1. Introduction
Amphipathic membrane-active peptides engage in selective interactions with a diverse range of membranes, utilizing various mechanisms to disrupt lipid bilayers [
1]. These interactions depend on specific peptide attributes and the composition of the membrane. This class of compounds encompasses both cell-penetrating peptides (CPPs) and antimicrobial peptides (AMPs) [
2].
CPPs, typically composed of 5–30 amino acids, have the capacity to transport a wide range of biomolecules into cells, including small drugs, small interfering RNA, proteins, peptide–nucleic acid, imaging agents, and vaccines [
2]. They hold promise as therapeutic agents and function as carriers for delivering therapeutic payloads across cell membranes. Because the most important factor determining the functioning of CPPs is related to their interaction with the cell membrane, the focus in studies of these peptides is made on the delineation of the correlations between their structure/structure-based properties (e.g., amphiphilicity, charge, conformational flexibility) and the membrane activity. Such structure-activity relationships (SAR) are indispensable for the rational design of new CPPs with predefined properties [
3,
4]. These studies underscore the significance of optimizing SAR components for CPPs in enhancing their transfection efficacy. The same strategy also works in the case of membrane-active AMPs.
AMPs represent a class of peptides consisting of 5-50 amino acid residues that target bacterial membranes. AMPs typically have an excess of positively charged and hydrophobic residues, which are frequently separated, thus making the peptides highly amphiphilic. This property is important for the interaction of AMPs with membranes [
5]. Shared characteristics with CPPs. such as amphipathicity or the presence of arginine-rich regions, suggest the potential for dual functionality for some of these molecules [
6].
AMPs offer a promising alternative to conventional antibiotics in the treatment of drug-resistant pathogenic microbes [
7,
8]. They possess unique characteristics, such as distinct sequences, short length, cationic and amphiphilic characters, high affinity, and strong interaction with negatively charged bacterial cell membranes. Furthermore, they exhibit a broad spectrum of antimicrobial activity while preserving the integrity of host cells. The utilization of AMPs provides advantages such as their natural occurrence in combating infections and reduced likelihood of resistance development compared to traditional antibiotics [
9].
The therapeutic applicability of AMPs is often constrained by their interaction with eukaryotic cell membranes, which can be detrimental to host cells. Researchers are thus focused on designing new AMPs with robust antimicrobial action and minimal cytotoxicity toward eukaryotic cells [
10].
AMPs isolated from natural sources face various challenges, including low stability, limited salt tolerance, and high toxicity, impeding their widespread therapeutic application. Alterations in structural and physicochemical factors can impact the antimicrobial activity of these peptides. Therefore, studies of SAR profiles for both naturally occurring and artificially created peptides are very important and pave the way for the development of peptides with increased stability and a broad spectrum of action [
11].
Cyclic AMPs have demonstrated enhanced membrane permeability compared to linear peptides. Specific amino acids play a pivotal role in influencing both the antibacterial action and cell cytotoxicity of AMPs. Peptides with increased net charge and hydrophobicity exhibit a heightened capacity to penetrate microbial membranes [
12]. However, elevated hydrophobicity is often associated with increased mammalian cell toxicity and a loss of antimicrobial specificity [
12].
We have previously shown that amphiphilic cyclic peptide [W
4R
4] (
Figure 1) containing both hydrophobic tryptophan (W) and positively charged arginine (R) residues acts as a promising lead AMP with a minimum inhibitory concentration (MIC) value of c.a. 3 μg/ml against MRSA [
13,
14]. Recently, we reported synthesizing and evaluating cyclic peptides containing R and W residues with antimicrobial properties against multidrug-resistant and nonresistant Gram-positive and Gram-negative bacterial strains [
