Submitted:
13 November 2023
Posted:
13 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Phenolic compounds isolated from ALE (Cynara scolymus L.)
3. Biological activities of sesquiterpene lactones derived from artichoke.
4. Antimicrobial activity of ALE (Cynara scolymus L.)
5. Pharmacological Studies of ALE and Health Benefits
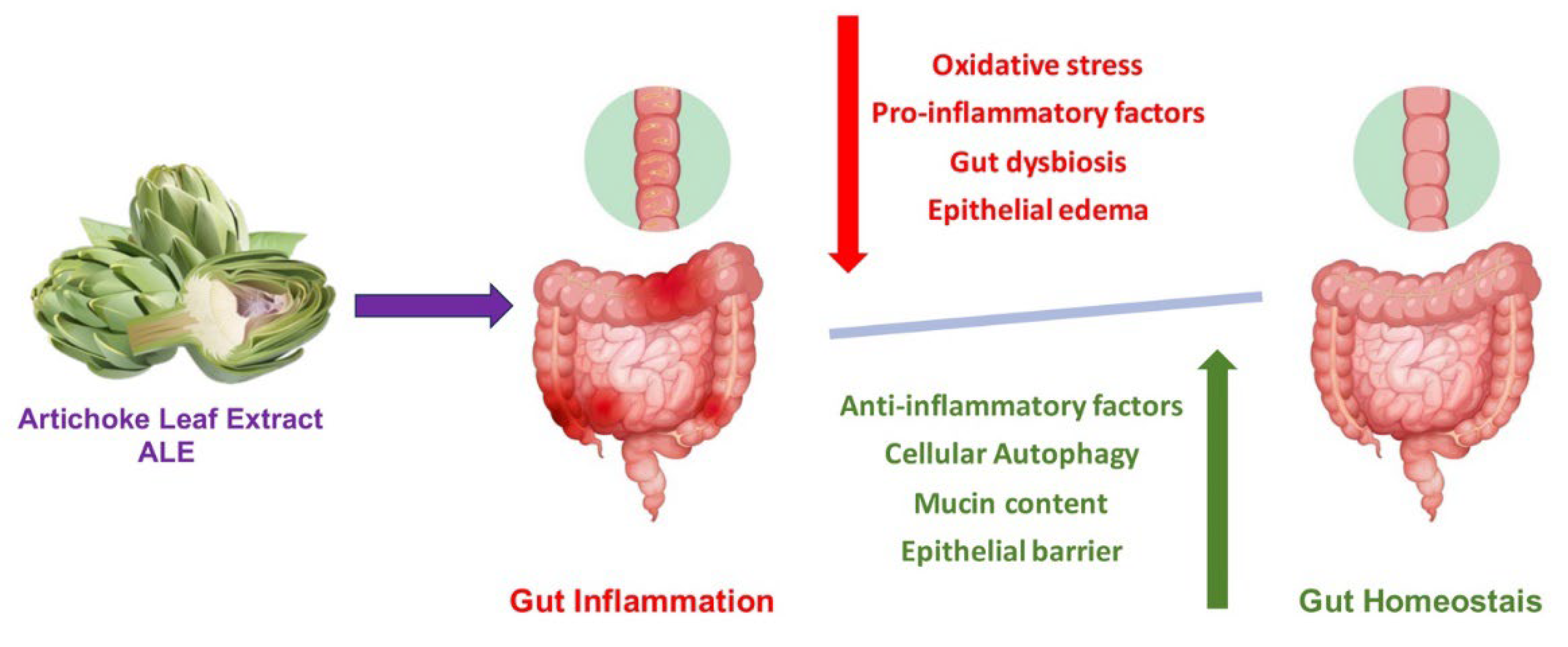
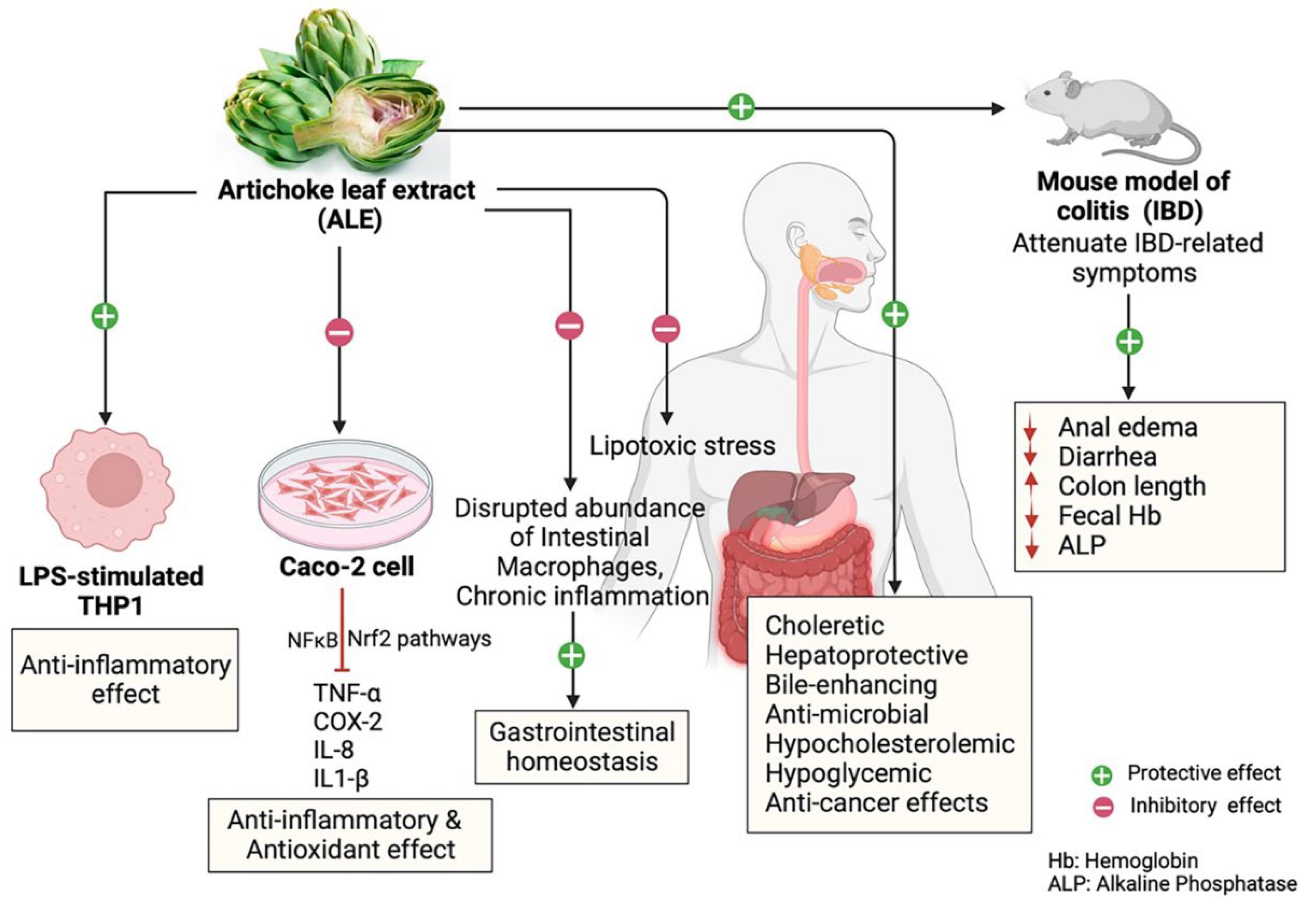
6. Beneficial Health Effects of Artichoke on Inflammatory and Gastrointestinal Diseases
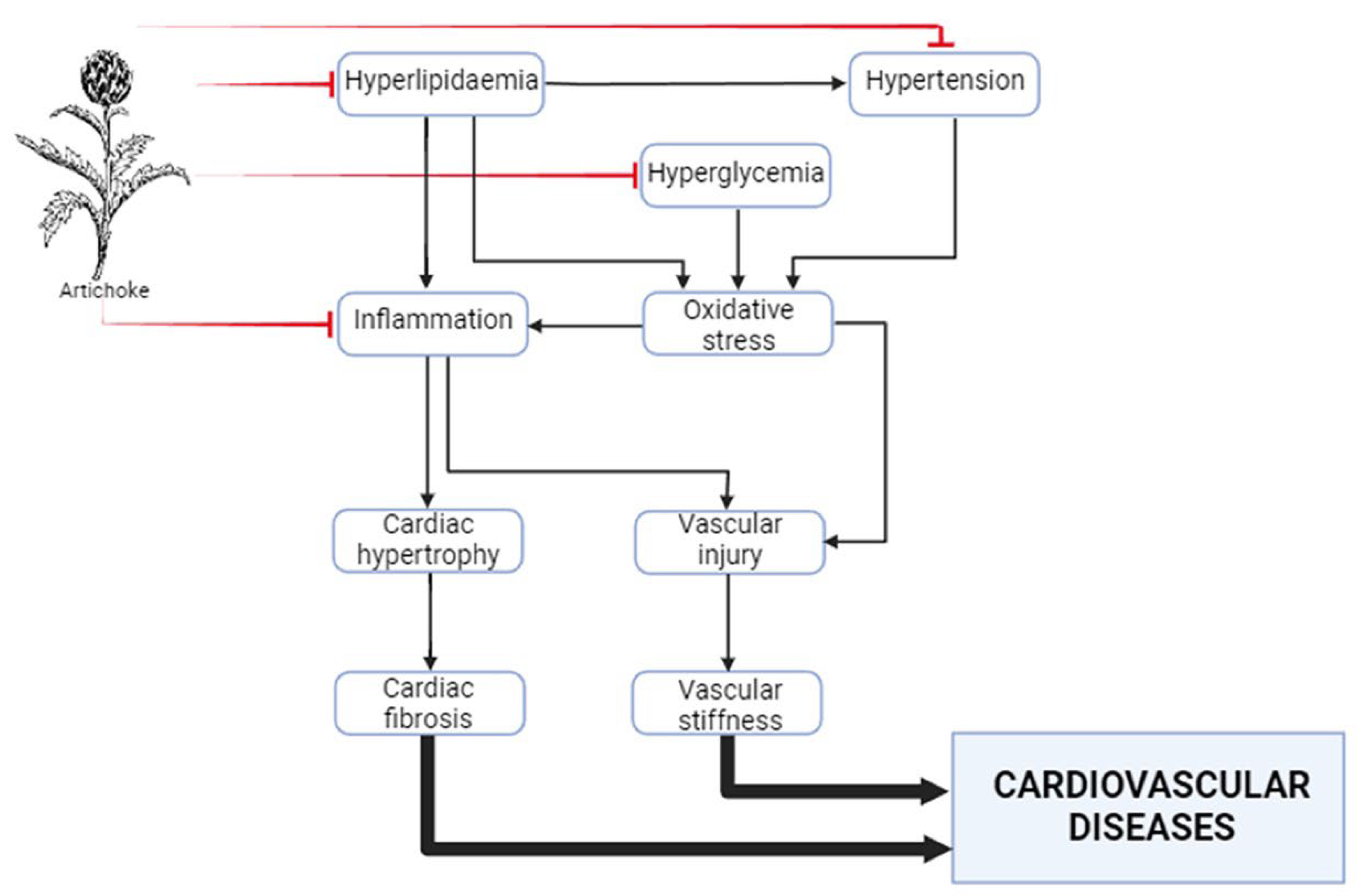
7. Anti-inflammatory effects in the cardiovascular system
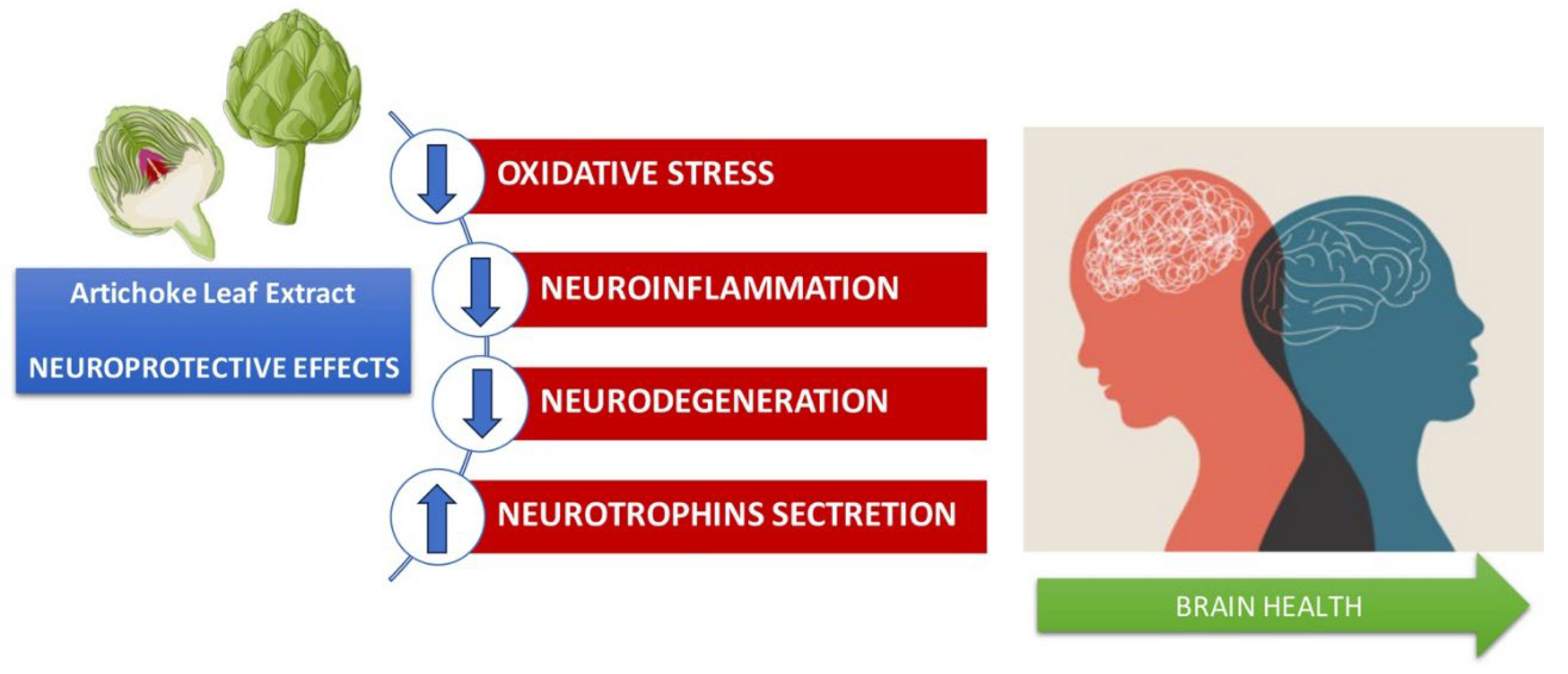
8. Anti-inflammatory effects in the brain and protective effects in neurodegenerative diseases
9. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications. Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Gatto, A.; De Paola, D.; Bagnoli, F.; Vendramin, G.G.; Sonnante, G. Population structure of Cynara cardunculus complex and the origin of the conspecific crops artichoke and cardoon. Ann. Bot. 2013, 112, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Jacinto, T.A.; Coutinho, P. Bioactive Compounds from Cardoon as Health Promoters in Metabolic Disorders. Foods. 2022, 11, 336. [Google Scholar] [CrossRef]
- Salem, M.B.; Affes, H.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Pharmacological Studies of Artichoke Leaf Extract and Their Health Benefits. Plant Foods Hum. Nutr. 2015, 70, 441–453. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Cimminelli, M.J.; Volpe, F.; Ansó, R.; Esparza, I.; Mármol, I.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Phenolic Composition of Artichoke Waste and its Antioxidant Capacity on Differentiated Caco-2 Cells. Nutrients. 2019, 11, 1723. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Wojdyło, A.; Tkacz, K.; Nowicka, P.; Hernández, F. Antidiabetic, Anticholinesterase and Antioxidant Activity vs. Terpenoids and Phenolic Compounds in Selected New Cultivars and Hybrids of Artichoke Cynara scolymus L. Molecules. 2019, 24, 1222. [Google Scholar] [CrossRef]
- Wang, M.; Simon, J.E.; Aviles, I.F.; He, K.; Zheng, Q.-Y.; Tadmor, Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2003, 51, 601–608. [Google Scholar] [CrossRef]
- Küskü-Kiraz, Z.; Mehmetçik, G.; Dogru-Abbasoglu, S.; Uysal, M. Artichoke leaf extract reduces oxidative stress and lipoprotein dyshomeostasis in rats fed on high cholesterol diet. Phytother. Res. 2010, 24, 565–570. [Google Scholar] [CrossRef]
- Magielse, J.; Verlaet, A.; Breynaert, A.; Keenoy, B.M.; Apers, S.; Pieters, L.; Hermans, N. Investigation of the in vivo antioxidative activity of Cynara scolymus (artichoke) leaf extract in the streptozotocin-induced diabetic rat. Mol. Nutr. Food Res. 2014, 58, 211–215. [Google Scholar] [CrossRef]
- Tang, X.; Wei, R.; Deng, A.; Lei, T. Protective Effects of Ethanolic Extracts from Artichoke, an Edible Herbal Medicine, against Acute Alcohol-Induced Liver Injury in Mice. Nutrients. 2017, 9, 1000. [Google Scholar] [CrossRef]
