1. Nuclear Distribution Genes and their Functional Significance across Eukaryotes
The nuclear distribution (
Nud) genes encode evolutionary conserved proteins, which play an important role in various cellular processes. A notable example of
Nud function, which inspired their name is nuclear migration, a precisely regulated process that holds particular importance in highly elongated cells [1,2].
Nud genes were originally discovered in the filamentous fungus
Aspergillus nidulans [3-6]. Xiang et al. [3] have identified four
Nud genes, namely
NudA,
NudC,
NudF and
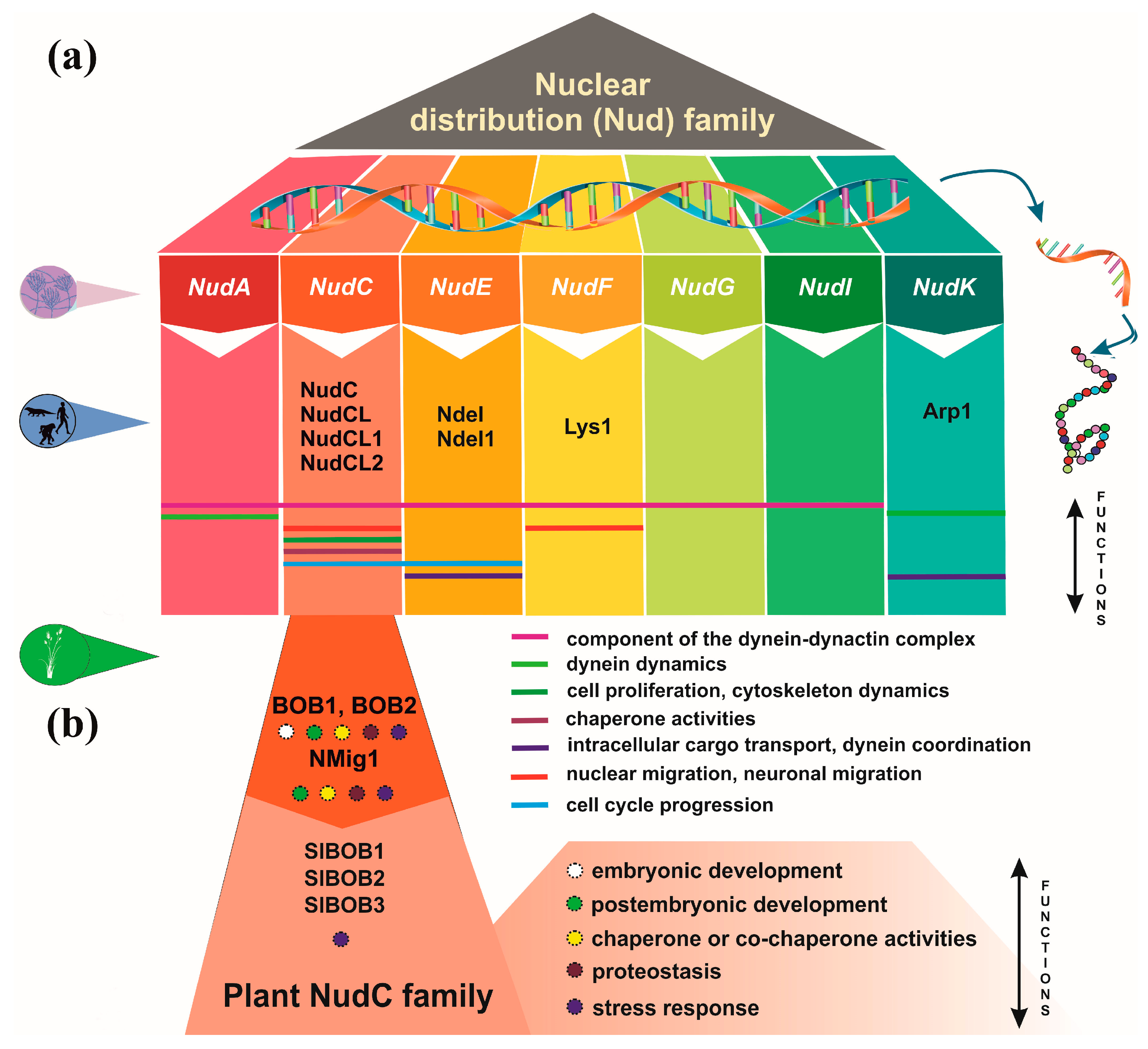
NudG, which are responsible for components of the cytoplasmic dynein-dynactin complex. NudC proteins are known for their compact structure and versatile functions, encompassing the facilitation of cell division, the regulation of gene expression and contribution to various essential biological processes (
Figure 1). Deletions in the
NudC gene lead to a more severe phenotype compared to other nuclear distribution mutants, and can result in lethality [4].
NudC acts in concert with the molecular motor dynein and other
Nud genes to regulate dynein-mediated processes, including vesicle transport in neurons [7,8], Golgi apparatus positioning, lysosomes and vesicles transport [9], kinetochore localization, spindle organization [10], phagosome movement [3], nuclear transport and membrane organelle organization [7]. The
NudA,
NudI and
NudG genes encode the heavy, intermediate and light chains of cytoplasmic dynein, respectively [5,11,12] The NudF protein is required for nuclear migration through the fungal mycelium, and interacts with microtubule-related proteins, such as a-tubulin and dynein [13-15]. The
NudF gene encodes a protein that exhibits a 42% sequence similarity with the human
Lis1 gene, which is associated with Miller-Dieker syndrome. The hemizygous deletion or mutation of
Lis1 gene results in type I lissencephaly, a condition that obstructs proper neuronal migration during brain development and leads to a smooth brain surface and disorganized cortical layering [16,17].
Lis1 plays a role in mitosis both through direct mechanisms [18-20], as well as indirectly, by impeding the movement of nuclei in radial glial progenitor cells towards the ventricular surface [21]. The NudE gene was subsequently identified as a multicopy suppressor of the NudF phenotype, demonstrating its capacity to rescue the aberrant characteristics associated with nudF mutations through the introduction of multiple NudE copies [22]. Furthermore, NudE possesses two homologs, Ndel and Ndel1, which play a role in recruiting dynein to mitotic kinetochores, facilitating the progression of mitosis [23,24]. Mutant mice lacking both Ndel and Ndel1 genes can exhibit varying neurological conditions, contingent upon the gene dosage. In particular, they may develop microcephaly due to mitotic abnormalities [25], or lissencephaly, which aligns with their role in the cytoplasmic dynein pathway [26]. Noteworthy, mutations in human NudE can lead to the co-occurrence of microcephaly and lissencephaly [27]. It has been shown that Lis1, in conjunction with NudE, orchestrates the assembly of an intricate molecular complex involving cytoplasmic dynein [28]. Within this assembly, Lis1, under the guidance of NudE, transforms dynein into a state of sustained force production, which proves to be a vital factor in facilitating the transport of heavy-load structures, including cell nuclei. Interestingly, NudE appears to be intricately connected within the same genetic pathway as NudF and NudA. The phenotypes observed in nudE homozygous mutants closely resemble those of nudF and nudA heterozygous mutants, suggesting that NudF, NudE and NudA operate within the same functional pathways [29]. Crucial element in the complex network of Nud proteins is the actin-related protein Arp1, encoded by the NudK gene. Arp1 assumes a pivotal function in the dynactin complex [30] by activating dynein and consequently enhancing the intracellular cargo transport [31]. NudC also regulates the level of NudF protein in A. nidulans [6]. A nudC mutation leads to a significant decrease in NudF, primarily at the restrictive temperature. On the other hand, additional copies of the NudF gene can effectively complement the temperature-sensitive phenotype of nudC mutants [5,32,33].
