1. Introduction
Tracheal agenesis (TA) is a congenital anomaly in which the whole or partial segment of the trachea is absent [
1]. The incidence is estimated 1 in 50,000 live births [
2]. Floyd et al. classified tracheal agenesis into three categories according to the type of fistulous connection with the esophagus [
3]. Although TA is associated with premature delivery and polyhydramnios, prenatal diagnosis is difficult. VACTERL association (vertebral defects anal atresia, cardiac defects, tracheoesophageal fistula, radial or renal anomalies, and limb abnormalities) is known associated anomalies. Most neonates with this condition die within hours of the birth owing to difficulties in diagnosis and treatment, in particular, due to the failure of the securement of the airway derived from the absence of the trachea. Mortality is high: 85% of cases die within 2 days [
2]. Initial prompt esophageal intubation and the long-term management of the “esophageal airway” is a crucial part of the management. However, in most cases, the management is challenged by the collapsing of the fragile esophagus wall and severe tracheomalacia-like symptoms.
External stenting technique which provides artificial cartilages to the esophagus to support the floppy esophageal wall has been reported [
4,
5,
6,
7]. The technique was initially invented for severe tracheomalacia and one of the authors (MA) of this article has been extensively involved in the surgical management of tracheomalacia with this technique [
8,
9]. Although a ringed polytetrafluoroethylene (PTFE) has been already used for tracheal external stent in patients with tracheomalacia, compressive injury on the adjacent structure is possible and may become fatal [
9]. Several surgical efforts are necessary to prevent injury of important structures such as large vessels caused by a ringed PTFE graft.
In this case report, we present a case of TA in which external esophageal stenting was effective to stabilize the respiratory status. Trachealization of the esophagus with external stenting have been already reported in several articles and this paper presents an additional case to corroborate the effectiveness of the stenting and the important surgical techniques for the success of the procedure based a lesson learned from the experience for tracheomalacia.
2. Case Description
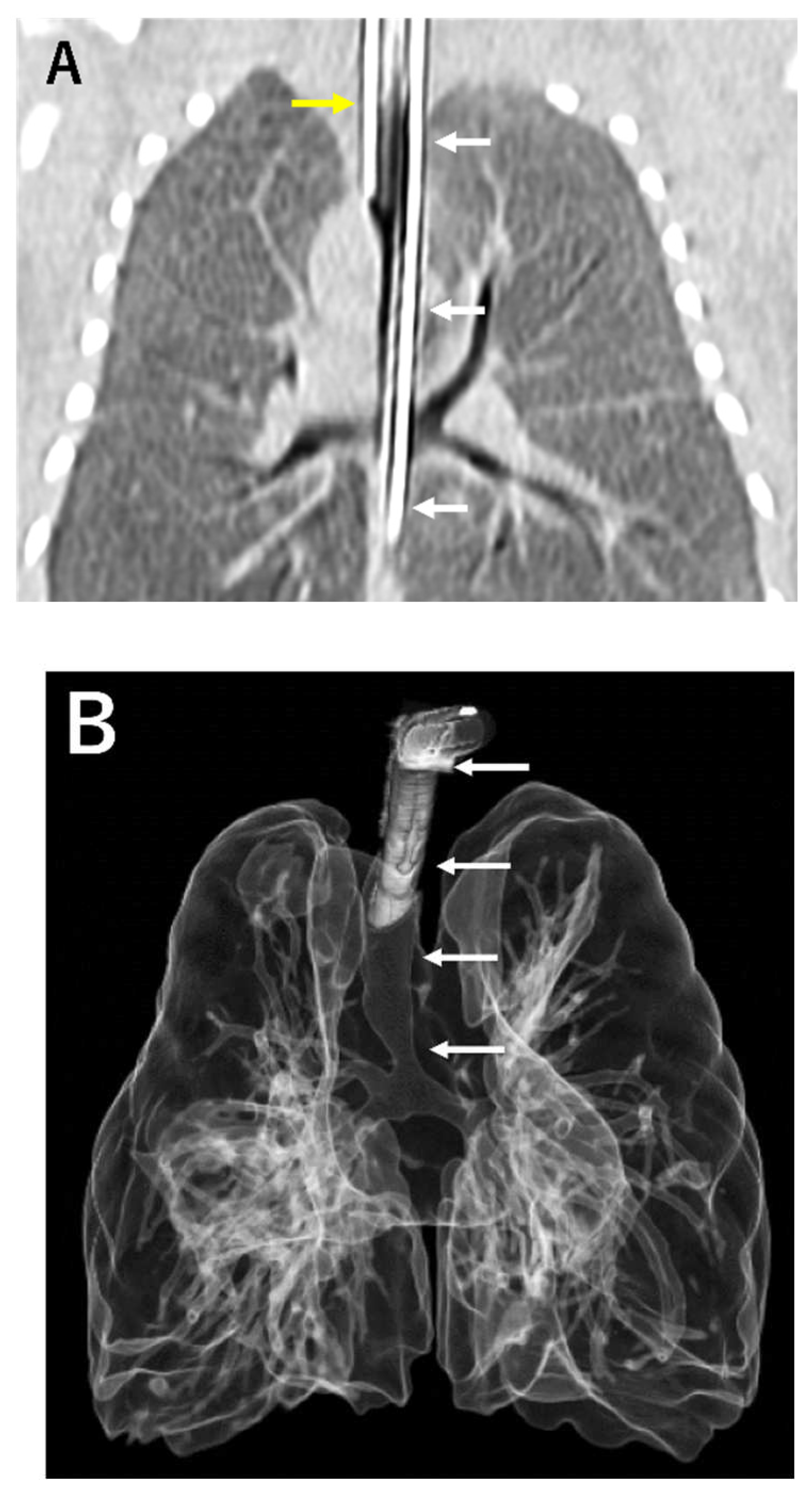
A male infant with a history of polyhydramnios was born at 36 weeks gestational age with birth weight of 2,822 g. Respiratory distress developed immediately after the birth, and therefore, endotracheal intubation was attempted. The larynx was normally visible, but an endo-tracheal tube could not go further beyond the subglottic space. TA was suspected and deliberate esophageal intubation finally succeeded resuscitation of the patient. Computed tomography (CT) scan confirmed Floyd type III TA (
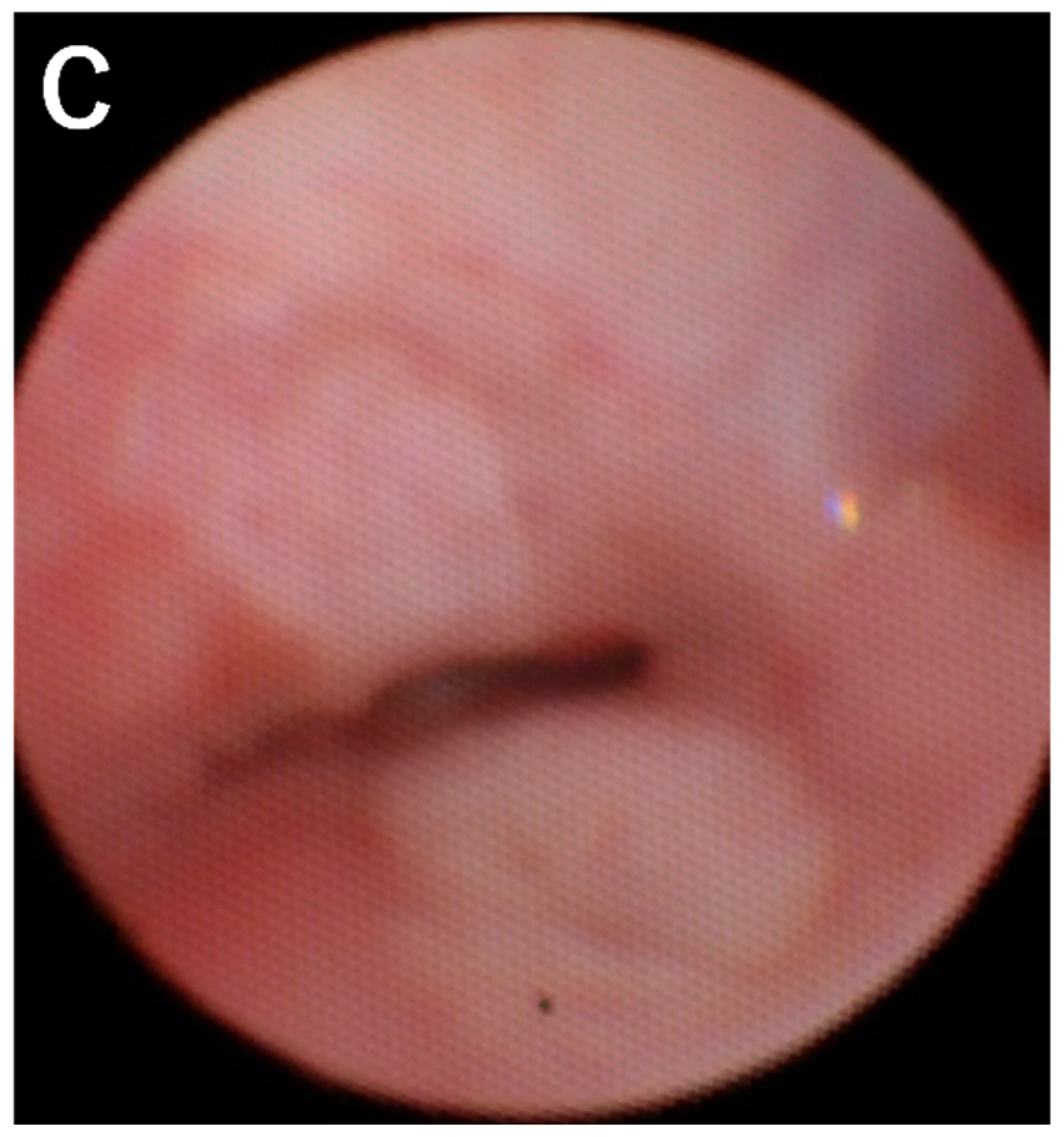
