1. Introduction
Lipolytic enzymes (esterases and lipases) have received notable attention owing to their vast distribution in biological systems and environments and their significance in a wide range of industrial and biotechnological applications [
1,
2]. Esterases (E.C 3.1.1.x where x depends on the substrate) are hydrolases that catalyze the hydrolysis and formation of ester bonds. They have a wide range of industrial and biotechnological applications. Efforts are aimed at gaining a better understanding of the biochemical properties of esterases that could help in devising strategies for regulating and improving their catalytic efficiency and stability.
Esterases and lipases belong to the α/β hydrolase superfamily, which exhibit common parallel β strands surrounded by α helical connections. Their active sites share characteristic sequence motif GXSXG pentapeptides and a catalytic triad consisting of serine-histidine-aspartic or glutamic acid [
3]. Most lipases and esterases are relatively small and they exhibit broad substrate specificities without cofactor requirement. Their unique properties such as catalytic versatility, robustness, and high specificity have attracted enormous attention as industrial biocatalysts for a wide range of applications [
4,
5]. Therefore, the growing interest and demand as well as the wide spectrum of applications have spurred extensive research to gain more insight into the properties of the enzyme which could be exploited for other specific applications of esterase for optimized industrial and biotechnological applications.
Thermoanaerobacter tengcongensis esterase (LipA3, EstA3) is a thermophilic hydrolytic enzyme from a thermophilic eubacterium
T. tengcongensis isolated from a hot spring in Tengchong, Yunnan Province, China with high potential for industrial and biotechnological applications [
6].
T. tengcongensis is an anaerobic, Gram-negative, rod-shaped bacterium, and can survive in temperatures ranging from 50 to 80 °C [
7].
T. tengcongensis, like other thermophilic microorganisms usually survive in extreme environmental conditions like hot temperatures and can produce thermophilic enzymes with adaptation difference, structural difference, and higher hydrophobicity [
8]. In an attempt to search for novel thermostable esterase for optimized industrial applications, a novel esterase gene, ORFs lipA (NP_622227) from a thermophilic eubacterium species,
T. tengcongensis genome was reported by Zhang et al. [
9].
T. tengcongensis esterase gene was heterologously expressed in
E. coli and its biochemical properties characterized leading to its classification as the first member of the XIV family of lipolytic enzymes [
6]. The biochemical characterization data also suggests that
T. tengcongensis esterase is a promising candidate for industrial and biotechnological applications because of its inherent thermal stability.
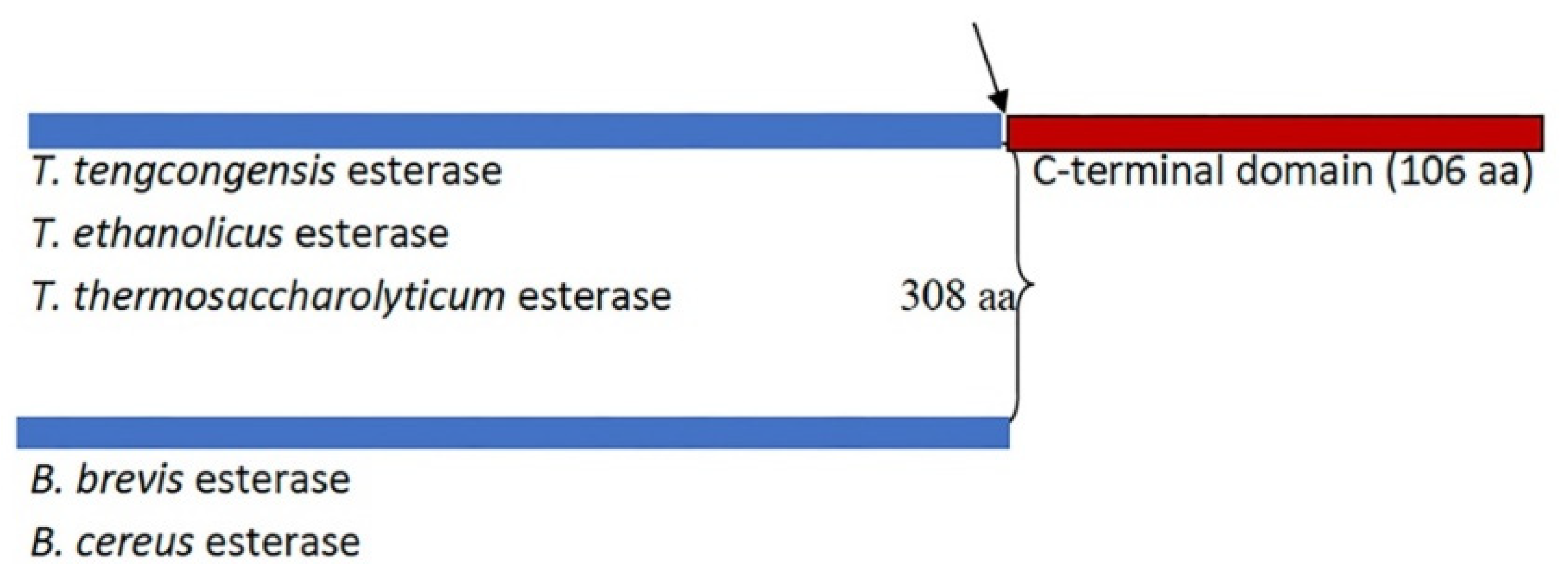
A remarkable variation in the primary sequence length among members of the family of XIV of lipolytic enzymes (esterases) was reported (
Figure 1) [
6]. The sequence alignment of
T. tengcongensis esterase with homologous esterases had earlier revealed an apparent tail of 106 non-conserved residues at the C-terminus with an unknown function [
6]. The identified apparent tail at the C-terminal of
T. tengcongensis esterase from sequence alignment of
T. tengcongensis esterase with other four homologous esterases of the same family revealed that this region is not conserved across all family members. Multiple sequence analysis also revealed that the identified apparent tail at the C-terminal region of
T. tengcongensis esterase is not conserved across all family members (
Figure 1). Due to its lack of conservation across the family, it is unlikely that it plays an essential structural or catalytic role. An understanding of the role of the non-conserved C-terminal tail could provide some insight into how the properties of the enzyme could be engineered for improved activity. Specifically, we investigated if the C-terminal tail has a role in determining the substrate preference of the enzyme. To achieve this, the wild-type
T. tengcongensis esterase (EstA3_
Tt) and the truncated version of the
T. tengcongensis esterase, without the apparent tail (EstA3_
TtΔ), as well as a naturally shorter version from
B. brevis (EstA3_
Bb) were synthesised and their biochemical properties were compared.
2. Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
2.1. Alphafold-Predicted Structure of T. tengcongensis EstA3
Although
Thermoanaerobacter tengcongensis esterase (EstA3_
Tt) has been characterized and reported in the literature [
6], there are no experimentally determined crystal structure in the database. Sequence alignment published by Rao
et al. [
6], suggests that the enzyme belongs to the α/β hydrolase superfamily of lipases (
Figure 1). All figures and tables should be cited in the main text as
Figure 1,
Table 1, etc.
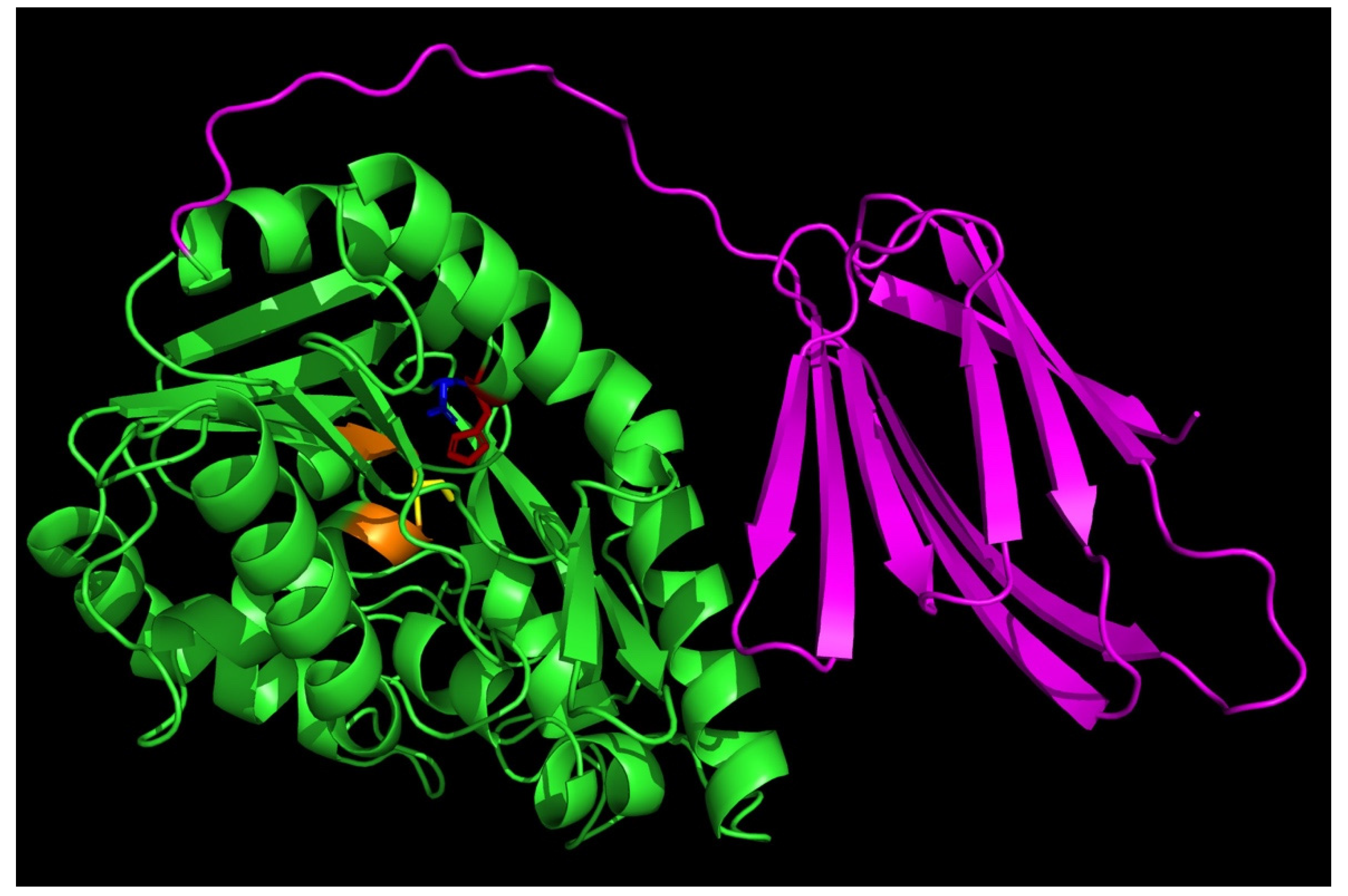
To better understand structure-function aspects of the enzyme, we searched through the AlphaFold Protein Database and downloaded the predicted structure of the enzyme (AF-Q8R7S7).
Figure 2 shows a PyMOL rendering of the predicted structure. The sequence alignment of
T. tengcongensis esterase with homologous esterases had earlier revealed an apparent tail containing 106 non-conserved residues at the C-terminus with an unknown function [
6]. The C-terminal domain contains more than 50% hydrophobic residues, that could be predicted to play some relevant role in the interaction with lipidic substrates as well as enzyme structural stabilization and regulation. Structure prediction using Phyre2 reveals a close structural similarity with
Bacillus licheniformis lipase BlEst2, another member of the α/β hydrolase superfamily. An interesting feature is that the 106 amino acid residue C-terminal tail folds into a β sandwich that packs next to the catalytic domain.
2.2. Design, Cloning, Expression, and Purification of Esterases
To probe the role of the C-terminal domain on the catalytic property and substrate preference of Thermoanaerobacter tengcongensis esterase, we expressed, purified, and compared the substrate preference and kinetic properties of the truncated enzyme against those of the full-length enzyme and a naturally shorter version from B. brevis. Probing the role of the non-conserved C-terminal domain (apparent tail) will allow a better understanding of its possible role in catalytic activity and substrate selectivity and kinetics of these family proteins.
The three recombinant proteins containing 6xHis-tag were purified to homogeneity from the supernatants of cell lysates by single-step affinity chromatography on a Ni–NTA column [
10]. Such high-level expression and efficient purification were similarly obtained for several esterases and other lipolytic enzymes using
E. coli expression system and Ni
2+-affinity chromatography as reported by Rao et al. [
11], Jensen et al. [
12], Latip et al. [
13], and López-Fernández et al. [
14]. Samples were taken during purification and assayed for esterase activity before and after dialysis, and all samples from the three purifications showed esterase activity.
