Submitted:
29 November 2023
Posted:
30 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
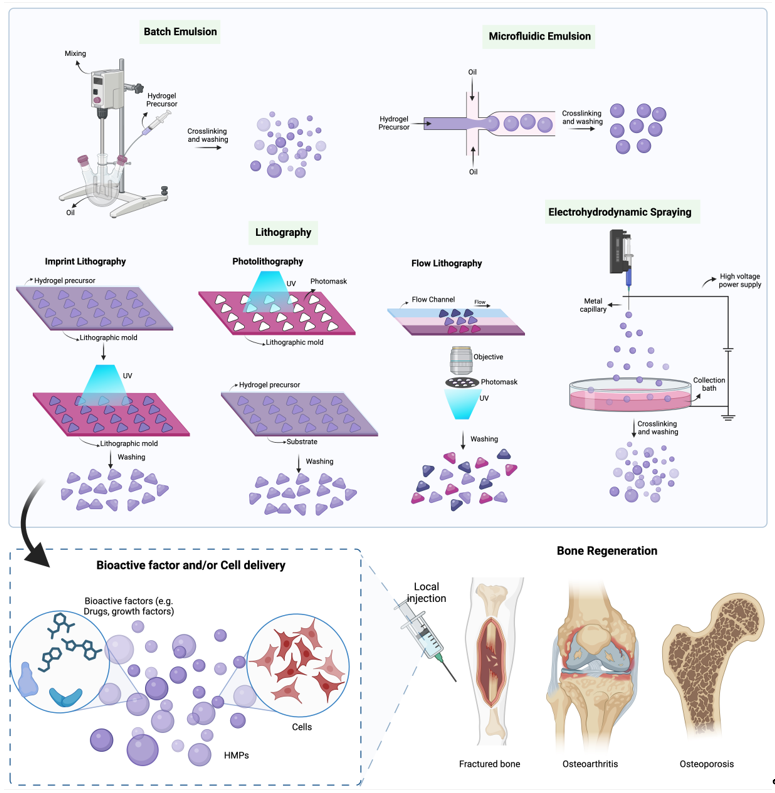
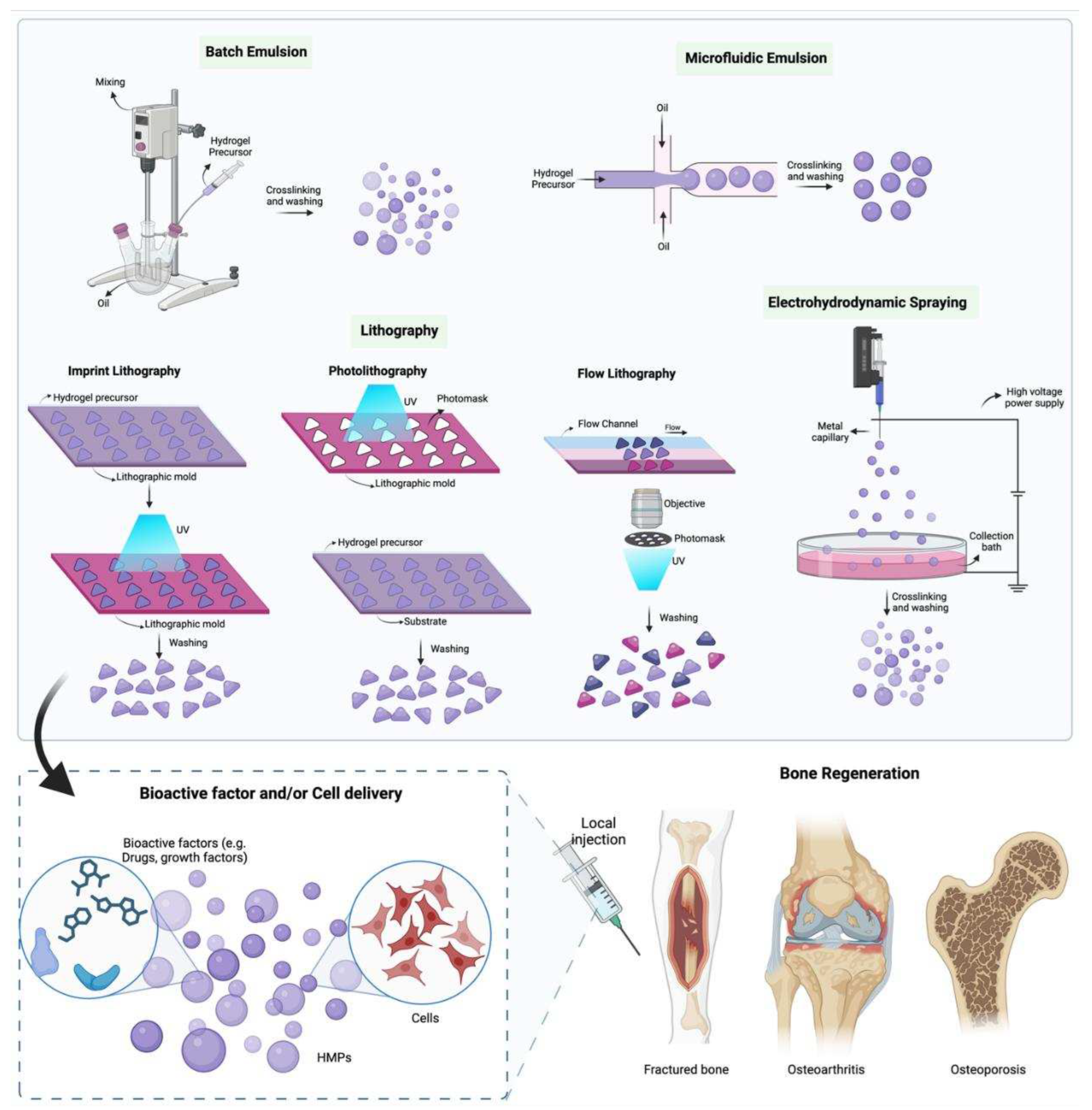
2. Fabrication of HMPs
2.1. Batch Emulsions
2.2. Microfluidic Emulsion
2.3. Lithography
2.4. Electrohydrodynamic spraying
| Fabrication Method | Particle Size | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Batch Emulsion | From a few micrometers to several millimeters | Simple and easily scalable, compatible with wide range of materials | Batch-to-batch variations, limited control over the size of HMPs, uneven drug/cell encapsulation | [27,29,30,31] |
| Microfluidics | From a few micrometers to several millimeters | Reproducible, well controlled HMP size, even drug/cell loading, ease in production of composite HMPs, aseptic | Low throughput, time-consuming | [36,38,39,40,43,44,47] |
| Lithography | From a few micrometers to several hundred micrometers | Control over size and shape, monodisperse particles, does not require surfactant or oil to form the particles | Low throughput, non-scalable, cost for photolithography masks | [50,51,52,53] |
| Electrohydrodynamic Spraying | From a few micrometers to several hundred micrometers | Simple, high encapsulation rate, no additional solvents | Difficult to control particle size and shape | [55,56,57,58] |
3. Composition of HMPs
3.1. Natural Polymers
3.2. Collagen HMPs
3.3. Gelatin HMPs
3.4. Alginate HMPs
3.5. Chitosan HMPs
3.6. Synthetic Materials
3.7. Poly(ethylene glycol) (PEG) HMPs
Poly(vinyl alcohol) (PVA) HMPs
| Biomaterial Classification | Biomaterial | Advantages | Disadvantages | HMP Fabrication Techniques | References |
|---|---|---|---|---|---|
| Natural | Collagen | Biocompatible, degradable, good bone conduction activity | Low mechanical features, suboptimal processing conditions, risk of denaturation during processing | Batch emulsion, EHD spraying | [60,61,65,66,128] |
| Gelatin | Biocompatible, nontoxic, tunable degradation, tailored crosslinking conditions, ease of functionalization and modification | Risk of triggering immunogenic reactions | Batch emulsion, microfluidics, EHD spraying, lithography | [75,76,77,78,79,80,129] | |
| Alginate | Biocompatible, lack of immunogenicity, cost-effective, gentle crosslinking, tunable mechanical properties | Lack of cell adhesion sites, slow degradation | Microfluidic emulsion, EHD Spraying, Batch emulsion | [87,91,93,94,95,96,130] | |
| Chitosan | Biocompatible, ease of processing, antibacterial nature, tunable degradation rates | Suboptimal mechanical properties, batch to batch variation | Batch emulsion, microfluidics, EHD Spraying | [99,100,101,102,103,105] | |
| Synthetic | Poly(ethylene glycol) (PEG) | Biocompatible, Non-toxic, ease of functionalization and modification | Slow degradation rates, resist protein and cell adhesion | Batch emulsion, microfluidic emulsion, lithography, EHD spraying | [109,110,111,112,113,114,117,118] |
| Poly(vinyl alcohol) (PVA) | Biodegradable, biocompatible, FDA approved, ease of functionalization | Lack of cell-adhesion sites | Microfluidics, batch emulsion, lithography | [36,119,120,121,122,131] |
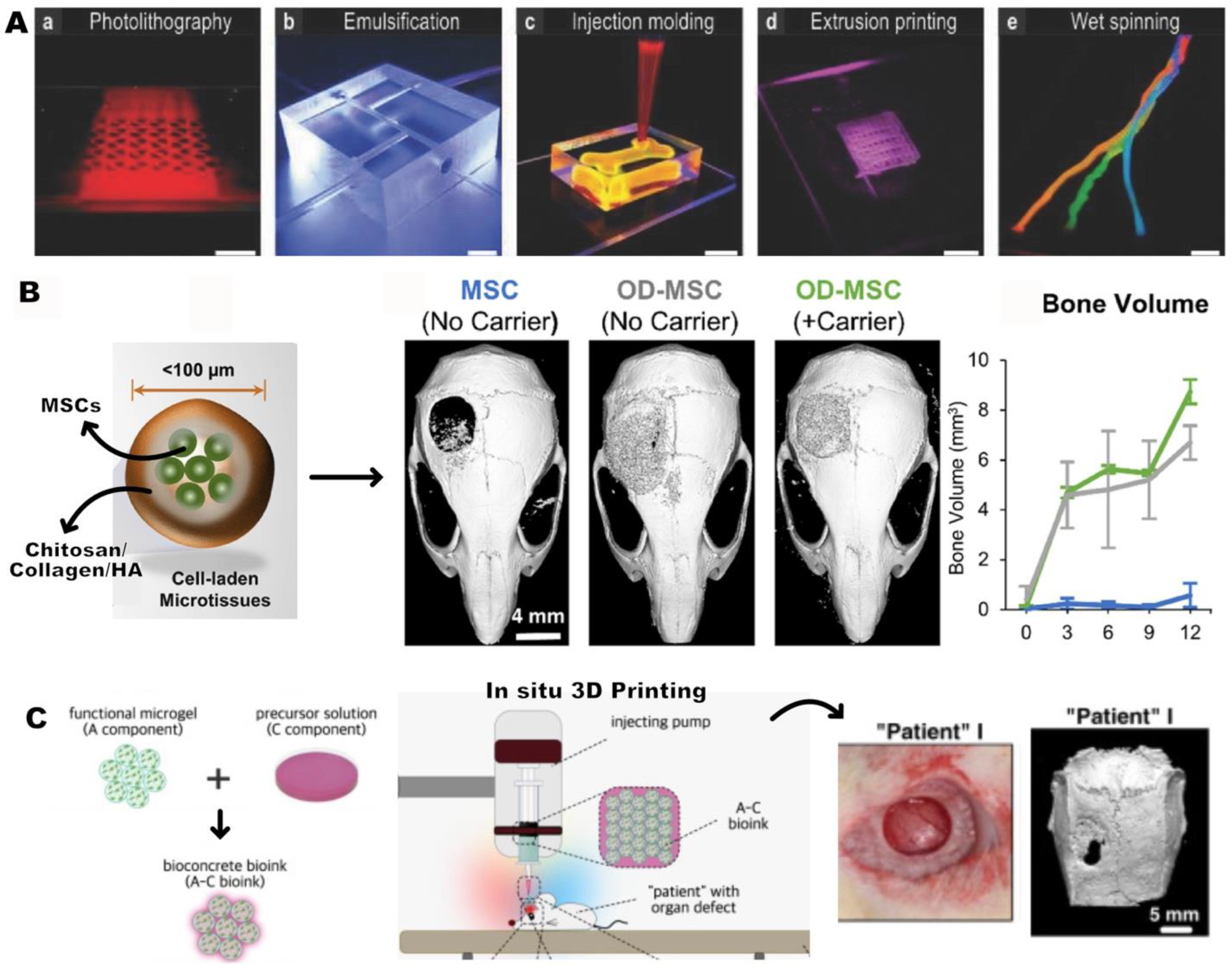
4. Applications of HMPs for Bone regeneration
4.1. Bioactive-factor delivery
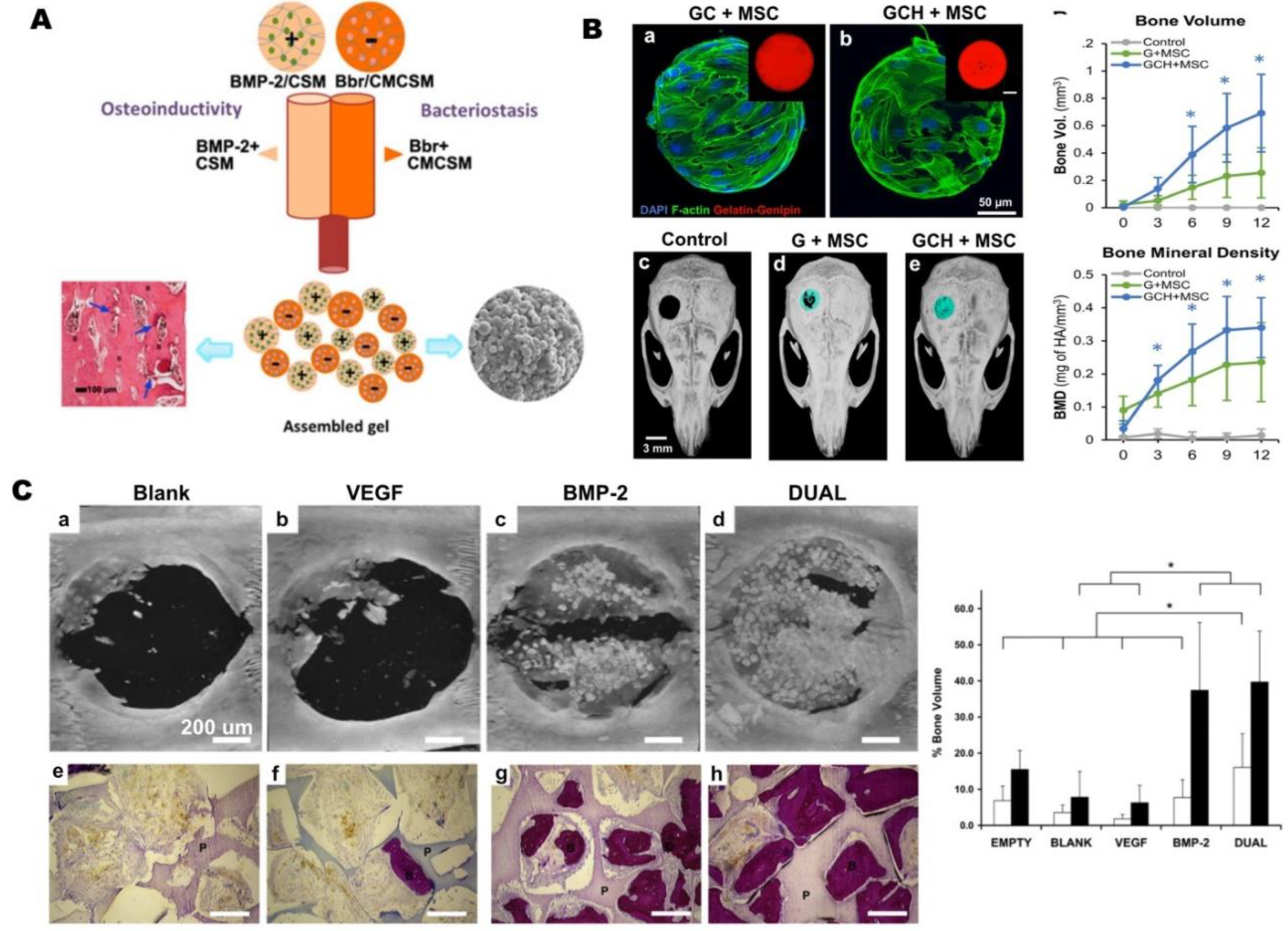
4.2. Cell Delivery
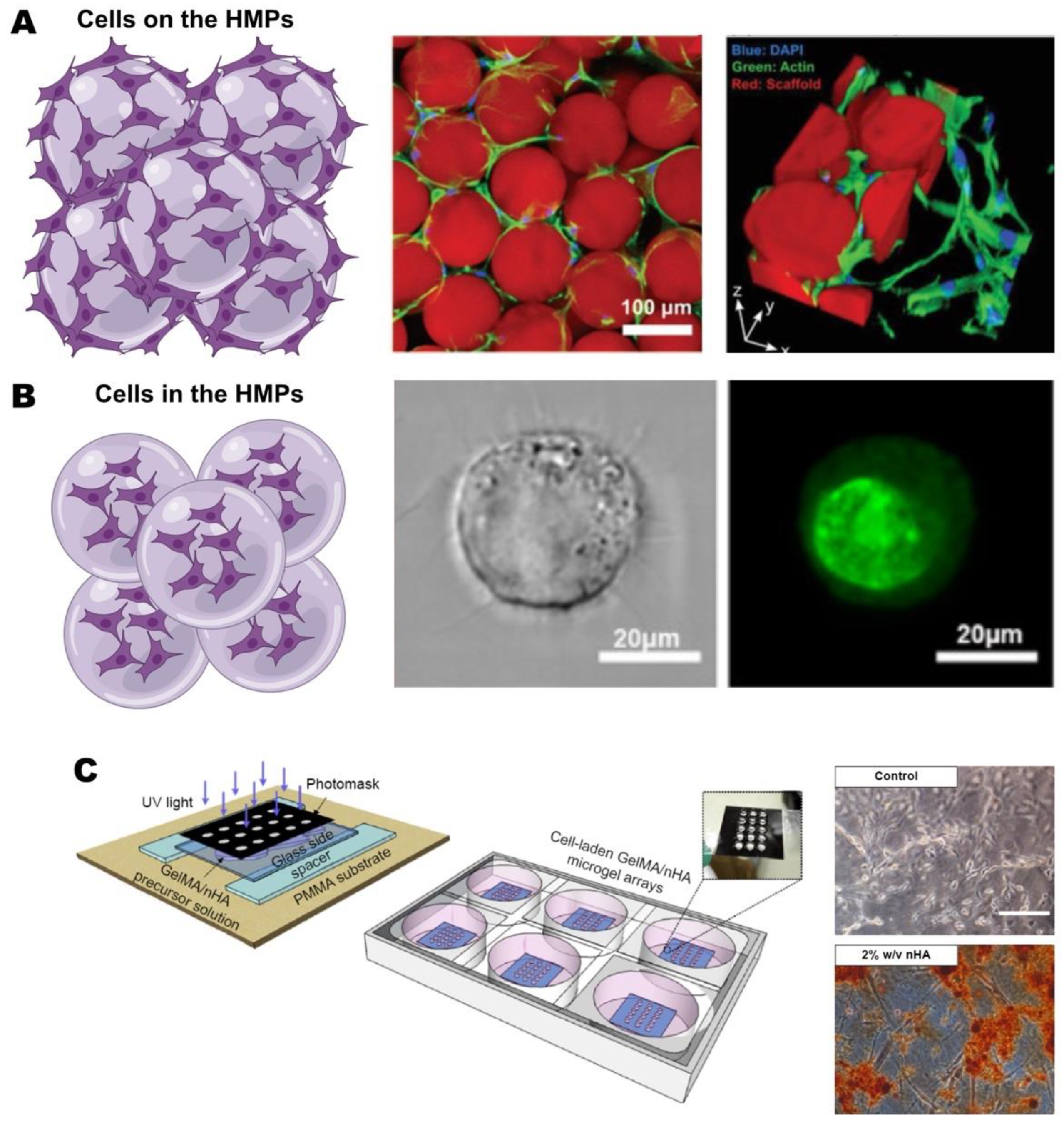
4.3. Scaffold Design with HMPs
4.3.1. HMP-based scaffolds
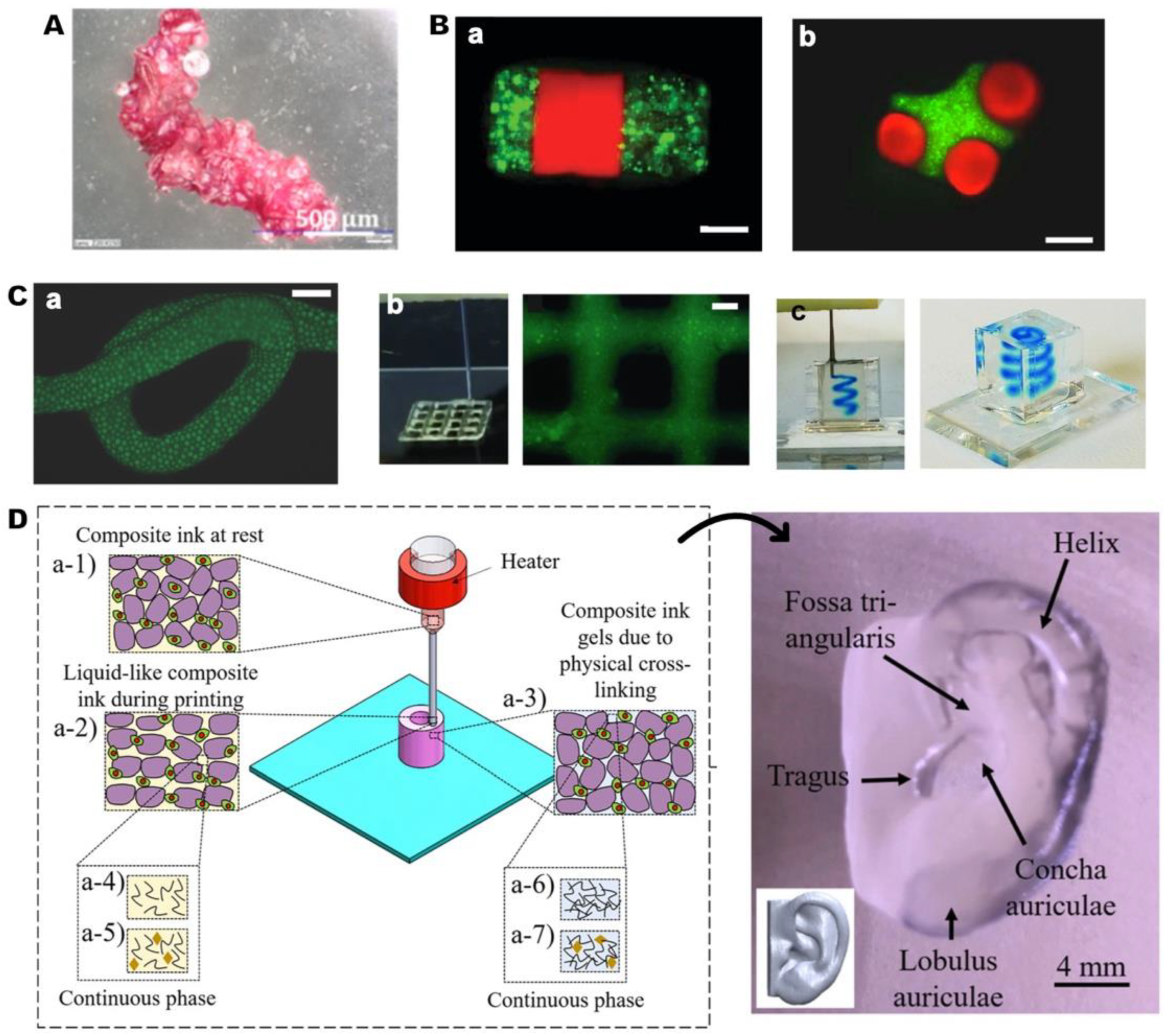
4.3.2. Reinforcing scaffolds: HMPs incorporated scaffolds
5. Conclusion
References
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Baroli, B. From natural bone grafts to tissue engineering therapeutics: Brainstorming on pharmaceutical formulative requirements and challenges. J Pharm Sci 2009, 98, 1317–1375. [Google Scholar] [CrossRef] [PubMed]
- Gazdag, A.R.; Lane, J.M.; Glaser, D.; Forster, R.A. Alternatives to Autogenous Bone Graft: Efficacy and Indications. J Am Acad Orthop Surg 1995, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.S.; et al. Postoperative Morbidity and Complications in Elderly Patients after Harvesting of Iliac Crest Bone Grafts. Medicina-Lithuania.
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of iliac crest bone graft harvesting. Clin Orthop Relat R 1996, 329, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL-TCP wet spun scaffolds carrying antibiotic-loaded microspheres for bone tissue engineering. J Biomat Sci-Polym E 2018, 29, 805–824. [Google Scholar]