15]. Furthermore, several amphiphilic membrane-active peptides composed of R and hydrophobic residues, such as W, 4,4′-biphenyl-l-alanine (Bip), 3,3-diphenyl-l-alanine (Dip), or 3-(2-naphthyl)-l-alanine (Nal), were evaluated by us as antimicrobial agents [
16].
The accumulated extensive data on the antimicrobial activity of these cyclic peptides, along with the information on their potential undesirable properties such as hemolytic activity, provides an opportunity to elucidate the molecular mechanisms of their actions and delineate the key SAR parameters. This knowledge forms a strong foundation for further rational design of novel antimicrobials that possess higher values of the selectivity indices (SI). According to our previous experience working with similar peptides [
16,
17], this problem can be efficiently addressed through a comprehensive approach that combines experimental and computational methods.
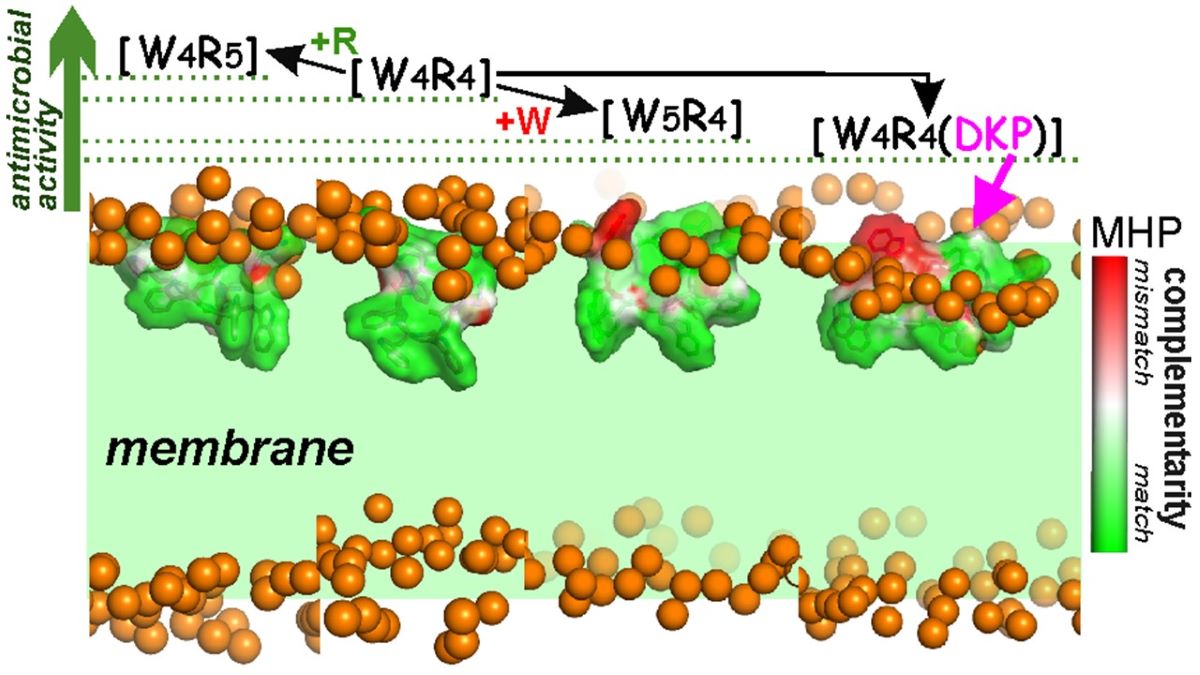
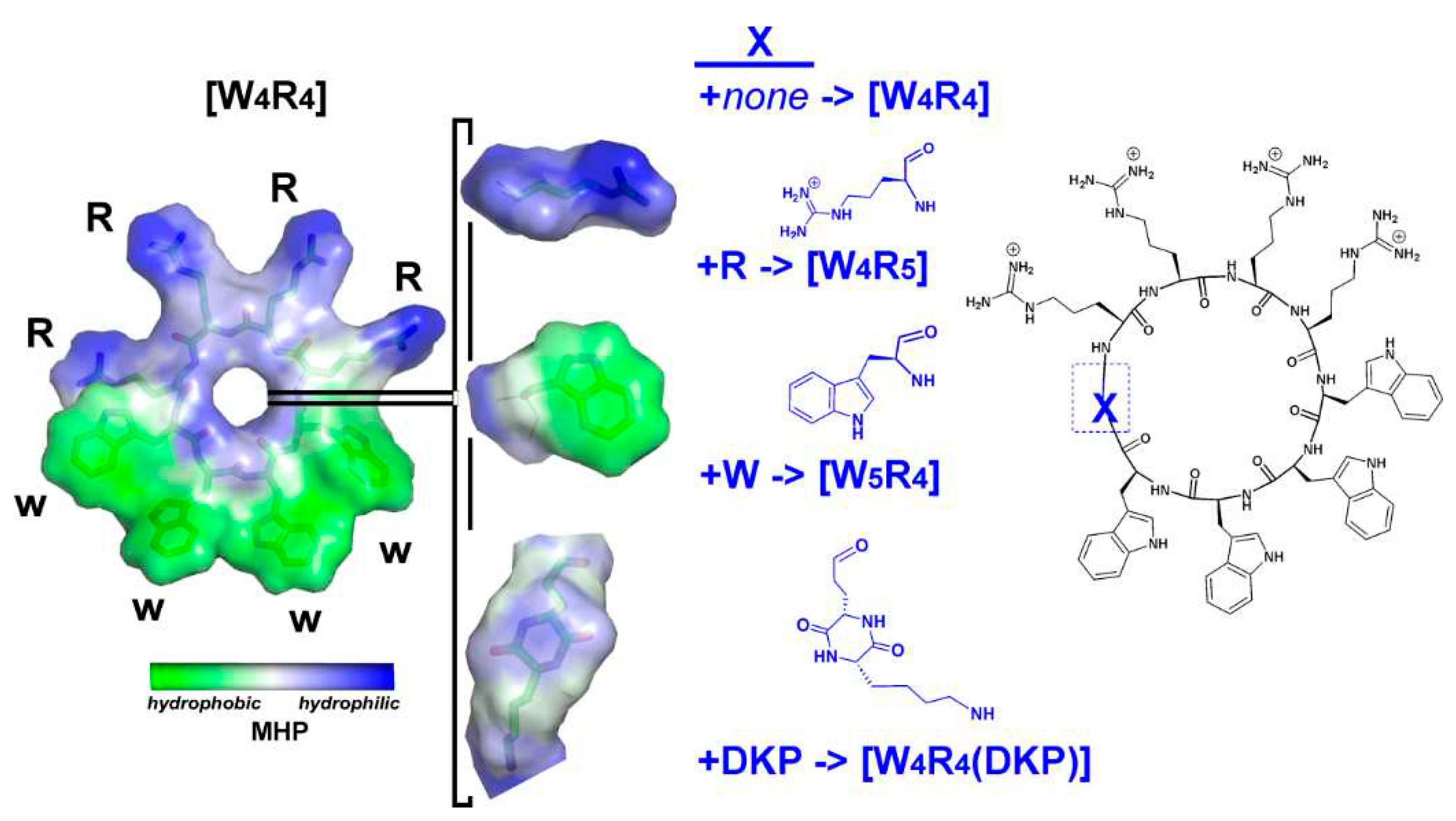
As part of our ongoing efforts to design AMPs, we conducted a study investigating the mechanisms of several cyclic amphiphilic AMPs and their interactions with membranes. The objective was to elucidate the nature of the interaction and understand how the sequence and shape of the peptides influence antimicrobial activity. This was accomplished via introducing in the peptide [W
4R
4] either an additional aromatic hydrophobic residue (W), a positively charged residue (R), or a residue with "intermediate" properties - 2,5-Diketopiperazine (DKP) (
Figure 1). DKP was chosen due to its unique polar characteristics, making it an ideal residue for this modification. DKPs are the simplest naturally occurring cyclic peptides, commonly biosynthesized by a large variety of living organisms, including 90% of Gram-negative bacteria, Gram-positive bacteria, fungi, and higher marine organisms [18-21]. Since the earliest report on DKP in 1924 [
22], they were found to span various biological activities such as antitumor, antiviral, antifungal, and antibacterial, and to interfere with quorum sensing in bacteria [
23,
24]. The DKP scaffold, characterized by a six-membered ring, offers several advantages such as increased rigidity, enhanced resistance against enzymatic degradation, and the ability to form intermolecular H-bonds with target sites. These features make DKPs an attractive class of cyclic peptides with high potential as therapeutic agents [
25]. A comprehensive overview of their biological and structural profile has been recently published [
26].
Thus, the study aims to investigate the structural and dynamic properties of cyclic peptides derived from [W4R4] with minimal modifications, incorporating additional aromatic hydrophobic (W) or polar positively charged (R) residues. This research seeks to understand the SAR of these peptides and their interactions with a model membrane. Specifically, the study introduces an "intermediate" substitution by incorporating DKP into the cyclic peptide [W4R4]. This approach combines peptide synthesis, in vitro antibacterial assays, and molecular modeling to inform the rational design of novel antimicrobial molecules with improved selectivity indices. The research also explores the binding of these peptides to a model membrane through molecular dynamics (MD) simulations and maps their hydrophobic properties to gain further insights into their interactions with the membrane.
3. Discussion
The findings from the antimicrobial activity tests and molecular modeling data yield several conclusions about the relationship between structure and activity for a group of four cyclic peptides that were studied. It is important to note that these peptides have very small structural differences with three of them being modifications of the original peptide [W4R4], each with the addition of one residue W, R, or a residue containing a DKP group that exhibits “intermediate” hydrophobic properties between W and R. By comparing these derivatives to the parent peptide, it was observed that [W4R5] showed increased antimicrobial activity while [W5R4] and [W4R4(DKP)] displayed decreased activity. Through the analysis of their structural-dynamic and hydrophobic properties, as well as the ability to interact with the model bacterial membrane, key SAR were identified.
