1. Introduction
In the Andean countries over the last decade, the consumption of non-conventional animal proteins such as guinea pig meat (
Cavia porcellus) has been increasing, which has led to a significant increase in the production of this species in the rural sector in Ecuador [
1]. In Ecuador, in the last two decades, per capita consumption of guinea pig meat has increased from 0.7 to 2.5 kg/year/person). The increase in demand for this meat has been due to its nutritional value [
2,
3]. However, the feed efficiency of guinea pigs reared in Ecuador is still not ideal, because producers in this sector have not yet implemented feeding strategies to improve animal performance [
1,
3].
In most developing countries, the use of additives such as antibiotic growth promoters still persist in animal production, with the aim of improving the health and increasing the productive parameters of animals [
2,
4]; however, the inappropriate use of these products has led to bacterial resistance, attributed to antibiotic residuals in the carcass, which creates a public health problem [
2,
5]. Probiotics consist of lactic and non-lactic bacteria, yeasts and fungi, which is important when supplementing monogastric or ruminants [
6,
7]. Probiotics also have beneficial properties as growth promoters, feed conversion improvers, gut flora modifiers, helping the animal to be more resistant to disease and reducing the use of antibiotics [
8,
9].
The use of microbial bioactive such as probiotics, prebiotics and symbiotic, emerges as a viable alternative to antibiotic growth promoters [
10], with the premise of maintaining meat safety, improving animal welfare, the development of the gastrointestinal tract and the immune system [
11], improving carcass yield, without leaving residues in the carcass [
12]. Recent studies provide encouraging data on the inclusion of diets with beneficial microorganisms in the ability to balance the microbiota in the different segments of the gastrointestinal tract, which has attracted much attention from researchers [
2]. However, scientific information on the positive effect of beneficial microorganisms and the mechanism of action is still insufficient (9, 13).
Previous studies on the use of agro-industrial substrates (molasses-vinasse) fermented with lactic acid bacteria and yeasts have shown significant (p<0.05) increases in weight gain in pre-weaned and post-weaned piglets, chickens and cattle [
7,
13,
14]. It has also been shown to improve health by reducing diarrhea disorders and deaths. However, there is still no scientific data showing a positive action on productive parameters, health and the ability to balance the natural microbiota in the different segments of the organs of the gastrointestinal tract in growing guinea pigs (
cavia porcillus) [
15,
16].
However, the impact of these additives differs according to the studies, although there are studies related to the use of probiotics in animals, which report no benefits on the productive parameters of the animals [
17]; while other studies attribute them benefits on animal performance, being probiotics the most promising feed additives of the future, postulating themselves as a good alternative for the substitution of antibiotic growth promoters [
2,
7,
18]. Therefore, the aim of the study was to evaluate the impact of probiotics obtained from agro-industrial substrates fermented with lactic acid bacteria and/or yeasts on the health and changes in the microbiota of the digestive tract of guinea pigs.
2. Materials and Methods
2.1. Animal Bioethics
All experimental procedures applied in this study were reviewed and approved by the Commission of Scientific Degrees by Agreement N° 189/13-14. Faculty of Agricultural Sciences, University of Zulia.
The procedures related to the handling, management and health care of live guinea pigs (
cavia porcillus) complied with the standards applicable to laboratory animals used for scientific purposes and were applied in accordance with the minimum standards for the protection of animals described in Council Directive 2008/120/EC on minimum standards for the protection of pigs (Council of the European Union, 2008) [
19].
2.2. Study Location
The experimental study with animals was carried out at the "Irquis" farm, located at Km 20 via Salado-Lentag, Cuenca, belonging to the Universidad de Cuenca.
2.3. Design and Dosage of Probiotics Used in the Study
Using a completely randomised design, four treatments, four replicates per treatment, each replicate consisting of 5 animals. The treatments evaluated are shown in
Table 1.
2.4. Animals and Basal Diet Used
The study used 80 male guinea pigs (cavia porcillus), Kury breed, 30 ± 5 days old and 250 ± 30 grams (g) live weight.
The feed offered to the study animals was a mixture of 20, 25, 30, 24.97 and 0.03 % alfalfa (Medicago sativa), maralfalfa (Pennisetum spp), king grass (Pennisetum purpureun x P. typhoides), balanced for guinea pig fattening (15, 4, 12, 7 and 87 % crude protein, fat, crude fibre, ash and dry matter, respectively) and 0.03 % vitamin C, respectively. The diet was designed for three phases: phase I from 30 to 60, phase II from 61 to 90 and phase III from 91 to 120 days of age.
Feed was provided in two equal rations per day (07:00 am and 04:00 pm), as recommended in a previous study by Miranda [
13], in accordance with the recommendations described in the NRC [
20] that meet the minimum requirements established for guinea pigs. In addition, 50 mL of water was offered daily in automatic waterers (Plasson, SKU: 885B722-8, Argentina).
Table 2.
Amount of feed at each stage of production and bromatological composition of the diet used for guinea pigs.
Table 2.