2.3. Hydrolytic Activities of Purified Esterases Reveal Different Substrate Preferences
Upon successfully achieving expression and purification and activity determination of the three recombinant enzymes, it was necessary to assess the comparative substrate preference of the three recombinant enzymes. The idea behind this study was to unravel the role of the non-conserved C-terminal domain of a recombinant
T. tengcongensis esterase, EstA3_
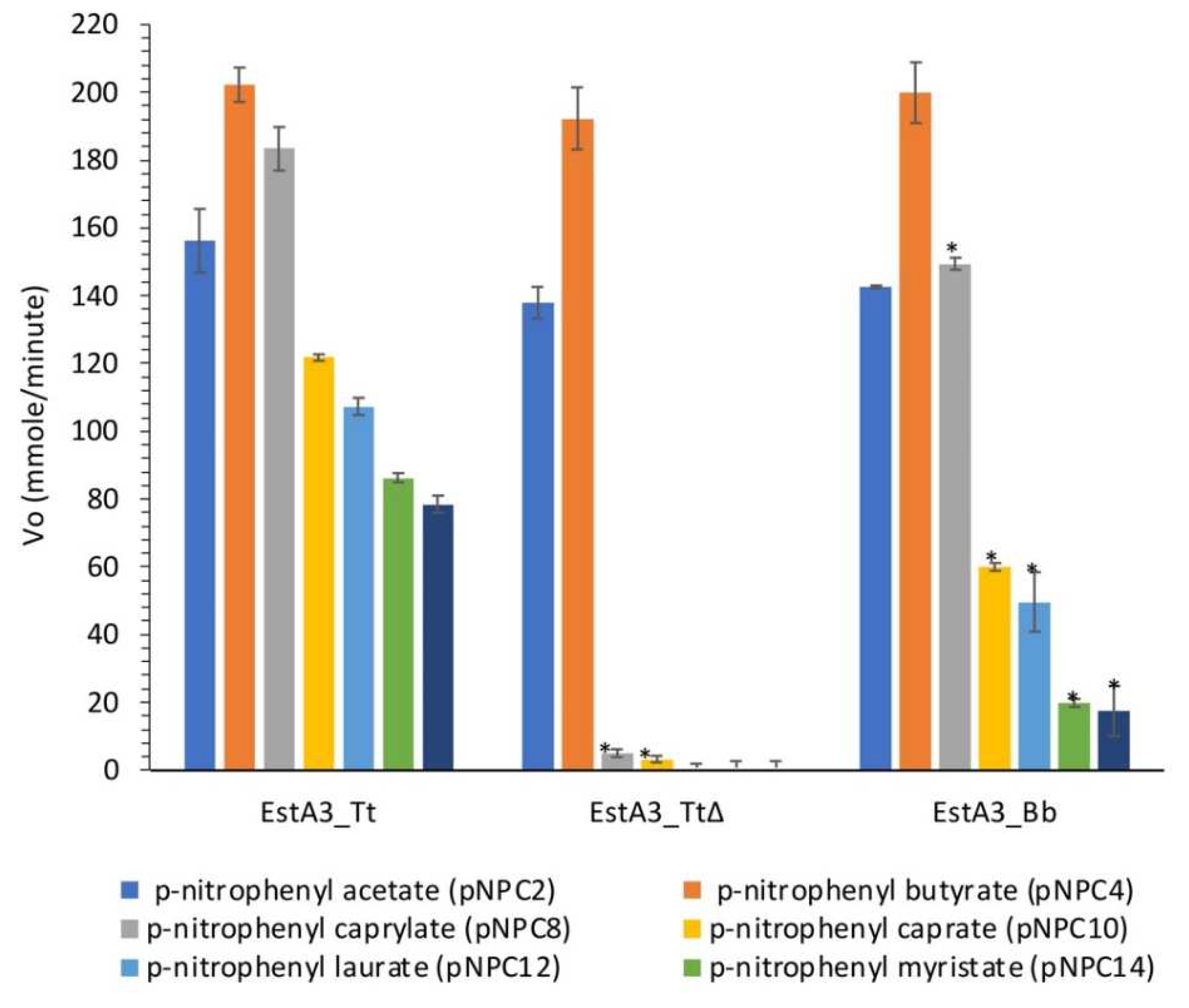
Tt in distinct substrate preference. The effect of para-nitrophenyl acyl substrates of varying chain lengths (pNPC2, pNPC4, pNPC8, pNPC10, pNPC12, pNPC14, and pNPC16) on the activities of the three recombinant esterases are shown in
Figure 3.
No significant variation was observed between the three enzymes in their catalytic hydrolysis of p-nitrophenyl esters with short chain lengths (pNPC2 and pNPC4), which suggests that the truncated C-terminal domain enhanced the recognition of long acyl chain substrates by the wild-type enzyme (EstA3_
Tt), thereby broadening the substrate preference of the enzyme. Previous reports of López-Fernández et al. on truncated prosequence of
Rhizopus oryzae lipase also revealed the involvement of the 28 amino acids in the N-terminal domain of
Rhizopus oryzae lipase in substrate specificity [
14]. A noteworthy observation is that truncation of the C-terminus 106 amino acid tail of EstA3_
Tt resulted in decrease in esterase catalytic hydrolysis pNPC8 substrate by 4.2-fold, an indication that the tail may also be involved in the catalytic process through enhanced substrate binding during the hydrolysis reaction.
2.4. Kinetics of Activities of Purified Esterases on C4 and C8 Substrates
The kinetics of any enzyme is critical to understanding how enzymes behave in cellular systems and help predict the enzyme's performance in industrial settings [
15,
16]. To further understand the role of the truncated C-terminal domain in the substrate utilization, the effect of varying substrate concentration on the activities and the kinetic parameters (K
m, K
cat, and V
max) of the three enzymes-mediated hydrolyses of a short (pNPC4) and a medium (pNPC8) acyl chain substrates were investigated and compared. Due to the lack of esterase activity of EstA3_
TtΔ against substrates with acyl chain longer than pNPC8, the kinetic study focused on only pNPC4 and pNPC8 substrates.
