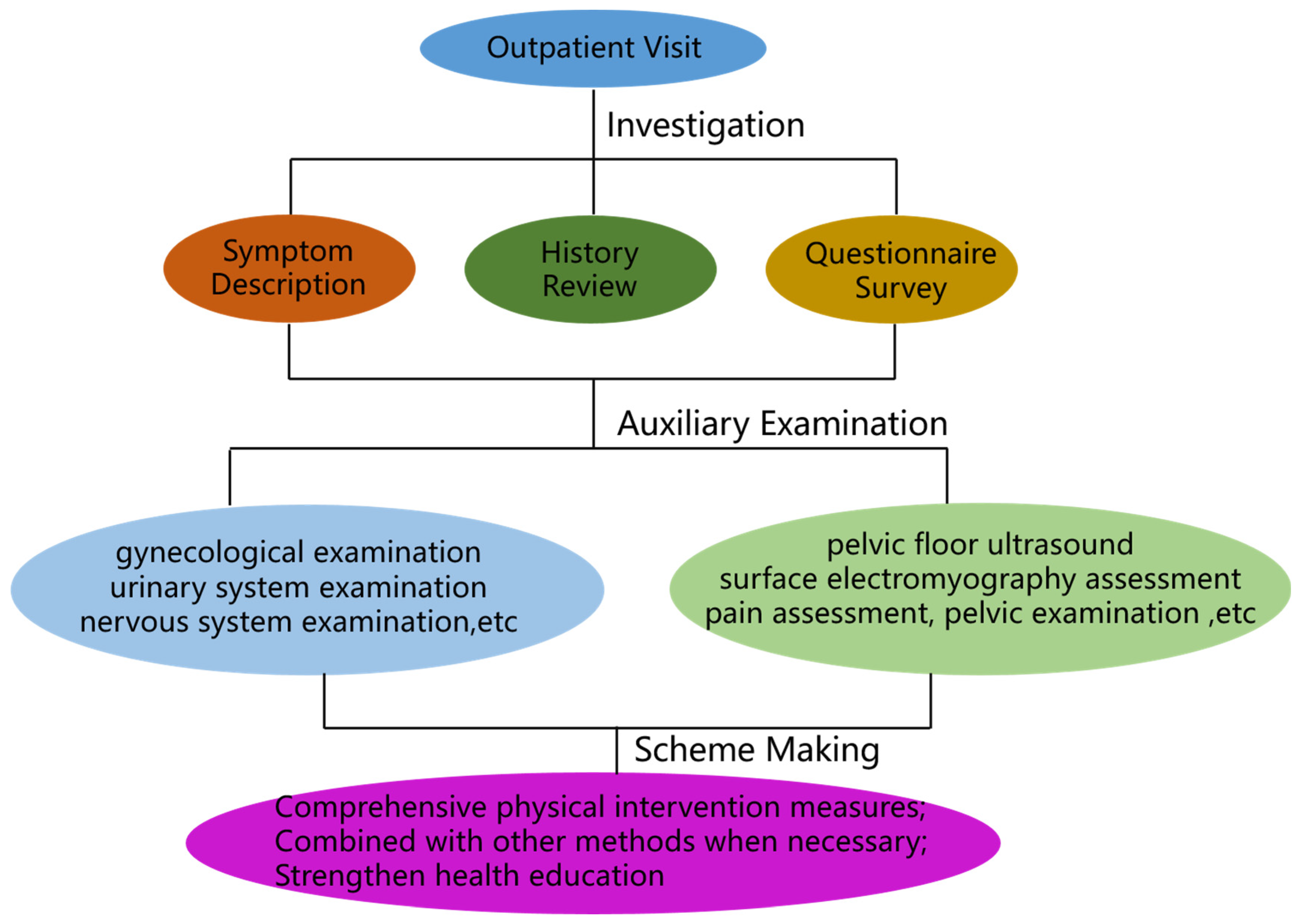
IV. Principles and contents of treatment for pelvic floor dysfunction
Physical therapy techniques such as electric stimulation, biofeedback, magnetic stimulation, radiofrequency, laser, etc. can be used alone or in combination to solve the problem of pelvic floor dysfunction. The pathogenesis of pelvic floor dysfunction is complex, and its occurrence may be one or two or more types of pelvic floor supportive tissue damage or function decline, therefore, the key to the implementation of the program is to focus on the pathogenesis and causes of its pathogenesis, and to solve the problem in a systematic and targeted manner.
Physiotherapy measures are formulated on the basis of clinical diagnosis by integrating relevant medical information from obstetrics and gynecology, anorectal medicine, urology, imaging, and clinical examination departments, etc., and then personalized treatment plans are formulated according to the type of disease, severity, and combined complications.
- (I)
Indications, contraindications and points of intervention
Pregnancy, childbirth, age, long-term increase in abdominal pressure, menopause, obesity and other causes of muscle, connective tissue, fascia and other abnormalities or injuries, which in turn induce a series of conventional pelvic floor dysfunction, such as lower urinary symptoms, pelvic organ prolapse, defecation disorders, chronic pelvic pain, sexual dysfunction; pelvic floor dysfunction of non-surgical indications [
29]; post-pelvic surgery urinary / defecation disorders, postpartum pelvic floor dysfunction of prevention and treatment.
- 2.
Active infection at the site of irritation; bladder stones; pregnant women; pelvic tumors; menstruation, puerperium; neurological or mental diseases; cardiac pacemakers. Neurological diseases; cardiac pacemakers; cognitive disorders; genitourinary inflammation in the acute stage.
- 3.
Intervention points
Physiotherapy intervention should grasp the following key points: a) systematic assessment before intervention (combining symptoms, medical history, physical examination, auxiliary examination, etc.); b) timing of therapeutic intervention, selection of parameters of the treatment program, frequency of intervention, treatment cycle and intensity of stimulation; c) adjustment of the treatment program if the effect of the phase of treatment is unsatisfactory; d) patient adherence: clinical practitioners should pay attention to the incidence of FPFD and the related factors, and identify and prevent FPFD as early as possible. Meta-analysis results showed that patients with a large degree of pelvic floor injury and a high number of deliveries had better adherence, which may be related to a higher need for treatment of the disease [
36], in addition to maintaining active communication in the treatment to provide timely feedback to patients; e) Professional guidance: pelvic floor muscle training should be supervised by a qualified person. A systematic review found that, in the treatment of SUI 58.8% of patients achieved significant improvement after 12 months of supervision. After 12 months, PFMT improved by 17% in patients with UUI and 28% in patients with MUI, and symptoms and quality of life improved after 6 months [
37].
- 4.
Precautions
a) Pay attention to improving lifestyle and controlling weight, such as eating a balanced diet, quitting smoking and limiting alcohol, and drinking less or no caffeinated beverages [
38,
39]; b) Necessary at-home pelvic floor muscle training, behavioral training [
38], etc., such as Kegel exercises, vaginal dumbbell training, and bladder exercises; c) Good and positive psychological communication; and d) If necessary, physiotherapy combined with medication [
40], uterine support, surgery, and other modalities for comprehensive treatment [
38].
- (II)
Physiotherapy interventions
Patients with pelvic floor dysfunction should be intervened early, especially menopausal and postmenopausal women, postpartum population, pelvic preoperative/postoperative and other high-risk groups should be intervened as early as possible for treatment and prevention. Some studies have shown that pelvic floor muscle training can be used as an adjunct to pelvic organ prolapse surgery [
29] to help alleviate the degree of prolapse and improve the quality of life, as well as to effectively improve prolapse symptoms. One to three months postpartum is the key period for recovery of pelvic floor function and prevention of pelvic floor dysfunction; three months to one year postpartum is the key period for recovery of pelvic floor function and prevention of pelvic floor dysfunction; and one year postpartum is the key period for targeting pelvic floor dysfunction and preventing further progression and complications.
- 2.
-
Principles of intervention
- (1)
Distinguish between types of pelvic floor dysfunction
Explanations of the occurrence and pathogenesis of pelvic floor dysfunction mainly involve the 1992 DeLancey vaginal support structure “three-level” theory [
41], DeLancey’s “hammock” hypothesis in 1994 [
42], Petros and Ulmsten’s “Integral Theory” in 1990 [
43], and the 1993 Norton “dry dock theory” [
44] pelvic floor theories. These theories well explain the pathogenesis of pelvic floor dysfunction such as urinary incontinence, pelvic organ prolapse, pelvic floor pain, etc., focusing on the fact that muscles, connective tissues, nerves, and blood vessels are interconnected and inextricably linked and work together to maintain the anatomical position of the pelvic organs and normal physiological function. Therefore, the solution of pelvic floor dysfunction often cannot be solved by one physical therapy modality alone, but should be integrated with a variety of physical therapy modalities, choosing the appropriate magnetic stimulation, electrical stimulation, radiofrequency, biofeedback, and other technological treatment programs. In the case of stress incontinence, for example, the pelvic floor muscle support is insufficient or weakened, the urethral closure function decreases, and collagen synthesis decreases [
45], and the integrated treatment modality should use different types of physical therapy techniques according to the damage of muscles and fascia.
- (2)
Types and methods of intervention
Physiotherapy programs for pelvic floor dysfunction should combine prevention and treatment, delay further disease progression, prevent complications, and combine prevention and treatment.
The types of intervention can be divided into targeted treatment programs, focused prevention programs, and universal clinical advice and guidance. Targeted treatment programs are mainly applicable to patients with conventional pelvic floor dysfunction, post-pelvic surgery urinary/defecation/sexual dysfunction, and post-partum pelvic floor dysfunction, etc. Detailed medical history, systematic structural (e.g., pelvic floor ultrasonography) and functional assessment (e.g., pelvic floor electromyography assessment), comprehensive auxiliary examinations, comprehensive clinical diagnosis, intensive pelvic floor intervention for type of disease, severity, and accompanying disorders, etc., formulate a stage-by-stage treatment program, and adjust the specific program at the right time according to the patient’s stage-by-stage recovery. Focused prevention program, mainly applicable to the screening and prevention of diseases in key and high-risk groups, such as pelvic floor screening after 1-3 months after pelvic surgery, pelvic floor function recovery after pelvic surgery, pelvic floor function screening in middle-aged, elderly and post-partum and other high-risk groups, and formulate personalized treatment plans according to the patient’s medical history and other auxiliary examinations. Universal clinical guidance and advice is given to prevent the occurrence of pelvic floor dysfunction through health education, pelvic floor muscle training (PFMT), pelvic floor muscle rehabilitation device (vaginal dumbbell) and other regular pelvic floor muscle training.
For pelvic floor dysfunction, we should do early detection, early diagnosis and early treatment.
- (3)
Selection of physical therapy modalities
With the development of physical therapy technology so far, electric stimulation, biofeedback, magnetic stimulation, radiofrequency and other technologies have been widely used in the treatment of various pelvic floor dysfunction, and some of the technologies have gained certain guideline or consensus recommendations and have been confirmed by a large number of literature. Pelvic floor muscle training (PFMT) can be used as a first-line therapy to address pelvic floor dysfunction, and electrical stimulation, and magnetic stimulation can be used as second-line therapies to address pelvic floor dysfunction when necessary. It has been shown that pelvic floor muscle training with equipment, such as biofeedback, is more effective than pelvic floor muscle training without equipment [
46].
Pelvic floor muscle training enhances muscle strength, coordination, speed of muscle recruitment, and endurance in order to increase intravesical pressure, maintain urethral closure pressure, and support pelvic organs during increased intra-abdominal pressure [
47]. Electrical stimulation on weakened muscles passively activates muscles and nerve fibers. Electrical stimulation using electrodes is an effective and conservative treatment to improve leakage and contractile strength [
48,
49]. It has been noted that magnetic stimulation stimulates the sacral nerves and the pelvic floor in a non-invasive, convenient, acceptable, and flexible way, which can provide options for incontinent patients who are not motivated to perform pelvic floor muscle training (PFMT) by stimulating the pelvic floor in order to allow the muscles to gain contraction [
50]. Radiofrequency stimulates the pelvic floor and elastin neoplastic remodeling to improve the elasticity and strength of fascia and ligaments [
51]. Laser is an energy-based treatment modality that stimulates neovascularization, collagen deposition, and elastic fiber proliferation and promotes epithelial cell proliferation, allowing deep collagen remodeling and neogenesis [
52,
53], and has been used in the treatment of pelvic floor dysfunction in recent years [
54].Different physical techniques address pelvic floor dysfunction at different levels from the mechanism of action.
V. Clinical value of AI system for pelvic floor dysfunction
- (I)
Challenges in the clinical diagnosis of pelvic floor dysfunction
Pelvic floor dysfunction is a complex set of diseases that involve the interaction of multiple systems and functions of the body, such as urinary tract, intestinal tract, and reproductive system. In recent years, with increased awareness of pelvic floor function and advances in medical technology, some progress has been made in the diagnosis and treatment of pelvic floor disorders. However, there are still some urgent problems in diagnosis.
