1. Introduction
Silicon and germanium remain the most popular materials in microelectronics [
1], photovoltaics [
2,
3,
4,
5], infrared optics [
6,
7,
8], as well as when creating ionizing radiation detectors [9, 10]. Modern technology for obtaining these materials is based on the processes of processing their high-purity chlorides and hydrides [
11,
12,
13].
Virtually all silane used in hydride technology is obtained by the catalytic disproportionation reaction of trichlorosilane (TCS) [14, 15]. In this reaction, in addition to silane, a sixteen-fold mass amount of silicon tetrachloride is obtained, which is subject to disposal. The most attractive disposal option is related to the conversion of silicon tetrachloride (STC) to TCS.
In addition to the hydride technology for the production of high-purity silicon, the Siemens process is mainly used, consisting of hydrogen reduction of TCS, where STC is formed as a by-product [
1], for germanium – hydrolysis of germanium tetrachloride to its oxide and subsequent high-temperature reduction to germanium with hydrogen [
16].
In addition, in the process of TCS synthesis, 50–60 mol% STC are formed by the silicon hydrochlorination reaction as a by-product [
17], so an important task is the conversion of the STC(SiCl
4) by-product to TCS(SiHCl
3), which makes the production of silicon both by hydride technology and using the Siemens process closed-loop and environmentally friendly [
18].
The chemical high-temperature method of processing STC into TCS, used in technological schemes for the production of silicon, has a low percentage of TCS output (20%) and high energy costs (according to Silicon Products Bitterfeld GmbH&Co. KG, more than 10 kW/h per 1 kg of STC). In this regard, it is promising to develop alternative methods for converting STC into chlorosilanes and silicon.
From the point of view of increasing the degree of conversion of STC, plasma-chemical methods for converting STC to chlorosilanes (SiHCl3, SiH2Cl2) and silicon are attractive.
In order to obtain silicon in the form of bulk samples in the works [19, 20], the hydrogen reduction process of STC was carried out under microwave (2.45 GHz) discharge conditions at a pressure of 100 kPa. Agglomerated µ-Si dendritic structures were obtained. However, the samples obtained were powdered silicon, making it difficult to obtain high-purity Si ingots. In [
21], a microwave discharge of atmospheric pressure in a mixture of SiCl
4 with H
2 and Ar at a pressure of 26 to 40 kPa was used to obtain SiHCl
3. In [22, 23], TCS was obtained in an arc, and in [
24] in a high-frequency (13.56 MHz) plasmatron. It should be noted that the content of impurities in STC is at the level of purity of the initial TCS. Keeping this level of purity is necessary to obtain high-purity silicon. This aspect was not considered in [
21,
22,
23,
24].
The production of germanium by the traditional chemical method using GeCl
4 is characterized by multistage and high energy costs. In this regard, it is attractive to develop a method for obtaining germanium from GeCl
4 in one step. Plasma chemical methods can also be promising here. By analogy with STC, with plasma-chemical hydrogen reduction of GeCl
4, one can also expect the formation of chlorogermanes of the composition of GeH
nCl
m. These compounds are used in pharmacology to develop a variety of biologically active germanium-containing drugs [
25,
26,
27].
Works devoted to plasma-chemical hydrogen reduction of GeCl
4 are isolated. Nanoparticles of germanium were obtained in the works [
28,
29] in the annular discharge with capacitive coupling in the GeCl
4/H
2/Ar mixture. In [
30], a 300 W HF discharge with a frequency of 13.56 MHz at a pressure of 2.5 Torr was used to obtain chalcogenide films of the GeS
2 composition from a mixture of GeCl
4 + H
2S in a GeCl
4/H
2S ratio of 1.5 to 50. In [
31], it is reported that when processing liquid GeCl
4 under the conditions of HF pulsed discharge, in the frequency range of 0.001–100 MHz, with a pulse repetition frequency and amplitude of 0.05–50 MHz and 1–8 kV, respectively, at atmospheric pressure, treating liquid GeCl
4, Ge
2Cl
6 is formed.
In this regard, the purpose of the present work was: 1) an experimental study of the hydrogen reduction processes of STC and GeCl4 under the conditions of HF-arc discharge, stabilized by two electrodes and determination of the formation of possible products; 2) thermodynamic analysis of Si/H/Cl and Ge/H/Cl systems and comparison, where possible, of the results of calculations with the results of experiments; 3) study of the main active plasma particles and determination of the mechanisms responsible for the hydrogen reduction of volatile silicon and germanium chlorides; 4) characterization of the obtained substances.
2. Materials and Methods
In this work, the following initial substances were used: STC and germanium tetrachloride (99.999%, Firm HORST Ltd., Russia) and hydrogen (99.99998%, “Monitoring”, Russia). In the plasma-chemical reactor, W and Si electrodes with a purity of material 99.999% (Sernia Inc., Russia) were used.
Experimental Conditions
Experiments on the study of the processes of reduction of STC and germanium in hydrogen plasma were carried out on the installation, the schematic diagram of which is shown in
Figure 1. The power of the high-frequency oscillator was 340 W, frequency 40.68 MHz. The flow rate of the plasma-forming gas H
2+SiCl
4(GeCl
4) was varied in the range of 400–500 cm
3/min. The pressure during the experiment was 760 Torr. The molar ratios of H
2/SiCl
4 and GeCl
4 were 6.9 and 15 correspondingly. The study of the process of plasma-chemical reduction of SiCl
4(GeCl
4) in hydrogen plasma of the RF-arc discharge was carried out at a constant energy input of 300±15 kJ/mol.
The plasma-chemical reactor was a tube of quartz glass with a diameter of 60 mm, along the axis of which electrodes were placed. High-frequency voltage was applied to the electrodes from the generator through the matching device and a discharge was ignited between the electrodes. A vapor-gas mixture of STC (germanium) and hydrogen was fed into the discharge volume. The power supplied to the gas discharge zone was determined by the calorimetric method according to the method [
32]. In the study of the process of reducing STC, silicon electrodes (Ø 6 mm) were used, in the study of the process of reducing germanium tetrachloride – tungsten electrodes (Ø 4 mm).
The dependence of the degree of conversion of SiCl4 (GeCl4), the yield of chlorosilanes and chlorogermanes, as well as silicon and germanium, on the pressure and molar ratio of reagents was experimentally investigated. The study of the process of plasma-chemical reduction of SiCl4 (GeCl4) in hydrogen plasma of the RF-arc discharge was carried out at a constant energy level of 300 kJ/mol. The value of the energy deposit was determined as the ratio of the power supplied to the gas discharge zone to the flow rate of the plasma-forming gas.
The yield of silicon and germanium was determined by the gravimetric method with an accuracy of 1·10-4 g.