- Yasukawa, K.; Matsubara, H.; Sano, Y. Inhibitory effect of the flowers of artichoke (Cynara cardunculus) on TPA-induced inflammation and tumor promotion in two-stage carcinogenesis in mouse skin. J. Nat. Med. 2010, 64, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, R. Inhibition of cholesterol biosynthesis in HepG2 cells by artichoke extracts is reinforced by glucosidase pretreatment. Phytother. Res. 2002, 16, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Sabater, C.; Molina-Tijeras, J.A.; Vezza, T.; Corzo, N.; Montilla, A.; Utrilla, P. Intestinal anti-inflammatory effects of artichoke pectin and modified pectin fractions in the dextran sulfate sodium model of mice colitis. Artificial neural network modelling of inflammatory markers. Food Funct. 2019, 10, 7793–7805. [Google Scholar] [CrossRef] [PubMed]
- Carloni, S.; Rescigno, M. Unveiling the gut-brain axis: Structural and functional analogies between the gut and the choroid plexus vascular and immune barriers. Semin. Immunopathol. 2022, 44, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Functional Foods. J. Funct. Foods. 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Robards, K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A. 2003, 1000, 657–691. [Google Scholar] [CrossRef] [PubMed]
- Crascì, L.; Cardile, V.; Longhitano, G.; Nanfitò, F.; Panico, A. Anti-degenerative effect of Apigenin, Luteolin and Quercetin on human keratinocyte and chondrocyte cultures: SAR evaluation. Drug Res. (Stuttg). 2018, 68, 132–138. [Google Scholar] [CrossRef]
- Negro, D.; Montesano, V.; Grieco, S.; Crupi, P.; Sarli, G.; De Lisi, A.; Sonnante, G. Polyphenol compounds in artichoke plant tissues and varieties. J. Food Sci. 2012, 77, C244–C252. [Google Scholar] [CrossRef] [PubMed]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Gambacorta, G.; Elia, A. Morphological and qualitative characterization of globe artichoke head from new seed-propagated cultivars. J. Sci. Food Agric. 2010, 90, 2689–2693. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G.; Knödler, M.; Carle, R.; Schieber, A. Influence of genotype, harvest time and plant part on polyphenolic composition of globe artichoke [Cynara cardunculus L. var. scolymus (L.) Fiori]. Food Chem. 2010, 119, 1175–1181. [Google Scholar] [CrossRef]
- Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A.; Ferreres, F. Artichoke (Cynara scolymus L.) byproducts as a potential source of health-promoting antioxidant phenolics. J. Agric Food Chem. 2002, 50, 3458–3464. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, B.; Künne, M.; Ma, L.; Xu, M.; Yan, F.; Piepho, H.P.; Honermeier, B. Optimization of the extraction procedure for the determination of phenolic acids and flavonoids in the leaves of globe artichoke (Cynara cardunculus var. scolymus L.). J. Pharm. Biomed. Anal. 2020, 177, 112879. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Mayor, L.; Calvo-Ramirez, A.; Ruiz-Melero, R.; Rodriguez-Lopez, A.D. Response Surface Optimization of Inulin and Polyphenol Extraction from Artichoke (Cynara scolymus (L.) Solid Wastes. Appl. Sci. 2022, 12, 7957. [Google Scholar] [CrossRef]
- Abd El-Ghany, Z. Evaluation of Antibacterial Activity, Gas Chromatography Analysis and Antioxidant Efficacy of Artichoke (Cynara scolymus L.). J. Agric. Chem. Biotech. 2017, 8, 265–280. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Mocan, A.; Atanasov, A.G. Cynaropicrin: A Comprehensive Research Review and Therapeutic Potential As an Anti-Hepatitis C Virus Agent. Front. Pharmacol. 2016, 7, 472. [Google Scholar] [CrossRef] [PubMed]