Amount of feed at each stage of production and bromatological composition of the diet used for guinea pigs.
| Production stage |
A Quantity of food offered, g/animal/day |
Nutritional composition*, %. |
| CP |
EE |
CF |
Ash |
DM |
| I |
100 |
19 |
5.88 |
12.5 |
5.85 |
87.82 |
| II |
150 |
17 |
5.84 |
12.8 |
5.67 |
88.52 |
| III |
200 |
16 |
5.85 |
12.6 |
5.68 |
87.64 |
2.5. Management and Conditioning System
The biosecurity measures on the farm were conditioned prior to the reception of the guinea pigs, as recommended by Vivas [
21], which allowed animal health control of the animals during the study. Site disinfection was performed at a dose of 3 cc/L with glutarhaldehyde, quaternary ammonium and isopropyl alcohol (Viroguard® Lima, Peru), as recommended by the manufacturer.
Guinea pigs were housed in group cages of 1.50 x 1.00 m
2, with five animals per cage. The temperature of the house was maintained at 14 ±2 °C. The cages for each treatment were placed 1.50 m apart on both sides of the aisle to avoid self-inoculation. All animals subjected in the study received the relevant veterinary care according to the guinea pig (
Cavia porcellus) husbandry manual [
21].
2.6. Obtaining and Administering Microbial Bioadditives to Guinea Pigs
All the bioadditives evaluated in the study were obtained following the methodology described by Miranda [
22]. T1, agro-industrial substrate (molasses-vinasse) fermented with
L. acidophilus and
L. bulgaricus. T2, agro-industrial substrate (molasses-vinasse) fermented with
S. cerevisiae and
K. fragilis. T3, agro-industrial substrate (molasses-vinasse) fermented with L. acidophilus,
L. bulgaricus, S. cerevisiae and
K. fragilis.
The administration of the microbial bioaditives under study was carried out according to the dose and group indicated in
Table 1, the first dose was in single doses orally, and from the second dose onwards it was administered every 3 days, according to the assigned group, while the control group received 0.25 mL of distilled water.
2.7. Productive Indicators
The guinea pigs (cavia porcillus) under study were weighed on a digital scale (Camry, China) of 5.00 kg capacity with an error of ± 5 g, at the beginning (30 d of age) and at 60, 90 and 120 days of age, with this information the weight gain (WG) was calculated.
2.8. Continuous Physical Evaluation of the Animals
All guinea pigs under study were monitored daily for macroscopic lesions, behavioural changes and health status. The presence of diarrhea and deaths were recorded daily on an individual basis, as animals were identified by a code on the eartag. This information was used to determine the presence of diarrhea and percentage mortality as described by Thrusfield [
23].
2.9. Slaughter and Scalding of the Animals
At the end of the study (90 days), six animals from each treatment were fasted for a period of 12:00 h, prior to slaughter, as recommended in the methodology used by Cornejo [
24]. At the end of this period the guinea pigs were transferred to the slaughter room. Sanitary slaughter of the animals was performed with prior stunning using the denudation technique at the atlanto-occipital joint, according to the methodology described by Sánchez [
25], previously established in the Mexican Official Standard NOM-033-ZOO-1995, Humane Slaughter of Domestic and Wild Animals (Humane Slaughter Association, 2016) [
26].
Exsanguination was performed after stunning, exsanguination was performed by unilateral cutting of the jugular vein and carotid artery, according to the methodology described by Sanchez [
25]. In a surgical cauldron of 15 L capacity, 12 L of water was tempered to 70 ºC. The previously obtained guinea pig carcasses were immersed for 20 seconds in cranio-cutaneous position. Finally, all fur was removed.
2.10. Evisceration of the Animals
Prior to evisceration, the atlanto-occipital joint and cervical vertebrae, as well as the carpal-metacarpal and tarsal-metatarsal joints were cut until a carcass without autopods and head was obtained. Evisceration was performed by laparotomy to isolate the organs of the gastrointestinal tract.
Digestive segments (pancreas, liver, stomach, small and large intestines), lungs, spleen, thymus and kidneys were carefully separated from the mesentery, stomach, small and large intestines were washed with sterile distilled water after removal of digesta contents and finally weighed on a digital electric scale (KAMRY, model EK5055-11, Hong Kong, China) with a capacity of 5 ± 0.1 kg.
2.11. Gross Pathological Examination of the Organs of the Gastrointestinal Tract
In lesions of the stomach, small intestine, colon and cecum the following parameters were assessed: thickness of the intestinal wall, using a tape measure, presence of circulatory disorders in the mucosa (oedema, congestion, haemorrhages), consistency of the intestinal contents (watery, mucous, foamy) and pH was measured with a digital pH meter (Hanna®, HI 99163. USA).
2.12. Collection of the Intestinal Mucosa
After isolation of the organs of the gastrointestinal tract, a 2 cm2 longitudinal incision was made in the stomach, small intestine, cecum and colon after washing with sterile distilled water and saline phosphate buffer (BFS) (NaCl 8, 0 g, KCl 0. 2 g, Na2HPO4-2H2O 1.44 g, 0.2 g, KH2PO4, in 1.0 L sterile distilled H2O) with 0.01% gelatin pH 7.4 according to the methodology used by Cueto [
27].