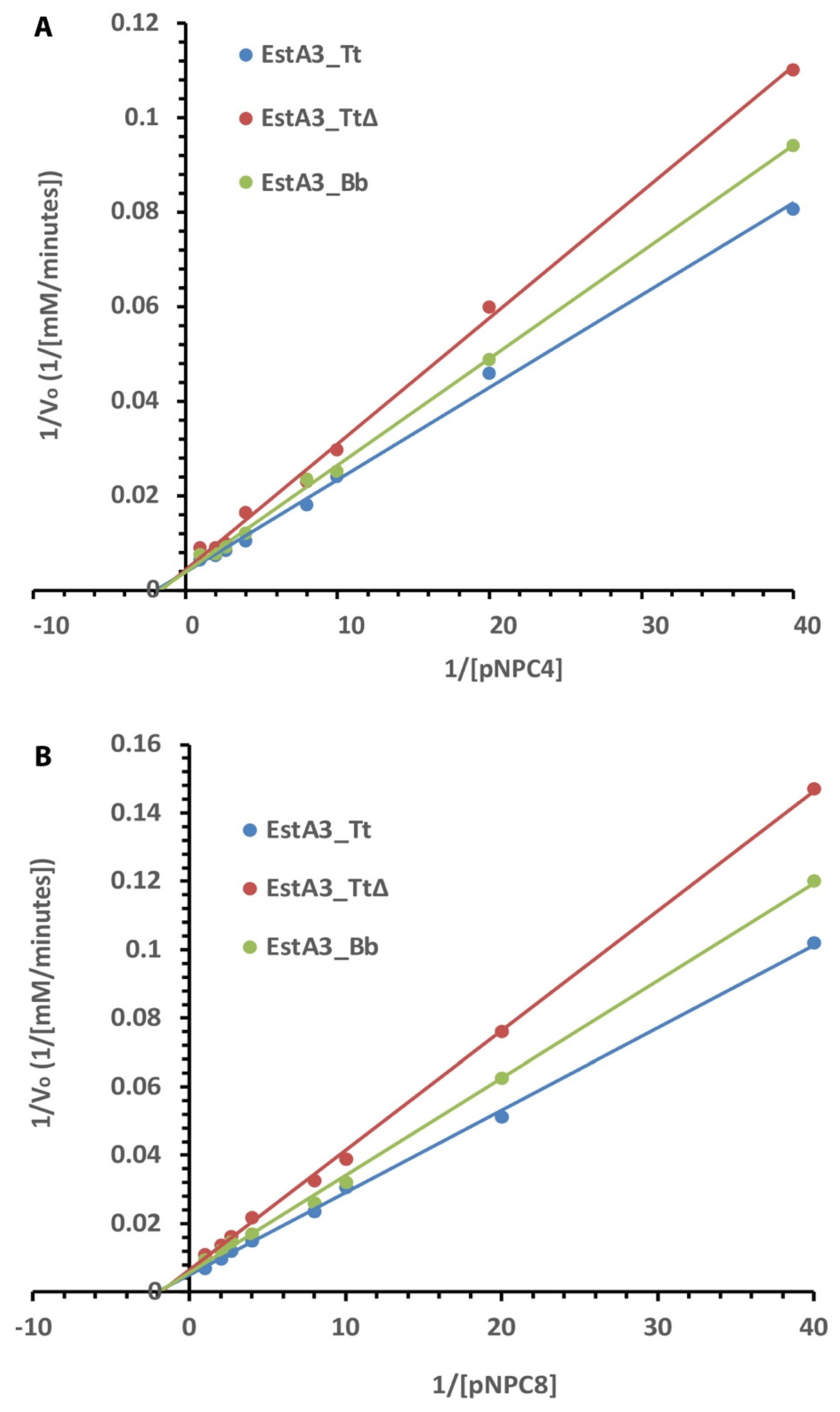
All the three recombinant esterases obey a typical Michaelis-Menten kinetics for the tested substrates (
Figure 4). The results of the effects of substrate concentration on the activities of the recombinant esterases show that the full-length enzyme (EstA3_
Tt) was more active than the truncated and naturally shorter enzymes. Consistently, full-length enzyme EstA3_
Tt performed better than the truncated and naturally shorter enzymes in all the tested substrates in the following order EstA3_
Tt ˃ EstA3_
Bb ˃ EstA3_
TtΔ. As summarized in
Table 1, the result shows a decrease in maximum velocity and turnover rate with increasing chain length for all the three recombinant esterases.
In contrast, there was no massive change in K
m values with increasing chain length for all the three recombinant esterases (
Table 1). The order of V
max for pNPC4 hydrolysis catalysed by the enzymes was EstA3_
Tt (263.1741 mM/min.) > EstA3_
Bb (256.41 mM/min.) > EstA3_
TtΔ (238.10 mM/min.) while the order of substrate affinity (Km) was EstA3_
TtΔ (0.64 mM) > EstA3_
Tt (0.53 mM) = EstA3_
Bb (0.53 mM). A similar trend of Vmax was observed for pNPC8 hydrolysis catalysed by the three recombinant enzymes. On the other hand, the order of substrate affinity (Km) was EstA3_Tt∆ (0.56 mM) > EstA3_
Bb (0.51 mM) > EstA3_
Tt (0.49 mM). The turnover number (K
cat) of EstA3_
Tt for pNPC4 substrate is approximately 2-fold and 1.9-fold higher than that of EstA3_
TtΔ and EstA3_
Bb, respectively. The K
cat value of EstA3_
Tt (2.28 x 10
5 s
-1) for pNPC8 substrate is approximately 2.3 and 2.1-fold higher than that of EstA3_
TtΔ (1.0 x 10
5 s
-1) and EstA3_
Bb (1.1 x 10
5 s
-1), respectively.
Table 1.
Kinetic parameters of the wild-type Thermoanaerobacter tengcongensis esterase, truncated Thermoanaerobacter tengcongensis esterase, and Brevibacillus brevis esterase for the hydrolyses of various p-nitrophenyl esters. Kinetic parameters (Km and Vmax) were calculated from the equation of the straight line of the Lineweaver-Burk plots shown in Figure 4.
Table 1.
Kinetic parameters of the wild-type Thermoanaerobacter tengcongensis esterase, truncated Thermoanaerobacter tengcongensis esterase, and Brevibacillus brevis esterase for the hydrolyses of various p-nitrophenyl esters. Kinetic parameters (Km and Vmax) were calculated from the equation of the straight line of the Lineweaver-Burk plots shown in Figure 4.
| Enzymes |
Km (mM) |
Vmax (mM/minutes) |
Kcat x 105 (S-1) |
| |
pNPC4 |
|
|
| EstA3 |
0.53 |
263.17 |
2.94 |
| EstA3.bb |
0.59 |
256.41 |
1.51 |
| EstA3∆ |
0.64 |
238.10 |
1.50 |
| |
|
|
|
| |
pNPC8 |
|
|
| EstA3 |
0.49 |
204.08 |
2.28 |
| EstA3.bb |
0.51 |
181.82 |
1.07 |
| EstA3∆ |
0.56 |
158.73 |
1.00 |
3. Discussion
3.1. Substrate Preference of EstA3_Tt, EstA3_Tt∆, and EstA3_Bb
E. coli is the most frequently used expression host system for laboratory and industrial scale production of enzymes and other proteins in biotechnology industry [
17]. In this work, the custom-made recombinant plasmid DNA carrying esterase genes of interest were synthesized using the services of Invitrogen GeneArt (a gene synthesis commercial company). The three custom-built vector pET28a (+) with a T7 promoter from Invitrogen-GeneArt each carrying different esterase genes namely EstA3, EstA3∆, and EstA3.bb were transformed into
E. coli BL21 (DE3) pLysS and a high-level expression was achieved in the first round of recombinant protein expression under predetermined optimization of cultivation and induction conditions. This indicates that all the three recombinant esterases (EstA3_
Tt, EstA3_
TtΔ and EstA3_
Bb) were inherently amenable to the expression system used for this study. Ni-NTA affinity chromatography matrix allows rapid and efficient purification of recombinant proteins carrying a histidine tag [
6,
10,
17].
