1. INTRODUCTION
Malignant pleural mesothelioma (MPM) is a rare, highly lethal cancer associated with asbestos exposure, with peak incidence expected in the 2020s in developed countries [
1]. MPM can be classified according to histology from best to worst prognosis as epithelioid, biphasic and sarcomatoid. The prognosis of patients with MPM is poor, with a median survival of 9-18 months.
For many years chemotherapy based in platinum plus pemetrexed has been the standard treatment for patients with MPM, but recently the combination of nivolumab and ipilimumab showed improvements in overall survival compared to chemotherapy [
2,
3]. After first line there is not any option of systemic treatment approved for MPM.
Genomic studies have shown that mesothelioma is mainly defined by loss of function in tumor suppressor genes with mutations in
BAP1,
CDKN2A and
NF2 being the most common alterations [
4,
5]. The chronic inflammatory response to asbestos causes a unique tumor environment composed primarily of immunosuppressive cells (regulatory T cells, macrophages, and myeloid-derived suppressor cells) [
6]. MPM expresses multiple immune checkpoint inhibitors. VISTA expression in MPM is much higher compared to other tumors and is only expressed in tumors of the epithelioid subtype [
5]. PD-L1 expression is positive in approximately 40% of patients [
7,
8,
9,
10], it is more common in the non-epithelioid subtype and is associated with a worse prognosis [
7,
8,
9,
10,
11].
In this review we provide an overview of current therapeutic options for patients with MPM, and discuss potential biomarkers of response to immunotherapy and new future options.
2. TREATMENT
2.1. SURGERY
Most patients with mesothelioma are not candidates for surgery due to the extent of the disease and comorbidities. The MARS study, which included 50 patients, demonstrated that extrapleural pneumonectomy was associated with severe complications and did not improve survival compared to patients who did not undergo surgery [
12]. In 2023, the results of the MARS-2 study were presented comparing the treatment of chemotherapy and pleuro-decortication versus chemotherapy without surgery [
13]. The study found that survival was superior for patients who did not receive surgery. Therefore, considering the results of these two studies, the role of surgery is currently reserved for diagnostic procedures and treatment of pleural effusion.
2.2. FIRST LINE
Regarding systemic treatment, the standard treatment for almost two decades has been antifolate and platinum chemotherapy [
2]. The pivotal EMPHACIS trial demonstrated that the combination of cisplatin and pemetrexed produces an increase in survival (OS) of 9.3 to 12.1 months compared to cisplatin alone. The addition of bevacizumab to chemotherapy with cisplatin and pemetrexed demonstrated in the MAPS study an increase in OS from 16.1 to 18.8 months and progression-free survival (PFS) from 7.3 to 9.2 months [
14]. However, bevacizumab is not approved by the FDA or EMA for MPM.
After almost two decades without new relevant advances in the first-line treatment in MPM, in May 2019 the FDA approved the NovoTTF therapy (alternating electric field therapy, TTFields) in combination platinum pemetrexed [
15]. The phase II STELLAR trial demonstrated an OS of 18.2 months and PFS of 7.6 months, both favorable compared to historical cohorts.
The third regulatory approval of first-line treatments took place in 2020 with the combination of nivolumab plus ipilimumab (reviewed later) [
3]. The results of this study have marked a change in the standard treatment of mesothelioma and have opened the doors to new research studies of other immunotherapy molecules in the first line.
2.3. SECOND LINE
There is currently no approved therapy when patients progress to first-line platinum-based treatment. The most widely adopted treatment options include single-agent chemotherapy with vinorelbine, gemcitabine, or retreatment with pemetrexed. These options are primarily based on retrospective or phase II studies reporting an overall response rate (ORR) of 0-19%, a disease control rate of 38-84%, a PFS of 1.6-3.8 months, with an OS ranging from 2.5 to 12 months [
16]. The results of the RAMES trial, the study with the largest number of second-line patients, have recently been published, which compared the combination of ramucirumab and gemcitabine versus gemcitabine and demonstrated an improvement in OS for the combination [
17].
3. IMMUNOTHERAPY
3.1. SECOND AND FUTHER LINES
The first studies with anti-CTLA-4 were negative. Tremelimumab was evaluated in the phase 2b DETERMINE study in a large cohort of patients versus placebo and did not demonstrate an improvement in OS (7.7 and 7.3 months in tremelimumab and placebo respectively) [
18].
Subsequently, anti-PD-1 demonstrated promising results in phase II studies, but they were not confirmed in all phase III studies (
Table 1). Pembrolizumab achieved a disease control rate of 72% in the phase Ib KEYNOTE-028 study [
19]. Unfortunately, these good results were not confirmed in the randomized PROMISE-meso study that compared pembrolizumab versus chemotherapy in patients who progressed to platinum and achieved a PFS of 2.5 months with pembrolizumab versus 3.4 months with chemotherapy [
20].
However, with nivolumab, an anti-PD-1 monoclonal antibody, the preliminary results of activity were confirmed in a phase III. The phase II NivoMes and MERIT studies demonstrated a response rate of 24-29% and OS of 11.8 and 17.3 months [
21,
22]. Based on the results of this study, nivolumab was approved by the Japanese health authorities in August 2018 in patients who progress to chemotherapy. Subsequently, the phase III CONFIRM trial evaluated nivolumab versus placebo in the second or third line [
23]. Most patients had epithelioid histology (88%) and were PD-L1 negative (66%). The study met its primary objectives with a median PFS of 3.0 months for nivolumab versus 1.8 months for placebo and a median OS of 9.2 with nivolumab and 6.6 months with placebo. The results of this study have not led to the approval of nivolumab in the second line.
Finally, avelumab, an anti-PD-L1 monoclonal antibody, was explored in a phase 1b study achieving a response rate of 9%, PFS of 4.1 months, and OS of 10.7 months [
24].
Regarding combinations of immunotherapy drugs, several studies have published the results of the combination of anti-PD-1 therapy with anti-CTLA-4 (
Table 2). The NIBIT-MESO-1 study was a phase II, open-label, non-randomized trial of patients with pleural or peritoneal mesothelioma in second or third line that administered tremelimumab in combination with durvalumab [
25]. The study achieved a response rate of 28% with a duration of response of 16.6 months, a PFS of 5.7 months, and median OS was 16.5 months. In a recent 4-year study update, it was reported that the combination of tremelimumab with durvalumab was associated with durable survival (20% at 36 months and 15% at 48 months) [
26].
Two studies have explored the combination of nivolumab with ipilimumab in patients who relapse after the first line of treatment, the INITIATE study and the MAPS-2 [
27,
28]. The studies reported a response rate of around 30%, a PFS of 6 months, and an OS of 15.9 months.
3.2. IMMUNOTHERAPY IN FIRST LINE
CheckMate-743 is an open-label, randomized phase III study comparing first-line treatment with nivolumab plus ipilimumab versus chemotherapy in patients with previously untreated unresectable MPM with an ECOG score of 1 or less [
3]. A total of 605 patients were included, randomized 1:1 to receive nivolumab at a dose of 3 mg/kg every 2 weeks with ipilimumab 1 mg/kg every 6 weeks for two years, versus chemotherapy with platinum and pemetrexed. The study was positive demonstrating a 4-month survival advantage for the immunotherapy combination (18.1 vs 14.1 months). The 3-year survival rate was 23.2% for immunotherapy versus 15.4% for chemotherapy [
29]. Median PFS was 6.8 months with nivolumab plus ipilimumab versus 7.2 months with chemotherapy and there was no difference in response rate (40% and 43% for immunotherapy and chemotherapy).
