Submitted:
13 November 2023
Posted:
14 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
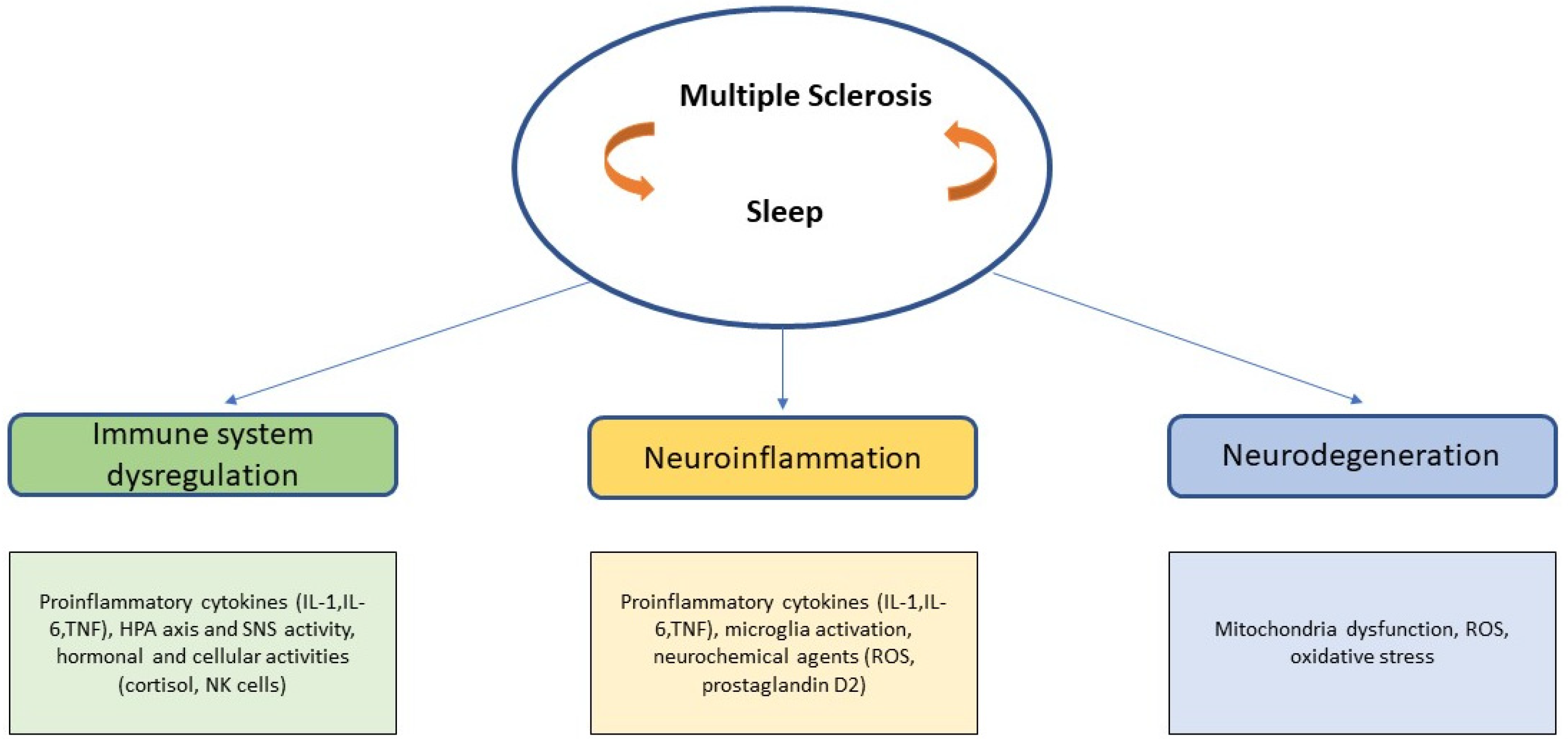
2. Etiopathogenesis of MS: A New Multifactorial Approach
3. Disturbed Sleep in MS
4. MS and Sleep: A Strong Indirect Relationship
4.1. Immune System and Sleep: Basic Principles Related to MS
4.2. Possible Cellular and Molecular Links between Sleep and MS in Neuroinflammation
IL-1
TNF-α
IL-6
4.3. Neurodegeneration: Mytochondria Dysfunction and Oxidative Stress
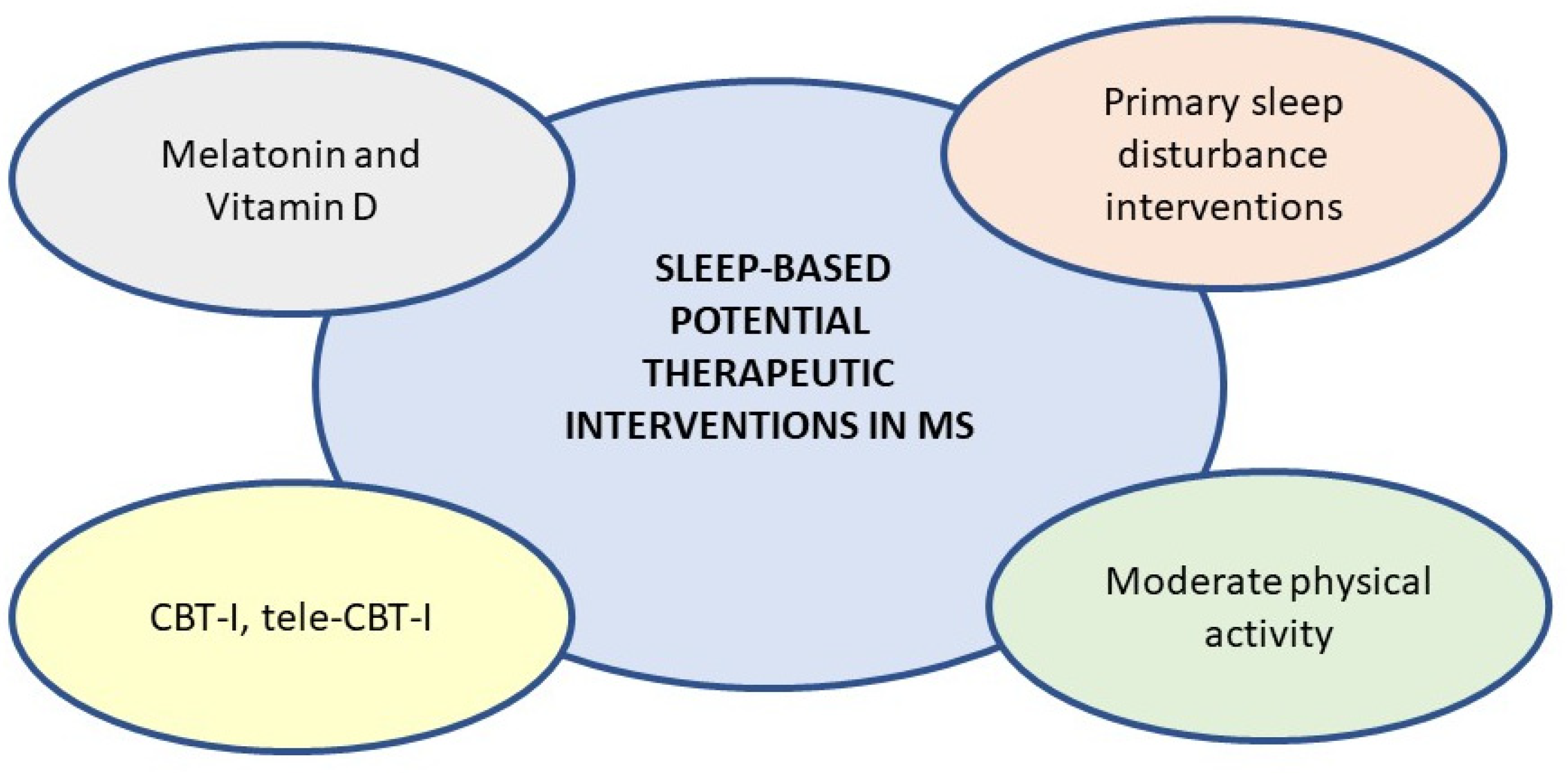
5. New Potential Evaluative and Therapeutic Scenario
5.1. Melatonin and Vitamin D
5.2. Sleep Management and Care in MS Clinical Routine
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Wallin M.T., Culpepper W.J., Campbell J.D., Nelson L.M., Langer-Gould A., Marrie R.A., Cutter G.R., Kaye W.E., Wagner L., Tremlett H., Buka S.L., Dilokthornsakul P., Topol B., Chen L.H., LaRocca N.G.; US. Multiple Sclerosis Prevalence Workgroup. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurol. 2019, 92, e1029-e1040. [CrossRef]
- Loma I., Heyman R. Multiple sclerosis: pathogenesis and treatment. Curr Neuropharmacol. 2011, 9, 409-16. [CrossRef]
- Tobore T.O. Towards a comprehensive etiopathogenetic and pathophysiological theory of multiple sclerosis. Int J Neurosci. 2020, 130, 279-300. [CrossRef]
- Mohammed E.M. Environmental Influencers, MicroRNA, and Multiple Sclerosis. J. of Cent. Nerv. System Dis. 2020;12. [CrossRef]
- Lassmann H., Brück W., Lucchinetti C.F. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007, 17, 210-8. [CrossRef]
- Al-Sharman A., Khalil H., El-Salem K., Aldughmi M., Aburub A. The effects of aerobic exercise on sleep quality measures and sleep-related biomarkers in individuals with Multiple Sclerosis: A pilot randomised controlled trial. NeuroRehab. 2019;45:107-115. [CrossRef]
- Lashley F.R. A review of sleep in selected immune and autoimmune disorders. Holist Nurs Pract. 2003, 17, 65-80. [CrossRef]
- Lazibat I., Rubinić Majdak M., Županić S. Multiple Sclerosis: New Aspects of Immunopathogenesis. Acta Clin Croat. 2018, 57, 352-361. [CrossRef]
- Filippi M., Rocca M.A., Barkhof F., Brück W., Chen J.T., Comi G., DeLuca G., De Stefano N., Erickson B.J., Evangelou N., Fazekas F., Geurts J.J., Lucchinetti C., Miller D.H., Pelletier D., Popescu B.F., Lassmann H.; Attendees of the Correlation between Pathological MRI findings in MS workshop. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2012, 11, 349-60. [CrossRef]
- Lucchinetti C.F., Popescu B.F., Bunyan R.F., Moll N.M., Roemer S.F.., Lassmann H, Brück W., Parisi J.E., Scheithauer B.W., Giannini C., Weigand S.D., Mandrekar J., Ransohoff R.M. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011, 365, 2188-97. [CrossRef]
- das Neves S.P., Sousa J.C., Sousa N., Cerqueira J.J., Marques F. Altered astrocytic function in experimental neuroinflammation and multiple sclerosis. Glia. 2021, 69, 1341-1368. [CrossRef]
- Titus H.E., Chen Y., Podojil J.R., Robinson A.P., Balabanov R., Popko B., Miller S.D. Pre-clinical and Clinical Implications of "Inside-Out" vs. "Outside-In" Paradigms in Multiple Sclerosis Etiopathogenesis. Front Cell Neurosci. 2020 Oct 27;14:599717. [CrossRef]
- Yong H., Chartier G., Quandt J. Modulating inflammation and neuroprotection in multiple sclerosis. J Neurosci Res. 2018 Jun;96:927-950. [CrossRef]
- Papiri G., D'Andreamatteo G., Cacchiò G., Alia S., Silvestrini M., Paci C., Luzzi S., Vignini A. Multiple Sclerosis: Inflammatory and Neuroglial Aspects. Curr Issues Mol Biol. 2023, 45, 1443-1470. [CrossRef]
- Jarius S., König F., Metz I., Ruprecht K., Paul F., Brück W., Wildemann B. Pattern II and pattern III MS are entities distinct from pattern I MS: evidence from cerebrospinal fluid analysis. J Neuroinflamm. 2017; 14, 171. [CrossRef]