The previously obtained fragments were deeply scraped with the help of a 75 mm spatula to obtain 2.00 mL of mucus, which were collected in 15 mL Falcon plastic tubes (Henso, Germany) with sterile screw cap and 5 mL of BFS were added according to the methodology described by Kandler and Weiss [
28]. Finally, they were centrifuged in a digital centrifuge (Yingtai, China) at 4582 x g at 8°C for 10 minutes and the supernatant was removed; this procedure was performed three times.
2.13. Microbial Growth on Selective Culture Media
1.0 mL of the previously obtained mucus content was taken, added to a 150 mL erlenmeyer flask containing 50 mL of nutrient broth and MRS separately. It was then incubated at 37 °C for 6 h in an incubator with an orbital shaker (Inkubationshaube TH 15, Germany) at 15 rpm. After this time, 5.0 mL of each culture was taken and homogenised with physiological saline at a ratio of 1/10 (v/v), followed by serial dilutions of 1/10, (v/v) to the 0.5 scale of the MacFarland scheme.
The microbial culture was then processed on the surface of Petri dishes containing sterile selective media (MRS and M17 agar, Sabouraud Dextrose agar and MacConkey agar) and a general medium (nutrient agar), using the streaking method. They were then incubated at 37 °C and at 30 °C for AS. Petri dishes containing MRS and M17 were incubated under anaerobic conditions in a GasPak Plus™ jar with 5% Co2. After this time, the process of identification of typical colonies was carried out. In addition, Gram staining was performed and observed with a binocular optical microscope (BA310 MOTIC, China) to differentiate morphosynthesis characteristics according to the Manual of Systematic Bacteriology [
28].
2.14. Biochemical Tests
Catalase and coagulase tests of scraping samples from digestive tract organs were performed with apiWeb version 5.1. Colonies grown on agar (MRS, M17, AN, AS and MacConkey) from subcultures were inoculated into miniaturised biochemical test kits: API® 50 CHL, API® 20 E and API® ID 23 C (BioMerieux), which were incubated for 24 and 48 h at 37 and 30 °C for bacteria and yeast, respectively, according to the manufacturer's recommendations. Genus confirmation and species definition were performed with the commercial API 20NE system (BioMerieux, St Louis, MO, USA), following the manufacturer's instructions. After this time, the carbohydrate fermentation profile of each strain was compared with the database provided by the manufacturer.
2.15. Statistical Analysis
Experimental data were processed with the statistical package Statgraphics plus ver. XV. II for Windows. Experimental variables such as weight gain, relative weight of digestive tract organs and microbial load (CFU. mL
-1) obtained in the culture media were subjected to a simple rank analysis of variance (ANOVA) according to a completely randomised design [
29]. When the P-value was <0.05, Duncan [
30] comparison test was applied to discriminate differences between treatments.
For the variables occurrence of diarrhoea and percentage mortality, a multiple comparison analysis of proportions was performed in the statistical package SAS version 17.
3. Results
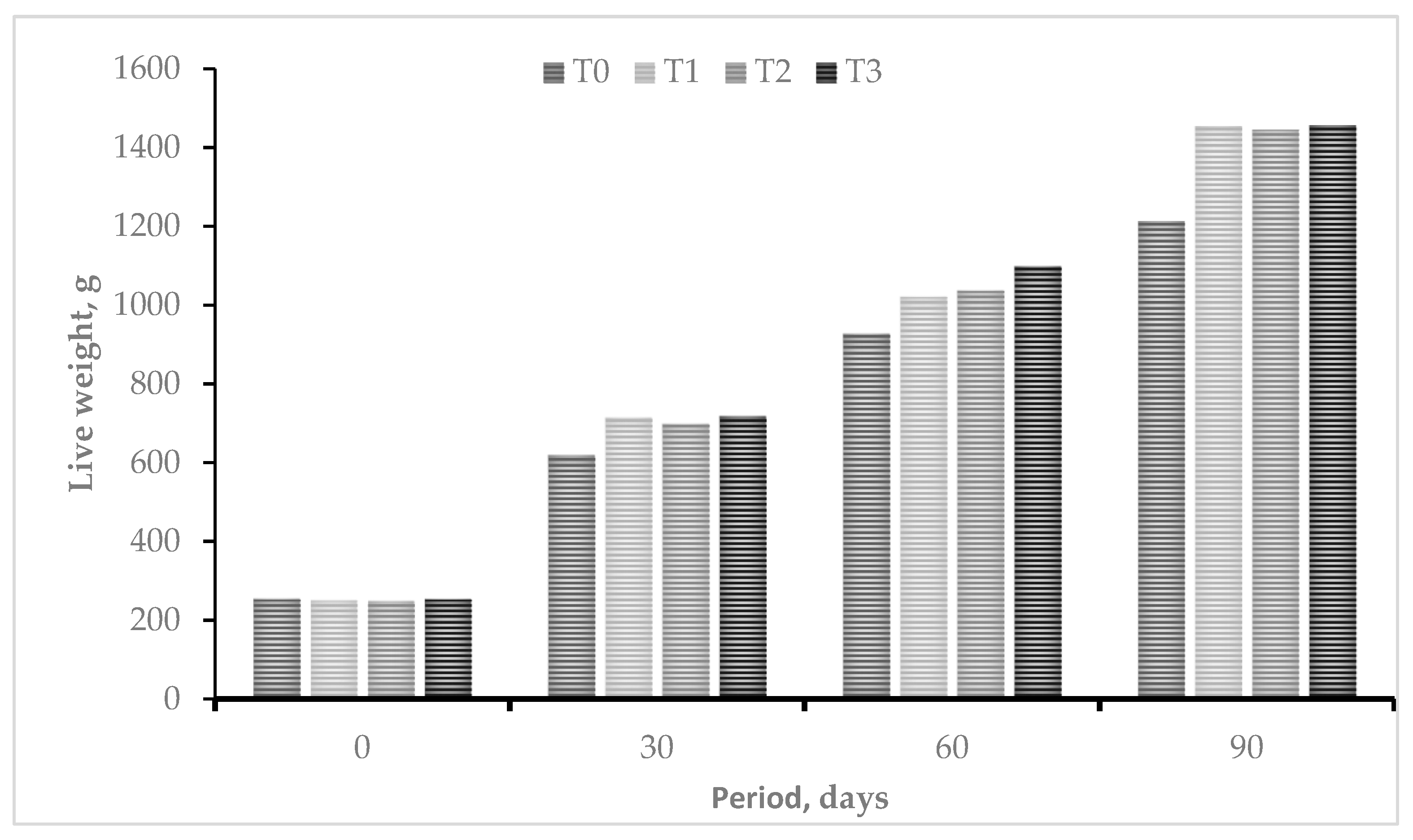
Figure 1 summaries the responses in weight gain in guinea pigs. When diets containing agro-industrial substrate fermented with lactic acid bacteria and yeasts were included, weight gain increased, but the animals in T3 gained more weight (p<0.05) in relation to the other treatments (T0, T1 and T2) in the evaluations carried out at 30, 60 and 90 days of study, which shows that probiotic microorganisms have a positive action on the utilization of the diet offered.