These findings provide valuable insights into the intricate network of Nud proteins and underscore their vital roles in various cellular processes (
Figure 1a). The involvement of
Nud gene family in nuclear migration, the cytoplasmic dynein-dynactin complex and their connections to human conditions, like Miller-Dieker syndrome and lissencephaly, emphasize their dual significance in both normal cellular functions and the pathophysiology of diseases.
2. Structural and Functional Features of NudC Proteins
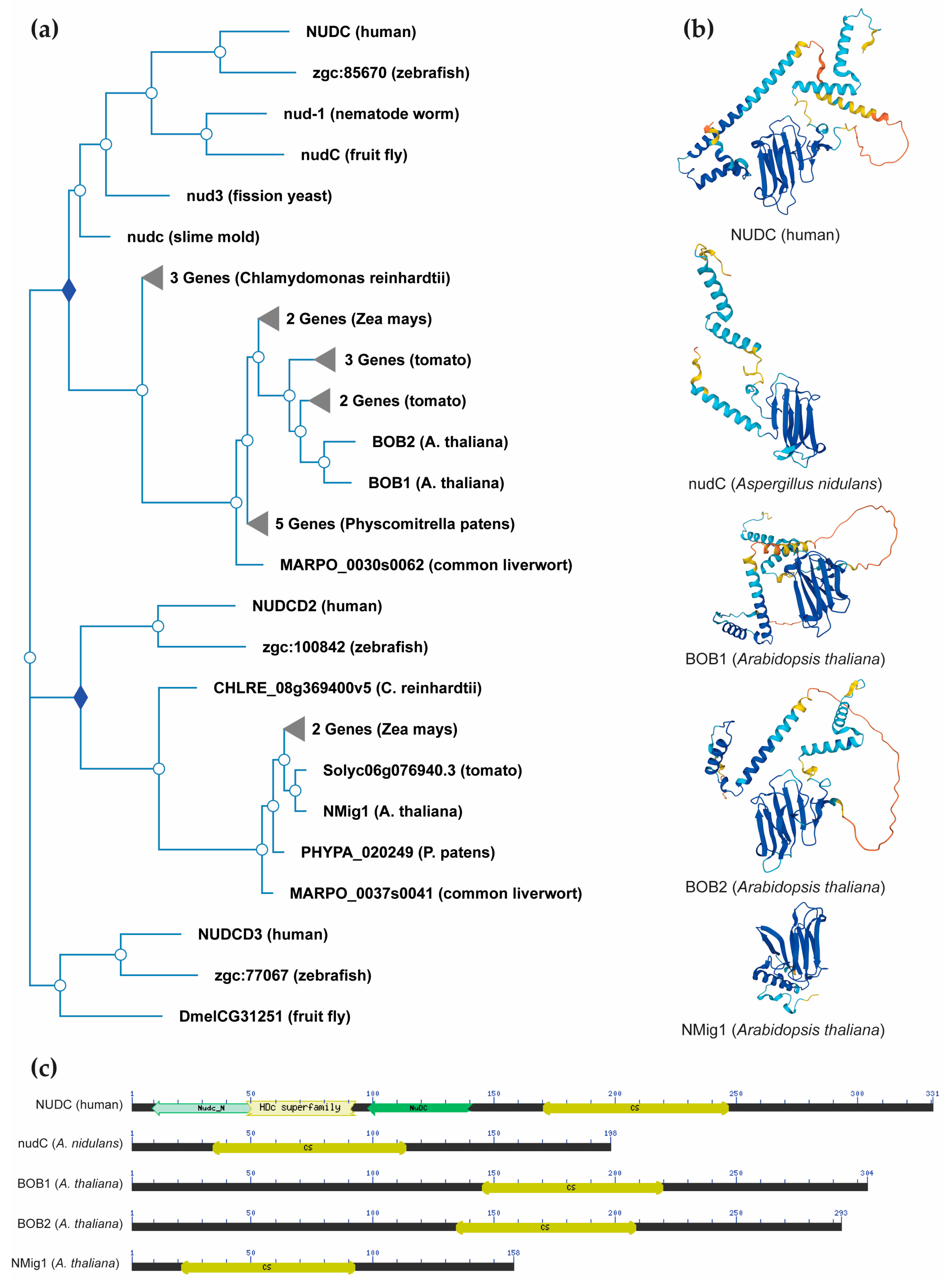
NudC proteins display a remarkable degree of conservation across a broad spectrum of organisms, spanning from fungi and plants to animals [34] (
Figure 2a). The
NudC homologous genes or proteins are found in a number of higher eukaryotes, including
Caenorhabditis elegans [35],
Drosophila melanogaster [36], newts [37], plants [38-40] and mammals [41]. The NudC family consists of four proteins: NudC [42-44], NudC-like (NudCL) also known as NudC domain-containing protein 3 (NUDCD3) [45,46], NudC-like 2 (NudCL2/NUDCD2) [47], and more distant NudC domain-containing 1 (NudCD1) also known as CML66 (Chronic Myeloid Leukemia 66).
NudC proteins typically exhibit a conserved domain architecture and contain specific motifs that are crucial for their functional roles in intracellular transport, nuclear positioning, cell cycle progression and stress responses (
Figure 2b,c). They include a coiled-coil region at the N-terminus serving as the dimerization module, and also possess a central globular domain, a CS domain (a domain shared by CHORD-containing proteins and SGT1) resembling p23 and other small heat shock proteins (sHSPs), and two conserved α-helices downstream [48-50]. This architecture is maintained across various species, albeit with some variations in the N-terminus. Higher eukaryotes possess an extended N-terminal segment compared to fungi. In vertebrates, the most substantial amino acid conservation is observed in a short N-terminal segment and the C-terminal α-helices [50]. It is essential to note that NudC molecules operate as dimers, and their biological roles may depend on this dimeric state. Common structural features between NudC and NudCL imply a potential functional overlap, despite the relatively low amino acid sequence similarity. NudCL is unique to the animal kingdom, whereas NudCL2 is found across all eukaryotes, distinguishing itself by the absence of an N-terminal extension and featuring a distinct C-terminal fragment [50].