- de Barcelos I.P., Troxell R.M., Graves J.S. Mitochondrial Dysfunction and Multiple Sclerosis. Biology (Basel). 2019, 8, 37. [CrossRef]
- Steinman R.M. Dendritic cells and the control of immunity: enhancing the efficiency of antigen presentation. Mt Sinai J Med. 2001, 68, 160-6. , Zipp F. Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Exp Neurol. 2014 Dec;262 Pt A:8-17. [CrossRef]
- Sospedra M., Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005 ;23:683-747. [CrossRef]
- Friese M.A., Schattling B., Fugger L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat Rev Neurol. 2014, 10, 225-38. [CrossRef]
- Romee R., Schneider S.E., Leong J.W., Chase J.M., Keppel C.R., Sullivan R.P., Cooper M.A., Fehniger T.A. Cytokine activation induces human memory-like NK cells. Blood. 2012, 120, 4751-60. [CrossRef]
- Peferoen L., Kipp M., van der Valk P., van Noort J.M., Amor S. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology. 2014, 141, 302-13. [CrossRef]
- Braley T.J. Overview: A Framework for the Discussion of Sleep in Multiple Sclerosis. Curr Sleep Med Rep. 2017, 3, 263-271.
- Hughes A.J., Dunn K.M., Chaffee T. Sleep Disturbance and Cognitive Dysfunction in Multiple Sclerosis: a Systematic Review. Curr Neurol Neurosci Rep. 2018, 18, 2. [CrossRef]
- Kołtuniuk A., Chojdak-Łukasiewicz J. Adherence to Therapy in Patients with Multiple Sclerosis-Review. Int J Environ Res Public Health. 2022, 19, 2203. [CrossRef]
- Sater R., Gudesblatt M., Kresa-Reahl K., Brandes D., Sater P. The relationship between objective parameters of sleep and measures of fatigue, depression, and cognition in multiple sclerosis. Multiple Sclerosis J. - Experimental, Translational and Clinical. 2015;1. [CrossRef]
- Bakalidou D., Giannopapas V., Giannopoulos S. Thoughts on Fatigue in Multiple Sclerosis Patients. Cureus. 2023, 15, e42146. [CrossRef]
- Arand D.L., Bonnet M.H. The multiple sleep latency test. Handb Clin Neurol. 2019;160:393-403. [CrossRef]
- Braley T.J., Shieu M.M., Zaheed A.B., Dunietz G.L. Pathways between multiple sclerosis, sleep disorders, and cognitive function: Longitudinal findings from The Nurses' Health Study. Mult Scler. 2023, 29, 436-446. [CrossRef]
- Chinnadurai S.A., Gandhirajan D., Pamidimukala V.., Kesavamurthy B, Venkatesan S.A. Analysing the relationship between polysomnographic measures of sleep with measures of physical and cognitive fatigue in people with multiple sclerosis. Mult Scler Relat Disord. 2018 Aug;24:32-37. [CrossRef]
- Cederberg K.L.J., Mathison B.G., Schuetz M.L., Motl R.W. Discrepancies between self-reported and device-measured sleep parameters in adults with multiple sclerosis. J Clin Sleep Med. 2022 Feb 1;18:415-421. https://doi.org/10.5664/jcsm.9586. J., Chervin R.D., Segal B.M. Fatigue, tiredness, lack of energy, and sleepiness in multiple sclerosis patients referred for clinical polysomnography. Mult Scler Int. 2012;2012:673936. doi:10.1155/2012/673936.
- Laslett L.L., Honan C., Turner J.A., Dagnew B., Campbell J..A, Gill T.K., Appleton S., Blizzard L., Taylor B.V., van der Mei I. Poor sleep and multiple sclerosis: associations with symptoms of multiple sclerosis and quality of life. J Neurol Neurosurg Psychiatry. 2022, jnnp-2022-329227. [CrossRef]
- Foschi M., Rizzo G., Liguori R., Avoni P., Mancinelli L., Lugaresi A., Ferini-Strambi L. Sleep-related disorders and their relationship with MRI findings in multiple sclerosis. Sleep Med. 2019, 56, 90-97. https://doi.org/10.1016/j.sleep.2019.01.010. , Howard R.S., Hirsch N.P., Miller D.H., Moseley I.F., Fish D. Sleep problems in multiple sclerosis. Eur Neurol. 1994;34:320-3. doi:10.1159/000117070.