First of all, we must realize that there are technical deficiencies in the current diagnostic means. Among the many diagnostic means, most are based on patients’ subjective evaluation indicators. This category of reporting instruments is collectively known in the clinic as Patient-Reported Outcome Measures (PROMs) [
55]. These subjective indicators, such as symptom self-assessment scales, functional assessments, and physical examinations, while providing information about the severity and impact of the disease, suffer from a high degree of individual variability and subjectivity. For example, in the assessment of stress urinary incontinence, the common clinical symptom scales mainly use the Ingelman-Sundberg scale [
56], which is a relatively subjective means of assessment that categorizes patients’ clinical scores into mild, moderate and severe according to their symptoms. Some other scales include the Chinese-validated incontinence impact questionnaire short form (IIQ-7), which was developed by the International Consultation on Incontinence (ICI) in 2005 [
6]. ICI) in 2005 [
57], and the impact on the patient’s sexual life would be done using the pelvic organ prolapsed- urinary incontinence sexual questionnaire short form (PISQ-12) [
58]. This type of questionnaire also contains a lot of subjective and symptomatic descriptions, but the same lacks some objective indicators such as MRI, ultrasound, etc. to describe the structural changes. In the diagnosis of pelvic organ prolapse, common examination methods include POP-Q, pelvic floor muscle strength examination and some scales, which are similar to the examination of stress urinary incontinence, but also these conventional methods are more subjective, which makes it more difficult to assess the objective pelvic floor structural changes of the patient, and brings some challenges to the subsequent treatment. Overall, in practical application, subjective evaluation indicators may be affected by a variety of factors such as patient’s mood, cultural background and socioeconomic status, thus affecting the accuracy of clinical diagnosis. Therefore, in the diagnosis of pelvic floor dysfunction, objective examination tools such as electromyography and imaging are very important for the selection of subsequent physiotherapy techniques and understanding the changes in pelvic floor structure.
In recent years, pelvic floor surface electromyography has been widely used in the diagnosis of pelvic floor dysfunction [
59]. The technique assesses the functional status of the pelvic floor muscles by measuring the electrical activity of the muscles, and can provide an objective diagnostic basis for the clinic. However, the myoelectric diagnostic technique has limitations and is susceptible to interference by a variety of physiologic and pathologic factors, etc. Pelvic floor ultrasound has been increasingly used in the diagnosis and treatment of female pelvic floor dysfunction, and has been recommended as the preferred examination method by the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) and the International Society of Gynecologic and Urologic Association (IUGA) because of its advantages of easy operation, no radiation, high resolution, and real-time dynamic observation [
60,
61,
62]. Pelvic floor ultrasound is widely used worldwide, especially in Europe and the United States.
In the field of gynecology, pelvic floor ultrasound is commonly used to evaluate the structures of a woman’s pelvic floor, including the uterus, vagina, bladder and rectum. It helps physicians diagnose and treat uterine prolapse, bladder prolapse, urinary incontinence, and other gynecologic conditions. In urology, pelvic floor ultrasound is used to evaluate the urethra and bladder to diagnose and treat problems such as urinary incontinence and urethral strictures. Despite its wide application worldwide, pelvic floor ultrasound is relatively generally practiced in China. The following are some of the main reasons for this: a) Pelvic floor ultrasound requires a high degree of training and experience on the part of specialized physicians and technicians in order to perform and interpret the examination correctly. In China, the standard of training and qualification may be high, which limits the use of pelvic floor ultrasound. b) Healthcare resources in China are unevenly distributed, with large cities and developed areas likely to have better access to high levels of healthcare and rural areas likely to face a lack of healthcare resources, which may affect the promotion and popularization of pelvic floor ultrasound. c) Pelvic floor ultrasound is also globally characterized by a dependence on the experience of the examiner in clinical practice. d) Pelvic floor ultrasound has a high level of clinical experience and is not widely used in China. It is characterized by a high degree of dependence on the examiner’s experience and shortcomings such as time-consuming and cumbersome measurements, which limits its use in the diagnosis of pelvic floor disorders. We selected 40 hospitals to conduct a survey on common examination means in the pelvic floor field and the application of pelvic floor ultrasound, which included a total of 97 pelvic floor specialists, in which most of the doctors chose questionnaires, muscle strength assessment and electromyographic assessment for common assessment means of the pelvic floor, with more than 95% of them choosing electromyographic assessment, and only 27.84% of the doctors chose ultrasound as the routine assessment (
Table 1). When asked, “Is pelvic floor ultrasound helpful in the development of the patient’s initial treatment plan and the benefit of treatment outcomes?” more than 95% of physicians agreed that it was helpful in diagnosis (
Table 2). When asked, “Would you be willing to utilize pelvic floor ultrasound to assist in the initial treatment planning of a patient?” Nearly 90% of physicians found it helpful in determining treatment options (
Table 3). From this survey, it can be determined that for the majority of pelvic floor physicians, electromyographic evaluation and ultrasound both have significant value in diagnosis, but ultrasound has a significant value due to a variety of subjective and objective reasons. However, due to various subjective and objective reasons, the development of ultrasound for the pelvic floor in China is relatively general, and most hospitals do not use ultrasound as a routine diagnostic tool, which greatly hampers the “accurate diagnosis” of pelvic floor dysfunction, and creates a problem that makes it extremely difficult to determine whether or not to carry out surgical treatments and the choice of physical techniques for non-surgical treatments. This makes the subsequent judgment of the need for surgical treatment and the selection of physical techniques for non-surgical treatment extremely difficult. Currently, physical therapy equipment is mainly based on the improvement of disease symptoms, such as biofeedback, electrical stimulation, magnetic stimulation and radiofrequency technology, etc. If a clear image of the pelvic floor structure can be provided, doctors can more accurately select and use physical therapy techniques, thus improving the effectiveness of the treatment and patient satisfaction.
- (II)
Prospects of AI in the diagnosis and treatment of pelvic floor dysfunction
Artificial intelligence (AI) can not only achieve the same diagnostic efficacy as that of senior physicians through feature extraction and automatic segmentation of images, but also be efficient and time-saving, which is conducive to the standardization and promotion of pelvic floor ultrasound.
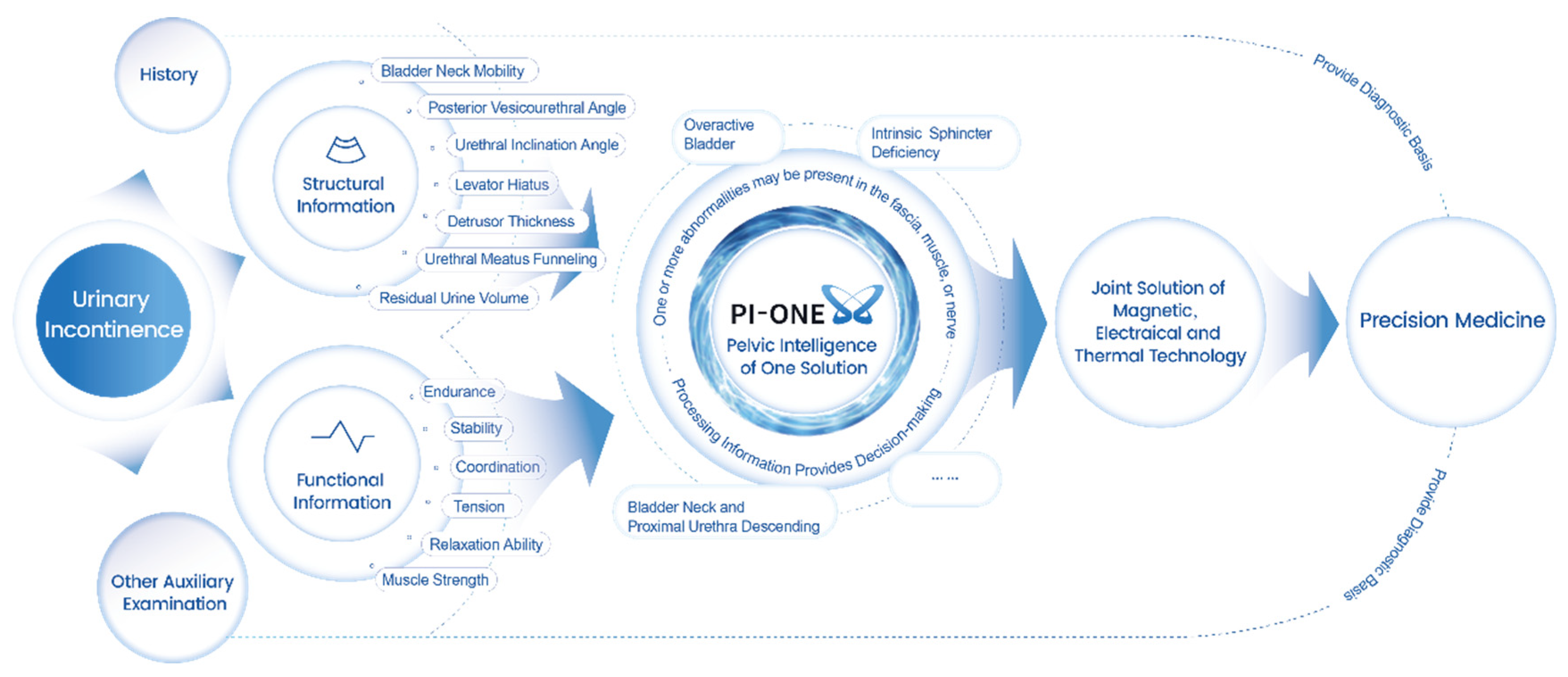
Patients often have two or more pelvic floor dysfunction occurring at the same time. Accurate and systematic assessment is necessary, and comprehensive and personalized treatment plans based on comprehensive clinical diagnosis is an important way to effectively address pelvic floor dysfunction. However, in fact, specialized clinicians are often unable to provide comprehensive medical information on gynecology and obstetrics, urology, anus and intestines, imaging and other specialties, and the systematic and comprehensive diagnosis and treatment of the disease will be limited.