The architectural variations seen in NudC protein family across different species (
Figure 2b) present an intriguing spectrum of structural diversity. This diversity highlights the remarkable adaptability of NudC protein family to different biological contexts, providing compelling insights into the potential existence of variable functional roles played by the NudC protein family in various organisms. Understanding their structural adaptability contributes to unraveling the complexities of their functions, positioning the NudC protein family as dynamic contributors to biological processes across the evolutionary landscape.
The expression of NudC is markedly associated with the rate of proliferation in diverse cell types and tissues. The human NudC homolog displays robust expression in actively proliferating cells [51] with central role to spindle formation during mitosis [12]. Depletion of NudC results in the presence of multiple spindles during metaphase and lagging chromosomes during anaphase [52]. Remarkably, both the depletion and overexpression of NudC components can induce cytokinesis defects in mammalian cells [52,53]. NudC deacetylation is an important player in the regulation and coordination of mitotic progression [54]. Additionally, NudC is a pivotal regulator of actin polymerization and depolymerization [55]. In A. nidulauns, the NudC gene is involved in the movement of nuclei during the asexual reproductive cycle and deposition of the cell wall, colony growth and overall viability [4,6]. NudC homologs from D. melanogaster, C. elegans, Arabidopsis thaliana and mammals are capable of complementing the nudC3 mutation in A. nidulans, restoring the normal movement of nuclei and promoting colony growth [35,36,43,56]. These findings strongly suggest that the role of NudC in nucleus movement is preserved throughout the evolution of eukaryotes.
As already noted, all NudC members share a common CS domain, which is typical for proteins with chaperone or co-chaperone activities [50,57]. The CS domain in the NudC family mirrors the molecular architecture of sHSPs, such as HSP20/α-crystallin and p23 proteins [49]. It is important to mention that HSP20 and p23 serve as central structures responsible for interacting with HSP90 and specific client proteins [49,57-59]. This implies that proteins containing these structures are instrumental in recruiting HSPs to multiprotein complexes [57]. Taipale et al. [60] has shown that co-chaperones of the NudC family specifically associate with protein partners that contain β-propeller domains. Experimental evidences support both the HSP90 co-chaperone and inherent chaperone functions of the NudC family. For instance, the microtubule-associated C. elegans NudC homolog, NUD-1, has demonstrated in vitro chaperone activity by preventing the heat-induced aggregation and precipitation of citrate synthase and luciferase [61]. Likewise, human NudC stabilizes Lis1 through HSP90-mediated pathways and exhibits intrinsic chaperone activity in vitro, inhibiting the aggregation of citrate synthase [47,62]. Utilizing a combination of Nuclear Magnetic Resonance, crystallography and modeling techniques, it has been demonstrated that NudC interacts with HSP40, HSP90 and the client receptor [63]. NudC facilitates the transfer of clients to HSP90 by recruiting substrates bound to HSP40 while excluding HSP70, ultimately expediting client activation [63-65].
Therefore, the NudC family members collectively play a vital role in cell functions, involving microtubule regulation, chaperone activities and various protein interactions. Their substantial conservation and occurrence in all tissues and cells during development and maturity underscore their significance in upholding cell integrity and various processes, highlighting their adaptability across species.
3. NudC Proteins in Plants
Plant biologists have increasingly recognized the importance of
NudC family members, due to their involvement in fundamental cellular processes critical for plant growth, development and response to environmental challenges (
Figure 1b). Nevertheless, to date, few studies have explored NudC members in plants and provided information for their roles in the plant kingdom [38-40,66-68].
3.1. Developmental role of plant NudC proteins
In the plant model species
A. thaliana, the gene known as
BOBBER1 (
BOB1), encodes a small 34.5 kDa protein possessing the NudC domain, which encodes a non-canonical sHSP with functions in development and high temperature responses [38,39,67]. Besides, two more genes from the Arabidopsis genome have been identified as CS domain-containing NudC family members. BOBBER2 (BOB2) is a paralog of BOB1, while NUCLEAR MIGRATION 1 (NMig1) is more distantly related and is characterized by shorter protein sequence (
Figure 2a,c).
The importance of the BOB1 gene becomes evident in its contribution to the precise partitioning and patterning of the apical domain in Arabidopsis embryos [38]. The loss of BOB1 results in embryo lethality, linked to developmental arrest at the globular stage without progressing to the transition stage. The bob1 null mutants exhibit an expansion of meristematic identity into the region that would typically give rise to cotyledons, leading to the failure of cotyledon formation. These developmental anomalies coincide with atypical gene expression patterns, particularly the overexpression of the meristem-specific SHOOTMERISTEMLESS (STM) gene in the upper half of the embryo. At the same time, the expected expression of the AINTEGUMENTA (ANT) transcripts in cotyledons is absent. It is noteworthy that bob1 mutant embryos do not establish auxin gradients [38], which suggests a potential interaction between BOB1 and the auxin-mediated developmental processes.
These findings underscore the critical role of this NudC gene in orchestrating key aspects of embryonic development and its potential involvement in regulating auxin-related developmental pathways.
The importance of
NudC genes extend beyond embryonic development to postembryonic phases (
Figure 1b). The hypomorphic
bob1-3 allele introduces a broad spectrum of developmental abnormalities, including shorter roots, smaller serrated leaves, stunted branched inflorescences, and irregularities in inflorescence and floral meristem formation, leading to pin-formed meristems and abnormal floral organ numbers [39]. It is noteworthy to mention that many of these phenotypic anomalies closely correspond to those seen in mutants associated with impaired auxin signaling or transport. Moreover, the formation of serrations on the edges of
bob1-3 leaves is contingent upon the activity of the PIN1 protein, underscoring the influence of BOB1 on this central player in the regulation of auxin efflux [38,70]. These observations provide compelling evidence for the critical involvement of BOB1 in shaping the auxin-mediated developmental landscape.