- Koblinger K., Füzesi T., Ejdrygiewicz J., Krajacic A., Bains J.S., Whelan P.J. Characterization of A11 neurons projecting to the spinal cord of mice. PLoS One. 2014, 9, e109636. [CrossRef]
- Clark C.M., Fleming J.A., Li D., Oger J., Klonoff H., Paty D. Sleep Disturbance, Depression, and Lesion Site in Patients With Multiple Sclerosis. Arch Neurol. 1992;49:641–643. [CrossRef]
- Ferini-Strambi L., Filippi M., Martinelli V., Oldani A., Rovaris M., Zucconi M., Comi G., Smirne S. Nocturnal sleep study in multiple sclerosis: correlations with clinical and brain magnetic resonance imaging findings. J Neurol Sci. 1994, 125, 194-7. [CrossRef]
- Benarroch E.E. The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology. 2009, 73, 1699-704. [CrossRef]
- Polak P.E., Kalinin S., Feinstein D.L. Locus coeruleus damage and noradrenaline reductions in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain. 2011 Mar;134(Pt 3):665-77. [CrossRef]
- Marien M.R., Colpaert F.C., Rosenquist A.C. Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res Brain Res Rev. 2004, 45, 38-78. [CrossRef]
- Biernacki T., Kokas Z., Sandi D., Füvesi J., Fricska-Nagy Z., Faragó P., Kincses T.Z., Klivényi P, Bencsik K., Vécsei L. Emerging Biomarkers of Multiple Sclerosis in the Blood and the CSF: A Focus on Neurofilaments and Therapeutic Considerations. Int. J. of Mol. Sci. 2022; 23:3383. [CrossRef]
- Whibley D., Goldstein C., Kratz A.L., Braley T.J. A multidimensional approach to sleep health in multiple sclerosis. Mult Scler Relat Disord. 2021, 56, 103271. [CrossRef]
- Irwin M.R., Opp M.R. Sleep Health: Reciprocal Regulation of Sleep and Innate Immunity. Neuropsychopharmacology. 2017, 42, 129-155. [CrossRef]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008, 9, 46-56. [CrossRef]
- Olofsson P.S., Rosas-Ballina M., Levine Y.A., Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev. 2012, 248, 188-204. [CrossRef]
- Imeri L., Opp M.R. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009, 10, 199-210. [CrossRef]
- Pan W., Kastin A.J. The Blood-Brain Barrier: Regulatory Roles in Wakefulness and Sleep. Neuroscientist. 2017, 23, 124-136. [CrossRef]
- He J., Hsuchou H., He Y., Kastin A.J., Wang Y., Pan W. Sleep restriction impairs blood-brain barrier function. J Neurosci. 2014, 34, 14697-706. [CrossRef]
- Dickstein J.B., Moldoksky. Sleep, cytokines and immune function. Sleep Med Rev. 1999. Vol.3, No.3, pp 219-228.
- Irwin M.R. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015, 66, 143-72. [CrossRef]
- Besedovsky L., Lange T., Born J. Sleep and immune function. Pflugers Arch. 2012, 463, 121-37. [CrossRef]
- Born W., Cady C., Jones-Carson J., Mukasa A., Lahn M., O'Brien R. Immunoregulatory functions of gamma delta T cells. Adv Immunol. 1999;71:77-144.
- Haus E.L., Smolensky M.H. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. 2013, 17, 273-84. [CrossRef]
- Kronfol Z., Nair M., Zhang Q., Hill E.E., Brown M.B. Circadian immune measures in healthy volunteers: relationship to hypothalamic-pituitary-adrenal axis hormones and sympathetic neurotransmitters. Psychosom Med. 1997, 59, 42-50. [CrossRef]
- Irwin M., McClintick J., Costlow C., Fortner M., White J., Gillin J.C. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996, 10, 643-53. [CrossRef]
- Redwine L., Dang J., Irwin M. Cellular adhesion molecule expression, nocturnal sleep, and partial night sleep deprivation. Brain Behav Immun. 2004, 18, 333-40. [CrossRef]
- Faraut B., Boudjeltia K.Z., Vanhamme L., Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2012, 16, 137-49. [CrossRef]
- Dinges D.F., Douglas S.D., Hamarman S., Zaugg L., Kapoor S. Sleep deprivation and human immune function. Adv Neuroimmunol. 1995;5:97-110. [CrossRef]
- Palmblad J., Petrini B., Wasserman J., Akerstedt T. Lymphocyte and granulocyte reactions during sleep deprivation. Psychosom Med. 1979, 41, 273-8. [CrossRef]
- Faraut B., Boudjeltia K.Z., Dyzma M., Rousseau A., David E., Stenuit P., Franck T., Van Antwerpen P., Vanhaeverbeek M., Kerkhofs M. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction. Brain Behav Immun. 2011, 25, 16-24. [CrossRef]
- Boudjeltia K.Z., Faraut B., Stenuit P., Esposito M.J., Dyzma M., Brohée D., Ducobu J., Vanhaeverbeek M., Kerkhofs M. Sleep restriction increases white blood cells, mainly neutrophil count, in young healthy men: a pilot study. Vasc Health Risk Manag. 2008;4:1467-70. [CrossRef]
- van Leeuwen W.M., Lehto M., Karisola P., Lindholm H., Luukkonen R., Sallinen M., Härmä M., Porkka-Heiskanen T., Alenius H. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4:e4589. [CrossRef]
- Schmid S.M., Hallschmid M., Jauch-Chara K., Wilms B., Lehnert H., Born J., Schultes B. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep. 2011, 34, 371-7. [CrossRef]
- Stamatakis K.A., Punjabi N.M. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010, 137, 95-101. [CrossRef]
- Shearer W.T., Reuben J.M.., Mullington J.M., Price N.J., Lee B.N., Smith E.O., Szuba M.P., Van Dongen H.P., Dinges D.F. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001, 107, 165-70. [CrossRef]
- Vgontzas A.N., Pejovic S., Zoumakis E., Lin H.M., Bixler E.O., Basta M., Fang J., Sarrigiannidis A., Chrousos G.P. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab. 2007, 292, E253-61. [CrossRef]
- Selter R.C., Hemmer B. Update on immunopathogenesis and immunotherapy in multiple sclerosis. Immunotargets Ther. 2013, 2, 21-30. [CrossRef]
- Gold R., Wolinsky J.S. Pathophysiology of multiple sclerosis and the place of teriflunomide. Acta Neurol Scand. 2011, 124, 75-84. [CrossRef]
- Blagov A.V., Grechko A.V., Nikiforov N.G., Borisov E.E., Sadykhov N.K., Orekhov A.N. Role of Impaired Mitochondrial Dynamics Processes in the Pathogenesis of Alzheimer's Disease. Int J Mol Sci. 2022, 23, 6954. [CrossRef]
- Koriem M. "Corrigendum to ‘Multiple sclerosis: New insights and trends’" Asian Pacific Journal of Tropical Biomedicine, 2017 vol. 7, no. 5,. [CrossRef]
- Bjartmar C., Wujek J.R., Trapp B.D. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003, 206, 165-71. [CrossRef]
- Brück W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol. 2005 Nov;252 Suppl 5:v3-9. [CrossRef]
- Gudkov S.V., Burmistrov D.E., Kondakova E.V., Sarimov R.M., Yarkov R.S., Franceschi C., Vedunova M.V. An emerging role of astrocytes in aging/neuroinflammation and gut-brain axis with consequences on sleep and sleep disorders. Ageing Res Rev. 2023, 83, 101775. [CrossRef]
- Morais L.H., Schreiber H.L. 4th, Mazmanian S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021, 19, 241-255. [CrossRef]
- Pak V.M., Onen S.H., Bliwise D.L., Kutner N.G., Russell K.L., Onen F. Sleep Disturbances in MCI and AD: Neuroinflammation as a Possible Mediating Pathway. Front Aging Neurosci. 2020, 12, 69. [CrossRef]
- Vaidyanathan T.V., Collard M., Yokoyama S., Reitman M.E., Poskanzer K.E. Cortical astrocytes independently regulate sleep depth and duration via separate GPCR pathways. Elife. 2021, 10, e63329. [CrossRef]
- Jewett K.A., Krueger J.M. Humoral sleep regulation; interleukin-1 and tumor necrosis factor. Vitam Horm. 2012;89:241-57. [CrossRef]
- Krueger J.M., Rector D.M., Roy S., Van Dongen H.P., Belenky G., Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008, 9, 910-9. [CrossRef]
- Obal F. Jr, Krueger J.M. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003, 8, d520-50. [CrossRef]
- Roky R., Obál F. Jr, Valatx J.L., Bredow S., Fang J., Pagano L.P., Krueger J.M. Prolactin and rapid eye movement sleep regulation. Sleep. 1995, 18, 536-42.
- Kilduff T.S., Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000, 23, 359-65. [CrossRef]
- Barun B. Pathophysiological background and clinical characteristics of sleep disorders in multiple sclerosis. Clin Neurol Neurosurg. 2013 Dec;115 Suppl 1:S82-5. [CrossRef]
- Motivala, S.J., & Irwin, M.R. Sleep and Immunity: Cytokine Pathways Linking Sleep and Health Outcomes. Current Directions in Psychological Science, 2007, 16(1), 21-25. [CrossRef]
- Opp M.R. Cytokines and sleep. Sleep Med Rev. 2005, 9, 355-64. [CrossRef]
- Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002, 105, 1135-43. [CrossRef]
- Born J., Lange T., Hansen K., Mölle M., Fehm H.L. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997, 158, 4454-64.