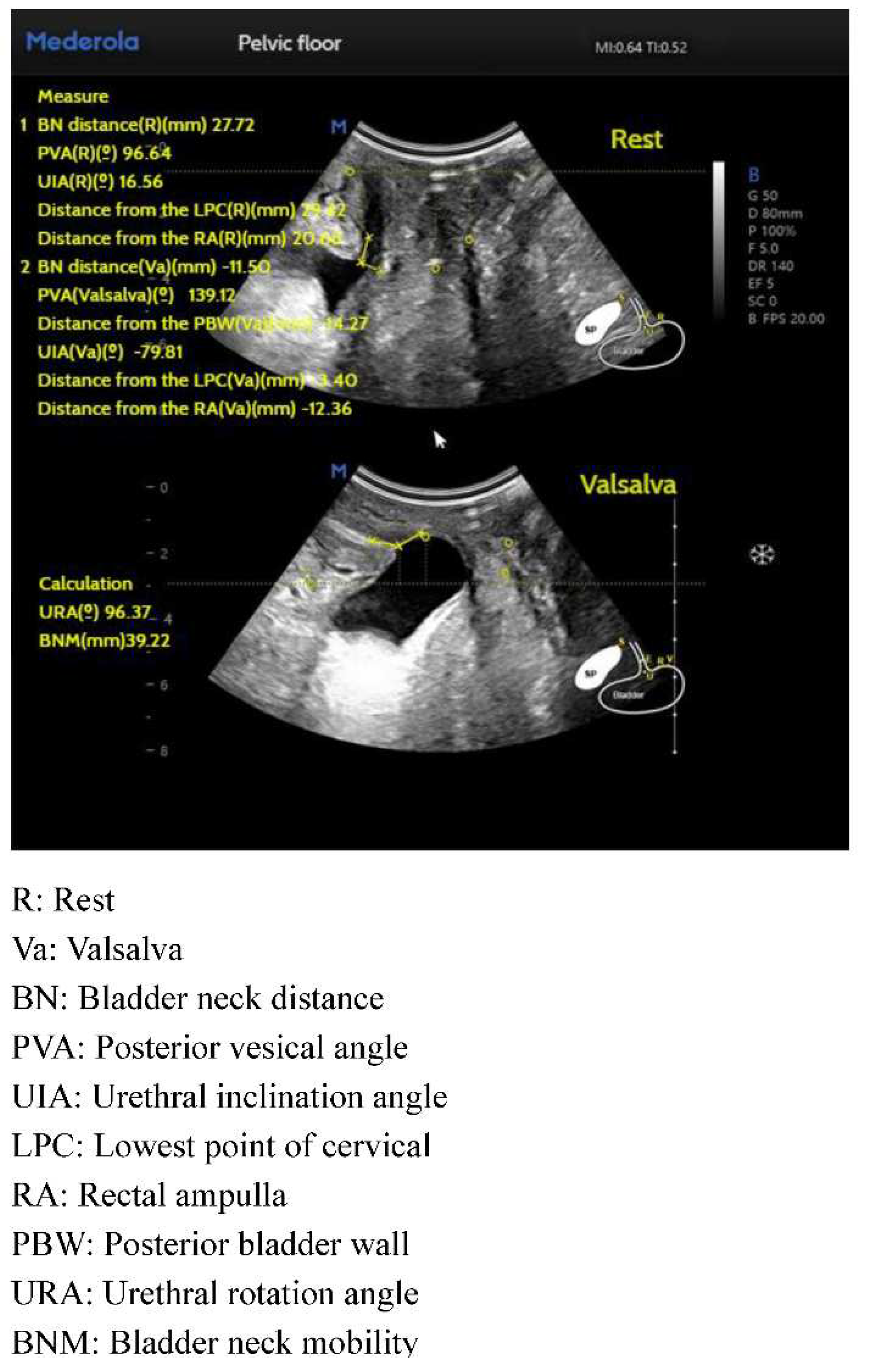
Conventional pelvic floor ultrasound assessment of female pelvic floor structure and function relies on the three dimensions of pelvic floor median sagittal section at maximal Valsalva state, tomographic ultrasound imaging of the anal raphe and anal sphincter and anal raphe fissure at maximal Valsalva state; however, the manual identification is highly empirically dependent, and AI automated identification and measurement can reduce the empirical dependence of the manual identification. The software/algorithms are used to extract features from the labeled images to enable automatic computer recognition. Currently, AI can be used in ultrasound applications to automatically measure and recognize some of the data of pelvic floor structures. For example, in anterior pelvic measurement, the main parameters of the bladder neck in the resting state, including bladder neck distance, bladder posterior angle, urethral inclination angle, etc., are automatically measured, and the above parameters are measured synchronously in the Valsalva state, so as to calculate the angle of rotation of the urethra and the degree of movement of the bladder meridian, etc., and to automatically obtain the bladder bulge and the biological indicators related to stress incontinence, so as to reduce the reliance on the operator’s experience, and to obtain the standardized section and measurement value quickly. The standardized cut surface and measurement value can be obtained quickly by reducing the operator’s experience. (
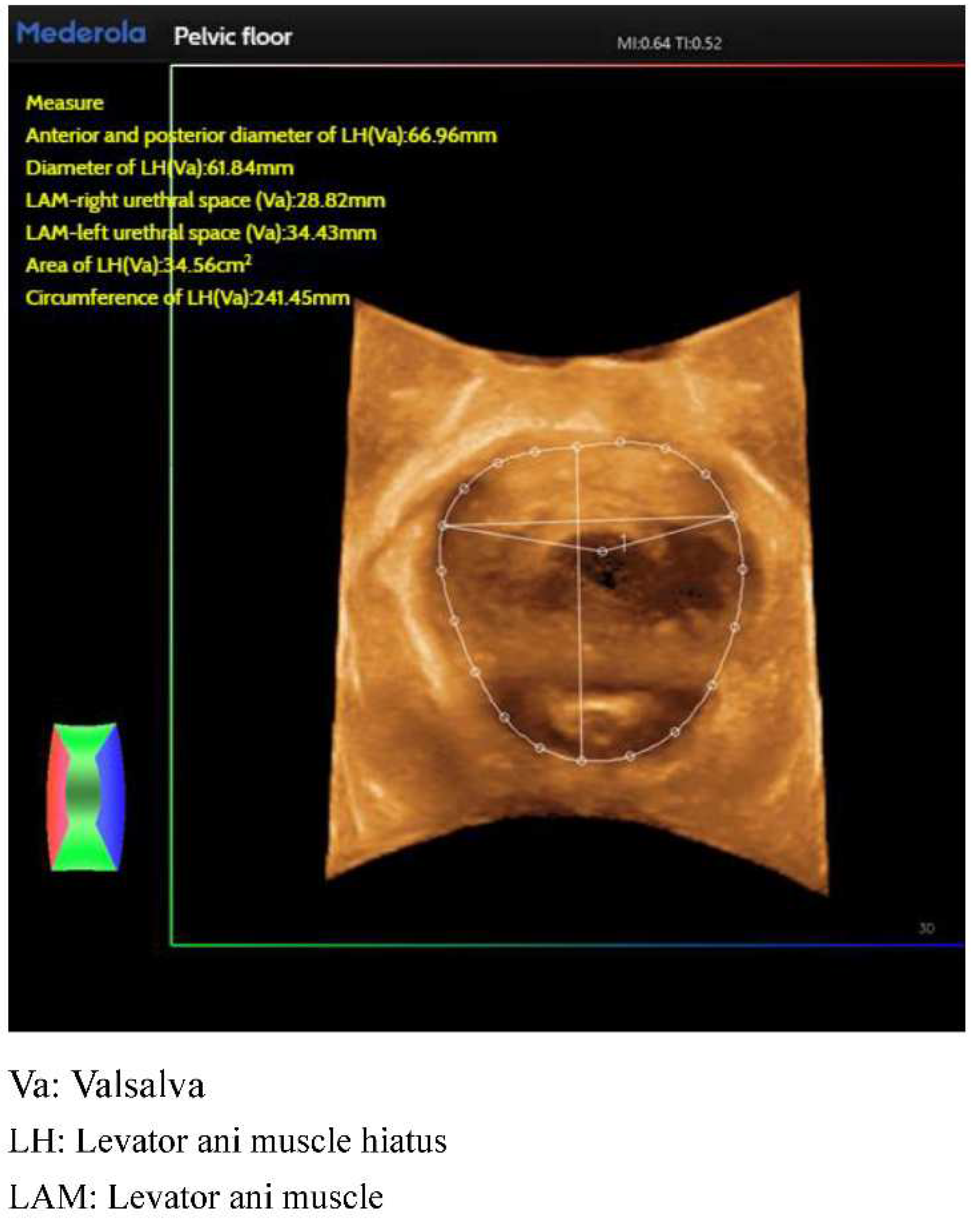
Figure 2). For the anorectal fissure, biological measurements of anorectal fissure dilatation and anorectal muscle damage were automatically obtained by localizing the level of the anorectal fissure in the maximal Valsalva state (
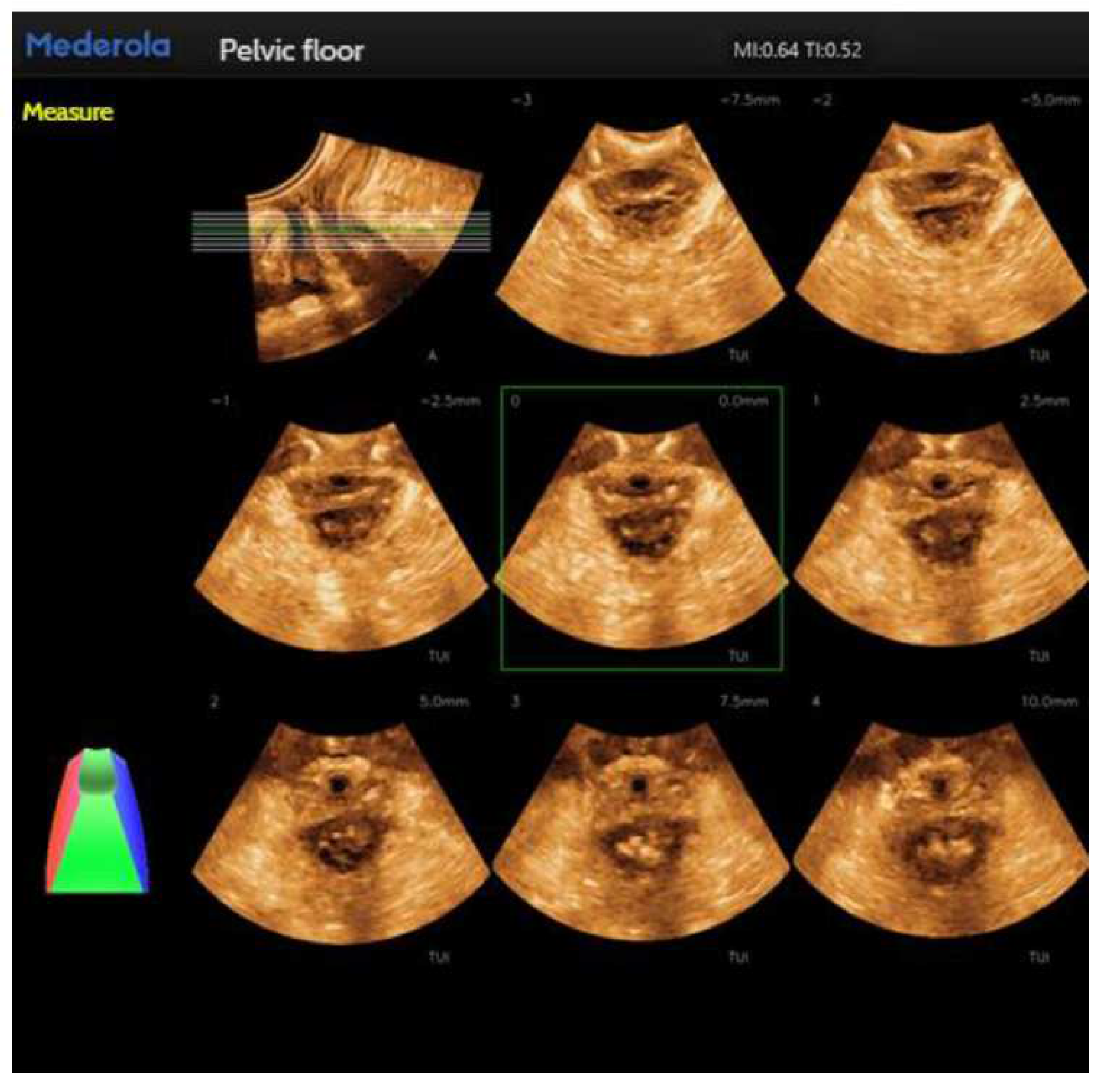
Figure 3). For automatic localization of the TUI tomography of the anal retinaculum muscle, operator experience dependence was reduced, and standardized views of the integrity of the anal retinaculum fissure were automatically obtained (
Figure 4). Automatically obtained parameters of interest include bladder neck mobility, anal reticulum fissure, urethral rotation angle, and posterior vesicourethral angle, which greatly reduces measurement discrepancies due to physician experience.
Pelvic floor diagnosis encompasses a variety of diagnostic tools such as patient history, physical examination, scales, electromyographic assessment of the pelvic floor surface, and pelvic floor imaging. Currently, the diagnosis of pelvic floor dysfunction has been relatively easy through these diagnostic tools, but the choice of physical technology, physical technology-related parameters, and the choice of course of treatment after encountering different structural changes still poses a great challenge to the pelvic floor physician. In the case of urinary incontinence, for example, for patients whose diagnosis does not reveal the presence of pathology, the combination of pelvic floor surface electromyography and pelvic floor ultrasound-related parameters can clarify the presence of specific pathologic structural changes in the patient, so that the appropriate physiotherapeutic interventions can be selected. After clarifying the appropriate physical end of the patient, the AI algorithm combined with the relevant literature reports (machine learning), to develop a specific physical therapy program that is appropriate for the patient. At the root, the intelligent system solves the problem for the diagnosis of the numerous parameters of electromyography, which need to be identified and analyzed one by one; ultrasound examination for the pelvic floor of the numerous parameters of measurement can not be standardized due to differences in the experience of physicians. For doctors who are initially exposed to pelvic floor ultrasound. The problem of not being able to standardize the pelvic floor ultrasound examination. Therapeutically, the intelligent system solves the problem of choosing physical technology. There are many choices of traditional physical therapy means (including biostimulation feedback, electrical stimulation, magnetic stimulation, radiofrequency technology, etc.), but the current status quo lacks the identification of the patient’s pathology and structure, which leads to a lack of basis for the selection of physical therapy means, and the effect is also uneven. With the increased use of intelligent systems, more diagnostic and treatment data will be optimized through automatic learning to develop better and more accurate solutions.
Pelvic floor intelligent diagnosis and treatment is based on a large number of evidence-based basis, fused with the concept of artificial intelligence AI, integrating pelvic floor big data, adding pelvic floor structure (such as pelvic floor ultrasound technology) and function assessment (such as pelvic floor surface electromyography technology) information, which can be used to assist in the diagnosis of pelvic floor dysfunction, to comprehensively assess the state of the pelvic floor, and to provide a reference for the treatment plan of pelvic floor dysfunction. At the same time, it also provides a diagnostic basis for the selection of physical therapy modalities such as electrical stimulation, biofeedback, radiofrequency, pelvic floor muscle training, or combined therapy, etc. Taking urinary incontinence as an example, it describes how pelvic floor intelligent diagnosis and therapy plays a clinical value in the diagnosis and treatment of pelvic floor dysfunction (
Figure 5). Although the pelvic floor intelligent diagnosis and treatment system can to some extent solve the problems of high dependence on the experience of ultrasonographers and the difficulty of choosing physical treatment means, more data are needed to improve its accuracy.
VI. Clinical diagnosis and assessment of common pelvic floor dysfunction
The diagnosis and assessment of urinary incontinence and pelvic organ prolapse, as the most common pelvic floor dysfunction, will be the focus of this content. The clinical diagnosis and assessment of sexual dysfunction, defecation disorders and chronic pelvic pain will be briefly described.
- (I)
Urinary incontinence
The clinical diagnosis of urinary incontinence requires a detailed history and appropriate physical examination. The clinical diagnosis of urinary incontinence requires a detailed history and physical examination. Incontinence is clearly typed and categorized by symptoms, signs, history, and ancillary tests for targeted treatment. If the patient has combined pelvic organ prolapse, the prolapsed uterus or vagina should be repositioned before urinary incontinence assessment and examination, in order to avoid missing the diagnosis of occult urinary incontinence.
- (1)
Medical history
A careful and detailed history is necessary and is the first step in the diagnosis and management of urinary incontinence. Present medical history (time of first onset, frequency of leakage, amount of leakage, predisposing factors, etc.), symptoms (the presence of urinary frequency and urgency, the presence of nocturia, the presence of symptoms of defecation, symptoms of sexual dysfunction, prolapse-specific symptoms, etc.), pregnancy and childbirth history (pregnancy, labor, abortion, induced abortion; cis-sections/cis-sections), menstruation history (menstrual cycle, duration of the line, color (normal/color dark red/color dark violet/color black), the degree of abdominal pain (none/mild pain/moderate pain/ severe pain), whether menopausal), surgical history (post spinal cord injury, pelvic surgery, anti-incontinence surgery, pelvic floor reconstruction, prolapse surgery, etc.), comorbidities (constipation, diabetes mellitus, etc.), family history (whether mothers, sisters have a history of pelvic floor dysfunction), therapeutic history (rehabilitation therapy, etc.), medication history (hormonal, antipsychotic drugs, diuretics, etc.), lifestyle habits (caffeine, strong tea, alcohol, smoking) [
63], physical exercise [
63], quality of life, presence of obesity, mental health status, sexual function [
40,
63], number of leaks during sex [
63], nutritional status, skin symptoms (ulcers, rashes, etc.), and vaginal laxity score [
64].