The content of chlorosilanes and chlorogermanes was determined by gas chromatography with a detection limit of 0.1% and IR spectroscopy with a resolution of 0.1 cm-1. In the study of the process of plasma-chemical reduction of SiCl4 (GeCl4), the content of metal impurities and electroactive impurities in the initial silicon and germanium chlorides, as well as in chlorosilanes, silicon and germanium, was controlled.
Gas chromatography analysis
Quantitative analysis of compounds available from the experiment was carried out by a gas chromatography method on GC “Tsvet-800” (Tsvet, Russia) equipped with a thermal conductivity detector (TCD) and a vacuum sample inlet system. For separation of components after synthesis, a 3 m long packed column with Chromaton N-AW-HMDS (0.16–0.20 mm) was used with a 15% liquid phase of E-301 at 373 K for 6 minutes. The results obtained were processed with the Tsvet – Analytic software.
IR Spectroscopy of Exhaust Gases
IR absorption spectra of the reactor gases were measured in the range 450–7000 cm
−1 by an IR spectrometer (Bruker Vertex 80v) equipped with a DTGS detector. The resolution and aperture were 1 cm
−1 and 5 mm, respectively. The gas mixture at the outlet of the reactor was taken into a cuvette with an optical path length of 10 cm. The pressure in the IR cuvette was 50 Torr. The identification of the components by the location of absorption bands was done using a database [
33]. The quantitative determination of the components was carried out using the Bouguer-Lambert-Baire equation:
where B is the integrated intensity of the spectral line or band in cm
−1; R is the universal gas constant; T is the temperature in K; с
0 is the speed of light in vacuum; A is the integral absorption coefficient; l is the optical path length in cm; and Na is Avogadro’s constant. The concentrations of gas components were calculated from:
where p is the partial pressure of the component and P is the total pressure in the cell.
Emission Spectroscopy
Optical emission spectroscopy (OES) was used to detect reaction products in plasma. Emission spectra were recorded by an AvaSpec-ULS2048CLEVO-RM-USB3 multichannel 2048-pixel fiber optic spectrometerwith ultra-lowlight scattering. The spectrawere recorded in the range 217.5–710 nm with a resolution of 0.17 nm.
Gravimetric analysis
All of the electrodes used in the experiments were weighed before and after the plasmochemical process of tetrachloride of silicon and germanium reduction. Measurement of the mass of the electrode was carried out with an accuracy of 1·10-4 g by analytical balance DV114C (Ohaus Discovery, Switzerland). The yield of silicon and germanium was determined by the difference between the masses of the electrodes before and after the experiments considering the amount of SiCl4 and GeCl4 passed through the plasma.
Methods for determining impurities in SiCl4, GeCl4, and chlorosilanes
Determination of impurities of metals and electroactive impurities in the initial SiCl4, GeCl4, and chlorosilanes was carried out by atomic emission spectroscopy on the spectrograph STE-1. Preliminary, by the method of double distillation, the impurities were concentrated on a coal weight of 100 g. Subcathode radiation of inter-electrode plasma was recorded. The detection limit of this method is 10-7–10-9 wt.%.
Characterization of Deposits
Morphological studies and analysis of elemental composition
Morphological studies and analysis of the elemental composition of the samples were carried out using the methods of raster electron microscopy and x-ray microanalysis.
The SEM image of the silicon electrode microrelief was obtained by a Tescan Vega II electron microscope at a voltage of 20 kV with a backscattered electron (BSE) detector and a detector of reflected electrons (RE detector) with zooming from 500 to 8000 and 20,000. The use of BSE and RE detectors allows obtaining more contrast images in comparison with an SE detector.
A transmission electron microscope (TEM) JEM 2100, JEOL, Japan, was used for structural studies of the powdered Ge. The TEM EDXINCAx-sight Energy attachment made by OXFORD instruments was used for elemental analysis.
Determination of impurity in Si and Ge
Impurity studies were performed on a high-resolution sector mass spectrometer with ionization in inductively coupled plasma ELEMENT-2 (Thermo Scientific). The device has three modes of operation of low, medium, and high resolution. For analysis, the samples are transferred to a solution that is transported by an argon stream to an induction discharge plasma. The cold plasma mode (generator power = 550 W) and high-resolution mode (R > 10,000) were used.
Thermodynamic calculation of chemical equilibrium
The calculations were performed using open-source software [
34]. The software implies the local thermodynamic equilibrium (LTE) and is based on the Gibbs free energy minimization algorithm:
where μ
j and n
j are the chemical potential and number of gram-moles of species j per gram of mixture, and N is the number of molecular species. The minimization is subject to the mass balance and non-negative concentration constraints; the minimization is performed by the Lagrangian multipliers method. Abundant literature exists on the topic [
35]. The authors of the present study have also recently developed an algorithm based on the mass action, charge, and mass conservation laws [
36,
37]; however, this model does not include phase transformation, which is of primary interest in plasma deposition methods.
The model [
34] assumes the equation of state for an ideal gas even when a small amount of the condensed phase is present. This assumption is acceptable because of the negligible volume of the condensed phase relative to the gaseous species [
34]. Another simplification is the purity of the condensed phase; the phase transitions are allowed between liquid and gas, solid and gas, solid and liquid, and between stable solid phases.
First, the plasma composition as a function of temperature is calculated for steady-state plasma at a fixed pressure of 1 atmosphere, a so-called t-p option in [
34]. The plasma is assumed to be in LTE, the assumption admissible for atmospheric RF-arc plasmas.
A similar approach was used in [
38] and adjusted in [
39,
40,
41] to calculate the equilibrium composition of hydrogen and argon-hydrogen plasma of halides considered in this paper. The coincidence of the results obtained with the algorithm [
34] and algorithm [
39,
40,
41] confirms the validity of both algorithms.