- Possart, K.; Herrmann, F.C.; Jose, J.; Costi, M.P.; Schmidt, T.J. Sesquiterpene Lactones with Dual Inhibitory Activity against the Trypanosoma brucei Pteridine Reductase 1 and Dihydrofolate Reductase. Molecules. 2021, 27, 149. [Google Scholar] [CrossRef]
- Rocchetti, G.; Giuberti, G.; Lucchini, F.; Lucini, L. Polyphenols and Sesquiterpene Lactones from Artichoke Heads: Modulation of Starch Digestion, Gut Bioaccessibility, and Bioavailability following In Vitro Digestion and Large Intestine Fermentation. Antioxidants (Basel). 2020, 9, 306. [Google Scholar] [CrossRef]
- Ingallina, C.; Di Matteo, G.; Spano, M.; Acciaro, E.; Campiglia, E.; Mannina, L.; Sobolev, A.P. Byproducts of Globe Artichoke and Cauliflower Production as a New Source of Bioactive Compounds in the Green Economy Perspective: An NMR Study. Molecules. 2023, 28, 1363. [Google Scholar] [CrossRef]
- De Cicco, P.; Busà, R.; Ercolano, G.; Formisano, C.; Allegra, M.; Taglialatela-Scafati, O.; Ianaro, A. Inhibitory effects of cynaropicrin on human melanoma progression by targeting MAPK, NF-κB, and Nrf-2 signaling pathways in vitro. Phytother. Res. 2021, 35, 1432–1442. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, M.; Wang, Y.; Xie, F.; Zhang, G.; Qin, X. Nrf2-a Promising Therapeutic Target for Defensing Against Oxidative Stress in Stroke. Mol. Neurobiol. 2017, 54, 6006–6017. [Google Scholar] [CrossRef]
- Jin, T.; Leng, B. Cynaropicrin Averts the Oxidative Stress and Neuroinflammation in Ischemic/Reperfusion Injury Through the Modulation of NF-kB. Appl. Biochem. Biotechnol. 2023, 195, 5424–5438. [Google Scholar] [CrossRef]
- Boulos, J.C.; Omer, E.A.; Rigano, D.; Formisano, C.; Chatterjee, M.; Leich, E.; Klauck, S.M.; Shan, L.T.; Efferth, T. Cynaropicrin disrupts tubulin and c-Myc-related signaling and induces parthanatos-type cell death in multiple myeloma. Acta Pharmacol. Sin. 2023, 44, 2265–2281. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nakashima, S.; Nakamura, S.; Hattori, Y.; Ando, T.; Matsuda, H. Inhibitory effects of cynaropicrin and related sesquiterpene lactones from leaves of artichoke (Cynara scolymus L.) on induction of iNOS in RAW264.7 cells and its high-affinity proteins. J. Nat. Med. 2021, 75, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Hayata, M.; Watanabe, N.; Kamio, N.; Tamura, M.; Nodomi, K.; Tanaka, K.; Iddamalgoda, A.; Tsuda, H.; Ogata, Y.; Sato, S.; Ueda, K.; Imai, K. Cynaropicrin from Cynara scolymus L. suppresses Porphyromonas gingivalis LPS-induced production of inflammatory cytokines in human gingival fibroblasts and RANKL-induced osteoclast differentiation in RAW264.7 cells. J. Nat. Med. 2019, 73, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms. 2021, 9, 2041. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 94391–1. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants (Basel). 2021, 10, 188. [Google Scholar] [CrossRef]