- Hasirci, N.; Kilic, C.; Kömez, A.; Bahcecioglu, G.; Hasirci, V. GELS HANDBOOK: Fundamentals, Properties and Applications Volume 2: Applications of Hydrogels in Regenerative Medicine. World Scientific, 2016; pp. 1–52. [Google Scholar]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv Mater 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; et al. Doxorubicin loaded hydrogel microparticles from microfluidics for local injection therapy of tumors. Colloid Surface B 2022, 220. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lin, S.; Nune, K.C.; Misra, R.D.K. Chitosan-gelatin-based microgel for sustained drug delivery. J Biomat Sci-Polym E 2016, 27, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Kilic Bektas, C.; Hasirci, V. Cell loaded 3D bioprinted GelMA hydrogels for corneal stroma engineering. Biomater Sci-Uk 2020, 8, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Flégeau, K.; Puiggali-Jou, A.; Zenobi-Wong, M. Cartilage tissue engineering by extrusion bioprinting utilizing porous hyaluronic acid microgel bioinks. Biofabrication 2022, 14. [Google Scholar] [CrossRef]
- Thangavel, P.; Vilvanathan, S.P.; Kuttalam, I.; Lonchin, S. Topical administration of pullulan gel accelerates skin tissue regeneration by enhancing collagen synthesis and wound contraction in rats. Int J Biol Macromol 2020, 149, 395–403. [Google Scholar] [CrossRef]
- Cui, T.T.; et al. Micro-Gel Ensembles for Accelerated Healing of Chronic Wound via pH Regulation. Adv Sci 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Semitela, A.; et al. Electrospinning of bioactive polycaprolactone-gelatin nanofibres with increased pore size for cartilage tissue engineering applications. J Biomater Appl 2020, 35, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bhat, S.; Nayak, V.; Kumar, A. Efficacy of supermacroporous poly(ethylene glycol)-gelatin cryogel matrix for soft tissue engineering applications. Mat Sci Eng C-Mater 2015, 47, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Beaumont, M.; Rosenau, T.; Tamada, Y. Porous Silk Fibroin/Cellulose Hydrogels for Bone Tissue Engineering via a Novel Combined Process Based on Sequential Regeneration and Porogen Leaching. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hu, Y.; Deng, Y.H.; Su, J.C. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv Funct Mater 2021, 31. [Google Scholar] [CrossRef]
- Kessler, M.; Nassisi, Q.; Amstad, E. Does the Size of Microgels Influence the Toughness of Microgel-Reinforced Hydrogels? Macromol Rapid Comm 2022, 43. [Google Scholar]
- Raemdonck, K.; Demeester, J.; De Smedt, S. Advanced nanogel engineering for drug delivery. Soft Matter 2009, 5, 707–715. [Google Scholar] [CrossRef]
- Newsom, J.P.; Payne, K.A.; Krebs, M.D. Microgels: Modular, tunable constructs for tissue regeneration. Acta Biomaterialia 2019, 88, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat Rev Mater 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Nguyen, T.P.T.; et al. Cell-laden injectable microgels: Current status and future prospects for cartilage regeneration. Biomaterials 2021, 279. [Google Scholar] [CrossRef]
- Vanderhoff, J.W.; Bradford, E.B.; Tarkowski, H.L.; Shaffer, J.B.; Wiley, R.M. Polymerization and polycondensation processes; American Chemical Society, 1962; Volume 34, pp. 32–51. [Google Scholar]
- Kilic Bektas, C.; et al. Self-Assembled Hydrogel Microparticle-Based Tooth-Germ Organoids. Bioengineering (Basel) 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.L.; Price, J.; West, J.L. Development and optimization of a dual-photoinitiator, emulsion-based technique for rapid generation of cell-laden hydrogel microspheres. Acta Biomaterialia 2011, 7, 3267–3276. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; et al. Emulsion-based encapsulation of pluripotent stem cells in hydrogel microspheres for cardiac differentiation. Biotechnol Progr 2020, 36. [Google Scholar] [CrossRef]
- Patel, Z.S.; Yamamoto, M.; Ueda, H.; Tabata, Y.; Mikos, A.G. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomaterialia 2008, 4, 1126–1138. [Google Scholar] [CrossRef] [PubMed]