Figure 1A). Tetralogy of Fallot (TOF) and anorectal malformation was also diagnosed. An emergency surgery to make the esophagus as the airway was performed 12 hours after the birth. The ligation of the esophagus distal to the origin of the tracheal and the resection of the esophageal stenosis just proximal to the bifurcation was carried out through the extrapleural approach. Gastrostomy and colostomy were created simultaneously. To prevent aspiration pneumonia due to the drooling of saliva into the airway via the esophagus, the esophagus was divided at its midway and the proximal esophagostomy for saliva drainage and the distal esophagostomy as the airway entrance was performed at day 45 of the life. His respiratory status was improved after the surgery but he still suffered from multiple episodes of tracheomalacia-like symptom due to the floppy esophageal wall including dying spell (
Figure 1B, C).
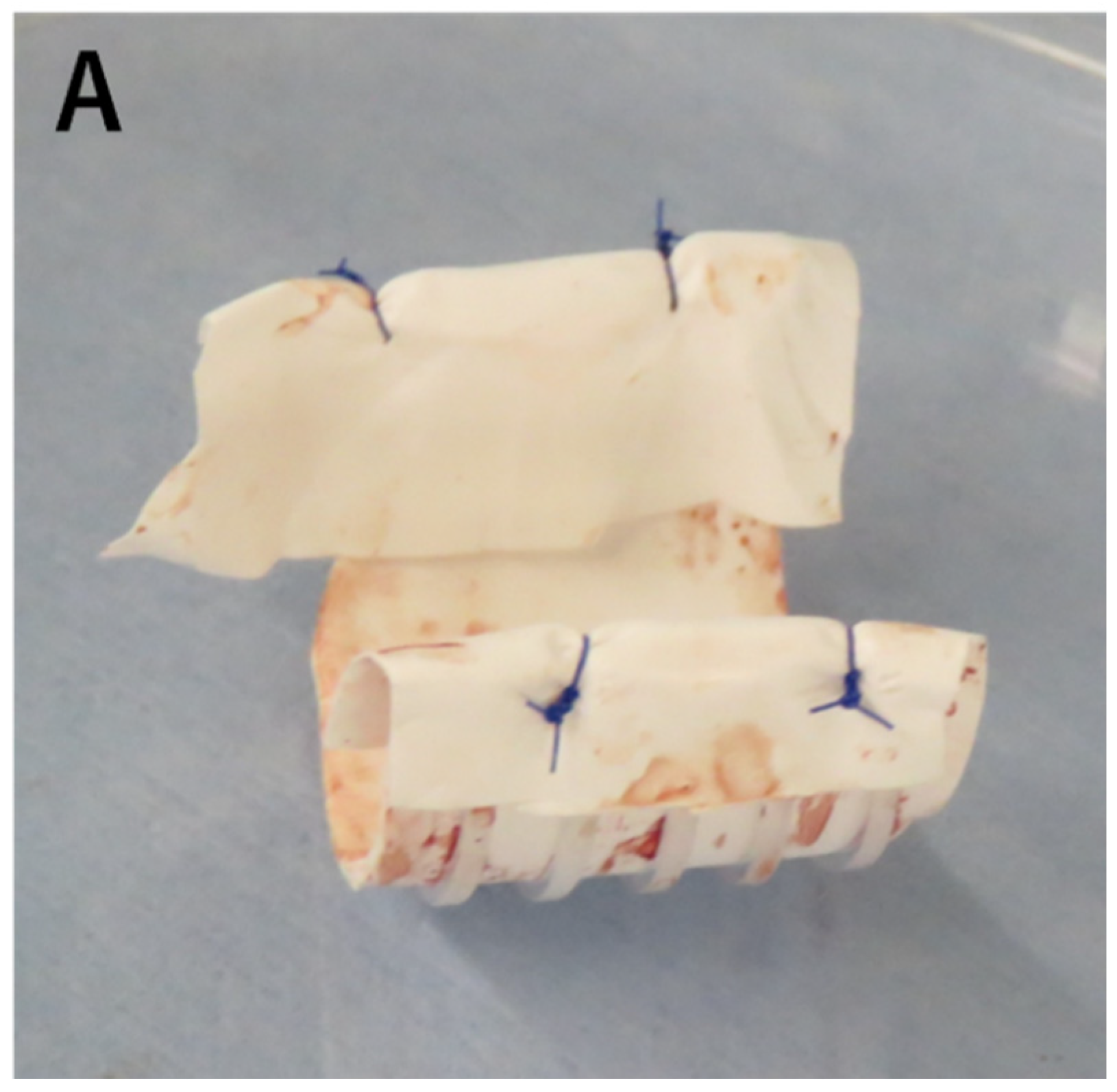
To reinforce the floppy esophageal wall and improve tracheomalacia-like symptom, external stenting of the esophagus was planned at 7 months of the life. The surgery was performed in supine position with median sternotomy. Cardiopulmonary bypass was established with an out-flow cannula in aortic arch and in-flow cannulas in superior and inferior vena cava. Right pulmonary artery ran just in front of the esophagus and was mobilized to expose the anterior aspect of the esophagus. A ringed PTFE (16mm in size) prosthesis was longitudinally cut in half and then adjusted to the length corresponding to that of the collapsed esophageal airway segment determined with intraoperative bronchoscopy. The length of PTFE graft involved 5 plastic rings. The edge of the prosthesis was covered with 0.1mm Gore-Tex PTFE sheet to prevent contact injury and perforation of surrounding organs (
Figure 2A). Then, it was placed around the anterior aspect of the esophagus. Using radial traction sutures, the prosthesis served as an external stent to structurally support the esophagus (trachealization) (
Figure 2B, C). Guided by intraoperative bronchoscopy, re-expansion of the collapsed segments was achieved by gentle traction and tying of the 5-0 polypropylene sutures.
After the operation, re-expansion of the collapsed segment was confirmed on repeat bronchoscopies (Figure. 2D). The respiratory status of the patient became stable and the ventilatory support could be weaned within a week after the surgery. At 12 months of age, he still needed ventilator support however was free from episodes of dying spell and enjoyed off-ventilator time on multiple occasions a day. His neurological milestones had been normal for his age. Enteral nutrition was fully given via gastrostomy whereas he ate various foods appropriate for his age orally with the well-working proximal esophagostomy. We thought it was possible to undergo surgical repair for TOF, and performed ventricular septal defect (VSD) closure and enlargement of the right ventricular outflow tract at 377 days of age. Postoperatively, pulmonary hypertension worsened, and he underwent removal of the VSD patch and pulmonary artery banding at 7th day after surgery. But pulmonary hypertension didn’t improve, and he died at 386 days of age.