- Donadio, G.; Mensitieri, F.; Santoro, V.; Parisi, V.; Bellone, M.L.; De Tommasi, N.; Izzo, V.; Dal Piaz, F. Interactions with Microbial Proteins Driving the Antibacterial Activity of Flavonoids. Pharmaceutics. 2021, 13, 660. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, H.; Zhang, H.; Lo, R.; Lu, Y. Antimicrobial Activities of Cynara scolymus L. Leaf, Head, and Stem Extracts. J. Food. Sci. 2006, 70, M149–M152. [Google Scholar] [CrossRef]
- Yildirim, A.B.; Basay, S.; Turker, A.U. A comparison of organically and conventionally grown artichokes: Phenolic constituents, antioxidant and antibacterial activities. Acta Alimentaria. 2020, 49, 69–75. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral Properties of Polyphenols from Plants. Foods. 2021, 10, 2277. [Google Scholar] [CrossRef] [PubMed]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Deng, A.; Liu, F.; Tang, X.; Wang, Y.; Xie, P.; Yang, Q.; Xiao, B. Water extract from artichoke ameliorates high-fat diet-induced non-alcoholic fatty liver disease in rats. 2022. BMC Complement Med. Ther. 2022, 22, 308. [Google Scholar] [CrossRef]
- Xia, N.; Pautz, A.; Wollscheid, U.; Reifenberg, G.; Förstermann, U.; Li, H. Artichoke, cynarin and cyanidin downregulate the expression of inducible nitric oxide synthase in human coronary smooth muscle cells. Molecules. 2014, 19, 3654–3668. [Google Scholar] [CrossRef]
- Kim, D.B.; Unenkhuu, B.; Kim, G.J.; Kim, S.W.; Kim, H.S. Cynarin attenuates LPS-induced endothelial inflammation via upregulation of the negative regulator MKP-3. Anim. Cells Syst. (Seoul). 2022, 26, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Benameur, T.; Porro, C.; Twfieg, M.-E.; Benameur, N.; Panaro, M.A.; Filannino, F.M.; Hasan, A. Emerging Paradigms in Inflammatory Disease Management: Exploring Bioactive Compounds and the Gut Microbiota. Brain Sci. 2023, 13, 1226. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ. 2018, 361, k2179. [Google Scholar] [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Viret, A.; Guilhaudis, L.; Oulyadi, H.; Bourafai-Aziez, A.; Charpentier, G.; Rousselot, G.; Cassin, E.; Descamps, S.; et al. Metabolic and Anti-Inflammatory Protective Properties of Human Enriched Serum Following Artichoke Leaf Extract Absorption: Results from an Innovative Ex Vivo Clinical Trial. Nutrients. 2021, 13, 2653. [Google Scholar] [CrossRef]
- Yip, J.L.K.; Balasuriya, G.K.; Spencer, S.J.; Hill-Yardin, E.L. The Role of Intestinal Macrophages in Gastrointestinal Homeostasis: Heterogeneity and Implications in Disease. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1701–1718. [Google Scholar] [CrossRef]
- Carpentieri, S.; Augimeri, G.; Ceramella, J.; Vivacqua, A.; Sinicropi, M.S.; Pataro, G.; Bonofiglio, D.; Ferrari, G. Antioxidant and Anti-Inflammatory Effects of Extracts from Pulsed Electric Field-Treated Artichoke By-Products in Lipopolysaccharide-Stimulated Human THP-1 Macrophages. Foods (Basel, Switzerland). 2022, 11, 2250. [Google Scholar] [CrossRef]
- Speciale, A.; Muscarà, C.; Molonia, M.S.; Toscano, G.; Cimino, F.; Saija, A. In Vitro Protective Effects of a Standardized Extract From Cynara Cardunculus L. Leaves against TNF-α-Induced Intestinal Inflammation. Front. Pharmacol. 2022, 13, 809938. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, B.; Moraes, L.; Magnusson, M.K.; Öhman, L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand. J. Gastroenterol. 2018, 53, 379–389. [Google Scholar] [CrossRef]