It is also important to note the presence of the following voiding symptoms: weakness of urination, difficulty in urination, sensation of incomplete urination, interruption of urination, leakage of urine after urination, abdominal pressure voiding, thin/divergent urinary line, multiple repetitions of urination within a short period of time, and position-dependent voiding, which are the common clinical manifestations of complex stress incontinence, to differentiate between non-complicated stress incontinence and complex stress urinary incontinence.
- (2)
Questionnaires
Questionnaires are often used in clinical studies to assist in the assessment of urinary incontinence. These questionnaires reflect the extent to which urinary incontinence affects the patient’s life, and can also be used as an important indicator of outcome. These questionnaires reflect the impact of incontinence on the patient’s life, and can also be used as one of the important indicators for the evaluation of treatment efficacy. Commonly used incontinence-related questionnaires include the Impact of Incontinence Questionnaire (IIQ-7, validated in Chinese), the Pelvic Organ Prolapse-Incontinence Sexuality Questionnaire (PISQ-12), the International Consultative Committee on Incontinence Questionnaire (ICI-Q-SF), the Incontinence Quality of Life Scale (I-QoL), and the Visual Analog Scale (VAS) [
60] (which allows patients to rate the quality of their lives on a scale from 0 to 1), as well as on a scale that allows patients to rate their quality of life. quality of life by rating symptoms on a scale of 0 to 10, with 0 representing “not at all troublesome” and 10 representing very troublesome), and these questionnaires have been adopted and used in much of the clinical literature.
- (3)
Physical examination
Combined with the gynecological examination, bimanual diagnosis to understand the position of the uterus, the morphology of the vulva, skin status, etc.; whether there is pelvic organ prolapse, type and degree of prolapse [
65]; pain examination, pelvic floor muscles and fascia with or without pressure pain, pain level (combined with visual analog scoring VAS); abdominal examination, the presence of abdominal masses; anal finger examination of anal sphincter muscle strength and rectal bulge; neurological examination, including perineal sensitivity, bulbocavernous muscle reflexes and so on.
Uncomplicated stress urinary incontinence was characterized as follows: no history of disease affecting the function of the lower urinary tract, no vaginal mass beyond the hymenal ring, and no vaginal deformity was found. Complex stress incontinence was characterized as follows: presence of neurogenic, diabetic, or Alzheimer’s disease; presence of a genitourinary basket urethral diverticulum; presence of a vaginal mass beyond the hymenal ring or pelvic organ prolapse. In addition, semi-quantitative assessment of pelvic floor muscle function using the modified Oxford muscle strength classification is necessary [
40].
- (4)
Urinary pad test
The urinary pad test is an important tool to assist in the diagnosis and typing of urinary incontinence, and can accurately measure the amount of urine leakage, indirectly affecting the choice of treatment and monitoring the effectiveness of treatment. It indirectly influences the choice of treatment and the monitoring of treatment effects. Depending on the time of testing, there are 20-minute, 1-hour, 24-hour and 48-hour pad tests [
66], of which the 1-h pad test is highly accurate but has poor reproducibility compared with the 24-hour pad test, which has relatively high sensitivity and specificity [
66]. The 1-hour urine pad test is mostly used in the literature [
63]. Specific methods: the whole test lasts for 1 hour, and the bladder is kept full for 1 hour, during which no urination is performed. The weighed urine pads were pre-positioned in the corresponding location, and in the 0th-15th minutes, the patient drank 500 ml of plain water; in the 16th-45th minutes, the patient walked and went up and down one flight of stairs, and in the last 15 minutes, the patient operated according to the following steps, sat and stood up 10 times, coughed forcefully for 10 times, ran on the spot for 1 minute, picked up the ground objects repeatedly for 5 times, and washed his hands with running water for 1 minute again. At the end of all the procedures, the pads were weighed, and the patient urinated and the urine output was recorded.
- (5)
Pelvic floor ultrasound
Pelvic floor ultrasound is characterized by real-time dynamic imaging, visualization, and multi-planar display of the location of anorectal muscle injuries, etc. It is mainly applied to Postpartum pelvic floor function assessment, diagnosis and severity assessment of pelvic floor dysfunction, evaluation of pelvic floor treatment efficacy, preoperative/postoperative assessment, and diagnosis of symptomatic urinary incontinence [
63]. The examination position is usually in the bladder lithotomy position. Pelvic floor ultrasound observations include bladder filling, urethral length, bladder neck position (at rest, in the Valsalva state, and in the pelvic floor contracted state), urethral mobility, leakage phenomenon (which also occurs when the urethra is unstable or in some cases (e.g., with an overactive bladder), bladder wall thickness, and genitourinary fistulae/ureteral ectasia.
Traditional pelvic floor ultrasound requires a high degree of professionalism on the part of the operator. Pelvic floor ultrasound based on the core algorithm of Artificial Intelligence (AI) can simulate the real ultrasound doctor’s pelvic floor related index measurement path and logic, summarize the huge amount of big data, and integrate the cloud computing logic into the pelvic floor intelligent medical care, and there are pelvic floor ultrasound that can realize automatic measurement of the distance from the neck of the bladder, the bladder posterior angle, the distance of the posterior wall of the bladder, the angle of inclination of the urethra, the angle of rotation of the urethra, urethral mobility and other indicators, providing intelligent measurements for the clinical diagnosis of lower urinary tract symptoms and improving the efficiency and level of diagnosis and treatment.
Posterior vesicourethral angle, funneling, urethral rotation angle, and bladder neck mobility are routinely observed in pelvic floor ultrasound in patients with urinary incontinence, and it has been shown that pelvic floor ultrasound can be used for transperineal ultrasound of the urethral rotation angle, the position of the bladder neck in the maximum Valsalva state, and the bladder neck mobility have high value in the diagnosis of female stress incontinence, which can help in the diagnosis and assessment of stress incontinence [
67], and it also can be used to assess urinary incontinence, voiding abnormalities, and periurethral lesions.3D/4D ultrasound is used to visualize urethral mobility, which increases after delivery, especially after instrumental assisted delivery, indicating a change in urethral support [
68]. Ultrasound is the method of choice for residual urine volume determination [
40], and whether the residual urine volume exceeds 100 mL is used as one of the indicators for observation of uncomplicated and complicated stress urinary incontinence, and preoperative and postoperative residual urine volume can be examined by catheterization or pelvic floor ultrasound. In addition, ultrasound is the method of choice for diagnosing postoperative complications such as postoperative urge incontinence, bladder dysfunction, and sling position. Post-colonoscopy ultrasound ultrasound imaging can be used to show bladder stability behind the neck of the sling.
- (6)
Pelvic floor surface electromyographic assessment
According to the recommendations of the International Continence Society (ICS), pelvic floor muscles are categorized into four types: normal, overactive (muscle hypertonicity), underactive (decreased activity or muscle laxity), and non-functional [
69]. Surface electromyography is used to detect pelvic floor electromyographic activity in response to the state of muscle activity. The pelvic floor surface EMG Glazer assessment was proposed by Prof. Glazer in 1997 [
70], and the Glazer assessment process was officially released in 2012 [
71] to assess muscle strength, endurance, muscle recruitment velocity, muscle stability, and muscle tone at rest. In a study on the correlation between pelvic floor electromyographic assessment and pelvic floor muscle function in patients with stress urinary incontinence, it was found [
72] that surface electromyography based on the Glazer assessment suggests that pelvic floor muscle contractility is decreased in patients with stress urinary incontinence, that the mean electromyographic value of the endurance contraction phase (reflecting muscular endurance) correlates with stress urinary incontinence, and that there is a trend towards correlation of the mean electromyographic value of the tension contraction phase (slow muscle strength) with stress urinary incontinence. It suggests that pelvic floor EMG assessment can be used for quantitative assessment of stress urinary incontinence. Another retrospective study that included 3027 cases of postpartum stress urinary incontinence found [
73] that the mean electromyographic value during the tension contraction phase (slow muscle muscle force) was correlated with postpartum stress urinary incontinence, and could be used as a tool to quantitatively analyze pelvic floor function in postpartum stress urinary incontinence. The conclusions of the results of these two clinical studies were consistent. Therefore, pelvic floor electromyographic assessment can be applied to the clinical assessment of stress urinary incontinence as an important tool for quantitative pelvic floor function assessment.
Pelvic floor ultrasound and pelvic floor surface electromyography assessment determine the pelvic floor status of patients with urinary incontinence from different perspectives, and comprehensively assess the pelvic floor from both anatomical and electrophysiological perspectives, which is important for the prediction, diagnosis, clinical typing, and guidance on the selection of therapeutic modalities for stress urinary incontinence [
74]. Some data show that pelvic floor muscle electrophysiological parameters and pelvic floor ultrasound parameters have a certain correlation with postpartum stress urinary incontinence, which provides a more objective imaging basis for clinical screening and diagnosis of postpartum stress urinary incontinence [
75], and experts suggest that the reasonable selection of the two in the clinical, combined with the two, can be given to the patients in the clinical diagnostic and treatment of stress urinary incontinence to give a comprehensive examination and assessment of the work [
76]. The relative amount of change in the size of the pelvic diaphragm fissure during the anal retraction period is positively correlated with the electromyographic value of the pelvic floor surface, therefore, three-dimensional pelvic floor ultrasonography and pelvic floor electromyographic assessment are of great significance in the evaluation of the contractile function of the anal retractor muscles [
77]. In addition, three-dimensional pelvic floor ultrasonography combined with pelvic floor electromyography is important for the realization of early detection, early treatment and early recovery of maternal pelvic floor function after childbirth [
78]. The combined assessment of pelvic floor ultrasound and electromyography can play different roles in the diagnosis of urinary incontinence, which is an important guide for the precise diagnosis and treatment of urinary incontinence.
- (7)
Voiding diary
Voiding diary, also known as bladder diary, refers to the continuous recording of the amount of fluid intake, amount of urine leakage, number of leakage, time of leakage, frequency/urgency of urination during the day/night, amount of voluntary urination, and activities engaged in during the time of leakage, etc., every 24 hours, on the basis of no change in the living status and urination habits, and this index is used as one of the observations of therapeutic efficacy in most of the literatures, but the results do not correlate with the seriousness of the symptoms of urinary incontinence [
63]. Voiding diaries can be applied to all patients with urinary incontinence as well as to those with atypical incontinence [
63,
79]. Commonly used are 3-day/7-day voiding diaries, and the EAU recommends the use of at least 3-day voiding diary [
80].
- (8)
Urodynamic examination
Urodynamic testing is an effective method of diagnosing the type of incontinence and is categorized into noninvasive and minimally invasive urodynamic tests, depending on whether or not manometry requires bladder placement of a manometric tube. Routine testing is not recommended before conservative treatment or before untreated overactive bladder [
40]. Urodynamic examination is mainly used in patients with uncomplicated stress incontinence who have failed conservative treatment, in pre-intervention evaluation of surgical intervention for urinary incontinence, in the presence or absence of forced urethral overactivity, in patients with poor compliance, in patients with bladder dysfunction prior to planned surgical procedures, in patients with suspicion of mixed incontinence or neurogenic incontinence, and in the monitoring of the effectiveness of clinical treatment [
40], with exclusion of urinary tract infection prior to the examination. The urodynamic examination includes urinary flow rate, bladder capacity, residual urine volume, bladder contraction index, bladder outlet obstruction coefficient, and perfusion rate, etc. These tables can determine the bladder sensitivity, compliance, and stability, provide objective information about the urinary tract and pelvic floor, identify potential bladder and urethral dysfunction, and determine the presence of detrusor muscle insufficiency, bladder outlet obstruction, and the severity of stress urinary incontinence.
Urodynamic examination is not recommended for uncomplicated stress incontinence, and ultrasound or catheterization can be used to determine the residual urine, which is not exceed 150 ml; urodynamic examination for complicated stress incontinence shows stable forced urethral muscle during storage, forceful contraction of the forced urethral muscle during voiding, and ≥150 ml; urodynamic examination for urgency incontinence shows a small bladder capacity, poor compliance, and absence of residual urine from the bladder; Mixed incontinence urodynamic examination is not clearly characterized [
81]. Urodynamic examination can assist in ruling out urge incontinence and mixed incontinence, as well as determining whether the patient has an intrinsic urethral sphincter defect and ruling out bladder dysfunction or obstruction.
- (9)
Urine routine
Urinary incontinence patients are recommended to perform routine urinalysis, especially patients with symptoms of urinary frequency and urgency, to rule out acute urinary tract infections. If the urine culture is positive, the first drug sensitivity test, and then targeted antibiotic treatment. In addition, it is recommended to exclude hematuria, proteinuria, blood sugar, etc..