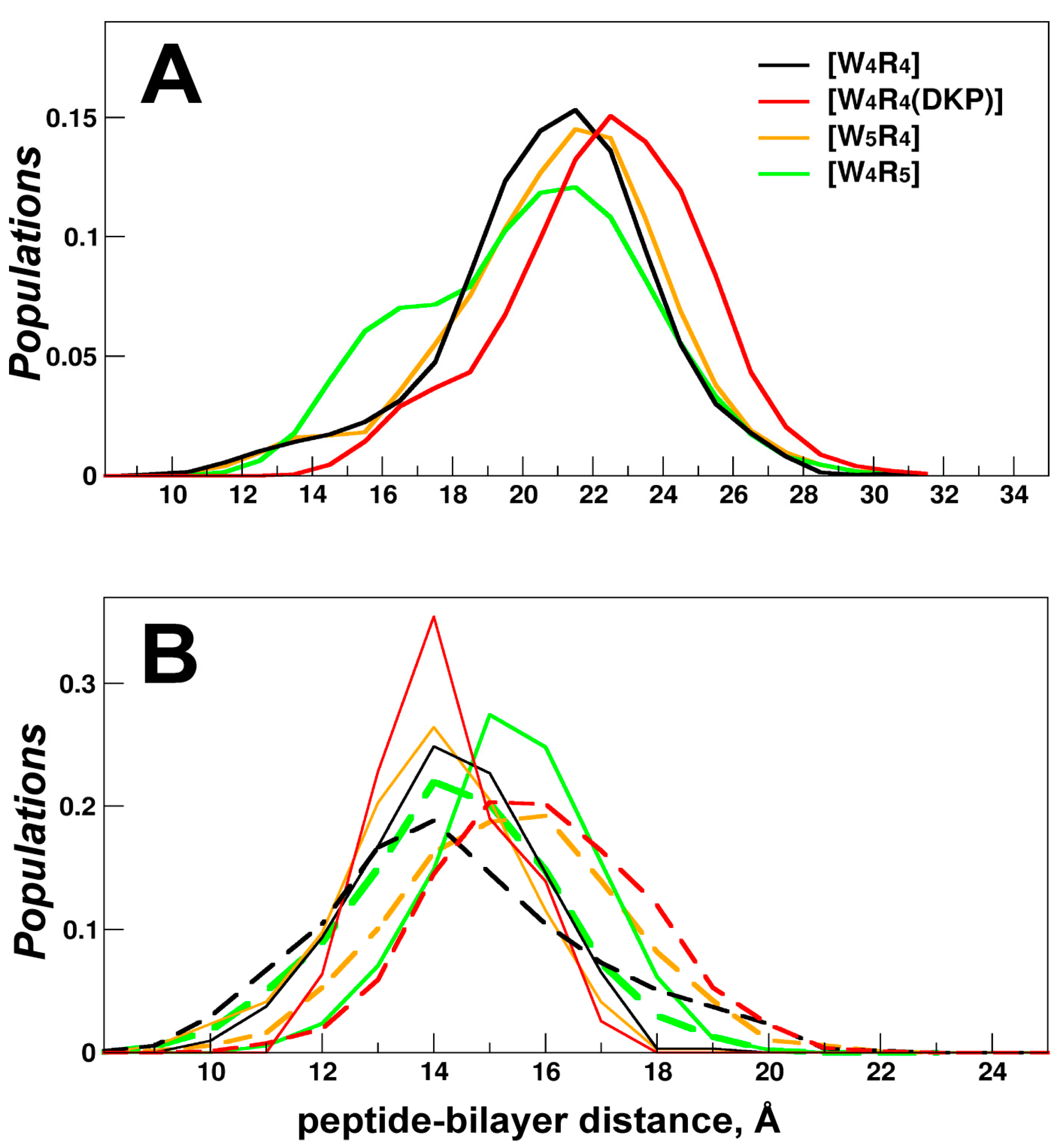
By utilizing all-atom MD simulations, we validate the hypothesis that the enhanced antimicrobial activity of the peptide is linked to its capability to penetrate deeper into the membrane, ultimately reaching the hydrophobic core. Moreover, such a peptide demonstrates a large population of these MD-states, indicating greater thermodynamic stability in this binding mode. The latter proposal is supported by multiple independent MD runs, during which the peptide successfully penetrated the bilayer. The process of protein embedding encompasses numerous intermediate states including those resulting from the hydrophobic mismatch between the protein and the membrane, as well as energetically favorable interactions between charged lipids and arginine residues. The degree of hydrophobic insertion of the peptide becomes less as the energy of the transition states increases.
Among the studied peptides, the most active [W4R5] consistently achieves the successful incorporation of its entire hydrophobic pattern into the membrane across a greater number of independent MD runs than other peptides. Analysis of the membrane-bound states of the less active peptides [W5R4] and [W4R4(DKP)] within the initial nanosecond timeframe (200-400 ns) and in the more equilibrated microsecond range (after 0.5 µs) indicates a more pronounced inclination towards instability in the apolar mode. The instability contributes to a decrease in the depth at which these peptides are inserted into the membrane.
Due to the structural constraints of cyclic peptides, their conformational possibilities for distinguishing polar and nonpolar regions are limited. The larger the hydrophobic pattern, the more the backbone of Trp-motif bends and the higher the probability of simultaneous embedding of charged Arg residues. Consequently, this leads to energetically unfavorable interactions of polar/charged groups (backbone atoms and Arg side chains) embedded into the fatty acyl chain region of the membrane. As a result, the equilibrium distribution of membrane-bound states shifts towards less buried states. The "breathing" behavior of the peptide, where it alternates between sinking and floating up within the equilibrated segments of long-term MD trajectories is observed. As depicted in
Figure 2B and
Figure 4B, the distribution of insertion depths for all peptides remains broad.
Based on the MD data, we assume that an increase in the size of the hydrophobic motif ([W
5R
4] compared to [W
4R
5]), restricts its ability to penetrate deeply into the membrane. This limitation arises from the simultaneous insertion of functional peptide groups with physicochemical properties that are not complementary to those of the water/lipid environment. Indeed, a shift of amphipathy towards an increase in the size of the hydrophobic pattern (addition of W versus R) while maintaining the total number of residues in the cyclic peptide leads to a reduction in antimicrobial activity as accentuated with larger cyclic peptides. The larger the size of the apolar pattern, the more significant this dependence becomes [
15]. A similar trend was observed in peptides containing the same number of R residues but varying numbers of W residues. For instance, comparing [W
7R
3] to [W
4R
3]; [W
7R
4] to [W
5R
4] and [W
4R
4]; [W
7R
5] to [W
4R
5]; and [W
7R
6] to [W
4R
6], peptides with a higher number of W residues exhibited reduced antimicrobial activity. The observed structure-functional patterns also do not exclude the next assumption. Deep penetration of the continuous hydrophobic pattern into the hydrophobic core may not be advantageous or less effective for their antimicrobial function, likely due to stronger hydrophobic interactions and consequent generation of membrane stability.
Furthermore, we found a correlation between the decrease in the plasticity of cyclic structure (attributed to the formation of turns and their stabilization by intramolecular hydrogen bonds) and a decrease in antimicrobial activity ([W
5R
4], [W
4R
4(DKP)] versus [W
4R
5] and [W
4R
4]; see
Table 2). Considering the sharp decline in conformational capabilities of peptides observed during MD simulations when interacting with the membrane, an augmentation in the backbone rigidity further diminishes the ability of the peptide to adapt to the lipid environment.