- Mateus, V.; Estarreja, J.; Silva, I.; Barracosa, P.; Teixeira-Lemos, E.; Pinto, R. Effect of Cynara cardunculus L. var. altilis (DC) in Inflammatory Bowel Disease. Appl. Sci. 2021, 11, 1629. [Google Scholar] [CrossRef]
- Ebrahimi-Mameghani, M.; Asghari-Jafarabadi, M. Rezazadeh KTCF7L2-rs7903146 polymorphism modulates the effect of artichoke leaf extract supplementation on insulin resistance in metabolic syndrome: A randomized double-blind placebo-controlled trial. J. Integr. Med. 2018, 16, 329–334. [Google Scholar] [CrossRef]
- Al-Ahdab, M.J. Protective effect of artichoke (Cynara scolymus L.) leaves and pulp extracts against carbon tetrachloride-induced acute hepatotoxicity in rats. World Appl. Sci. J. 2014, 32, 1004–1014. [Google Scholar]
- El Sohaimy, S.A. Chemical composition, antioxidant and antimicrobial potential of artichoke. Open Nutraceuticals J. 2014, 7, 15–20. [Google Scholar] [CrossRef]
- Rezazadeh, K.; Rahmati-Yamchi, M.; Mohammadnejad, L.; Ebrahimi-Mameghani, M.; Delazar, A. Effects of artichoke leaf extract supplementation on metabolic parameters in women with metabolic syndrome: Influence of TCF7L2-rs7903146 and FTO-rs9939609 polymorphisms. Phytother Res. 2018, 32, 84–93. [Google Scholar] [CrossRef]
- Rondanelli, M.; Opizzi, A.; Faliva, M.; Sala, P.; Perna, S.; Riva, A.; Morazzoni, P.; Bombardelli, E.; Giacosa, A. Metabolic management in overweight suIs withIe impaired fasting glycaemia by means of a highly standardized extract from Cynara scolymus: A double-blind, placebo-controlled, randomized clinical trial. Phytother. Res. 2014, 28, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.R.; Sheikhhossein, F.; Talebyan, A.; Bazshahi, E.; Djafari, F.; Hekmatdoost, A. Effects of Artichoke Supplementation on Liver Enzymes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. Res. 2022, 11, 228–239. [Google Scholar] [CrossRef]
- Sabater, C.; Corzo, N.; Olano, A.; Montilla, A. Enzymatic extraction of pectin from artichoke (Cynara scolymus L.) by-products using Celluclast®1.5L. Carbohydr. Polym. 2018, 190, 43–49. [Google Scholar] [CrossRef]
- Jin, M.; Wang, Y.; Yang, X.; Yin, H.; Nie, S.; Wu, X. Structure characterization of a polysaccharide extracted from noni (Morinda citrifolia L.) and its protective effect against DSS-induced bowel disease in mice. Food Hydrocolloids. 2019, 90, 189–197. [Google Scholar] [CrossRef]
- Pacheco, M.T.; Vezza, T.; Diez-Echave, P.; Utrilla, P.; Villamiel, M.; Moreno, F.J. Anti-inflammatory bowel effect of industrial orange by-products in DSS-treated mice. Food Funct. 2018, 9, 4888–4896. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens. 2019, 8, 126. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Hold, G.L.; Smith, M.; Grange, C.; Watt, E.R.; El-Omar, E.M.; Mukhopadhya, I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: What have we learnt in the past 10 years? World J. Gastroenterol. 2014, 20, 1192–1210. [Google Scholar] [CrossRef]
- Sasaki, H.; Lyu, Y.; Nakayama, Y.; Nakamura, F.; Watanabe, A.; Miyakawa, H.; Nakao, Y.; Shibata, S. Combinatorial Effects of Soluble, Insoluble, and Organic Extracts from Jerusalem Artichokes on Gut Microbiota in Mice. Microorganisms. 2020, 8, 954. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Masciangelo, S.; Saturni, L. Celiac disease, inflammation and oxidative damage: A nutrigenetic approach. Nutrients. 2012, 4, 243–257. [Google Scholar] [CrossRef]