- (10)
Swab test
When abdominal pressure increases in patients with stress incontinence, the bladder neck moves downward and the posterior angle of the urinary bladder changes, if the posterior angle of the urinary bladder angle disappears, and when abdominal pressure increases, the urethral sphincter is unable to control urine, resulting in incontinence. The swab test determines whether the anatomical position of the bladder has changed by the angle of the urethra. Cough, labor, bladder filling, urethral mobility, and prolapse may all have an effect on the results of the swab test [
82]. Specific methods: the length of the swab is about 10 cm, the position is cystotomy position, and the vulva is sterilized; a smooth sterilized and lubricated swab is taken and gently inserted into the urethra for about 4 cm, and the angle between the exposed portion of the swab and the horizontal line is measured for the patient at rest and in the Valsalva state, respectively. If this angle is <15° it indicates good anatomy; >30° or 2-3 cm upward indicates decreased function of anatomical support structures and greater urethral mobility; if this angle is 15-30°, the results do not determine the degree of anatomical support. The swab test is >30° for uncomplicated stress incontinence and >30° for patients with complicated stress incontinence and urge incontinence. Further urodynamic testing should be done in those <30° with stress incontinence.
- (11)
Stress test is the most common diagnostic method for urinary incontinence [
63]. Specific methods: the position is truncated, the patient is instructed to cough forcefully three times, urine from the external urethral orifice, it is positive; if there is no urine outflow, it is negative. In addition, the patient is instructed to cough in the standing position to observe whether there is leakage of urine from the external urethral orifice to avoid leakage, and to exclude poor bladder filling at the same time. Negative stress test in patients with urge incontinence, some patients with urge incontinence coughing will cause contraction of the urethral muscle, there will be delayed outflow of urine from the external urethra, which is still considered a negative stress test. Therefore, with increased abdominal pressure, attention should be paid to the time of emergence of urine leakage.
- (12)
Finger-pressure test [
81]
The finger pressure test is performed on the basis of a positive pressure test. Specific method: the index finger and middle finger are placed transvaginally at the bladder neck level, on both sides of the urethra, the patient coughs to increase the abdominal pressure, then urine flows out, elevate the bladder neck, the leakage stops, then the finger pressure test is positive, and vice versa is negative, but the negative result can not rule out the intrinsic sphincter of the urethra defects or other causes of incontinence, then it is recommended to further examination and to explore the cause of the disease.
- (13)
Other
Other ancillary tests, such as urethrocystoscopy, X-ray, MRI, and CT. Urethrocystoscopy [
40,
63] is indicated in cases of positive urine cytology results, bladder or urethral pain, recurrent urinary tract infections, suspicion of uroepithelial bladder cancer. This test is recommended after unsuccessful conservative treatment (physiotherapy, medication, etc.), hematuria, suspicion of fistula, suspicion of foreign body or tumor in the bladder, sling displacement, etc. X-rays and MRI can be used in congenital cases, postoperative urinary incontinence, and diagnosis of clinically ambiguous morphology. mri can also show the entire pelvic structure, but is static, and the use of mri is not recommended for routine diagnosis, and it is of reference value in complex cases [
63,
83,
84]. CT is recommended in special cases, such as suspicion of renal tumors and urethral fistulae [
63,
85]. Patients with perimenopausal and postmenopausal urinary incontinence should also be noted for estrogen changes and the presence of symptoms of genitourinary syndrome of menopause (GSM) [
63].
- 2.
Grading of urinary incontinence [
65]
(1) Subjective grading: according to the Ingelman-Sundberg grading method, combined with its clinical manifestations, stress urinary incontinence is classified as mild, moderate and severe. Mild: leakage of urine under sudden increase of abdominal pressure such as coughing, sneezing, etc., but does not require the use of urinary pads; Moderate: leakage of urine occurs during daily activities such as running, jumping, fast walking, etc., and requires the use of urinary pads; Severe: leakage of urine under minor activities such as turning over, position change, slow walking, etc., which will seriously affect the patient’s quality of life.
(2) Objective grading: using the 1h urine pad test, according to the amount of urine leakage, urinary incontinence is divided into mild, moderate, severe and very severe. Leakage ≥2g is positive, 2g≤leakage <5g is mild, 5g≤leakage <10g is moderate, 10g≤leakage <50g is severe, and ≥50g is extremely severe.
- 3.
Incontinence classification
Stress incontinence typing is not necessary for diagnosis, but in patients who do not respond well to initial treatment options, abdominal leakage point (ALPP) and imaging urodynamics can be used to typify stress incontinence and to identify urethral hypermobility stress incontinence.
- (II)
Pelvic organ prolapse
The diagnosis of pelvic organ prolapse is mainly based on symptoms, signs and auxiliary examination, detailed history, anatomical assessment, concomitant symptoms, etc. for a comprehensive and thorough evaluation of the patient with prolapse. Detailed history, anatomical evaluation, concomitant symptoms, etc. are used to assess the patient with prolapse in a comprehensive and holistic manner.
Symptom assessment is of utmost importance and is part of the assessment of pelvic organ prolapse [
86]. In general, the lowest point of prolapse reaches or exceeds the level of the hymen before specific clinical symptoms are manifested. However, patients with pelvic organ prolapse tend to present with a cluster of symptoms, mostly involving lower urinary tract symptoms, defecation disturbances, and symptoms of sexual dysfunction.
Patients with prolapse often present with a foreign body sensation in the vagina, seeing or feeling an organ prolapse out of the vaginal opening or a bulge in the vagina (which is the most specific clinical symptom of prolapse), and a feeling of abdominal distension or a feeling of falling down; in severe cases of prolapse, the organ is unable to retract, and may be accompanied by an increase in secretions, ulcers, and hemorrhage. The degree of prolapse may vary with body position, activity level and weight bearing.
Lower urinary tract symptoms are mostly related to defects in the supporting structures of the bladder and urethra, and patients with prolapse associated with bulging of the anterior vaginal wall often have stress incontinence, which is relieved as the degree of prolapse worsens, and the bladder can be emptied completely when the prolapse is relieved. In addition, prolapse may may also present with lower urinary tract symptoms such as dysuria, urinary retention, urinary frequency, urgency, nocturia, and urge incontinence. After prolapse surgery, prolapse symptoms resolve and urinary incontinence may occur [
39]. Posterior pelvic support defects or bulging posterior vaginal wall are mostly characterized by defecation disorders such as constipation, difficulty in defecation, straining to defecate, and fecal incontinence. In some patients with posterior vaginal wall dilatation, the perineum and posterior vaginal wall can be compressed by hand to assist defecation. In addition, patients with pelvic organ prolapse may be accompanied by symptoms of sexual dysfunction, such as difficulty, pain, and decreased libido during sexual intercourse [
39,
87].
- 2.
Physical signs
Physical examination should include abdominal examination (presence of hard lumps in the abdomen), pelvic examination (gynecologic specific examination, presence of masses, presence of tenderness, etc.). Gynecological examination, external genitalia and vaginal epithelium, the presence of vaginal atrophy, skin irritation or ulceration [
86,
87]; vaginal secretions, cleanliness, the presence of bilateral adnexa, uterine body, cervix and other abnormalities; vulvar skin morphology, color, the presence of mucosal atrophy, ulceration of the vagina and cervix. Before the pelvic examination, the bladder should be emptied and the position should be cystotomy, observing the maximum degree of prolapse that can be achieved by relaxation and Valsalva state prolapse, respectively, and other positions such as the standing position can be chosen if necessary. It is recommended that pelvic organ prolapse be specifically described using the POP-Q, which is currently one of the most recommended methods in national and international clinical studies, guidelines or consensus. In addition, attention should also be paid to observing the integrity of anal sphincter, muscle strength, defecation, neurological examination mainly for the lumbosacral innervated area of perineum and perianal sensation, mons pubis and labia majora sensation, bulbocavernosus muscle reflexes, anal reflexes; vaginal laxity classification, and so on.
- 3.
Medical history
Patients with prolapse often have lower urinary tract symptoms, defecation symptoms, and sexual dysfunction, so the history should be detailed and comprehensive. Current medical history (when the first symptoms appeared, when the prolapse-specific symptoms appeared/remitted), the presence of the following risk factors, race, vaginal delivery, age, obesity, connective tissue disease, menopause, chronic constipation, surgery, increased abdominal pressure, etc. [
86]; lifestyle habits (e.g., smoking, etc.); pregnancy and childbirth history (pregnancy, labor, miscarriage, induced abortion, cis-analysis); menstruation history; surgical history (pelvic surgery, anti-incontinence surgery, etc.); treatment history (history of rehabilitation therapy, medications, etc.); and history of treatment (history of rehabilitation therapy, medications, etc.). incontinence surgery, etc.); treatment history (history of rehabilitation therapy, history of medication, etc.); comorbidities (chronic constipation, etc.), family history (history of pelvic floor dysfunction such as prolapse, etc.); lower urinary tract symptoms (urinary incontinence, urinary difficulty, urinary frequency and urgency, etc.); defecation symptoms (difficulty in defecation, incontinence of bowel movement, straining to defecate, constipation, etc.); other concomitant symptoms (prolapses ulcers, bleeding, etc.); sexual symptoms (difficulty in sexual intercourse, pain, etc.); quality of life (difficulty in sexual intercourse) (difficulty in sexual intercourse, pain, etc.); quality of life (can be assessed by combining with questionnaires); mental health status (the presence of anxiety, depression), etc.
- 4.
Questionnaires
In women with pelvic organ prolapse, a validated pelvic floor symptom questionnaire is considered to aid assessment and decision-making [
88]. The Pelvic Floor Dysfunction Impact Questionnaire (PFDI-20) focuses on pelvic, bowel, and bladder symptoms in patients with prolapse in the last 3 months; the Pelvic Organ Prolapse, Urinary Incontinence Sexual Function Questionnaire (PISQ-12) assesses the symptoms of sexual dysfunction in patients with prolapse; and the Pelvic Floor Dysfunction Impact Questionnaire Short Questionnaire 7 (PFIQ-7) is used to assess the effects of pelvic, bowel, and bladder symptoms of patients with prolapse on their daily life, moods, and so forth. The PFIQ-7 was used to assess the impact of pelvic, bowel and bladder symptoms on daily life and mood.
- 5.
POP-Q
Quantitative assessment of pelvic organ prolapse (POP-Q) is necessary to obtain the organs and severity of anterior, middle and posterior pelvic prolapse. This test should be performed after emptying the bladder and rectum. Description of pelvic organ prolapse grading: degree 0, no prolapse; degree I, the most distal part of the prolapse is more than 1 cm above the level of the hymen, with measurements < -1 cm; degree II, the most distal part of the prolapse is less than 1 cm above the plane of the hymen, with measurements ≥ -1 cm, ≤ +1 cm; degree III, the most distal part of the prolapse is more than 1 cm above the plane of the hymen, but less than the full length of the vagina - 2 cm, with measurements > +1 cm, and but < (full vaginal length -2) cm; Degree IV, prolapse of the full length of the vagina, measured value ≥ (full vaginal length -2) cm.
- 6.
Pelvic floor ultrasound
Pelvic floor ultrasound is inexpensive, noninvasive, highly maneuverable, and has high consistency of repeated measurements [
89]. It is now a routine preoperative assessment tool for prolapse. Pelvic organ prolapse can be assessed by two-dimensional gray. Pelvic organ prolapse can be assessed using two-dimensional gray-scale transperineal ultrasound at maximum Valsalva. The size of the anal retinaculum fissure is closely related to the degree of pelvic organ prolapse, with the larger the fissure in Valsalva, the more severe the prolapse. If the area of the anal retinaculum cleft is ≥35 m2, it suggests moderate prolapse and may be accompanied by prolapse symptoms [
90,
91].
Transperineal ultrasound can be used as visual biofeedback to interpret the effects of pelvic floor muscle contraction on the bladder neck, such as coughing to alleviate prolapse. Clinical screening for postpartum POP can be performed by quantitative parameters of transperineal pelvic floor ultrasound to prevent or minimize the occurrence of postpartum POP. Another study showed that altered morphology of the anal retinaculum fissure, bladder neck movement, and altered posterior vesicourethral angle were closely associated with the development of postpartum POP [
92]. Preoperative pelvic floor ultrasound is helpful in clinical diagnosis and treatment, and transvaginal ultrasound can assess cervical length, uterine body and cervix, and rule out uterine pathology before performing uterine retention [
93]. Therefore, pelvic floor ultrasound should be actively used in the diagnosis and evaluation of pelvic organ prolapse.
- 7.
Pelvic floor electromyography
Pelvic floor electromyographic assessment can quantitatively assess muscle function such as muscle strength, endurance, stability, and resting muscle tone in patients with prolapse, but it cannot determine changes in anatomical function. Specific methods: in the supine position of 120°, the prolapsed organ is retracted into the vagina before assessment, and according to voice guidance, the average of the pre-resting phase, rapid contraction (fast muscle test), sustained contraction and relaxation (slow muscle test), sustained contraction (endurance test), and the average of the post-resting phase are measured. It has been shown [
94] that some of the pelvic floor EMG values of patients with prolapse showed abnormalities, such as fast muscle (89.20% abnormal rate), slow muscle (99.65% abnormal rate) and endurance (99.30% abnormal rate), in addition to some of the patients in the pre-resting phase and post-resting phase, which showed abnormal EMG values, with an abnormal rate of 28.22% and 26.48%, respectively. In addition, EMG values in the pre-resting phase were negatively correlated with age, mean values in the post-resting phase were positively correlated with BMI, and muscle variability in endurance testing was positively correlated with years of menopause, age, and number of births, and these correlations were statistically significant.
