Submitted:
09 November 2023
Posted:
14 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. West Nile Virus
2.1. West Nile Virus in vultures
| Species | WNV diagnosis | Geographical area | Year | Study | |
|---|---|---|---|---|---|
|
Gypaetus barbatus |
ELISA; VNT |
Austria |
NM |
[56] |
|
|
Gypaetus barbatus |
RT-PCR |
Austria |
2008 |
[57] |
|
|
Aegypius monachus Neophron percnopterus |
ELISA | Spain | 2006-2009 | [58] | |
|
Gypaetus barbatus |
ELISA; SNT |
Spain |
2017 |
[59] |
|
| Neophron percnopterus | ELISA; MNT | Iran | 2017-2018 | [60] | |
|
Aegypius monachus Gyps fulvus |
ELISA; VNT; RT-PCR |
Spain | 2017-2019 | [63] | |
|
Gyps fulvus |
RT-PCR | Serbia | 2018-2022 | [64] |
3. Discussion
4. Conclusion
Author Contributions
Conflicts of Interest
References
- Houston, D.C.; Cooper, J.E. The digestive tract of the whiteback griffon vulture and its role in disease transmission among wild ungulates. J. Wildl. Dis. 1975, 11, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Moleón, M.; Sánchez-Zapata, J.A.; Margalida, A.; Carrete, M.; Owen-Smith, N.; Donázar, J.A. Humans and scavengers: The evolution of interactions and ecosystem services, BioScience. 2014, 64, 394–403. [CrossRef]
- Blumstein, D.T.; Rangchi, T.N.; Briggs, T.; De Andrade, F.S.; Natterson-Horowitz, B. A systematic review of carrion eaters' Adaptations to Avoid Sickness. J. Wildl. Dis. 2017, 53, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Arbulu, S., Jiménez, J.J., Gútiez, L. et al. Evaluation of bacteriocinogenic activity, safety traits and biotechnological potential of fecal lactic acid bacteria (LAB), isolated from Griffon Vultures (Gyps fulvus subsp. fulvus). BMC Microbiol. 2016, 16, 228. [CrossRef]
- Plaza, P.; Blanco, G.; Lambertucci, S. Implications of bacterial, viral and mycotic microorganisms in vultures for wildlife conservation, ecosystem services and public health. Ibis 2020, 162, 1109–1124. [Google Scholar] [CrossRef]
- BirdLife International and Handbook of the Birds of the World 2021. The IUCN Red List of Threatened Species, Version 2022-2. Available online: https://www.iucnredlist.org/ (accessed on 27 September 2023).
- Hiraldo, F.; Delibes, M.; Calderón, J. El quebrantahuesos Gypaetus barbatus (L.). Sistemática, Taxonomía, Biología, Distribución y Protección. Monografías, 22. Instituto para la Conservación de la Naturaleza, Madrid, Spain. 1979.
- Angelov, I.; Hashim, I.; Oppel, S. Persistent electrocution mortality of Egyptian Vultures Neophron percnopterus over 28 years in East Africa. Bird Conserv. Int. 2013, 23, 1–6. [Google Scholar] [CrossRef]
- Thiollay, J-M. The decline of raptors in West Africa: long-term assessment and the role of protected areas. Ibis 2006, 148, 240-254. [CrossRef]
- Donázar, J.A.; Palacios, C.J.; Gangoso, L.; Ceballos, O.; González, M.J.; Hiraldo, F. Conservation status and limiting factors in the endangered population of Egyptian vulture (Neophron percnopterus) in the Canary Islands. Biol. Conserv. 2002, 107, 89–97. [Google Scholar] [CrossRef]
- Pirastru, M.; Mereu, P.; Manca, L.; Bebbere, D.; Naitana, S.; Leoni, G.G. Anthropogenic drivers leading to population decline and genetic preservation of the Eurasian griffon vulture (Gyps fulvus). Life 2021, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Avizanda, A.; Jovani, R.; Carrete, M.; Donázar, J.A. Resource unpredictability promotes species diversity and coexistence in an avian scavenger guild: A field experiment. Ecology 2012, 93, 2570–2579. [Google Scholar] [CrossRef] [PubMed]
- Virani, M.Z.; Kendall, C.; Njoroge, P.; Thomsett, S. Major declines in the abundance of vultures and other scavenging raptors in and around the Masai Mara ecosystem, Kenya. Biol. Conserv. 2011, 144, 746–752. [Google Scholar] [CrossRef]
- Cabral, M.J.; Almeida, J.; Almeida, P.R.; Delliger, T.; Ferrand de Almeida, N.; Oliveira, M.E.; Palmeirim, J.M.; Queirós, A.I.; Rogado, L.; Santos-Reis M. Livro Vermelho dos Vertebrados de Portugal. Publisher: Instituto da Conservação da Natureza, Lisboa, Portugal, 2005; pp.215-216.
