Introduction
Staphylococcus aureus is found in normal human flora and causes serious diseases associated with high morbidity and mortality worldwide (Balasubramanian et al., 2017). This type of bacterium is a ubiquitous microorganism that commonly colonizes the skin of the human body and mucous membranes, mainly in the nasal cavity of healthy individuals (Sakr et al., 2018).
Transmission of S. aureus between individuals occurs through direct skin-to-skin contact or indirect contact with contaminated inanimate objects (Brown et al., 2013). Frequently, carriers of S. aureus can be branched into persistent and intermittent nasal carriers. Globally, about 20% of the general population is S. aureus persistent, while 30–50% are intermittent carriers of S. aureus (Ritchie et al., 2016; Sakr et al., 2018).
S. aureus can be transferred to food through manual contact by hands as well as respiratory secretions during the food preparation and handling process, resulting from poor hygiene practices of food handlers. Certainly, during food handling, nasal carriers' hands constitute the major vector for S. aureus dissemination, and unhygienic people are the leading cause of contamination. Asymptomatic food handlers are the main vector for contaminating food with S. aureus during food handling (Ghasemzadeh-Moghaddam et al., 2015; Sakr et al., 2018). In addition, food can also be contaminated through manual contact or via respiratory secretions resulting from asymptomatic food handlers, which eventually become the source of staphylococcal food poisoning (Leibler et al., 2016; Bencardino and Vitali, 2019).
Foodborne diseases are major global health difficulties, particularly in low-income nations that use insecure water for food cleaning and processing, and have poor food production and handling (WHO, 2015). Based on the World Health Organization estimation, nearly 600 million people globally become infected with foodborne illnesses, and at least 420 thousand people die each year (WHO, 2022).
In an investigation on foodborne illness outbreaks conducted among food services, a global team of food safety investigators recorded 816 outbreaks associated with the practices of food handlers, resulting in 80,682 cases of foodborne illness (Greig et al., 2007). Food handlers with poor practices appear to be the most important source of contaminating food, particularly food prepared to eat, such as that served in restaurants (McIntyre et al., 2013).
Globally, the overall rate of S. aureus nasal carriage was reported among food handlers to be 26.6% in Kuwait, (Al-Bustan et al., 1996), 21.6% in Sudan (Saeed and Hamid, 2010), 38–60% in Nigeria (Eke et al., 2015; Okareh and Erhahon, 2015), 23.8% in Lebanon (Osman et al., 2019), 7.1% in Bosnia and Herzegovina (Šegalo et al., 2020), and 50% in Malaysia (Seow et al., 2021).
Yemen is a developing country where endemic diseases are commonly prevalent among the population. This can be due to factors such as overcrowding, lack of safe water, poor disposal of waste, poor hygienic practices and attitudes, and inadequate environmental sanitation (Edrees and Al-Awar, 2020; Edrees and Anbar, 2021). In addition, the absence of rules regulating restaurants and their employees, unqualified restaurant workers, and unhygienic cooking and storing of food can lead to increased risks of staphylococcal food poisoning.
Data on nasal carriers of S. aureus and its antibacterial susceptibility tests among food handlers employed in Yemen are inadequate. Therefore, this study is the first to determine the prevalence of nasal carriage of S. aureus and its antibacterial susceptibility test among food handlers working in restaurants in Sana’a City, Yemen.
Study area and period
This cross-sectional study was carried out at restaurants located in districts in Sana’a city which includes ten official districts. This study was carried out for a period of five months, from November 2022 to March 2023.
Study population
A total of four hundred and twenty (420) specimens were collected from restaurant workers aged between 17 and 44 years old who are working in different districts belonging to Sana’a capital. The districts subjected to this study were Al-Sabain, Al-Wahdah, Assafi'ya, Az'zal, Ma'ain, Ath'thaorah, and Shu'aub districts. The participating food handlers include cooks, waiters, and cleaning workers.