- Sollid, L.M.; Jabri, B. Triggers and drivers of autoimmunity: Lessons from coeliac disease. Nat. Rev. Immunol. 2013, 13, 294–30. [Google Scholar] [CrossRef]
- Barone, M.V.; Auricchio, R.; Nanayakkara, M.; Greco, L.; Troncone, R.; Auricchio, S. Pivotal Role of Inflammation in Celiac Disease. Int. J. Mol. Sci. 2022, 23, 7177. [Google Scholar] [CrossRef]
- Vacca, M.; Pinto, D.; Annunziato, A.; Ressa, A.; Calasso, M.; Pontonio, E.; Celano, G.; De Angelis, M. Gluten-Free Bread Enriched with Artichoke Leaf Extract In Vitro Exerted Antioxidant and Anti-Inflammatory Properties. Antioxidants. 2023, 12, 845. [Google Scholar] [CrossRef]
- Kim, H.C. Epidemiology of cardiovascular disease and its risk factors in Korea. Glob. Health Med. 2021, 3, 134–141. [Google Scholar] [CrossRef]
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines. 2022, 10, 1938. [Google Scholar] [CrossRef]
- Ożarowski, M.; Karpiński, T.M.; Szulc, M.; Wielgus, K.; Kujawski, R.; Wolski, H.; Seremak-Mrozikiewicz, A. Plant Phenolics and Extracts in Animal Models of Preeclampsia and Clinical Trials-Review of Perspectives for Novel Therapies. Pharmaceuticals. 2021, 14, 269. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020 83, 770–803. [CrossRef]
- D’Antuono, I.; Carola, A.; Sena, L.M.; Linsalata, V.; Cardinali, A.; Logrieco, A.F.; Colucci, M.G.; Apone, F. Artichoke Polyphenols Produce Skin Anti-Age Effects by Improving Endothelial Cell Integrity and Functionality. Molecules. 2018, 23, 2729. [Google Scholar] [CrossRef]
- Mandim, F.; Petropoulos, S.A.; Fernandes, Â.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Chemical Composition of Cynara Cardunculus L. var. altilis Heads: The Impact of Harvesting Time. Agronomy. 2020, 10, 1088. [Google Scholar] [CrossRef]
- Zayed, A.; Serag, A.; Farag, M.A. Cynara cardunculus L.: Outgoing and potential trends of phytochemical, industrial, nutritive and medicinal merits. J. Funct. Foods. 2020, 69, 103937. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Phenolic composition and antioxidant capacity of cultivated artichoke, Madeira cardoon and artichoke-based dietary supplements. Food Res. Int. 2012, 48, 712–724. [Google Scholar] [CrossRef]
- Ceccarelli, N.; Curadi, M.; Picciarelli, P.; Martelloni, L.; Sbrana, C.; Giovannetti, M. Globe artichoke as functional food. Mediterr. J. Nutr. Metab. 2010, 3, 197–201. [Google Scholar] [CrossRef]
- Liu, J.; Rajendram, R.; Zhang, L. Effects of Oleanolic Acid and Maslinic Acid on Glucose and Lipid Metabolism: Implications for the Beneficial Effects of Olive Oil on Health. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1423–1429. [Google Scholar] [CrossRef]
- Avci, E.; Dolapoglu, A.; Akgun, D. Role of Cholesterol as a Risk Factor in Cardiovascular Diseases. In Cholesterol—Good, Bad and the Heart; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Nirosha, K.; Divya, M.; Vamsi, S.; Sadiq, M. A review on hyperlipidemia. Int. J. Novel Trends Pharm. Sci. 2014, 4, 81–92. Available online: https://scienztech.org/index.php/ijntps/article/view/112.