- Ives, A.M.; Brenn-White, M.; Buckley, J.Y.; Kendall, C.J.; Wilton, S.; Deem, S.L. A global review of causes of morbidity and mortality in free-living vultures. EcoHealth 2022, 19, 40–54. [Google Scholar] [CrossRef]
- Garcês, A.; Pires, I.; Sargo, R.; Sousa, L.; Prada, J.; Silva, F. Admission causes, morbidity, and outcomes in scavenger birds in the North of Portugal (2005–2022). Animals 2023, 13, 2093. [Google Scholar] [CrossRef] [PubMed]
- Hugh-Jones, M.E.; De Vos, V. Anthrax and wildlife. Rev Sci Tech. 2002, 21, 359–383. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, I.; Sakaguchi, G.; Riemann, H.; Behymer, D.; Hurvell, B. Antibodies to Clostridium botulinum toxins in free-living birds and mammals. J Wildl Dis. 1979, 15, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Triana, L.M.; Jeffries, C.L.; Mansfield, K.L.; Carnell, G.; Fooks, A.R.; Johnson, N. Emergence of West Nile virus lineage 2 in Europe: a review on the introduction and spread of a mosquito-borne disease. Front Public Health. 2014, 2, 271. [Google Scholar] [CrossRef] [PubMed]
- May, F.J.; Davis, C.T.; Tesh, R.B.; Barrett, A.D. Phylogeography of West Nile virus: from the cradle of evolution in Africa to Eurasia, Australia, and the Americas. J Virol. 2011, 85, 2964–2974. [Google Scholar] [CrossRef] [PubMed]
- García-Carrasco, J.M.; Muñoz, A.R.; Olivero, J.; Segura, M.; Real, R. Mapping the risk for West Nile virus transmission, Africa. Emerg Infect Dis. 2022, 28, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Habarugira, G.; Suen, W.W.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. West Nile virus: an update on pathobiology, epidemiology, diagnostics, control and “One Health” implications. Pathogens 2020, 9, 589. [Google Scholar] [CrossRef]
- Watts, M.J.; Monteys, V.S.I.; Mortyn, P.G.; Kotsila, P. The rise of West Nile virus in Southern and Southeastern Europe: A spatial-temporal analysis investigating the combined effects of climate, land use and economic changes. One Health. 2021, 13, 100315. [Google Scholar] [CrossRef]
- Young, J.J.; Haussig, J.M.; Aberle, S.W.; Pervanidou, D.; Riccardo, F.; Sekulić, N.; Bakonyi, T.; Gossner, C.M. Epidemiology of human West Nile virus infections in the European Union and European Union enlargement countries, 2010 to 2018. Euro Surveill. 2021, 26, 2001095. [Google Scholar] [CrossRef]
- Pachler, K.; Lebl, K.; Berer, D.; Rudolf, I.; Hubalek, Z.; Nowotny, N. Putative new West Nile virus lineage in Uranotaenia unguiculata mosquitoes, Austria, 2013. Emerg Infect Dis. 2014, 20, 2119–2122. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Ebel, G.D.; Deubel, V.; Kerst, A.J.; Murri, S.; Meyer, R.; Bowen, M.; McKinney, N.; Morrill, W.E.; Crabtree, M.B.; Kramer, L.D.; Roehrig, J.T. Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East. Virology. 2002, 298, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Gamino, V.; Höfle, U. Pathology and tissue tropism of natural West Nile virus infection in birds: a review. Vet Res. 2013, 44, 39. [Google Scholar] [CrossRef] [PubMed]
- Savini, G.; Capelli, G.; Monaco, F.; Polci, A.; Russo, F.; Di Gennaro, A.; Marini, V.; Teodori, L.; Montarsi, F.; Pinoni, C.; Pisciella, M.; Terregino, C.; Marangon, S.; Capua, I.; Lelli, R. Evidence of West Nile virus lineage 2 circulation in Northern Italy. Vet Microbiol. 2012, 158, 267–73. [Google Scholar] [CrossRef] [PubMed]
- Valiakos, G.; Touloudi, A.; Iacovakis, C.; Athanasiou, L.; Birtsas, P.; Spyrou, V.; Billinis, C. Molecular detection and phylogenetic analysis of West Nile virus lineage 2 in sedentary wild birds (Eurasian magpie), Greece, 2010. Euro Surveill. 2011, 16, 19862. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J. Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014, 6, 606–623. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.A.; Diamond, M.S. Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J Virol. 2006, 80, 9349–9360. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile virus. Lancet Infect Dis. 2002, 2. 519-529. [CrossRef]
- van der Meulen, K.M.; Pensaert, M.B.; Nauwynck, H.J. West Nile virus in the vertebrate world. Arch Virol. 2005, 150, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Vidaña, B.; Busquets, N.; Napp, S.; Pérez-Ramírez, E.; Jiménez-Clavero, M.Á.; Johnson, N. The role of birds of prey in West Nile virus epidemiology. Vaccines (Basel). 2020, 8, 550. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, N.M.; Kratz, G.E.; Bates, R.; Scherpelz, J.A.; Bowen, R.A.; Komar, N. Clinical evaluation and outcomes of naturally acquired West Nile virus infection in raptors. J Zoo Wildl Med. 2009, 40, 51–63. [Google Scholar] [CrossRef]
- Kritzik, K.L.; Kratz, G.; Panella, N.A.; Burkhalter, K.; Clark, R.J.; Biggerstaff, B.J.; Komar, N. Determining raptor species and tissue sensitivity for improved West Nile virus surveillance. J Wildl Dis. 2018, 54, 528–533. [Google Scholar] [CrossRef]