In terms of toxicity, grade 3-4 adverse events occurred in 31% of patients treated with immunotherapy and 32% of patients treated with chemotherapy. However, in patients who discontinued immunotherapy due to toxicity, survival was not affected reaching a median OS of 25.4 months from randomization.
The magnitude of survival benefit with nivolumab plus ipilimumab was greater in patients with PD-L1 expression ≥1%, but it should be noted that PD-L1 expression was not a stratification factor in the study.
In a prespecified histology-based exploratory analysis, subgroups of patients with epithelioid histology had an OS of 18.7 months with immunotherapy versus 16.2 months with chemotherapy (HR 0.85). However, in the subgroup of patients with non-epithelioid histology the differences were greater with an OS of 18.1 months versus 8.8 months for immunotherapy versus chemotherapy, respectively (HR 0.46). Although the benefit of immunotherapy seems greater in non-epithelioid tumors, after adjusting for treatment it is observed that the OS of patients treated with immunotherapy in the group of epithelioid and non-epithelioid tumors was similar (median OS 18.7 months and 18.1 months in epithelioid and non-epithelioid). It is postulated that non-epithelioid tumors have a worse prognosis and are less sensitive to chemotherapy treatment.
Exploratory biomarker analysis included a 4-gene gene expression signature (
CD8A,
STAT1,
LAG3, and
CD27), TMB, and pulmonary immune prognostic index (LIPI) measured by LDH levels and neutrophil-lymphocyte ratio (NLR) in peripheral blood [
29]. A positive correlation was detected in patients with high inflammatory gene signature expression with a survival benefit from immunotherapy (21.8 months in patients with high inflammatory signature versus 16.8 months in patients with low score). Of the other two markers studied, neither the TMB nor the LIPI score were predictive for survival.
Regarding the analysis of quality of life, immunotherapy demonstrated an improvement in symptoms accompanied by the maintenance of general condition and a reduction in the risk of definitive deterioration of symptoms related to the disease during treatment [
30].
The results of this study led to the approval by regulatory agencies (FDA and EMA) of the combination of nivolumab plus ipilimumab as first-line treatment in unresectable MPM.
3.3. COMBINATION OF IMMUNOTHERAPY WITH CHEMOTHERAPY
Prior to the publication of the CheckMate-743 study, the results of two phase II studies that analyzed the effectiveness of the combination of immunotherapy with durvalumab added to the first line of chemotherapy were presented [
31,
32] (
Table 3). The DREAM study included 54 patients who received durvalumab in combination with cisplatin and pemetrexed for a total of 6 cycles followed by durvalumab until completion of one year or progression [
31]. The trial exceeded its prespecified objective with a 6-month PFS rate of 57%. Median PFS was 7 months and OS was 18.4 months. In a post-hoc analysis, responses were observed in all histological types and no significant association was detected between PD-L1 expression and PFS. The same treatment scheme was used in the PrE0505 study finding a response rate of 56.4% and a PFS of 6.7 months [
32]. The median OS of 20.4 months was significantly longer than historical control Regarding analysis by histology, patients with epithelioid tumors had a higher response rate than patients with non-epithelioid tumors (65.9% versus 28.6%, P = 0 ,03). Based on these results, a randomized phase III study (DREAM3R) has been completed to compare whether there are survival differences with the addition of durvalumab to standard platinum treatment, the results of which are pending.
The third study of combination chemotherapy plus immunotherapy in the first line is the phase 2 JME-01 study [
33]. This is a single-arm treatment study combining cisplatin with pemetrexed and nivolumab for 4-6 cycles, followed by a maintenance phase with nivolumab until progression or unacceptable toxicity. The objective response rate was 77%, including responses in all histologies, with a duration of response of 6.7 months, PFS was 8 months and OS was 20.8 months.
During the year 2023, the results of the first phase III study of chemotherapy with immunotherapy in the first line were presented [
34]. The IND.227 study is a phase 2-3 study that evaluates the addition of pembrolizumab to chemotherapy. In the phase II the trial did not demonstrated differences in PFS (6.7 and 6.8 months) but it did in OS (19.8 versus 8.9 months for combination and chemotherapy respectively) [
35]. Despite not meeting the primary endpoint of PFS, numerically superior survival data for the combination with pembrolizumab led to completion of enrollment. In the phase III, 440 patients were included and stratified by histology. The study demonstrated improved OS for the chemo-immunotherapy combination with a median OS of 17.3 months versus 16.1 months in favor of the pembrolizumab group. Three-year survival was 25% vs 17% for immunotherapy. However, as in the CheckMate-743 study, no differences were observed in PFS (7.1 months in both treatment groups) and a greater survival benefit was observed in patients with non-epithelioid histology (OS 12.3 versus 8.1 months in favor of pembrolizumab in non-epithelioid tumors and 19.8 vs 18.2 months in epithelioid tumors). No significant differences were observed according to the level of PD-L1 expression.
4. PREDICTIVE FACTORS OF RESPONSE TO IMMUNOTHERAPY
The identification of biomarkers that allow us to know which patients with MPM may respond to immunotherapy could improve the cost-benefit ratio and potentially facilitate the rational development of novel combination strategies to overcome primary resistance.
4.1. PD-L1
The predictive role of PD-L1 for immunotherapy response in MPM is unclear. Early phase I and II studies have found conflicting results regarding a higher response rate in PD-L1 positive tumors treated with immunotherapy compared to negative ones. However, in the phase 2 KEYNOTE-158 study and in the phase III PROMISE study, no differences were demonstrated in PD-L1 expressing patients compared to PD-L1 negative patients [
20,
36].
Regarding nivolumab, four trials have shown contradictory results. In the NivoMes trial, no differences in response or survival were demonstrated when stratifying patients by PD-L1 status [
21]. In contrast, in the Japanese MERIT trial, which tested nivolumab in a similar pretreated population, an interesting (not statistically significant) trend was reported in favor of PD-L1 positivity compared to PD-L1 negativity in terms of response rate, PFS and OS [
22]. Finally, in the randomized phase 3 CONFIRM study, PD-L1 expression ≥1% was not related to survival [
23].
Results from the combination of nivolumab with ipilimumab have shown a modest association between PD-L1 protein expression and outcomes. In the INITIATE trial, a post-hoc analysis of 12-week response and duration of response by PD-L1 status suggested greater benefit in PD-L1-positive compared to negative tumors [
28]. The MAPS2 trial also reported an advantage in terms of response rate, but not in terms of duration of response at 12 weeks for patients with PD-L1-positive tumors [
27]. In the CheckMate-743 trial, in which patients were not stratified by PD-L1, PD-L1 positivity appeared to predict better outcomes with nivolumab plus ipilimumab over chemotherapy [
3]. Patients with tumors expressing PD-L1 < 1% had an OS of 17.3 months vs. 18 months for PD-L1 ≥ 1% patients.
When checkpoint inhibitors are combined with chemotherapy, the predictive value of PD-L1 for response to treatment is even lower. Neither the DREAM trial nor IND227 found any association with OS or PFS with PD-L1 >1% [
31].
Finally, in a meta-analysis of 29 publications PD-L1 positive patients who did not receive immunotherapy appeared to have a worse prognosis compared to PD-L1 negative patients [
38]. In contrast, among patients who received immunotherapy, similar or better survival was observed in PD-L1 positive individuals. These findings suggest that the initial prognosis of patients with PD-L1-positive tumors may be worse than that of patients with negative tumors and that PD-L1-targeted therapies may improve survival.
