1. Introduction
The World Health Organization (WHO) declared the end of coronavirus disease 2019 (COVID-19) as a public health emergency on May 5th, 2023 [
1]. Nevertheless, there is no doubt that the COVID-19 pandemic between 2019 and 2022 resulted in high morbidity and mortality on a global scale, and that community-level immunity, either acquired through SARS-CoV-2 infection or vaccination, played a crucial role in controlling the pandemic.
At present, analyzing global immunity is still of interest in order to clearly understand how and who to test for protecting fragile patients, such as patients with immunosuppressive status [
2]. Indeed, mRNA COVID-19 vaccines have been demonstrated to induce a strong cellular [
3] and humoral [
4,
5] response to SARS-CoV-2, with a progressive decline observed over six months post-vaccination, regardless of previous COVID-19 disease [
6]. However, it has been documented that in some groups of patients, immunity is not effectively stimulated neither by vaccines nor direct viral infection, leading to a possible reinfection of these patients [
7]. Moreover, it has been shown that circulating antibodies against SARS-CoV-2 can persist for up to 18 months, but it is not clear whether there could be a decline in immunological memory, especially in asymptomatic infected individuals [
8]. Therefore, the measurement of antibody titers developed against SARS-CoV-2 infection or vaccine-induced can be useful to facilitate the understanding of community-level immunity. The concomitant measurement of T-cell immunity has been helpful in defining individuals with a poor immunological response and in identifying previously asymptomatic infected children in case of suspicious multi-systemic inflammatory syndrome (MIS-C) [
9].
To facilitate large-scale serosurveillance it is essential to employ robust and well-characterized assays that can be performed on easily accessible self-collected samples. In the last ten years, saliva reached increasingly high importance for the evaluation of many aspects of human health. The use of the salivary matrix to detect active COVID-19 infection is now well established both with the molecular real-time RT-PCR technique and with chemiluminescent enzyme immunoassays in highly automated platforms [
10,
11]: it is easy and non-invasive to collect, and fast and cost-effective to analyze, allowing for widespread execution of the tests. Moreover, it is suitable for large-scale serial sampling for epidemiological studies and screening tests, since it may be well-accepted as a sampling method also by fragile, geriatric, and pediatric patients [
12,
13,
14]. Furthermore, the opportunity to measure both antibodies and viral RNA in one single specimen makes saliva a valuable specimen to monitor individual and population SARS-CoV-2 transmission, infection, and seropositivity [
15], in addition to being used for molecular diagnosis for viral RNA detection.
Since SARS-CoV-2 is a respiratory virus, the detection of antibodies at the sites of primary viral infection needs further investigation [
16]. In this study, we aimed to investigate the clinical, pre-analytical, and analytical performances of an enzyme-linked immunosorbent assay (ELISA) to detect IgG antibodies against SARS-CoV-2 in saliva. In order to test the analytical performance of the assay, both salivary and serum samples of adult and pediatric patients were evaluated, who were infected and/or received COVID-19 mRNA vaccination.
2. Materials and Methods
2.1. Study design and sample collection
We conducted a single-center, observational study on pediatric patients (age ≤ 18 yrs) and adults who attended the COVID-19 Family Cluster Follow-up (CASE cohort) at the Department of Women’s and Children’s Health, University Hospital of Padova enrolled from January to October 2022. Parents or legally authorized representatives were informed of the research proposal and provided their written informed consent. In addition, a cohort of healthcare workers (HCW) was included.
SARS-CoV-2 positivity among individuals, including both children and adults within the CASE cohort, was identified through molecular rRT-PCR testing. Upon enrollment, a pediatrician (CDC and DD) gathered demographic data, medical history, SARS-CoV-2 molecular rRT-PCR test results, and vaccination status of both children and their parents. Additionally, a clinical evaluation was conducted. The CASE cohort has been previously described [
17].
For participants in the HCW cohort, routine screenings were conducted to detect SARS-CoV-2 infection every 2 or 3 weeks, utilizing rapid antigen testing. Positive results from the rapid antigen tests were further confirmed through molecular rRT-PCR testing.