3. Discussion of the results.
Hydrogen reduction of SiCl4
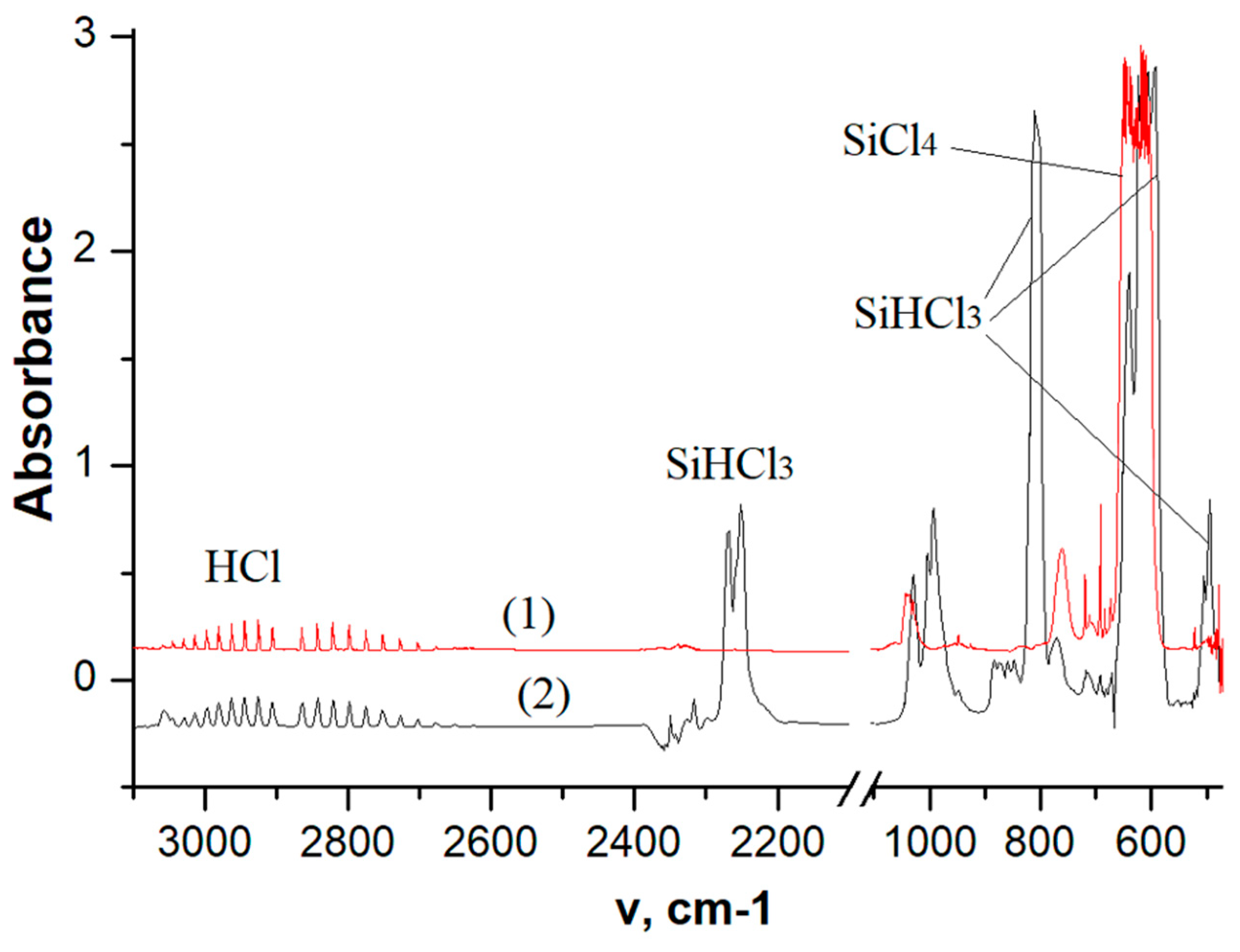
In studying the plasma chemical reduction process of silicon tetrachloride, the main gas-phase reaction products were shown to be trichlorosilane (SiHCl
3) and hydrogen chloride. SiHCl
3 absorption bands are recorded in the range of 2257, 806, 588 and 497 cm
-1 (
Figure 2). A band belonging to the HCl molecule is recorded in the 3000 cm
-1 region. At the ratio H
2/SiCl
4 = 6.9, the SiHCl
3 yield is 44%. This, as follows from thermodynamic analysis, corresponds to an equilibrium concentration of SiHCl
3 at a temperature of 1250 K. The equilibrium concentration of dichlorosilane at this temperature is much lower. It should be noted that the results for the yield of TCS are in good agreement with the data [
42].
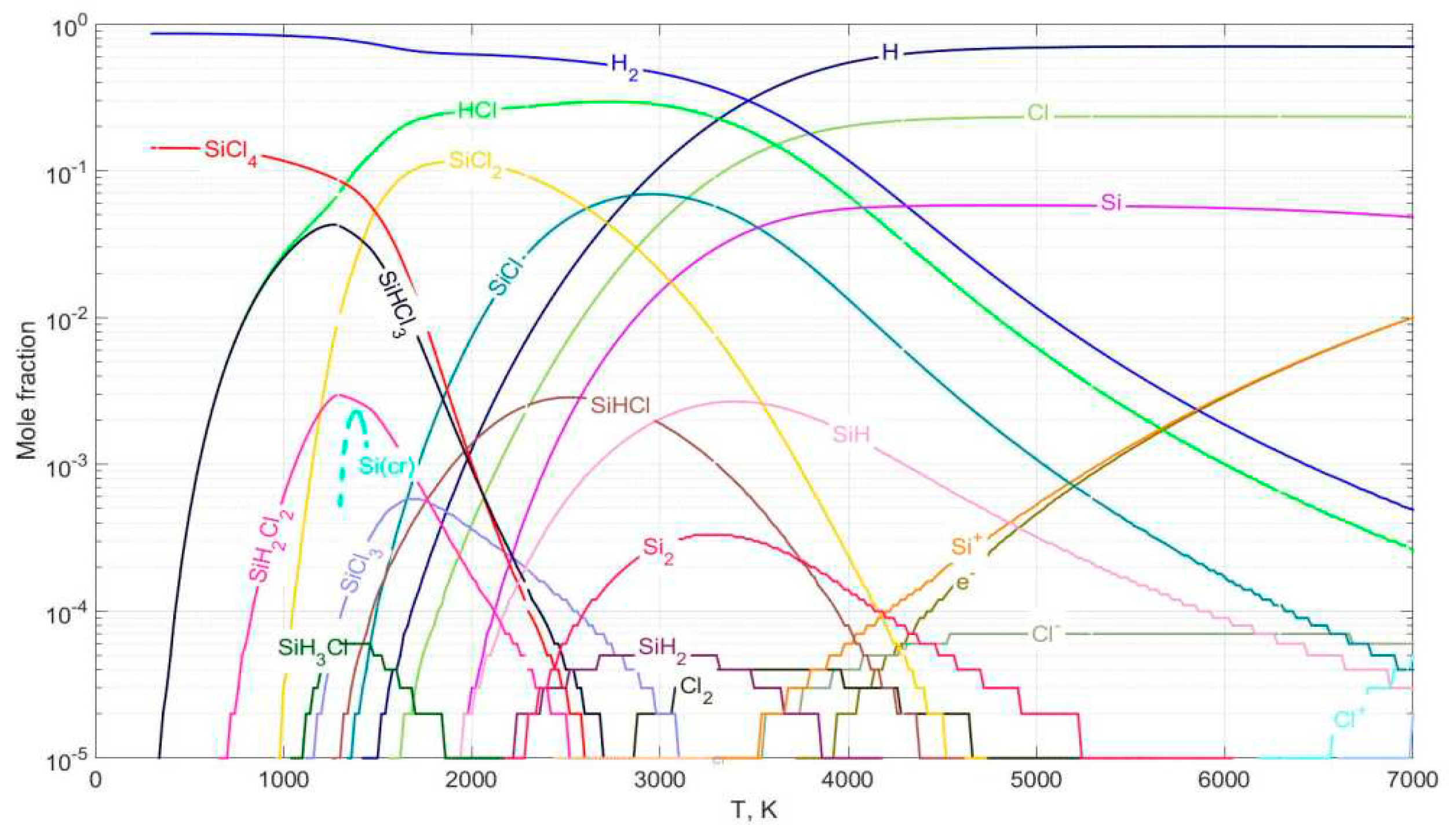
The equilibrium composition of the system SiCl
4/H
2 = 6.9, depending on the temperature is shown in
Figure 3.