Sample size
The Epi Info 7 program (version 7.2.5.0) was used to calculate the sample size as follows: The population size was 7500. The expected mean of S. aureus prevalence was 17.5%, according to a previous report (Osman et al., 2019; Šegalo et al., 2020; Vicar et al., 2023). Additionally, the acceptable margin of error was set at 5%, and the confidence level was set at 99%. Therefore, a sample size of 365 was determined by the program. Then, the sample size was increased to 420 to obtain more precise results.
Data collection
A structured questionnaire was used to collect the necessary data from participants. The items in the questionnaire were designed based on a past study, and few changes were made (Seow et al., 2021). The needed data such as age, gender (male/female), district name, educational level (illiterate/primary/repertory/secondary/graduate), job (cook/waiter/cleaning), experience years (< 2 years/between 2–5 years/ > 5 years), and level of personal hygiene (high/moderate/ ow) were gathered via face-to-face interviews and recorded in the questionnaire sheet which was initially constructed in Arabic and then translated into English after data collection. The age group was clustered into three categories: < 20 years, 20–30 years, and > 30 years.
Inclusion and exclusion criteria
All participants who worked in selected restaurants and who signed the informed consent, agreed to bring specimens, and did not take antimicrobial agents before sampling were included in this project. Restaurant workers who refused sample collection, signed the informed consent form, or used antibiotics within seven days before sampling were excluded from this study.
Specimen collection
Under aseptic conditions, the nasal swabs were sampled using a sterile swab moistened with a sterile saline solution. The tip of the swab was inserted into the nasal cavity (approximately 2–3 cm), rotated six times before swab withdrawal, and returned to its sterile container. Swab specimens were labeled with participating information and immediately transported to the microbiology lab at Queen Arwa University (QAU) for further processing and examination within 1–2 hours (Mahon et al., 2015; Luis et al., 2020).
Identification of S. aureus
Nasal swabs were inoculated on a mannitol salt agar plate and incubated at 37°C for 48 h. After incubation, the yellow colonies (mannitol fermentation) on mannitol salt agar were subcultured on nutrient agar. Additionally, further Gram staining and biochemical tests were used for S. aureus isolation and identification according to standard microbiological methods (Mahon et al., 2015; Luis et al., 2020).
Antimicrobial susceptibility test
The Kirby–Bauer disc diffusion method was used to determine the antibacterial tests of S. aureus isolates on Mueller–Hinton agar according to guidelines of the Clinical and Laboratory Standards Institute (CLSI, 2021). The antibiotic discs used included amoxicillin-clavulanic acid (AMC, 25 μg), ciprofloxacin (CIP, 5 µg), methicillin (MET, 5 µg), oxacillin (OX, 1 µg), and vancomycin (VA, 30 µg) (HiMedia Comp., India). The inhibition zone was measured, and the results were interpreted as resistant or sensitive according to CLSI (2021) guidelines.
Ethics considerations
The project proposal was primarily submitted to the Medical Sciences College of Queen Arwa University and ethical approval for this study was obtained from the Ethics Research Committee of Queen Arwa University on 15 October 2022. Additionally, QAU was formally communicated with restaurants in the selected districts by an official letter that explained the aims and purpose of the study delivered to the Health and Public Office in Sana’a Capital. Before the start of data and sample collection, the goals of this study were well explained to the study subjects for their agreement for inclusion in this study. Written informed consent was obtained from all the study subjects. Food handlers with positive results for S. aureus were advised for proper treatment at the nearest health center.
Statistical analysis
The obtained data are presented in the tables. The variables of values are described as frequencies and percentages in tables and figures. The SPSS Statistic program (26 version, IBM Co., Armonk, New York) was used for statistical analysis of the observed data. Chi-square (χ2) and confidence interval (CI 95%) tests were used to verify the existence of associations. Statistical significance (P-value) was considered at < 0.05.
Results
Approximately 420 food handlers were enrolled in this study. The majority of nasal swabs were collected from the age group of 20–30 years (51.7%), male (98.8%), those who had secondary school certificates (51.0%), and equally from each district (14.3%) (
Table 1).