All included subjects were educated in collecting saliva samples using Salivette (Sarstedt, Germany). In detail, the procedure for saliva collection included avoiding eating and drinking, performing normal oral hygiene at least 1 hour before collection, and keeping the swab contained inside the Salivette device in the mouth for 1 minute. On the same day of saliva collection, all subjects underwent blood sampling for SARS-CoV-2 S-RBD IgG serological test. Salivary samples were centrifuged at 4000 rpm for 5 minutes at room temperature (RT) and then frozen at -20°C until use. Blood contamination of saliva was excluded by visual assessment of all samples after centrifugation. Blood samples were left clotting for 30 min at RT, centrifuged at 4000 rpm for 5 minutes, and then frozen at -80°C until use.
2.2. Study design and sample collection
The N/S anti-SARS-CoV-2 salivary IgG (ELISA) (RayBiotech Parkway Lane Suite, Peachtree Corners, GA, USA) assay (anti-SARS-CoV-2 N/S sIgG) was used. This assay allowed us to quantitatively determine IgG against the nucleocapsid protein (N) and receptor-binding domain (RBD) (part of the S1 subunit of the spike protein). This method uses a plate coated with the SARS-CoV-2 N and S1-RBD proteins, which combine with the antibodies present in the sample. After one hour of incubation, the plate is washed and biotinylated IgG antibody is added to each well. This is followed by a short incubation of 30 minutes followed by a series of washings and the addition of the horseradish peroxidase (HRP)-streptavidin solution. After 30 more minutes of incubation and 5 washes, the TMB (3,3’,5,5’-tetramethylbenzidine) substrate solution is added and finally, after a 15-minute incubation, the solution is added acid to stop the reaction. The same procedure is performed in another plate coated with human albumin which is used as a blank. The results from the albumin-coated plate should be subtracted from those obtained from the N/S1-RBD SARS-CoV-2 IgG protein-coated plate.
Serum SARS-CoV-2 S-RBD IgG was performed using an already validated assay [
18], by an automated platform Maglumi 2000 Plus (Snibe Co. Ltd., Shenzhen, China) which exploits the principle of chemiluminescence with paramagnetic particles coated with recombinant S-RBD antigen.
2.3. Precision assessment
Precision was evaluated by using 6 saliva samples with different levels of anti-SARS-CoV-2 N/S sIgG. For 5 of these samples, 5 aliquots were prepared, pipetted in random wells in one plate, and then analyzed. The last sample, with a mean concentration of 2.01 kAU/L, was repeatedly analyzed 25 times, plated in consecutive wells of the same plate, to achieve a robust estimation of repeatability.
2.4. Linearity assessment
Linearity was assessed by using a series of six sample pools, prepared with different anti-SARS-CoV-2 N/S sIgG values, as specified in the CLSI EP06 A: 2003 guideline (paragraph 4.3.1). In brief, salivary pools were serially diluted with a pool of negative saliva with a value of anti-SARS-CoV-2 N/S sIgG < 0.5 kAU/L. All measurements were performed in triplicate. A second-order polynomial regression was used to detect deviation from linearity.
2.5. Impact of sample collection time on salivary Ab levels
In 19 subjects, salivary samples were collected using the same procedures specified above at three different times: 1) before breakfast, or immediately after waking up; 2) during the morning, between 10 and 11 am, and 3) after lunch, between 2.30 and 4.30 pm.
2.6. Statistical analyses
Statistical analyses were performed using Stata v 16.1 (Stata Corp, Lakeway drive, TX, USA). Descriptive statistics, the χ2 test, the Fisher exact test, and a 2-tailed, Kruskall-Wallis and unpaired t -test were used for categorical or continuous covariates. Linear regressions were used to assess the association between studied parameters and salivary or serum IgG after the transformation of the variables into base-10 logarithms. Friedman test for paired data was used to assess differences across the time of sample collection. Statistical significance was set at P < 0 .05. All P values were 2-sided. Graphs were made using GraphPad Prism, version 9.2 (GraphPad Software).
2.3. Ethical statement
The study protocol was approved by local Ethical Committee (Prot. N° 0070714 of November 24th, 2020; amendment N°71779 of November 26th, 2020).
3. Results
3.1. Performance verification and precision analysis
The five repetitions of the first 5 samples had mean values ranging from 1.61 kUA/L to 10.1 kAU/L; precision (CV%) ranged from 23.6% (at a value of 7.4 kAU/L) to 3.9% (at a value of 3.77 kAU/L), with mean precision being of 17.5% (SD, ± 8.5%) (Supplementary
Table 1). In the last sample, with a mean concentration of 2.01 kAU/L, the 25 repetitions presented a mean CV of 11%.