4. Discussion
Tracheal agenesis is a rare condition with 118 English reports of 175 cases since its discovery in 1900 [
10]. Most of the patient with this condition died within a few hours after the birth because of respiratory distress and unsuccessful resuscitation, and only ten patients have been reported as survived more than 12 months to date [
4,
5,
7,
10,
11,
12,
13,
14].
Our patient had Floyd’s type III TA. Floyd et al. classified TA based on the type of fistulous connection with the esophagus into three types [
3]. In type I, the stump of the distal trachea attaches to the anterior esophageal wall. Type II involves no trachea, but carina is present with a fistula to the esophagus. In type III, two mainstem bronchi arise individually from the esophagus. The relative incidence of these three types is 19%, 51% and 30% [
10].
From a viewpoint of the management of the near-lethal condition, initial securement of the airway plays a crucial role and prompt esophageal intubation would be life-saving [
2]. Emergent surgery after the temporary securement of the airway is mandatory to divide the esophagus just distal to the origin of the trachea to use the esophagus as a new airway tract. The resection of the stenotic part in the esophagus just proximal to the divergence of the trachea is also required if the stenotic part hampers stable ventilation [
13]. Further esophageal diversion at the cervical esophagus to prevent aspiration pneumonia due to the draining of saliva into the esophageal airway is carried out at the same time or later [
14]. These procedures are performed in acute or semi-acute setting to stabilize the respiratory status of the patient, whereas the management of tracheomalacia-like symptom would come out as a long-term issue afterwards since the esophageal wall lacks cartilages which keep the lumen patent. The symptom would be so severe that the patient may suffer “dying spell” in the worst scenario [
15]. External stenting for tracheomalacia was firstly described in 1997 and then implanted into the treatment of TA in 2008 [
4,
16]. In this procedure, artificial material such as PTFE vascular graft is sutured to the esophageal wall. The technique has been described in a limited number of articles but gradually acknowledged [
4,
5,
6,
7].
Five TA patients underwent external stenting at 2 to 3 months of age in 3 cases and 7 to 8 months of age in 2 case (
Table 1). Two Floyd I patients and one Floyd II patient (case 4) required resection of a tracheoesophageal fistula and reanastomosis with external stenting. In a Floyd II patient (case 3), the stenotic tracheoesophageal fistula was widened using a pericardial patch with external esophageal stenting. In case 1 and case 4, full circle PTFE grafts were placed around the esophagus, whereas a semicircle PTFE graft was placed on the anterior aspect of the esophagus in case 2 and case 5. In case 3, a 3/4 circle PTFE graft was placed around esophagus other than the part covered a pericardial patch. A semicircle graft for anterior wall allows the airway growth, keeps the adequate blood supply to esophagus and enables us to use a smaller prosthesis. This is important for avoiding contact injury and perforation of surrounding organs such as large vessels especially in pediatric patients whose organs are in the vicinity. A semicircle graft around anterior wall may be sufficient for re-expansion of the collapsed esophagus because the posterior wall is supported by vertebrae.
In the current case, the stenting was performed by one of the authors (MA) who has reported his 98 cases of experience of external stenting for tracheomalacia [
9]. Based on his substantial experience, several surgical techniques were introduced for successful stenting. Firstly, an approach for the exteriorization of the esophagus was via median sternotomy. Posterolateral thoracotomy is a familiar approach for pediatric surgeons to handle the thoracic esophagus, however in this case, extensive adhesions from the previous thoracotomy was expected and disturbance of blood supply after the adhesiolysis around the esophagus was concerned. In particular, lacking of blood supply after the adhesiolysis was considered to be detrimental since the esophagus was divided multiple times in the previous surgeries and it was difficult to anticipate normal vascular anatomy supplying the organ. Hence, the anterior aspect of the esophagus where it was intact from the previous surgeries was approached directly. Secondly, the anterior aspect of the esophagus where the external stent would be applied was dissected meticulously from the surrounding vessels like pulmonary artery guided by the intraoperative bronchoscopy. Intraoperative bronchoscopy was indispensable to determine the length of the esophagus which should be stented and directly confirm the patency of the esophagus after the application of the sutures between the esophagus and the stent. The last tip was covering of the edge of the PTFE stent with 0.1mm PTFE Gore-Tex sheet to prevent direct contact injury of the surrounding vessels and the esophagus by the stent.