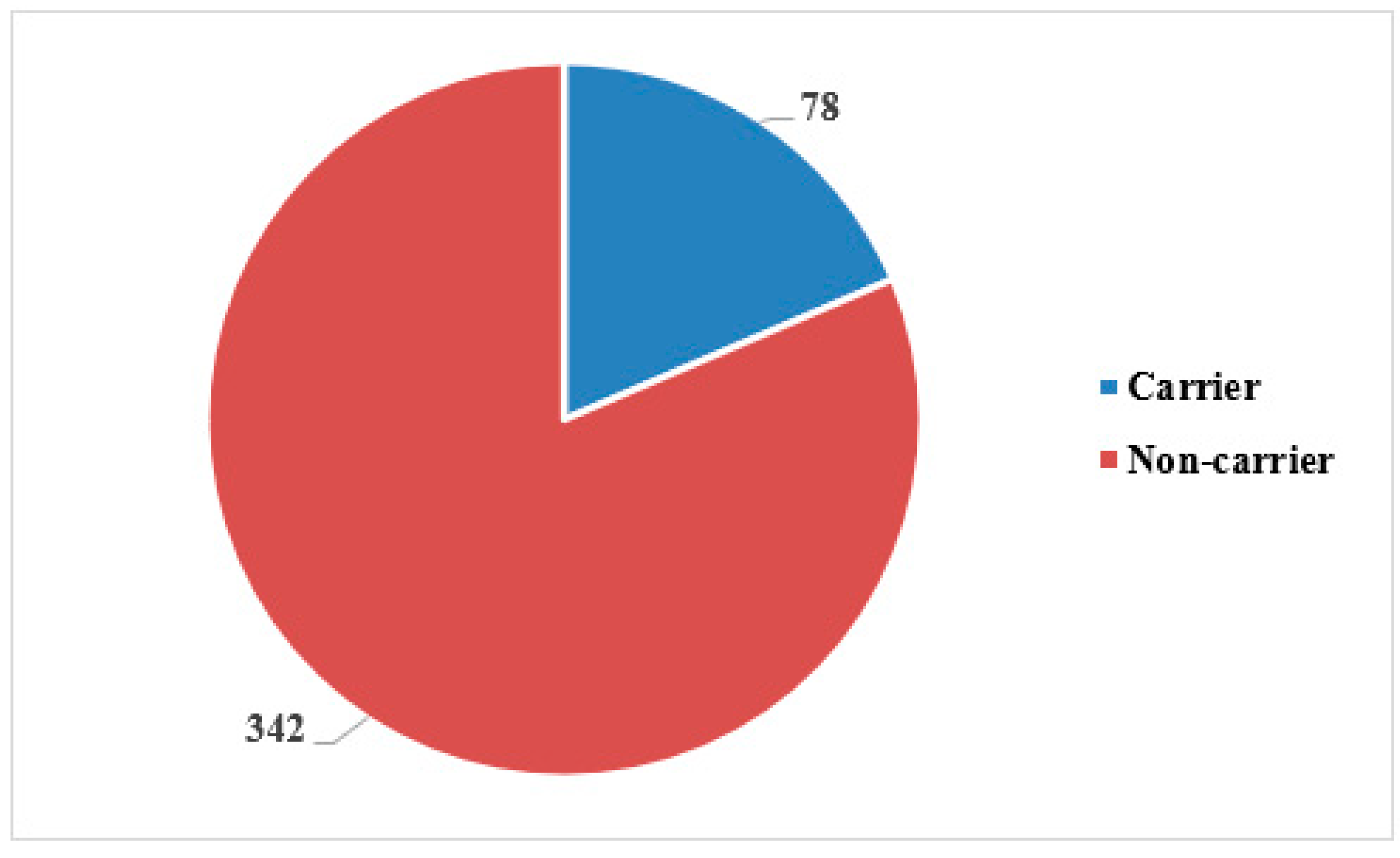
Frequency of S. aureus nasal carriage
The present study revealed that approximately 78 (18.6%) food handlers were
S. aureus nasal carriers while 342 (81.4%) food handlers were reported as noncarriers, as shown in
Figure 1.