- Gelli, R.; Mugnaini, G.; Bolognesi, T.; Bonini, M. Cross-linked Porous Gelatin Microparticles with Tunable Shape, Size, and Porosity. Langmuir 2021, 37, 12781–12789. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Khan, Y.; Berkland, C.J.; Laurencin, C.T.; Detamore, M.S. Microsphere-Based Scaffolds in Regenerative Engineering. Annual Review of Biomedical Engineering 2017, 19, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Varde, N.K.; Pack, D.W. Microspheres for controlled release drug delivery. Expert Opin Biol Th 2004, 4, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Blaker, J.J.; Knowles, J.C.; Day, R.M. Novel fabrication techniques to produce microspheres by thermally induced phase separation for tissue engineering and drug delivery. Acta Biomaterialia 2008, 4, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.B.; et al. Preparation of Monodisperse Biodegradable Polymer Microparticles Using a Microfluidic Flow-Focusing Device for Controlled Drug Delivery. Small 2009, 5, 1575–1581. [Google Scholar] [CrossRef]
- Lu, S.; Lee, E.J.; Lam, J.; Tabata, Y.; Mikos, A.G. Evaluation of Gelatin Microparticles as Adherent-Substrates for Mesenchymal Stem Cells in a Hydrogel Composite. Ann Biomed Eng 2016, 44, 1894–1907. [Google Scholar] [CrossRef]
- Cohen, N.; et al. PEG-fibrinogen hydrogel microspheres as a scaffold for therapeutic delivery of immune cells. Front Bioeng Biotech 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.Q.; Li, M.Q.; Chen, Z.Z.; Leong, K.W. Cell-laden microfluidic microgels for tissue regeneration. Lab Chip 2016, 16, 4482–4506. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.L.; Garcia, A.J. Methods for Generating Hydrogel Particles for Protein Delivery. Ann Biomed Eng 2016, 44, 1946–1958. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal Chem 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Carneiro, J.; Campos, J.B.L.M.; Miranda, J.M. Production of hydrogel microparticles in microfluidic devices: a review. Microfluid Nanofluid 2021, 25. [Google Scholar] [CrossRef]
- Li, W.; et al. Microfluidic fabrication of microparticles for biomedical applications. Chem Soc Rev 2018, 47, 5646–5683. [Google Scholar] [CrossRef] [PubMed]
- Lu, et al. Modular and Integrated Systems for Nanoparticle and Microparticle Synthesis-A Review. Biosensors-Basel 2020, 10. [CrossRef] [PubMed]
- Chen, C.H.; Abate, A.R.; Lee, D.Y.; Terentjev, E.M.; Weitz, D.A. Microfluidic Assembly of Magnetic Hydrogel Particles with Uniformly Anisotropic Structure. Adv Mater 2009, 21, 3201–3204. [Google Scholar] [CrossRef]
- Martinez, C.J.; et al. A microfluidic approach to encapsulate living cells in uniform alginate hydrogel microparticles. Macromol Biosci 2012, 12, 946–951. [Google Scholar] [CrossRef]
- Li, Q.; Chang, B.; Dong, H.; Liu, X. Functional microspheres for tissue regeneration. Bioact Mater 2023, 25, 485–499. [Google Scholar] [CrossRef]
- Li, Y.N.; et al. Composite core-shell microparticles from microfluidics for synergistic drug delivery. Sci China Mater 2017, 60, 543–553. [Google Scholar] [CrossRef]
- Zhao, X.; et al. Hierarchically porous composite microparticles from microfluidics for controllable drug delivery. Nanoscale 2018, 10, 12595–12604. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.W.; et al. New Hope for Treating Intervertebral Disc Degeneration: Microsphere-Based Delivery System. Front Bioeng Biotech 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Um, T.; Ahn, G.N.; Kim, D.P.; Lee, H.Y.M. Robust and scalable production of emulsion-templated microparticles in 3D-printed milli-fluidic device. Chem Eng J 2022, 431. [Google Scholar] [CrossRef]
- Jans, A.; et al. High-Throughput Production of Micrometer Sized Double Emulsions and Microgel Capsules in Parallelized 3D Printed Microfluidic Devices. Polymers-Basel 2019, 11. [CrossRef]
- Helgeson, M.E.; Chapin, S.C.; Doyle, P.S. Hydrogel microparticles from lithographic processes: Novel materials for fundamental and applied colloid science. Curr Opin Colloid In 2011, 16, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Acharya, G.; et al. The hydrogel template method for fabrication of homogeneous nano/microparticles. J Control Release 2010, 141, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Bin Hamzah, Y.; Hashim, S.; Abd Rahman, W.A.W. Synthesis of polymeric nano/microgels: a review. J Polym Res 2017, 24. [Google Scholar] [CrossRef]
- Li, B.; He, M.H.; Ramirez, L.; George, J.; Wang, J. Multifunctional Hydrogel Microparticles by Polymer-Assisted Photolithography. Acs Appl Mater Inter 2016, 8, 4158–4164. [Google Scholar] [CrossRef]
- Merkel, T.J.; et al. Scalable, Shape-Specific, Top-Down Fabrication Methods for the Synthesis of Engineered Colloidal Particles. Langmuir 2010, 26, 13086–13096. [Google Scholar] [CrossRef]
- Naqvi, S.M.; et al. Living Cell Factories - Electrosprayed Microcapsules and Microcarriers for Minimally Invasive Delivery. Adv Mater 2016, 28, 5662–5671. [Google Scholar] [CrossRef] [PubMed]