4.2. HISTOLOGY
In the CheckMate-743 study, a difference in efficacy according to histology was reported [
3]. In the study, patients were stratified by histology and 76% of the included patients had epithelioid histology. Immunotherapy showed greater benefit over chemotherapy in non-epithelioid than epithelioid histology with a median OS of 18.1 months in the immunotherapy arm and 8.8 months in the chemotherapy arm (HR 0.46, 95% CI: 0 .31–0.68) for non-epithelioid histology compared with 18.7 months and 16.5 months for epithelioid histology (HR 0.86, 95% CI 0.69–1.08), respectively. The difference in the benefit of immunotherapy by histology seems to be mainly related to a lower efficacy of chemotherapy in the non-epithelioid subtype since the median OS with immunotherapy was the same in all histology (18 months). It should be considered that the trial was not specifically designed to identify a difference according to histological subtype.
Identical results were found in the IND227 study that evaluated the addition of pembrolizumab to first-line chemotherapy [
34]. In this study, patients were stratified by histology and 78% of patients had epithelioid histology. As in the checkmate 743 study, the difference in survival was greater in patients with non-epithelioid histology who achieved an OS of 12.3 with pembrolizumab added to chemotherapy versus 8.2 months with chemotherapy. Patients with epithelioid histology had an OS of 19.8 with chemo-immunotherapy versus 18.2 months with chemotherapy alone. Despite the greater difference in survival for patients with non-epithelioid tumors, in this study, unlike in CheckMate-743, the magnitude of the overall effect of immunotherapy was greater for patients with epithelioid histology who showed an OS of 19.8 months vs. 12.3 months for patients with non-epithelioid tumors.
Initial second-line and subsequent immunotherapy studies that evaluated the efficacy of treatment by histology reported greater efficacy of immunotherapy in non-epithelioid tumors in the trials with nivolumab (NivoMes and MERIT) and durvalumab plus tremelimumab (MESO-TREM) [
18,
19,
20,
21,
22,
23]. However, in second-line studies with a larger number of patients, greater efficacy for immunotherapy in patients with non-epithelioid histology has not been demonstrated. The CONFIRM trial reported a significant improvement for PFS and OS with nivolumab in the epithelioid group [
23]. Similarly, in the PROMISE-meso trial with pembrolizumab patients with tumors with non-epithelioid histology showed worse PFS and OS, although these data were not statistically significant, probably due to the limited sample size [
20].
4.3. TMB
TMB is considered a potential predictive biomarker of response to immunotherapy in some tumors such as lung cancer and melanoma [
39,
40]. In KEYNOTE-158, a response rate advantage was found in the high TMB group [
36]. However, considering the mesothelioma cohort, in 85 evaluable cases, only 1 patient had high TMB and a response was reported in 9 of 84 patients with low TMB. The same tissue TMB score was observed in both responders and non-responders to pembrolizumab (1.26 mutations per megabase). An exploratory analysis regarding TMB was also performed in the Checkmate-743 trial [
15]. TMB assessment was feasible in 53% of patients treated with nivolumab plus ipilimumab and in 45% of patients treated with the chemotherapy arm, with a low mean TMB value (1.75 mut/Mb). In this analysis, higher mutational burden also did not correlate with better survival in the immunotherapy or chemotherapy arm.
4.4. CRHOMOSOMICS REARRENGEMENTS
Chromothripsis has been associated with a worse prognosis in patients with MPM [
41,
42]. However, this structural chromosomal variant is associated with the potential formation of neoantigens that facilitate intratumoral expansion of T cell clones, suggesting that chromothripsis could play a role in the response to immunotherapy. In a retrospective study that explored the correlation between chromosomal rearrangements and survival in patients with MPM who received nivolumab or ipilimumab in combination with nivolumab, it was detected that rearrangements were not predictors of efficacy, but genetic signatures associated with presentation and antigen processing predicted an OS difference of more than 1.5 years [
43]. Further work will be required to demonstrate whether these chromosomal rearrangements may present an opportunity for a biomarker of response to immune checkpoint inhibitors.
4.5. GENOMIC MARKERS
The most in-depth analysis to date of genomic and phenotypic factors correlated with immunotherapy outcome was performed in patients from the PrE0505 study treated with chemotherapy plus durvalumab [
32]. Chromosomal instability was identified to occur more frequently in epithelioid MPM with an OS of more than 12 months. The authors also demonstrated that a higher burden of immunogenic mutations in major histocompatibility complex (MHC) class I and MHC class II was significantly associated with a better response to durvalumab plus chemotherapy (p = 0.064 and p = 0.023, respectively), especially in the epithelioid. Furthermore, better survival was observed in patients with high variability in T cell receptor (TCR) clonality and worse survival in the APOBEC signature. They demonstrated that greater divergence of the human leukocyte antigen (HLA)-B locus was related to a better radiological response to chemo-immunotherapy, particularly in epithelioid MPM. Despite the limitations of being a small cohort study, this analysis has revealed a subset of genomic and immunological characteristics that could predict outcomes after chemo-immunotherapy in MPM.
BAP1 is a tumor suppressor gene that represents the most commonly mutated gene in MPM, especially in epithelioid tumors [
44]. The PrE0505 trial with durvalumab plus chemotherapy showed that germline mutations in
BAP1 were associated with significantly prolonged survival after chemo-immunotherapy [
32].
The second most common somatic mutation in MPM patients is the 9p21 deletion, which contains
CDK2N2A. A pan-cancer analysis of data from The Cancer Genome Atlas (TCGA) with eight immunotherapy trials, which did not include MPM patients, showed that loss of 9p21 is associated with a “cold” tumor microenvironment [
45]. Considering that almost 50% of TCGA samples in the MPM cohort present loss of 9p21, this mechanism represents an important explanation for immunotherapy resistance in MPM [
46].
The Checkmate-743 study showed that a high four-gene inflammatory signature score (
CD8A,
STAT1,
LAG3, and
CD274) was associated with improved OS in the nivolumab plus ipilimumab arm (21.8 versus 16.8 months in patients with low score) [
29]. In the chemotherapy arm, no correlation was identified between the inflammatory gene signature score and response to treatment. The inflammatory signature score could, therefore, be considered a positive predictive biomarker of response to immunotherapy.
4.6. TIME
It has been suggested that the type of TIME may affect the outcome of immunotherapy. In MPM a comprehensive immunoproteogenomic analysis defined two disparate TIMEs, TIME-I is characterized by a greater number of PD-1+ CTLA-4+ CD8+ T cells, while the TIME-II subtype contains more Tregs and naïve CD8+ T cells. TIME-I was reported to be associated with a better response to immunotherapy [
47]. In another study, TIME was classified according to NanoString analysis into group 1 with poor gene expression associated with the immune system; group 2 with moderate T cell effector gene expression and a high level of B cell gene expression; and group 3 which had high expression of PD-L1 and T cell effector gene [
48]. These comprehensive studies indicate the potential predictive value of TIME for MPM immunotherapy, but this hypothesis has not been verified in clinical trials with immunotherapy.
Preliminary data presented in the DREAM study have indicated that CD8 density within tumor biopsies does not predict response, but PFS and OS were significantly better when CD8 was quantified only within epithelial areas [
32].
5. NEW IMMUNOTHERAPY DRUGS UNDER INVESTIGATION
5.1. THERAPIES BASED ON MESOTHELIN
Mesothelin is a glycoprotein that is expressed in many solid tumors including MPM. Different drugs targeting mesothelin inhibition have been explored in previously treated MPM patients.
CRS-207 is a live, attenuated, non-virulent Listeria Monocytogenes that encodes human mesothelin. A phase Ib clinical trial found complete responses in 3% of patients and partial responses in 54% of patients [
49]. However, a subsequent phase II study did not show clinical activity of the combination of CRS-207 with PD-1 inhibition in the interim analysis, so clinical development of this therapy was discontinued.