Distribution of S. aureus concerning sociodemographic characteristics
The frequency of nasal carriage of
S.
aureus was higher among food handlers aged >30 years (23.1%), male food handlers (18.8%), and uneducated study subjects (25.0%) with a nonsignificant difference (
P >0.05) as revealed in
Table 2.
Regarding district names, it was noticed that the highest rate of
S.
aureus nasal carriage was isolated from food handlers working in Ma'ain (31.7%), while the lowest rate was found among food handlers working in Al-Wahdah (8.3%) with significant differences (
P =0.019) as shown in
Table 3.
Risk factors associated with nasal carriers of S. aureus
Table 4 reveals that a higher rate of
S.
aureus nasal carriers was observed among food handlers who worked as cooks (19.4%), had less than 2 years of experience (20.5%), and had low hygiene (29.0%;
P = 0.008).
Antibacterial susceptibility profile of S. aureus isolates
The
S. aureus isolates showed variable susceptibility patterns for the different antibiotics tested. It was found that
S. aureus isolates were highly sensitive to ciprofloxacin (71.8%), vancomycin (76.9%), and amoxicillin-clavulanic acid (61%). Conversely, a high resistance rate of
S. aureus isolates was only observed for oxacillin at 69.2% and methicillin at 66.7
% (
Table 5).
Discussion
To the best of our knowledge, this study is the first to determine the nasal carriage of S. aureus among food handlers in Yemen. The overall rate of S. aureus nasal carriage in this study was 18.6% among food handlers. This finding is significantly higher than that reported at 7.1% in Bosnia and Herzegovina by Šegalo et al. (2020). Additionally, this result is lower than previous results reported at 26.6% in Kuwait, (Al-Bustan et al., 1996), 21.6% in Sudan (Saeed and Hamid, 2010), 20.5% in Ethiopia (Dagnew et al., 2012), 60% in Ekpoma (Eke et al., 2015), 38% in Benin City-Nigeria (Okareh and Erhahon, 2015), 20% in Iran (Nasrolahei et al., 2017), 53% in Brazilian (da Silva et al., 2018), 23.8% in Tripoli, Lebanon (Osman et al., 2019),15.5% in Nigeria (Anowai et al., 2019), 50% in Malaysia (Seow et al., 2021), and 21.7 % in Ghana (Vicar et al., 2023).
The variation in results could be attributed to numerous factors such as demographic location, sample size, collection and diagnostic methods, personal hygiene habits, education level, hygiene of the environment, and rules employed in each country.
In this result, the nasal carrier of S. aureus was significantly higher among participants aged >30 years (23.1%) compared with the other age groups. This study is in agreement with the report by Beyene et al. (2019), which revealed that the age group of 30–39 years had a higher rate of S. aureus (30.7%). A different report by Eke et al. (2015) noted that the age range of 26–30 years had the highest prevalence of 67%. Conversely, several studies revealed that nasal carriage of S. aureus was associated with younger age (Skramm et al., 2011; Holtfreter et al., 2016; Akinnola et al., 2022).
The current results showed that S. aureus carriage was detected only in males handling food at 18.8%. A similar study by Eke et al. (2015) noticed that males had a higher prevalence rate than females. Additionally, in numerous reports, nasal carriage of S. aureus was correlated with males (Holtfreter et al., 2016). In dissimilar findings, a high carriage rate of S. aureus was detected among females (30.5%) compared to males (25.2%) by Beyene et al. (2019) and 63.49% for females and 56.75% for males by Akinnola et al. (2022).
The traditions and culture of the Yemeni community restrict the employment of women in public places such as restaurants (Edrees and Anbar, 2021). Yemeni women rarely work in restaurants. This explains why only a few samples were collected from females in this study.