The animals that consumed diets with microbial additives presented less (p<0.05) number of animals with diarrhoea, of these the treatments T1 and T2, were with less (p<0.015 and p<0.014) occurrence of diarrhea, in the evaluation carried out at 30 and 60 days respectively. However, the aetiology of the possible agents associated with the occurrence of diarrhea was not identified. In terms of deaths, animals in the control group had a higher number (p>0.05) of animals dying from diarrheal disorders at 30 and 60 days of the study, but there were no guinea pig deaths at 90 days of the experiment (see
Table 3).
Table 4 shows the general weight status of the digestive tract organs of guinea pigs at 120 days of age. The relative weight of the small intestine with cecal contents, liver, lungs and kidneys was higher (p<0.05) in animals consuming diets containing agro-industrial substrate fermented with lactic acid bacteria and yeast. However, in the other organs evaluated there were no significant differences (p>0.05).
Table 5 shows the results of macroscopic lesions of the digestive tract organs in 120-day-old guinea pigs. Animals in the control treatment (T0) showed a higher number of animals with lesions at the intestinal level. However, guinea pigs that consumed the agro-industrial substrates fermented with lactic acid bacteria and yeast (T1, T2 and T3), did not present macroscopic lesions to be considered physiological alterations at the level of the digestive tract organs.
Macroscopic changes in the stomach, small intestine, colon and cecum showed significant changes in relation to the thickness of the intestinal wall in the animals of the control group (T0) compared to guinea pigs consuming agro-industrial substrates fermented with lactic acid bacteria and yeast. A similar situation occurred with circulatory disorders at the level of the intestinal mucosa and contents in the T0 treatment, as can be seen in
Table 5.
Table 6 presents the microbial load cultured on MRS, M17, Nutrient, Sabouraud Dextrose and MacConkey Agar. In stomach contents samples (post-mortem) cultured on MRS, M17, Nutrient and Sabouraud Dextrose agar, there was no significant growth (p>0.05) between treatments. However, on MacConkey agar, higher microbial growth (p=0.003) was observed in samples from the control treatment (T0) compared to the other treatments (T1, T2 and T3).
Samples of small intestine, colon and cecum scrapings from animals consuming diets containing agro-industrial substrates fermented with lactic acid bacteria and yeast (T1, T2 and T3), showed increased microbial growth (p<0.05) when cultured on Petri dishes containing MRS, M17, Nutrient and Sabouraud Dextrose agar, compared to guinea pigs from the control treatment. In contrast, on MacConkey agar, higher microbial growth was observed in the control treatment samples compared to T1, T2 and T3 treatments (see
Table 6).
Table 7 reports the main mycoorganisms detected in the different organs of the digestive tract in guinea pigs at 120 days of age. In the samples from animals that consumed diet containing agro-industrial substrates fermented with lactic acid bacteria and yeasts (T1, T2 and T2), a higher presence of microorganisms with numerical profiles corresponding to
L. acidophilus,
L. bulgariccus,
Saccharomyces spp. and
K. fragilis was observed, compared to animals in the control group.
In samples from control treatment animals, a higher presence of numerical profiles corresponding to pathogens such as E. coli was observed.