- Jiménez-Clavero, M.Á. Animal viral diseases and global change: bluetongue and West Nile fever as paradigms. Front Genet. 2012, 3, 105. [Google Scholar] [CrossRef] [PubMed]
- Hull, J.; Hull, A.; Reisen, W.; Fang, Y.; Ernst, H. Variation of West Nile virus antibody prevalence in migrating and wintering hawks in central California. Condor 2006, 108, 435–439. [Google Scholar] [CrossRef]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, N.M.; Gould, D.H.; Bowen, R.A.; Komar, N. Natural and experimental West Nile virus infection in five raptor species. J. Wildl. Dis. 2006, 42, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, F.; Fischer, D.; Fischer, L.; Maisch, H.; Risch, T.; Dreyer, S.; Sadeghi, B.; Geelhaar, D.; Grund, L.; Merz, S.; et al. Vaccination of zoo birds against West Nile Virus—A Field Study. Vaccines 2023, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Angenvoort, J.; Fischer, D.; Fast, C.; Eiden, M.; Rodriguez, A.V.; Revilla-Fernández, S.; Nowotny, N.; de la Fuente, J.G.; Lierz, M.; Groschup, M.H. Pathogenesis of West Nile virus lineage 1 and 2 in experimentally infected large falcons. Vet Microbiol. 2013, 161, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Jiménez de Oya, N.; Escribano-Romero, E.; Blázquez, A.B.; Martín-Acebes, M.A.; Saiz, J.C. Current progress of avian vaccines against West Nile virus. Vaccines (Basel). 2019, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- D'Agostino, J.J.; Isaza, R. Clinical signs and results of specific diagnostic testing among captive birds housed at zoological institutions and infected with West Nile virus. J Am Vet Med Assoc. 2004, 224, 1640–1643. [Google Scholar] [CrossRef]
- Steele, K.E.; Linn, M.J.; Schoepp, R.J.; Komar, N.; Geisbert, T.W.; Manduca, R.M.; Calle, P.P.; Raphael, B.L.; Clippinger, T.L., Larsen, T.; Smith, J.; Lanciotti, R.S.; Panella, N.A.; McNamara, T.S. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet Pathol. 2000, 37, 208–24. [CrossRef]
- Joyner, P.H.; Kelly, S.; Shreve, A.A.; Snead, S.E.; Sleeman, J.M.; Pettit, D.A. West Nile virus in raptors from Virginia during 2003: clinical, diagnostic, and epidemiologic findings. J. Wildl. Dis. 2006, 42, 335–344. [Google Scholar] [CrossRef]
- Saggese, M.D. West Nile virus in Neotropical raptors: should we be concerned? In Neotropical Raptors; Bildstein et al., Eds.; United States of America, 2007, pp. 149-173.
- Ferraguti, M.; De La Puente, J.M.; Soriguer, R.; Llorente, F.; Jiménez-Clavero, M.Á.; Figuerola, J. West Nile virus-neutralizing antibodies in wild birds from southern Spain. Epidemiol. Infect. 2016, 144, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.C.; Nemeth, N.M.; Edwards, E.; Bright, P.R.; Komar, N. Passive West Nile virus antibody transfer from maternal Eastern screech-owls (Megascops asio) to progeny. Avian Dis. 2006, 50, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, N.M.; Kratz, G.E.; Bates, R.; Scherpelz, J.A.; Bowen, R.A.; Komar, N. Naturally induced humoral immunity to West Nile virus infection in raptors. Ecohealth. 2008, 5, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Marra, P.P.; Griffing, S.; Caffrey, C.; Kilpatrick, M.A.; McLean, R.; Brand, C.; Saito, E.; Dupuis, A.P.; Kramer, L.; Novak, R. West Nile virus and wildlife. BioScience. 2004, 54, 393–402. [Google Scholar] [CrossRef]
- Wünschmann, A.; Shivers, J.; Bender, J.; Carroll, L.; Fuller, S.; Saggese, M.; Van Wettere, A.; Redig, P. Pathologic and immunohistochemical findings in Goshawks (Accipiter gentilis) and Great Horned Owls (Bubo virginianus) naturally infected with West Nile virus. Avian Dis. 2005, 49, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, C.; Franca, M.; Uzal. F.; Anderson, M.; Barr, B.; Woods, L.; Moore, J.; Woolcock, P.; Shivaprasad, H.L. Pathology and immunohistochemical findings of West Nile virus infection in Psittaciformes. Vet Pathol. 2011, 48, 975–984. [CrossRef]
- Jones, M.P. Selected infectious diseases of birds of prey. J. Exot. Pet Med. 2006, 15, 5–17. [Google Scholar] [CrossRef]
- Phalen, D.N.; Dahlhausen, B. West Nile virus. Semin. Avian Exot. Pet Med. 2004, 13, 67–78. [Google Scholar] [CrossRef]
- Wodak, E.; Richter, S.; Bagó, Z.; Revilla-Fernández, S.; Weissenböck, H.; Nowotny, N.; Winter, P. Detection and molecular analysis of West Nile virus infections in birds of prey in the eastern part of Austria in 2008 and 2009. Vet. Microbiol. 2011, 149, 358–366. [Google Scholar] [CrossRef]
- Bakonyi, T.; Ferenczi, E.; Erdelyi, K.; Kutasi, O.; Csörgö, T.; Seidel, B.; Weissenböck, H.; Brugger, K.; Bán, E.; Nowotny, N. Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Vet. Microbiol. 2013, 165, 61–70. [Google Scholar] [CrossRef]
- García-Bocanegra, I.; Busquets, N.; Napp, S.; Alba, A.; Zorrilla, I.; Villalba, R.; Arenas, A. Serosurvey of West Nile virus and other flaviviruses of the Japanese encephalitis antigenic complex in birds from Andalusia, southern Spain. Vector Borne Zoonotic Dis. 2011, 11, 1107–1113. [Google Scholar] [CrossRef]