These data showed that the highest carriage rate of S. aureus was recovered from uneducated subjects (25%), while the lower rate was recovered from study subjects holding a bachelor’s certificate (8.8%). In contrast, there was a high carriage rate of S. aureus (35.7%) among workers who had college certificates (Beyene et al., 2019) and high school-leavers (44%) (Akinnola et al., 2022).
The participants in this study who worked as cooks were found to have the highest rate of S. aureus nasal carriers at 19.4% compared with the others, and there was a significant difference (P = 0.008). This result is consistent with the study by Beyene et al. (2019) which revealed that the high carriage rate of S. aureus was 36.3% among cook participants. Additionally, approximately 50% of cooked food was reported as a nasal carrier of S. aureus (Seow et al., 2021).
Regarding years of experience, it was found that the highest rate of S. aureus nasal carriage was isolated from food handlers who had less than 2 years of experience at 20.5%. This is in line with reports performed in Ethiopia showing that S. aureus prevalence was higher among food handlers with work experience (Assefa, et al., 2015; Beyene et al., 2019). In addition, Seow et al. (2021) showed a high rate among food handlers working between 1 and 5 years (44%). The dissimilar report by Akinnola et al., 2022) documented that the highest rate was among food handlers with service experience of 1 –10 years (59%).
Furthermore, it demonstrated that food handlers who had many years of work experience were less exposed to bacterial contamination by hand (Assefa, et al., 2015). The prevalence of S. aureus was found to be significantly correlated with years of working in food service. This anticipated outcome could be elucidated by the statement that the food handlers who practiced in the restaurant for a long time had improved their awareness of food safety knowledge about personal hygiene, prompting them to accomplish better food hygiene practices (Ismail et al., 2016). An investigation conducted in some settings among food handlers documented that many years of work are remarkably positively correlated with their practices and attitudes (P < 0.05) in the workplace (Bou-Mitri et al., 2018). Therefore, it can be implied that food handlers with many years of experience have better personal hygiene practices than food handlers without experience.
This finding showed that food handlers with low hygiene had a higher rate of S. aureus nasal carriage at 28.2%. It has been demonstrated that inadequate personal hygiene habits on the part of handlers can have major consequences for food safety, encouraging the spread of S. aureus strains during manufacturing, processing, and distribution (Al-Bahry et al., 2014).
In this result, it was found that S. aureus isolates were sensitive to ciprofloxacin (71.8%), vancomycin (76.9%), and amoxicillin-clavulanic acid (61%). A report by Eke et al. (2015) documented that the isolated S. aureus was completely sensitive to ciprofloxacin (100%). Additionally, it was recorded that S. aureus isolates were sensitive to vancomycin (100%), and ciprofloxacin (70%) (Dagnew et al. 2012). A study by Beyene et al. (2019) detected the sensitivity of S. aureus isolates to ciprofloxacin (96.5%), cefoxitin (95.3%), amoxicillin-clavulanic acid (94.2%), oxacillin (93%), vancomycin (93%), methicillin (93%), and vancomycin (93%) Recently, it was observed that 100% of isolated S. aureus strains were methicillin-susceptible S. aureus (Seow et al., 2021).
In this result, a high resistance rate of S. aureus isolates was observed only for oxacillin at 69.2% and methicillin at 66.7%. In a similar investigation, it was reported that the isolates of S. aureus were resistant to penicillin (51.2%), ampicillin (46.3%), amoxicillin (39%), cotrimoxazole (31.7%), and methicillin and ciprofloxacin (9.8% in each) (Dagnew et al., 2012). Another study by Eke et al. (2015) revealed that all S. aureus isolates were resistant to ampicillin and 75% to Ampiclox. Moreover, a report by Beyene et al. (2019) indicated that 90.7% of the isolates were resistant to ampicillin and penicillin. Isolated strains of S. aureus were found to be resistant to penicillin (99.5%), ampicillin (97.9%), and amoxicillin (94.9%) (Šegalo et al., 2020). Additionally, approximately 93.75% of S. aureus isolates were resistant to penicillin and 50% to oxacillin (Akinnola et al., 2022). Moreover, Al-Khawlany et al. (2021) revealed that vancomycin was the most effective antibacterial against all isolated Methicillin-Resistant Staphylococcus aureus (MRSA) strains. Additionally, S. aureus isolates presented high resistance to broad-spectrum antibiotics such as ampicillin (40%-75%), tetracycline (40%-80%), amoxiclav (20%-80%) and chloramphenicol (7.7%-50%) (Vicar et al., 2023).