The introduction of a DKP group, which is a flexible moiety with amphiphilic properties, into a cyclic structure ([W
4R
4(DKP)] peptide) results in the emergence of unproductive modes of membrane binding. The DKP group tends to localize at the interface of polar and nonpolar zones of the bilayer. As a result, the hydrophobic pattern of the peptide can either become "trapped” near the bilayer surface (Supporting Information,
Figure S3) or be inserted less deeply (
Figure 3).
A comprehensive summary of the obtained results can be clearly illustrated through SAR profiles for each peptide, which are strings of characters denoting specific features (designated as S1-S8 in
Table 3). The numerical value of each of them can be either 1 (represented by the symbol "●" in
Table 3) or 0 (represented by the symbol "○"). These features are determined based on the peptide’s “Activity” (S1-S5) and its effectiveness in embedding into the non-polar region of the bilayer (described in the “Modeling” section of
Table 3, S6–S8).
For the peptide where these parameters reach their extreme values: highest antimicrobial (S1-S4), lowest hemolytic (S5) activities as well as the maximum values of MD-elucidated features (S6-S8), the respective signs are set to 1. The MD-related features were evaluated by comparing the four studied peptides, while activity parameters were determined using four additional analogs with an increased content of W and R residues (
Table 3).
It is evident that the peptide [W4R5] possesses the optimal pattern in terms of "activity/binding to the bacterial membrane” and exhibits the best characteristics in both sections of the profile – the "experimental" (S1-S5) and the "calculated" ones (S6-S8). Among others, the peptide [W4R5] demonstrated most favorable HC50 value of 340 µg/mL and displayed low MIC values of 4 µg/mL, 32 µg/mL, 32 µg/mL, and 16 µg/mL against MRSA (ATCC BAA-1556), K. pneumoniae (ATCC BAA-1705), P. aeruginosa (ATCC 27883), and E. coli (ATCC 25922), respectively. A very similar SAR profile (most of the features equal to “1”) was found for [W4R4] that shows almost identical antimicrobial activity but has a lower SI value (43.8 instead of 85 for [W4R5]) due to being more hemolytically active.
Comparing [W
4R
4] with peptides [W
5R
4], [W
6R
4], and [W
7R
4] which have the same number of R residues and an increased number of W residues, differences in both SAR profile and activity of these peptides become pronounced [
15]. As the number of W residues increased (from 4 to 7), a significant loss of antimicrobial activity of these peptides was observed showing MIC values of 4 µg/mL, 8 µg/mL, 128 µg/mL, and 256 µg/mL against MRSA (ATCC BAA-1556), respectively. Also, a noticeable decrease in the HC
50 values was also demonstrated (from 175 to 60 µg/mL) in this set of peptides.
Considering the previous information regarding the impact of adding residues W and R to the parent peptide [W
4R
4], it is reasonable to assume that the introduction of another R into the latter will enhance the interaction between positively charged guanidine groups of arginine residues and negatively charged groups of polar lipid heads and with water molecules, making it unprofitable to embed the peptide deeply into the membrane. The predominance of arginine residues over tryptophan residues in a cyclic peptide impairs the activity profile, although not so clearly. Thus, [W
4R
6] and [W
4R
7] were generally less active than [W
4R
5] and [W
4R
4] (SI values of 33 and 12 versus 85 and 43.8) [
15].
Based on the correlation between MD results and the activity of the four "basic" peptides, it is possible to extrapolate the SAR profiles to 10- / 11-residue peptides listed in
Table 3, which only have activity indicators available. In summary, these 10/11-residue analogs do not exhibit both high antimicrobial activity and low hemolytic activity compared to the most promising peptide [W
4R
5].
The SAR model showed a strong correlation with our previous experimental results [
15]. Comparatively [W
4R
6] and [W
4R
7], which had a similar number of W residues but a higher number of R residues, exhibited lower HC
50 values, when compared with [W
4R
5]. [W
4R
6] was generally less active than [W
4R
5] against three bacterial strains, MRSA (ATCC BAA-1556), K. pneumoniae (ATCC BAA-1705), P. aeruginosa (ATCC 27883), and E. coli (ATCC 25922). [W
4R
7] was also less active than [W
4R
6] against these bacteria. When comparing [W
4R
4] with peptides [W
5R
4], [W
6R
4], and [W
7R
4], which had similar numbers of R residues and different numbers of W residues, the differences became more pronounced. Thus, the addition of W residues led to a noticeable increase in hemolytic activity and a significant decrease in antimicrobial one. In general, the order of antimicrobial activity was observed as follows: [W
4R
4] > [W
5R
4] > [W
6R
4] > [W
7R
4], which is reflected in the SAR profiles (
Table 3).
4. Materials and Methods
4.1. Materials
Fmoc-L-Trp(Boc)-2-chlorotrityl resin (loading 0.37 mmol/g) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Protected Amino acids were purchased from AAPPTec LLC (Louisville, KY, USA). TFA, phenylsilane, acetic acid, DMF, HBTU, tetrakis(triphenylphosphine)palladium (0), thioanisole, EDT, DIPEA, hydrazine hydrate, piperidine, triisoproylsilane (TIS), HOAt, DIC, and all other reagents were bought from MilliporeSigma (St. Louis, MO, USA). All bacterial experiments were carried out under a laminar flow hood Labconco (Kansas City, MO, USA). All bacterial strains except MRSA (Los Angeles County (LAC) clone) employed in this study were procured from the American Type Culture Collection (ATCC).