The observation that all the three recombinant enzymes were more active on short acyl chain substrates (pNPC4) supports the previous findings that all the three recombinant enzymes are true esterases that favour the hydrolysis of short acyl-chain substrates (≤ C10) [9,19–21]. The three recombinant enzymes had the highest turn-over rate (K
cat) with the short-acyl chain substrate (pNPC4), and the highest affinities (low K
m values) for long-acyl chain substrate. The fact that the esterase activity and substrate preference was influenced massively by the truncated C-terminal domain with little effect on the substrate affinity (K
m), suggests that such influence of the C-terminal domain may not be through facilitating substrate binding to the active site of the enzyme alone [
22].
Our data from the catalytic activity, substrates preferences, and kinetics suggest that truncated C-terminal domain of non-conserved residues may contribute significantly to the catalytic activity and substrate promiscuity of EstA3_
Tt. Martínez-Martínez
et al. earlier predicted that the molecular mechanisms by which some esterases exhibit more substrate promiscuity than others remain unclear and yet to be elucidated [
2]; findings from this study however, revealed that the non-conserved residues such as the ones depicted C-terminal domain could be one of the key contributory factors for the substrate promiscuity of the family of XIV of lipolytic enzymes (esterases) and possibly other esterases in general. This substrate promiscuity is an important and indisputable property of the esterase enzyme [
3]. A narrow substrate spectrum is usually one of the most frequently encountered challenges for industrial applications of enzymes [
23].
3.2. The C-Terminal Tail Contributes to the Stability of EstA3
Findings from catalytic property suggest that the truncated C-terminal domain accounted for the drastic decrease in catalytic activity and instability. One possible explanation for this is that it is likely that the C-terminal domain plays major role in folding of an enzyme to adopt its functionally active conformation during or following translation [
17]. Our result implies that although the 106 amino acid residues at the C-terminal domain of EstA3 are not conserved across the family members, their absence in truncated enzyme (EstA3_
TtΔ) interferes with post-expression catalytic activity and stability.
3.3. Role of the C-Terminal β Sandwich in Binding Long Chain Acyl Substrates
Findings from this study suggest that the apparent tail (C-terminal domain) plays a critical role in substrate preference. The three enzymes displayed higher activity towards short acyl chain substrates than the long-chain substrates with the highest preference for p-nitrophenyl butyrate (pNPC4). The fact that the three recombinant lipolytic enzymes preferentially favour the hydrolysis of short acyl chain ester (pNPC4) suggest they are classified as esterases rather than lipases [
24]. A similar trend with pNPC4 as the most preferred substrate has also been reported for esterases from
Sulfolobus shibatae [
25]
, Sulfolobus tokodaii [
26],
Bacillus subtilis (RRL 1789) [
27], and
Geobacillus sp. HBB [
28].
The preference of the three recombinant enzymes towards p-nitrophenyl substrates (pNPC2, pNPC4, pNPC8, pNPC10, pNPC12, pNPC14, and pNPC16) was established and showed that with increase in substrate acyl chain length, a corresponding decrease in esterase was observed. Furthermore, comparing the acyl chain substrates utilization of the wild-type enzyme (EstA3_
Tt) with the truncated enzyme (EstA3_
TtΔ) and the naturally shorter enzyme (EstA3_
Bb) revealed an abrupt and significant decrease in catalytic activity toward p-nitrophenyl esters with chain lengths ranging from C8 to C16. Such an abrupt decrease in the activity of the truncated enzyme toward p-nitrophenyl esters with chain lengths ranging from C8 to C16 was also reported for another naturally shorter esterase designated as EstEP16 [
29]. The EstEP16 was a new and thermostable esterase characterized from a metagenomic library derived from a deep-sea hydrothermal field in the east Pacific.
It is notable that full-length enzyme (EstA3_
Tt) hydrolyzed a broader spectrum of p-nitrophenyl esters (from C2 to C16) than the truncated enzyme (EstA3_
TtΔ) and the naturally shorter enzyme (EstA3_
Bb). Findings from the studies revealed that there was no detectable esterase activity towards acyl substrates bearing ≥ C10 for the truncated version of
T. tengcongensis esterase. A similar result was reported for
Bacillus aryabhattai esterase (BaCE) by Zhang et al. [
30]. BaCE was active against esters with short-chain fatty acids (from pNPC2 to pNPC8) with high substrate specificity toward pNP butyrate (pNPC4) but no detectable activity was reported for long acyl chain substrates (≥ C10) [
30].
The β sandwich sequence did not retrieve any structure from the NCBI Conserved Domain Architecture Retrieval tool (
https://www.ncbi.nlm.nih.gov/Structure/lexington/lexington.cgi). We ran the predicted sandwich through the Dali server (
http://ekhidna2.biocenter.helsinki.fi/dali/) and most of the structural matches were to integrins. However, the closest match is a crystal structure (6WPX) of
Bacillus licheniformis lipase BlEst2 in propetide form [
31]. Structure prediction using Phyre2 identified a close structural alignment of LipA3 with the C-terminal domain of BlEst2, an indication that this feature is common among the α/β hydrolase superfamily. It is likely that the β sandwich is involved in binding the hydrophobic part of the long-chain fatty acid moiety since its deletion resulted in loss of activity of C12-16 substrates. Due to the high hydrophobicity of the tail region, it is conceivable that the domain could facilitate interaction of lipidic substrates hence its contribution to the ability of EstA3_
Tt to bind and hydrolyse long chain fatty acids.
Similar observations have been made in other lipases in which hydrophobic domains are attributed to catalytic specificity for long chain substrates [
14,
32]. The findings from this work, coupled with recent developments in protein structure prediction and modelling, can be used to alter the specificity and catalytic efficiency of the enzyme as was done recently for a
Rhizopus oryzae lipase [
33].