Figure 3 shows that condensed silicon is created only in the temperature range of 1210–1625 K. The formation of SiHCl
3 is observed in the temperature range of 300–2700 K, SiH
2Cl
2 in the range of 750–2500 K, and SiH
3Cl in the range of 1150–1850 K. The formation of SiCl
2 and SiHCl radicals is observed in the range of 1000–4500 K and 1230–4300 K, respectively.
In [
43,
44], it is noted that in the thermodynamic analysis of the Si-H-Cl system, five independent, equilibrium reactions should be taken into account, but in the case of low concentrations of H
2(H
2/SiCl
4˂10) and temperatures below 1123–1173K, the number of independent reactions is reduced to two:
as the equilibrium concentrations of SiH
2Cl
2, SiH
3Cl and SiCl
2 become negligible. This is confirmed experimentally.
To clarify the temperature range of the hydrogen reduction of SiCl
4 using mathematical modeling, the distribution of thermal and concentration fields in the reaction chamber was investigated in [
45]. It was shown that the gas temperature in the reaction zone is in the range of 900 – 1700 K. This also confirms that under the experimental conditions implemented, chemical reactions close to equilibrium occur.
Hydrogen reduction of GeCl4
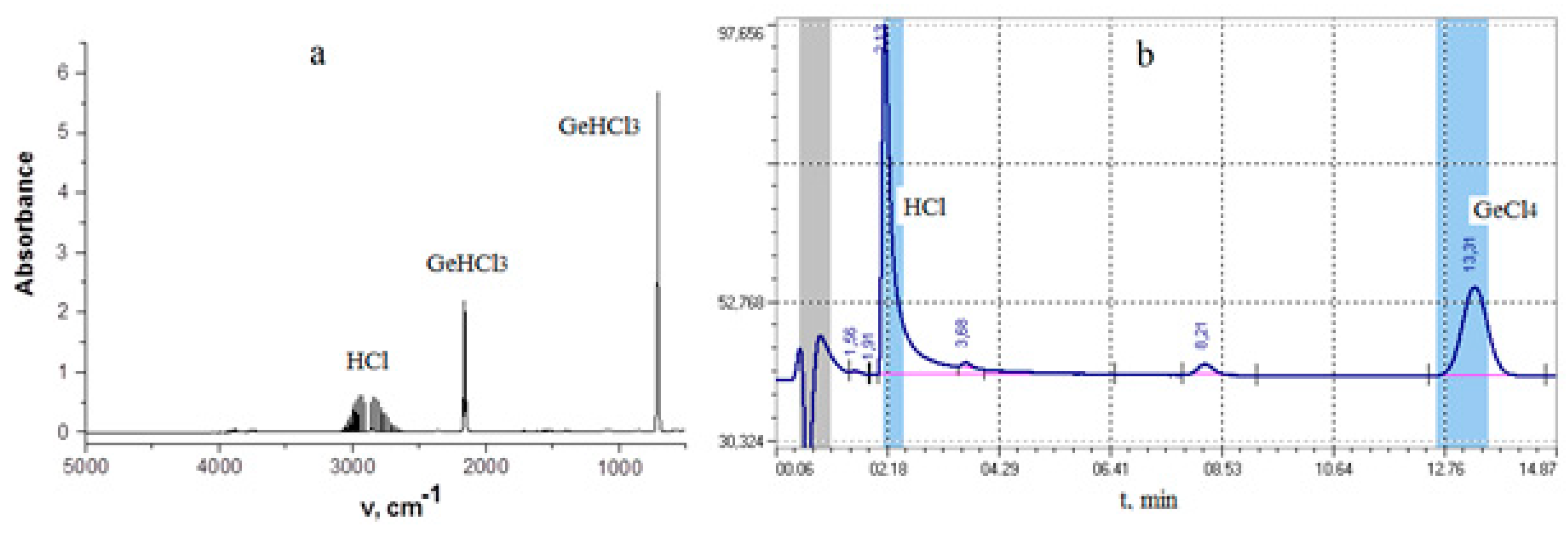
When studying the process of plasma-chemical reduction of germanium tetrachloride, it was necessary to determine into which products GeCl
4 can transform in a hydrogen plasma of this type of discharge. It was shown that in the gas phase leaving the gas mixture reactor, according to IR analysis, the formation of trichlorogermane (GeНCl
3) is observed. Its absorption bands are recorded in the range of 2155 and 708 cm
-1 (
Figure 4 a). Also in the region of 3000 cm
-1 a band related to the HCl molecule is recorded.
Germanium tetrachloride GeCl
4 has no absorption bands in this frequency range. On the contrary, the GeCl
4 and HCl peaks are well recorded in the chromatogram (
Figure 4 b), but the GeНCl
3 peak cannot be separated from the GeCl
4 peak on the gas column adsorbent. Thus, the formation of trichlorogermane and hydrogen chloride is confirmed by IR spectroscopy. The overall conversion of GeCl
4 can be seen from the peak in the chromatogram.