- Imaninezhad, M.; Jain, E.; Zustiak, S.P. Cell Microencapsulation in Polyethylene Glycol Hydrogel Microspheres Using Electrohydrodynamic Spraying. Organoids 2019, 1576, 313–325. [Google Scholar]
- Qayyum, A.S.; et al. Design of electrohydrodynamic sprayed polyethylene glycol hydrogel microspheres for cell encapsulation. Biofabrication 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.W.; Jiang, J.; Davoodi, P.; Srinivasan, M.P.; Wang, C.H. Electrohydrodynamic atomization: A two-decade effort to produce and process micro-/nanoparticulate materials. Chem Eng Sci 2015, 125, 32–57. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat Rev Mater 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Wang, H.A.; Leeuwenburgh, S.C.G.; Li, Y.B.; Jansen, J.A. The Use of Micro- and Nanospheres as Functional Components for Bone Tissue Regeneration. Tissue Eng Part B-Re 2012, 18, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Chen, X. Fabrication, applications and challenges of natural biomaterials in tissue engineering. Appl Mater Today 2020, 20. [Google Scholar] [CrossRef]
- Wee, C.Y.; Yang, Z.J.; Thian, E.S. Past, present and future development of microspheres for bone tissue regeneration: a review. Mater Technol 2021, 36, 364–374. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomaterialia 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr Chem Biol 2009, 3, 189–196. [Google Scholar]
- Mano, J.F.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef] [PubMed]
- Rossler, B.; Kreuter, J.; Scherer, D. Collagen Microparticles - Preparation and Properties. Journal of Microencapsulation 1995, 12, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Chan, O.C.M.; So, K.F.; Chan, B.P. Fabrication of nano-fibrous collagen microspheres for protein delivery and effects of photochemical crosslinking on release kinetics. J Control Release 2008, 129, 135–143. [Google Scholar] [CrossRef] [PubMed]
- agai, N. et al. Preparation and characterization of collagen microspheres for sustained release of VEGF. J Mater Sci-Mater M 2010, 21, 1891–1898. [CrossRef] [PubMed]
- Khatami, N.; Khoshfetrat, A.B.; Khaksar, M.; Zamani, A.R.N.; Rahbarghazi, R. Collagen-alginate-nano-silica microspheres improved the osteogenic potential of human osteoblast-like MG-63 cells. Journal of Cellular Biochemistry 2019, 120, 15069–15082. [Google Scholar] [CrossRef]
- Chan, B.P.; Hui, T.Y.; Wong, M.Y.; Yip, K.H.K.; Chan, G.C.F. Mesenchymal Stem Cell-Encapsulated Collagen Microspheres for Bone Tissue Engineering. Tissue Eng Part C-Me 2010, 16, 225–235. [Google Scholar] [CrossRef]
- Seong, Y.J. et al. Porous calcium phosphate-collagen composite microspheres for effective growth factor delivery and bone tissue regeneration. Materials Science and Engineering C-Materials for Biological Applications 2020, 109. [CrossRef]
- Hou, J.; et al. Segmental bone regeneration using rhBMP-2-loaded collagen/chitosan microspheres composite scaffold in a rabbit model. Biomed Mater 2012, 7. [Google Scholar] [CrossRef]
- Yang, C.L.; et al. The application of recombinant human collagen in tissue engineering. Biodrugs 2004, 18, 103–119. [Google Scholar] [CrossRef]
- Wang, T.; Lew, J.; Premkumar, J.; Poh, C.L.; Win Naing, M. Production of recombinant collagen: state of the art and challenges. Engineering Biology 2017, 1, 18–23. [Google Scholar] [CrossRef]
- Liu, D.S.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu Rev Food Sci T 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.E.; Nuge, T.; Yeow, T.K.; Nordin, N.; Prasad, R. Gelatin based scaffolds for tissue engineering-a review. Polym. Res. J 2015, 9, 15. [Google Scholar]
- Dong, Z.X. et al. Progress of gelatin-based microspheres (GMSs) as delivery vehicles of drug and cell. Mat Sci Eng C-Mater 2021, 122. [CrossRef] [PubMed]
- Patel, Z.S.; Ueda, H.; Yamamoto, M.; Tabata, Y.; Mikos, A.G. In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm Res 2008, 25, 2370–2378. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Kim, C.; Nam, J.O.; Kang, S.M.; Lee, C.S. Synthesis and characterization of thermosensitive gelatin hydrogel microspheres in a microfluidic system. Macromol Res 2016, 24, 529–536. [Google Scholar] [CrossRef]
- Foox, M.; Zilberman, M. Drug delivery from gelatin-based systems. Expert Opin Drug Del 2015, 12, 1547–1563. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J Control Release 2014, 190, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.J. et al. Cross-linked gelatin microsphere-based scaffolds as a delivery vehicle of MC3T3-E1 cells: and evaluation. Materials Science and Engineering C-Materials for Biological Applications 2020, 108. [CrossRef] [PubMed]