Findings from the substrate preference studies indicate that the non-conserved C-terminal domain of EstA3_
Tt play a noticeable role in selective substrate preference, especially towards long acyl chain substrates. Hence, the widely accepted assertion that the variable sequence/non-conserved sequence does not play a catalytic role in a group or family of enzymes, may not be the case for lipolytic enzymes. The involvement of the truncated non-conserved C-terminal domain on the catalytic activity and substrate preference of EstA3_
Tt as revealed in this work matches the findings from Kovacic
et al. that non-catalytic residues may contribute notably to substrate specificity, protein activity, and stability through yet to be elucidated mechanisms [
34]. Although this finding is relevant to the family of XIV of lipolytic enzymes (esterases), it has provided new openings for deeper research to understand the actual role and mechanism of non-conserved residues of the general lipolytic enzymes (esterases and lipases). More subtle experimental designs and targeted mutagenesis studies are required to gain a better understanding of how these systems work and may reflect a new paradigm in the regulation of enzyme activity.
The proposed contribution of the C-terminal domain to broad substrate preference could open a range of industrial applications of esterases. Therefore, such observed broad substrate preference implies that the full-length enzyme could also perform some specific industrial applications where lipases are usually used in the catalysis of medium and long acyl chain substrates. This might consequently reduce the cost of producing multiple enzymes for different industrial applications. Acquisition of new specificities without compromising existing ones is the major driving force for the natural evolution of enzymes with novel specificities through microbial adaptation to extreme ecosystems [
35]. The presence of the C-terminal domain in the wild-type enzyme (EstA3_
Tt) confer broad substrate spectrum, whereas its absence narrowed the substrate spectrum of the truncated enzyme (EstA3_
TtΔ). The molecular mechanism by which the C-terminal domain facilitates more activity towards the long acyl chain substrates remains unknown and needs further investigation.
3.4. Structure-Guided Engineering of Esterases Could Generate New Catalytic Flexibility for Industrial Applications
Exploiting protein engineering in attaching the C-terminal domain to other naturally short esterase family members broadens their industrial applications where long acyl chain lipids are used as substrates. On the other hand, since the truncation did not massively affect esterase activity towards short acyl esters pNPC2 and pNPC4, industrial applications that require hydrolysis of short acyl chain substrates might prefer truncated esterase enzymes to cut the cost of production.
The overall objective of this study is to decipher the outcome of truncation of an apparent tail sequence with non-conserved residues, and findings from the experimental data revealed the involvement of the truncated C-terminal domain in the catalytic activity and preference towards long-chain substrates. The truncation resulted in a decrease in esterase catalytic activity by 4.2-fold. The full-length enzyme showed more preference for long acyl chain substrates compared to two short-length enzymes, which indicate significant role of the of the truncated C-terminal domain in broad substrate preference.
The findings represent a significant improvement in our understanding and suggests that the widely accepted trademark about the involvement of the non-conserved residues/sequence in enzyme catalysis may not necessarily be the case in esterases. This study particularly provides a material basis on the role of non-conserved sequence in catalytic activity and substrate preference among the XIV family of lipolytic enzymes which can be explored for more efficient industrial applications. This study also provides useful information that would spur curiosity for further research to better understand the mechanism through which the truncated apparent C-terminal domain contributes to the observed properties of T. tengcongensis esterase.
More subtle experimental designs and targeted mutagenesis studies would be needed to gain better understanding of how these systems work and may reflect a new paradigm in regulation of enzyme activity. The understanding of the role played by the non-conserved C-terminal domain on the properties of this enzyme could open new insight into effective utilization of esterases and other lipolytic enzymes for optimized industrial and biotechnological applications.
4. Materials and Methods
4.1. Plasmids and Bacterial Strains
E. coli BL21(DE3) plysS host strain (Novagen, Germany), pET-28a (+) (Novagen), the protein overexpression vector, and all other reagents for molecular cloning were made available through the research group of Dr. Femi Olorunniji, School of Pharmacy and Biomolecular Science, Liverpool John Moores University, UK. Gene sequences were obtained from National Centre for Biotechnology Information (NCBI) database and codon optimized. Gene synthesis and custom-made plasmids each carrying different esterase genes namely FEM45 (EstA3_Tt), FEM103 (EstA3_Tt∆), and FEM170 (EstA3_Bb) were constructed by Synthetic Biology Company Invitrogen GeneArt, Thermo Fisher Scientific, England.
4.2. Chemicals
Para-nitrophenyl esters (C2-C16), p-nitrophenyl acetate (pNPC2), p-nitrophenyl butyrate (pNPC4), p-nitrophenyl caprylate (pNPC8), p-nitrophenyl caprate (pNPC10) p-nitrophenyl laurate (pNPC12), p-nitrophenyl myristate (pNPC14), p-nitrophenyl palmitate (pNPC16), isopropyl-β-D-thiogalactopyranoside (IPTG), chloramphenicol, kanamycin, and all other reagents were obtained from Sigma and New England Biolab (UK) and were of analytical grade.
4.3. Protein Expression and Purification
The custom-made plasmids each carrying different esterase genes namely pFEM45 (EstA3_Tt), pFEM103 (EstA3_TtΔ), and pFEM170 (EstA3_Bb) were transformed into E. coli BL21 (DE3) pLysS cells.
The transformed E. coli BL21(DE3)pLysS cells containing custom-made recombinant plasmids were cultured in Luria-Bertani (LB) medium containing 100 µl of µg/ml Chloramphenicol and 100 µl of 50 µg/ml Kanamycin with constant shaking at 225 rpm, 37 oC to mid log phase (when the cell density at OD600 reached 0.8). Isopropyl thio-β-D-galactoside (IPTG) was added (at the final concentration of 1 mM) to the cell cultures and allowed to grow overnight with constant shaking at 225 rpm, 20 oC in a refrigerated incubator (EES-60 Model). Induced cells were harvested by centrifugation at 5,000 ×g for 10 minutes using refrigerated centrifuge at 4 oC. The cell pellets were resuspended and incubated in 50 mM Tris–HCl buffer (pH 8.0) at 4 oC. The cell suspension was then lysed by ultrasonication using a Branson Sonifier®, Model-250 for 5 min (10 sec ON/OFF pulses). After ultrasonic cell disintegration, the cell debris was removed by centrifugation (4000 rpm, 30 min). Subsequently, the supernatant was purified with a Nickel affinity column (Ni-his NTA Novagen). Supernatant was loaded on Nickel affinity column and eluted using high concentration of imidazole. The fractions containing esterase activity were collected and concentrated by dialysis against glycerol storage/dialysis Tris-HCl pH buffer 7.5 (made up of a final concentration of the following compositions 25 mM Tri-HCl pH 7.5; 50% Glycerol; 1 mM DTT; 1000 mM NaCl) overnight with constant shaking using magnetic stirrer in the cold room. The Nickel affinity chromatography purification steps were carried out using ÄKTA Purifier according to standard protocol.