In addition, according to X-ray phase analysis, deposition of ingot-shaped, drop-shaped germanium was observed at the ends of the electrodes, and germanium powder was deposited on the walls of the reactor. Thus, the main reactions of plasma-chemical conversion are the formation of trichlorogermane and germanium according to the reactions:
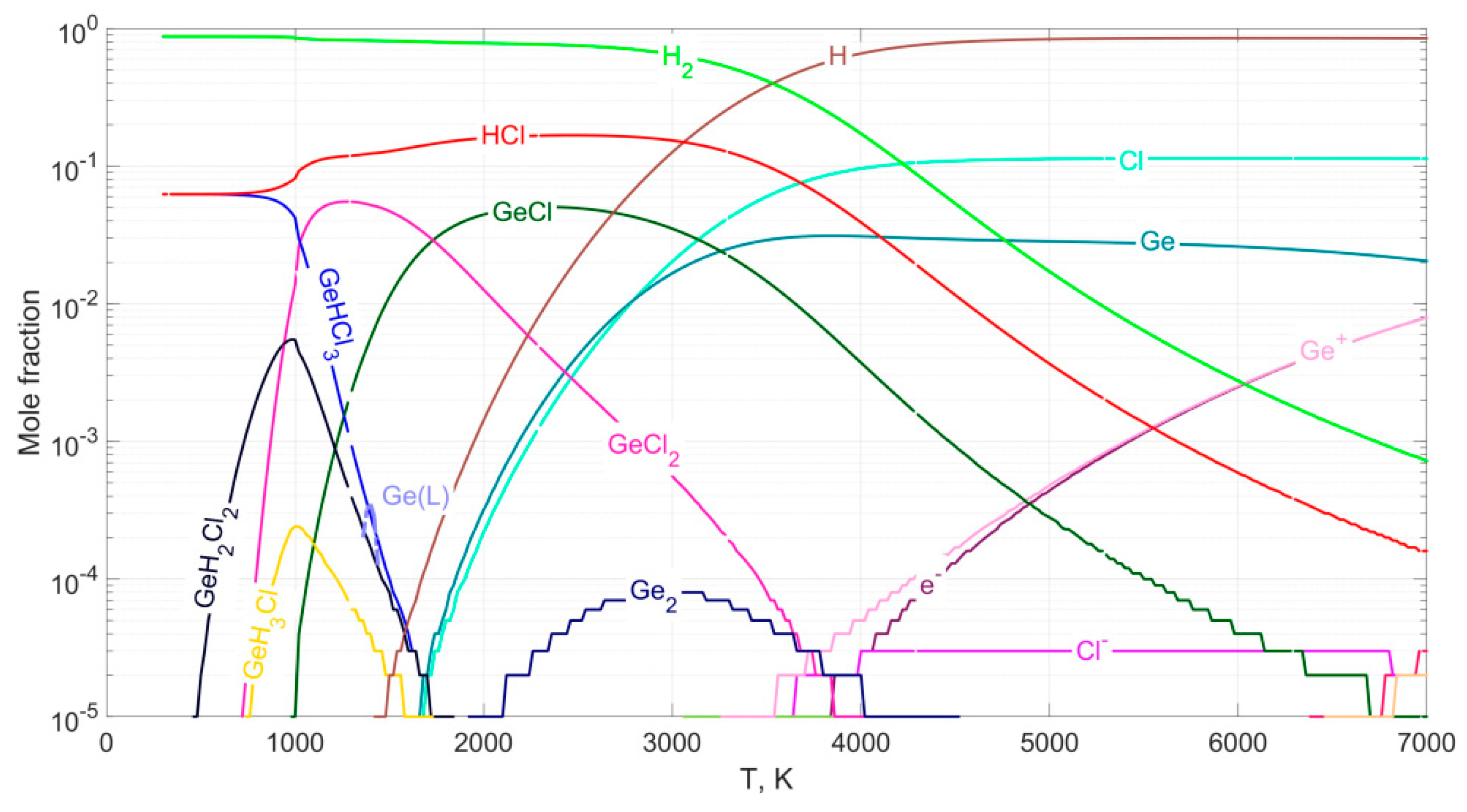
The equilibrium composition of the H
2/GeCl
4 = 15 system depending on the temperature at atmospheric pressure is shown in
Figure 5. This mixture stoichiometry was selected based on the maximum yields of Ge and GeHCl
3. Condensed germanium is formed from germanium tetrachloride, in the range of 1250–1500 K. Formation of the GeHCl
3 compound, according to
Figure 5, occurs in the range of 300–1580 K, GeH
2Cl
2 in the range of 500–1580 K, and GeH
3Cl in the range of 760–1500 K.
Studies of thermal and concentration fields in the process of hydrogen reduction of GeCl4 by the method of mathematical modeling were not carried out. However, on the basis of similar studies for the hydrogen reduction process of the STC, it can be argued that in this case, the reaction chamber also has a complete interaction of the gas mixture with the plasma. This makes it possible to assume the equilibrium of the process and estimate the temperature range of the reaction.
According to the experiment, polycrystalline germanium is deposited at the ends of the electrodes, an amorphous germanium powder is formed in the reactor volume, and the analysis of the gas phase confirms the formation of GeHCl
3. Equilibrium calculations shown in
Figure 3 also show the possibility of the formation of these substances, which indicates the proximity of the realized experimental conditions to the equilibrium. The GeH
2Cl
2 and GeH
3Cl compounds are not observed experimentally, due to their low equilibrium concentrations.
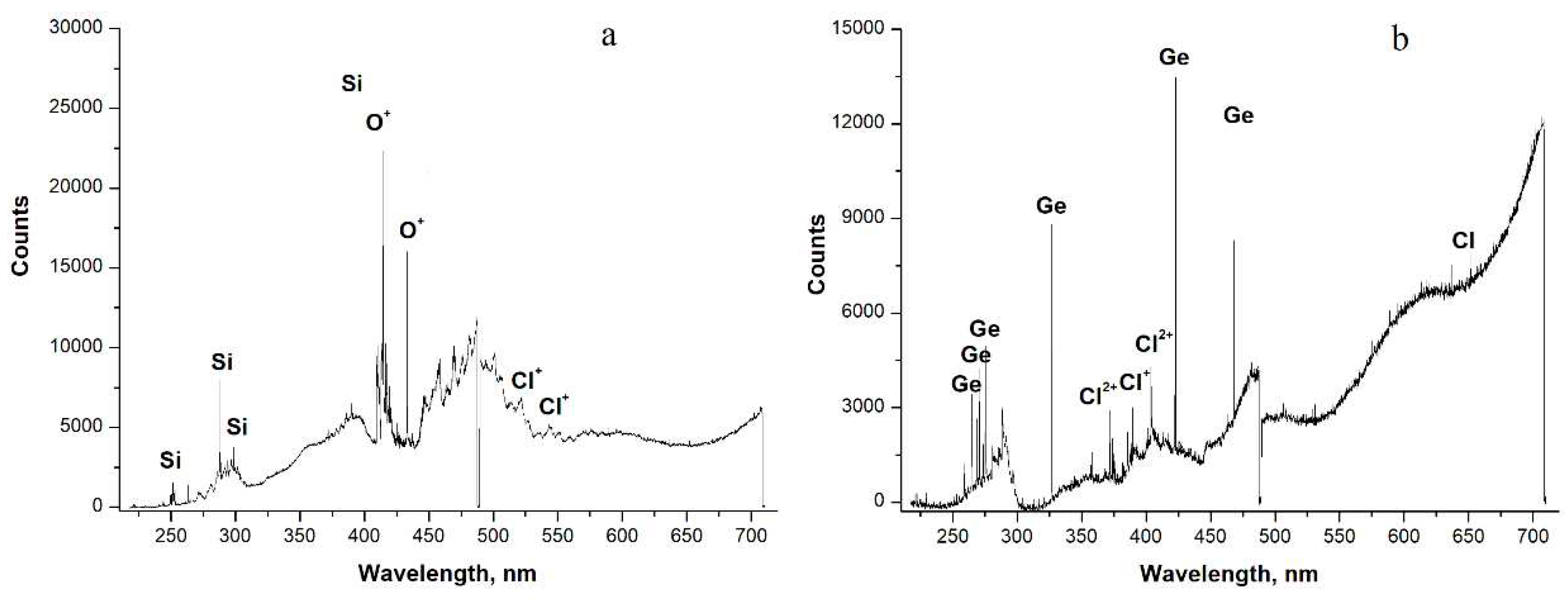
Figure 6 a, b) shows the emission spectra of mixtures of H
2 + SiCl
4 and H
2 + GeCl
4 during hydrogen reduction in RF-arc discharge. It can be seen that in the experimental conditions realized, the nature of the emission spectra is identical. Namely, in both cases, lines belonging to silicon or Germany are observed, as well as lines of chlorine ions. In both cases, the emission spectra lack bands of radicals such as Si-Cln, Si-Hn, Ge-Cln and Ge-Hn, as well as lines belonging to atomic hydrogen. This may indicate that the interaction of molecules with each other under these conditions occurs by a molecular mechanism, and plasma is a highly efficient source of heating. In this case, the reactions (4) to (7) describe this mechanism.
