1. Introduction
Polycystic ovary syndrome is a common reproductive and endocrinological disorder that affects 6–10 percent of women and is characterized by ovulatory dysfunction (chronic
anovulation), polycystic ovarian morphology, and biochemical and/or clinical hyperandrogenism when two out of three
Rotterdam criteria`s
(2003) are present. It is important to note that the diagnosis of polycystic ovary syndrome is established only after alternative causes of androgen excess have been ruled out, including hyperprolactinemia, non-classical congenital adrenal hyperplasia, androgen-secreting ovarian or adrenal tumors, hypothyroidism, Cushing's syndrome, and acromegaly. Polycystic ovary syndrome is typically identified when a patient experiences severe reductions in their quality of life due to hair loss, alopecia, acne, and problems related to infertility. Additionally, metabolic complications, including dyslipidemia, endothelial dysfunction, atherosclerosis, insulin resistance, obesity, fatty liver disease, coagulation disorders, and increased risk of cardiovascular disease, are associated with polycystic ovary syndrome [
1,
2]. Although its pathogenesis remains unclear, a significant amount of data shows that adipokines may play a role in the etiology of polycystic ovary syndrome [
3,
4].
In addition to serving as the body's largest endocrine organ, adipose tissue releases a myriad of adipokines that are involved in numerous pathological processes, including reproduction,
inflammation, glucose and lipid metabolism, regulation of energy metabolism, and insulin resistance [
5]. Oncostatin M is a novel adipokine and a member of the IL-6 cytokine family that stimulates the Janus kinase/signal transducer and activator of the transcription pathway by binding to a transmembrane receptor. Oncostatin M has many biological functions, including lipogenesis/adipogenesis, hematopoiesis, osteogenesis, and inflammation regulation [
6]. It has also been shown that human oocytes and granulosa cells express oncostatin M and its receptor. Additionally, it has been reported that oncostatin M has stimulatory effects on the number and growth of primordial germ cells in the ovaries [
7]. Oncostatin M may also stimulate the production of additional growth factors that support and facilitate the development of primordial follicles. The increase in oncostatin M signaling following human chorionic gonadotropin administration and subsequent ovulation indicates that oncostatin M signaling plays a fundamental role in ovulation [
8].
Therefore, in light of the current literature, we hypothesized that oncostatin M might contribute to the pathophysiology of polycystic ovary syndrome because of its relationship with ovulation, insulin resistance, and inflammation.
3. Results
Blood samples were collected from 64 women (polycystic ovary syndrome group, n = 32; control group, n = 32), and no significant differences were found in body mass index,
triglyceride, high-density lipoprotein cholesterol, white blood count, thyroid-stimulating hormone, prolactin, and estradiol levels between the study groups.
Table 1 summarizes the study groups' metabolic, anthropometric, and hormonal results.