Currently, the increasing resistance of pathogenic bacteria to commonly used antimicrobial agents, particularly in Yemen, could result from the availability of antibiotics as over-the-counter drugs. The widespread use and misuse of antibiotics, the absence of antimicrobial susceptibility tests, the empirical use of broad-spectrum antibacterial drugs, and the quality of antibiotics are also factors contributing to antibiotic resistance increasing (Al-Haik et al., 2017; Alhlale et al., 2019; Edrees et al., 2020; Alhlale et al., 2020; Elmanama et al., 2023).
Limitations of the study
The limitation of this investigation is representing on the study was a cross-sectional study conducted over a short period of time and in a one Yemeni governorate. Furthermore, the majority of individuals subjected to sample collection have difficulties getting consent mainly because of their fear that the results will be given to official authorities. In addition, there is a lack of modern technology that are used in the genetically identifying of bacteria.
Conclusion
The prevalence rate of S. aureus nasal carriers among food handlers, remains a health problem for restaurant consumers. The prevalence of S. aureus among study subjects could be attributed to factors such as poor personal hygiene, inadequate education, the absence of regular monitoring and control of food handlers’ hygiene and restaurants' environmental sanitation. In addition, there was a lack of routine diagnosis and treatment for food handlers. Therefore, food handlers must adhere strictly to restaurant policies and rules, and training programs are needed to improve food handlers’ food safety knowledge, attitudes, and practices through periodic medical checkups, and regular monitoring of food handlers’ hygiene.
Author’s Contributions
Nasser Al-Aomary, and Bashir Al-Ofairi conceived and designed the experimental study. Jameela Thabit and Nasser Al-Aomary shared with team workers for specimen collection and analysis. Wadhah Edrees and Bashir Al-Ofairi statistically analyzed and presented the data in tables and figures. Additionally, Wadhah Edrees and Nasser Al-Aomary prepared and wrote the manuscript. All authors have reviewed, revised, and accepted the manuscript for submission.
Date Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Funding
Authors received no financial support.
Acknowledgment
The authors would like to be grateful to team researchers Ahlam M. Mutair, Alia J. Al-Kamel, Amal A. Alodaini, Fatima A, Al-Aameri, Jehan A. Mogali, Reham Abdo Alsameai, Shaima H. Alameri, and Zainab M. Alasri for specimen collecting and examination in the lab. Additionally, many thanks go to Dr. Gader Hasan, Dr. Lamis Al-Ghouli, Dr. Tammam Al-Saqqaf, and Dr. Ahmed Al-Ruwaisi members of the staff of the medical laboratories at Queen Arwa University for their invariable help with specimen processing and analysis.
Conflicts of Interest
The authors declare that they have no competing interests.
References
-
Akinnola, O.O., Williams, A.N., Oniha, M.I., and Ogunleye, B.O. "Nasal carriage of Staphylococcus aureus and associated risk factors among food handlers in a Nigerian University." J Pure Appl Microbiol 16, no 4 (2022): 2507–2513. [CrossRef]
-
Al-Bahry, S.N., Mahmoud, I.Y., Al-Musharafi, S.K., and Sivakumar, N. (2014). "Staphylococcus aureus contamination during food preparation, processing and handling." International Journal of Chemical Engineering and Applications 5, no 2(2014): 388–390.