Investigation of the substances obtained
Characterization of samples obtained during the reduction of SiCl4
In the case of the hydrogen reduction of SiCl
4, the main conversion products are of trichlorosilane, as well as silicon.
Table 1 shows the content of metal impurities in the initial SiCl
4, as well as a mixture of reaction products and silicon. In addition, the content of electroactive impurities is given for precipitated silicon. It can be seen that the content of metal impurities in the reaction products mixture is at the level of the initial SiCl
4. This suggests that in this type of discharge, it is possible to obtain chlorosilanes and in particular TCS not only with a high yield but also with the preservation of the chemical purity of the original SiCl
4, which is the main requirement for the technology of obtaining high-purity silicon. This possibility appears due to the use of a high-purity silicon electrode as a material. The modes in this case are selected in such a way as to avoid melting the electrode.
Figure 7 shows an image of a silicon electrode before and after hydrogen reduction, as well as a view of the silicon formed on the electrodes in the form of drops Ø3-5 mm. In a silicon sample, on the contrary, there is a concentration of metal impurities. Apparently, impurities of metals are in the original SiCl
4 in the form of volatile compounds, possibly chlorides, with a binding energy less than that of STC and therefore more easily react with chemically active plasma, which leads to their concentration in the deposited silicon. However, the hydrogen reduction process of SiCl
4 under RF-arc discharge conditions can be used to obtain high-purity, monocrystalline samples, provided that the stages of zone recrystallization and growth of the Czochralski crystal are carried out. The concentration of electroactive impurities Al, B, P, As, and Sn in silicon is below the detection limit.
Samples of germanium obtained in RF-arc discharge from GeCl4
Experimentally, it was shown that under the conditions of RF-arc discharge at a pressure below atmospheric pressure, from a mixture of GeCl
4 + H
2, at the ends of the electrodes, an ingot Ge was formed. In addition, Ge was also formed as a powder deposited on the inner surface of the reactor. The ingot Ge, according to X-ray phase analysis, was polycrystalline.
Table 2 shows the impurity composition of the initial GeCl
4 and the resulting ingot Ge.
It can be seen that the content of impurities in Ge is at the level of purity of the original GeCl
4. Exceptions are impurities W and Fe coming from structural materials (the tungsten electrode and stainless steel nozzles). However, these impurities can be easily removed by zone recrystallization by the Bridgman method [
46].
Of particular interest is also the powdered Ge. Nanocrystals of germanium can be of interest for various electronic and optoelectronic applications, primarily due to the possibility of adjusting their bandwidth from the infrared to the visible range of the spectrum depending on the size [
28,
29].
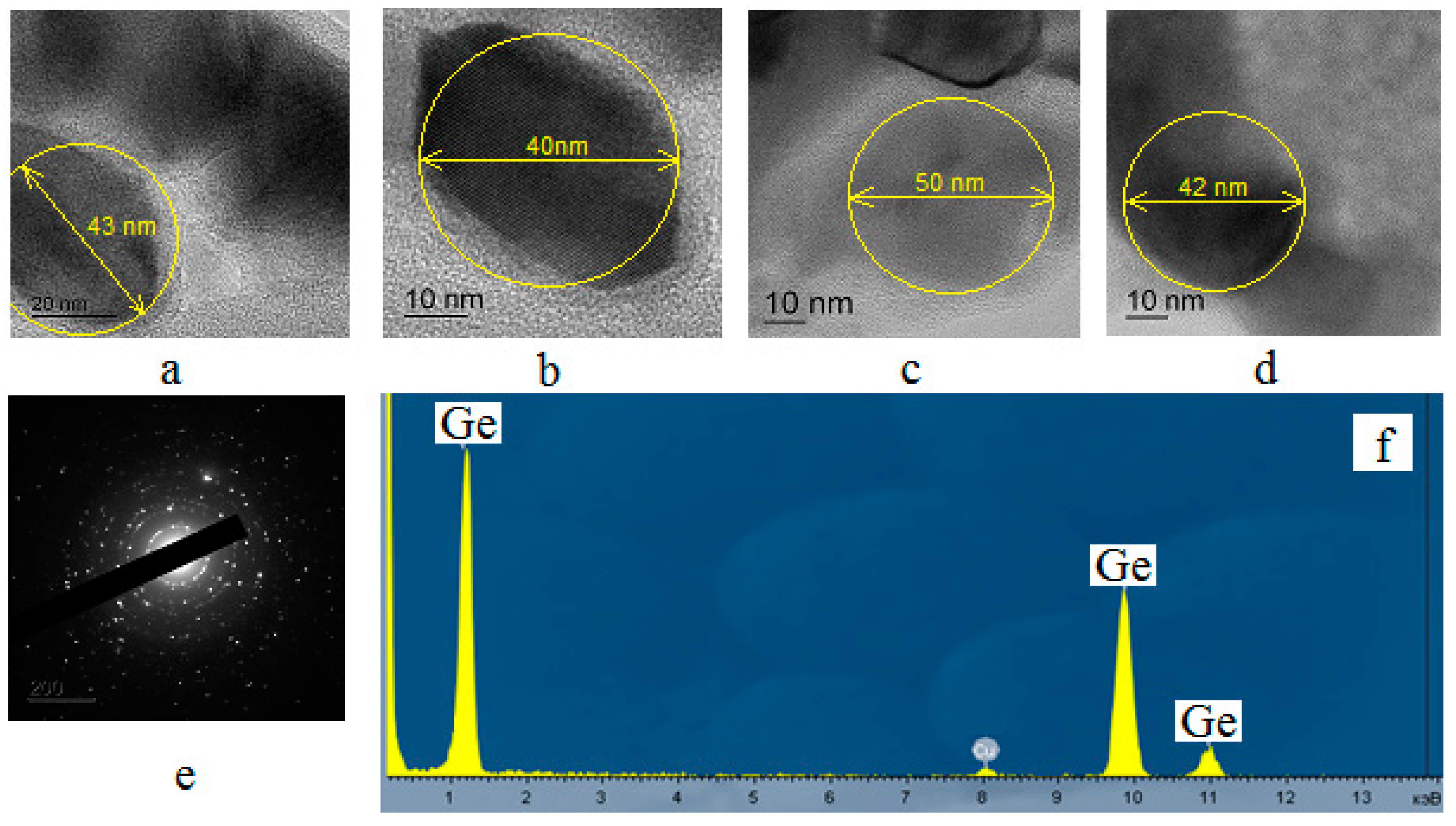
Figure 8 a-d) shows the TEM image of this sample.