- Redwine L., Hauger R..L, Gillin J.C., Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000, 85, 3597-603. [CrossRef]
- Zielinski M.R., McKenna J.T., McCarley R.W. Functions and Mechanisms of Sleep. AIMS Neurosci. 2016;3:67-104. [CrossRef]
- Dinarello C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018, 281, 8-27. [CrossRef]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int. J. Mol. Sci. 2020, 21, 6009. [CrossRef]
- Viola-Saltzman M., Watson N.F. Traumatic brain injury and sleep disorders. Neurol Clin. 2012, 30, 1299-312. [CrossRef]
- Boutin H., LeFeuvre R.A., Horai R.., Asano M, Iwakura Y., Rothwell N.J. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001, 21, 5528-34. Erratum in: J Neurosci 2001, 21, 1a. [CrossRef]
- Berger A.M., Parker K.P., Young-McCaughan S., Mallory G.A., Barsevick A.M., Beck S.L., Carpenter J.S., Carter P.A., Farr L.A., Hinds P.S., Lee K.A., Miaskowski C., Mock V., Payne J.K., Hall M. Sleep wake disturbances in people with cancer and their caregivers: state of the science. Oncol Nurs Forum. 2005, 32, E98-126. [CrossRef]
- Opp M.R., Obal F. Jr, Krueger J.M. Interleukin 1 alters rat sleep: temporal and dose-related effects. Am J Physiol. 1991 Jan;260(1 Pt 2):R52-8. [CrossRef]
- Krueger J.M., Obál F.J., Fang J., Kubota T., Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2001, 933, 211-21. [CrossRef]
- Imeri L., Ceccarelli P., Mariotti M., Manfridi A., Opp M.R., Mancia M. Sleep, but not febrile responses of Fisher 344 rats to immune challenge are affected by aging. Brain Behav Immun. 2004, 18, 399-404. [CrossRef]
- De Sarro G., Gareri P., Sinopoli V.A., David E., Rotiroti D. Comparative, behavioural and electrocortical effects of tumor necrosis factor-alpha and interleukin-1 microinjected into the locus coeruleus of rat. Life Sci. 1997;60:555-64. [CrossRef]
- Manfridi A., Brambilla D., Bianchi S., Mariotti M., Opp M.R., Imeri L. Interleukin-1beta enhances non-rapid eye movement sleep when microinjected into the dorsal raphe nucleus and inhibits serotonergic neurons in vitro. Eur J Neurosci. 2003, 18, 1041-9. [CrossRef]
- Zielinski M.R., Krueger J.M. Sleep and innate immunity. Front Biosci (Schol Ed). 2011, 3, 632-42. [CrossRef]
- Obal F. Jr, Opp M., Cady A.B., Johannsen L., Postlethwaite A.E., Poppleton H.M., Seyer J.M., Krueger J.M. Interleukin 1 alpha and an interleukin 1 beta fragment are somnogenic. Am J Physiol. 1990 Sep;259(3 Pt 2):R439-46. [CrossRef]
- Zielinski M.R., Krueger J.M. Sleep and innate immunity. Front Biosci (Schol Ed). 2011 Jan 1;3:632-42. [CrossRef]
- Schmidt E.M., Linz B., Diekelmann S., Besedovsky L., Lange T., Born J. Effects of an interleukin-1 receptor antagonist on human sleep, sleep-associated memory consolidation, and blood monocytes. Brain Behav Immun. 2015, 47, 178-85. [CrossRef]
- Probert L. TNF and its receptors in the CNS: The essential, the desirable and the deleterious effects. Neuroscience. 2015, 302, 2-22. [CrossRef]
- Zielinski M.R., Systrom D.M., Rose N.R. Fatigue, Sleep, and Autoimmune and Related Disorders. Front Immunol. 2019, 10, 1827. [CrossRef]
- Zielinski M.R., Gibbons A.J. Neuroinflammation, Sleep, and Circadian Rhythms. Front Cell Infect Microbiol. 2022, 12, 853096. [CrossRef]
- Leibowitz S.M, Yan J. NF-κB Pathways in the Pathogenesis of Multiple Sclerosis and the Therapeutic Implications. Front Mol Neurosci. 2016, 9, 84. [CrossRef]
- Opp M.R., Toth L.A. Neural-immune interactions in the regulation of sleep. Front Biosci. 2003, 8, d768-79. [CrossRef]
- Irwin M.R. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. 2019, 19, 702-715. [CrossRef]
- Irwin M.R., Wang M., Campomayor C.O., Collado-Hidalgo A., Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006, 166, 1756-62. [CrossRef]
- Nishimoto N. and Kishimoto T. Interleukin 6: From Bench to Bedside. Nature Clinical Practice Rheumatology 2006, 2, 619-626. [CrossRef]
- Mohamed-Ali V., Flower L., Sethi .J, Hotamisligil G., Gray R., Humphries S.E., York D.A., Pinkney J. beta-Adrenergic regulation of IL-6 release from adipose tissue: in vivo and in vitro studies. J Clin Endocrinol Metab. 2001, 86, 5864-9. [CrossRef]
- Sironi M., Breviario F., Proserpio P., Biondi A., Vecchi A., Van Damme J., Dejana E., Mantovani A. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989, 142, 549-53.
- Bruunsgaard H., Andersen-Ranberg K., Hjelmborg J.v., Pedersen B.K., Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med. 2003, 115, 278-83. [CrossRef]
- Rohleder N., Aringer M., Boentert M. Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci. 2012, 1261, 88-96. [CrossRef]
- Vgontzas A.N., Papanicolaou D.A., Bixler E.O., Lotsikas A., Zachman K., Kales A., Prolo P., Wong M.L., Licinio J., Gold P.W., Hermida R.C., Mastorakos G., Chrousos G.P. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999, 84, 2603-7. [CrossRef]
- Lange T., Dimitrov S., Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010, 1193, 48-59. [CrossRef]
- Dimitrov S., Besedovsky L., Born J., Lange T. Differential acute effects of sleep on spontaneous and stimulated production of tumor necrosis factor in men. Brain Behav Immun. 2015, 47, 201-10. [CrossRef]
- Redwine L., Dang J., Hall M., Irwin M. Disordered sleep, nocturnal cytokines, and immunity in alcoholics. Psychosom Med. 2003, 65, 75-85. [CrossRef]
- Vgontzas A.N., Zoumakis E., Bixler E.O., Lin H.M., Follett H., Kales A., Chrousos G.P. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004, 89, 2119-26. [CrossRef]
- Simpson N., Dinges D.F. Sleep and inflammation. Nutr Rev. 2007 Dec;65(12 Pt 2):S244-52. [CrossRef]
- Burgos I., Richter L., Klein T., Fiebich B., Feige B., Lieb K., Voderholzer U., Riemann D. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun. 2006, 20, 246-53. [CrossRef]
- Späth-Schwalbe E., Hansen K., Schmidt F., Schrezenmeier H., Marshall L., Burger K., Fehm H.L., Born J. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab. 1998, 83, 1573-9. [CrossRef]
- Bargiela D., Chinnery P.F. Mitochondria in neuroinflammation - Multiple sclerosis (MS), leber hereditary optic neuropathy (LHON) and LHON-MS. Neurosci Lett. 2019, 710, 132932. [CrossRef]
- Patergnani S., Morciano G., Carinci M., Leo S., Pinton P., Rimessi A. The "mitochondrial stress responses": the "Dr. Jekyll and Mr. Hyde" of neuronal disorders. Neural Regen Res. 2022, 17, 2563-2575. [CrossRef]
- Mao, P. and Reddy, P.H. Is multiple sclerosis a mitochondrial disease? Biochimica et Biophysica Acta, 2010 1802, 66-79.
- Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr Biol. 2014 May 19;24:R453-62. [CrossRef]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979, 59, 527-605. [CrossRef]
- Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014, 94, 909-50. [CrossRef]
- Michaličková D., Hrnčíř T., Canová N.K., Slanař O. Targeting Keap1/Nrf2/ARE signaling pathway in multiple sclerosis. Eur J Pharmacol. 2020, 873, 172973. [CrossRef]
- van Horssen J., Schreibelt G., Drexhage J., Hazes T., Dijkstra C.D., van der Valk P., de Vries H.E. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic Biol Med. 2008, 45, 1729-37. [CrossRef]
- Ortiz G.G., Pacheco-Moisés F.P., Bitzer-Quintero O.K., Ramírez-Anguiano A.C., Flores-Alvarado L.J., Ramírez-Ramírez V., Macias-Islas M.A., Torres-Sánchez E.D. Immunology and oxidative stress in multiple sclerosis: clinical and basic approach. Clin Dev Immunol. 2013;2013:708659. [CrossRef]
- Park J.S., Chyun J.H., Kim Y.K., Line L.L., Chew B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab (Lond). 2010, 7, 18. [CrossRef]
- Reimund E. The free radical flux theory of sleep. Med Hypotheses. 1994, 43, 231-3. [CrossRef]
- Teixeira, N.B., Picolo, G., Giardini, A.C. Alterations of peripheral nerve excitability in an experimental autoimmune encephalomyelitis mouse model for multiple sclerosis. J Neuroinflammation 2020; 17, 266. [CrossRef]
- Villafuerte G., Miguel-Puga A., Rodríguez E.M., Machado S., Manjarrez E., Arias-Carrión O. Sleep deprivation and oxidative stress in animal models: a systematic review. Oxid Med Cell Longev. 2015;2015:234952. [CrossRef]
- Richardson R.B., Mailloux R.J. Mitochondria Need Their Sleep: Redox, Bioenergetics, and Temperature Regulation of Circadian Rhythms and the Role of Cysteine-Mediated Redox Signaling, Uncoupling Proteins, and Substrate Cycles. Antioxidants (Basel). 2023, 12, 674. [CrossRef]
- Hartmann C., Kempf A. Mitochondrial control of sleep. Curr Opin Neurobiol. 2023, 81, 102733. [CrossRef]
- Hill V.M., O'Connor R.M., Sissoko G.B., Irobunda I.S., Leong S., Canman J.C., Stavropoulos N., Shirasu-Hiza M. A bidirectional relationship between sleep and oxidative stress in Drosophila. PLoS Biol. 2018, 16, e2005206. [CrossRef]
- Gulec M., Ozkol H., Selvi Y., Tuluce Y., Aydin A., Besiroglu L., Ozdemir P.G. Oxidative stress in patients with primary insomnia. Prog Neuropsychopharmacol Biol Psychiatry. 2012, 37, 247-51. [CrossRef]
- Silva R.H., Abílio V.C., Takatsu A.L., Kameda S.R., Grassl C., Chehin A.B., Medrano W.A., Calzavara M.B., Registro S., Andersen M.L., Machado R.B.., Carvalho R.C., Ribeiro Rde A., Tufik S., Frussa-Filho R. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004, 46, 895-903. [CrossRef]
- Vaccaro A., Kaplan Dor Y., Nambara K., Pollina E.A., Lin C., Greenberg M.E., Rogulja D. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell. 2020, 181, 1307-1328.e15. [CrossRef]
- Everson C.A., Henchen C.J., Szabo A., Hogg N. Cell injury and repair resulting from sleep loss and sleep recovery in laboratory rats. Sleep. 2014, 37, 1929-40. [CrossRef]
- Kempf A., Song S.M., Talbot C.B., Miesenböck G. A potassium channel β-subunit couples mitochondrial electron transport to sleep. Nature. 2019, 568, 230-234. [CrossRef]
- Shadel G.S., Horvath T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015, 163, 560-9. [CrossRef]
- Melhuish Beaupre L.M., Brown G.M., Braganza N.A., Kennedy J.L., Gonçalves V.F. Mitochondria's role in sleep: Novel insights from sleep deprivation and restriction studies. World J Biol Psychiatry. 2022, 23, 1-13. [CrossRef]
- Rodriguez A.M., Nakhle J., Griessinger E., Vignais M.L. Intercellular mitochondria trafficking highlighting the dual role of mesenchymal stem cells as both sensors and rescuers of tissue injury. Cell Cycle. 2018;17:712-721. [CrossRef]
- Somarajan B.I., Khanday M.A., Mallick B.N. Rapid Eye Movement Sleep Deprivation Induces Neuronal Apoptosis by Noradrenaline Acting on Alpha1 Adrenoceptor and by Triggering Mitochondrial Intrinsic Pathway. Front Neurol. 2016, 7, 25. [CrossRef]
- Singh M., Jadhav H.R. Melatonin: functions and ligands. Drug Discov Today. 2014, 19, 1410-8. [CrossRef]
- Blanco R.A., Ziegler T.R., Carlson B.A., Cheng P.Y., Park Y., Cotsonis G.A., Accardi C.J., Jones D.P. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr. 2007, 86, 1016-23. [CrossRef]
- Wilking M., Ndiaye M., Mukhtar H., Ahmad N. Circadian rhythm connections to oxidative stress: implications for human health. Antioxid Redox Signal. 2013, 19, 192-208. [CrossRef]
- Ramanathan L., Gulyani S., Nienhuis R., Siegel J.M. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport. 2002, 13, 1387-90. [CrossRef]
- Atrooz F., Salim S. Sleep deprivation, oxidative stress and inflammation. Adv Protein Chem Struct Biol. 2020;119:309-336. [CrossRef]
- Zhao D., Yu Y., Shen Y., Liu Q., Zhao Z., Sharma R., Reiter R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front Endocrinol (Lausanne). 2019, 10, 249. [CrossRef]
- Gunata M., Parlakpinar H., Acet H.A. Melatonin: A review of its potential functions and effects on neurological diseases. Rev Neurol (Paris). 2020, 176, 148-165. [CrossRef]
- Claustrat B., Leston J. Melatonin: Physiological effects in humans. Neurochirurgie. 2015 Apr-Jun;61(2-3):77-84. [CrossRef]
- Pardridge W.M., Mietus L.J. Transport of albumin-bound melatonin through the blood-brain barrier. J Neurochem. 1980, 34, 1761-3. [CrossRef]
- Dubocovich M.L., Delagrange P., Krause D.N., Sugden D., Cardinali D.P., Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010, 62, 343-80. [CrossRef]
- Cecon E., Oishi A., Jockers R. Melatonin receptors: molecular pharmacology and signalling in the context of system bias. Br J Pharmacol. 2018, 175, 3263-3280. [CrossRef]
- Weaver D.R., Liu C., Reppert S.M. Nature's knockout: the Mel1b receptor is not necessary for reproductive and circadian responses to melatonin in Siberian hamsters. Mol Endocrinol. 1996, 10, 1478-87. [CrossRef]
- Mazzucchelli C., Pannacci M., Nonno R., Lucini V., Fraschini F., Stankov B.M. The melatonin receptor in the human brain: cloning experiments and distribution studies. Brain Res Mol Brain Res. 1996 Jul;39(1-2):117-26. [CrossRef]
- Savaskan E., Wirz-Justice A., Olivieri G., Pache M., Kräuchi K., Brydon L., Jockers R., Müller-Spahn F., Meyer P. Distribution of melatonin MT1 receptor immunoreactivity in human retina. J Histochem Cytochem. 2002, 50, 519-26. [CrossRef]
- Blodgett T.J., Blodgett N.P. Melatonin and melatonin-receptor agonists to prevent delirium in hospitalized older adults: An umbrella review. Geriatr Nurs. 2021, 42, 1562-1568. [CrossRef]
- Ren W., Liu G., Chen S., Yin J., Wang J., Tan B., Wu G., Bazer F.W., Peng Y., Li T., Reiter R.J., Yin Y. Melatonin signaling in T cells: Functions and applications. J Pineal Res. 2017 Apr;62(3). [CrossRef]
- Reppert S.M., Godson C., Mahle C.D., Weaver D.R., Slaugenhaupt S.A., Gusella J.F. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci U S A. 1995, 92, 8734-8. [CrossRef]
- Al-Ghoul W.M., Herman M.D., Dubocovich M.L. Melatonin receptor subtype expression in human cerebellum. Neuroreport. 1998, 9, 4063-8. [CrossRef]
- Wu Y.H., Ursinus J., Zhou J.N., Scheer F.A., Ai-Min B., Jockers R., van Heerikhuize J., Swaab D.F. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord. 2013 Jun;148(2-3):357-67. [CrossRef]
- Esposito S., Laino D., D'Alonzo R., Mencarelli A., Di Genova L., Fattorusso A., Argentiero A., Mencaroni E. Pediatric sleep disturbances and treatment with melatonin. J Transl Med. 2019, 17, 77. [CrossRef]
- Sack R.L., Lewy A.J., Hughes R.J. Use of melatonin for sleep and circadian rhythm disorders. Ann Med. 1998, 30, 115-21. [CrossRef]
- Liebmann P.M., Wölfler A., Felsner P., Hofer D., Schauenstein K. Melatonin and the immune system. Int Arch Allergy Immunol. 1997, 112, 203-11. [CrossRef]
- Reiter R.J. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991 Aug;79(1-3):C153-8. [CrossRef]
- Minich D.M., Henning M., Darley C., Fahoum M., Schuler C.B., Frame J. Is Melatonin the "Next Vitamin D"?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements. Nutrients. 2022 Sep 22;14:3934. [CrossRef]
- Hardeland R., Cardinali D.P., Srinivasan V., Spence D.W., Brown G.M., Pandi-Perumal S.R. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011, 93, 350-84. [CrossRef]
- Akpinar Z., Tokgöz S., Gökbel H., Okudan N., Uğuz F., Yilmaz G. The association of nocturnal serum melatonin levels with major depression in patients with acute multiple sclerosis. Psychiatry Res. 2008, 161, 253-7. [CrossRef]
- Ghorbani A., Salari M., Shaygannejad V., Norouzi R. The role of melatonin in the pathogenesis of multiple sclerosis: a case-control study. Int J Prev Med. 2013 May;4(Suppl 2):S180-4.