4.4. Determination of Esterase Activity
Protein concentration was determined by measuring absorbance at 280 nm. Enzyme activity was monitored spectrophotometrically at 405 nm by using a CLARIOstar plate reader (BMG LABTECH) with p-nitrophenyl caprylate (pNPC8). The activities of the recombinant esterases were determined in reaction that contained 50 mM phosphate buffer pH 7.4, 20 mM pNPC8 and esterase enzyme. An aliquot of 10 μl of 20 mM pNPC4 was added to 100 μl of 50 mM Tris-HCl buffer (pH 8.5), and 70 μl of distilled water to form a “buffered substrate mixture”. Reactions were initiated by the addition of 20 μl of enzyme to “buffered substrate mixture” and incubated at 50 oC for 15 minutes. The change in absorbance at 405 nm was monitored immediately using a CLARIOstar plate reader (BMG LABTECH). One unit of enzyme activity was defined as the amount of enzyme that releases 1 μmol p-nitrophenol from p-nitrophenyl ester per minute.
4.5. Determination of Substrate Preference and Kinetic Parameters
Substrate specificity of the enzyme was determined using various p-nitrophenyl esters with different acyl chain (pNPC2, pNPC4, pNPC8, pNPC10, pNPC12, pNPC14 and pNPC16). The kinetic parameters (Km and Vmax) of the recombinant esterases using short and medium chain substrates (pNPC4 and pNPC8) were determined. The plot of esterase activity (V) versus substrate concentrations ([S]) (Michaelis-Menten plot) was used to show the kinetic behaviours of the esterases. On the other hand, the equation of the straight line of the reciprocal plot of esterase activity (V) against the substrate concentrations ([S]) (Lineweaver-Burk plot) was used to calculate the kinetic parameters (Km and Vmax). The turnover number (Kcat) was calculated as Vmax divided by the corresponding enzymatic concentration.
Author Contributions
Conceptualization, F.O. and S.M.; methodology, E.J., A.B., and F.O; software, F.O.; validation, E.J. and A.I.; formal analysis, E.J. and A.I.; investigation, E.J., A.A., and M.A.; resources, F.O. and S.M.; data curation, E.J., A.B., and F.O.; writing—original draft preparation, E.J., A.I. and F.O.; writing—review and editing, E.J., A.I., A.B., S.M., and F.O.; visualization, E.J. and A.A.; supervision, A.I., S.M., and F.O.; project administration, S.M. and F.O.; funding acquisition, S.M. and F.O.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andualema, B.; Gessesse, A. Microbial Lipases and Their Industrial Applications. Biotechnol. Faisalabad 2012, 11, 100–118. [Google Scholar] [CrossRef]
- Martínez-Martínez, M.; Coscolín, C.; Santiago, G.; Chow, J.; Stogios, P.J.; Bargiela, R.; Gertler, C.; Navarro-Fernández, J.; Bollinger, A.; Thies, S.; et al. Determinants and Prediction of Esterase Substrate Promiscuity Patterns. ACS Chem. Biol. 2018, 13, 225–234. [Google Scholar] [CrossRef]
- Holmquist, M. Alpha Beta-Hydrolase Fold Enzymes Structures, Functions and Mechanisms. Curr. Protein Pept. Sci. 2000, 1, 209–235. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.; Gupta, R. Medical Aspects of Esterases: A Mini Review. Int J Pharm Pharm Sci 2016, 8, 21–26. [Google Scholar]
- Panda, T.; Gowrishankar, B.S. Production and Applications of Esterases. Appl. Microbiol. Biotechnol. 2005, 67, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Xue, Y.; Zhou, C.; Tao, J.; Li, G.; Lu, J.R.; Ma, Y. A Thermostable Esterase from Thermoanaerobacter Tengcongensis Opening up a New Family of Bacterial Lipolytic Enzymes. Biochim. Biophys. Acta BBA-Proteins Proteomics 2011, 1814, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xu, Y.; Liu, Y.; Ma, Y.; Zhou, P. Thermoanaerobacter Tengcongensis Sp. Nov., a Novel Anaerobic, Saccharolytic, Thermophilic Bacterium Isolated from a Hot Spring in Tengcong, China. Int. J. Syst. Evol. Microbiol. 2001, 51, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Steel, D.M.; Walker, J.M. Thermostable Proteins. Life Chem. Rep. Ser. 1991, 8, 49–96. [Google Scholar]
- Zhang, J.; Liu, J.; Zhou, J.; Ren, Y.; Dai, X.; Xiang, H. Thermostable Esterase from Thermoanaerobacter Tengcongensis: High-Level Expression, Purification and Characterization. Biotechnol. Lett. 2003, 25, 1463–1467. [Google Scholar] [CrossRef]
- Crowe, J.; Döbeli, H.; Gentz, R.; Hochuli, E.; Stüber, D.; Henco, K. 6xHis-Ni-NTA Chromatography as a Superior Technique in Recombinant Protein Expressiod/Purification. In Protocols for Gene Analysis; Humana Press: New Jersey, 1994; Vol. 31, pp. 371–388. [Google Scholar] [CrossRef]
- Rao, L.; Xue, Y.; Zheng, Y.; Lu, J.R.; Ma, Y. A Novel Alkaliphilic Bacillus Esterase Belongs to the 13th Bacterial Lipolytic Enzyme Family. PloS One 2013, 8, e60645. [Google Scholar] [CrossRef]
- Jensen, M.-B. V.; Horsfall, L.E.; Wardrope, C.; Togneri, P.D.; Marles-Wright, J.; Rosser, S.J. Characterisation of a New Family of Carboxyl Esterases with an OsmC Domain. Plos One 2016, 11, e0166128. [Google Scholar] [CrossRef]
- Latip, W.; Raja Abd Rahman, R.N.Z.; Leow, A.T.C.; Mohd Shariff, F.; Kamarudin, N.H.A.; Mohamad Ali, M.S. The Effect of N-Terminal Domain Removal towards the Biochemical and Structural Features of a Thermotolerant Lipase from an Antarctic Pseudomonas Sp. Strain AMS3. Int. J. Mol. Sci. 2018, 19, 560. [Google Scholar] [CrossRef]