- Busquets, N.; Laranjo-González, M.; Soler, M.; Nicolás, O.; Rivas, R.; Talavera, S.; Villalba, R.; San Miguel, E.; Torner, N.; Aranda, C.; Napp, S. Detection of West Nile virus lineage 2 in North-Eastern Spain (Catalonia). Transbound Emerg. Dis. 2019, 66, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, H.; Beck, C.; Lecollinet, S.; Monier, M.; Mousson, L.; Zakeri, S.; Raz, A.; Arzamani, K.; Nourani, L.; Dinparast-Djadid, N.; Failloux, A.B. Serological evidence of West Nile virus infection among birds and horses in some geographical locations of Iran. Vet. Med. Sci. 2021, 7, 204–209. [Google Scholar] [CrossRef] [PubMed]
- García-Ripollés, C.; López-López, P.; Urios, V. First description of migration and wintering of adult Egyptian Vultures Neophron percnopterus tracked by GPS satellite telemetry. Bird study. 2010, 57, 261–265. [Google Scholar] [CrossRef]
- Phipps, W.L.; López-López, P.; Buechley, E.R.; Oppel, S.; Álvarez, E.; Arkumarev, V.; Bekmansurov, Rinur.; Berger-Tal, O.; Bermejo, A.; Bounas, A.; Alanís, I.C.; de la Puente, J.; Dobrev, V.; Duriez, O.; Efrat, R.; Fréchet, G.; García, J.; Galán, M.; García-Ripollés, C.; Gil, A.; Iglesias-Lebrija, J.J.; Jambas, J.; Karyakin, I.V.; Kobierzycki, E.; Kret, E.; Loercher, F.; Monteiro, A.; Morant, E.J.; Nikolov, S.C.; Pereira, J.; Peške, L.; Ponchon, C.; Realinho, E.; Saravia, V.; Sekercioğlu, C.H.; Skartsi, T.; Tavares, J.; Teodósio, J.; Urios, V.; Vallverdú, N. Spatial and temporal variability in migration of a soaring raptor across three continents. Front. Ecol. Evol. 2019, 7, 1-14. [CrossRef]
- Bravo-Barriga, D.; Aguilera-Sepúlveda, P.; Guerrero-Carvajal, F.; Llorente, F.; Reina, D.; Pérez-Martín, J.E.; Jiménez-Clavero, M.Á.; Frontera, E. West Nile and Usutu virus infections in wild birds admitted to rehabilitation centres in Extremadura, western Spain, 2017-2019. Vet. Microbiol. 2021, 255, 109020. [Google Scholar] [CrossRef] [PubMed]
- Marinković, D.; Nešić, V.; Davitkov, D.; Aničić, M. Causes of morbidity and mortality in European griffon vulture (Gyps fulvus) population in Serbia in the period of 2018–2022 – post-mortem findings. J. Comp. Pathol. 2023, 203, 52. [Google Scholar] [CrossRef]
- Nash, D.; Mostashari, F.; Fine, A.; Miller, J.; O’Leary, D.; Murray, K.; Huang, A.; Rosenberg, A.; Greenberg, A.; Sherman, M.; Wong, S.; Layton, M; 1999 West Nile Outbreak Response Working Group. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001, 344, 1807–1814. [CrossRef]
- Rideout, B.A.; Stalis, I.; Papendick, R.; Pessier, A.; Puschner, B.; Finkelstein, M.E.; Smith, D.R.; Johnson, M.; Mace, M.; Stroud, R.; Brandt, J.; Burnett, J.; Parish, C.; Petterson, J.; Witte, C.; Stringfield, C.; Orr, K.; Zuba, J.; Wallace, M.; Grantham, J. Patterns of mortality in free-ranging California Condors (Gymnogyps californianus). J. Wildl. Dis. 2012, 48, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Straub, M.H.; Kelly, T.R.; Rideout, B.A.; Eng, C.; Wynne, J.; Braun, J.; Johnson, C.K. Seroepidemiologic Survey of Potential Pathogens in Obligate and Facultative Scavenging Avian Species in California. PLoS One. 2015, 10, e0143018. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature. 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. Hyg. 1940, s1-20, 471–492. [Google Scholar] [CrossRef]
- Taylor, R.M.; Work, T.H.; Hurlbut, H.S.; Rizk, F. A study of the ecology of West Nile virus in Egypt. Am J Trop Med Hyg. 1956, 5, 579–620. [Google Scholar] [CrossRef]
- Mencattelli, G.; Ndione, M.H.D.; Rosà, R.; Marini, G.; Diagne, C.T.; Diagne, M.M.; Fall, G.; Faye, O.; Diallo, M.; Faye, O.; Savini, G.; Rizzoli, A. Epidemiology of West Nile virus in Africa: An underestimated threat. PLoS Negl Trop Dis. 2022, 16, e0010075. [Google Scholar] [CrossRef]
- Joubert, L.; Oudar, J.; Hannoun, C.; Beytout, D.; Corniou, B.; Guillon, J.C.; Panthier, R. Epidémiologie du virus West Nile: etude d'un foyer en Camargue. IV. La méningo-encéphalomyélite du cheval [Epidemiology of the West Nile virus: study of a focus in Camargue. IV. Meningo-encephalomyelitis of the horse]. Ann Inst Pasteur (Paris). 1970, 118, 239–247. [Google Scholar] [PubMed]
- Tsai, T.F.; Popovici, F.; Cernescu, C.; Campbell, G.L.; Nedelcu, N.I. West Nile encephalitis epidemic in Southeastern Romania. Lancet. 1998, 352, 767–771. [Google Scholar] [CrossRef] [PubMed]
- García-Carrasco, J.M.; Muñoz, A.R.; Olivero, J.; Figuerola, J.; Fa, J.E.; Real, R. Gone (and spread) with the birds: can chorotype analysis highlight the spread of West Nile virus within the Afro-Palaearctic flyway? One Health. 2023, 17, 100585. [Google Scholar] [CrossRef]
- Rappole, J.H., Derrickson, S.R.; Hubálek, Z. Migratory birds and spread of West Nile virus in the Western Hemisphere. Emerg Infect Dis. 2000, 6, 319–328. [CrossRef] [PubMed]
- Nir, Y.; Goldwasser, R.; Lasowski, Y.; Avivi, A. Isolation of arboviruses from wild birds in Israel. Am J Epidemiol. 1967, 86, 372–378. [Google Scholar] [CrossRef]
- Jupp, P.G. The ecology of West Nile virus in South Africa and the occurrence of outbreaks in humans. Ann N Y Acad Sci. 2001, 951, 143–152. [Google Scholar] [CrossRef]