In addition, the genetic interplay of BOB1 with AS1 and AS2 provide valuable insights into a developmental pathway that relies on the function of this NudC gene. AS1 and AS2 serve as transcriptional regulators, contributing to the establishment of meristem boundaries by suppressing KNOX gene expression and reinforcing adaxial polarity during leaf development [71-73]. An allelic variant of BOB1, known as eal-1 and sharing the same mutation as bob1-3, offers a unique perspective in this context. Remarkably, eal-1 represents the sole viable allele of BOB1 with well-documented phenotypes [39]. When combined with as1 and as2 mutations, eal-1 demonstrates leaf morphology characterized by abaxialized filamentous structures, along with an upregulation of KNOTTED-like homeobox (KNOX) and ETTIN (ETT)/AUXIN RESPONSE FACTOR3 (ARF3) genes. ETT plays a role in enhancing abaxial identity and is directly regulated by the AS1-AS2 complex [72]. The observed polarity abnormalities in eal-1; as2 plants are mitigated in an ett genetic background, suggesting that ETT acts downstream of BOB1, AS1 and AS2 [66]. These findings illuminate the intricate interactions among BOB1, AS1 and AS2 within the context of leaf development.
Recent studies conducted by Velinov et al. [40,69] have advanced the understanding of the functions of NudC members in postembryonic root development in Arabidopsis. These studies have provided substantial insights into an uncharacterized Arabidopsis homolog of NudC genes, designated as NMig1 (Nuclear Migration 1). In-depth exploration of NMig1 has revealed that its constitutive overexpression leads to enhanced root growth and increased lateral root development under a range of conditions, including both optimal and abiotic stress scenarios [40]. Conversely, NudC mutant plants have exhibited a noticeable reduction in primary root growth and lateral root development when compared to the wild-type Col-0 [69]. Furthermore, GUS expression analysis has revealed prominent expression of NMig1 in the root meristem, emphasizing its importance for root development. This expression has been detected at various stages of lateral root primordia formation. During the initial phases, NMig1 expression encompassed the entire lateral root primordia, while as development progressed, the highest expression levels became concentrated in the central, actively dividing cells that constitute the core of these primordia.
These findings underscore the fundamental importance of NudC genes in leaf development and their role in guiding the initial phases of lateral root formation and ultimately determining the final root architecture. Furthermore, these investigations emphasize the promising potential of NudC family members as valuable targets for crop improvement, especially when focused on optimizing plant architecture to enhance crop performance.
3.2. Unveiling the Role of NudC Proteins in Plant Stress Response and Resilience
The structural similarities between the NudC domain and α-crystallin domain (ACD)/p23 proteins [49] suggest the potential for shared functions with ACD-containing sHSPs [74,75]. The ACD, initially identified in the chaperone of the vertebrate eye lens, spans approximately 80–100 amino acids and is primarily located in the C-terminal domain [76]. It encompasses two conserved regions that form a pleated β-sheet sandwich, separated by a variable-length hydrophilic domain [77,78]. These structural features closely resemble those observed in the human and mouse NudC homologs [50], suggesting the possibility of common functions with sHSPs. In plants, ACD proteins serve diverse roles, from responding to abiotic stresses and hormones to regulating transcription, virus movement and DNA demethylation [79]. The coordinated expression of different ACD proteins can collectively function as a chaperone network, safeguarding cellular machinery under a variety of stress conditions.
The Arabidopsis protein BOB1 demonstrates characteristics that align with typical sHSPs. It is induced under heat stress, possesses a NudC domain that shares structural homology with ACD-containing sHSPs, and exhibits in vitro chaperone activity, effectively preventing the aggregation of model protein substrates [39,77,80]. Under normal conditions, BOB1 is primarily localized in the plant cell cytoplasm, but during heat stress, it translocates to heat shock granules in association with HSP17.6 [39,81,82]. Previous studies have documented the presence of sHSPs, Hsp70s and heat shock factors within these granules, which are hypothesized to serve as locations for interactions between misfolded proteins and chaperones [39,80,83-85]. It is interesting to note that heat shock granules can form in response to protein misfolding even in the absence of elevated temperatures [86]. The partial loss-of-function mutant bob1-3 displays reduced thermotolerance, which can be rescued by introducing a functional BOB1 transgene, underscoring the pivotal role of this NudC family member in enhancing plant thermotolerance [39].
BOB1, with its sHSP-like characteristics and crucial role in heat stress response, is not only involved in thermotolerance but also contributes to proteostasis. In conjunction with the 26S proteasome (26SP), it forms part of a genetic network linking proteostasis to the AS1-AS2 developmental pathway [67]. The interactions within this network are believed to rely on BOB1 chaperone activities. Importantly, this network plays a vital role in repressing KNOX gene expression, which is critical for normal plant development. These studies highlight the intricate link between heat stress response, proteostasis and developmental processes in plants.
The second NudC gene in Arabidopsis, NMig1, also shares structural homology with ACD-containing sHSPs, and exhibits significant upregulation in response to various abiotic stressors, including heat shock [40]. Constitutive overexpression of NMig1 results in enhanced root growth and lateral root development, even under adverse abiotic stress conditions. NMig1-overexpressing plants display reduced susceptibility to the inhibitory effects of abiotic stress on root morphology. Delving into the molecular mechanisms underlying these effects reveals elevated expression levels of genes encoding antioxidant enzymes and other genes closely associated with stress responses. The increased antioxidant activity, coupled with lower levels of reactive oxygen species (ROS) and reduced lipid peroxidation, underscores the critical role of NMig1 in mitigating the impact of abiotic stress. Furthermore, NMig1 overexpression coincides with an upregulation of heat shock proteins and genes related to abiotic stress responses, indicating its multifaceted role in enhancing plant stress resilience.
The involvement of tomato NudC homologs in the context of tomato immune responses is notably intriguing, given the broader context of the role of NudC genes in enhancing plant stress resilience as discussed earlier. In a study conducted by Liu et al. [68], a compelling connection between tomato orthologues of NudC domain proteins and SlSAP3, a member of the stress-associated protein family, came to light. SlSAP3 serves as a positive regulator of tomato immunity against Pseudomonas syringae pv. tomato (Pst) DC3000. The three identified tomato SlBOB proteins, namely SlBOB1, SlBOB2 and SlBOB3, share a common NudC domain at their C termini, with variations in regions outside the NudC domain [68]. Silencing SlBOB1 or the simultaneous silencing of all three SlBOB genes, which act as negative regulators of immunity, leads to enhanced resistance against Pst DC3000. Hence, it appears that plant BOB proteins play diverse roles in responding to biotic and abiotic stress.
These findings further emphasize the vital role played by NudC genes in modulating plant stress resilience, and provide valuable insights into their potential as targets for the development of stress-tolerant crops.