3.2. Linearity assessment
The six samples evaluated for linearity ranged from 20.1 kAU/L to 248 kAU/L (
Figure 1). With the exception of the samples at values of 20.9 kAU/L (sample 1) and 27.9 kAU/L (sample 4), in which linearity was confirmed by polynomial regression, the other four samples demonstrated a marked absence of linearity.
3.3. Clinical study
Table 1 summarizes the characteristics of the studied population.
A total of 95 (50.8%) pediatric patients and 92 (49.2%) adults were included in the study, with a ratio between females/males not significantly different for children (51.6%) and adults (60.8%). A small part of studied individuals (17.9% for pediatrics and 14.1% for adults) presented comorbidities, with ongoing prolonged therapy (17% for pediatrics and 10.1% for adults). Across major comorbidities, there were rheumatic diseases, inflammatory bowel diseases, renal diseases, chronic cephalea, and metabolic diseases (e.g. Hashimoto’s disease and hypothyroidism). Salivary anti-SARS-CoV-2 N/S sIgG differed between pediatric and adults, being higher in the latter group (χ2 = 6.4, p = 0.0188,
Table 1).
Table 2 reports the univariate linear regression analyses of anti-SARS-CoV-2 N/S sIgG with respect to all studied variables, subdivided by pediatric and adult individuals.
Considering pediatric subjects with and without previous SARS-CoV-2 infection, anti-SARS-CoV-2 N/S sIgG median (and IQR) levels were similar, being 2.3 kAU/L (0.5-51.4 kAU/L) and 3.6 kAU/L (0.5-68.4 kAU/L) (p = 0.770), respectively. In adults, anti-SARS-CoV-2 N/S sIgG median (and IQR) levels did not significantly differ between subjects with or without previous COVID-19 [29.5 kAU/L (0.5-92.1 kAU/L) and 51.8 kAU/L (0.5-137.2 kAU/L), p = 0.749]. Anti-SARS-CoV-2 N/S sIgG did not differ in subjects with or without comorbidities (χ2 = 2.98, p = 0.083 for children and χ2 = 2.94, p = 0.086 for adults) or in subjects with or without prolonged therapy (χ2 = 3.064, p = 0.080 for pediatric and χ2 = 0.042, p = 0.838 for adults) (
Table 2).
Table 3 reports the univariate analyses of serum anti-SARS-CoV-2 S-RBD IgG with respect to all studied variables.
Serum anti-SARS-CoV-2 S-RBD IgG median levels (and IQR) significantly differ between subjects with or without previous COVID-19 in pediatric patients [306.2 kBAU/L (3.9-1554.9 kBAU/L) and 1523.7 kBAU/L (410.5-2739.2 kBAU/L), respectively, p < 0.001], but the difference is not statistically significant in adults [426.9 kBAU/L (297.5-4993.4) and 2171.3 (1101.3-4081.7), p = 0.121]. The presence of comorbidities was significantly associated with anti-SARS-CoV-2 S-RBD IgG in pediatric patients and adults (
Table 3); prolonged therapy was related to the presence of antibodies in serum in children, but not in adults (
Table 3). The time from the last COVID-19 infection or vaccination was not associated with anti-SARS-CoV-2 N/S sIgG or with anti-SARS-CoV-2 S-RBD IgG, both in pediatric and adult patients (
Table 2 and
Table 3).
Table 4 reports multivariate analyses performed on salivary and serum of adult and pediatric samples for what concerns all the studied variables (the presence or absence of comorbidities, prolonged therapy, previous SARS-CoV-2 infection and time from last COVID-19 infection or vaccination). Statistically significant differences were found in salivary and serum antibody titers for what concerns age in pediatric patients (p < 0.001 for both). However, none of the studied variables showed statistically significant association with the presence of antibodies, with the exception of the presence or absence of comorbidities for serum anti-SARS-CoV-2 S-RBD IgG in adults.