In the small intestine scraping samples, a higher presence of numerical profiles of microorganisms known as probiotics was observed mainly in the T1, T2 and T3 treatments, compared to samples from animals in the control group, as shown in
Table 7.
4. Discussion
4.1. Productive Behaviour
The use of probiotics as growth promoters in animal feed is mainly due to the described improvement in production associated with increased digestion and nutrient absorption [
8,
31]. In fattening guinea pigs fed agro-industrial substrates fermented with lactic acid bacteria and yeast in the diet, a significant increase in weight gain was observed. On the other hand, [
9,
18,
32] observed no significant variations in weight gain, feed intake and feed conversion in broilers fed a commercial probiotic in their diet. In pigs receiving probiotics based on different Lactobacillus species, a significant increase in daily weight gain, a reduction in the incidence of diarrhea compared to the control group [
33], and an increase in nutrient digestibility [
32] were observed.
The increase in nutrient digestibility could be due to the increased enzyme activity in the gut caused by the administered probiotics [
10]. Research has reported increased amylase enzyme activity when Lactobacillus is added to piglets diets [
14], and increased sucrase and lactase activity when Lactobacillus is added to pig diets [
7].
Bacillus amyloliquefaciens, which has been used as a probiotic in animal feed, produces extracellular enzymes such as amylase, cellulase, proteases and metalloproteases, which can improve nutrient digestion [
34]. It has also been reported that probiotic consumption increases the height of intestinal villi in rabbits, increasing the area of nutrient absorption [
35].
Probiotics reduce the symptoms of lactose maldigestion. This effect was observed in people who ingested fermented dairy products [
36]. This effect is due, on the one hand, to the fact that the bacteria contained in these products possess lactose-metabolising enzymes such as β-galactosidase (lactase) [
37]. On the other hand, these strains increase intestinal enzyme activity (intestinal lactase) [
11].
In other species evaluated, production parameters improved with the inclusion of probiotics in the diet, as is the case reported by Cornejo [
24] evaluating the probiotic Bioplus 2B in rabbits. While Miranda [
7], found a 12% improvement when including Lactobacillus in the diet of piglests. This result is probably due to the increase in enzyme activity in the intestinal tract caused by probiotics, which results in an increase in nutrient digestibility, in addition, the consumption of agro-industrial substrates fermented with lactic acid bacteria and yeast increases the ratio of villus height/crypt depth and duodenal villus height [
6,
18], this improvement increases the area of nutrient absorption and translates into higher weight gain in guinea pigs (5, 11).
4.2. Health and Diarrhea
One of the most studied yeasts has been Saccharomyces, which reduced the risk of diarrhoea. Other studies show a reduction in diarrhoea caused by Clostridium difficile, although this was only significant in puppies [
9,
11]. Therefore, its use can be considered with a strong recommendation (based on a moderate level of evidence).
In the mucous membranes, the epithelium plays a barrier function that prevents the entry of toxic substances and pathogens [
16]. To this end, the intestinal epithelium synthesises mucins to form a physical mucus barrier [
17]. Probiotics protect the epithelium through a cytoprotective effect and have the ability to increase the expression of mucins by cells in the ileum and colon, which is a highly effective mechanism in the antibacterial fight against bacteria [
6,
38].
Other reports agree with the data obtained in the present study, with the inclusion of different species of microorganisms (usually Lactobacillus alone or in combination with
bifidobacteria,
enterococci or
Saccharomyces boulardii) [
4,
8]. The use of mixed cultures of lactic acid bacteria and yeasts decreases the risk of
E. coli associated diarrhoea [
7]. Although overall positive results were found with all probiotics used, it is recognised that there is significant heterogeneity in the studies, so there is insufficient evidence to say whether the effect varies systematically between populations, including the probiotic preparation usedb [
8,
16,
27].
In controlled studies in which probiotic bioaditoves fermented with
Lactobacillus spp,
L. bulgaricus and
Streptococcus thermophilus were administered, the incidence of Clostridium diarrhoea was reduced and is therefore recommended, although the level of recommendation would be weak with low evidence [
31,
33].
The administration of fermented substrates containing lactic acid bacteria and yeasts to guinea pigs is effective in the prevention of diarrhoea associated with diarrhoea. However, the results are not homogeneous and, in addition, there is a great variability in the strains studied and in the nutritional formulas used [
18]. In the present study, different strains have been used, such as
L acidophilus, L bulgaricus S cerevisiae and K
fragilis. The microorganisms used in the present study have been shown to have a probiotic effect with positive results, only in less than half of the studies were significant benefits observed in terms of reduction of diarrhoea [
27,
36]. Other probiotics may be effective, but the paucity of studies precludes drawing conclusions [
38]. Also, for secondary prevention of recurrent infections by pathogens with a low level of evidence, mixed cultures of lactic acid bacteria and yeasts could be used, although not all authors conclude the same [
6,
7].
