging Stud 2008, 22, 295–303. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Lukowski, J.K.; Anderton, C.R. Spatially resolved mass spectrometry at the single cell: recent innovations in proteomics and metabolomics. J Am Soc Mass Spectrom 2021, 32, 872–894. [Google Scholar] [CrossRef] [PubMed]
- Shami-Shah, A.; Norman, M.; Walt, D.R. Ultrasensitive protein detection technologies for extracellular vesicle measurements. Mol Cell Proteomics 2023, 22, 100557. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Ho, M.S.; Zorec, R.; Parpura, V. The concept of neuroglia. Adv Exp Med Biol 2019, 1175, 1–13. [Google Scholar] [PubMed]
- Banerjee, S.; Bhat, M.A. Neuron-glial interactions in blood-brain barrier formation. Annu Rev Neurosci 2007, 30, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Mira, R.G.; Lira, M.; Cerpa, W. Traumatic brain injury: mechanisms of glial response. Front Physiol 2021, 12, 740939. [Google Scholar] [CrossRef] [PubMed]
- Huebner, E.A.; Strittmatter, S.M. Axon Regeneration in the Peripheral and Central Nervous Systems. In Cell Biology of the Axon. Results and Problems in Cell Differentiation; Koenig, E. (Eds), Springer: Berlin, Heidelberg, Germany, 2009; Volume 48, pp. 305–360. [Google Scholar] [CrossRef]
- Simon, M.J.; Iliff, J.J. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim Biophys Acta 2016, 1862, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.I.; Anjo, S.I.; de Castro, J.V; Serra, S.C.; Salgado, A.I.; Manadas, B.; Costa, B.M. Crosstalk between glial and glioblastoma cells triggers the “go-or-grow” phenotype of tumor cells. Cell Comm Signaling 2017, 15, 37. [Google Scholar] [CrossRef]
- Angara, K.; Borin, T.F.; Arbab, A.S. Vascular mimicry: a novel neovascularization mechanism driving anti-angiogenic therapy (AAT) resistance in glioblastoma. Transl Oncol 2017, 10, 650–660. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, W.; Zhang, X.; Huang, B.; Chen, A.; He, Y.; Wang, J.; Li, X. Galunisertib inhibits glioma vasculogenic mimicry formation induced by astrocytes. Sci Rep 2016, 6, 23056. [Google Scholar] [CrossRef] [PubMed]
- Cocola, C.; Magnaghi, V.; Abeni, A.; Pelucchi, P.; Martino, V.; Vilardo, L.; Piscitelli, E.; Consiglio, A.; Grillo, G.; Mosca, E.; Gualtierotti, R.; Mazzaccaro, D.; La Sala, G.; Di Pietro, C.; Palizban, M.; Liuni, S.; DePedro, G.; Morara, S.; Nano, G.; Kehler, J.; Greve, B.; Noghero, A.; Marazziti, D.; Bussolino, F.; Bellipanni, G.; D'Agnano, I.; Götte, M.; Zucchi, I.; Reinbold, R. Transmembrane protein TMEM230, a target of glioblastoma therapy. Front Cell Neurosci 2021, 15, 703431. [Google Scholar] [CrossRef] [PubMed]
- Scheib, J.; Höke, A. Advances in peripheral nerve regeneration. Nat Rev Neurol 2013, 9, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Carra, S.; Sangiorgio, L.; Pelucchi, P.; Cermenati, S.; Mezzelani, A.; Martino, V.; Palizban, M.; Albertini, A.; Götte, M.; Kehler, J.; Deflorian, G.; Beltrame, M.; Giordano, A.; Reinbold, R.; Cotelli, F.; Bellipanni, G; Zucchi, I. Zebrafish Tmem230a cooperates with the Delta/Notch signaling pathway to modulate endothelial cell number in angiogenic vessels. J Cell Physiol 2018, 233, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, I.; Reinbold, R. Agents that modulate TMEM230 as angiogenesis regulators and that detect TMEM230 as markers of metastasis. EU Patent N. EP3576763B1 (05/02/2018) and US Patent N. US20200247882A1 (08/06/2020) granted on 31/01/2023.
- Magnaghi, V.; Veiga, S.; Ballabio, M.; Gonzalez, L.C.; Garcia-Segura, L.M.; Melcangi, R.C. . Sex-dimorphic effects of progesterone and its reduced metabolites on gene expression of myelin proteins by rat Schwann cells. J Peripher Nerv Syst 2006, 11, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Manfredi, A.; Ranucci, E.; Procacci, P.; Laus, M.; Antonioli, D.; Mantovani, C.; Magnaghi; V. ; Ferruti, P. Degradable poly(amidoamine) hydrogels as scaffolds for in vitro culturing of peripheral nervous system cells. Macromol Biosci 2013, 13, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Melfi, S.; Montt Guevara, M.M.; Bonalume, V.; Ruscica, M.; Colciago, A.; Simoncini, T.; Magnaghi, V. Src and phospho-FAK kinases are activated by allopregnanolone promoting Schwann cell motility, morphology and myelination. J Neurochem 2017, 141, 165–178. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Magnaghi, V.; Cavarretta, I.; Riva, M.A.; Piva, F.; Martini, L. Effects of steroid hormones on gene expression of glial markers in the central and peripheral nervous system: variations induced by aging. Exp Gerontol 1998, 33, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Magnaghi, V.; Parducz, A.; Frasca, A.; Ballabio, M.; Procacci, P.; Racagni, G.; Bonanno, G.; Fumagalli, F. GABA synthesis in Schwann cells is induced by the neuroactive steroid allopregnanolone. J Neurochem 2010, 112, 980–990. [Google Scholar] [CrossRef]
- Urzì, O.; Bagge, R.O.; Crescitelli, R. The dark side of foetal bovine serum in extracellular vesicle studies. Journal of Extracellular Vesicles 2022, 11, 12271. [Google Scholar] [CrossRef]
- Tewari, B.P.; Chaunsali, L.; Prim, C.E.; Sontheimer, H. A glial perspective on the extracellular matrix and perineuronal net remodeling in the central nervous system. Front Cell Neurosci 2022, 16, 1022754. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.; Bolívar, S.; Navarro, X.; Udina, E. New insights into peripheral nerve regeneration: the role of secretomes. Exp Neurol 2022, 354, 114069. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Bergmann, S.; Lawler, S.E.; Qu, Y.; Fadzen, C.M.; Wolfe, J.M.; Regan, M.S.; Pentelute, B.L.; Agar, N.Y. R; Cho, C-F. Blood-brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat Protoc 2018, 13, 2827–2843. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).