After external esophageal stenting, it is important to maintain the tip of endotracheal tube within the esophagus stabilized with the graft. Custom-ordered long tracheostomy tube would be one of the solutions if ready-made tube could not fit well into the newly formed trachea. Careful follow-up bronchoscopy is necessary to prevent luminal obstruction due to the ulceration of the esophageal mucosa or the formation of granulation tissue. Management of TA requires a multidisciplinary approach. TA patients have a stormy postoperative course including multiple surgeries and prolonged postoperative course. An experienced anesthetist, a specialized pediatric bronchoscopist, a dedicated surgical team and a trained intensive care unit team are essential in the management of this lethal and precipitous condition.
Regarding the long-term prognosis of TA, mechanical respiratory support and oxygen administration were not necessary in a few long-term survivors [
14], whereas some patients died because of airway troubles such as accidental extubation [
10], airway obstruction due to the esophageal ulcer and granulation at the distal end of the airway tube [
12], and a total collapse of the reconstructed trachea [
6]. In TA patients achieving stabilization of the respiratory status, esophageal/alimentary reconstruction using a gastric tube, a jejunum or the patient’s own esophagus was performed to enable oral feeding. More surprisingly, one of these patients has gained esophageal speech technique despite the congenital absence of trachea [
14]. These managements are necessary to improve the quality of life of long-term survivors with TA.
Tracheal replacement therapy, other than using esophagus, could be proposed as a solution for patients with TA. Autologous tissue transplantation [
17], tissue flaps [
18], tracheal allotransplantation [
19], bioprosthetic reconstruction and tissue-engineered medicine [
20] are potential approaches. However, these are currently inadequate. The absence of a ciliated epithelial lining to provide mucociliary clearance remains a limiting aspect of using autologous tissue which has no ciliated epithelium. Allotransplantation also involves long-term immunosuppression with the concomitant risks. The ideal repair would be replacement by bioengineered tracheal graft, but it has not yielded a reliable solution.
5. Conclusions
Tracheal agenesis seems rare but is important as differential diagnosis for respiratory distress in a case of a newborn with inaudible crying and inability to intubate into the trachea in spite of good visualization of the glottis. External stenting of the esophagus would be effective to relieve tracheomalacia-like symptom in the patient with the esophageal airway. Several surgical tips including surgical approach, intraoperative bronchoscopy, and the covering of the edge of ringed PTEF graft were learned from the experience of external stenting for tracheomalacia and they worked as well in the current case of tracheal agenesis with the floppy esophageal airway. It would be worthwhile considering the employment of these techniques if surgeons encounter this rare and catastrophic condition.
Author Contributions
T.H.: conception and design of study, data acquisition, data interpretation and drafting of the manuscript; R.T.: data interpretation and critical revision; M.A.: data interpretation and critical revision; H.O.: data interpretation and critical revision. All authors approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The datasets used and/or analyzed during this study are available from the author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Payne, W. Congenital absence of the trachea. Brooklyn Med. J. 1900, 14, 568–570. [Google Scholar]
- de Groot-van der Mooren, M.; Haak, M.C.; Lakeman, P.; Cohen-Overbeek, T.E.; van der Voorn, J.P.; Bretschneider, J.H. Tracheal agenesis: approach towards this severe diagnosis. Case report and review of the literature. Eur. J. Pediatr. 2012, 171, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Floyd, J.; Campbell, DC.; Dominy, D. Agenesis of the trachea. Am. Rev. Respir. Dis. 1962, 86, 557–560. [Google Scholar] [PubMed]