The powdered Ge is a nanoparticle. The diffraction pattern of the sample (
Figure 8 e) indicates that these particles are monocrystalline, and according to X-ray microanalysis (
Figure 8 f), the Ge sample obtained does not contain Cl and O impurities.
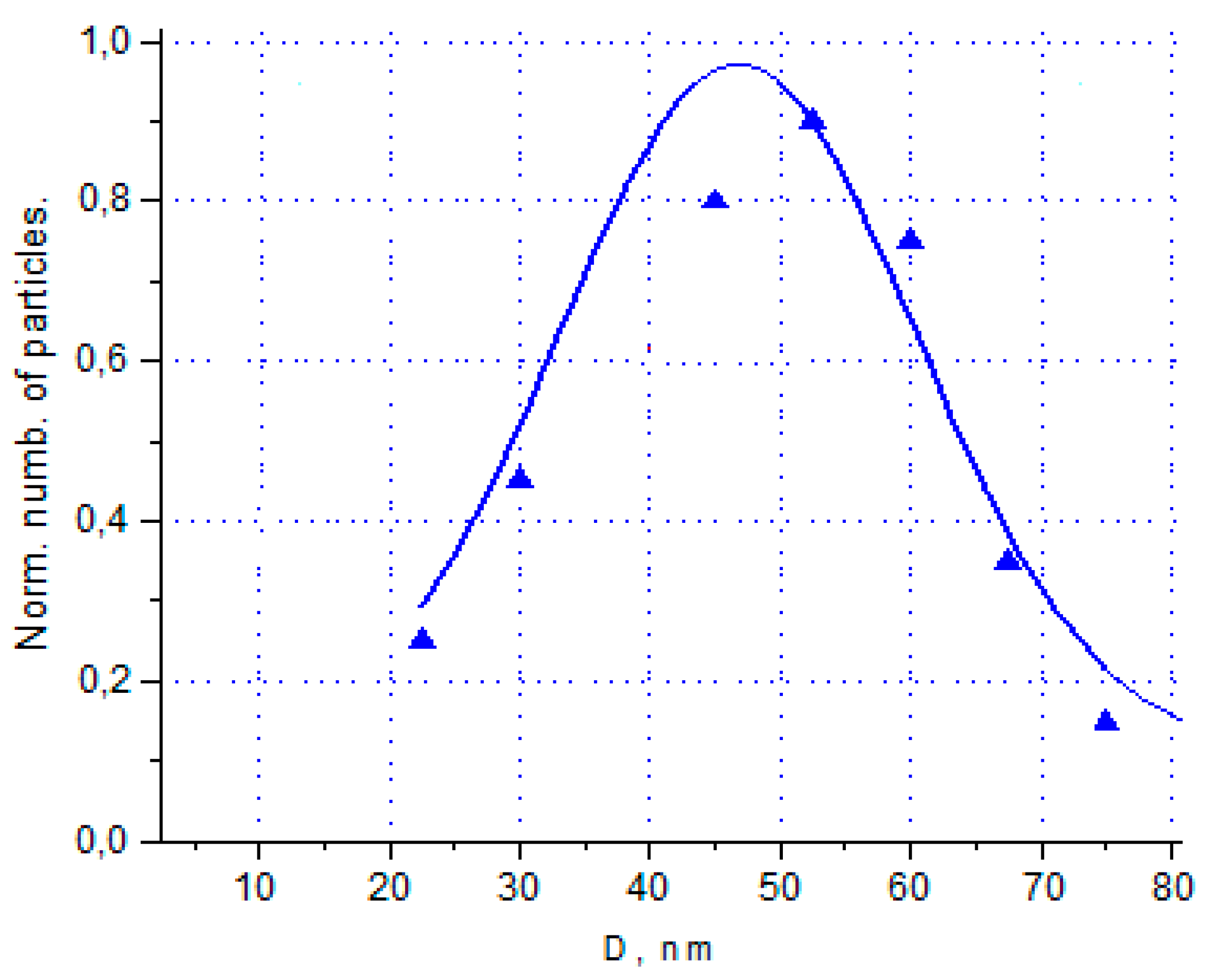
In the study of nanostructured germanium samples by laser diffraction using dispersion, it was impossible to carry out a complete separation of agglomerates, which did not make it possible to correctly estimate the particle size distribution. Therefore, the evaluation of this parameter was carried out on the basis of photographs obtained on TEM. The particle size distribution is shown in
Figure 9. It can be seen that the particle size has a range from 22 to 75 nm with an average size of 40–50 nm. Thus, in the RF-arc discharge, in the pressure range of 30–760 Torr, conditions are created for obtaining not only ingot-like, compacted Ge but also Ge in the form of nanoparticles.
4 Conclusions
Based on the studies conducted, it can be concluded that RF-arc discharge can be used to obtain high-purity trichlorosilane and silicon from the by-product of silicon production of STC. The content of the target products in the realized experimental conditions is close to equilibrium, which is confirmed by thermodynamic analysis. The temperature ranges in the zone of STC hydrogen reduction reactions determined using numerical modeling correspond to the temperatures at which a thermodynamically equilibrium yield of products is observed. RF-arc discharge can also be used to obtain high-purity germanium in the form of both polycrystalline ingots and nanopowder with an average particle size of 40–50 nm, as well as for the synthesis of high-purity trichlorogermane with a high yield. The main mechanism responsible for the hydrogen reduction of silicon and germanium tetrachloride is molecular.
Author Contributions
R.A. Kornev - statement of the problem, experimental research, formulation of conclusions. I.B. Gornushkin - calculation of thermodynamic equilibrium concentrations. L.V. Shabarova - preparation and editing of the manuscript and the figures. A.A. Ermakov - recording emission and IR spectra. G.M. Mochalov - gas chromatographic and IR analysis. N.V.Rekunov, V.A.Medov - preparation and conduct of an plasmachemical experiment. A.D. Chaschina - analysis of the received products. All authors reviewed the manuscript, A.A Kalinina – writing – review & editing.