The study with the largest number of patients with a mesothelin inhibitor published to date did not demonstrate efficacy against chemotherapy [
50]. Anetumab ravtansine, an anti-mesothelin antibody conjugate linked to the tubulin inhibitor DM4, was evaluated in a phase II trial and patients were randomized to receive anetumab ravtansine or vinorelbine. No differences were found in PFS or OS (4.3 versus 4.5 months and 10.1 versus 11.6 months for anetumab ravtansine and vinorelbine, respectively).
5.2. CHECKPOINT INHIBITORS
In preclinical models the combination of anti-LAG-3 and anti-PD1 reduced tumor size and provided a survival benefit and a recent phase I study with tebotelimab found a partial response in a patient with MPM [
51,
52]. There is currently an ongoing clinical trial with Ieramilimab in combination with the anti-PD1 PDR001 in patients with advanced tumors including a cohort of patients with MPM.
VISTA is an immune checkpoint gene that has strong expression in epithelioid MPM, above the levels of other solid tumors [
5,
53,
54]. A phase I clinical trial with CA-170, a small molecule inhibitor of VISTA, which included a cohort of 12 patients with MPM did not show partial or complete responses in any of the patients. However, to date, VISTA has not been investigated as a potential predictive biomarker of response to immunotherapy.
Due to the high expression of B7-H3 in patients with MPM, it has been proposed as a potential therapeutic target [
55]. In a series of 44 MPM samples, B7-H3 was found to be expressed in 41 of the 44 patients. In another study considering histology, it was confirmed that B7-H3 is expressed in almost all patients (90% of epithelioid tumors and 89% of non-epithelioid tumors) [
56]. Regarding its prognostic value, the TCGA analysis showed that B7-H3 expression was associated with worse survival.
Several studies have shown that TIM-3 expression was high in tumor-infiltrating lymphocytes (TILs) in different tumors, including MPM [
57]. A study conducted in 54 patients with MPM found that TIM-3 expression occurs in up to 40% of patients and correlates with PD-L1 [
58]. A phase I study is currently underway including MPM patients treated with the monoclonal antibody INCAGN02390 targeting TIM-3.
5.3. VACCINES
Dendritic cell (DC) therapy aims to induce the proliferation of T cells and promote the activation of CD4+ and CD8+T-cells by presenting them with tumor antigens, allowing CD8+T-cells to infiltrate the tumor microenvironment [
59]. In mesothelioma, clinical studies with dendritic cells have shown notable antitumor activity in two studies with a small number of patients, with a survival of up to 24 months in some patients [
60,
61]. To validate these promising results, a European randomized phase II/III trial (DENIM study) evaluating dendritic cell immunotherapy as maintenance treatment after standard first-line chemotherapy is underway.
Other immunotherapeutic strategies based on the local delivery of cytokines through genetically modified viruses have been evaluated. Adenovirus-delivered interferon alfa-2b (Ad-IFN) is a replication-defective adenoviral vector containing the human interferon-alpha2b gene. Intrapleural administration of Ad-IFN in combination with celecoxib and chemotherapy was evaluated in a cohort of 40 patients, achieving a response rate of 25% and survival of 21.5 months [
62]. Following these promising results, the phase III INFINITE trial was launched, which evaluates the efficacy of intrapleural Ad-IFN in combination with celecoxib and gemcitabine in the second or third line context and the results are expected in 2024. ONCOS -102 is a GM-CSF oncolytic adenovirus that demonstrated some activity in a phase II study in combination with cisplatin and pemetrexed [
63].
In the second line of treatment, the NIPU study evaluated the addition of the UV1 anti-telomerase vaccine in combination with ipilimumab and nivolumab [
64]. The study did not demonstrate improvement in the primary endpoint of PFS for patients who received the UV1 vaccine but did find an improvement in survival (15.4 months vs. 11.1 months for patients who received UV1 vs. those who did not).
5.4. CAR-T
CAR-Ts were investigated in two phase I clinical trials that showed moderate clinical responses [
65,
66]. A third phase I clinical study was designed to investigate the safety and feasibility of CART-mesothelin cells administered intravenously or intrapleurally with or without cyclophosphamide in solid tumors, including epithelioid MPM. The study is ongoing and data is not yet available. Finally, the intrapleural administration of CAR-T targeting mesothelin followed by the administration of pembrolizumab has been explored, finding median survival of 23.9 months in previously treated patients [
67]. Although the response rate found was lower than expected (2 partial responses out of 16 treated patients), these responses were maintained for more than six months.
6. DISCUSSION
MPM is characterized as an aggressive tumor, related to exposure to asbestos and with a decreasing incidence in recent years in developed countries. Genomic alterations in MPM are not as frequent as in other tumors, being dominated by the loss of suppressor genes that lack a therapeutic target [
4]. However, the chronic inflammatory response generated by asbestos generates a tumor microenvironment equivalent to that of an immunologically active tumor.
For two decades the only treatment available for patients with advanced MPM has been chemotherapy with cisplatin and pemetrexed which achieved a median survival of 12 months [
2]. For patients who progress to platinum-pemetrexed combination treatment there is no other approved chemotherapy option. However, in recent years there have been significant improvements in the knowledge of the biology of mesothelioma. The greatest advance has been made in the field of immunotherapy which has improved the survival of patients with different types of cancer and now also in mesothelioma. The combination of two immunotherapy agents with nivolumab plus ipilimumab in the CheckMate-743 study has demonstrated an increase in survival positioning the immunotherapy combination as a valid alternative to first-line chemotherapy for patients with MPM [
3]. In this study, the magnitude of the benefit of immunotherapy was greater for patients with PD-L1 expression greater than 1% and for patients with non-epithelioid histology.
Another first-line treatment option is the combination of chemotherapy with immunotherapy. To date, two single-arm phase II studies have reported an interesting benefit from the combination of durvalumab with chemotherapy (DREAM and PRE0505) while the results of the randomized phase III study are pending [
31,
32]. In 2023, the results of the first randomized trial that evaluates the role of chemo-immunotherapy versus chemotherapy in the first line of treatment were presented [
34]. The IND227 study showed that the combination treatment achieved a survival of 17.3 months compared to 16.1 months with chemotherapy. In this study, as in CheckMate-743, better results were found for patients with non-epithelioid histology due to the lower efficacy of chemotherapy in non-epithelioid tumors, but in this case the overall benefit of immunotherapy was better for patients with epithelioid tumors (OS of 19.8 months vs. 12.3 months for patients with epithelioid and non-epithelioid tumors treated with pembrolizumab). In this trial no significant differences were found according to the level of PD-L1 expression. Currently, another phase III trial with chemo-immunotherapy (BEAT-MESO) has completed its recruitment and is awaiting results.