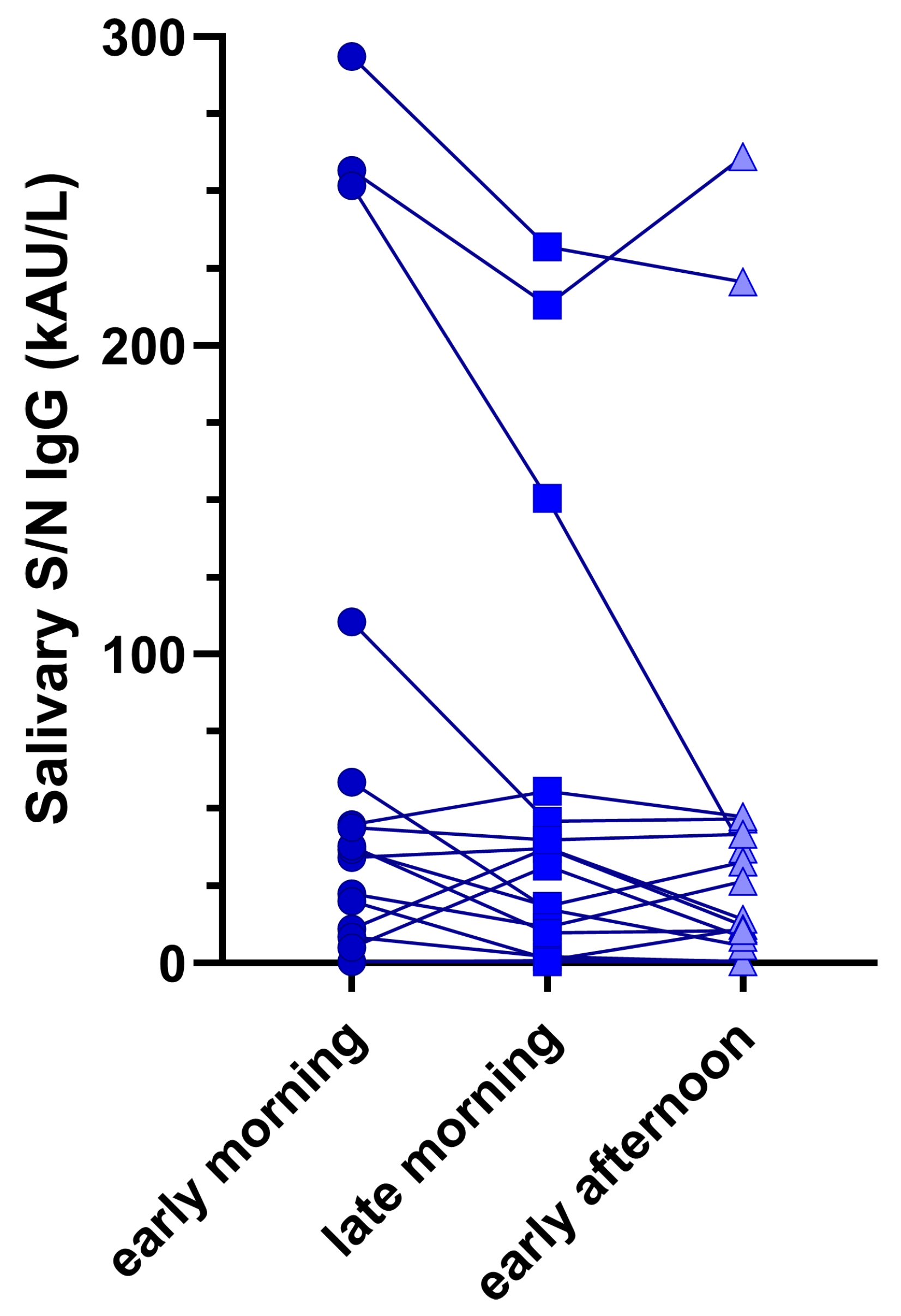
3.4. Impact of sample collection time on salivary Ab
Figure 2 shows the intra-subject differences in anti-SARS-CoV-2 N/S sIgG across all collected samples. Although differences were observable, they were not statistically significant (Friedmans’ test p = 0.327).
4. Discussion
COVID-19 vaccination represents the standard care for preventing SARS-CoV-2 severe infection, and vaccines have been shown to elicit a strong cellular and immunological response, which protects against viral infection [
19]. Thus, the assessment of SARS-CoV-2 specific antibodies is not typically sought, even if this analysis could be of relevance in evaluating the immunological status of fragile and immunocompromised patients [
20]. Differently from adults, SARS-CoV-2 infection is notably mild in children [
21]. However, in fragile children, such as immunosuppressed patients, transplant recipients, or with comorbidities [
22] and/or prolonged therapies, COVID-19 might increase the risk of hospitalization [
17,
23]. In such situations, both in adults and children, identifying the existence of SARS-CoV-2 antibodies could hold clinical relevance; additionally, in multi-system inflammatory syndrome in children (MIS-C) diagnosis, serologic testing for SARS-CoV-2 is useful for the detection of past infection and thus for prescribing the correct therapy [
24].