- Chao, S.C.; Wang, M.J.; Pai, N.S.; Yen, S.K. Preparation and characterization of gelatin-hydroxyapatite composite microspheres for hard tissue repair. Materials Science and Engineering C-Materials for Biological Applications 2015, 57, 122. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Hasirci, V. Mimicking corneal stroma using keratocyte-loaded photopolymerizable methacrylated gelatin hydrogels. J Tissue Eng Regen M 2018, 12, E1899–E1910. [Google Scholar] [CrossRef]
- Yue, K. et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [CrossRef] [PubMed]
- Xiao, S.N. et al. Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: an Effective Strategy for Tissue Engineering. Stem Cell Rev Rep 2019, 15, 664–679. [CrossRef] [PubMed]
- Chai, N.W. et al. Construction of 3D printed constructs based on microfluidic microgel for bone regeneration. Compos Part B-Eng 2021, 23. [CrossRef]
- Jalandhra, G.K.; Molley, T.G.; Hung, T.T.; Roohani, I.; Kilian, K.A. In situ formation of osteochondral interfaces through bone-ink printing in tailored microgel suspensions. Acta Biomaterialia 2023, 156, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.L. et al. 3D bioassembly of cell-instructive chondrogenic and osteogenic hydrogel microspheres containing allogeneic stem cells for hybrid biofabrication of osteochondral constructs. Biofabrication 2022, 14. [CrossRef] [PubMed]
- Tuin, A.; Kluijtmans, S.G.; Bouwstra, J.B.; Harmsen, M.C.; Van Luyn, M.J.A. Recombinant Gelatin Microspheres: Novel Formulations for Tissue Repair? Tissue Eng Pt A 2010, 16, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J Pharm Sci 2021, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog Polym Sci 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. International Journal of Biological Macromolecules 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Kong, Y.; Zhao, Y.; Li, D.; Shen, H.W.; Yan, M.M. Dual delivery of encapsulated BM-MSCs and BMP-2 improves osteogenic differentiation and new bone formation. Journal of Biomedical Materials Research Part A 2019, 107, 2282–2295. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. Sn Appl Sci 2021, 3. [Google Scholar] [CrossRef]
- Sun, J.C.; Tan, H.P. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Grellier, M.; et al. The effect of the co-immobilization of human osteoprogenitors and endothelial cells within alginate microspheres on mineralization in a bone defect. Biomaterials 2009, 30, 3271–3278. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, A.; et al. Bone Regeneration Potential of Stem Cells Derived from Periodontal Ligament or Gingival Tissue Sources Encapsulated in RGD-Modified Alginate Scaffold. Tissue Eng Pt A 2014, 20, 611–621. [Google Scholar] [CrossRef]
- Croisier, F.; Jérome, C. Chitosan-based biomaterials for tissue engineering. Eur Polym J 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Levengood, S.K.L.; Zhang, M.Q. Chitosan-based scaffolds for bone tissue engineering. Journal of Materials Chemistry B 2014, 2, 3161–3184. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; et al. Chitosan microspheres as a potential carrier for drugs. Int J Pharmaceut 2004, 274, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; et al. Glucose-Responsive Microgels Integrated with Enzyme Nanocapsules for Closed-Loop Insulin Delivery. Acs Nano 2013, 7, 6758–6766. [Google Scholar] [CrossRef]
- Sartipzadeh, O.; Naghib, S.M.; Haghiralsadat, F.; Shokati, F.; Rahmanian, M. Microfluidic-assisted synthesis and modeling of stimuli-responsive monodispersed chitosan microgels for drug delivery applications. Sci Rep-Uk 2022, 12. [Google Scholar] [CrossRef]
- Chen, J.D.; Pan, P.P.; Zhang, Y.J.; Zhong, S.N.; Zhang, Q.Q. Preparation of chitosan/nano hydroxyapatite organic-inorganic hybrid microspheres for bone repair. Colloid Surface B 2015, 134, 401–407. [Google Scholar] [CrossRef]
- Cai, B.; et al. Injectable Gel Constructs with Regenerative and Anti-Infective Dual Effects Based on Assembled Chitosan Microspheres. ACS Appl Mater Interfaces 2018, 10, 25099–25112. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Khoulenjani, S.; Mirzadeh, H.; Etrati-Khosroshahi, M.; Shokrgozar, M.A. Particle size modeling and morphology study of chitosan/gelatin/nanohydroxyapatite nanocomposite microspheres for bone tissue engineering. Journal of Biomedical Materials Research Part A 2013, 101, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Wise, J.K.; Alford, A.I.; Goldstein, S.A.; Stegemann, J.P. Synergistic enhancement of ectopic bone formation by supplementation of freshly isolated marrow cells with purified MSC in collagen-chitosan hydrogel microbeads. Connect Tissue Res 2016, 57, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.W. et al. Reconstruction of Large-scale Defects with a Novel Hybrid Scaffold Made from Poly(L-lactic acid)/Nanohydroxyapatite Alendronate-loaded Chitosan Microsphere: in vitro and in vivo Studies. Sci Rep-Uk 2017, 7. [CrossRef] [PubMed]