- López-Fernández, J.; Barrero, J.J.; Benaiges, M.D.; Valero, F. Truncated Prosequence of Rhizopus Oryzae Lipase: Key Factor for Production Improvement and Biocatalyst Stability. Catalysts 2019, 9, 961. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Lee, B. Culture Conditions for the Production of Esterase from Lactobacillus Casei CL96. Bioprocess Biosyst. Eng. 2001, 24, 59–63. [Google Scholar] [CrossRef]
- Samoylova, Y.V.; Sorokina, K.N.; Romanenko, M.V.; Parmon, V.N. Cloning, Expression and Characterization of the Esterase estUT1 from Ureibacillus Thermosphaericus Which Belongs to a New Lipase Family XVIII. Extremophiles 2018, 22, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Fakruddin, M.; Mohammad Mazumdar, R.; Bin Mannan, K.S.; Chowdhury, A.; Hossain, M.N. Critical Factors Affecting the Success of Cloning, Expression, and Mass Production of Enzymes by Recombinant E. Coli. Int. Sch. Res. Not. 2013, 2013. [Google Scholar] [CrossRef]
- Olorunniji, F.J.; Buck, D.E.; Colloms, S.D.; McEwan, A.R.; Smith, M.C.M.; Stark, W.M.; Rosser, S.J. Gated Rotation Mechanism of Site-Specific Recombination by ϕC31 Integrase. Proc. Natl. Acad. Sci. 2012, 109, 19661–19666. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Bessler, C.; Srinivas, R.; Krishna, S.H. Optimizing Lipases and Related Enzymes for Efficient Application. TRENDS Biotechnol. 2002, 20, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Levisson, M.; Van Der Oost, J.; Kengen, S.W.M. Characterization and Structural Modeling of a New Type of Thermostable Esterase from Thermotoga Maritima. FEBS J. 2007, 274, 2832–2842. [Google Scholar] [CrossRef]
- López-López, O.; E Cerdan, M.; I Gonzalez Siso, M. New Extremophilic Lipases and Esterases from Metagenomics. Curr. Protein Pept. Sci. 2014, 15, 445–455. [Google Scholar] [CrossRef]
- Igunnu, A.; Joel, E.B.; Ezetulugo, L.N.; Malomo, S.O. Modulatory Effects of Mg2+ and Zn2+ Ions on Monoesterase Activity of Wild-Type and Mutant E. Coli Alkaline Phosphatases. Ilorin J. Sci. 2018, 5, 36–47. [Google Scholar] [CrossRef]
- Ferrer, M.; Bargiela, R.; Martínez-Martínez, M.; Mir, J.; Koch, R.; Golyshina, O.V.; Golyshin, P.N. Biodiversity for Biocatalysis: A Review of the α/β-Hydrolase Fold Superfamily of Esterases-Lipases Discovered in Metagenomes. Biocatal. Biotransformation 2015, 33, 235–249. [Google Scholar] [CrossRef]
- Arpigny, J.L.; JAEGER, K.-E. Bacterial Lipolytic Enzymes: Classification and Properties. Biochem. J. 1999, 343, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ejima, K.; Liu, J.; Oshima, Y.; Hirooka, K.; Shimanuki, S.; Yokota, Y.; Hemmi, H.; Nakayama, T.; Nishino, T. Molecular Cloning and Characterization of a Thermostable Carboxylesterase from an Archaeon, Sulfolobus Shibatae DSM5389: Non-Linear Kinetic Behavior of a Hormone-Sensitive Lipase Family Enzyme. J. Biosci. Bioeng. 2004, 98, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Miyamoto, K.; Ohta, H. A Novel Thermostable Esterase from the Thermoacidophilic Archaeon Sulfolobus Tokodaii Strain 7. FEMS Microbiol. Lett. 2004, 236, 97–102. [Google Scholar] [CrossRef]
- Kaiser, P.; Raina, C.; Parshad, R.; Johri, S.; Verma, V.; Andrabi, K.I.; Qazi, G.N. A Novel Esterase from Bacillus Subtilis (RRL 1789): Purification and Characterization of the Enzyme. Protein Expr. Purif. 2006, 45, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Metin, K.; Burcu Bakir Ateslier, Z.; Basbulbul, G.; Halil Biyik, H. Characterization of Esterase Activity inGeobacillus Sp. HBB-4. J. Basic Microbiol. 2006, 46, 400–409. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, J.; Cai, H.; Ni, H.; Xiao, A.; Hou, L. Characterization of a New and Thermostable Esterase from a Metagenomic Library. Microbiol. Res. 2013, 168, 589–597. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Chen, C.-S.; Liu, H.-T.; Chen, J.-L.; Xia, Y.; Wu, S.-J. Purification, Identification and Characterization of an Esterase with High Enantioselectivity to (S)-Ethyl Indoline-2-Carboxylate. Biotechnol. Lett. 2019, 41, 1223–1232. [Google Scholar] [CrossRef]
- Nakamura, A.M.; Godoy, A.S.; Kadowaki, M.A.S.; Polikarpov, I. The first structure of Bacillus licheniformis lipase BlEst2 in its propeptide and mature form revealing molecular details of inhibition by its C-terminal domains. (Department of Biotechnology and Biomedicine, Technical University of Denmark, Søltofts Plads 224, 2800 Kongens Lyngby, Denmark), (2021). (manuscript in preparation; to be submitted). [CrossRef]
- Sayari, A.; Frikha, F.; Miled, N.; Mtibaa, H.; Ali, Y.B.; Verger, R.; Gargouri, Y. N-Terminal Peptide of Rhizopus Oryzae Lipase Is Important for Its Catalytic Properties. FEBS Lett. 2005, 579, 976–982. [Google Scholar] [CrossRef]
- Huang, J.; Dai, S.; Chen, X.; Xu, L.; Yan, J.; Yang, M.; Yan, Y. Alteration of Chain-Length Selectivity and Thermostability of Rhizopus Oryzae Lipase via Virtual Saturation Mutagenesis Coupled with Disulfide Bond Design. Appl. Environ. Microbiol. 2023, 89, e01878–22. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, F.; Babic, N.; Krauss, U.; Jaeger, K.-E. Classification of Lipolytic Enzymes from Bacteria. Aerob. Util. Hydrocarb. Oils Lipids 2019, 24, 255–289. [Google Scholar] [CrossRef]
- Lan, T.; Wang, X.-R.; Zeng, Q.-Y. Structural and Functional Evolution of Positively Selected Sites in Pine Glutathione S-Transferase Enzyme Family. J. Biol. Chem. 2013, 288, 24441–24451. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).