Rather than relying on serological tests for SARS-CoV-2 antibodies, an alternative approach is represented by the use of salivary samples. This method could improve overall patient compliance, encompassing even those who are fragile, elderly, and children [
25]. Saliva testing serves as a non-invasive source of antibodies for immunoassays, and it facilitates cost-effective epidemiological monitoring of infections [
26]. However, antibody titers tend to be lower in saliva than in serum [
27] and thus assays should be carefully evaluated before use.
In the present study, an ELISA immunoassay was used to detect antibodies against S/N peptides of SARS-CoV-2 (anti-SARS-CoV-2 N/S sIgG) in salivary samples; results were then compared to serum anti-SARS-CoV-2 S-RBD IgG through the use of an established chemiluminescence assay detecting the RBD portion of the viral spike protein [
28]. Precision analysis of the assay demonstrated a mean CV of 17.5% in five salivary samples tested five times each. One additional sample, with a mean concentration of 2.01 kAU/L was tested 25 times, reporting a CV of 20.9%, further supporting the good repeatability of the assay, also for low anti-SARS-CoV-2 N/S sIgG levels. However, the assay lacked linearity for four out of six samples analyzed, suggesting that it has some limitations in accurately quantifying anti-SARS-CoV-2 N/S sIgG antibodies within certain concentration ranges. This fact should be considered especially when patients undergo antibody monitoring during time [
29].
For what concerns the clinical performances, some differences were found between saliva and serum evaluations. Firstly, adult patients reported higher SARS-CoV-2 antibody titers than children. Differences between saliva and serum have been described by some studies [
30,
31], underlining that the persistence of elevated SARS-CoV-2 antibodies in plasma may not indicate the persistence of antibodies at mucosal sites such as the nose or mouth [
31]. Other authors demonstrated a high concordance between saliva and serological findings [
26,
32], while partial discordant results are reported by other studies [
30], meaning that salivary matrix still requires further investigation and validation as a source of biological analytes. Age-related factors may influence antibody titers both in children and in adults since age was found to be significantly associated with anti-SARS-CoV-2 antibody levels. In addition, no statistically significant differences were found for anti-SARS-CoV-2 N/S sIgG and for anti-SARS-CoV-2 S-RBD IgG between children and adults with and without previous SARS-CoV-2 infection at multivariate analyses (
Table 4). These results are unexpected since a wane of Ab levels is well described in the literature [
33,
34,
35]. This could be explained by the limited time from samples collection and vaccination or SARS-CoV-2 infection. Lastly, even though small antibody variations were found depending on the collection time of saliva, they were not statistically significant in either patient, suggesting the possibility of collecting samples at different time points during the day, including outpatient visits.
Saliva has already been tested by other authors, demonstrating a high concordance with serological findings in some studies [
26,
32], and partial discordant results in other cases [
30], meaning that the salivary matrix still requires further investigation and validation as a source of biological analytes. Although the humoral response to SARS-CoV-2 by specific antibodies has been extensively investigated in serum samples of COVID-19-infected patients and vaccinated patients, local humoral immunity in the oral cavity and its relationship to systemic antibody levels needs to be further addressed [
36].
This study presents several limitations, such as the small sample size, and the large variability of the time from the last SARS-CoV-2 infection or last COVID-19 vaccination; on the other hand, a strength of the study is represented by the well characterized cohort of pediatric patients.
5. Conclusions
In conclusion, mucosal immunity could provide valuable data that are useful to deepen the understanding of SARS-CoV-2 immune response and antibody presence [
37]. The use of alternative matrices found at the site of viral entry (i.e. oral cavity) may provide further information and novel analytical methods for antibody testing in the general population, in order to monitor immunity, induced either by previous SARS-CoV-2 infection or vaccination, especially for fragile patients.
Supplementary Materials
Supplementary Table S1: Performance verification and precision analysis for six evaluated samples.
Author Contributions
Study design: A.P., C.D.; Investigation, A.P., C.C., G.F., I.T.; Sample Collection: C.D., S.G., G.F., I.T.; Statistical analysis: A.P.; Writing—original draft, I.T, A.P., C.C., C.D; Writing—review & editing, S.G., D.D., D.B., C.G., M.P.; Supervision: C.G., M.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors sincerely thank the UniCredit Foundation, Italy for their generous grant, crucial in advancing this research. The invaluable support provided not only made this project possible but also significantly contributed to the pursuit of knowledge and innovation in the field.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local Ethical Committee (Prot. N° 0070714 of November 24th, 2020; amendment N°71779 of November 26th, 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is unavailable due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Statement on the fourteenth meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic.