- Wang, Y.T.; Guo, L.X.; Dong, S.L.; Cui, J.W.; Hao, J.C. Microgels in biomaterials and nanomedicines. Adv Colloid Interfac 2019, 266, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm Res-Dordr 2009, 26, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.J.; Wyman, O.M.; Alge, D.L. Assembly of PEG Microgels into Porous Cell-Instructive 3D Scaffolds via Thiol-Ene Click Chemistry. Advanced Healthcare Materials 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.; et al. Stop-flow lithography to generate cell-laden microgel particles. Lab Chip 2008, 8, 1056–1061. [Google Scholar] [CrossRef]
- Olabisi, R.M.; et al. Hydrogel Microsphere Encapsulation of a Cell-Based Gene Therapy System Increases Cell Survival of Injected Cells, Transgene Expression, and Bone Volume in a Model of Heterotopic Ossification. Tissue Eng Pt A 2010, 16, 3727–3736. [Google Scholar] [CrossRef]
- Chung, S.E.; et al. Optofluidic maskless lithography system for real-time synthesis of photopolymerized microstructures in microfluidic channels. Appl Phys Lett 2007, 91. [Google Scholar] [CrossRef]
- Sonnet, C.; et al. Rapid healing of femoral defects in rats with low dose sustained BMP2 expression from PEGDA hydrogel microspheres. J Orthop Res 2013, 31, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Dreifke, M.B.; Ebraheim, N.A.; Jayasuriya, A.C. Investigation of potential injectable polymeric biomaterials for bone regeneration. Journal of Biomedical Materials Research Part A 2013, 101, 2436–2447. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B-Re 2008, 14, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Nuttelman, C.R.; Tripodi, M.C.; Anseth, K.S. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol 2005, 24, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int J Polym Mater Po 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Young, C.; Rozario, K.; Serra, C.; Poole-Warren, L.; Martens, P. Poly(vinyl alcohol)-heparin biosynthetic microspheres produced by microfluidics and ultraviolet photopolymerisation. Biomicrofluidics 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Bezemer, J.M.; et al. Microspheres for protein delivery prepared from amphiphilic multiblock copolymers 1. Influence of preparation techniques on particle characteristics and protein delivery. J Control Release 2000, 67, 233–248. [Google Scholar] [PubMed]
- Piacentini, E.; Yan, M.Y.; Giorno, L. Development of enzyme-loaded PVA microspheres by membrane emulsification. J Membrane Sci 2017, 524, 79–86. [Google Scholar] [CrossRef]
- Hou, Y.; et al. Injectable degradable PVA microgels prepared by microfluidic technology for controlled osteogenic differentiation of mesenchymal stem cells. Acta Biomaterialia 2018, 77, 28–37. [Google Scholar] [CrossRef]
- Xue, K.; Teng, S.H.; Niu, N.; Wang, P. Biomimetic synthesis of novel polyvinyl alcohol/hydroxyapatite composite microspheres for biomedical applications. Mater Res Express 2018, 5. [Google Scholar] [CrossRef]
- Sinha, A.; Mishra, T.; Ravishankar, N. Polymer assisted hydroxyapatite microspheres suitable for biomedical application. J Mater Sci-Mater M 2009, 19, 2009–2013. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-H.; Ventura, R.; Min, Y.-K.; Lee, B.-T. Genipin cross-linked polyvinyl alcohol-gelatin hydrogel for bone regeneration. Journal of Biomedical Science and Engineering 2016, 9, 419–429. [Google Scholar] [CrossRef]
- García-García, P. et al. Alginate-hydrogel versus alginate-solid system. Efficacy in bone regeneration in osteoporosis. Mat Sci Eng C-Mater 2020, 115. [CrossRef] [PubMed]
- Lama, M.; et al. Self-Assembled Collagen Microparticles by Aerosol as a Versatile Platform for Injectable Anisotropic Materials. Small 2020, 16. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Hernández, J.A.; et al. Micro- and Nanoparticles by Electrospray: Advances and Applications in Foods. J Agr Food Chem 2015, 63, 4699–4707. [Google Scholar] [CrossRef]