4.3. Modification of the Microbiota and the Environment of the Digestive Tract
One of the mechanisms of probiotics is to change microbial population dynamics, decrease the growth of pathogenic microorganisms and promote the growth of beneficial microflora [
1,
6]. Beneficial microbial populations in the digestive tract have been associated with increased animal performance, reflecting more efficient digestion and improved immunity [
5]. The ability of probiotics to reduce pathogenic microorganisms in the digestive tract may be due to the production of bacteriocins [
18], exclusion by competition as probiotics adhere to the intestinal epithelium, change in pH and induction of the immune system response [
31].
In guinea pigs that received probiotics as dietary additives, increases in the population of
Lactobacillus and
Bifidobacteria decreases in the population of
E. coli [
32] and decreases in the population of
Clostridium spp. [
34] were reported. The bacteria
L. reuteri,
B. subtillis and
B. licheniformis, have shown in piglets the ability to decrease the amount of
E. coli and
Salmonella sp. in faeces [
36]. This decrease in the excretion of pathogenic microorganisms reduces the risk of infection of other animals in livestock production and cross-contamination. Modification in the digestive tract population has been seen with commonly used probiotic bacteria both gram negative and gram positive, lactic acid bacteria and spore-forming bacteria such as
Bacillus spp [
37,
38].
Some of the bacterial species commonly used as probiotics, including lactic acid bacteria such as
Bifidobacterium,
Bacillus and
Lactobacillus, produce substances (bacteriocins) that have the ability to inhibit the growth of pathogenic microorganisms such as
Staphylococcus,
Enterococcus,
Listeria and
Salmonella in the gut of animals [
18,
27,
31]. Bacteriocins inhibit bacterial cell wall synthesis, resulting in the production of pores on the bacterial surface [
1,
2,
6]. The genera
Lactobacillus and
Bifidobacterium produce organic acids such as lactic and acetic acids, which can be taken up by other bacteria, including pathogens, in the gastrointestinal tract, reducing their intracellular pH to lethal levels [
7,
8,
16]. These organic acids can also contribute to the lowering of pH in the digestive tract, creating favorable conditions for the existing microbiota and decreasing the risk of being colonized by pathogenic microorganisms [
11,
38].
Microorganisms supplied as probiotics could colonise the digestive tract of young and adult animals, preventing colonization by pathogenic microorganisms. Some strains of
Lactobacillus and
Bifidobacterium have hydrophobic surface proteins that promote non-specific adhesion to animal cells, cover receptor binding sites and prevent the intestinal epithelium from binding to pathogenic microorganisms [
27]. In guinea pig, adhesion of
Lactobacillus spp. to the intestinal epithelium has been observed, which could exclude pathogenic microorganisms by competition [
31].
One of the possible mechanisms to reinforce the integrity of the intestinal barrier and prevent the entry of pathogens and toxins into the animal is to increase the expression of genes involved in the transmission of signals between the tight junctions of the cells of the intestinal epithelium [
31,
37]. In some probiotics, such as
Lactobacillus, modulation of genes encoding adhesion proteins within these tight junctions, such as
E. cadherin and E-
catenin, has been observed [
38].
Some bacteria used as probiotics have the ability to reduce the permeability of the intestinal epithelium, decreasing the translocation of intestinal pathogens from the gastrointestinal tract to other organs such as the liver, spleen and lymph nodes in mice [
32] and pigs [
7]. For probiotics to effectively contribute to maintaining the barrier function of the intestinal wall, it is crucial that they are administered before pathogens have multiplied in the gastrointestinal tract. This effect depends on the species used as a probiotic and the type of pathogen (virus, bacterium or fungus) [
1,
6,
9,
11].
Some bacteria used as probiotics secrete chemical signals called autoinducers that affect the behaviour of other bacteria and even the host (18). This process of bacterial communication is called quorum sensing [
27]. These signals sent by probiotic bacteria can have an effect on the virulence of pathogenic bacteria by affecting them.
5. Conclusions
The inclusion of 1.00 mL of agro-industrial substrates fermented with lactic acid bacteria and yeasts tends to improve the productive performance and weight gain of guinea pigs in the growth stage increases significantly. On the other hand, it helps to significantly reduce the occurrence of diarrhoea and death of the animals, especially in young animals. In addition, there are no macroscopic lesions in the digestive tract organs and, consequently, the digestive organs have a higher weight and a better appearance. Furthermore, an improvement in the presence of beneficial microorganisms in the different segments of the digestive tract is observed in the T1, T2 and T3 treatments.
These results are the basis for the use of bioadditives with probiotic capacity in commercial guinea pig farms.
Author Contributions
José Miranda: animal experimentation, feed formulation, laboratory experiment, statistical analysis, study design and writing; Jose Miranda, Juan Taboada: study design, feed formulation, animal experimentation, data evaluation, manuscript revision; Veronica Once, Wilfrido Briñez, José Miranda, Juan Taboada: coordination, study design, data collection, critical revision of the manuscript.
Funding
This research was financed by the research project "Evaluation of the effect of the inclusion of bioadditives in the diet on the bioproductive indexes, carcass quality and meat quality of guinea pigs (Cavia porcellus), registered in the research department, UNACH, Ecuador.
Data Availability Statement
Acknowledgments
Thanks are due to all the students who were involved in the study.
Conflicts of Interest
We declare that we have no financial or personal relationships with other persons or organizations that could unduly influence our work, and that we have no professional or personal interest of any kind or nature in any product, service and/or company that could be construed as influencing the content of this document.