- Craig, K.J.T.; Rizvi, R.; Willis, V.C.; Kassler, W.J.; Jackson, G.P. Effectiveness of Contact Tracing for Viral Disease Mitigation and Suppression: Evidence-Based Review. JMIR Public Health and Surveillance 2021, 7, e32468. [Google Scholar] [CrossRef] [PubMed]
- Cosma, C.; Galla, L.; Padoan, A.; Furlan, G.; Marchioro, L.; Zaninotto, M.; Basso, D.; Plebani, M. SARS-CoV-2 specific T-cell humoral response assessment after COVID-19 vaccination using a rapid direct real-time PCR amplification. Clinical Chemistry and Laboratory Medicine (CCLM) 2023, 61, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Cosma, C.; Bonfante, F.; Della, R.F.; Barbaro, F.; Santarossa, C.; Dall’Olmo, L.; Pagliari, M.; Bortolami, A.; Cattelan, A.; et al. Neutralizing antibody titers six months after Comirnaty vaccination: kinetics and comparison with SARS-CoV-2 immunoassays. Clin Chem Lab Med 2022, 60, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Cosma, C.; Bonfante, F.; Rocca, F. della; Barbaro, F.; Santarossa, C.; DallOlmo, L.; Pagliari, M.; Bortolami, A.; Cattelan, A.; et al. SARS-CoV-2 neutralizing antibodies after one or two doses of Comirnaty (BNT162b2 BioNTech/Pfizer): Kinetics and comparison with chemiluminescent assays. Clinica Chimica Acta 2021, 523, 446–453. [Google Scholar] [CrossRef]
- Bonfante, F.; Costenaro, P.; Cantarutti, A.; Chiara, C.D.; Bortolami, A.; Petrara, M.R.; Carmona, F.; Pagliari, M.; Cosma, C.; Cozzani, S.; et al. Mild SARS-CoV-2 Infections and Neutralizing Antibody Titers. Pediatrics 2021, 148. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Mohamed, S.; Sanders, E.C.; Gommerman, J.L.; Tal, M.C. Guardians of the oral and nasopharyngeal galaxy: <scp>IgA</scp> and protection against <scp>SARS-CoV</scp>-2 infection. Immunological Reviews 2022, 309, 75–85. [Google Scholar] [PubMed]
- Di Chiara C, Cantarutti A, Costenaro P, et al. Long-term Immune Response to SARS-CoV-2 Infection Among Children and Adults After Mild Infection. JAMA Netw Open. 2022;5(7):e2221616. Published 2022 Jul 1. [CrossRef]
- Lin, J.E.; Asfour, A.; Sewell, T.B.; Hooe, B.; Pryce, P.; Earley, C.; Shen, M.Y.; Kerner-Rossi, M.; Thakur, K.T.; Vargas, W.S.; et al. Neurological issues in children with COVID-19. Neuroscience Letters 2021, 743, 135567. [Google Scholar] [CrossRef]
- Sharma, A.; Farouk, I.A.; Lal, S.K. COVID-19: A Review on the Novel Coronavirus Disease Evolution Transmission, Detection, Control and Prevention. Viruses 2021, 13, 202. [Google Scholar] [CrossRef]
- Rai, P.; Kumar, B.K.; Deekshit, V.K.; Karunasagar, I.; Karunasagar, I. Detection technologies and recent developments in the diagnosis of COVID-19 infection. Applied Microbiology and Biotechnology 2021, 105, 441–455. [Google Scholar] [CrossRef]
- Wang, Y.; Upadhyay, A.; Pillai, S.; Khayambashi, P.; Tran, S.D. Saliva as a diagnostic specimen for SARS-CoV-2 detection: A scoping review. Oral Diseases 2022, 28, 2362–2390. [Google Scholar] [CrossRef]
- Basso, D.; Aita, A.; Padoan, A.; Cosma, C.; Navaglia, F.; Moz, S.; Contran, N.; Zambon, C.-F.; Cattelan, A.M.; Plebani, M. Salivary SARS-CoV-2 antigen rapid detection: A prospective cohort study. Clinica Chimica Acta 2021, 517, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Kaczor-Urbanowicz, K.E.; Carreras-Presas, C.M.; Aro, K.; Tu, M.; Garcia-Godoy, F.; Wong, D.T.W. Saliva diagnostics Current views and directions. Experimental Biology and Medicine 2016, 242, 459–472. [Google Scholar] [CrossRef]