- Hubálek, Z.; Halouzka, J. West Nile fever—a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 1999, 5, 643–650. [Google Scholar] [CrossRef] [PubMed]
- López, G.; Jiménez-Clavero, M.A.; Tejedor, C.G.; Soriguer, R.; Figuerola, J. Prevalence of West Nile virus neutralizing antibodies in Spain is related to the behavior of migratory birds. Vector Borne Zoonotic Dis. 2008, 8, 615–621. [Google Scholar] [CrossRef]
- Lord, R.D.; Calisher, C.H. Further evidence of southward transport of arboviruses by migratory birds. Am J Epidemiol. 1970, 92, 73–78. [Google Scholar] [CrossRef]
- Malkinson, M.; Banet, C.; Weisman, Y.; Pokamunski, S.; King, R.; Drouet, M.T.; Deubel, V. Introduction of West Nile virus in the Middle East by migrating white storks. Emerg Infect Dis. 2002, 8, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Höfle, U.; Blanco, J.M.; Crespo, E.; Naranjo, V.; Jiménez-Clavero, M.A.; Sanchez, A.; de la Fuente, J.; Gortazar, C. West Nile virus in the endangered Spanish imperial eagle. Vet Microbiol. 2008, 129, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Casades-Martí, L.; Holgado-Martín, R.; Aguilera-Sepúlveda, P.; Llorente, F.; Pérez-Ramírez, E.; Jiménez-Clavero, M.Á.; Ruiz-Fons, F. Risk factors for exposure of wild birds to West Nile virus in a gradient of wildlife-livestock interaction. Pathogens. 2023, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Brugueras, S.; Fernández-Martínez, B.; Martínez-de la Puente, J.; Figuerola, J.; Porro, T.M.; Rius, C.; Larrauri, A.; Gómez-Barroso, D. Environmental drivers, climate change and emergent diseases transmitted by mosquitoes and their vectors in southern Europe: a systematic review. Environ Res. 2020, 191, 110038. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency (EUNIS). Available online: https://eunis.eea.europa.eu/habitats/393 (accessed on 23 October 2023).
- Díaz, M.; Campos, P.; Pulido, F.J. The Spanish dehesas: a diversity of land use and wildlife. In Farming and birds in Europe: The Common Agricultural Policy and its implications for bird conservation; Pain, D., Pienkowski, M., Eds.; Academic Press: London, England, 1997; pp. 178–209. [Google Scholar]
- Delgado-González, A.; Cortés-Avizanda, A.; Serrano, D.; Arrondo, E.; Duriez, O.; Margalida, A.; Carrete, M.; Oliva-Vidal, P.; Sourp, E.; García-Barón, I.; de La Riva, M.; Sánchez-Zapata, J.A.; Donázar, J.A. Apex scavengers from different European populations converge at threatened savannah landscapes. Sci Rep. 2022, 12, 2500. [Google Scholar] [CrossRef]
- Guerrero-Carvajal, F.; Bravo-Barriga, D.; Martín-Cuervo, M.; Aguilera-Sepúlveda, P.; Ferraguti, M.; Jiménez-Clavero, M.Á.; Llorente, F.; Alonso, J.M.; Frontera, E. Serological evidence of co-circulation of West Nile and Usutu viruses in equids from western Spain. Transbound Emerg Dis. 2021, 68, 1432–1444. [Google Scholar] [CrossRef]
- Morant, J.; Arrondo, E.; Sánchez-Zapata, J.A.; Donázar, J.A.; Cortés-Avizanda, A.; de La Riva, M.; Blanco, G.; Martínez, F.; Oltra, J.; Carrete, M.; Margalida, A.; Oliva-Vidal, P.; Martínez, J.M.; Serrano, D.; Pérez-García, J.M. Large-scale movement patterns in a social vulture are influenced by seasonality, sex, and breeding region. Ecol Evol. 2023, 13, e9817. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.; de Langarica, F.M.Z.G.; Molina, M.G. Spring migration of eurasian griffon vultures across the strait of Gibraltar: number, timing and age composition. Ardeola 2019, 66, 113–118. [Google Scholar] [CrossRef]
- Adelman, J.S.; Tokarz, R.E.; Euken, A.E.; Field, E.N.; Russell, M.C.; Smith, R.C. Relative influence of land use, mosquito abundance, and bird communities in defining West Nile virus infection rates in Culex mosquito populations. Insects. 2022, 13, 758. [Google Scholar] [CrossRef]
- Palomar, A.M.; Veiga, J.; Portillo, A.; Santibáñez, S.; Václav, R.; Santibáñez, P.; Oteo, J.A.; Valera, F. Novel genotypes of nidicolous argas ticks and their associated microorganisms from Spain. Front Vet Sci. 2021, 8, 637837. [Google Scholar] [CrossRef]
- Moraga-Fernández, A.; Oliva-Vidal, P.; Sánchez-Sánchez, M.; Muñoz-Hernández, C.; Martínez, J.M.; Margalida, A.; de la Fuente, J.; de Mera, I.G.F. Health risks associated with argasid ticks, transmitted pathogens, and blood parasites in Pyrenean griffon vulture (Gyps fulvus) nestlings. Eur J Wildl Res. 2023, 69, 112. [Google Scholar] [CrossRef]
- Blahove, M.R.; Carter, J.R. Flavivirus persistence in wildlife populations. Viruses 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.R.; Stallknecht, D.E.; Willis, J.; Conroy, M.J.; Davidson, W.R. Wild bird mortality and West Nile virus surveillance: biases associated with detection, reporting, and carcass persistence. J Wildl Dis. 2006, 42, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Cacho, I.M. Exposure and carriage of West Nile virus in feathered Iberian scavengers. Master’s thesis: Uppsala University, Uppsala. 2022.