Funding
The work was carried out within the framework of the state task in the field of scientific activity (subject No. FSWE-2022-0008).
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Acknowledgments
The work was carried out within the framework of the state task in the field of scientific activity (subject No. FSWE-2022-0008).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. .
References
- Zulehner W (2000) Materials Science and Engineering B 73:7-15. [CrossRef]
- McHugo SA, Thompson AC, Mohammed A, Lamble G, Périchaud I, Martinuzzi S, Werner M, Rinio M, Koch W,. Hoefs HU, Haessler C (2001) Journal of Applied Physics. 89:4282-4288.
- Zhang X, Gong L, Wu B, Zhou M, Dai B (2015) Solar Energy Materials and Solar Cells 139:27-33.
- Cariou R, Tang J, Ramay N, Ruggeri R, RocaiCabarrocas P (2015) Solar Energy Materials & Solar Cells 134:15–21.
- Leal R, Dornstettera JC, Haddad F, Poulain G, Maurice JL, Cabarrocas PR (2015) 42nd Photovoltaic Specialist Conference (PVSC) New Orleans.
- Kim W, Matsuhara H, Onaka T (2005) Cryogenic Optical Systems and Instruments XI 5904:590418(12). [CrossRef]
- Fonollosa. J, Rubio R, Hartwig S, Marco S, Santander J, Fonseca L, Wollenstein J, Moreno M (2008) Sensors and Actuators B 132:498-507.
- Houssa M (2007) Germanium-Based Technologies: From Materials to Devices, Oxford:Elsevier.
- Raudorf TW, Trammel RC, Darken LS (1976) IEEE Trans. on Nucl. Sci 26:297-302.
- Pehl RH, Madden NW, Elliott JH (1979) IEEE Trans. on Nucl. Sci. 26:321-323.
- Eaglesham DJ, Cerullo M (1990) Phys. Rev. Lett. 64:1943–1947.
- Chu S, Majumdar A (2012) Nature 488:294–303.
- Bathey, BR, Cretella MC (2005) J. Mater Sci 17:3877–3896.
- Vorotyntsev A, Markov A, Petukhov A, Atlaskina M, Atlaskin A, Kapinos A, Vorotyntsev V, Pryakhina V (2021) Catal Ind 13:1–11.
- Matveev AK, Mochalov GM, Suvorov SS (2015) Method of obtaining silane and chlorosilanes RU Patent No 2608523.
- E; Claeys С (2007) Germanium-Based Technologies: From Materials to Devices Oxford:Elsevier Science.
- Jarkin VN, Kisarin OA, Kritskaya TV (2021) Izvestiya Vysshikh Uchebnykh Zavedenii. Materialy Elektronnoi Tekhniki 24:5-26. [In Russian].
- Sennikov PG, Kornev RA, Nazarov VV (2020) Sposob poluchenia visokochistogo polikrastallicheskogo kremnia Patent RF (RU) № 2739312. [In Russian].
- Lifeng W, Zhibin M, Aihua H, Jianhua W (2009) Inorganic Materials 45:1403-1407.
- Lifeng W, Zhibin M, Aihua H, Jianhua W (2010) Journal of Hazardous Materials 173:305-309.
- Zhenxi LU, Weigang Z (2014) Chinese Journal of Chemical Engineering 22: 227-233.
- Qingyon W, Hanbin C, Yuliang L, Xumei T, Zhijun H, Shuyong S, Yongxiang Y, Xiaoyan D (2010) Inorganic Materials 3:299-302.
- Gromov GN, Bolgov MV, Muravitski SA (2009) Sposob poluchenia trihlorsilana plazmohimichekim gidrirovaniem tetrahlorida kremnia I ustroistvo dlia ego osushestvlenia Patent RF (RU) № № 2350558. 685 [In Russian].
- Sarma KR, Rice MJ (1982) High pressure plasma hydrogenation of silicon tetrachloride US Patent № 4,309,259.
- Fang YZ, Ma WY, Zhou JH, Lu C, Wu JG (2008) Journal of Molecular Structure: THEOCHEM, 857:51-56.
- Menchikov LG, Ignatenko MA Pharmaceutical Chemistry Journal 46: 635 – 638.
- Gielen M, Tiekink ERT (2005) Metallotherapeutic drugs and metal-based diagnostic agents. The use of metals in medicine, Wiley.
- Ahadi AM, Hunter KI, Kramer NJ, Strunskus T, Kersten H, Faupel F, Kortshagen UR (2016) Physics Letters 108:093105.
- Gresback R, Holman Z, Kortshagen U (2007) Applied physics letters 91:093119(3).
- Whitham PJ, Strommen DP, Lundell S, Lau LD, Rodriguez R (2014) Plasma Chem Plasma Process 34:755-766.
- Lang JE, Rauleder H, Muh E (2013) Method for producing higher silanes with improved yield WO Patent № 007426 A1.
- Gusev AV, Kornev RA, Sukhanov AYu (2006) Inorganic Materials 42:1123-1026.
- Gribov LA, Smirnov VN (1961) Usp Fiz Nauk 527:527–567 [in Russian].
-
https://cearun.grc.nasa.gov. Accessed on 15 April 2020.
- Smith WR, Missen RW (1982) Chemical reaction equilibrium analysis: theory and experiment, Wiley:New York.
- Shabanov SV, Gornushkin IB (2018) Appl Phys A 124:716.
- Shabanov SV, Gornushkin IB (2014) Spectrochim Acta B 100:147–172.
- Belov GV, Iorish VS, Yungman VS (2000) High Temp 38:191–196.
- Shabarova LV, Sennikov PG, Kornev RA, Plekhovich AD, Kutyin AM (2019) High Energy Chem 53:482-489.
- Shabarova LV, Plekhovich AD, Kutyin AM, Sennikov PG, Kornev RA (2019) High Energy Chem 53:482–489.
- Shabarova LV, Plekhovich AD, Kutyin AM, Sennikov PG, Kornev RA (2020) Theor Found Chem Eng 54:504–513 [in Russian].
- Sarma KR (1985) Hydrogenation Method silicon tetrachloride, Chanley C.S. Patent 4542004 USA.
- Wolf WE, Teichman R (1980) Z. anorg. allg Chem 460: 65-80.
- Sirtl E, Hunt LP, Sawyer DH (1974) J. Electrochem. Soc.: Solid-State Science and Technology 121:919-925.
- Kornev R, Sennikov P, Nazarov V, Sukhanov A, Shabarova L (2019) Plasma Physics and Technology 6:127-130.
- Sennikov PG, Kornev RA, Abrosimov NV, Bulanov AD, Gavva VA, Potapov AM (2019) Materials Science & Engineering B 244:1–5.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).