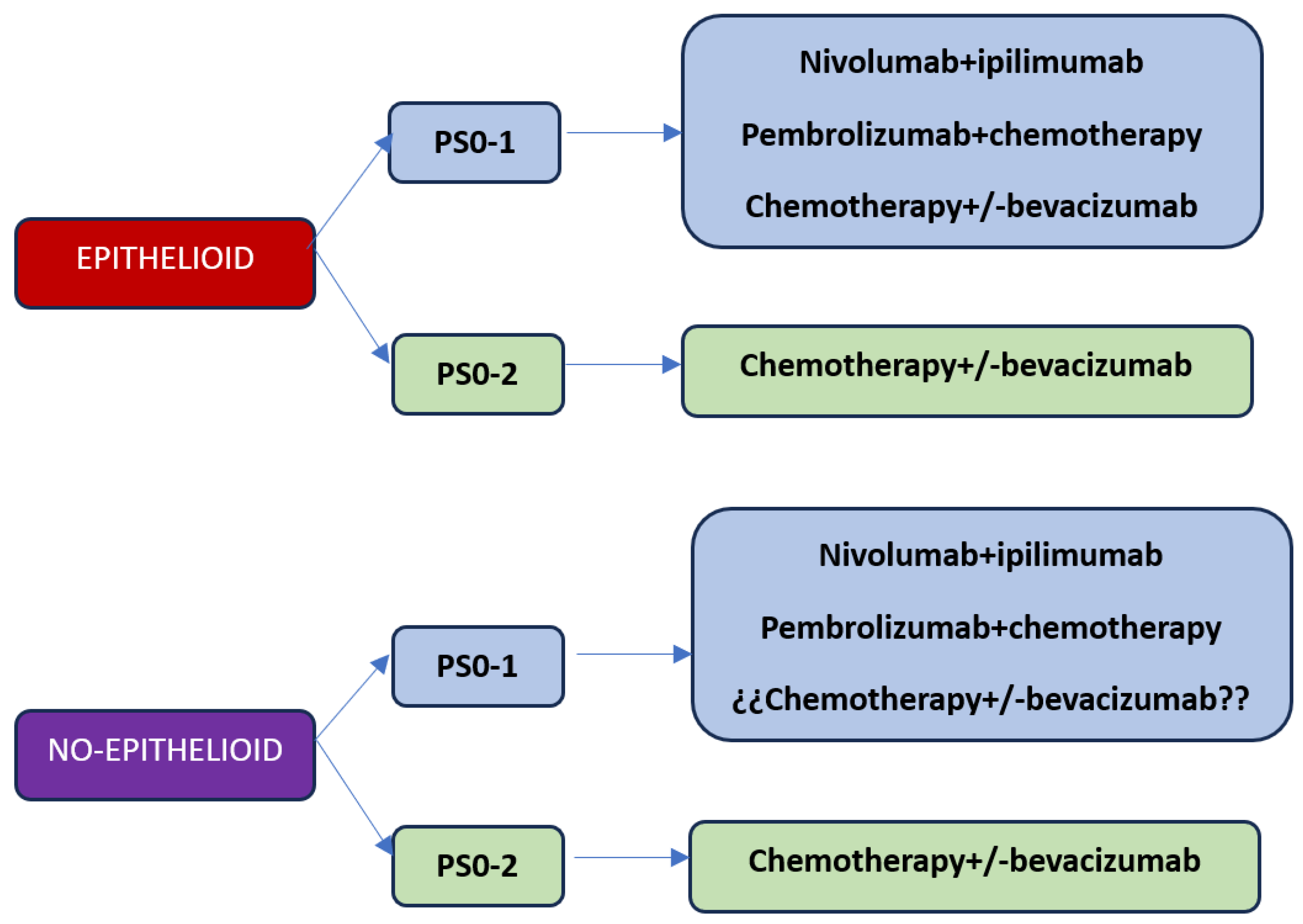
With the current results of the first-line studies, we are faced with three treatment options: chemotherapy, combination of immunotherapy with nivolumab plus ipilimumab or chemotherapy plus immunotherapy (
Figure 1). Based on what has been reported in immunotherapy studies, the results are superior with chemotherapy, so the option of first-line chemotherapy should be reserved for patients with contraindications to receiving immunotherapy treatment or patients with performance status 2. Regarding the best choice of first-line treatment considering the options of nivolumab plus ipilimumab versus chemo-immunotherapy, at the moment there is no study that answers this question. On the one hand, the combination of chemotherapy plus immunotherapy releases neoantigens that could potentially make a better response with immunotherapy, but on the other hand the addition of chemotherapy significantly increases toxicity. The EVOLVE clinical trial exploring the combination of anti-CTLA4 therapy with anti-PD-1 plus chemotherapy versus chemotherapy alone or the combination of nivolumab plus ipilimumab could answer whether chemo-immunotherapy is superior to the immunotherapy doublet, although the study will use double CTLA-4 and PD-1 blockade as a combination to chemotherapy.
The identification of predictive biomarkers of response to immunotherapy that can help us select patients with the greatest possibility of benefiting from treatment is an unmet need. Compared with other cancers, biomarker research has made limited progress. Although the predictive role of PD-L1 is well established in different tumors, its role in MPM is more controversial.
Histology has been postulated as a potential predictor of response to immunotherapy. In the Chekmate-743 trial and in the IND227, greater benefit of immunotherapy was reported in patients with non-epithelioid histology [
15,
34]. It should be noted that in these studies the magnitude of the benefit with immunotherapy was similar in both histologies in the nivolumab plus ipilimumab treatment, but the efficacy of pembrolizumab was clearly lower in non-epithelioid than in epithelioid tumors. The randomized CONFIRM study with nivolumab demonstrated opposite results with better survival in patients with epithelioid histology [
23]. Analysis of future randomized immunotherapy trials will be necessary to discern the possible predictive role of histology.
Regarding other biomarkers, the TMB in patients with MPM is low and so far, no greater efficacy has been demonstrated with immunotherapy in patients with a higher TMB. Promising results were found with the inflammatory gene signature in the Checkmate-743 study [
15].
In conclusion, fortunately the growing research in the field of MPM has culminated in the approval of new therapies that improve patient survival. However, new combinations of immunotherapy with chemotherapy could further improve patient outcomes. Although MPM is a disease with a globally decreasing incidence, there is still a long way to go to optimize the first line of treatment and approve therapies after the progression of immunotherapy.
Author Contributions
Conception and design: all authors; Administrative support: SC, PG; Provision of study materials or patients: all authors; Collection and assembly of data: all authors; Data analysis and interpretation: SC. Manuscript writing: all authors; Final approval of manuscript: all authors.
Acknowledgments
this manuscript has been carried out as a final master’s project in cancer immunotherapy carried out by the University of Alcalá under the direction of doctors Melchor Alvarez de Mon, Agusto Silva, Jorge Monserrat, Angel Asunsolo, Ignacio Duran, Juan Arevalo and Pilar Garrido.