- Salem, M.B.; Affes, H.; Dhouibi, R.; Charfi, S.; Turki, M.; Hammami, S.; Ayedi, F.; Sahnoun, Z.; Zeghal, K.M.; Ksouda, K. Effect of Artichoke ynaraa scolymus) on cardiac markers, lipid profile and antioxidants levels in tissue of HFD-induced obesity. Arch. Physiol. Biochem. 2022, 128, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, E.; Rafieian-Kopaei, M. Protective effect of artichoke (Cynara scolymus) leaf extract against lead toxicity in rat. Pharm. Biol. 2013, 51, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Giacosa, A.; Opizzi, A.; Faliva, M.A.; Sala, P.; Perna, S.; Riva, A.; Morazzoni, P.; Bombardelli, E. Beneficial effects of artichoke leaf extract supplementation on increasing HDL-cholesterol in subjects with primary mild hypercholesterolaemia: A double-blind, randomized, placebo-controlled trial. Int. J. Food Sci. Nutr. 2013, 64, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.Y.; Kim, S.Y.; Choi, M.S. Luteolin-Enriched Artichoke Leaf Extract Alleviates the Metabolic Syndrome in Mice with High-Fat Diet-Induced Obesity. Nutrients. 2018, 10, 979. [Google Scholar] [CrossRef]
- Santos, H.O.; Bueno, A.A.; Mota, J.F. The effect of artichoke on lipid profile: A review of possible mechanisms of action. Pharmacol. Res. 2018, 137, 170–178. [Google Scholar] [CrossRef]
- Davidson, M.H.; Maki, K.C. Effects of Dietary Inulin on Serum Lipids. J. Nutr. 1999, 129, 1474S–1477S. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, R.; Fausel, M. Antioxidant and hepatoprotective effects of artichoke extracts and constituents in cultured rat hepatocytes. Toxicol In Vitro. 1997, 11, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.; De Smet, P.A.; Shaw, D.; Muraay, V. Traditional remedies and the test of time. Eur. J. Clin. Pharmacol. 1998, 54, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, N.; Brausch, I.; Yao, Y.; Förstermann, U. Flavonoids from artichoke (Cynara scolymus L.) up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J. Pharmacol. Exp. Ther. 2004, 310, 926–932. [Google Scholar] [CrossRef]
- Roghani-Dehkordi, F.; Kamkhah, A.F. Artichoke leaf juice contains antihypertensive effect in patients with mild hypertension. J. Diet Suppl. 2009, 6, 328–341. [Google Scholar] [CrossRef]
- Perez-Garcia, F.; Adzet, T.; Caniqueral, S. Activity of artichoke leaf extract on reactive oxygen species in human leukocytes. Free Radic. Res. 2000 33, 661–665. [CrossRef]
- Valerio, F.; De Bellis, P.; Lonigro, S.L.; Morelli, L.; Visconti, A.; Lavermicocca, P. In vitro and in vivo survival and transit tolerance of potentially probiotic strains carried by artichokes in the gastrointestinal tract. Appl. Environ. Microbiol. 2006, 72, 3042–3045. [Google Scholar] [CrossRef] [PubMed]
- Mocelin, R.; Marcon, M.; Santo, G.D.; et al. Hypolipidemic and antiatherogenic effects of Cynara scolymus in cholesterol-fed rats. Rev. Bras. Farmacogn. 2016, 26, 233–239. [Google Scholar] [CrossRef]
- Vidal-Itriago, A.; Radford, R.A.W.; Aramideh, J.A.; Maurel, C.; Scherer, N.M.; Don, E.K.; Lee, A.; Chung, R.S.; Graeber, M.B.; Morsch, M. Microglia morphophysiological diversity and its implications for the CNS. Front. Immunol. 2022, 13, 997786. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Porro, C.; Calvello, R.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Microglia Mediated Neuroinflammation: Focus on PI3K Modulation. Biomolecules. 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K. Microglia mediated neuroinflammation in neurodegenerative diseases: A review on the cell signaling pathways involved in microglial activation. J. Neuroimmunol. 2023, 383, 578180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, S.; Fan, X. Role of Polyphenols as Antioxidant Supplementation in Ischemic Stroke. Oxid. Med. Cell. Longev. 2021, 2021, 5471347. [Google Scholar] [CrossRef] [PubMed]
- Parrella, E.; Gussago, C.; Porrini, V.; Benarese, M.; Pizzi, M. From Preclinical Stroke Models to Humans: Polyphenols in the Prevention and Treatment of Stroke. Nutrients. 2020, 13, 85. [Google Scholar] [CrossRef]
- Číž, M.; Dvořáková, A.; Skočková, V.; Kubala, L. The Role of Dietary Phenolic Compounds in Epigenetic Modulation Involved in Inflammatory Processes. Antioxidants (Basel). 2020, 9, 691. [Google Scholar] [CrossRef]
- Kong, L.; An, X.; Hu, L.; Zhang, S.; Liu, L.; Zhao, S.; Wang, R.; Nan, Y. Resveratrol ameliorates nutritional steatohepatitis through the mmu-miR-599/PXR pathway. Int. J. Mol. Med. 2022, 49, 47. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Pu, C.; Yang, E.; Zhang, H.; Feng, Y.; Luo, P.; Yang, Y.; Zhang, L.; Li, X.; Jiang, X.; Dai, S. Macrophage/Microglia Sirt3 Contributes to the Anti-inflammatory Effects of Resveratrol Against Experimental Intracerebral Hemorrhage in Mice. Cell. Mol. Neurobiol. 2023, 43, 2871–2882. [Google Scholar] [CrossRef] [PubMed]
- Koronowski, K.B.; Khoury, N.; Saul, I.; Loris, Z.B.; Cohan, C.H.; Stradecki-Cohan, H.M.; Dave, K.R.; Young, J.I.; Perez-Pinzon, M.A. Neuronal SIRT1 (Silent Information Regulator 2 Homologue 1) Regulates Glycolysis and Mediates Resveratrol-Induced Ischemic Tolerance. Stroke. 2017, 48, 3117–3125. [Google Scholar] [CrossRef]