References
- Cano, J.; Carcelén, F.; Ara, M.; Quevedo, W.; Alvarado, A.; Jiménez, R. Effect of supplementation with a probiotic mixture on the productive behavior of guinea pigs (Cavia Porcellus) during the growth and finishing phase. Rev Inv Vet Perú 2016, 27, 51–58. [Google Scholar]
- Ardoino, S.; Toso, R.; Toribio, M.; Álvarez, H.; Mariani, E.; Cachau, P.; Mancilla, M.; Oriani, D. Antimicrobials as growth promoters (AGP) in balanced poultry feed: use, bacterial resistance, new alternatives and replacement options. Sci. Vet 2017, 19, 50–66. [Google Scholar] [CrossRef]
- Condoy, M.; Iñiguez, F.; Naula, J.; Vega, L. Garlic on productive parameters and intestinal morphometry in guinea pigs. Rev. Inv. Cs. Agro. Vet 2022, 6, 310–316. [Google Scholar]
- Astaiza, J.; Benavides, J.; Chaves, C.; Arciniegas, A.; Quiroz, C. Standardization of the necropsy technique in guinea pigs (Cavia porcellus) at the University of Nariño. Revest Invest Pecua 2014, 2, 79–83. [Google Scholar]
- Frias, H.; Murga, N.; Flores, G.; Cornejo, V.; Del Solar, J.; Romani, A.; Bardales, W.; Segura, G.; Polveiro, R.; Vieira, D.; Lopez, R.; Maicelo, J. An analysis of the cecum microbiome of three breeds of the guinea pig: Andina, Inti, and Per. Res Vet Sci. 2023, 161, 50–61. [Google Scholar] [CrossRef]
- Jurado, H.; Zambrano, E.; Fajardo, C. Addition of microencapsulated Lactobacillus plantarum on intestinal, immune, productive parameters and blood biochemistry in chickens. Biotec Sect Agropec Agroindustrial 2021, 19, 11–21. [Google Scholar]
- Miranda, J.; Marin, A.; Garcia, Y. Impact of microbial additives on the productive, zoometric behavior and diarrheal incidence of piglets. Rev MVZ Córdoba 2018, 23, 6617–6627. [Google Scholar]
- Paredes, M. ; Goicochea, EEffect of five diets with different proportions of neutral detergent fiber and starch on the productive performance, ingestive behavior and weight of digestive organs of guinea pig (Cavia porcellus). Rev Inv Vet Perú 2021, 32, e19495. [Google Scholar]
- Guevara, J.; Carcelén, F.; García, T.; Bravo, N.; Núñez, O.; Reyna, L.; Erazo, R.; Vílchez, C. Effect of supplementation of natural and commercial probiotics on the productive performance of growing guinea pigs. Agroind. sci 2022, 12, 215–220. [Google Scholar] [CrossRef]
- Guevara, J.; Carcelén, F.; García, T. Productive behavior of growing guinea pigs (Cavia porcellus) supplemented with natural prebiotics and probiotics. Cienc Tecnol Agropecuaria 2021, 22, e1920. [Google Scholar]
- Oso, A.; Idowu, O.; Haastrup, A.; Ajibade, A.; Olowonefa, K.; Aluko, A.; Ogunade, I.; Osho, S. : Bamgbose, A. Growth performance, apparent nutrient digestibility, caecal fermentation, ileal morphology and caecal microflora of growing rabbits fed diets containing probiotics and prebiotics. Livest Sci 2013, 157, 184–190. [Google Scholar] [CrossRef]
- Mínguez, C.; Calvo, A.; Zeas, V.; Sánchez, D. A comparison of the growth performance, carcass traits, and behavior of guinea pigs reared in wire cages and floor pens for meat production. Meat Science 2019, 152, 38–40. [Google Scholar] [CrossRef]
- Miranda, J.; Taboada, J.; Briñez, W. Effect of bioadditives on bioproductive indicators of nulliparous guinea pigs (Cavia porcellus) and their offspring. Rev MVZ Córdoba 2022, 27, e2547. [Google Scholar]
- Miranda, J.; Marin, A.; Marrero, L.; Lazo, L.; Sánchez, D.; Pérez, A. Effect of bioprepared on the bioproductive behavior of sows and their offspring. Rev Cient, FCV-LUZ 2018, 28, 298–305. [Google Scholar]
- Bochmann, M.; Knell, S.; Dennler, M.; Grest, P.; Wenger, S. Clinical Presentation, Diagnosis, and Treatment of Cholelithiasis in a Pet Guinea Pig (Cavia porcellus). J Exotic Pet Med 2016, 25, 327–331. [Google Scholar] [CrossRef]
- Chamorro, S.; de Blas, C.; Grant, G.; Badiola, I.; Menoyo, D.; Carabaño, R. Effect of dietary supplementation with glutamine and a combination of glutamine-arginine on intestinal health in twenty-five-day-old weaned rabbits. J Anim Sci 2009, 88, 170–180. [Google Scholar] [CrossRef]
- Castillo, W.; Huaman, A.; Sánchez, A. Evaluation of glutamine and glutamic acid in diets of guinea pigs (Cavia porcellus) on the structure and intestinal enzymatic activity and productive and economic performance. Rev Inv Vet Perú 2022, 33, 1–15. [Google Scholar]
- Guevara, J.; Carcelén, F. Effect of probiotic supplementation on the productive parameters of guinea pigs. Rev. Per. Quím. Ing. Quím 2014, 17, 69–74. [Google Scholar]
- Council of the European Union. Council Directive 2008/120/EC of 18 December (2008). Laying down minimum standards for the protection of pigs. Official Journal of the European Union.