References
- Peto, J.; Decarli, A.; La Vecchia, C.; Levi, F.; Negri, E. The European mesothelioma epidemic. Br J Cancer 1999, 79, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; Niyikiza, C.; Paoletti, P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003, 21, 2636–2644 Corrected and republished in: J Clin Oncol 2023 Apr 20;41, 2125. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; Oulkhouir, Y.; Bautista, Y.; Cornelissen, R.; Greillier, L.; Grossi, F.; Kowalski, D.; Rodríguez-Cid, J.; Aanur, P.; Oukessou, A.; Baudelet, C.; Zalcman, G. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Bueno, R.; Stawiski, E.; Goldstein, L.D.; et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet 2016, 48, 407–416. [Google Scholar] [CrossRef]
- Hmeljak, J.S.-V.F.; Hoadley, K.A.; Shih, J.; et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov 2018, 8, 1548–1565. [Google Scholar] [CrossRef]
- Ujiie, H.; Kadota, K.; Nitadori, J.I.; Aerts, J.G.; Woo, K.M.; Sima, C.S.; Travis, W.D.; Jones, D.R.; Krug, L.M.; Adusumilli, P.S. The tumoral and stromal immune microenvironment in malignant pleural mesothelioma: A comprehensive analysis reveals prognostic immune markers. Oncoimmunology 2015, 4, e1009285. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.; et al. The immune microenvironment, genome-wide copy number aberrations, and survival in mesothelioma. J. Thorac. Oncol. 2017, 12, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.S.; Roden, A.C.; Peikert, T.; Sheinin, Y.M.; Harrington, S.M.; Krco, C.J.; Dong, H.; Kwon, E.D. B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol. 2014, 9, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Cedrés, S.; Ponce-Aix, S.; Zugazagoitia, J.; Sansano, I.; Enguita, A.; Navarro-Mendivil, A.; Martinez-Marti, A.; Martinez, P.; Felip, E. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). PLoS One 2015, 10, e0121071. [Google Scholar] [CrossRef]
- Brosseau, S.; Danel, C.; Scherpereel, A.; Mazières, J.; Lantuejoul, S.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Gounant, V.; Antoine, M.; et al. Shorter survival in malignant pleural mesothelioma patients with high PD-L1 expression associated with sarcomatoid or biphasic histology subtype: a series of 214 cases from the Bio-MAPS cohort. Clin Lung Cancer 2019, 20, e564–e575. [Google Scholar] [CrossRef]
- Pasello, G.; Zago, G.; Lunardi, F.; Urso, L.; Kern, I.; Vlacic, G.; Grosso, F.; Mencoboni, M.; Ceresoli, G.L.; Schiavon, M.; et al. Malignant pleural mesothelioma immune microenvironment and checkpoint expression: correlation with clinical-pathological features and intratumor heterogeneity over time. Ann Oncol. 2018, 29, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Treasure, T.; Lang-Lazdunski, L.; Waller, D.; Bliss, J.M.; Tan, C.; Entwisle, J.; Snee, M.; O’Brien, M.; Thomas, G.; Senan, S.; O’Byrne, K.; Kilburn, L.S.; Spicer, J.; Landau, D.; Edwards, J.; Coombes, G.; Darlison, L.; Peto, J.; MARS trialists. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011, 12, 763–772. [Google Scholar] [CrossRef]
- Lim, E. MARS2, a multicentre randomized trial comparing (extended) pleurectomy decortication versus no (extended) decortication for patients with malignant pleural mesothelioma. Plenary Sesion. World Conference Lung Cancer 2023.
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; Rivière, F.; Janicot, H.; Gervais, R.; Locher, C.; Milleron, B.; Tran, Q.; Lebitasy, M.P.; Morin, F.; Creveuil, C.; Parienti, J.J.; Scherpereel, A.; French Cooperative Thoracic Intergroup (IFCT). Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Ceresoli, G.L.; Aerts, J.G.; Dziadziuszko, R.; Ramlau, R.; Cedres, S.; van Meerbeeck, J.P.; Mencoboni, M.; Planchard, D.; Chella, A.; Crinò, L.; Krzakowski, M.; Rüssel, J.; Maconi, A.; Gianoncelli, L.; Grosso, F. Tumour Treating Fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (STELLAR): a multicentre, single-arm phase 2 trial. Lancet Oncol. 2019, 20, 1702–1709. [Google Scholar] [CrossRef]
- Kindler, H.L.; Ismaila, N.; Armato, S.G., 3rd; Bueno, R.; Hesdorffer, M.; Jahan, T.; Jones, C.M.; Miettinen, M.; Pass, H.; Rimner, A.; Rusch, V.; Sterman, D.; Thomas, A.; Hassan, R. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018, 36, 1343–1373. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Zucali, P.A.; Pagano, M.; Grosso, F.; Pasello, G.; Garassino, M.C.; Tiseo, M.; Soto Parra, H.; Grossi, F.; Cappuzzo, F.; de Marinis, F.; Pedrazzoli, P.; Bonomi, M.; Gianoncelli, L.; Perrino, M.; Santoro, A.; Zanelli, F.; Bonelli, C.; Maconi, A.; Frega, S.; Gervasi, E.; Boni, L.; Ceresoli, G.L. Gemcitabine with or without ramucirumab as second-line treatment for malignant pleural mesothelioma (RAMES): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2021, 22, 1438–1447. [Google Scholar] [CrossRef]
- Maio, M.; Scherpereel, A.; Calabrò, L.; Aerts, J.; Perez, S.C.; Bearz, A.; Nackaerts, K.; Fennell, D.A.; Kowalski, D.; Tsao, A.S.; Taylor, P.; Grosso, F.; Antonia, S.J.; Nowak, A.K.; Taboada, M.; Puglisi, M.; Stockman, P.K.; Kindler, H.L. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017, 18, 1261–1273. [Google Scholar] [CrossRef]
- Alley, E.W.; Lopez, J.; Santoro, A.; Morosky, A.; Saraf, S.; Piperdi, B.; van Brummelen, E. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017, 18, 623–630. [Google Scholar] [CrossRef]
- Popat, S.; Curioni-Fontecedro, A.; Dafni, U.; Shah, R.; O’Brien, M.; Pope, A.; Fisher, P.; Spicer, J.; Roy, A.; Gilligan, D.; Gautschi, O.; Nadal, E.; Janthur, W.D.; López Castro, R.; García Campelo, R.; Rusakiewicz, S.; Letovanec, I.; Polydoropoulou, V.; Roschitzki-Voser, H.; Ruepp, B.; Gasca-Ruchti, A.; Peters, S.; Stahel, R.A. A multicentre randomised phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: the European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Ann Oncol. 2020, 31, 1734–1745. [Google Scholar] [CrossRef]
- Quispel-Janssen, J.; van der Noort, V.; de Vries, J.F.; Zimmerman, M.; Lalezari, F.; Thunnissen, E.; Monkhorst, K.; Schouten, R.; Schunselaar, L.; Disselhorst, M.; Klomp, H.; Hartemink, K.; Burgers, S.; Buikhuisen, W.; Baas, P. Programmed Death 1 Blockade With Nivolumab in Patients With Recurrent Malignant Pleural Mesothelioma. J Thorac Oncol. 2018, 13, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kijima, T.; Aoe, K.; et al. Clinical efficacy and safety of nivolumab: results of a multicenter, open-label, single-arm, Japanese phase II study in malignant pleural mesothelioma (MERIT). Clin Cancer Res. 2019, 25, 5485–5492. [Google Scholar] [CrossRef] [PubMed]
- Fennell, D.A.; Ewings, S.; Ottensmeier, C.; Califano, R.; Hanna, G.G.; Hill, K.; Danson, S.; Steele, N.; Nye, M.; Johnson, L.; Lord, J.; Middleton, C.; Szlosarek, P.; Chan, S.; Gaba, A.; Darlison, L.; Wells-Jordan, P.; Richards, C.; Poile, C.; Lester, J.F.; Griffiths, G.; CONFIRM trial investigators. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1530–1540. [Google Scholar] [CrossRef]
- Hassan, R.; Thomas, A.; Nemunaitis, J.J.; et al. Efficacy and safety of avelumab treatment in patients with advanced unresectable mesothelioma: phase 1b results from the JAVELIN Solid Tumor Trial. JAMA Oncol. 2019, 5, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, L.; Morra, A.; Giannarelli, D.; et al. Tremelimumab combined with durvalumab in patients with mesothelioma (NIBIT-MESO-1): an open-label, non-randomised, phase 2 study. Lancet Respir Med. 2018, 6, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, L.; Rossi, G.; Morra, A.; Rosati, C.; Cutaia, O.; Daffinà, M.G.; Altomonte, M.; Di Giacomo, A.M.; Casula, M.; Fazio, C.; Palmieri, G.; Giannarelli, D.; Covre, A.; Maio, M. Tremelimumab plus durvalumab retreatment and 4-year outcomes in patients with mesothelioma: a follow-up of the open label, non-randomised, phase 2 NIBIT-MESO-1 study. Lancet Respir Med. 2021, 9, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Scherpereel, A.; Mazieres, J.; Greillier, L.; et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019, 20, 239–253. [Google Scholar] [CrossRef]