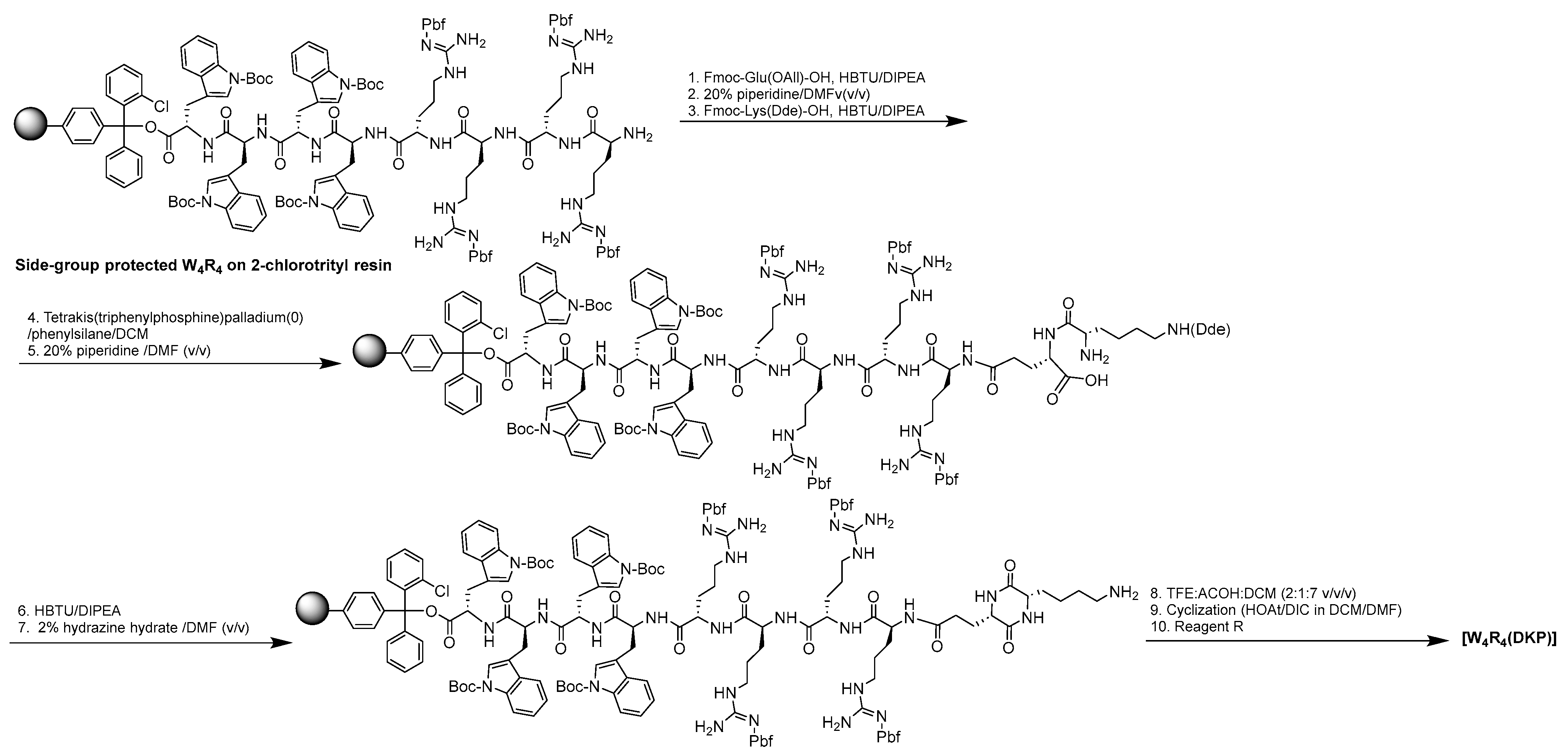
4.2. Chemical synthesis of [W4R4(DKP)]
We have previously reported the synthesis of [W
4R
4], [W
5R
4], [W
4R
5] [
15]. The sequence W
4R
4 was assembled on the resin by Fmoc/tBu solid-phase peptide synthesis using tryptophan preloaded trityl resin (Fmoc-L-Trp(Boc)-2-chlorotrityl resin, 0.37 mmol/g, 0.3 mmol, 810 mg) as previously reported [
13]. A solution of Fmoc-Glu(OAll)-OH (369 mg), HBTU (341 mg), and DIPEA (315 µL) in DMF (20.0 mL) was added to the resin. The reaction was continued under nitrogen for 2 h, followed by draining of the solution and washing with DMF (2 × 10.0 mL). A solution of 20% piperidine in DMF (20 mL, v/v) was added to remove N-terminal Fmoc. The reaction was continued for 20 min. followed by washing with DMF (2 × 10.0 mL). The deprotection with 20% piperidine in DMF (v/v) and washing with DMF (2 × 10.0 mL) was repeated. A solution of Fmoc-Lys(Dde)-OH (480 mg), HBTU (341 mg), and DIPEA (315 µL) in DMF (20.0 mL) was added to the resin. The reaction was continued under nitrogen for 2 h, followed by draining of the solution and washing with DMF (2 × 10.0 mL). A solution of 20% piperidine in DMF (20.0 mL, v/v) was added, and the reaction was continued for 20 min followed by washing with DMF (2 ×10.0 mL). Then another addition of 20% piperidine in DMF (v/v) was repeated, followed by washing with DMF (2 × 10.0 mL) to deprotect the Fmoc of lysine. The resin was washed with anhydrous DCM (3 × 10 mL). Tetrakis(triphenylphosphine)palladium catalyst (0.1 equiv., 34.7 mg) was dissolved in 10 ml DCM, and then 20 equiv. of phenylsilane (740 µL) was added. The whole mixture was added to the resin. Agitation was done for 30 min under nitrogen. The same previous steps were repeated again followed by washing with DCM (3 × 10 mL) to remove the allyl group of the glutamic acid. A solution of HBTU (341 mg), DIPEA (315 µL) in DMF (20.0 mL) was added to the resin to accomplish the cyclization. The reaction was continued under nitrogen for 2 h. It was followed by draining the solution and washing with DMF (5 × 10.0 mL). A solution 2% hydrazine was prepared by dissolving 3.0 mL of 98% solution of hydrazine monohydrate in 103.4 mL of DMF. The solution (10 mL) was added to the resin and agitated for 15 min, followed by washing with DMF (3 × 10 mL). The same previous steps were repeated once to remove the Dde of lysine. The side chain-protected peptide was cleaved from the resin by using a solution mixture of trifluoroethanol (TFE)/acetic acid/dichloromethane (DCM) (20 mL, 2:1:7 v/v/v) for 2 h to cleave the resin. The resin was filtered off, and the liquid was evaporated to dryness to get side-chain-protected linear peptide. The side chain-protected peptide was taken in 500 mL round bottom flask under nitrogen. DMF (200 ml) and DCM (50 ml) were added to the protected peptide. A solution of HOAt (136 mg) in 4 mL DMF and 208 µL of DIC were added to cyclize the peptide. The reaction was stirred for 18 h under nitrogen. The solvents were evaporated under a vacuum. Removal of functional protecting groups was performed by stirring the peptide in a 15 mL solution of TFA/thioanisole/EDT/anisole (90:5:3:2 v/v/v/v) for 2 h. After filtration of the resin, cold diethyl ether (15.0 mL) was added to the filtrate to precipitate peptide that was purified by RP-HPLC (Shimadzu LC-20AP), using a gradient system from 0 to 100% acetonitrile (CH
3CN) and water containing 0.1% (v/v) TFA using a preparative C18 column (Gemini, 5 μm particle size, 100 Å pore size, 21.2 mm × 250 mm) from Phenomenex. The peptide had an HPLC purity of >95%.
MALDI-TOF(m/z): C79H105N27O11: calculated, 1608.9168; found, 1609.3731 [M + H]+.
4.3. Antibacterial assay
The selected strains of bacteria were cultured according to the guidelines of the Clinical Laboratory Standards Institute (CLSI) to determine MIC values using a microbroth dilution assay. The MIC values were determined to be at concentrations in wells in which no visible bacterial growth was present. An aliquot of an overnight culture of bacteria was grown according to the guidelines of CLSI, diluted in 1 mL normal saline to achieve 0.5 McFarland turbidity (1.5 × 108 bacterial cell CFU/mL). 60 µL of the 0.5 McFarland solution was added to 8,940 µL of MH media (a 1/150 dilution). The tested peptides (256 µg/ml) were prepared from a stock solution of the samples for testing in Mueller Hinton Broth MH media. An amount of 100 µL MH media was pipetted into the sterile 96-well plate except for the first well. An amount of 200 µL of 256 µg/mL compound samples was added by pipette into the first well and serially diluted with the MH media along sterile 96 wells using a multi-channel pipette except the last well. An amount of 100 µL aliquot of bacteria solution was added to each well, and the plate was incubated at 37 ⁰C for 24 h. All experiments were conducted in triplicate.