- Pisanic, N.; Randad, P.R.; Kruczynski, K.; Manabe, Y.C.; Thomas, D.L.; Pekosz, A.; Klein, S.L.; Betenbaugh, M.J.; Clarke, W.A.; Laeyendecker, O.; et al. COVID-19 Serology at Population Scale: SARS-CoV-2-Specific Antibody Responses in Saliva. J Clin Microbiol 2020, 59. [Google Scholar] [CrossRef] [PubMed]
- Klingler, J.; Lambert, G.S.; Itri, V.; Liu, S.; Bandres, J.C.; Enyindah-Asonye, G.; Liu, X.; Simon, V.; Gleason, C.R.; Kleiner, G.; et al. Detection of Antibody Responses Against SARS-CoV-2 in Plasma and Saliva From Vaccinated and Infected Individuals. Frontiers in Immunology 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Di Chiara C, Boracchini R, Sturniolo G, et al. Clinical features of COVID-19 in Italian outpatient children and adolescents during Parental, Delta, and Omicron waves: a prospective, observational, cohort study. Front Pediatr. 2023;11:1193857. Published 2023 Aug 10. [CrossRef]
- Padoan, A.; Bonfante, F.; Cosma, C.; Chiara, C.D.; Sciacovelli, L.; Pagliari, M.; Bortolami, A.; Costenaro, P.; Musso, G.; Basso, D.; et al. Analytical and clinical performances of a SARS-CoV-2 S-RBD IgG assay: comparison with neutralization titers. Clinical Chemistry and Laboratory Medicine (CCLM) 2021, 59, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Cosma, C.; Bonfante, F.; Rocca, F. della; Barbaro, F.; Santarossa, C.; Dall’Olmo, L.; Pagliari, M.; Bortolami, A.; Cattelan, A.; et al. Neutralizing antibody titers six months after Comirnaty vaccination: kinetics and comparison with SARS-CoV-2 immunoassays. Clinical Chemistry and Laboratory Medicine (CCLM) 2021, 60, 456–463. [Google Scholar] [CrossRef]
- Civita, E.L.; Zannella, C.; Brusa, S.; Romano, P.; Schettino, E.; Salemi, F.; Carrano, R.; Gentile, L.; Punziano, A.; Lagnese, G.; et al. BNT162b2 Elicited an Efficient Cell-Mediated Response against SARS-CoV-2 in Kidney Transplant Recipients and Common Variable Immunodeficiency Patients. Viruses 2023, 15, 1659. [Google Scholar] [CrossRef]
- Du, W.; Yu, J.; Wang, H.; Zhang, X.; Zhang, S.; Li, Q.; Zhang, Z. Clinical characteristics of COVID-19 in children compared with adults in Shandong Province China. Infection 2020, 48, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Müller, G.Y.; Olivo-Gutierrez, M.; Favela-Aragon, K.L.; Hernández-Castillo, P.A.; Esquivel-Gomez, V.; Camacho-Ortiz, A. Prevalence of antibodies against SARS-CoV-2 in hemodialysis patients. International Urology and Nephrology 2021, 54, 457–458. [Google Scholar] [CrossRef]
- Turtle, L.; Thorpe, M.; Drake, T.M.; Swets, M.; Palmieri, C.; Russell, C.D.; Ho, A.; Aston, S.; Wootton, D.G.; Richter, A.; et al. Outcome of COVID-19 in hospitalised immunocompromised patients: An analysis of the WHO ISARIC CCP-UK prospective cohort study. PLOS Medicine 2023, 20, e1004086. [Google Scholar] [CrossRef]
- Oyeniran, S.J.; Wang, H.; Everhart, K.; Mack, K.; Harvey, K.; Leber, A.L. Performance comparison of three commercial tests for the detection of SARS-CoV-2 antibodies in a common set of pediatric samples. Journal of Immunological Methods 2023, 520, 113536. [Google Scholar] [CrossRef]
- Torul, D. ; omezli, mehmet Is saliva a reliable biofluid for the detection of COVID-19? Dental and Medical Problems 2021, 58, 229–235. [Google Scholar] [CrossRef]