The combination of electromyographic assessment, which quantitatively assesses pelvic floor muscle function, and pelvic floor ultrasound, which provides feedback on anatomical location and structure, allows for simultaneous assessment of the pelvic status of patients with pelvic organ prolapse at both the structural and functional levels.
- 8.
Other
Urodynamic tests, MRI and other programs should be carried out according to the actual need for these tests. Pelvic organ prolapse has a certain effect on the contraction of the urethra muscle and bladder compliance. Urodynamic examination can observe the effect on bladder function. Preoperative urodynamic examination is recommended to evaluate the urinary flow rate and the determination of residual urine volume, and for patients with prolapse combined with urinary incontinence, preoperative clinical examination related to urinary incontinence is recommended. Preoperative pelvic floor MRI is helpful for diagnosis and choice of treatment modality. In addition, the Modified Oxford Muscle Strength Grading System can be referred to determine the pelvic floor muscle strength in patients with prolapse, and cough and stress tests are also needed to exclude occult incontinence [
93].
- (III)
Defecation disorders
Defecation disorders are dominated by constipation and fecal incontinence, and chronic constipation is used as an example to illustrate clinical diagnosis and assessment. The diagnosis of chronic constipation is based on history and clinical symptoms, while drawing on the Rome IV criteria [
95], in addition, the following assessments may be included: anorectal palpation to help exclude organic anorectal disease and understand anal sphincter function; colonoscopy, for first-time patients ≥40 years of age; gastrointestinal transport test to help in the diagnosis of slow-transmission constipation; pelvic floor electromyography as an aid in myogenic and neurogenic identification [
96]; pelvic floor ultrasound can observe the morphology and structure of the vesicourethra, cervix-vagina, anorectum and real-time functional status during Valsalva or simulated fecal maneuvers, providing a multi-parameter reference for clinical selection of treatment modalities and assessment of therapeutic efficacy, which is easy to perform in real time and reproducible, and is of high diagnostic value [
95]; psychological assessment, balloon forcing out test, anorectal pressure and fecography can also be used for the diagnosis of chronic constipation [
96].
- (IV)
Sexual dysfunction
Female sexual dysfunction identifies five types according to the American College of Obstetricians and Gynecologists (ACOG) Guidelines for the Management of Female Sexual Dysfunction [
97], female sexual interest/arousal disorders, female orgasmic disorders, genital pelvic pain and penetrative disorders, and substance- or drug-induced sexual dysfunction. The clinical diagnosis of female sexual dysfunction combines history, physical examination, and diagnostic criteria [
97,
98]. A comprehensive medical history is assessed, including the time of symptom onset and duration; partner status; past sexual experiences; some possible predisposing factors, such as sedentary behavior and genital-pelvic-related injuries; history of medications; in addition, questionnaires are necessary to assess symptoms, such as the Female Sexual Function Index (FSFI). Physical examination, targeted gynecological examination, to assess whether sexual dysfunction is induced or related to the primary disease. Diagnostic criteria: the patient’s symptoms meet the DSM-5 diagnostic criteria for sexual dysfunction, the symptoms persist for at least 6 months (except for substance- and drug-induced sexual dysfunction) and are sufficient to cause significant personal distress, and psychological disorders, life stresses, and factors of the sexual partner are excluded.
- (V)
Chronic pelvic pain
Chronic pelvic pain is associated with chronic or persistent pain perceived in structures associated with the female pelvis that lasts for at least 6 months [
99]. It is often associated with negative cognitive, behavioral, sexual, and emotional consequences as well as symptoms suggestive of lower urinary tract (LUT), sexual, bowel, pelvic floor, or gynecologic dysfunction [
100]. The diagnosis of chronic pelvic pain syndrome is based [
100] on a combination of history (psychological profile, holistic assessment of urologic, gynecologic, gastrointestinal, and fascial aspects), and physical examination (fascial, neurologic examination, impactology, laboratory, and pelvic floor function).
VII. Clinical application of physiotherapy techniques in pelvic floor dysfunction
Conventional physical therapy for pelvic floor dysfunction includes pelvic floor muscle training (PFMT) [
101,
102], biofeedback therapy [
103], pelvic floor electrical stimulation [
104], magnetic stimulation therapy [
105], radiofrequency therapy [
106]and laser therapy. These therapeutic means have different degrees of therapeutic efficacy for different types of pelvic floor dysfunction, and the following describes the common physical therapeutic means and their clinical applications in pelvic floor dysfunction.
- (I)
Overview of physiotherapy techniques
Pelvic floor muscle training (PFMT) is also known as Kegel exercise. Specific methods [
65]: continuous contraction of the pelvic floor muscles (i.e., anal contraction exercise) for no less than 3 s, relaxation and rest for 2-6 s, 15-30 min, repeated 3 times a day; or 150-200 times a day anal contraction exercise.
According to EAU guideline recommendations, for women with stress and mixed incontinence, pelvic floor muscle training is effective in relieving incontinence and improving quality of life for patients (level A evidence), and starting pelvic floor muscle training in the early postnatal period reduces symptoms of maternal stress incontinence for more than 12 months (level A evidence) [
107].The NICE guideline recommends that women with stress or mixed incontinence should be offered guided pelvic floor muscle training for at least three months as a first-line treatment [
108]. A systematic review published in 2018 reviewed the effectiveness of pelvic floor muscle training for patients with urinary incontinence from 2001-2014, which included 31 studies covering 1,817 women from 14 countries, and suggested that PFMT was eight times more likely to be curative in female patients with stress urinary incontinence compared to placebo control (56% vs. 6%; risk ratio (RR) 8.38, 95% confidence interval (CI) 3.68 to 19.07; 4 trials, 165 women; high quality evidence). For women with any type of UI, the PFMT group was five times more likely to report a cure (35% versus 6%; RR 5.34, 95% CI 2.78 to 10.26; 3 trials, 290 women; moderate quality evidence). The results suggest a favorable effect of PFMT for the treatment of urinary incontinence [
109]. Pelvic floor muscle training is characterized by individual difficulty in mastery, and the traditional model is 1-to-1 under the guidance of a rehabilitation therapist, but this undoubtedly increases the healthcare burden and is not conducive to the popularization of this training modality.A 2020 randomized controlled study comparing the cost-effectiveness assessment of group PFMT with individual PFMT involved 362 women aged ≥60 years with stress or mixed incontinence A total of 362 women aged ≥60 years with stress or mixed incontinence participated. Grouped into 12 one-hour weekly PFMT sessions delivered individually (one physiotherapist per woman) or in groups (one physiotherapist per eight women), the results suggested that group-based PFMT was ≥ 60% less costly than individual therapy, and there was no statistically significant difference in effectiveness between the two groups [
110]. Overall, group-based PFMT has better outcomes for UI’s and is more cost effective.
According to NICE guideline recommendations, patients with symptomatic pelvic organ prolapse that does not exceed 1 cm outside the hymen on exertion may be considered for guided pelvic floor muscle training for a minimum of 4 months [
111]. A meta-analysis published in 2022, which reviewed the effectiveness of pelvic floor muscle training for the treatment of patients with pelvic organ prolapse evaluated up to 2021 and included 13 studies, suggested that compared with the placebo group, women in the PFMT group had a greater reduction in prolapse symptom scores ((POP-SS; mean difference [MD] -1.66, 95% CI -2.36 to -0.97, p < 0.00001]) and POP score (risk [RR] 1.51, 95% CI 1.14-2.01, p = 0.004), were significantly improved. However, in terms of long-term outcomes, there was no difference in symptomatic improvement for patients [
112]. A multicenter randomized controlled trial conducted in three centers in New Zealand and the United Kingdom evaluated the effect of PFMT as secondary prevention in women with stage 1-3 prolapse, and the results suggested a reduction in prolapse symptoms in the PFMT group [
113]. Overall, PFMT is effective in mild to moderate pelvic organ prolapse.
A program of guided pelvic floor muscle training (PFMT) for a period of at least 4 months is considered for women with fecal incontinence and concomitant pelvic organ prolapse, as recommended by NICE guidelines [
114]. A systematic review published in 2020 reviewed articles related to evaluating the effectiveness of PFMT in preventing or treating urinary incontinence and fecal incontinence in pregnant or postpartum women up to 2017, which included eight studies suggesting that there is no evidence that antenatal PFMT reduces the risk of fecal incontinence in late pregnancy, and additionally no evidence that PFMT reduces the risk of fecal incontinence in late postpartum[
111].There is a lack of high-quality studies on the efficacy of PFMT for the prevention and treatment of fecal incontinence, and further evaluation of the efficacy of PFMT for the treatment of fecal incontinence is needed.
The use of PFMT in other types of pelvic floor dysfunction includes chronic pelvic pain and sexual dysfunction, but the efficacy of PFMT in the treatment of this type of pelvic floor dysfunction has not been confirmed by high-quality studies and has not been recommended in major guidelines. A study that included 77 postmenopausal women presenting with sexual dysfunction evaluated the value of PFMT for sexual function improvement, and the results suggested that a higher proportion of women in the PFMT group were free of sexual dysfunction compared with the placebo control group [
115].
- 2.
Biofeedback
Pelvic floor biofeedback (BF) is a behavioral therapy technique used to improve pelvic floor muscle dysfunction or decline, commonly used in the treatment of stress urinary incontinence, pelvic organ prolapse and other pelvic floor dysfunction. It works by placing sensors to monitor the activity of the patient’s pelvic floor muscles and providing visual or auditory feedback to let the patient know the strength of the muscle contraction and the recovery time, thus helping the patient to locate and train the pelvic floor muscles. Biofeedback can improve the patient’s perception and control of muscle contraction compared to muscle training alone. According to the recommendation of NICE guidelines for pelvic floor dysfunction, biofeedback can be considered as a complementary technique for women who are unable to perform effective pelvic floor muscle training [
111].
According to the EAU guideline recommendations, for patients with urinary incontinence, the addition of biofeedback therapy may provide greater clinical benefit for women undergoing PFMT [
107]. A systematic review published in 2021 reviewed studies of PFMT with and without Electromyogram-Biofeedback (EMG-BF) for the treatment of SUI up to 2021, which included a total of 21 clinical studies, and suggested that in the management of SUI, the combination of PFMT with EMG-BF achieved better results than PFMT alone to achieve better results, but at the same time the paper also suggested that the heterogeneity of the included studies was relatively high [
47].
Biofeedback is mainly used in the treatment of mild to moderate pelvic organ prolapse. A small-sample study published in 2017 evaluated the efficacy of biofeedback in patients with mild-to-moderate POP, and the results suggested that the intervention improved patients’ quality of life [
116]. Another small-sample study included 77 patients with POP degrees I-II for evaluating the effect of PFMT combined with biofeedback, and the results suggested that PFMT combined with biofeedback significantly reduced the degree of prolapse in POP and enhanced pelvic floor muscle extension in postmenopausal women with elevated intra-abdominal pressure [
117]. Overall, there is a lack of high-quality research on the use of biofeedback in pelvic organ prolapse, and studies with larger sample sizes are needed to verify its efficacy.
Biofeedback can be used as a primary treatment for patients with fecal incontinence, as recommended by the Italian guidelines on the management of fecal incontinence [
118]. The efficacy of biofeedback in the treatment of fecal incontinence was assessed in a systematic review published in 2012, which included 21 studies, and the results suggested that biofeedback has a therapeutic effect on fecal incontinence, but most of the studies were of low quality and larger studies are needed to confirm the effect [
119]. A large-scale randomized controlled study in which 377 fecal incontinence were included evaluated the therapeutic effect of biofeedback in patients with fecal incontinence, and the results suggested that medication combined with biofeedback and behavioral guidance could achieve the best therapeutic effect [
120].
Biofeedback in pelvic floor dysfunction also includes chronic pelvic pain and sexual dysfunction. A systematic review published in 2022 assessed the efficacy of biofeedback for chronic pelvic pain, which included 37 studies, and the results suggested that biofeedback-assisted training had a positive effect on pain reduction, overall symptom relief, and improved quality of life [
121].
- 3.
Electrical Stimulation
Electrical Stimulation (ES) is a passive treatment for pelvic floor dysfunction, in which a weak pulse of electricity is applied to the pelvic floor muscles to stimulate their contraction, thus enhancing and remodeling the function of the pelvic floor muscles.
Electrical stimulation of the pelvic floor muscles may have some efficacy in urinary incontinence, as recommended by the EAU guideline on urinary incontinence [
107].The NICE guideline on the non-surgical treatment of pelvic floor dysfunction suggests that electrical stimulation may be considered as a complementary treatment for women who are unable to perform effective pelvic floor muscle contractions [
108]. A meta-analysis published in 2022 evaluating the short- and long-term efficacy of electrical stimulation for the treatment of stress urinary incontinence through 2020 suggested that in the short term (< 3 months), ES significantly improved incontinence-specific quality of life (I-QOL) and reduced leakage but did not significantly reduce frequency of incontinence episodes compared with sham ES or no intervention. In the long term (3-7.5 months), ES significantly improved quality of life and reduced the frequency of incontinence episodes, but did not significantly reduce urine leakage [
122]. Another meta-analysis published in 2022 evaluated nine randomized controlled studies of electrical stimulation for urinary incontinence up to 2021 and suggested significant improvements in the pad test (P=0.01), frequency of incontinence (P=0.04), and some indicators of quality of incontinence, compared with the control group [
123]. A series of clinical studies have confirmed that for urinary incontinence, electrical stimulation has a certain therapeutic effect, but its specific effect is highly differentiated and needs to be judged comprehensively according to the patient’s situation.