- Disselhorst, M.J.; Quispel-Janssen, J.; Lalezari, F.; et al. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single-arm, phase 2 trial. Lancet Respir Med. 2019, 7, 260–270. [Google Scholar] [CrossRef]
- Peters, S.; Scherpereel, A.; Cornelissen, R.; Oulkhouir, Y.; Greillier, L.; Kaplan, M.A.; Talbot, T.; Monnet, I.; Hiret, S.; Baas, P.; Nowak, A.K.; Fujimoto, N.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Zhang, X.; Hu, N.; Balli, D.; Spires, T.; Zalcman, G. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol. 2022, 33, 488–499. [Google Scholar] [CrossRef]
- Scherpereel, A.; Antonia, S.; Bautista, Y.; Grossi, F.; Kowalski, D.; Zalcman, G.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Sun, X.; Lawrance, R.; Zhang, X.; Daumont, M.J.; Bennett, B.; McKenna, M.; Baas, P. First-line nivolumab plus ipilimumab versus chemotherapy for the treatment of unresectable malignant pleural mesothelioma: patient-reported outcomes in CheckMate 743. Lung Cancer 2022, 167, 8–16. [Google Scholar] [CrossRef]
- Nowak, A.K.; Lesterhuis, W.J.; Kok, P.-S.; et al. Durvalumab with first-line chemotherapy in previously untreated malignant pleural mesothelioma (DREAM): a multicentre, single-arm, phase 2 trial with a safety run-in. Lancet Oncol 2020, 21, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Anagnostou, V.; Sun, Z.; et al. Durvalumab with platinumpemetrexed for unresectable pleural mesothelioma: survival, genomic and immunologic analyses from the phase 2 PrE0505 trial. Nat Med 2021, 27, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Kozuki, T.; Aoe, K.; Wada, S.; Harada, D.; Yoshida, M.; Sakurai, J.; Hotta, K.; Fujimoto, N. JME-001 phase II trial of first-line combination chemotherapy with cisplatin, pemetrexed, and nivolumab for unresectable malignant pleural mesothelioma. J Immunother Cancer 2021, 9, e003288. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Perrone, F.; Greillier, L.; Tu, W.; Piccirillo, M.C.; Grosso, F.; Lo Russo, G.; Florescu, M.; Mencoboni, M.; Morabito, A.; Cecere, F.L.; Ceresoli, G.L.; Dawe, D.E.; Zucali, P.A.; Pagano, M.; Goffin, J.R.; Sanchez, M.L.; Gridelli, C.; Zalcman, G.; Quantin, X.; Westeel, V.; Gargiulo, P.; Delfanti, S.; Tu, D.; Lee, C.W.; Leighl, N.; Sederias, J.; Brown-Walker, P.; Luo, Y.; Lantuejoul, S.; Tsao, M.S.; Scherpereel, A.; Bradbury, P.; Laurie, S.A.; Seymour, L. Pembrolizumab plus chemotherapy versus chemotherapy in untreated advanced pleural mesothelioma in Canada, Italy, and France: a phase 3, open-label, randomised controlled trial. Lancet. Lancet 2023, S0140-6736(23)01613-6. [Google Scholar] [CrossRef]
- Piccirillo, M.C.; Chu, Q.; Bradbury, P.; Tu, W.; Coschi, C.H.; Grosso, F.; Florescu, M.; Mencoboni, M.; Goffin, J.R.; Pagano, M.; Ciardiello, F.; Cecere, F.L.; Vincent, M.; Ferrara, R.; Dawe, D.E.; Hao, D.; Lee, C.W.; Morabito, A.; Gridelli, C.; Cavanna, L.; Iqbal, M.; Blais, N.; Leighl, N.B.; Wheatley-Price, P.; Tsao, M.S.; Ugo, F.; El-Osta, H.; Gargiulo, P.; Gaudreau, P.O.; Tu, D.; Sederias, J.; Brown-Walker, P.; Perrone, F.; Seymour, L.; Laurie, S.A. Brief Report: Canadian Cancer Trials Group IND.227: A Phase 2 Randomized Study of Pembrolizumab in Patients With Advanced Malignant Pleural Mesothelioma (NCT02784171). J Thorac Oncol. 2023, S1556-0864(23)00128-4. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Nakagawa, K.; Fujimoto, N.; Kuribayashi, K.; Guren, T.K.; Calabrò, L.; et al. Efficacy and safety of pembrolizumab in patients with advanced mesothelioma in the open-label, single-arm, phase 2 KEYNOTE-158 study. Lancet Respir Med 2021, 9, 613–621. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Brown, R.J.; Sammon, C.; Daumont, M.J.; McKenna, M.; Sanzari, J.K.; Forde, P.M. The Predictive and Prognostic Nature of Programmed Death-Ligand 1 in Malignant Pleural Mesothelioma: A Systematic Literature Review. JTO Clin Res Rep. 2022, 3, 100315. [Google Scholar] [CrossRef]
- Gemelli, M.; Cortinovis, D.L.; Baggi, A.; di Mauro, P.; Calza, S.; Berruti, A.; Grisanti, S.; Rota, M. Immune Checkpoint Inhibitors in Malignant Pleural Mesothelioma: A Systematic Review and Meta-Analysis. Cancers (Basel) 2022, 14, 6063. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Frampton, G.M.; Rioth, M.J.; Yusko, E.; Xu, Y.; Guo, X.; et al. Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol Res 2016, 4, 959–967. [Google Scholar] [CrossRef]
- Nowak, A.K.; Chin, W.L.; Keam, S.; Cook, A. Immune checkpoint inhibitor therapy for malignant pleural mesothelioma. Lung Cancer 2021, 162, 162–168. [Google Scholar] [CrossRef]
- Hiltbrunner, S.; Mannarino, L.; Kirschner, M.B.; Opitz, I.; Rigutto, A.; Laure, A.; et al. Tumor immune microenvironment and genetic alterations in mesothelioma. Front Oncol 2021, 11, 660039. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.S.; Peikert, T.; Smadbeck, J.B.; Udell, J.B.M.; Garcia-Rivera, E.; Elsbernd, L.; et al. Neoantigenic Potential of Complex Chromosomal Rearrangements in Mesothelioma. J Thorac Oncol. 2019, 14, 276–287. [Google Scholar]
- Kosari, F.; et al. Tumor junction burden and antigen presentation as predictors of survival in mesothelioma treated with immune checkpoint inhibitors. J. Thorac. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Nastase, A.; Mandal, A.; Lu, S.K.; Anbunathan, H.; Morris-Rosendahl, D.; Zhang, Y.Z.; et al. Integrated genomics point to immune vulnerabilities in pleural mesothelioma. Sci Rep 2021, 11, 19138. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Siemers, N.O.; Pandya, D.; Chang, H.; Sanchez, T.; Harbison, C.; et al. Analyses of PD-L1 and inflammatory gene expression association with efficacy of nivolumab ± ipilimumab in gastric Cancer/Gastroesophageal junction cancer. Clin Cancer Res 2021, 27, 3926–3935. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]
- Patil, N.S.; Righi, L.; Koeppen, H.; et al. Molecular and histopathological characterization of the tumor immune microenvironment in advanced stage of malignant pleural mesothelioma. J Thorac Oncol. 2018, 13, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Creaney, J.; Patch, A.M.; Addala, V.; Sneddon, S.A.; Nones, K.; Dick, I.M.; et al. Comprehensive genomic and tumor immune profiling reveals potential therapeutic targets in malignant pleural mesothelioma. Genome Med 2022, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Alley, E.; Kindler, H.; Antonia, S.; Jahan, T.; Honarmand, S.; et al. Clinical response of live-attenuated, listeria monocytogenes expressing mesothelin (CRS-207) with chemotherapy in patients with malignant pleural mesothelioma. Clin Cancer Res. 2019, 25, 5787–5798. [Google Scholar] [CrossRef]
- Kindler, H.L.; Novello, S.; Bearz, A.; Ceresoli, G.L.; Aerts, J.G.J.V.; Spicer, J.; Taylor, P.; Nackaerts, K.; Greystoke, A.; Jennens, R.; Calabrò, L.; Burgers, J.A.; Santoro, A.; Cedrés, S.; Serwatowski, P.; Ponce, S.; Van Meerbeeck, J.P.; Nowak, A.K.; Blumenschein, G., Jr.; Siegel, J.M.; Kasten, L.; Köchert, K.; Walter, A.O.; Childs, B.H.; Elbi, C.; Hassan, R.; Fennell, D.A. Anetumab ravtansine versus vinorelbine in patients with relapsed, mesothelin-positive malignant pleural mesothelioma (ARCS-M): a randomised, open-label phase 2 trial. Lancet Oncol. 2022. [Google Scholar] [CrossRef]
- Marcq, E.; Waele, J.; Audenaerde, J.V.; Lion, E.; Santermans, E.; Hens, N.; Pauwels, P.; van Meerbeeck, J.P.; Smits, E.L.J. Abundant expression 485 of TIM-3, LAG-3, PD-1 and PD-L1 as immunotherapy checkpoint targets in effusions of mesothelioma patients. Oncotarget. 486 2017, 8, 89722–89735. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J. A phase I, frst-in-human, open-label, dose-escalation study of MGD013, a bispecifc DART molecule binding PD-1 and LAG-3, in patients with unresectable or metastatic neoplasms. In: 2020; ASCO Virtual Scientifc Program. American Society of Clinical Oncology.