- Lee, S.M.; Choi, G.; Yang, Y.J.; Il Joo, K.; Cha, H.J. Visible light-crosslinkable tyramine-conjugated alginate-based microgel bioink for multiple cell-laden 3D artificial organ. Carbohydrate Polymers 2023, 313. [Google Scholar] [CrossRef] [PubMed]
- Baudis, S.; et al. Modular Material System for the Microfabrication of Biocompatible Hydrogels Based on Thiol-Ene-Modified Poly(vinyl alcohol). J Polym Sci Pol Chem 2016, 54, 2060–2070. [Google Scholar] [CrossRef]
- Bysell, H.; Månsson, R.; Hansson, P.; Malmsten, M. Microgels and microcapsules in peptide and protein drug delivery. Adv Drug Deliver Rev 2011, 63, 1172–1185. [Google Scholar] [CrossRef]
- Sultana, A.; Zare, M.; Thomas, V.; Kumar, T.S.S.; Ramakrishna, S. Nano-based drug delivery systems: Conventional drug delivery routes, recent developments and future prospects. Medicine in Drug Discovery 2022, 15, 100134. [Google Scholar] [CrossRef]
- Malmsten, M.; Bysell, H.; Hansson, P. Biomacromolecules in microgels - Opportunities and challenges for drug delivery. Curr Opin Colloid In 2010, 15, 435–444. [Google Scholar] [CrossRef]
- Meena, L.K.; Rather, H.; Kedaria, D.; Vasita, R. Polymeric microgels for bone tissue engineering applications - a review. Int J Polym Mater Po 2020, 69, 381–397. [Google Scholar] [CrossRef]
- Gillman, C.E.; Jayasuriya, A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Materials Science and Engineering C-Materials for Biological Applications 2021, 130. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.R.; et al. Advances in Growth Factor Delivery for Bone Tissue Engineering. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Ramly, E.P. et al. Safety and Efficacy of Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) in Craniofacial Surgery. Prs-Glob Open 2019, 7. [CrossRef] [PubMed]
- Li, M.; Liu, X.Y.; Liu, X.D.; Ge, B.F. Calcium Phosphate Cement with BMP-2-loaded Gelatin Microspheres Enhances Bone Healing in Osteoporosis: A Pilot Study. Clin Orthop Relat R 2010, 468, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.S.; et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; et al. Fabrication of gelatin methacrylate/nanohydroxyapatite microgel arrays for periodontal tissue regeneration. Int J Nanomed 2016, 11, 4707–4718. [Google Scholar]
- Patrick, M.D.; Keys, J.F.; Kumar, H.S.; Annamalai, R.T. Injectable nanoporous microgels generate vascularized constructs and support bone regeneration in critical-sized defects. Sci Rep-Uk 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B. et al. Bioactive apatite incorporated alginate microspheres with sustained drug-delivery for bone regeneration application. Mat Sci Eng C-Mater 2016, 62, 779–786. [CrossRef]
- Leeuwenburgh, S.C.G.; et al. Mineralization, Biodegradation, and Drug Release Behavior of Gelatin/Apatite Composite Microspheres for Bone Regeneration. Biomacromolecules 2010, 11, 2653–2659. [Google Scholar] [CrossRef]
- Labusca, L.; Herea, D.D.; Mashayekhi, K. Stem cells as delivery vehicles for regenerative medicine-challenges and perspectives. World Journal of Stem Cells 2018, 10, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; et al. Stem cell therapy: old challenges and new solutions. Mol Biol Rep 2020, 47, 3117–3131. [Google Scholar] [CrossRef] [PubMed]
- Lastra, M.L.; Ribelles, J.L.G.; Cortizo, A.M. Design and characterization of microspheres for a 3D mesenchymal stem cell culture. Colloid Surface B 2020, 196. [Google Scholar] [CrossRef] [PubMed]
- de Rutte, J.M.; Koh, J.; Di Carlo, D. Scalable High-Throughput Production of Modular Microgels for In Situ Assembly of Microporous Tissue Scaffolds. Adv Funct Mater 2019, 29. [Google Scholar] [CrossRef]
- Moshaverinia, A.; et al. Co-encapsulation of anti-BMP2 monoclonal antibody and mesenchymal stem cells in alginate microspheres for bone tissue engineering. Biomaterials 2013, 34, 6572–6579. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; et al. Injectable Stem Cell-Laden Photocrosslinkable Microspheres Fabricated Using Microfluidics for Rapid Generation of Osteogenic Tissue Constructs. Adv Funct Mater 2016, 26, 2809–2819. [Google Scholar] [CrossRef]
- An, C.F.; et al. Continuous microfluidic encapsulation of single mesenchymal stem cells using alginate microgels as injectable fillers for bone regeneration. Acta Biomaterialia 2020, 111, 181–196. [Google Scholar] [CrossRef]
- Alkhursani, S.A. et al. Application of Nano-Inspired Scaffolds-Based Biopolymer Hydrogel for Bone and Periodontal Tissue Regeneration. Polymers-Basel 2022, 14. [CrossRef] [PubMed]
- Alzanbaki, H.; Moretti, M.; Hauser, C.A.E. Engineered Microgels-Their Manufacturing and Biomedical Applications. Micromachines-Basel 2021, 12. [Google Scholar] [CrossRef]