NICE guidelines have the same recommendations for pelvic organ prolapse as for urinary incontinence. A randomized controlled study evaluated the efficacy of electrical stimulation biofeedback therapy combined with pelvic floor exercises in the treatment of postpartum pelvic organ prolapse. 104 patients with postpartum pelvic organ prolapse were enrolled in the study, and the total effective rate was higher in the combination group than in the pelvic floor muscle training group alone (P<0.05). The degree of pelvic prolapse in both groups tended to be 0~I degree, and the improvement in the combined group was better than that in the control group (P<0.05). Also, the improvement in sexual function-related treatment was better in the combined group than in the control group in this study [
124]. Another study evaluated the therapeutic effect of electrical stimulation biofeedback combined with proprioceptive training on the prevention of postpartum pelvic organ prolapse, which included a total of 108 postpartum women, and the results suggested that the incidence of pelvic organ prolapse was significantly lower in the electrical stimulation biofeedback combined with proprioceptive training group compared with the control group [
125]. It was concluded that there is still a lack of meta-analysis to analyze the effect of electrical stimulation in the treatment of pelvic organ prolapse, as well as a lack of high-quality studies to confirm the efficacy.
The role of electrical stimulation in chronic pelvic pain has been validated by some evidence. A systematic review published in 2023 assessed the efficacy of transcutaneous electrical stimulation techniques in women suffering from chronic pelvic pain, which included a total of 10 studies, and the results suggested that transcutaneous electrical stimulation could mildly reduce pain in women with primary dysmenorrhea [
126]. Another systematic assessment of the efficacy of all nerve stimulation for chronic pelvic pain was conducted in a review that included a total of 36 studies, with results suggesting that percutaneous tibial nerve stimulation and percutaneous electrical nerve stimulation showed improvement in pain. Other modalities (sacral nerve stimulation, spinal cord stimulation, intravaginal electrical stimulation, and pubic nerve stimulation) may only mildly reduce pain in patients with CPP. All treatments generally improved quality of life [
127]. More clinical studies are needed to clarify the appropriate parameters of percutaneous electrical stimulation and intravaginal electrical stimulation to guide clinical treatment.
The effectiveness of electrical stimulation has also been validated for a number of other pelvic floor dysfunction such as sexual dysfunction. A systematic review published in 2023 evaluated the efficacy of multiple physical therapy treatments for patients with sexual dysfunction, which included 19 studies, and the results suggested that the interventions of electrical stimulation and electrical stimulation combined with pelvic floor muscle training had significant effects on pain and quality of life improvement [
128]. One randomized controlled study published in 2021 evaluated the efficacy of biofeedback versus electrical stimulation for the treatment of female dysfunction, which included 22 patients, and the results suggested that both groups showed good results in improving the mean score of the Female Sexual Function Index and its domains. Both interventions reduced pain similarly during or after vaginal penetration (p = 0.985) [
129].
- 4.
Magnetic Stimulation
Magnetic stimulation is a painless, non-invasive and non-invasive technique, in which the changing magnetic field generates induced currents that act on the corresponding tissues to increase muscle fiber recruitment, enhance muscle strength, promote blood circulation in local tissues, and regulate the pelvic nerves and sacral nerves, thus improving pelvic floor function [
130,
131].
Magnetic stimulation has variable therapeutic efficacy for different incontinence categories. A systematic review published in 2023 reviewed studies evaluating the efficacy of magnetic stimulation for the treatment of urge incontinence up to 2022, with a total of five studies included in the review, and the results suggested that all five studies confirmed that MS is an effective and noninvasive treatment for UUI [
132]. Another systematic review published in 2020 assessed studies up to 2019 on the efficacy of extracorporeal magnetic stimulation for the treatment of stress urinary incontinence, which included a total of 20 studies, with results suggesting that magnetic stimulation had an effect on quality of life, number of leaks, pad test results, and reduction in the number of incontinence events; however, due to a high degree of heterogeneity among the studies, the results need to be treated with caution [
133]. A systematic review published in 2023 reviewed studies on the efficacy of magnetic stimulation for urinary incontinence up to 2021.The systematic review included a total of seven studies with urinary incontinence types including stress incontinence, urge incontinence and mixed incontinence, and the results suggest that magnetic stimulation is an effective treatment option for women with urinary incontinence. However, more large-scale, high-quality RCTs with extended follow-up periods are needed to determine the therapeutic efficacy of magnetic stimulation [
134]. The therapeutic efficacy of magnetic stimulation has been validated to some extent, but there is heterogeneity in the treatment regimen, and effective treatment parameters need to be clarified, which is the direction of subsequent research.
The evidence for magnetic stimulation for the treatment of pelvic organ prolapse is currently relatively scarce. A randomized controlled study published in 2019 assessed the efficacy of pelvic floor magnetic stimulation for the treatment of postpartum pelvic organ prolapse, which included a total of 60 patients, and the results suggested that the magnetic stimulation combined with pelvic floor muscle training group had a statistically significant difference in the POP-Q scale efficiency, with a significantly higher reduction in PFIQ-7 scores than that with the pelvic floor muscle training group [
135]. Another retrospective study published in 2020 evaluated the effect of pelvic floor magnetic stimulation combined with electrical stimulation on postpartum pelvic organ prolapse, which included a total of 90 patients with pelvic organ prolapse, and resulted in an improvement in the POP-Q scores in all patients, and some improvement in pelvic floor muscle strength in 93.3% of the patients [
14]. From the current study, the evidence for the use of magnetic stimulation technology in pelvic organ prolapse is relatively limited, and large-scale, high-quality studies are needed to determine its efficacy in treating pelvic organ prolapse.
Magnetic stimulation is recommended as an optional physiotherapy option for the treatment of chronic pelvic pain according to the EAU guidelines on chronic pelvic pain syndrome [
100]. A systematic review published in 2022 assessed the therapeutic efficacy of magnetic stimulation therapy for the treatment of chronic pelvic pain, which included a total of five clinical trials, and the results suggested that magnetic stimulation therapy improved pain, quality of life, and urinary symptoms. The review also suggested that a combination with electrical stimulation and other behavioral therapies could be considered for optimal results in this patient population [
136]. There are fewer studies on magnetic stimulation for the treatment of chronic pelvic pain in women, and most of them have focused on chronic pelvic pain in men, and more studies are needed to confirm its therapeutic efficacy and appropriate parameters.
Other types of pelvic floor dysfunction in which magnetic stimulation has been applied include sexual dysfunction and fecal incontinence. A retrospective study evaluated the efficacy of magnetic stimulation therapy combined with pelvic floor muscle training on postpartum sexual function in primiparous women, and the results suggested that, compared with the single pelvic floor muscle training group, the combined group had significantly higher scores of desire, sexual arousal, vaginal lubrication, orgasm, sexual satisfaction, and pain of sexual intercourse than the control group, and the difference was statistically significant [
137]. A randomized controlled study evaluating the efficacy of lumbosacral nerve magnetic stimulation in the treatment of fecal incontinence, which was categorized into three frequencies of 1, 5, or 15 Hz, suggested that there was a reduction in the incidence of fecal incontinence in all three groups, with the 1 Hz frequency being overall superior to the 5 and 15 Hz frequencies. Significant improvements in anorectal neuropathy and physiology were noted, suggesting an improvement in the mechanism [
138]. Overall, the magnetic stimulation technique is effective for sexual dysfunction and fecal incontinence, but most of the current studies have small sample sizes and larger studies are needed to find appropriate parameters to verify its efficacy.
- 5.
Radiofrequency
Radiofrequency technology differs from pelvic floor muscle training, electrical stimulation, biofeedback and magnetic stimulation in that it is a thermal energy technology. The principle of non-ablative radiofrequency is to act on the target tissues of the human body, causing the directional or vortex movement of electrons and ions in the tissues as well as the high-frequency vibration of polar molecules to produce a thermal effect [
139] (40 °C~50 °C), the hydrogen bond in the collagen triple helix is broken, collagen fibers shorten and thicken, and the microinjury induces a cascade reaction [
140] to stimulate the neonatal remodeling of collagen and elastin to enhance the fascia and the ligament elasticity and strength, promote blood circulation in the pelvic region, improve cell function and tissue metabolic function, and stimulate the regeneration of small blood vessels and small nerves in the local region [
51,
141,
142].
The therapeutic efficacy of nonablative radiofrequency techniques in urinary incontinence has been confirmed in several clinical studies. A meta-analysis published in 2023 reviewed the efficacy of physical techniques in the treatment of urinary incontinence, which included a total of 11 randomized controlled studies, of which there were three studies analyzing the improvement of radiofrequency techniques on ICIQ-SF in stress urinary incontinence, and all three studies suggested that in patients with urinary incontinence, radiofrequency had an ameliorative effect [
143]. Slongo et al. published in 2022 A study evaluated the efficacy of radiofrequency in the treatment of urinary incontinence in 117 women and showed that ICIQ-SF scores decreased from 13.6 (±3.8) to 8.2 (±5.2) in the radiofrequency-combined pelvic floor muscle training group, and from 14.2 (±3.1) to 9.3 (±5.2) in the single pelvic floor muscle training group, with the difference between the two groups being statistically significant (P < 0.05) [
144]. Seki et al. evaluated the efficacy of CO2 laser versus radiofrequency for the treatment of female stress urinary incontinence in a randomized controlled trial published in 2022, which included 139 female patients with stress urinary incontinence, and the study was divided into the radiofrequency group, the laser treatment group, and the placebo group, and the results suggested that the objective cure rates were 63.6%, 66.7%, and 22.2% for the three groups, respectively, and the results suggested that laser and The results suggest that both laser and radiofrequency show better therapeutic effects on stress urinary incontinence [
143]. Overall, radiofrequency has a good therapeutic effect on urinary incontinence, but patients need to be screened for changes in pelvic floor structure to determine the need for radiofrequency.
In recent years, with the development of science and technology, non-ablative radiofrequency (RF) technology has been used clinically for the treatment of vaginal laxity and vaginal atrophy. A prospective randomized single-blind study including nine research centers in Canada, Italy, Spain, and Japan evaluated the therapeutic effect of radiofrequency technology on vaginal laxity, in which the percentage of no vaginal laxity after treatment in the radiofrequency treatment group and the placebo group were 43.5% and 19.6%, respectively, and the difference between the two groups of the FSFI scores and the FSDS-R scores were 1.8 and -2,42 which proved that the radiofrequency treatment group improvement was superior to the placebo group [
145]. This study also provided preliminary evidence of the efficacy of radiofrequency technology in the treatment of vaginal laxity.Fu et al. evaluated the improvement of vaginal laxity by radiofrequency technology.A total of 102 patients with vaginal laxity were enrolled in the study.The VLQ scores showed a gradual improvement in the VLQ, FSFI total scores at 1, 3, 6, and 12 months after the radiofrequency treatments.At 1, 3, 6, and 12 months, 24.51% (25/102), 48.04% (49/102), 66.67% (68/102) and 79.41% (81/102) of the patients reported no symptoms of vaginal laxity, in addition to significant improvement in symptoms related to sexual functioning (libido, sexual arousal, lubrication, orgasm, satisfaction, pain) [
106]. Preliminary validation of radiofrequency in vaginal laxity has been obtained, and more subsequent studies are needed to elucidate the therapeutic effects at the mechanistic level.
The efficacy of non-ablative radiofrequency in sexual dysfunction has also gradually attracted the attention of various researchers.Desai et al. evaluated the efficacy of radiofrequency technology in improving female genital appearance, sexual function, etc.A total of 41 patients with sexual dysfunction were enrolled in the study.After undergoing radiofrequency treatment, the patients’ FSFI scores improved from 21.77 to 25.79.Sexual desire (from 2.99 to 3.54), arousal (from 3.14 to 3.83), and orgasm (from 3.14 to 4.39) [
146]. This study confirms to some extent the effectiveness of radiofrequency in the treatment of female sexual dysfunction. In another study, Wang et al. evaluated the efficacy of radiofrequency technology for the treatment of female sexual dysfunction, with a total of 31 women completing follow-up, and the results suggested that there was a significant improvement in FSFI scores [
146]. Overall, radiofrequency treatment for female dysfunction has been demonstrated in a small sample of studies, but high-quality evidence is needed to determine its efficacy and safety.
- 6.
Laser
Laser is a kind of energy-based physical therapy technology, the thermal effect temperature is generally controlled at 60-70 °C. Through thermal response, laser accelerates cellular metabolism, causes overexpression of heat shock proteins, triggers a repair response, stimulates the regeneration of collagen fibers, small blood vessels, and micro-neurons, improves the elasticity of connective tissues around the vagina, and enhances the supportive function of the pelvic floor [
147]. Vaginal lasers can be widely used in menopausal genitourinary syndrome, stress urinary incontinence [
148] and pelvic organ prolapse [
149]. The therapeutic principle in stress urinary incontinence is mainly that the thermal effect of laser promotes perivaginal collagen remodeling, stimulates collagen neogenesis, increases the thickness and elasticity of the anterior vaginal wall, improves the function of urethral support of the anterior vaginal wall, and enhances the stability of the urethra [
150], and ameliorates or cures stress urinary incontinence. The results of a clinical study of vaginal laser in urinary incontinence found that the cure rate of stress incontinence was 78.6%, and the cure rate of mixed incontinence with predominantly urinary incontinence was 70% [
151]. A study by Fistoni’c et al. found [
152] that urinary incontinence symptoms improved in 72.3% of patients from baseline to months 2-6 of follow-up, while similar results were obtained in another study, with 75.5% of patients showing similar improvement after 6 months of intervention [
153].In a study by Isaza et al. it was found that the ICIQ-UI-SF, the 1-hour urinary pads, had an effective improvement at follow-ups of 12, 24 and 36 months were effectively improved [
154]. In a review that included 30 clinical studies with 2053 patients, it was found that the 1-hour urinary pad test, ICIQ-SF, remained significantly improved at 3-12 months follow-up in patients with urinary incontinence [
155].Gaspar et al. reported that urinary pad weights were reduced by more than 50% in 82% and 50% of the patients 3 and 6 months after the start of treatment, respectively [
156], and Tien came to the aforementioned consistent conclusions [
157]. In a systematic review that included 64 clinical studies on GSM in menopausal genitourinary syndromes, improvements in the sexual function index (FSFI), and vaginal health score (VHI) were found to varying degrees [
158].