Non-high-density
lipoprotein cholesterol, low
-density lipoprotein cholesterol, total testosterone, and luteinizing hormone/follicle-stimulating hormone ratio were considerably higher in the polycystic ovary syndrome group than in the control group (p < 0.001;
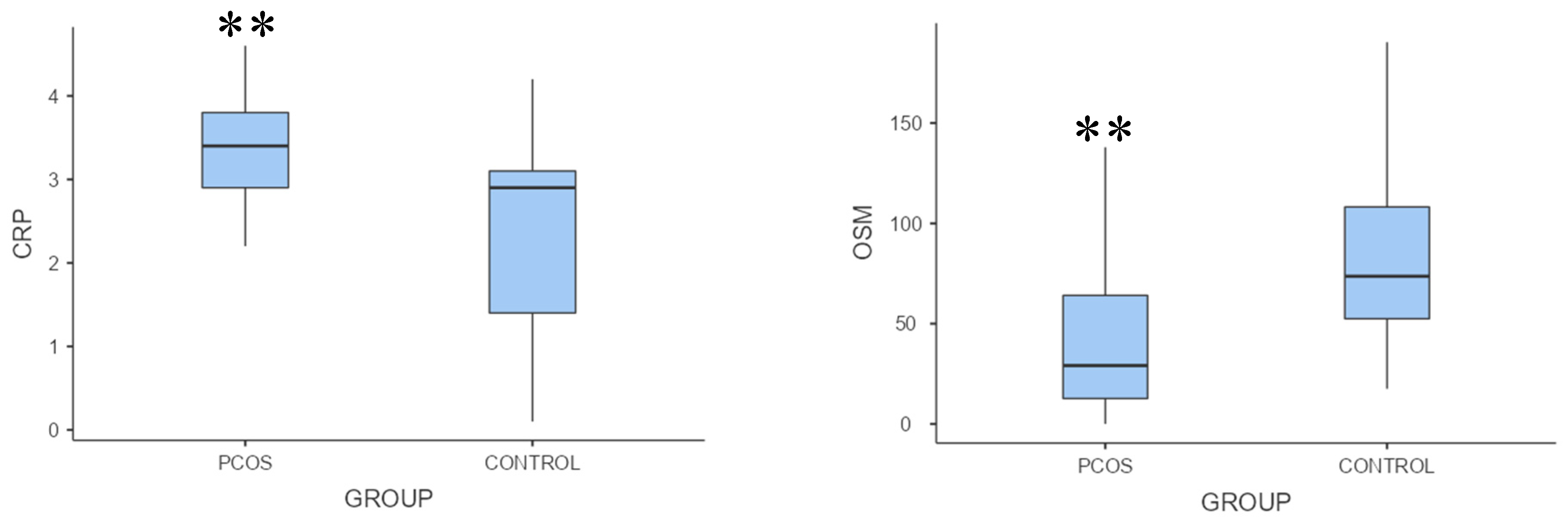
Table 1). Oncostatin M levels were significantly lower, but C-reactive protein levels were substantially higher in the polycystic ovary syndrome group than in the control group (p = 0.002, p = 0.001, respectively;
Table 1,
Figure 1).
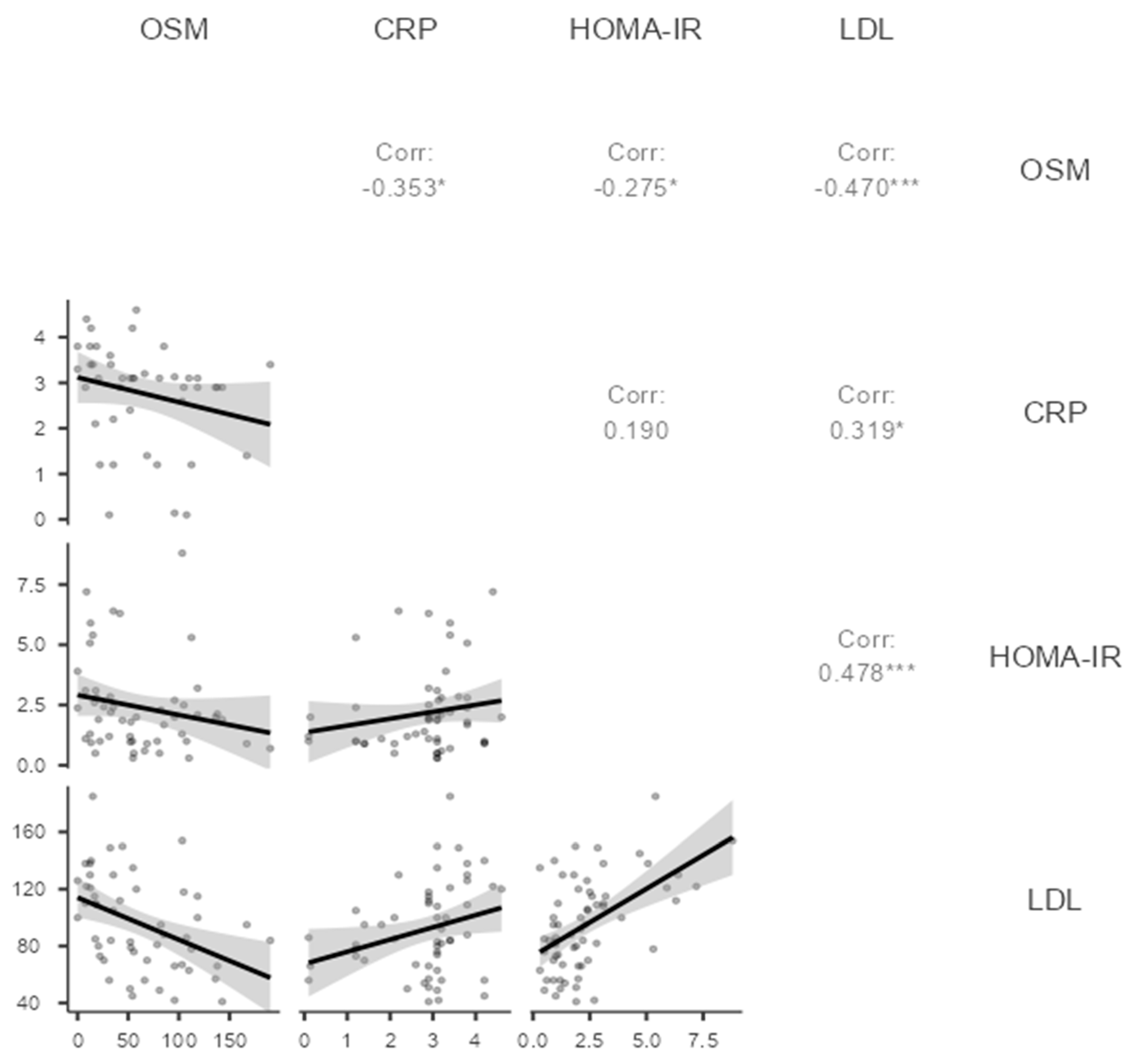
Regarding the association between oncostatin M and biochemical variables, oncostatin M was inversely correlated with fasting glucose, total cholesterol, non-high-density
lipoprotein cholesterol, and luteinizing hormone / follicle-stimulating hormone ratio (ρ = -0.329, p = 0.017; ρ = -0.386, p = 0.005; ρ = -0.440, p = 0.001; ρ = -0.316, p = 0.023, respectively). Conversely, there was no correlation between oncostatin M and
homeostasis model assessment of insulin resistance and total testosterone level, respectively (ρ = 0.275, p = 0.048; ρ = 0.220, p = 0.118).
Table 2 summarizes the correlation between oncostatin M and biochemical markers.
In the context of the inflammation and metabolic parameters, oncostatin M was inversely correlated with C-reactive protein,
homeostasis model assessment of insulin resistance, and low-density lipoprotein cholesterol (ρ = -0.353, p = 0.019; ρ = -0.275, p = 0.048; ρ = -0.470, p < 0.001, respectively;
Table 2,
Figure 2). The correlation of oncostatin M with C-reactive protein, homeostasis model assessment of insulin resistance, and low-density lipoprotein was plotted in
Figure 2.