- Du, Y.; Lo, E.; Vidula, M.K.; Khabiry, M.; Khademhosseini, A. Method of Bottom-Up Directed Assembly of Cell-Laden Microgels. Cell Mol Bioeng 2008, 1, 157–162. [Google Scholar] [CrossRef]
- Mielan, B.; et al. Polymeric Microspheres/Cells/Extracellular Matrix Constructs Produced by Auto-Assembly for Bone Modular Tissue Engineering. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Lemperle, G.; Morhenn, V.B.; Pestonjamasp, V.; Gallo, R.L. Migration studies and histology of injectable microspheres of different sizes in mice. Plast Reconstr Surg 2004, 113, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Arimura, H.; Ouchi, T.; Kishida, A.; Ohya, Y. Preparation of a hyaluronic acid hydrogel through polyion complex formation using cationic polylactide-based microspheres as a biodegradable cross-linking agent. J Biomat Sci-Polym E 2005, 16, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Nisal, A. et al. Silk fibroin micro-particle scaffolds with superior compression modulus and slow bioresorption for effective bone regeneration. Sci Rep-Uk 2018, 8. [CrossRef]
- Feng, Q.; Li, D.G.; Li, Q.T.; Cao, X.D.; Dong, H. Microgel assembly: Fabrication, characteristics and application in tissue engineering and regenerative medicine. Bioactive Materials 2022, 9, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.A.; Lo, E.; Ali, S.; Khademhosseini, A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. P Natl Acad Sci USA 2008, 105, 9522–9527. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, F. et al. Directed assembly of cell-laden microgels for building porous three-dimensional tissue constructs. Journal of Biomedical Materials Research Part A 2011, 97, 93–102. [CrossRef] [PubMed]
- Zamanian, B.; et al. Interface-Directed Self-Assembly of Cell-Laden Microgels. Small 2010, 6, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Dinh, N.D.; et al. Effective Light Directed Assembly of Building Blocks with Microscale Control. Small 2017, 13. [Google Scholar] [CrossRef]
- Morley, C.D.; Tordoff, J.; O'Bryan, C.S.; Weiss, R.; Angelini, T.E. 3D aggregation of cells in packed microgel media. Soft Matter 2020, 16, 6572–6581. [Google Scholar] [CrossRef]
- Gurkan, U.A.; Tasoglu, S.; Kavaz, D.; Demirel, M.C.; Demirci, U. Emerging Technologies for Assembly of Microscale Hydrogels. Advanced Healthcare Materials 2012, 1, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Song, K.H.; Daly, A.C.; Burdick, J.A. Jammed Microgel Inks for 3D Printing Applications. Adv Sci 2019, 6. [Google Scholar] [CrossRef]
- Xin, S.J.; et al. Generalizing hydrogel microparticles into a new class of bioinks for extrusion bioprinting. Sci Adv 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Song, K.D.; Compaan, A.M.; Chai, W.X.; Huang, Y. Injectable Gelatin Microgel-Based Composite Ink for 3D Bioprinting in Air. Acs Appl Mater Inter 2020, 12, 22453–22466. [Google Scholar] [CrossRef]
- Huang, W.; Li, X.L.; Shi, X.T.; Lai, C. Microsphere based scaffolds for bone regenerative applications. Biomater Sci-Uk 2014, 2, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Kamperman, T.; et al. Single Cell Microgel Based Modular Bioinks for Uncoupled Cellular Micro- and Macroenvironments. Advanced Healthcare Materials 2017, 6. [Google Scholar] [CrossRef]
- Annamalai, R.T.; et al. Injectable osteogenic microtissues containing mesenchymal stromal cells conformally fill and repair critical-size defects. Biomaterials 2019, 208, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.J.; et al. In situ 3D bioprinting with bioconcrete bioink. Nature Communications 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Basmanav, F.B.; Kose, G.T.; Hasirci, V. Sequential growth factor delivery from complexed microspheres for bone tissue engineering. Biomaterials 2008, 29, 4195–4204. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.X. Injectable alginate/hydroxyapatite gel scaffold combined with gelatin microspheres for drug delivery and bone tissue engineering. Mat Sci Eng C-Mater 2016, 63, 274–284. [Google Scholar] [CrossRef]
- Zhuang, W.D.; et al. A 3D-printed bioactive polycaprolactone scaffold assembled with core/shell microspheres as a sustained BMP2-releasing system for bone repair. Biomater Adv 2022, 133. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Urena, P.; et al. Development of a Cell-Based Gene Therapy Approach to Selectively Turn Off Bone Formation. Journal of Cellular Biochemistry 2017, 118, 3627–3634. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