- (II)
Clinical application of physiotherapy technical parameters in pelvic floor dysfunction
According to information published by the International Continence Society, physical therapy should be used as the first-line conservative treatment for female stress incontinence [
87].
A systematic review of pelvic floor muscle training for stress urinary incontinence showed that to achieve maximal pelvic floor muscle training (PFMT) effect, PFMT should be continued for 6-12 weeks, with a frequency of no less than three times per week, and the duration of each training session should be more than 45 minutes [
46].
The use of biofeedback in the form of pelvic floor muscle training can increase muscle nutrition and function, promote urethral closure when intra-abdominal pressure increases, improve quality of life, and help guide the contraction of the pelvic floor muscles to achieve therapeutically effective stimulation [
159]. The improvement of urinary incontinence by electrical stimulation may be related to the occurrence of urinary incontinence symptoms with the urethra and bladder neck anatomical support structures and pelvic floor muscles. Electrical stimulation can promote blood circulation and tissue nutrition around the urethra and bladder neck. Long-term electrical stimulation can increase the strength of slow muscle fibers around the urethra [
160]. A Meta-analysis study concluded that electrical stimulation for urinary incontinence was performed at a frequency of 50 Hz, a pulse width of 200-500 μs, and a stimulation time of 15-30 minutes, twice a day or 2-5 times a week, for a period of 8-24 weeks, with stimulation intensity at maximum tolerance [
160]. Transvaginal electrical stimulation can directly or indirectly stimulate pubic nerves [
160], improve muscle sensitivity and motor function, significantly reduce the number of leaks and the amount of leaks, improve subjective healing and satisfaction, and can be used as a unique type of biofeedback to improve muscle perception, especially in patients who are unable to contract their pelvic floor muscles correctly [
161]. The results of another meta-analysis showed that transvaginal electrical stimulation was administered at a frequency of 10-50 Hz and a pulse width of 200-700 μs for 20-30 minutes at a time, at least once a week, for 6-12 weeks [
123]. The results of myoelectric biofeedback combined with pelvic floor muscle training showed that the combined treatment could improve the treatment efficiency of urinary incontinence patients, enhance the muscle strength, and improve the symptoms of urinary incontinence [
47], and it was more effective than pelvic floor muscle training alone [
47,
162].
Magnetic stimulation depolarizes motor nerves to generate action potentials that stimulate muscle contraction. Magnetic stimulation is an effective, virtually side effect-free and more privacy-focused than traditional methods [
163,
164], and the technique is an intervention that incorporates pelvic floor muscle training and neuromodulation to enhance muscle strength and endurance by stimulating the pelvic floor nerves to elicit contractions [
163,
165]. The results of a Meta-analysis showed that extracorporeal magnetic stimulation was effective in improving quality of life (QoL), reducing the number of leaks, the amount of leaks and the occurrence of urinary incontinence [
133]. For stress urinary incontinence, the results of two Meta-analyses showed that stimulation parameters of 2-3 times per week for 2-8 weeks, a frequency of 1-50 Hz [
50,
133], and a treatment time of 15-50 min [
50,
133], and sacral nerve stimulation, with a frequency of 15 Hz and a stimulation time of 15 min, improved patients’ quality of life QoL, and the ICIQ-UI-S, number of leaks and 1-hour urinary pad test were all significantly different [
50,
131,
166].
Radiofrequency therapy is an alternative to conservative treatment for patients with stress urinary incontinence [
45]. A clinical study of radiofrequency in stress urinary incontinence [
12] found sustained and effective relief of frequency and degree of incontinence, significant improvement in quality of life (reduction in I-QoL and ICIQ-SF scores), and a significant reduction in the number of occurrences of urinary urgency, nocturia, and sexual intercourse incontinence, with the stimulation parameters of: once per month for 3 months.
For stress incontinence, combined magnetic and electric therapy is recommended, supplemented by radiofrequency if necessary, to improve the abnormalities of mucous membranes, fascia, ligaments, etc. The parameters of the treatment are: electrical stimulation frequency of 50 Hz, pulse width of 200-700 μs, each time for 20-30 minutes; magnetic stimulation frequency of 50 Hz, the duration of the treatment is 20 minutes; biofeedback can be carried out after the end of the treatment of the electrical stimulation, 2-3 times a week, 10-12 times A course of treatment. Radiofrequency is added to the magnetic-electrical combination program after assessing the presence or possible presence of mucosal damage (e.g., urethral hypermobility, combined with pelvic floor ultrasound assessment), once every 3-4 weeks [
12,
167], for a course of 3-5 treatments. Stress incontinence can also be treated with combined magneto-electric therapy to strengthen the pelvic floor muscles supplemented with vaginal laser therapy at 4-week intervals for a course of 3 treatments [
155,
168].
- 2.
Pelvic organ prolapse
Pelvic floor muscle training alone, through muscle contraction, can increase muscle strength and endurance, help organs return to their anatomical position [
29], improve the degree of prolapse, and improve quality of life. The results of a meta-analysis concluded that pelvic floor muscle training alone for 4 weeks-24 weeks and longer could provide better results with 20-60 repetitive contractions per day. A study of electrical stimulation combined with biofeedback compared to pelvic floor muscle training alone showed pelvic floor muscle training 2-3 days per week for 12 weeks, electrical stimulation for biofeedback with electrical stimulation frequency of 30-50 Hz, pulse width of 300-500 μs, and stimulation time of 10 minutes, followed by 15 minutes of biofeedback training twice a week, for a total of 24 sessions [
169]. Clinical studies related to combined magneto-electricity treatment of pelvic organ prolapse are limited. One clinical study related to magnetic-electric combination for postmenopausal pelvic organ prolapse showed a combination of magnetic stimulation and electrical stimulation biofeedback every other day, with 10 sessions of electrical stimulation biofeedback + 10 sessions of magnetic stimulation as 1 session for a total of 2 sessions [
132], and another clinical study related to post-partum pelvic organ prolapse showed a slightly different regimen for the 5 sessions of the treatment, with stimulation alone for 30 min for the first 5 sessions, followed by 15 sessions of magnetic stimulation and electrical stimulation biofeedback combined at intervals, 2 times per week, a total of 20 treatments, after some recovery of pelvic floor muscle strength or according to the course of treatment, neuromuscular electrical stimulation was adjusted to myoelectric triggered electrical stimulation [
14].
According to the mechanism of the occurrence of pelvic organ prolapse, fascia and ligament damage in patients with prolapse, especially in middle-aged and elderly women, the level of estrogen decreases, and the collagen composition in the fascia and ligament changes, and the addition of radiofrequency among the physical therapy measures is necessary, but there is a lack of clinical literature. Magnetic and electric radiofrequency combined with radiofrequency treatment is recommended, with electrical stimulation frequency of 30Hz, pulse width of 300-500μs, stimulation time of 10-30min, magnetic stimulation stimulation frequency of 50Hz, stimulation time of 20min, 2-3 times per week, biofeedback and/or pelvic floor muscle training 2-3 times per week, 20-24 times (total number of treatments for both magnetic and electrical stimulation) in one course of treatment, and radiofrequency treatment once every 3-4 weeks (Refer to urinary incontinence program), 3-5 times a course of treatment.
- 3.
Functional constipation
Biofeedback training is the first-line non-surgical treatment for functional constipation. Biofeedback training can significantly relieve the clinical symptoms of functional constipation patients, improve the physiological function of the gastrointestinal tract, and promote the physical and mental health of patients [
170,
171]. The results of a Meta-analysis of the intervention effect of biofeedback training on patients with functional constipation showed [
172] that biofeedback 30-60 min/times, 2-3 times per week for 4-5 weeks, and intensive training can also be done 1 time/day for 30 min each time for 5 consecutive days, and ultimately concluded that biofeedback training can significantly reduce the proportion of manually assisted defecation, the force of anal pressure during defecation, and significantly increase the rate of intestinal marker expulsion. Regarding sacral neuromodulation for constipation, the stimulation parameters chosen in most studies were pulse width of 210 μs and frequency of 10-15 Hz, which generally take effect in 2-4 weeks [
173,
174,
175,
176].
The recommended treatment plan biofeedback or biofeedback combined with sacral neuromodulation, biofeedback 30 min/times. 2-3 times per week and magnetic stimulation at a frequency of 10-15 Hz 2-3 times per week for at least 2 weeks.
- 4.
Other
Several clinical studies have shown that 1-2 sessions of electromyographic biofeedback can improve pelvic floor muscle strength, frequency of sexual intercourse, and reduce sexual intercourse pain with 6-18 interventions at a frequency of 2-3 times per week [
177].
A comprehensive meta-analysis showed that despite the use of transcutaneous electrical nerve stimulation for the treatment of pelvic floor pain, there is no consistency in the effective parameters that can be addressed, with stimulation frequencies ranging from 2-120 Hz, and that in principle, high-frequency TENS may transmit stronger afferent inputs to the CNS and stronger segmental inhibition of stronger nociceptive-causing transmitters from secondary neurons [
178], whereas low-frequency TENS (10hz or lower) may activate μ-opioid receptors on the ventral side of the medullary anastomosis [
179]. The results of one meta-analysis showed that the frequency of electrical stimulation for pelvic floor pain was concentrated above 50 Hz, and that high-frequency TENS (50 Hz or more) activated δ-opioid-like substances for the relief of pain in patients with CPP that may have an anti-ischemic effect, and was thought to be more effective in relieving pain in patients with CPP, and that high-frequency TENS with the maximum tolerable intensity would result in more prolonged relief rather than suppression of the pain event with a pulse width of 50 -400 μs with a stimulation time of at least 20 min [
126], but this result has not been consistently recognized.
Electromagnetic therapy non-invasively passes through neuromuscular tissues to depolarize nerve cells, thus changing the resting state membrane potential and reducing pain transmission [
180].Meta-analysis results show that electromagnetic treatment of pelvic floor pain, with a frequency ranging from 1-50Hz, stimulates an increase in the blood flow of local tissues and improves the ischemic state of the tissues, with a frequency of 10Hz acting as a neural analgesic, and with a frequency of 50Hz it can achieve tissue decongestion, stimulate repair, from 1 time per day to 2 times per week, duration of 2-16 weeks, treatment ranging from 10min-24 hours, but mostly in the treatment of 20-30 minutes can play a satisfactory effect [
136], but there are also clinical studies show that the use of different frequencies to treat pelvic floor pain, 2 times per week, for 4 consecutive weeks, 10Hz 15min, 50Hz 15min [
181].
Radiofrequency, as a thermal technique, relieves pain by inhibiting ischemia and spasm, and stimulates thermoreceptors to enhance vasodilation and reduce ischemia-induced pain [
182,
183]. The frequency of radiofrequency treatment for pelvic floor pain was mentioned in two randomized controlled studies as once a week for 6-10 sessions [
184,
185].
Recommended treatment regimen for electromagnetic stimulation can be considered 10 Hz, or 10/50 Hz variable frequency treatment with a view to obtaining better therapeutic results, 2-5 times per week, for 20-30 minutes, at least for 2 consecutive weeks. Radiofrequency can be used alone or in combination with magnetic stimulation, the magnetic stimulation treatment program refers to the aforementioned electromagnetic treatment for pelvic floor pain, while radiofrequency is recommended once a week, 6-10 times a course of treatment.
Laser is one of the commonly used non-physical treatment techniques with menopausal genitourinary syndromes and urinary incontinence, and its frequency and duration of treatment are basically consistent across clinical studies and reviews, with most recommending a course of 3 treatments, with an interval cycle of at least 4 weeks between two treatments.
- (III)
Evaluation of the effectiveness of physiotherapy and follow-up visits
1. Indicators of treatment effect: The effectiveness of each type of pelvic floor dysfunction sexual treatment, in addition to the effective rate, should be combined with the symptomatic Relief, the presence or absence of complications, adverse reactions, quality of life, relevant questionnaires and other comprehensive assessment.
Subjective evaluation: symptomatic relief or improvement. Objective evaluation: urinary pad test, voiding diary, POP-Q score, visual analog score, and improvement of auxiliary examination indexes. Efficacy: cure, improvement, effective, ineffective.
2. Follow-up
(1) Follow-up content: Whether the symptoms recur or not, if they recur or new conditions appear, etc. should go to the hospital again, and pelvic floor assessment should be carried out in time.
(2) Follow-up methods: Telephone, WeChat or SMS, etc., and hospital follow-up if necessary.
Follow-up time: It is recommended that 1-3 months after the intervention, if necessary, according to the patient’s situation, regular follow-up.