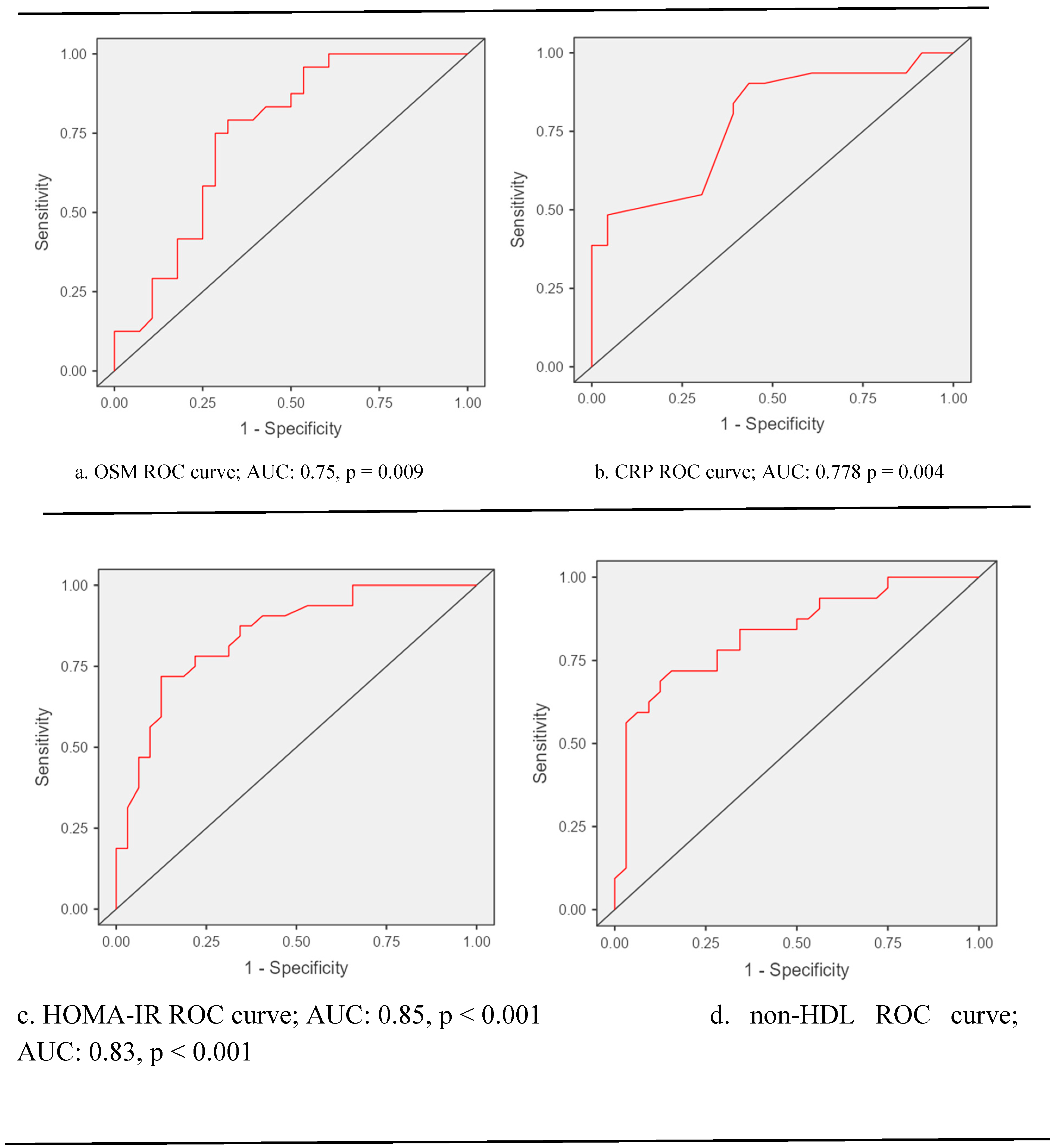
ROC curve analysis unveiled that the performance of biochemical markers for diagnosing polycystic ovary syndrome is as follows: for oncostatin M Sensitivity: 50%, Specificity: 75%, Accuracy: 63%, p = 0.009; for C-reactive protein, Sensitivity: 84%, Specificity: 61%, Accuracy: 74%, p = 0.004; for
homeostasis model assessment of insulin resistance, Sensitivity: 72%, Specificity: 81%, Accuracy: 77%, p < 0.001; and for non-high-density lipoprotein cholesterol; Sensitivity: 72%, Specificity: 75%, Accuracy: 73%, p < 0.001 (
Figure 3).