4.4. Molecular Dynamics Simulations
To study the peptide-membrane interactions, a series of MD simulations of the peptides [W4R4], [W5R4], [W4R5], and [W4R4(DKP)] was conducted in both water and in a hydrated pre-equilibrated negatively charged lipid bilayers. The latter were composed of DOPC and DOPG lipids and their ratio (7:3) was chosen to match our previous experiments (16) and accurately represent the lipid composition of bacterial plasma membrane. Specifically, the two-component bilayer consisted of 96 DOPC and 32 DOPG molecules.
Preparation of Starting Configurations. The peptides’ initial spatial arrangements were constructed using the molecular builder Avogadro version 1.2.0 [
27] (
http://avogadro.cc/). To probe the conformational mobility in water, the peptides were subjected to simulation in an aqueous solution at 310 K. For all peptides, three independent simulations were performed, thus giving a total MD time of 600 ns in each case. The preparation and production stages of the MD process were consistent for all peptides and were described previously [
16].
Two representatives of the most populated clusters (root-mean-square deviation (RMSD)-based clustering algorithm) that dominated in water simulations were selected as initial configurations for conducting MD simulations of each peptide in the water-bilayer environment. To clarify how the initial protein conformation affects the resulting mode of peptide-membrane binding, we performed a series of 200 ns MD calculations with three starting models for each peptide. Two models were taken from MD data in water and the third one was the original model (close to the structure of “ideal ring”) whose energy was only minimized in water.
A minimum of 12 (16 for [W
4R
5]) independent MD runs (4 for each starting model) were carried out in the DOPC/DOPG bilayer. In all MD starts, the peptides were initially fully exposed to water and positioned adjacent to the membrane surface. A complete description of MD trajectories (number, length, starting structures, etc.) can be seen in
Table S1. For the peptides with a membrane-embedded hydrophobic pattern (Trp-motif), the corresponding MD runs (see
Table S1 for details) were extended to 400 ns. Additionally, for each peptide, at least two MD trajectories were further prolonged to 1 μs for 8-mer peptides or 2 μs for 9-mer peptides. This was done to assess the stability of the peptide-membrane binding mode when the Trp-motif was embedded into the membrane. Overall, the total simulation times were 7.6 µs, 6.8 µs, 4.2 µs, and 4.4 µs for peptides [W
4R
5], [W
5R
4], [W
4R
4], and [W
4R
4(DKP)], respectively.
MD Protocols. MD simulations were performed using the GROMACS
[28] package version 2020.4 and the all-atom CHARMM36 [
29] force field. Molecular topology for the peptide [W
4R
4(DKP)] was generated using the set of CHARMM36 force field parameters (bonded and nonbonded) for proteins [
30]. In all calculations, the tip3p [
31] water model and 3D periodic boundary conditions were employed. After peptide insertion, the peptide-bilayer systems were resolvated (ca. 5.500 water molecules) within boxes ca. 66 × 66 × 77 Å
3 in size. To keep the system electrically neutral, Na
+ counterions were added. A spherical cutoff function (12 Å) and particle mesh Ewald (PME) algorithm [
32] (with a 12 Å cutoff) were used to treat van der Waals and electrostatic interactions, respectively. The preparation and production MD stages were the same for all peptides and described elsewhere [
16]. Finally, MD production runs (at least 200 ns each) were conducted in an NPT ensemble at a semi-isotropic pressure and a constant temperature of 310 K with an integration time step of 2 fs.
Data Analysis. MD trajectories were sampled for analysis at time intervals of 100-1000 ps. MD data were analyzed and averaged over the accumulated sets of MD trajectories. The conformational mobility of the peptides was evaluated using standard GROMACS utilities (
gmx rmsd, gmx cluster). Lipid (phosphorus atoms) and peptide density profiles were obtained using
gmx density utility. Intermolecular interactions (electrostatic, hydrophobic contacts, and hydrogen bonds), secondary structure, as well as the depth of peptide insertion into the membrane were evaluated using GROMACS tools (
gmx distance, gmx hbond) and original in-house software utilities. Geometric criteria used to detect electrostatic and hydrophobic intermolecular interactions are described elsewhere [
33]. The total and non-polar accessible surface areas of the peptides were estimated by
naccess v. 2.1.1 software [
34].
The distribution of hydrophobic/hydrophilic properties on the molecular surfaces of the peptides was calculated using the molecular hydrophobicity potential (MHP) approach [
35]. For the analysis, MHP values were calculated in log
P units, where
P is the octanol−water distribution coefficient.
Mapping the hydrophobic properties of the peptides’ surfaces (
Figure S1) was done employing the Protein Surface Topography technique [
36] and its complementarities to the water-membrane environment were estimated with the freely available Platinum software [
33]. Molecular graphics were rendered using PyMOL v. 2.5 (
http://pymol.org).
Figure 1.
Chemical structures and hydrophobic/hydrophilic surface properties of [W4R4], [W4R5], [W5R4], and [W4R4(DKP)]. The insertion site of positively charged arginine, hydrophobic tryptophan and weakly polar “DKP moiety” group is indicated by a blue rectangle in the chemical structure of the parent [W4R4] which is highly amphiphilic. The amphiphilic “DKP moiety” group includes the polar DKP ring and an apolar hydrocarbon chain. Surface properties of the molecules are color-coded according to the values of their molecular hydrophobicity potential (MHP, see Methods section for details).
Figure 1.
Chemical structures and hydrophobic/hydrophilic surface properties of [W4R4], [W4R5], [W5R4], and [W4R4(DKP)]. The insertion site of positively charged arginine, hydrophobic tryptophan and weakly polar “DKP moiety” group is indicated by a blue rectangle in the chemical structure of the parent [W4R4] which is highly amphiphilic. The amphiphilic “DKP moiety” group includes the polar DKP ring and an apolar hydrocarbon chain. Surface properties of the molecules are color-coded according to the values of their molecular hydrophobicity potential (MHP, see Methods section for details).
Scheme 1.
Synthesis of [W4R4(DKP)].
Scheme 1.
Synthesis of [W4R4(DKP)].
Figure 2.
Depth of membrane insertion for cyclic peptides [W4R4], [W5R4], [W4R5] and [W4R4(DKP)]). The peptide indication color scheme (see the legend on panel A) is the same for both panels. (A) Distributions of the distances between the peptide center of mass and the bilayer center (“depth distribution”) were calculated over all MD states in 200-400-ns runs. (B) Comparison of depth distributions for MD states of peptides in A-mode (observed in the initial MD time period (up to 400 ns)) and all MD states of µs-length MD runs (collected after the first 0.5 µs, dotted lines).
Figure 2.
Depth of membrane insertion for cyclic peptides [W4R4], [W5R4], [W4R5] and [W4R4(DKP)]). The peptide indication color scheme (see the legend on panel A) is the same for both panels. (A) Distributions of the distances between the peptide center of mass and the bilayer center (“depth distribution”) were calculated over all MD states in 200-400-ns runs. (B) Comparison of depth distributions for MD states of peptides in A-mode (observed in the initial MD time period (up to 400 ns)) and all MD states of µs-length MD runs (collected after the first 0.5 µs, dotted lines).