- Egorov, A.I.; Griffin, S.M.; Fuzawa, M.; Kobylanski, J.; Grindstaff, R.; Padgett, W.; Simmons, S.; Hallinger, D.R.; Styles, J.N.; Wickersham, L.; et al. A Multiplex Noninvasive Salivary Antibody Assay for SARS-CoV-2 Infection and Its Application in a Population-Based Survey by Mail. Microbiology Spectrum 2021, 9. [Google Scholar] [CrossRef]
- Guerra, E.N.S.; Castro, V.T. de; Santos, J.A. dos; Acevedo, A.C.; Chardin, H. Saliva is suitable for SARS-CoV-2 antibodies detection after vaccination: A rapid systematic review. Frontiers in Immunology 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Bonfante, F.; Cosma, C.; Di, C.C.; Sciacovelli, L.; Pagliari, M.; Bortolami, A.; Costenaro, P.; Musso, G.; Basso, D.; et al. Analytical and clinical performances of a SARS-CoV-2 S-RBD IgG assay: comparison with neutralization titers. Clin Chem Lab Med 2021, 59, 1444–1452. [Google Scholar] [CrossRef]
- Casian, J.G.; Angel, A.N.; Lopez, R.; Bagos, C.; MacMullan, M.A.; Bui, M.L.; Chellamathu, P.; Das, S.; Turner, F.; Slepnev, V.; et al. Saliva-Based ELISAs for Effective SARS-CoV-2 Antibody Monitoring in Vaccinated Individuals. Frontiers in Immunology 2021, 12. [Google Scholar] [CrossRef]
- Azzi, L.; Gasperina, D.D.; Veronesi, G.; Shallak, M.; Maurino, V.; Baj, A.; Gianfagna, F.; Cavallo, P.; Dentali, F.; Tettamanti, L.; et al. Mucosal immune response after the booster dose of the BNT162b2 COVID-19 vaccine. eBioMedicine 2023, 88, 104435. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I.; Dropulic, L.; Wang, K.; Gangler, K.; Morgan, K.; Liepshutz, K.; Krogmann, T.; Ali, M.A.; Qin, J.; Wang, J.; et al. Comparison of Levels of Nasal Salivary, and Plasma Antibody to Severe Acute Respiratory Syndrome Coronavirus 2 During Natural Infection and After Vaccination. Clinical Infectious Diseases 2022, 76, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Pisanic, N.; Randad, P.R.; Kruczynski, K.; Manabe, Y.C.; Thomas, D.L.; Pekosz, A.; Klein, S.L.; Betenbaugh, M.J.; Clarke, W.A.; Laeyendecker, O.; et al. COVID-19 Serology at Population Scale: SARS-CoV-2-Specific Antibody Responses in Saliva. Journal of Clinical Microbiology 2020, 59. [Google Scholar] [CrossRef]
- Liew, F.; Talwar, S.; Cross, A.; Willett, B.J.; Scott, S.; Logan, N.; Siggins, M.K.; Swieboda, D.; Sidhu, J.K.; Efstathiou, C.; et al. SARS-CoV-2-specific nasal IgA wanes 9 months after hospitalisation with COVID-19 and is not induced by subsequent vaccination. eBioMedicine 2023, 87, 104402. [Google Scholar] [CrossRef]
- Evans, J.P.; Zeng, C.; Carlin, C.; Lozanski, G.; Saif, L.J.; Oltz, E.M.; Gumina, R.J.; Liu, S.-L. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Science Translational Medicine 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.R.; Holm, B.E.; Pérez-Alós, L.; Bayarri-Olmos, R.; Rosbjerg, A.; Fogh, K.; Pries-Heje, M.M.; Møller, D.L.; Hansen, C.B.; Heftdal, L.D.; et al. Short-Lived Antibody-Mediated Saliva Immunity against SARS-CoV-2 after Vaccination. Microbiology Spectrum 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Garziano, M.; Utyro, O.; Poliseno, M.; Santantonio, T.A.; Saulle, I.; Strizzi, S.; Caputo, S.L.; Clerici, M.; Introini, A.; Biasin, M. Natural SARS-CoV-2 Infection Affects Neutralizing Activity in Saliva of Vaccinees. Frontiers in Immunology 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- MacMullan, M.A.; Ibrayeva, A.; Trettner, K.; Deming, L.; Das, S.; Tran, F.; Moreno, J.R.; Casian, J.G.; Chellamuthu, P.; Kraft, J.; et al. ELISA detection of SARS-CoV-2 antibodies in saliva. Scientific Reports 2020, 10. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).