- Watanabe, T.; Okuyama, H.; Kubota, A.; Kawahara, H.; Hasegawa, T.; Ueno, T. A case of tracheal agenesis surviving without mechanical ventilation after external esophageal stenting. J. Pediatr. Surg. 2008, 43, 1906–1908. [Google Scholar] [CrossRef] [PubMed]
- Usui, N.; Kamiyama, M.; Tani, G.; Takama, Y.; Soh, H.; Uehara, S. Three-stage reconstruction of the airway and alimentary tract in a case of tracheal agenesis. Ann. Thorac. Surg. 2010, 89, 2019–2022. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Kim, M.S.; Yang, J.H.; Jun, T.G. Tracheal agenesis reconstruction with external esophageal stenting: postoperative results and complications. Korean J. Thorac. Cardiovasc. Surg. 2015, 48, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Densmore, J.C.; Oldham, K.T.; Dominguez, K.M.; Berdan, E.R.; McCormick, M.E.; Beste, D.J. Neonatal esophageal trachealization and esophagocarinoplasty in the treatment of flow-limited Floyd II tracheal agenesis. J. Thorac. Cardiovasc. Surg. 2017, 153, e121–e125. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, Y.; Ando, M.; Hasegawa, H. External Stenting for Tracheobronchomalacia Associated With Congenital Heart Disease. Circulation 2014, 130, A12872. [Google Scholar] [CrossRef]
- Ando, M.; Nagase, Y.; Hasegawa, H.; Takahashi, Y. External stenting: A reliable technique to relieve airway obstruction in small children. J. Thorac. Cardiovasc. Surg. 2017, 153, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Straughan, A.J.; Mulcahy, C.F.; Sandler, A.D.; Bauman, N.M.; Steinhorn, D.; Gitman, L. Tracheal Agenesis: Vertical Division of the Native Esophagus–A Novel Surgical Approach and Review of the Literature. Ann. Otol. Rhinol. Laryngol. 2021, 130, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, E.; Yokoyama, T.; Ichikawa, T.; Matsuura, Y. Surgical management of tracheal agenesis. J. Thorac. Cardiovasc. Surg. 1994, 108, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Soh, H.; Kawahawa, H.; Imura, K.; Yagi, M.; Yoneda, A.; Kubota, A. Tracheal agenesis in a child who survived for 6 years. J. Pediatr. Surg. 1999, 34, 1541–1543. [Google Scholar] [CrossRef] [PubMed]
- Fuchimoto, Y.; Mori, M.; Takasato, F.; Tomita, H.; Yamamoto, Y.; Shimojima, N. A long-term survival case of tracheal agenesis: management for tracheoesophageal fistula and esophageal reconstruction. Pediatr. Surg. Int. 2011, 27, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Tazuke, Y.; Okuyama, H.; Uehara, S.; Ueno, T.; Nara, K.; Yamanaka, H. Long-term outcomes of four patients with tracheal agenesis who underwent airway and esophageal reconstruction. J. Pediatr. Surg. 2015, 50, 2009–2011. [Google Scholar] [CrossRef] [PubMed]
- Varela, P.; Torre, M.; Schweiger, C.; Nakamura, H. Congenital tracheal malformations. Pediatr. Surg. Int. 2018, 34, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Hagl, S.; Jakob, H.; Sebening, C.; van Bodegom, P.; Schmidt, K.; Zilow, E. External stabilization of long-segment tracheobronchomalacia guided by intraoperative bronchoscopy. Ann. Thorac. Surg. 1997, 64, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Fabre, D.; Kolb, F.; Fadel, E.; Mercier, O.; Mussot, S.; Chevalier, T.L.; Dartevelle, P. Successful tracheal replacement in humans using autologous tissues: an 8-year experience. Ann. Thorac. Surg. 2013, 96, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Z. Airway reconstruction with autologous pulmonary tissue flap and an elastic metallic stent. World J. Surg. 2015, 39, 1981–1985. [Google Scholar] [CrossRef]
- Delaere, P.R.; Vranckx, J.J.; Verleden, G.; Leyn, P.D.; Raemdonck, D.V. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N. Engl. J. Med. 2014, 370, 1568–1570. [Google Scholar] [CrossRef]
- Elliott, M.J.; Butler, C.R.; Varanou-Jenkins, A.; Partington, L.; Carvalho, C.; Samuel, E.; Crowley, C.; Lange, P.; Hamilton, N.J.; Hynds, R.E.; et al. Tracheal replacement therapy with a stem cell-seeded graft: lessons from compassionate use application of a GMP-compliant tissue-engineered medicine. Stem Cells Transl. Med. 2017, 6, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).