- Yan, X.; Liu, K.; Sun, X.; Qin, S.; Wu, M.; Qin, L.; Wang, Y.; Li, Z.; Zhong, X.; Wei, X. A cross-sectional study of blood selenium concentration and cognitive function in elderly Americans: National Health and Nutrition Examination Survey 2011-2014. Ann. Hum. Biol. 2020, 47, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lei, T.; Zhang, J.; Yan, Y.; Wang, N.; Song, C.; Li, C.; Sun, M.; Li, J.; Guo, Y.; Yang, J.; Kang, T. Longan (Dimocarpus longan Lour.) Aril ameliorates cognitive impairment in AD mice induced by combination of D-gal/AlCl3 and an irregular diet via RAS/MEK/ERK signaling pathway. J. Ethnopharmacol. 2021, 267, 113612. [Google Scholar] [CrossRef] [PubMed]
- Tressera-Rimbau, A.; Arranz, S.; Eder, M.; Vallverdú-Queralt, A. Dietary Polyphenols in the Prevention of Stroke. Oxid. Med. Cell. Longev. 2017, 2017, 7467962. [Google Scholar] [CrossRef] [PubMed]
- Cicek, B.; Genc, S.; Yeni, Y.; Kuzucu, M.; Cetin, A.; Yildirim, S.; Bolat, I.; Kantarci, M.; Hacimuftuoglu, A.; Lazopoulos, G.; Tsatsakis, A.; Tsarouhas, K.; Taghizadehghalehjoughi, A. Artichoke (Cynara Scolymus) Methanolic Leaf Extract Alleviates Diethylnitrosamine-Induced Toxicity in BALB/c Mouse Brain: Involvement of Oxidative Stress and Apoptotically Related Klotho/PPARγ Signaling. J. Pers. Med. 2022, 12, 2012. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Piccinini, A.; Oliveira, M.P.; Silva, M.R.; Bett, G.S.; Becker, I.B.; Mendes, T.F.; Salla, D.H.; Silva, L.E.; Vilela, T.C.; et al. Effects of Ethanolic Extract of Cynara cardunculus (Artichoke) Leaves on Neuroinflammatory and Neurochemical Parameters in a Diet-Induced Mice Obesity Model. Neurochem. Res. 2022, 47, 1888–1903. [Google Scholar] [CrossRef]
- Klöting, N.; Blüher, M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 277–287. [Google Scholar] [CrossRef]
- Guo, Y.J.; Luo, T.; Wu, F.; Mei, Y.W.; Peng, J.; Liu, H.; Li, H.R.; Zhang, S.L.; Dong, J.H.; Fang, Y.; Zhao, L. Involvement of TLR2 and TLR9 in the anti-inflammatory effects of chlorogenic acid in HSV-1-infected microglia. Life Sci. 2015, 127, 12–18. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Tyagi, E. Diet and cognition: Interplay between cell metabolism and neuronal plasticity. Curr. Opin. Clin. Nutr. Metab. Care. 2013, 16, 726–733. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants (Basel). 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.A.; O'Connor, J.C. Brain-derived neurotrophic factor and inflammation in depression: Pathogenic partners in crime? World J. Psychiatry. 2022, 12, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Sun-Waterhouse, D.; Ouyang, F.; Tan, X.; Li, D.; Xu, L.; Li, B.; Wang, Y.; Li, F. Apple phenolic extracts ameliorate lead-induced cognitive impairment and depression- and anxiety-like behavior in mice by abating oxidative stress, inflammation and apoptosis via the miR-22-3p/SIRT1 axis. Food Funct. 2022, 13, 2647–2661. [Google Scholar] [CrossRef]
- Liu, X.; Huang, K.; Niu, Z.; Mei, D.; Zhang, B. Protective effect of isochlorogenic acid B on liver fibrosis in non-alcoholic steatohepatitis of mice. Basic Clin. Pharmacol. Toxicol. 2019, 124, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Shi, Y.; Wu, H.; Niu, C.; Sun, X.; Wang, K. Inhibition of temperature-sensitive TRPV3 channel by two natural isochlorogenic acid isomers for alleviation of dermatitis and chronic pruritus. Acta Pharm. Sin. B. 2022, 12, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R. Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 6827. [Google Scholar] [CrossRef]
- Wang, X.L.; Gardner, W.; Yu, S.Y.; Serchov, T. A Pattern to Link Adenosine Signaling, Circadian System, and Potential Final Common Pathway in the Pathogenesis of Major Depressive Disorder. Mol. Neurobiol. 2022, 59, 6713–6723. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants (Basel). 2020, 9, 743. [Google Scholar] [CrossRef]
- Cianciulli, A.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A. Folic Acid Is Able to Polarize the Inflammatory Response in LPS Activated Microglia by Regulating Multiple Signaling Pathways. Mediators Inflamm. 2016, 2016, 5240127. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Jenner, P.; Przedborski, S. Pathogenesis of Parkinson's disease. Mov. Disord. 2013, 28, 24–30. [Google Scholar] [CrossRef]
- Maccioni, R.B.; Rojo, L.E.; Fernández, J.A.; Kuljis, R.O. The role of neuroimmunomodulation in Alzheimer's disease. Ann. N. Y. Acad. Sci. 2009, 1153, 240–246. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Abbas, H.; Zewail, M.; Noureldin, M.H.; Ali, M.M.; Shamaa, M.M.; Khattab, M.A.; Ibrahim, N. Neuroprotective Effect of Artichoke-Based Nanoformulation in Sporadic Alzheimer's Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceuticals (Basel). 2022, 15, 1202. [Google Scholar] [CrossRef] [PubMed]
- Mekkey, S.M.; Raghif, A.R.A.; Alkafaj, H.A.R.; Hadi, N.R. The Anti-Parkinson Effects of Cyanara Scoluymus (Artichoke) Extract in Rat Model of Rotenone Induced Parkinsonism. Annals of the Romanian Society for Cell Biology. 2021, 25, 2318–2329. Available online: https://www.annalsofrscb.ro/index.php/journal/article/view/5845/4508.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