- NRC (National Research Council). 1995. Nutrient requirements of laboratory animals. 4th ed. Washington, USA: National Academy Press.
- Vivas, J. ) “Especies alternativas”: Manual de Crianza de cobayos (Cavia porcellus). 1ra ed. Managua: UNA. 2013; 81p. [Google Scholar]
- Miranda, J.; Marin, A.; Sánchez, D.; García, Y. Obtaining, characterization and evaluation of two candidate preparations for probiotics developed with agroindustrial waste. Rev MVZ Cordoba 2018, 23, 6487–6499. [Google Scholar]
- Thrusfield, M. Veterinary Epidemiology. Third edition. BlacK well Publishing. 2005, pp. 269–270.
- Cornejo, J.; Rodríguez, L.; Pro, A.; González, F.; Conde, V.; Ramírez, M.; Hernández, A. Efecto del ayuno ante mortem en el rendimiento de la canal y calidad de la carne de conejo. Arch Zootec. 2016, 65, 171–175. [Google Scholar] [CrossRef]
- Sánchez, D.; Cevallos, L.; Nuñez, D.; Morales, A. First report of postmortem pH evolution and rigor mortis in guinea pigs. Livest Sci 2019, 229, 22–27. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana NOM-033-ZOO-1995, Humane slaughter of domestic and wild animals, Mexico. 2015.
- Cueto C, Acuña Y, Valenzuela J. in vitro evaluation of probiotic potential of lactic bacteria acid isolated from coastal serum. Actual Biol 2010, 32, 129–138.
- Kandler, O. ; Weiss. N. Regular nonsporing Gram-positive rods. P. 1208-1260. En P. H. A. Sneath, M. S. Mair, M. E. Sharpe y J. G. Holt (Editor). Bergey´s Manual of Systematic Bacteriology, 10th edition, vol. 2. The Williams and Wilkins Co; Baltimore. 1992.
- Steel RG, Torrie JH, Dickey, D.A. Principles and procedures of statistics: A iometrical approach. 3th ed. McGraw Hill Book Co. Inc. New York, 1997.
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11. [Google Scholar] [CrossRef]
- Puente, J.; Carcelén, F.; Ara, M.; Bezada, S.; Huamán, A.; Santillán, G.; Perales, R.; Guevara, J.; Asencios, A. Effect of supplementation with increasing levels of probiotics on the histomorphometry of the small intestine of the guinea pig (Cavia porcellus). Rev Inv Vet Perú 2019, 30, 624–633. [Google Scholar]
- Valdizán, C.; Carcelén, F.; Ara, M.; Bezada, S.; Jiménez, R.; Asencios, A.; Guevara, J. Effect of the inclusion of probiotic, prebiotic and synbiotic in the diet on the productive parameters of the guinea pig (Cavia porcellus). Rev Inv Vet Perú 2019, 30, 590–597. [Google Scholar] [CrossRef]
- Jahuira, M.; Arias, J.; Diaz, F.; Chauca, L. Analysis of the temperature-humidity index on mortality and body weight of guinea pigs (Cavia porcellus) from the synthetic line in Moquegua, Peru. Cienc Tecnol Agropecuaria 2014, 23. [Google Scholar]
- Melanie, T.; Fabienne, B.; Ulrike, F. Successful Treatment of a Gastric Trichobezoar in a Peruvian Guinea Pig (Cavia aperea porcellus). J Exotic Pet Med 2008, 17, 148–151. [Google Scholar]
- De Cubellis, J.; Graham, J. Gastrointestinal Disease in Guinea Pigs and Rabbits. Vet Clin Exot Anim 2013, 16, 421–435. [Google Scholar] [CrossRef]
- Carcelén, F.; San Martín, F.; Ara, M.; Bezada, S.; Asencios, A.; Jimenez, R.; Guevara, J. Effect of the inclusion of different levels of probiotic on the productive parameters and intestinal morphology in fattening guinea pigs (Cavia porcellus). Rev Inv Vet Perú 2020, 31, e18735. [Google Scholar]
- Andía, V.; Ángeles, A. Effect of feed supplemented with a probiotic mixture on the productive parameters of Cavia porcellus, guinea pig. TAYACAJA 2021, 4, 13–21. [Google Scholar]
- Torres, C.; Carcelén, F.; Ara, M.; San Martín, F.; Jiménez, R.; Quevedo, W.; Rodríguez, J. Effect of supplementation of a probiotic strain on the productive parameters of guinea pig (Cavia porcellus). Rev Inv Vet Perú 2013, 24, 433–440. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).