4. Future Avenues of Research on NudC Proteins in Plants
Although some progress has been achieved in deciphering the molecular mechanisms underpinning the impact of NudC proteins on plants, many key questions remain on the horizon for forthcoming investigations. Research efforts are poised to examine the intricate mechanisms through which NudC members contribute to various aspects of plant morphology, growth, development and stress responses. This exploration may encompass their involvement in diverse metabolic pathways, signal transduction processes or interactions with other cellular constituents, extending beyond their currently recognized roles. Our research findings thus far have shed light on the vital role of NudC family members in the development and branching of the primary root in Arabidopsis, as well as in enhancing plant resistance against adverse abiotic stress factors [40,69]. Such studies rely on the development of an exhaustive genetic toolbox comprising transcriptional and translational reporters for NMig1 and BOB1, as well as Arabidopsis lines with gene overexpression or downregulation in combination with tissue- and developmental stage-specific promoters. Investigating the NudC interactions with specific protein partners, which play an essential role in plant growth, development and stress responses is of utmost importance. The dissection of NudC protein interactome requires the generation of NudC gene versions with affinity purification tags, as well as fluorescent protein-tagged versions for further validation of the putative protein-protein interactions. Besides the interactomic studies, other systems biology approaches should also provide an added value to our knowledge on the role of plant NudC proteins. As outlined above, there are already some experimental evidences for the direct involvement of BOB1 in auxin-mediated processes. Hence, hormone profiling in lines with altered NudC expression will associate the observed growth phenotypes with the changes in the composition and quantity of endogenous phytohormones and their precursors, conjugates and degradation products. Such findings, particularly their connection to temperature fluctuations and hormonal responses, offer the potential to unveil novel and essential aspects of plant functioning.
An additional avenue of research may involve in-depth structural analyses of NudC domains in plant species to elucidate differences in structural features of plant NudC domains compared to other organisms, and how these distinctions correlate with plant-specific functions. A possible approach in this respect could be the design of chimeric NudC versions with swapped protein domains from other eukaryotic organisms. The expression of such gene constructs in Arabidopsis mutants with impaired NudC gene expression would be instrumental in addressing the capability for functional complementation based on conserved protein signatures. Besides, expression of plant NudC genes in heterologous systems, such as yeast and mammalian cells, would shed light on the extent of functional homology when it comes to cell cycle progression.
The distinctive chaperone activities of NudC proteins in plants warrant comprehensive exploration. It appears that the members of this protein family might orchestrate the functioning of many HSP proteins through differential interactions depending on the environmental context. Further experiments with truncated protein versions and with the aforementioned chimeric constructs would elucidate the significance of specific protein motifs for the composition of the NudC protein interactome within the heat shock granules. A comprehensive understanding of the plant-specific chaperone network involving NudC proteins will further illuminate the molecular mechanisms of plant stress resilience.
In summary, the ongoing studies on NudC proteins in plants hold the promise of revealing a wealth of knowledge that can significantly advance our comprehensive understanding of regulatory mechanisms at the whole-plant level. The obtained information could ultimately fuel novel strategies for enhancing crop productivity and strengthening plant resilience in response to environmental challenges. Notably, the structural and functional conservation of NudC proteins across species hints at their potential applications in medical research. Investigating NudC roles in cell division, microtubule regulation and related pathways may lead to insights into human diseases and therapeutic opportunities. Realizing the full potential of NudC proteins necessitates further research and interdisciplinary collaboration. These endeavors hold the key to unlocking the profound benefits that NudC proteins can offer to plant biology and medical science.
Author Contributions
The idea was conceived by V.V. The manuscript was written by V.V. and K.M. The figures were created by M.G., D.T. and K.M. The final draft of the manuscript was read and approved by all authors.
Acknowledgments
This study was funded by the Bulgarian National Science Fund (BNSF), Grant No. DN11/8/15.12.2017.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morris, N. R. Nuclear migration. From fungi to the mammalian brain. J. Cell Biol. 2000, 148, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Fischer, R.; Xiang, X. Accumulation of cytoplasmic dynein and dynactin at microtubule plus ends in Aspergillus nidulans is kinesin dependent. Mol. Biol. Cell 2003, 14, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Beckwith, S.M.; Morris, N.R. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 1994, 91, 2100–2104. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Xiang, X.; Dawe, A.L.; Morris, N.R. Deletion of nudC, a nuclear migration gene of Aspergillus nidulans, causes morphological and cell wall abnormalities and is lethal. Mol. Biol. Cell 1997, 8, 1735–1749. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Roghi, C.; Morris, N.R. Characterization and localization of the cytoplasmic dynein heavy chain in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 1995, 92(21), 9890–9894. [Google Scholar] [CrossRef] [PubMed]
- Osmani, A.H.; Osmani, S.A.; Morris, N.R. The molecular cloning and identification of a gene product specifically required for nuclear movement in Aspergillus nidulans. J. Cell Biol. 1990, 111(2), 543–551. [Google Scholar] [CrossRef] [PubMed]
- Fath, K.R.; Trimbur, G.M.; Burgess, D.R. Molecular motors and a spectrin matrix associate with Golgi membranes in vitro. J. Cell Biol. 1997, 139, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Goldstein, L.S. Principles of cargo attachment to cytoplasmic motor proteins. Curr. Opin. Cell Biol. 2002, 14, 63–68. [Google Scholar] [CrossRef]
- Aniento, F.; Emans, N.; Griffiths, G. , Gruenberg, J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J. Cell Biol 1993, 123(6) 1373. [Google Scholar] [CrossRef]
- Pfarr, C.M.; Coue, M.; Grissom, P.M.; Hays, T.S.; Porter, M.E.; McIntosh, J.R. Cytoplasmic dynein is localized to kinetochores during mitosis. Nature 1990, 345(6272), 263–265. [Google Scholar] [CrossRef]