Figure 3.
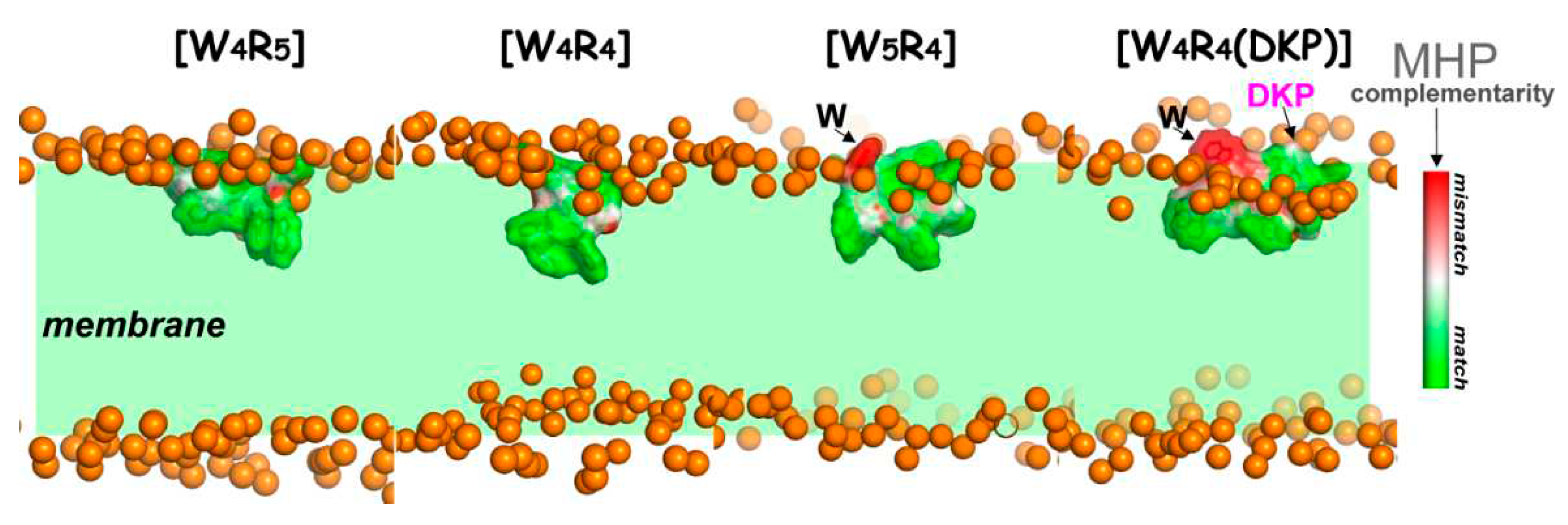
Complementarity of hydrophobic/hydrophilic surface properties for the membrane-bound states of the peptides [W4R5], [W4R4], [W5R4] and [W4R4(DKP)]. The molecular hydrophobicity potential (MHP) values induced by both peptide and water-lipid medium are calculated in the surface points of the peptide molecule. Color-coded scale for MHP match/mismatch values (peptide-induced to environment-induced) is given on the right. Phosphate groups of lipids are given as golden spheres. Water and lipid molecules are omitted for clarity, and the membrane region is indicated with a green background.
Figure 3.
Complementarity of hydrophobic/hydrophilic surface properties for the membrane-bound states of the peptides [W4R5], [W4R4], [W5R4] and [W4R4(DKP)]. The molecular hydrophobicity potential (MHP) values induced by both peptide and water-lipid medium are calculated in the surface points of the peptide molecule. Color-coded scale for MHP match/mismatch values (peptide-induced to environment-induced) is given on the right. Phosphate groups of lipids are given as golden spheres. Water and lipid molecules are omitted for clarity, and the membrane region is indicated with a green background.
Figure 4.
Membrane binding of four cyclic peptides via tryptophan motif and/or DKP moiety. (A) Apolar and intermediate membrane binding modes (in % MD time) realized in each 200-ns MD run (total 12-16 for each peptide). The intensity of bin shading corresponds to a few of the incorporated tryptophan residues: at least one (light color), two (medium intensity), or all tryptophan motifs (dark, A-mode). Additional violet bin shows a fraction of MD states with tightly membrane-bound DKP moiety of the [W4R4(DKP)] peptide. (B) Insertion depth of the peptides in A-mode as well as [W4R4(DKP)] peptide in locked-state. The density distribution of phosphorous (P) atoms of lipids and Trp residues along the bilayer normal (Z) for the DOPC/DOPG membrane is shown. For the locked states of [W4R4(DKP)], localization of the W-motif and DKP atoms is indicated by cyan and violet dashed lines, respectively. MD-averaged (over the last 500 ns) atomic density distributions for the DKP moiety and the entire peptides in A-mode are shown by a violet continuous line and dotted lines, respectively.
Figure 4.
Membrane binding of four cyclic peptides via tryptophan motif and/or DKP moiety. (A) Apolar and intermediate membrane binding modes (in % MD time) realized in each 200-ns MD run (total 12-16 for each peptide). The intensity of bin shading corresponds to a few of the incorporated tryptophan residues: at least one (light color), two (medium intensity), or all tryptophan motifs (dark, A-mode). Additional violet bin shows a fraction of MD states with tightly membrane-bound DKP moiety of the [W4R4(DKP)] peptide. (B) Insertion depth of the peptides in A-mode as well as [W4R4(DKP)] peptide in locked-state. The density distribution of phosphorous (P) atoms of lipids and Trp residues along the bilayer normal (Z) for the DOPC/DOPG membrane is shown. For the locked states of [W4R4(DKP)], localization of the W-motif and DKP atoms is indicated by cyan and violet dashed lines, respectively. MD-averaged (over the last 500 ns) atomic density distributions for the DKP moiety and the entire peptides in A-mode are shown by a violet continuous line and dotted lines, respectively.