- Melamud L., Golan D., Luboshitzky R., Lavi I., Miller A. Melatonin dysregulation, sleep disturbances and fatigue in multiple sclerosis. J Neurol Sci. 2012 Mar 15;314(1-2):37-40. [CrossRef]
- Sandyk R., Awerbuch G.I. Nocturnal melatonin secretion in multiple sclerosis patients with affective disorders. Int J Neurosci. 1993 Feb;68(3-4):227-40. [CrossRef]
- López-González A., Álvarez-Sánchez N., Lardone P.J., Cruz-Chamorro I., Martínez-López A., Guerrero J.M., Reiter R.J., Carrillo-Vico A. Melatonin treatment improves primary progressive multiple sclerosis: a case report. J Pineal Res. 2015, 58, 173-7. [CrossRef]
- Ghareghani M., Scavo L., Jand Y., Farhadi N., Sadeghi H., Ghanbari A., Mondello S., Arnoult D., Gharaghani S., Zibara K. Melatonin Therapy Modulates Cerebral Metabolism and Enhances Remyelination by Increasing PDK4 in a Mouse Model of Multiple Sclerosis. Front Pharmacol. 2019, 10, 147. [CrossRef]
- Damasceno A., Moraes A.S., Farias A., Damasceno B.P., dos Santos L.M., Cendes F. Disruption of melatonin circadian rhythm production is related to multiple sclerosis severity: A preliminary study. J Neurol Sci. 2015;353(1-2):166-8. [CrossRef]
- Gholipour T., Ghazizadeh T., Babapour S., Mansouri B., Ghafarpour M., Siroos B., Harirchian M.H. Decreased urinary level of melatonin as a marker of disease severity in patients with multiple sclerosis. Iran J Allergy Asthma Immunol. 2015, 14, 91-7.
- Adamczyk-Sowa M., Pierzchala K., Sowa P., Mucha S, Sadowska-Bartosz I., Adamczyk J., Hartel M. Melatonin acts as antioxidant and improves sleep in MS patients. Neurochem Res. 2014, 39, 1585-93. [CrossRef]
- Sánchez-López A.L., Ortiz G.G., Pacheco-Moises F.P., Mireles-Ramírez M.A., Bitzer-Quintero O.K., Delgado-Lara D.L.C., Ramírez-Jirano L.J., Velázquez-Brizuela I.E. Efficacy of Melatonin on Serum Pro-inflammatory Cytokines and Oxidative Stress Markers in Relapsing Remitting Multiple Sclerosis. Arch Med Res. 2018, 49, 391-398. [CrossRef]
- Yosefifard M., Vaezi G., Malekirad A.A., Faraji F., Hojati V. A Randomized Control Trial Study to Determine the Effect of Melatonin on Serum Levels of IL-1β and TNF-α in Patients with Multiple Sclerosis. Iran J Allergy Asthma Immunol. 2019, 18, 649-654. [CrossRef]
- Zarezadeh M., Khorshidi M., Emami M., Janmohammadi P., Kord-Varkaneh H., Mousavi S.M., Mohammed S.H., Saedisomeolia A., Alizadeh S. Melatonin supplementation and pro-inflammatory mediators: a systematic review and meta-analysis of clinical trials. Eur J Nutr. 2020, 59, 1803-1813. [CrossRef]
- Serada S., Fujimoto M., Mihara M., Koike N., Ohsugi Y., Nomura S., Yoshida H., Nishikawa T., Terabe F., Ohkawara T., Takahashi T., Ripley B., Kimura A., Kishimoto T., Naka T. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008, 105, 9041-6. [CrossRef]
- Michaud M., Balardy L., Moulis G., Gaudin C., Peyrot C., Vellas B., Cesari M., Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013, 14, 877-82. [CrossRef]
- Chen M., Wang Y.H., Wang Y., Huang L., Sandoval H., Liu Y.J., Wang J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. lFeb 24;311:1160-4. [CrossRef]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013, 39, 1-10. [CrossRef]
- 187. Farez MF, Mascanfroni ID, Méndez-Huergo SP, Yeste A, Murugaiyan G, Garo LP, Balbuena Aguirre ME, Patel B, Ysrraelit MC, Zhu C, Kuchroo VK, Rabinovich GA, Quintana FJ, Correale J. Melatonin Contributes to the Seasonality of Multiple Sclerosis Relapses. Cell. 2015, 162, 1338-52. [CrossRef]
- Álvarez-Sánchez N, Cruz-Chamorro I, Díaz-Sánchez M, Sarmiento-Soto H, Medrano-Campillo P, Martínez-López A, Lardone PJ, Guerrero JM, Carrillo-Vico A. Melatonin reduces inflammatory response in peripheral T helper lymphocytes from relapsing-remitting multiple sclerosis patients. J Pineal Res. 2017 Nov;63(4). [CrossRef]
- Muñoz-Jurado A, Escribano BM, Caballero-Villarraso J, Galván A, Agüera E, Santamaría A, Túnez I. Melatonin and multiple sclerosis: antioxidant, anti-inflammatory and immunomodulator mechanism of action. Inflammopharmacology. 2022 Oct;30:1569-1596. [CrossRef]
- Adamczyk-Sowa M, Sowa P, Adamczyk J, Niedziela N, Misiolek H, Owczarek M, Zwirska-Korczala K. Effect of melatonin supplementation on plasma lipid hydroperoxides, homocysteine concentration and chronic fatigue syndrome in multiple sclerosis patients treated with interferons-beta and mitoxantrone. J Physiol Pharmacol. 2016, 67, 235–42.
- Yosefifard, Masoomeh & Vaezi, Gholamhassan & Maleki-Rad, Ali & Faraji, Fardin & Hojati, Vida. (2020). Effect of Melatonin on Serum Levels of INF-1β and Vitamin B12 in Patients With Multiple Sclerosis: A Randomized Controlled Trial. Iranian Journal of Toxicology. 14. 19-24. [CrossRef]
- Miller E, Walczak A, Majsterek I, Kędziora J. Melatonin reduces oxidative stress in the erythrocytes of multiple sclerosis patients with secondary progressive clinical course. J Neuroimmunol. 2013 Apr 15;257(1-2):97-101. [CrossRef]
- Roostaei T, Sahraian MA, Hajeaghaee S, Gholipour T, Togha M, Siroos B, Mansouri S, Mohammadshirazi Z, Aghazadeh Alasti M, Harirchian MH. Impact of Melatonin on Motor, Cognitive and Neuroimaging Indices in Patients with Multiple Sclerosis. Iran J Allergy Asthma Immunol. 2015 Dec;14:589-95.