- Beckwith, S. M.; Roghi, C.H.; Liu, B.; Morris, N.R. The “8-kD” cytoplasmic dynein light chain is required for nuclear migration and for dynein heavy chain localization in Aspergillus nidulans. J. Cell Biol. 1998, 143, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M. Y.; Huang, N. N.; Clawson, G.A.; Osmani, S.A.; Pan, W.; Xin, P.; Razzaque, M.S.; Miller, B.A. Involvement of the fungal nuclear migration gene nudC human homolog in cell proliferation and mitotic spindle formation. Exp. Cell Res. 2002, 273, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Willins, D.A.; Xiang, X.; Morris, N.R. An alpha tubulin mutation suppresses nuclear migration mutations in Aspergillus nidulans. Genetics 1995, 141, 4, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Willins, D. A. , Liu, B.; Xiang, X.; Morris, N.R. Mutations in the heavy chain of cytoplasmic dynein suppress the nudF nuclear migration mutation of Aspergillus nidulans. Mol. Gen. Genet. 1997, 255, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Morris, N.R.; Efimov, V.P.; Xiang, X. Nuclear migration, nucleokinesis and lissencephaly. Trends Cell Biol. 1998, 8(12), 467–470. [Google Scholar] [CrossRef]
- Dobyns, W.B.; Reiner, O.; Carrozzo, R.; Ledbetter, D.H. Lissencephaly. A human brain malformation associated with deletion of the LIS1 gene located at chromosome 17p13. JAMA 1993, 270, 2838–2842. [Google Scholar] [CrossRef]
- Hirotsune, S.; Fleck, M.W.; Gambello, M.J.; Bix, G.J.; Chen, A.; Clark, G.D.; Ledbetter, D.H.; McBain, C.J.; Wynshaw-Boris, A. Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nat. Genet. 1998, 19, 4, 333–339. [Google Scholar] [CrossRef]
- Faulkner, N.E.; Dujardin, D.L.; Tai, C.Y.; Vaughan, K.T.; O'Connell, C.B.; Wang, Y.L.; Vallee, R.B. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat. Cell Biol. 2000, 2(11), 784–791. [Google Scholar] [CrossRef]
- Siller, K.H.; Serr, M.; Steward, R.; Hays, T.S.; Doe, C.Q. , 2005. Live imaging of Drosophila brain neuroblasts reveals a role for Lis1/dynactin in spindle assembly and mitotic checkpoint control. Mol. Biol. Cell 2005, 16, 11, 5127–5140. [Google Scholar] [CrossRef]
- Yingling, J.; Youn, Y.H.; Darling, D.; Toyo-Oka, K.; Pramparo, T.; Hirotsune, S.; Wynshaw-Boris, A. Neuroepithelial stem cell proliferation requires LIS1 for precise spindle orientation and symmetric division. Cell 2008, 132(3), 474–486. [Google Scholar] [CrossRef]
- Tsai, J.W.; Lian, W.N.; Kemal, S.; Kriegstein, A.R.; Vallee, R.B. Kinesin 3 and cytoplasmic dynein mediate interkinetic nuclear migration in neural stem cells. Nat. Neurosci. 2010, 13, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Efimov, V.P.; Morris, N.R. The Lis1-related NUDF protein of Aspergillus nidulans interacts with the coiled-coil domain of the NUDE/RO11 protein. J. Cell Biol. 2000, 150(3), 681–688. [Google Scholar] [CrossRef]
- Ma, L.; Tsai, M.Y.; Wang, S.; Lu, B.; Chen, R.; Iii, J.R.; Zhu, X.; Zheng, Y. Requirement for Nudel and dynein for assembly of the lamin B spindle matrix. Nat. Cell Biol. 2009, 11, 247–256. [Google Scholar] [CrossRef]
- Stehman, S.A.; Chen, Y.; McKenney, R.J.; Vallee, R.B. NudE and NudEL are required for mitotic progression and are involved in dynein recruitment to kinetochores. J. Cell Biol. 2007, 178, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Walsh, C.A. Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron 2004, 44, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Mori, D.; Toyo-Oka, K.; Chen, A.; Garrett-Beal, L.; Muramatsu, M.; Miyagawa, S.; Hiraiwa, N.; Yoshiki, A.; Wynshaw-Boris, A.; et al. Complete loss of Ndel1 results in neuronal migration defects and early embryonic lethality. Mol. Cell. Biol. 2005, 25, 17, 7812–7827. [Google Scholar] [CrossRef] [PubMed]
- Alkuraya, F.S.; Cai, X.; Emery, C.; Mochida, G.H.; Al-Dosari, M.S.; Felie, J.M.; Hill, R.S.; Barry, B.J.; Partlow, J.N.; Gascon, G.G. Human mutations in NDE1 cause extreme microcephaly with lissencephaly. Am. J. Med. Genet. 2011, 88(5), 536–547. [Google Scholar] [CrossRef] [PubMed]
- McKenney, R.J.; Vershinin, M.; Kunwar, A.; Vallee, R.B.; Gross, S.P. LIS1 and NudE induce a persistent dynein force-producing state. Cell 2010, 141(2), 304–314. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Olson, E.C.; Stukenberg, P.T.; Flanagan, L.A.; Kirschner, M.W.; Walsh, C.A. LIS1 regulates CNS lamination by interacting with mNudE, a central component of the centrosome. Neuron 2000, 28(3), 665–679. [Google Scholar] [CrossRef]
- Xiang, X.; Zuo, W.; Efimov, V.P.; Morris, N.R. Isolation of a new set of Aspergillus nidulans mutants defective in nuclear migration. Curr. Genet. 1999, 35, 626–630. [Google Scholar] [CrossRef]
- Xiang, X.; Han, G.; Winkelmann, D.A.; Zuo, W.; Morris, N.R. , 2000. Dynamics of cytoplasmic dynein in living cells and the effect of a mutation in the dynactin complex actin-related protein Arp1. Curr. Biol. 2000, 10(10), 603–606. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Morris, N.R. Extragenic suppressors of nudC3, a mutation that blocks nuclear migration in Aspergillus nidulans. Genetics 1995, 141, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Morris, N.R. Genetic and molecular analysis of a tRNALeu missense suppressor of nudC3, a mutation that blocks nuclear migration in Aspergillus nidulans. Genetics 1997, 145(3), 707–714. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Wang, W.; Zhou, T.; Yang, Y. Emerging roles of NudC family: from molecular regulation to clinical implications. Sci. China Life Sci. 2016, 59, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Dawe, A.L.; Caldwell, K.A.; Harris, P.M.; Morris, N.R.; Caldwell, G.A. Evolutionarily conserved nuclear migration genes required for early embryonic development in Caenorhabditis elegans. Dev. Genes Evol. 2001, 211, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Cunniff, J.; Chiu, Y.H.; Morris, N.R.; Warrior, R. Characterization of DnudC, the Drosophila homolog of an Aspergillus gene that functions in nuclear motility. Mech Dev. 1997, 66, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Moreau, N.; Aumais, J.P.; Prudhomme, C.; Morris, S.M.; Yu-Lee, L.Y. NUDC expression during amphibian development. Int. J. Dev. Biol. 2001, 45, 839–843. [Google Scholar]
- Jurkuta, R.J.; Kaplinsky, N.J.; Spindel, J.E.; Barton, M.K. Partitioning the apical domain of the Arabidopsis embryo requires the BOBBER1 NudC domain protein. Plant Cell 2009, 21, 1957–1971. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.E.; Hoyer, J.S.; Johnson, A.I.; Moody, Z.R.; Lopez, J.; Kaplinsky, N.J. BOBBER1 is a noncanonical Arabidopsis small heat shock protein required for both development and thermotolerance. Plant Physiol. 2009, 151, 241–252. [Google Scholar] [CrossRef]
- Velinov, V.; Vaseva, I.; Zehirov, G.; Zhiponova, M.; Georgieva, M.; Vangheluwe, N.; Beeckman, T.; Vassileva, V. Overexpression of the NMig1 gene encoding a NudC domain protein enhances root growth and abiotic stress tolerance in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 815. [Google Scholar] [CrossRef]
- Riera J, Lazo PS. The mammalian NudC-like genes: a family with functions other than regulating nuclear distribution. Cell. Mol. Life Sci. 66, 2383-90, (2009).