4. Discussion
Our study indicated that oncostatin M levels were markedly lower in polycystic ovary syndrome patients compared to the control group. We also discovered that patients with polycystic ovary syndrome had significantly higher levels of serum C-reactive protein, homeostasis model assessment of insulin resistance (HOMA-IR), and total testosterone compared to the control group. We observed a significant negative correlation between oncostatin M levels and fasting plasma glucose, homeostasis model assessment of insulin resistance, C-reactive protein, non-high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total cholesterol values, and luteinizing hormone/follicle-stimulating hormone ratios.
In a study published by Nikanfar S et al. [
8], patients with polycystic ovary syndrome and healthy controls were compared in terms of oncostatin M and its receptor levels in their follicular fluids. It was found that oncostatin M and its receptor levels in follicle fluid were significantly lower in patients with polycystic ovary syndrome than in the control group. In that study, Nikanfar et al. [
8] attributed the decrease in oocyte maturation and the increase in the number of immature oocytes in patients with polycystic ovary syndrome to the increased expression of SOCS3. This increase in expression interfered with the levels of oncostatin M and its receptor in the follicle fluid. Additionally, they showed that inflammatory cytokines mediate SOSC3 elevation in patients with polycystic ovary syndrome. Although we did not examine all inflammatory cytokines in this study, we discovered that C-reactive protein levels were notably elevated in patients with polycystic ovary syndrome compared to the control group. We also observed that plasma C-reactive protein levels were negatively correlated with the plasma levels of oncostatin M in patients with polycystic ovary syndrome. Based on this relationship, we can hypothesize that patients with polycystic ovary syndrome [
10] may experience low-level inflammation. This inflammation could be associated with oncostatin M, similar to the findings of the study conducted by Nikanfar S et al. [
8]
Another study from Elks CM et al. [
10], which evaluated the relationship between oncostatin M and inflammation, demonstrated that cancellation of adipocyte oncostatin M signaling disrupts adipose tissue homeostasis, resulting in inflammation and insulin resistance without significant changes in fat mass. Besides, Elks CM and colleagues[
10] also observed that treatment with oncostatin M induced gene expression of Timp1, Igfbp3, and Spp1 while decreasing insulin resistance and other metabolic dysregulations. We think that Elks CM et al.`s [
10] study is essential in providing solid proof for the relationship of oncostatin M with inflammation and insulin resistance. Similarly, we delineate the negative correlation between oncostatin M, homeostatic model assessment for insulin resistance, and C-reactive protein values.
In a different study published by Akarsu M and his colleagues [
11], 51 patients with insulin resistance and 33 healthy controls without insulin resistance were compared regarding oncostatin M, homeostasis model assessment of insulin resistance, C-reactive protein, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride levels, and waist circumference. As a result, Akarsu M and colleagues [
11] found that waist circumference, fasting glucose, insulin, C-reactive protein, high-density lipoprotein cholesterol, oncostatin M, and
homeostasis model assessment of insulin resistance values were statistically significantly different in the patients with high insulin resistance. In the same study, the researchers also noted a significant positive correlation between oncostatin M levels and both C-reactive protein and homeostasis model assessment of insulin resistance values. In our study, we found a negative correlation between oncostatin M levels and both homeostasis model assessment of insulin resistance and C-reactive protein levels. Including groups of obese patients and a relatively small control group in Akarsu M and colleagues' study may have resulted in different findings compared to our study. In their research, Akarsu M and colleagues suggested that oncostatin M may have contributed to the development of insulin resistance by interacting with specific adipokines, especially in obese patients. We believe that conducting additional studies to investigate the relationship between oncostatin M, inflammatory markers, and insulin resistance will help us gain a better understanding of the role of oncostatin M in metabolism.
In addition to metabolic and hormonal factors, neuroendocrine mechanisms play an essential and central role in the pathophysiology of polycystic ovary syndrome and remain up-to-date in recent research. An imbalance in the pattern of gonadotropin-releasing hormone production can lead to a disruption in the hypothalamic-pituitary-ovarian or adrenal axis, which in turn has been associated with the development of polycystic ovary syndrome. This imbalance causes a higher release of luteinizing hormone compared to follicle-stimulating hormone. Hypothalamic gonadotropin-releasing hormone neurons serve as the main connection point between reproductive function and metabolic status. They also play a crucial role as the final output pathway for the central regulation of the reproductive axis [
12].