-
Al-Bustan, M.A., Udo, E.E., and Chugh T.D. "Nasal carriage of enterotoxin-producing Staphylococcus aureus among restaurant workers in Kuwait City." Epidemiol Infect 116, no 3(1996): 319–322. [CrossRef]
-
Al-Haik, M.W., Al-Haddad, M.A., Al-Kaf, G.A., and Edrees, W.H. Antimicrobial activities for hadhrami honey on growth of some pathogenic bacteria. Universal J Pharm Res 2, no 6(2017): 7–12. [CrossRef]
-
Alhlale, F.M., Saleh, H.A., Alsweedi, S.K., and Edrees, W.H. The inhibitory effect of Euphorbia hirta extracts against some wound bacteria isolated from Yemeni patients. COPS 3, no 2 (2019): 780–786.
-
Alhlale, M.F., Humaid, A., Saleh, A.H., Alsweedi, K.S., and Edrees, W.H. "Effect of most common antibiotics against bacteria isolated from surgical wounds in Aden Governorate hospitals, Yemen. " Universal J Pharm Res 1, no 5(2020): 21-24.
-
Al-Khawlany, R.S., and et al. "Prevalence of methicillin-resistant Staphylococcus aureus and antibacterial susceptibility among patients with skin and soft tissue infection at Ibb City, Yemen." PSM Microbiol 6, no 1(2021): 1-11. https://psmjournals.org/index.php/microbiol/article/view/535.
-
Anowai, C.O., Agarry, O.O., and Akin-Osanaiye B.C. "Antibiotic susceptibility profile of Staphylococcus aureus isolated from food handlers in Abuja, Nigeria review. World Journal of Research and Review 9, no 1(2019): 5–11.
-
Assefa, T., Tasew, H., Wondafrash, B., and Beker, J. "Contamination of bacteria and associated factors among food handlers working in the student cafeterias of Jimma University Main Campus, Jimma, South West Ethiopia." Altern Integr Med 4, no 1 (2015):1–8.
-
Balasubramanian, D., Harper, L., Shopsin, B., and Torres, V.J. Staphylococcus aureus pathogenesis in diverse host environments." Pathogens and Disease 75, no1 (2017): 1–13.
-
Bencardino, D., and Vitali, L.A. "Staphylococcus aureus carriage among food handlers in a pasta company: Pattern of virulence and resistance to linezolid." Food Control 96(2019). 351–356. [CrossRef]
-
Beyene, G., Mamo, G., Kassa, T., Tasew, G., and Mereta, S.T. "Nasal and hand carriage rate of Staphylococcus aureus among food handlers working in Jimma Town, Southwest Ethiopia." Ethiop J Health Sci 29, no 5(2019): 605. [CrossRef]
-
Bou-Mitri, C., Mahmoud, D., El Gerges, N., and Abou Jaoude, M. "Food safety knowledge, attitudes and practices of food handlers in Lebanese hospitals: A cross-sectional study." Food Control 94 (2018):78–84. [CrossRef]
-
Brown, A.F., Leech, J.M., Rogers, T.R., and McLoughlin, R.M. "Staphylococcus aureus colonization: Modulation of host immune response and impact on human vaccine design." Frontiers in Immunology 4, no DEC (2013): 1–20. [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). "Performance standards for antimicrobial susceptibility testing." M100, 31st ed. Clinical and Laboratory Standards Institute, Wayne, PA. 2021.
-
da Silva, M., Nicolete, G., Lopes, B., Morita, C., de Souza, J., Winkelstroter, L. and Rodrigues, M. "Evaluation of antimicrobial susceptibility by Staphylococcus aureus isolated from the nutrition service of a teaching hospital." Advances in Microbiology 8, no 4 (2018): 270–285. [CrossRef]
-
Dagnew, M., Tiruneh, M., Moges, F., and Tekeste, Z. "Survey of nasal carriage of Staphylococcus aureus and intestinal parasites among food handlers working at Gondar University, Northwest Ethiopia." BMC Public Health 12 (2012): 837. [CrossRef]
-
Edrees, W.H., Al-Asbahi, A.A., Al- Shehari, W.A., and Qasem, E.A. "Vulvovaginal candidiasis prevalence among pregnant women in different hospitals in Ibb, Yemen." Universal J Pharm Res 5, no 4 (2020): 1–5. [CrossRef]
-
Edrees, W.H., and Al-Awar, S.M. "Bacterial contamination of mobile phones of medical laboratory workers at Sana’a city, Yemen and their antimicrobial susceptibility." Journal of Pharmacy and Pharmacognosy Research 8, no 6(2020): 591-599. WOS:000571733000010, Google Scholar.