- Axtell, S.M.; Truong, T.M.; O'Neal, K.D.; Yu-Lee, L.Y. Characterization of a prolactin-inducible gene, clone 15, in T cells. Mol. Endocrinol. 1995, 9, 3, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.A.; Zhang, M.Y.; Gocke, C.D.; De Souza, C.; Osmani, A.H.; Lynch, C.; Davies, J.; Bell, L.; Osmani, S.A. A homolog of the fungal nuclear migration gene nudC is involved in normal and malignant human hematopoiesis. Exp. Hematol. 1999, 27, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Ledbetter, D.H. Molecular cloning and characterization of the human NUDC gene. Hum Genet. 1999, 104, 498–504. [Google Scholar] [CrossRef]
- Shu, T.; Tseng, H.C.; Sapir, T.; Stern, P.; Zhou, Y.; Sanada, K.; Fischer, A.; Coquelle, F.M.; Reiner, O.; Tsai, L.H. Doublecortin-like kinase controls neurogenesis by regulating mitotic spindles and M phase progression. Neuron 2006, 49(1), 25–39. [Google Scholar] [CrossRef]
- Zhou, T.; Zimmerman, W.; Liu, X.; Erikson, R.L. A mammalian NudC-like protein essential for dynein stability and cell viability. Proc. Natl. Acad. Sci. U S A 2006, 103, 9039–9044. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yan, X.; Cai, Y.; Lu, Y.; Si, J.; Zhou, T. NudC-like protein 2 regulates the LIS1/dynein pathway by stabilizing LIS1 with Hsp90. Proc Natl Acad Sci U S A 2010, 107, 3499–504. [Google Scholar] [CrossRef] [PubMed]
- Lupas, A.; Van Dyke, M.; Stock, J. Predicting coiled coils from protein sequences. Science 1991, 252, 1162–1164. [Google Scholar] [CrossRef]
- Garcia-Ranea, J.A.; Mirey, G.; Camonis, J.; Valencia, A. p23 and HSP20/alpha-crystallin proteins define a conserved sequence domain present in other eukaryotic protein families. FEBS Lett. 2002, 529, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Cierpicki, T.; Burdette, A.J.; Utepbergenov, D.; Janczyk, P.; Derewenda, U.; Stukenberg, P.T.; Caldwell, K.A.; Derewenda, Z.S. Structural features and chaperone activity of the NudC protein family. J. Mol. Biol. 2011, 409(5), 722–741. [Google Scholar] [CrossRef]
- Gocke, C.D.; Osmani, S.A.; Miller, B.A. The human homologue of the Aspergillus nuclear migration gene nudC is preferentially expressed in dividing cells and ciliated epithelia. Histochem. Cell Biol. 2000, 114, 293–301. [Google Scholar] [CrossRef]
- Zhou, T.; Aumais, J.P.; Liu, X.; Yu-Lee, L.Y.; Erikson, R.L. A role for Plk1 phosphorylation of NudC in cytokinesis. Dev. Cell 2003, 5, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Aumais, J.P.; Williams, S.N.; Luo, W.; Nishino, M.; Caldwell, K.A.; Caldwell, G.A.; Lin, S.H.; Yu-Lee, L.Y. Role for NudC, a dynein-associated nuclear movement protein, in mitosis and cytokinesis. J. Cell Sci. 2003, 116(10), 1991–2003. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.; Pan, J.; Hawke, D.H.; Lin, S.-H.; Yu-Lee, L. NudC deacetylation regulates mitotic progression. PloS One 2013, 8, e73841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, W.; Lu, Y.; Yan, X.; Yan, X.; Zhu, X.; Liu, W.; Yang, Y.; Zhou, T. NudC regulates actin dynamics and ciliogenesis by stabilizing cofilin 1. Cell Res. 2016, 26(2), 239–253. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M.; Albrecht, U.; Reiner, O.; Eichele, G.; Yu-Lee, L.Y. The lissencephaly gene product Lis1, a protein involved in neuronal migration, interacts with a nuclear movement protein, NudC. Curr. Biol. 1998, 8(10), 603–606. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Jacob, J.; Michowski, W.; Nowotny, M.; Kuznicki, J.; Chazin, W.J. Human Sgt1 binds HSP90 through the CHORD-Sgt1 domain and not the tetratricopeptide repeat domain. J. Biol. Chem. 2004, 279(16), 16511–16517. [Google Scholar] [CrossRef] [PubMed]
- Botër, M.; Amigues, B.; Peart, J.; Breuer, C.; Kadota, Y.; Casais, C.; Moore, G.; Kleanthous, C.; Ochsenbein, F.; Shirasu, K.; et al. Structural and functional analysis of SGT1 reveals that its interaction with HSP90 is required for the accumulation of Rx, an R protein involved in plant immunity. Plant Cell 2007, 19, 11–3791. [Google Scholar] [CrossRef] [PubMed]
- Echtenkamp, F.J.; Zelin, E.; Oxelmark, E.; Woo, J.I.; Andrews, B.J.; Garabedian, M.; Freeman, B.C. Global functional map of the p23 molecular chaperone reveals an extensive cellular network. Mol. Cell 2011, 43(2), 229–241. [Google Scholar] [CrossRef]
- Taipale, M.; Tucker, G.; Peng, J.; Krykbaeva, I.; Lin, Z.Y.; Larsen, B.; Choi, H.; Berger, B.; Gingras, A.C.; Lindquist, S. A quantitative chaperone interaction network reveals the architecture of cellular protein homeostasis pathways. Cell 2014, 158(2), 434–448. [Google Scholar] [CrossRef]
- Faircloth, L.M.; Churchill, P.F.; Caldwell, G.A.; Caldwell, K.A. The microtubule-associated protein, NUD-1, exhibits chaperone activity in vitro. Cell Stress and Chaperones 2009, 14, 95–103. [Google Scholar] [CrossRef]
- Zhu, X.J.; Liu, X.; Jin, Q.; Cai, Y.; Yang, Y.; Zhou, T. The L279P mutation of nuclear distribution gene C (NudC) influences its chaperone activity and lissencephaly protein 1 (LIS1) stability. J. Biol. Chem. 2010, 285, 29903–29910. [Google Scholar] [CrossRef] [PubMed]
- Biebl, M.M.; Delhommel, F.; Faust, O.; Zak, K.M.; Agam, G.; Guo, X.; Mühlhofer, M.; Dahiya, V.; Hillebrand, D.; Popowicz, G.M.; et al. NudC guides client transfer between the Hsp40/70 and Hsp90 chaperone systems. Mol. Cell 2022, 82(3), 555–569. [Google Scholar] [CrossRef] [PubMed]