In a study conducted by Reynolds MF and colleagues [
13], it was found that valproic acid may be associated with polycystic ovary syndrome. In the same study, Reynolds MF and colleagues demonstrated that polycystic ovary syndrome is caused by impaired secretion of N-methyl-D-aspartate and impaired neuronal transmission of gamma-aminobutyric acid, which affects the secretion of gonadotropin-releasing hormone. In another study published by Igaz P and colleagues [
14], it was shown that oncostatin M could regulate gonadotropin-releasing hormone secretion through N-methyl-D-aspartate secretion, which is a glutamate agonist. In our current study, we observed that our polycystic ovary syndrome patients had a significantly higher luteinizing hormone / follicle-stimulating hormone ratio than the control group. We also found a significant negative correlation between luteinizing hormone / follicle-stimulating hormone ratio and oncostatin M levels. Therefore, based on both studies mentioned above and our study, we claim that oncostatin M may interact through the neuroendocrine system in polycystic ovary syndrome. We believe that conducting more extensive studies on the relationship between oncostatin M and gonadotropin-releasing hormone secretion could provide a clearer understanding of the role oncostatin M in the development of polycystic ovary syndrome.
We also found that patients with polycystic ovary syndrome had higher levels of low-density lipoprotein and non-high-density lipoprotein cholesterol than the control group. Additionally, we observed a negative correlation between these elevated cholesterol levels and oncostatin M levels. Some previous in vitro research suggested that oncostatin M directly causes dyslipidemia and atherosclerosis, which contradicts our study. For example, in their study, Liu et al. [
15] showed that oncostatin M plays a supportive role in ox-LDL-induced foam cell formation and inflammation. Therefore,
silencing oncostatin M
may be beneficial for treating atherosclerosis. In another study by Zhan et al., oncostatin M
receptor-β deficiency in macrophages was responsible for inhibiting atherogenesis. Zhan et al. [
16] also observed that despite a fat-rich diet, there was no difference in low-density and non-high-density lipoprotein cholesterol levels in mice, and these mice did not develop atherosclerosis. They attributed this effect to the inhibitory nature of the absence of oncostatin M receptors in the JAK2/STAT3 pathway. We believe both in vitro studies should be validated by in vivo studies, as the interaction between statins and serum lipids may differ in the human body [
17].
Various studies have also revealed that higher serum testosterone levels accompanying polycystic ovary syndrome may be responsible for dyslipidemia [
15,
18,
19]. Several papers in the literature show that hyperandrogenemia might be associated with dyslipidemia, especially in obese people with polycystic ovary syndrome [
20,
21]. In our study, we observed that patients with polycystic ovary syndrome had higher levels of serum total testosterone compared to the control group. However, we did not analyze the relationship between serum total testosterone and lipid levels in our study because the patients with polycystic ovary syndrome were not obese. We found that polycystic ovary syndrome patients had higher levels of serum total testosterone. Furthermore, we did not observe any significant correlation between oncostatin M and total testosterone levels. Although some studies have examined the link between oncostatin M and serum total testosterone levels, the results of these studies are controversial and have primarily been conducted on rats [
22,
23]. Subsequently, this relationship should be scrutinized in future studies.
The main limitation of this study is that the plasma levels of the oncostatin M receptor and suppressor of cytokine signaling were not evaluated because of the lack of funds that facilitated the drawing of a conclusion. Despite its inadequacies, this is one of the pioneer studies that has evaluated oncostatin M levels in patients with polycystic ovary syndrome. Considering that there have been only a few scientific papers investigating the relationship between polycystic ovary syndrome and oncostatin M, this could be seen as a limitation of this study when trying to make a comparison.