- Padureanu R, Albu CV, Mititelu RR, Bacanoiu MV, Docea AO, Calina D, Padureanu V, Olaru G, Sandu RE, Malin RD, Buga AM. Oxidative Stress and Inflammation Interdependence in Multiple Sclerosis. J Clin Med. 2019, 8, 1815. [CrossRef]
- Senthil Kumaran V, Arulmathi K, Srividhya R, Kalaiselvi P. Repletion of antioxidant status by EGCG and retardation of oxidative damage induced macromolecular anomalies in aged rats. Exp Gerontol. 2008, 43, 176-83. [CrossRef]
- Maes M, Landucci Bonifacio K, Morelli NR, Vargas HO, Barbosa DS, Carvalho AF, Nunes SOV. Major Differences in Neurooxidative and Neuronitrosative Stress Pathways Between Major Depressive Disorder and Types I and II Bipolar Disorder. Mol Neurobiol. 2019, 56, 141-156. [CrossRef]
- Holton KF, Kirkland AE. Moving past antioxidant supplementation for the dietary treatment of multiple sclerosis. Mult Scler. 2020, 26, 1012-1023. [CrossRef]
- Morris JL, Gillet G, Prudent J, Popgeorgiev N. Bcl-2 Family of Proteins in the Control of Mitochondrial Calcium Signalling: An Old Chap with New Roles. Int J Mol Sci. 2021, 22, 3730. [CrossRef]
- Maldonado MD, Mora-Santos M, Naji L, Carrascosa-Salmoral MP, Naranjo MC, Calvo JR. Evidence of melatonin synthesis and release by mast cells. Possible modulatory role on inflammation. Pharmacol Res. 2010, 62, 282-7. [CrossRef]
- Escribano BM, Colín-González AL, Santamaría A, Túnez I. The role of melatonin in multiple sclerosis, Huntington's disease and cerebral ischemia. CNS Neurol Disord Drug Targets. 2014;13:1096-119. [CrossRef]
- Túnez I, Montilla P, Del Carmen Muñoz M, Feijóo M, Salcedo M. Protective effect of melatonin on 3-nitropropionic acid-induced oxidative stress in synaptosomes in an animal model of Huntington's disease. J Pineal Res. 2004, 37, 252-6. [CrossRef]
- Bahamonde C, Conde C, Agüera E, Lillo R, Luque E, Gascón F, Feijóo M, Cruz AH, Sánchez-López F, Túnez I. Elevated melatonin levels in natalizumab-treated female patients with relapsing-remitting multiple sclerosis: relationship to oxidative stress. Eur J Pharmacol. 2014, 730, 26-30. [CrossRef]
- Alghamdi BS, AboTaleb HA. Melatonin improves memory defects in a mouse model of multiple sclerosis by up-regulating cAMP-response element-binding protein and synapse-associated proteins in the prefrontal cortex. J Integr Neurosci. 2020, 19, 229-237. [CrossRef]
- Campbell A, Sharman E, Bondy SC. Age-related differences in the response of the brain to dietary melatonin. Age (Dordr). 2014, 36, 49-55. [CrossRef]
- Lassmann H, van Horssen J. Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim Biophys Acta. 2016, 1862, 506-10. [CrossRef]
- Michaličková D, Šíma M, Slanař O. New insights in the mechanisms of impaired redox signaling and its interplay with inflammation and immunity in multiple sclerosis. Physiol Res. 2020, 69, 1-19. [CrossRef]
- Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J Pineal Res. 2010, 48, 297-310. [CrossRef]
- Anderson, G. 2019. Mitochondria and the Gut as crucial hubs for the interactions of melatonin with sirtuins, inflammation, butyrate, tryptophan metabolites, and alpha 7 nicotinic receptor across a host of medical conditions. Melatonin Research. 2, 2 (June 2019), 70-85. [CrossRef]
- Leon J, Acuña-Castroviejo D, Sainz RM, Mayo JC, Tan DX, Reiter RJ. Melatonin and mitochondrial function. Life Sci. 2004, 75, 765-90. [CrossRef]
- Martín M, Macías M, Escames G, Reiter RJ, Agapito MT, Ortiz GG, Acuña-Castroviejo D. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J Pineal Res. 2000, 28, 242-8. [CrossRef]
- Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002, 19, 695-707. [CrossRef]
- Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014, 71, 2997-3025. [CrossRef]
- Hsu WY, Anderson A, Rowles W, Peters KE, Li V, Stone KL, Ashbrook LH, Gelfand AA, Bove RM. Effects of melatonin on sleep disturbances in multiple sclerosis: A randomized, controlled pilot study. Mult Scler J Exp Transl Clin. 2021, 7, 20552173211048756. [CrossRef]
- Mocayar Marón FJ, Ferder L, Reiter RJ, Manucha W. Daily and seasonal mitochondrial protection: Unraveling common possible mechanisms involving vitamin D and melatonin. J Steroid Biochem Mol Biol. 2020, 199, 105595. [CrossRef]
- El-Salem K, Khalil H, Al-Sharman A, Al-Mistarehi AH, Yassin A, Alhayk KA, Qawasmeh MA, Bashayreh SY, Kofahi RM, Obeidat AZ. Serum vitamin d inversely correlates with depression scores in people with multiple sclerosis. Mult Scler Relat Disord. 2021, 48, 102732. [CrossRef]
- Munger KL, Zhang SM, O'Reilly E, Hernán MA, Olek MJ, Willett WC, Ascherio A. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004, 62, 60-5. [CrossRef]
- Ascherio A, Munger KL, White R, Köchert K, Simon KC, Polman CH, Freedman MS, Hartung HP, Miller DH, Montalbán X, Edan G, Barkhof F, Pleimes D, Radü EW, Sandbrink R, Kappos L, Pohl C. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol. 2014, 71, 306-14. [CrossRef]
- Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010, 9, 941-55. [CrossRef]
- Veldurthy V, Wei R, Oz L, Dhawan P, Jeon YH, Christakos S. Vitamin D, calcium homeostasis and aging. Bone Res. 2016, 4, 16041. [CrossRef]
- Hewer S, Lucas R, van der Mei I, Taylor BV. Vitamin D and multiple sclerosis. J Clin Neurosci. 2013, 20, 634-41. [CrossRef]
- McLaughlin L, Clarke L, Khalilidehkordi E, Butzkueven H, Taylor B, Broadley SA. Vitamin D for the treatment of multiple sclerosis: a meta-analysis. J Neurol. 2018, 265, 2893-2905. [CrossRef]
- Toell A, Polly P, Carlberg C. All natural DR3-type vitamin D response elements show a similar functionality in vitro. Biochem J. 2000 Dec 1;352 Pt 2(Pt 2):301-9.
- May E, Asadullah K, Zügel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004 Dec;3:377-93. [CrossRef]
- Montava M, Garcia S, Mancini J, Jammes Y, Courageot J, Lavieille JP, Feron F. Vitamin D3 potentiates myelination and recovery after facial nerve injury. Eur Arch Otorhinolaryngol. 2015, 272, 2815-23. [CrossRef]
- Matías-Guíu J, Oreja-Guevara C, Matias-Guiu JA, Gomez-Pinedo U. Vitamin D and remyelination in multiple sclerosis. Neurologia (Engl Ed). 2018, 33, 177-186. English, Spanish. [CrossRef]
- Pierrot-Deseilligny C, Souberbielle JC. Vitamin D and multiple sclerosis: An update. Mult Scler Relat Disord. 2017, 14, 35-45. [CrossRef]
- von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010, 11, 344-9. [CrossRef]
- Danner OK, Matthews LR, Francis S, Rao VN, Harvey CP, Tobin RP, Wilson KL, Alema-Mensah E, Newell Rogers MK, Childs EW. Vitamin D3 Suppresses Class II Invariant Chain Peptide Expression on Activated B-Lymphocytes: A Plausible Mechanism for Downregulation of Acute Inflammatory Conditions. J Nutr Metab. 2016;2016:4280876. [CrossRef]
- Fawaz L, Mrad MF, Kazan JM, Sayegh S, Akika R, Khoury SJ. Comparative effect of 25(OH)D3 and 1,25(OH)2D3 on Th17 cell differentiation. Clin Immunol. 2016 May;166-167:59-71. https://doi.org/10.1016/j.clim.2016.02.011. Vitamin D effects on B cell function in autoimmunity. Ann N Y Acad Sci. 2014 May;1317:84-91. doi:10.1111/nyas.12440.