- Biebl, M.M.; Lopez, A.; Rehn, A.; Freiburger, L.; Lawatscheck, J.; Blank, B.; Sattler, M.; Buchner, J. Structural elements in the flexible tail of the co-chaperone p23 coordinate client binding and progression of the Hsp90 chaperone cycle. Nat. Commun. 2021, 12(1), 828. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Elimelech, A.R.; Klimm, K.; Sattler, M. The charged linker modulates the conformations and molecular interactions of Hsp90. ChemBioChem 2021, 22(6), 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, N.; Kanamaru, K.; Ueno, Y.; Kojima, S.; Kobayashi, T.; Machida, C.; Machida, Y. ASYMMETRIC-LEAVES2 and an ortholog of eukaryotic NudC domain proteins repress expression of AUXIN RESPONSE-FACTOR and class 1 KNOX homeobox genes for development of flat symmetric leaves in Arabidopsis. Biol. Open 2012, 1, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Silverblatt-Buser, E.W.; Frick, M.A.; Rabeler, C.; Kaplinsky, N.J. Genetic interactions between BOB1 and multiple 26S proteasome subunits suggest a role for proteostasis in regulating Arabidopsis development. G3-Genes Genom. Genet. 2018, 8(4), 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, J.; Jiang, S.; Wang, H.; Gao, Y.; Zhang, H.; Li, D.; Song, F. Tomato SlSAP3, a member of the stress-associated protein family, is a positive regulator of immunity against Pseudomonas syringae pv. tomato DC3000. Mol. Plant Pathol. 2019, 20(6), 815–830. [Google Scholar] [CrossRef] [PubMed]
- Velinov, V.; Georgieva, M.; Zehirov, G.; Vassileva, V. NudC-like genes contribute to root growth and branching in Arabidopsis thaliana. C. R. Acad. Bulg. Sci. 2021, 74(12), 1767–1773. [Google Scholar] [CrossRef]
- Kaplinsky, N.J. Temperature compensation of auxin dependent developmental patterning. Plant Signal. Behav. 2009, 4(12), 1157–1158. [Google Scholar] [CrossRef] [PubMed]
- Iwakawa, H.; Ueno, Y.; Semiarti, E.; Onouchi, H.; Kojima, S.; Tsukaya, H.; Hasebe, M.; Soma, T.; Ikezaki, M.; Machida, C.; et al. 2002. The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana, required for formation of a symmetric flat leaf lamina, encodes a member of a novel family of proteins characterized by cysteine repeats and a leucine zipper. Plant Cell Physiol 2002, (43, 5), 467–478. [Google Scholar] [CrossRef]
- Iwasaki, M.; Takahashi, H.; Iwakawa, H.; Nakagawa, A.; Ishikawa, T.; Tanaka, H.; Matsumura, Y.; Pekker, I.; Eshed, Y.; Vial-Pradel, S.; et al. Dual regulation of ETTIN (ARF3) gene expression by AS1-AS2, which maintains the DNA methylation level, is involved in stabilization of leaf adaxial-abaxial partitioning in Arabidopsis. Development 2013, 140, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Machida, C.; Nakagawa, A.; Kojima, S.; Takahashi, H.; Machida, Y. The complex of ASYMMETRIC LEAVES (AS) proteins plays a central role in antagonistic interactions of genes for leaf polarity specification in Arabidopsis. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Studer, S.; Narberhaus, F. Chaperone activity and homo-and hetero-oligomer formation of bacterial small heat shock proteins. J. Biol. Chem. 2000, 275(47), 37212–37218. [Google Scholar] [CrossRef]
- Eisenhardt, B.D. Small heat shock proteins: recent developments. BioMol. Concepts 2013, 4(6), 583–595. [Google Scholar] [CrossRef] [PubMed]
- Boelens, W.C.; Croes, Y.; de Ruwe, M.; de Reu, L.; de Jong, W.W. Negative charges in the C-terminal domain stabilize the αB-crystallin complex. J. Biol. Chem. 1998, 273(43), 28085–28090. [Google Scholar] [CrossRef] [PubMed]
- Haslbeck, M.; Franzmann, T.; Weinfurtner, D.; Buchner, J. Some like it hot: the structure and function of small heat-shock proteins. Nat. Struct. Mol. Biol. 2005, 12(10), 842–846. [Google Scholar] [CrossRef] [PubMed]
- Bondino, G.H.; Valle, M.E.; Ten Have, A. Evolution and functional diversification of the small heat shock protein/α-crystallin family in higher plants. Planta 2012, 235, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Rao, S.; Mathur, S. The α-crystallin domain containing genes: identification, phylogeny and expression profiling in abiotic stress, phytohormone response and development in tomato (Solanum lycopersicum). Front. Plant Sci. 2016, 7, 426. [Google Scholar] [CrossRef] [PubMed]
- Wallace, E.W.J.; Kear-Scott, J.L.; Pilipenko, E.V; Schwartz, M.H.; Laskowski, P.R.; Rojek, A.E.; Katanski, C.D.; Riback, J.A.; Dion, M.F.; Franks, A.M.; et al. Reversible, specific, active aggregates of endogenous proteins assemble upon heat stress. Cell 2015, 162, 1286–1298. [Google Scholar] [CrossRef]
- Kirschner, M.; Winkelhaus, S.; Thierfelder, J.M.; Nover, L. Transient expression and heat-stress-induced co-aggregation of endogenous and heterologous small heat-stress proteins in tobacco protoplasts. Plant J. 2000, 24(3), 397–412. [Google Scholar] [CrossRef]
- Miroshnichenko, S.; Tripp, J.; Nieden, U.Z.; Neumann, D.; Conrad, U.; Manteuffel, R. Immunomodulation of function of small heat shock proteins prevents their assembly into heat stress granules and results in cell death at sublethal temperatures. Plant J. 2005, 41(2), 269–281. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: life on the verge of death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Scharf, K.D.; Heider, H.; Hohfeld, I.; Lyck, R.; Schmidt, E.; Nover, L. The tomato Hsf system: HsfA2 needs interaction with HsfA1 for efficient nuclear import and may be localized in cytoplasmic heat stress granules. Mol. Cell Biol. 1998, 18, 2240–2251. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.; Gernhard, S.; von Koskull-Doring, P.; Vierling, E.; Scharf, K.D. The plant sHSP superfamily: five new members in Arabidopsis thaliana with unexpected properties. Cell Stress Chaperones 2008, 13, 183–197. [Google Scholar] [CrossRef]
- Lawrence, R.; Kaplinsky, N. Arabidopsis Heat Shock Granules exhibit dynamic cellular behavior and can form in response to protein misfolding in the absence of elevated temperatures. MicroPubl Biol. 2020, 2020. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).