- Chung, Y.S.; Kim, M.; Cha, Y.J.; Kim, K.A.; Shim, H.S. Expression of V-set immunoregulatory receptor in malignant mesothelioma. Mod Pathol. 2020, 33, 263–270. [Google Scholar] [CrossRef]
- Muller, S.; Victoria Lai, W.; Adusumilli, P.S.; Desmeules, P.; Frosina, D.; Jungbluth, A.; Ni, A.; Eguchi, T.; Travis, W.D.; Ladanyi, M.; et al. V-domain Ig-containing suppressor of T-cell activation (VISTA), a potentially targetable immune checkpoint molecule, is highly expressed in epithelioid malignant pleural mesothelioma. Mod Pathol. 2020, 33, 303–311. [Google Scholar] [CrossRef]
- Alcala, N.; Mangiante, L.; Le-Stang, N.; Gustafson, C.E.; Boyault, S.; Damiola, F.; Alcala, K.; Brevet, M.; Thivolet-Bejui, F.; Blanc-Fournier, C.; et al. Redefning malignant pleural mesothelioma types as a continuum uncovers immune-vascular interactions. EBioMedicine. 2019, 48, 191–202. [Google Scholar] [CrossRef]
- Matsumura, E.; Kajino, K.; Abe, M.; Ohtsuji, N.; Saeki, H.; Hlaing, M.T.; Hino, O. Expression status of PD-L1 and B7-H3 in mesothelioma. Pathol Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541. [Google Scholar] [CrossRef]
- Klampatsa, A.; O’Brien, S.M.; Thompson, J.C.; Rao, A.S.; Stadanlick, J.E.; Martinez, M.C.; Liousia, M.; Cantu, E.; Cengel, K.; Moon, E.K.; et al. Phenotypic and functional analysis of malignant mesothelioma tumor-infiltrating lymphocytes. Oncoimmunology 2019, 8, e1638211. [Google Scholar] [CrossRef]
- Belderbos, R.A.; Baas, P.; Berardi, R.; Cornelissen, R.; Fennell, D.A.; van Meerbeeck, J.P.; Scherpereel, A.; Vroman, H.; Aerts, J.G.J.V. A mul- 532 ticenter, randomized, phase II/III study of dendritic cells loaded with allogeneic tumor cell lysate (MesoPher) in subjects with 533 mesothelioma as maintenance therapy after chemotherapy: DENdritic cell Immunotherapy for Mesothelioma (DENIM) trial. 534 Transl Lung Cancer Res. 2019, 8, 280–285. [Google Scholar]
- Hegmans, J.P.; Veltman, J.D.; Lambers, M.E.; et al. Consolidative Dendritic Cell-based Immunotherapy Elicits Cytotoxicity against Malignant Mesothelioma. Am J Respir Crit Care Med 2010, 181, 1383–1390. [Google Scholar] [CrossRef]
- Cornelissen, R.; Hegmans, J.P.; Maat, A.P.; Kaijen-Lambers, M.E.; Bezemer, K.; Hendriks, R.W.; et al. Extended tumor control after dendritic cell vaccination with low-dose cyclophosphamide as adjuvant treatment in patients with malignant pleural mesothelioma. Am J Respirat Crit Care Med. 2016, 193, 1023–1031. [Google Scholar] [CrossRef]
- Sterman, D.H.; Alley, E.; Stevenson, J.P.; Friedberg, J.; Metzger, S.; Recio, A.; Moon, E.K.; Haas, A.R.; Vachani, A.; Katz, S.I.; et al. Pilot and Feasibility Trial Evaluating Immuno-Gene Therapy of Malignant Mesothelioma Using Intrapleural Delivery of Adenovirus-IFNα Combined with Chemotherapy. Clin. Cancer Res. 2016, 22, 3791–3800. [Google Scholar] [CrossRef] [PubMed]
- Ponce, S.; Cedrés, S.; Ricordel, C.; Isambert, N.; Viteri, S.; Herrera-Juarez, M.; Martinez-Marti, A.; Navarro, A.; Lederlin, M.; Serres, X.; Zugazagoitia, J.; Vetrhus, S.; Jaderberg, M.; Hansen, T.B.; Levitsky, V.; Paz-Ares, L. ONCOS-102 plus pemetrexed and platinum chemotherapy in malignant pleural mesothelioma: a randomized phase 2 study investigating clinical outcomes and the tumor microenvironment. J Immunother Cancer 2023, 11, e007552. [Google Scholar] [CrossRef] [PubMed]
- Helland, A.; Haakensen, A.; Ojlert, A.; Thunold, S.; Nowak, A.; et al. First survival data from the NIPU trial: a randomized, open-label, phase II study evaluating nivolumab and ipilimumab combined with UV1 vaccination as second-line treatment in patients with malignant mesothelioma. Annals of Oncology 2023, 34 (Supp 2), S1-2, S1337. [Google Scholar] [CrossRef]
- Klampatsa, A.; Haas, A.R.; Moon, E.K.; Albelda, S.M. Chimeric Antigen Receptor (CAR) T Cell Therapy for Malignant Pleural Mesothelioma (MPM). Cancers 2017, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, P.S.; Zauderer, M.G.; Rivière, I.; Solomon, S.B.; Rusch, V.W.; O’Cearbhaill, R.E.; et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti–PD-1 agent pembrolizumab. Cancer Discov 2021, 11, 2748–2763. [Google Scholar] [CrossRef]
- Tolnay, E.; Kuhnen, C.; Wiethege, T.; König, J.E.; Voss, B.; Müller, K.M. Hepatocyte growth factor/scatter factor and its receptor c-Met are overexpressed and associated with an increased microvessel density in malignant pleural mesothelioma. J. Cancer Res. Clin. Oncol. 1998, 124, 291–296. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).