- da Costa DS, Hygino J, Ferreira TB, Kasahara TM, Barros PO, Monteiro C, Oliveira A, Tavares F, Vasconcelos CC, Alvarenga R, Bento CA. Vitamin D modulates different IL-17-secreting T cell subsets in multiple sclerosis patients. J Neuroimmunol. 2016, 299, 8-18. [CrossRef]
- Sotirchos ES, Bhargava P, Eckstein C, Van Haren K, Baynes M, Ntranos A, Gocke A, Steinman L, Mowry EM, Calabresi PA. Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis. Neurology. 2016, 86, 382-90. [CrossRef]
- Ashtari F, Toghianifar N, Zarkesh-Esfahani SH, Mansourian M. Short-term effect of high-dose vitamin D on the level of interleukin 10 in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled clinical trial. Neuroimmunomodulation. 2015;22:400-4. [CrossRef]
- Mosayebi G, Ghazavi A, Ghasami K, Jand Y, Kokhaei P. Therapeutic effect of vitamin D3 in multiple sclerosis patients. Immunol Invest. 2011;40:627-39. [CrossRef]
- Haas MJ, Jafri M, Wehmeier KR, Onstead-Haas LM, Mooradian AD. Inhibition of endoplasmic reticulum stress and oxidative stress by vitamin D in endothelial cells. Free Radic Biol Med. 2016, 99, 1-10. [CrossRef]
- Smolders J, Hupperts R, Barkhof F, Grimaldi LM, Holmoy T, Killestein J, Rieckmann P, Schluep M, Vieth R, Hostalek U, Ghazi-Visser L, Beelke M; SOLAR study group. Efficacy of vitamin D3 as add-on therapy in patients with relapsing-remitting multiple sclerosis receiving subcutaneous interferon β-1a: a Phase II, multicenter, double-blind, randomized, placebo-controlled trial. J Neurol Sci. 2011 Dec 15;311(1-2):44-9. [CrossRef]
- 236. Sandberg L, Biström M, Salzer J, Vågberg M, Svenningsson A, Sundström P. Vitamin D and axonal injury in multiple sclerosis. Mult Scler. 2016 Jul;22:1027-31. [CrossRef]
- Wagner CL, Hollis BW, Kotsa K, Fakhoury H, Karras SN. Vitamin D administration during pregnancy as prevention for pregnancy, neonatal and postnatal complications. Rev Endocr Metab Disord. 2017, 18, 307-322. [CrossRef]
- Pierrot-Deseilligny C, Rivaud-Péchoux S, Clerson P, de Paz R, Souberbielle JC. Relationship between 25-OH-D serum level and relapse rate in multiple sclerosis patients before and after vitamin D supplementation. Ther Adv Neurol Disord. 2012, 5, 187-98. [CrossRef]
- McCarty DE, Chesson AL Jr, Jain SK, Marino AA. The link between vitamin D metabolism and sleep medicine. Sleep Med Rev. 2014, 18, 311-9. [CrossRef]
- Dong X, Craig T, Xing N, Bachman LA, Paya CV, Weih F, McKean DJ, Kumar R, Griffin MD. Direct transcriptional regulation of RelB by 1alpha,25-dihydroxyvitamin D3 and its analogs: physiologic and therapeutic implications for dendritic cell function. J Biol Chem. 2003, 278, 49378-85. [CrossRef]
- Majde JA, Krueger JM. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005, 116, 1188-98. [CrossRef]
- Ryan S, Nolan GM, Hannigan E, Cunningham S, Taylor C, McNicholas WT. Cardiovascular risk markers in obstructive sleep apnoea syndrome and correlation with obesity. Thorax. 2007, 62, 509-14. [CrossRef]
- McCarty DE. Resolution of hypersomnia following identification and treatment of vitamin d deficiency. J Clin Sleep Med. 2010, 6, 605-8.
- McCarty DE, Reddy A, Keigley Q, Kim PY, Marino AA. Vitamin D, race, and excessive daytime sleepiness. J Clin Sleep Med. 2012, 8, 693-7. [CrossRef]
- Muscogiuri G, Barrea L, Savastano S, Colao A. Nutritional recommendations for CoVID-19 quarantine. Eur J Clin Nutr. 2020, 74, 850-851. [CrossRef]
- Gao Q, Kou T, Zhuang B, Ren Y, Dong X, Wang Q. The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-Analysis. Nutrients. 2018, 10, 1395. [CrossRef]
- Al-Shawwa B, Ehsan Z, Ingram DG. Vitamin D and sleep in children. J Clin Sleep Med. 2020, 16, 1119-1123. [CrossRef]
- Abboud M. Vitamin D Supplementation and Sleep: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients. 2022, 14, 1076. [CrossRef]
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005, 29, 21-30. [CrossRef]
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002, 13, 100-5. [CrossRef]
- Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011, 57, 63-9. [CrossRef]
- Vitaterna MH, Takahashi JS, Turek FW. Overview of circadian rhythms. Alcohol Res Health. 2001;25:85-93.
- Lucock M, Jones P, Martin C, Beckett E, Yates Z, Furst J, Veysey M. Vitamin D: Beyond Metabolism. J Evid Based Complementary Altern Med. 2015, 20, 310-22. [CrossRef]
- Jamilian H, Amirani E, Milajerdi A, Kolahdooz F, Mirzaei H, Zaroudi M, Ghaderi A, Asemi Z. The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Prog Neuropsychopharmacol Biol Psychiatry. 2019, 94, 109651. [CrossRef]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517-49. [CrossRef]
- Kaneko I, Sabir MS, Dussik CM, Whitfield GK, Karrys A, Hsieh JC, Haussler MR, Meyer MB, Pike JW, Jurutka PW. 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: implication for behavioral influences of vitamin D. FASEB J. 2015, 29, 4023-35. [CrossRef]
- Ghareghani M, Zibara K, Rivest S. Melatonin and vitamin D, two sides of the same coin, better to land on its edge to improve multiple sclerosis. Proc Natl Acad Sci U S A. 2023, 120, e2219334120. [CrossRef]
- Golan D, Staun-Ram E, Glass-Marmor L, Lavi I, Rozenberg O, Dishon S, Barak M, Ish-Shalom S, Miller A. The influence of vitamin D supplementation on melatonin status in patients with multiple sclerosis. Brain Behav Immun. 2013, 32, 180-5. [CrossRef]
- Sivertsen B, Omvik S, Pallesen S, Bjorvatn B, Havik OE, Kvale G, Nielsen GH, Nordhus IH. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006, 295, 2851-8. [CrossRef]
- Morin CM, Vallières A, Guay B, Ivers H, Savard J, Mérette C, Bastien C, Baillargeon L. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009, 301, 2005-15. [CrossRef]
- Morin CM, Benca R. Chronic insomnia. Lancet. 2012, 379, 1129-41. Erratum in: Lancet. 2012, 379, 1488. [CrossRef]
- Edinger JD, Means MK. Cognitive-behavioral therapy for primary insomnia. Clin Psychol Rev. 2005, 25, 539-58. [CrossRef]
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008, 4, 487-504.
- Baron KG, Corden M, Jin L, Mohr DC. Impact of psychotherapy on insomnia symptoms in patients with depression and multiple sclerosis. J Behav Med. 2011, 34, 92-101. [CrossRef]
- Williams-Cooke C, LeSuer L, Drerup M, Siengsukon C. The Impact of Cognitive Behavioral Therapy for Insomnia on Sleep Log and Actigraphy Outcomes in People with Multiple Sclerosis: A Secondary Analysis. Nat Sci Sleep. 2021, 13, 1865-1874. [CrossRef]
- Turkowitch D, Ludwig R, Nelson E, Drerup M, Siengsukon CF. Telehealth-Delivered Cognitive Behavioral Therapy for Insomnia in Individuals with Multiple Sclerosis: A Pilot Study. Mult Scler Int. 2022, 2022, 7110582. [CrossRef]
- Nadjar Y, Coutelas E, Prouteau P, Panzer F, Paquet D, Saint-Val C, Créange A. Injection of interferon-beta in the morning decreases flu-like syndrome in many patients with multiple sclerosis. Clin Neurol Neurosurg. 2011, 113, 316-22. [CrossRef]
- Bøe Lunde HM, Aae TF, Indrevåg W, Aarseth J, Bjorvatn B, Myhr KM, Bø L. Poor sleep in patients with multiple sclerosis. PLoS One. 2012;7:e49996. [CrossRef]
- Rocchi C, Pulcini A, Vesprini C, Totaro V, Viticchi G, Falsetti L, Danni MC, Bartolini M, Silvestrini M, Buratti L. Sleep in multiple sclerosis patients treated with interferon beta: an actigraphic study. Neurol Res. 2020, 42, 744-748. [CrossRef]
- Braley TJ, Chervin RD. A practical approach to the diagnosis and management of sleep disorders in patients with multiple sclerosis. Ther Adv Neurol Disord. 2015, 8, 294-310. [CrossRef]
- Morrison I, Riha RL. Excessive daytime sleepiness and narcolepsy--an approach to investigation and management. Eur J Intern Med. 2012, 23, 110-7. [CrossRef]
- Côté I, Trojan DA, Kaminska M, Cardoso M, Benedetti A, Weiss D, Robinson A, Bar-Or A, Lapierre Y, Kimoff RJ. Impact of sleep disorder treatment on fatigue in multiple sclerosis. Mult Scler. 2013, 19, 480-9. [CrossRef]
- Sivam S, Yee BJ. Role of gabapentin enacarbil XR in restless legs syndrome. Ther Clin Risk Manag. 2012;8:201-8. [CrossRef]
- Aburub A, Khalil H, Al-Sharman A, Alomari M, Khabour O. The association between physical activity and sleep characteristics in people with multiple sclerosis. Mult Scler Relat Disord. 2017, 12, 29-33. [CrossRef]
- Newland P, Lorenz RA, Smith JM, Dean E, Newland J, Cavazos P. The Relationship Among Multiple Sclerosis-Related Symptoms, Sleep Quality, and Sleep Hygiene Behaviors. J Neurosci Nurs. 2019, 51, 37-42. [CrossRef]
- Amtmann D, Askew RL, Kim J, Chung H, Ehde DM, Bombardier CH, Kraft GH, Jones SM, Johnson KL. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015, 60, 81-90. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