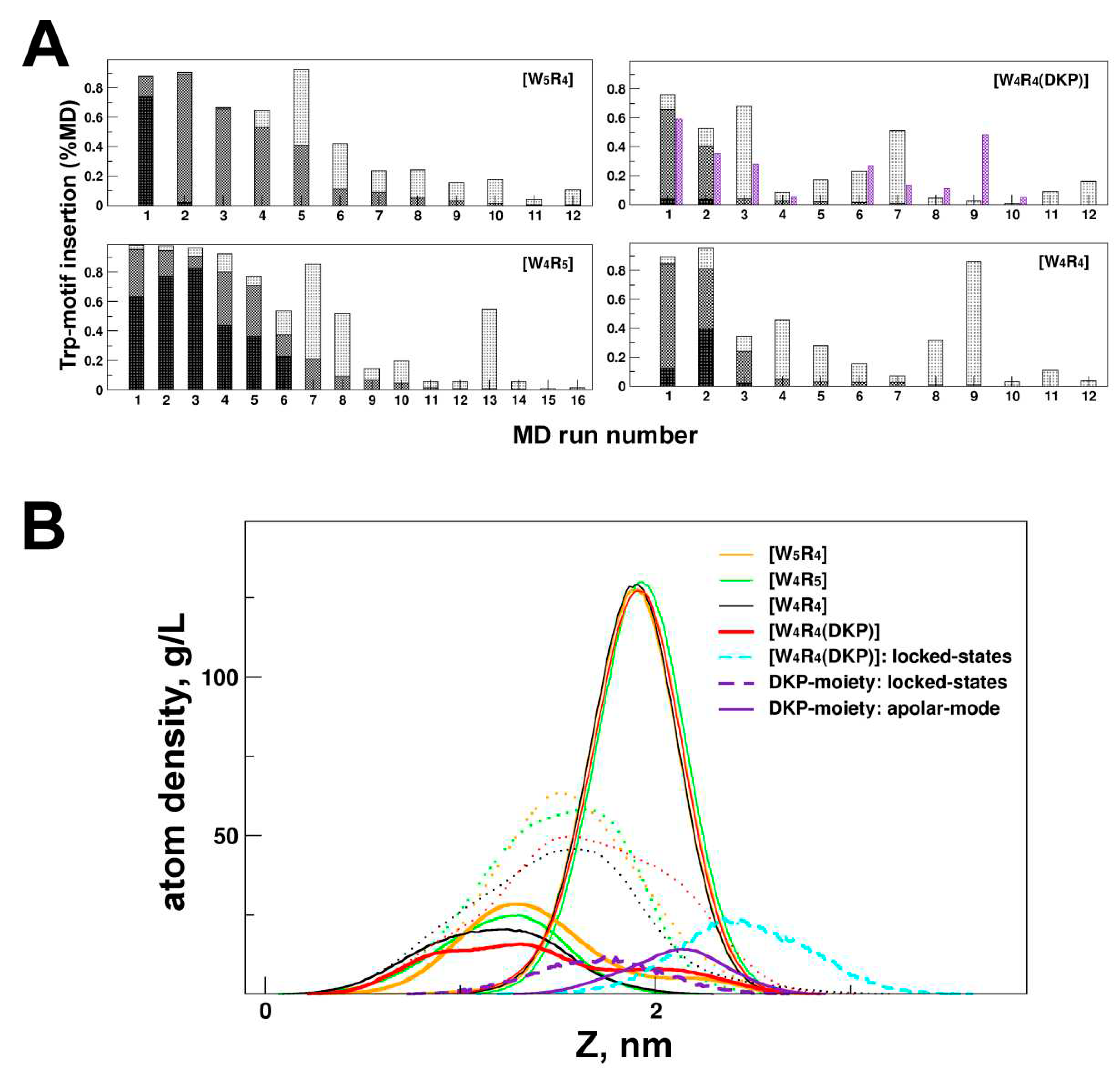
Table 1.
Antibacterial activity of [W4R4], [W4R5], [W5R4], and [W4R4(DKP)] against bacterial pathogens.
Table 1.
Antibacterial activity of [W4R4], [W4R5], [W5R4], and [W4R4(DKP)] against bacterial pathogens.
| |
|
MIC (µg/mL)a |
| |
[W4R4] |
[W4R5] |
[W5R4] |
[W4R4(DKP)] |
Meropenem |
Daptomycin |
MRSA (ATCC BAA-1556)
(LAC clone)
|
4 |
4 |
8 |
32 |
2 |
ND |
Klebsiella pneumoniae
(ATCC BAA 1705)
|
32 |
32 |
128 |
64 |
16 |
ND |
Pseudomonas aeruginosa
(ATCC 27883)
|
64 |
32 |
128 |
64 |
1 |
ND |
E. coli
(ATCC 25922)
|
16 |
16 |
64 |
32 |
1 |
ND |
S. aureus
(ATCC 29213)
|
8 |
4 |
NDb
|
32 |
ND |
1 |
E. faecium
(ATCC 700221)
|
4 |
4 |
ND |
16 |
ND |
2 |
E. faecalis
(ATCC 29212)
|
16 |
8 |
ND |
64 |
ND |
16 |
S. pneumoniae
(ATCC 51938)
|
4 |
2 |
ND |
4 |
ND |
8 |
Bacillus subitilis
(ATCC 6633)
|
1 |
4 |
ND |
64 |
ND |
0.5 |
Table 2.
Main parameters of membrane-bound states (A-mode) and conformational mobility in water for a set of cyclic peptides with different antimicrobial activities.
Table 2.
Main parameters of membrane-bound states (A-mode) and conformational mobility in water for a set of cyclic peptides with different antimicrobial activities.
| A-modea: |
[W4R5] |
[W4R4] |
[W5R4] |
[W4R4(DKP)] |
| number of 200 ns-MD runs |
6 from 16 |
3 from 12 |
2 from 12 |
2 from 12 |
| %MD states in 200 ns / 400 ns-MD runs |
20.4 / 30.0 |
6.7 / 11.0 |
6.3 / 10.8 |
0.8 / 2.8 |
| Binding Depth in µs-MD (Å)b
|
15.2±1.8 /
12.9±1.9 |
14.9±2.4 /
11.9±2.2 |
15.8±2.0 /
14.2±2.0 |
16.4±1.8 /
15.2±2.1 |
| Stability of deep membrane binding in µs-MDc
|
+ |
+ |
- |
- |
| Conformational behavior in water: |
| RMSD, Åd
|
1.7±0.5 |
0.9±0.3 |
1.6±0.7 |
2.1±0.8 |
| Backbone plasticity: number of h-bondse |
0.7±0.8 |
0.7±0.7 |
1.3±1.0 |
1.3±0.8 |
Table 3.
Structure-activity relationships for W/R-consisting cyclic peptides with varied numbers of W/R residues.
Table 3.
Structure-activity relationships for W/R-consisting cyclic peptides with varied numbers of W/R residues.
| |
Peptide |
SAR-profile |
| |
|
Activitya
|
Modelingb |
| S1 |
S2 |
S3 |
S4 |
S5 |
S6 |
S7 |
S8 |
| |
|
MRSA |
K.pneumoniae
|
P.aeruginosa
|
E. coli |
HC50
|
Backbone
plasticity
|
Binding Depth |
Inserted W-motif |
+R
↑ |
[W4R7] |
○c
|
○ |
○ |
○ |
○ |
|
|
|
| [W4R6] |
○ |
○ |
● |
○ |
○ |
|
|
|
| [W4R5] |
● |
● |
○ |
● |
● |
● |
● |
● |
| |
[W4R4] |
● |
● |
○ |
● |
○ |
● |
● |
○ |
↓
+W |
[W5R4] |
○ |
○ |
○ |
○ |
○ |
○ |
○ |
○ |
| [W6R4] |
○ |
○ |
○ |
○ |
○ |
|
|
|
| [W7R4] |
○ |
○ |
○ |
○ |
○ |
|
|
|
| |
[W4R4(DKP)] |
○ |
○ |
○ |
○ |
|
○ |
○ |
○ |