- Gangoso, L.; Cortés-Avizanda, A.; Sergiel, A.; Pudifoot, B.; Miranda, F.; Muñoz, J.; Delgado-González, A.; Moleón, M.; Sánchez-Zapata, J.A.; Arrondo, E.; Donázar, J.A. Avian scavengers living in anthropized landscapes have shorter telomeres and higher levels of glucocorticoid hormones. Sci. Total Environ. 2021, 782, 146920. [Google Scholar] [CrossRef]
- FAO & UNEP. The State of the World’s Forests 2020. Forests, Biodiversity and People. FAO & UNEP, Rome, Italy, 2020; pp. 72-79.
- Morand, S.; Lajaunie, C. Outbreaks of vector-borne and zoonotic diseases are associated with changes in forest cover and oil palm expansion at global scale. Front Vet Sci. 2021, 8, 661063. [Google Scholar] [CrossRef]
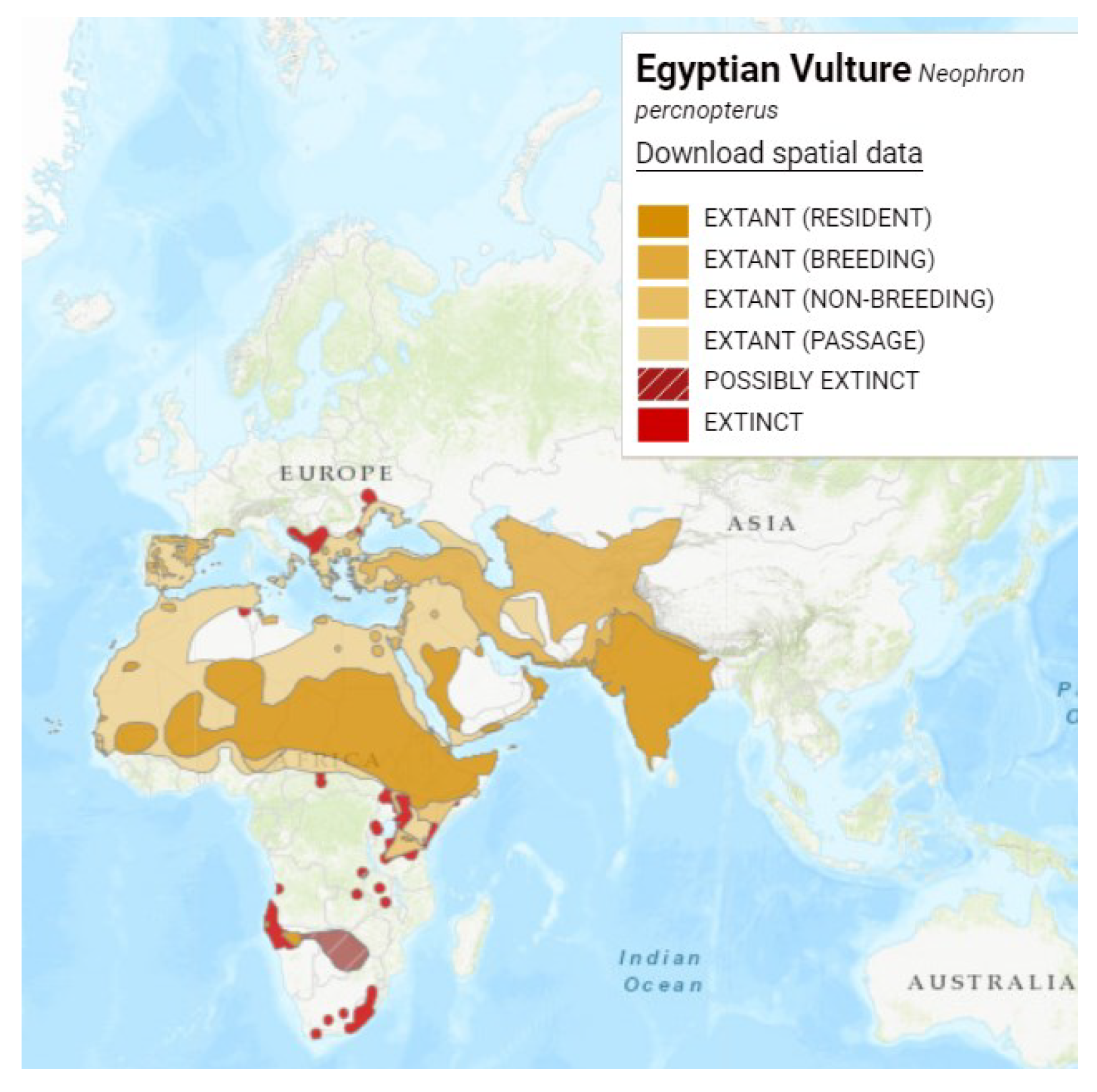
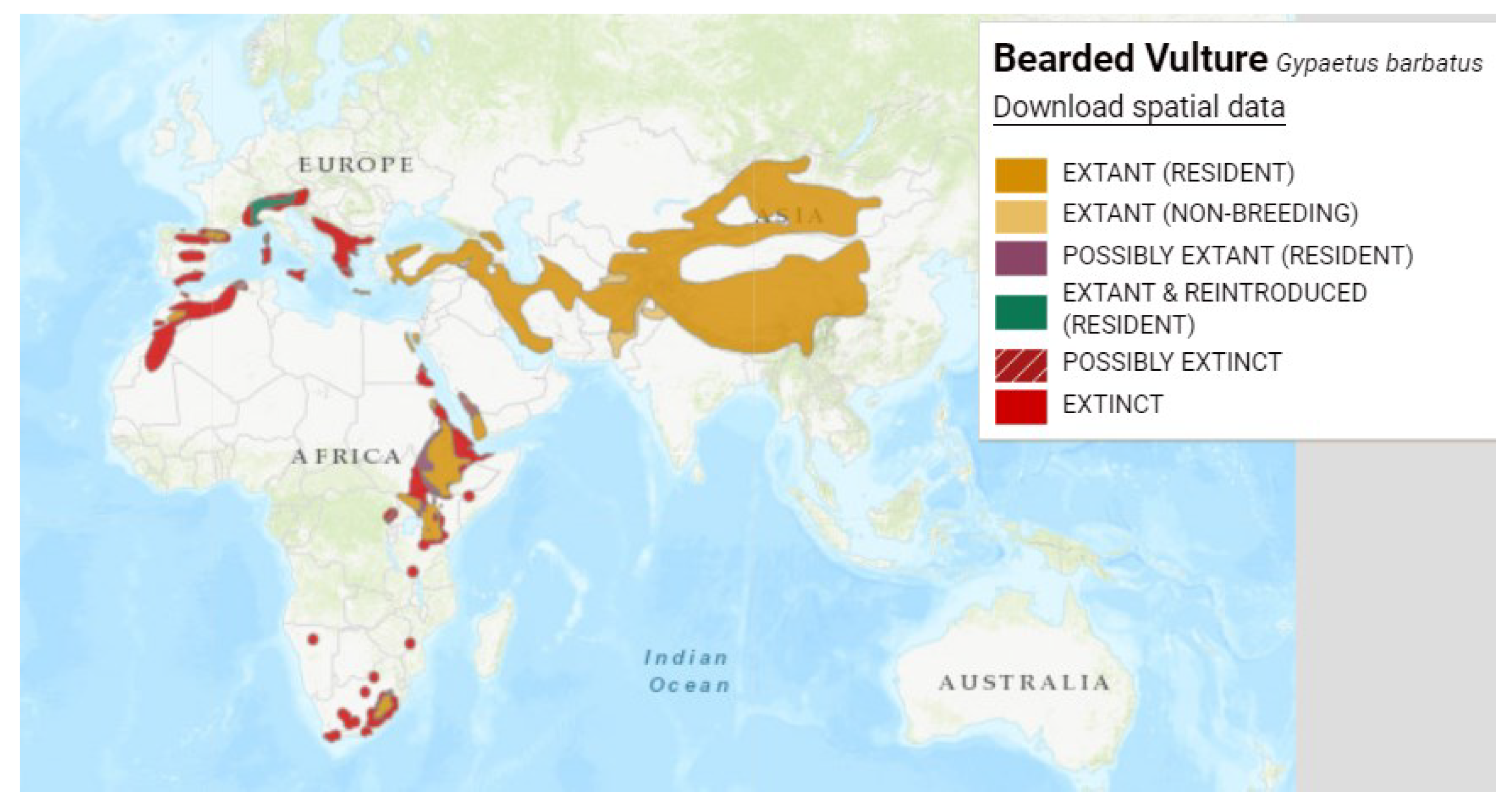
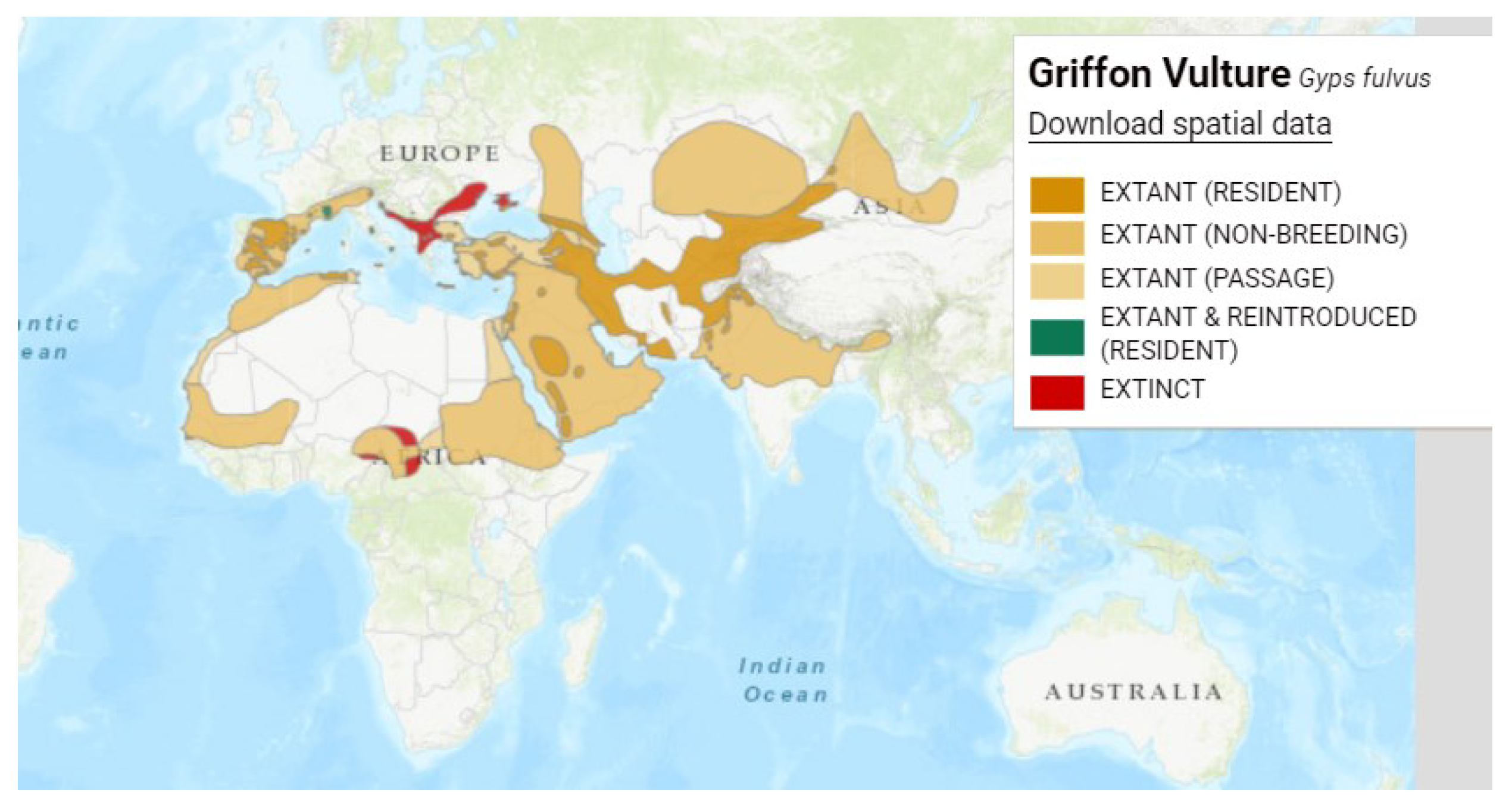
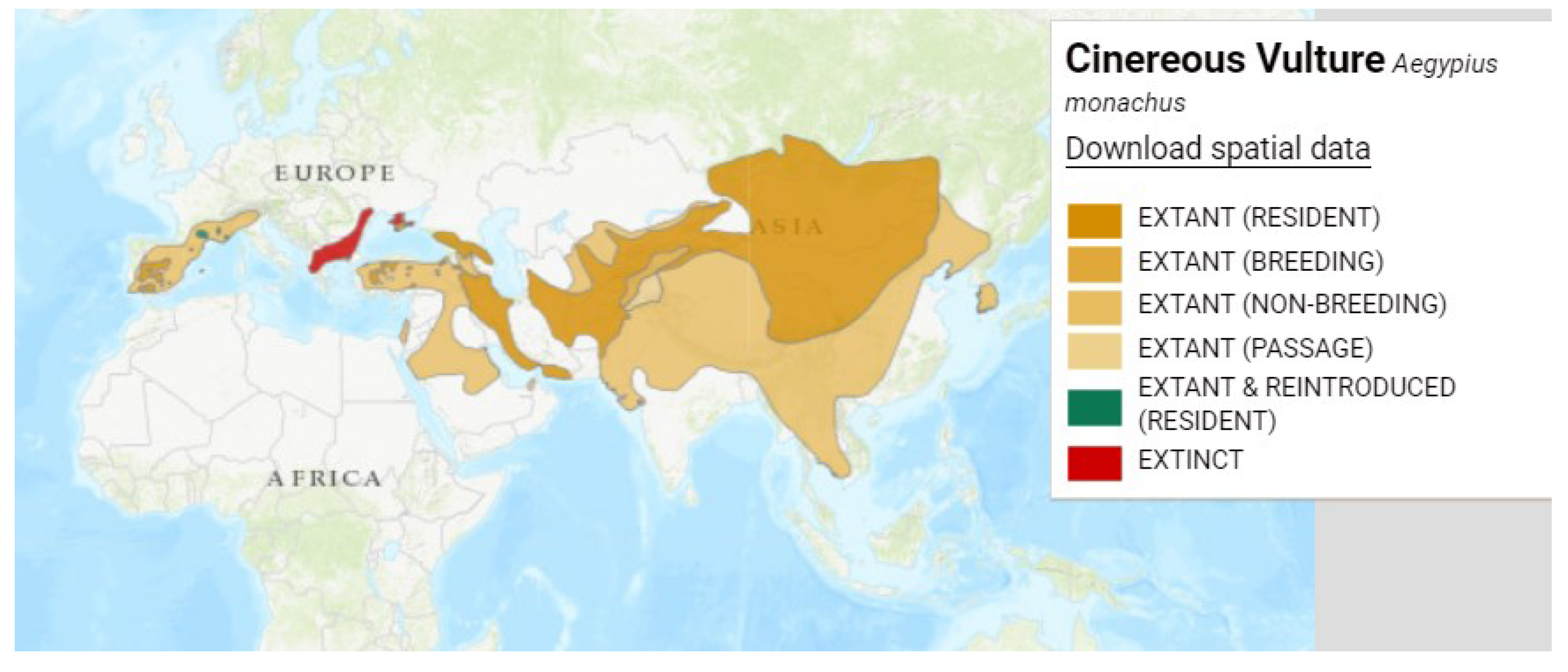
| Species | IUCN global statusa | Toxic | Trauma | Infectious | Idiopathic | Metabolic | Inflammatory | Totalb |
|---|---|---|---|---|---|---|---|---|
| Aegypius monachus | NT | 9 (485) |
1 (9) |
6 (46) |
1 (1) |
2 (16) |
1 (1) |
14 (542) |
|
Gypaetus barbatus |
NT |
8 (61) |
7 (80) |
1 (3) |
2 (2) |
0 |
1 (1) |
9 (143) |
|
Gyps fulvus |
LC |
18 (615) |
12 (978) |
5 (73) |
1 (5) |
1 (51) |
0 |
32 (1722) |
| Neophron percnopterus |
EN |
15 (500) |
13 (176) |
3 (38) |
0 |
0 |
0 |
25 (714) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