-
Edrees, W.H., and Anbar, A.M. "Prevalence and antibiotic susceptibility of Streptococcus pyogenes isolated from schoolchildren in Sana’a city, Yemen." PSM Vet Res 6, no 2(2021): 22–30. https://psmjournals.org/index.php/vetres/article/view/575.
-
Edrees, W.H., and Anbar, A.M. Prevalence and antibiotic susceptibility of Streptococcus pyogenes isolated from schoolchildren in Sana’a city, Yemen. PSM Vet. Res 6, no 2 (2021): 22–30.
-
Eke, S.O., Eloka, C.V., Mgbachi, N., Nwobodo, H.A., and Ekpen-Itamah, U.J. "Nasal carriage of Staphylococcus aureus among food handlers and restaurant workers in Ekpoma Edo state, Nigeria." International Journal of Community Research 5, no 1(2015): 7–14.
-
Elmanama, A., Shubair, E.M., Sharif, A.F., Marouf, M.A., Hassona, F.I., El-Hallaq, M.M., and Al-Reefi, R.M. "Methicillin-resistant Staphylococcus aureus isolated from nasal swab: Profiling of some virulence genes." Israa University Journal of Applied Science 6, no 2 (2023): 184–201.
-
Ghasemzadeh-Moghaddam, H., Neela, V., van Wamel, W., and et al. "Nasal carriers are more likely to acquire exogenous Staphylococcus aureus strains than non-carriers." Clinical Microbiology and Infections 21, no 11(2015): 998. [CrossRef]
-
Greig, J.D., Todd, C.D., Bartleson, C.A., and Michaels, B. "Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 1. Description of the problem, methods and agents involved." J Food Prot, 70, no 1 (2012):1752–1761. [CrossRef]
-
Holtfreter, S., Grumann, D., Balau, V., and et al. "Molecular epidemiology of Staphylococcus aureus in the general population in northeast Germany – results of the study of health in Pomerania (SHIP-TREND-0)." J Clin Microbiol 54, no 11(2016): 2774–2785. [CrossRef]
-
Ismail FH, Chik, C.T., Muhammad, R., and Yusoff, N.M. "Food safety knowledge and personal hygiene practices amongst mobile food handlers in Shah Alam." Selango. Procedia - Social and Behavioral Sciences 222 (2016): 290–298. [CrossRef]
-
Leibler, J.H., Jordan, J.A., Brownstein, K., Lander, L., Price, L.B., and Perry, M.J. "Staphylococcus aureus nasal carriage among beefpacking workers in a Midwestern United States Slaughterhouse." PloS One 11, no 2 (2016): 1–11. [CrossRef]
- Luis MD, Pezzlo, T.M., Bittencourt, C.E., and Ellena, M. "Color atlas of medical bacteriology." Third Edition. 2020. American Society for Microbiology and John Wiley and Sons, Inc. Washington, USA. Pp; 25–156.
-
Mahon, R.C., Lehman, C.D., and Manuselis, G. "Textbook of diagnostic microbiology." Fifth Edition. 2015. Saunders, An Imprint of Elsevier, Inc. USA. Pp; 37–115.
-
McIntyre, L., Vallaster, L., Wilcott, L., Henderson, S.B., and Kosatsky, T. "Evaluation of food safety knowledge, attitudes and self-reported hand washing practices in FOODSAFE trained and untrained food handlers in British Columbia. Canada." Food Control 30, no 1 (2013): 150–156. [CrossRef]
-
Nasrolahei, M., Mirshafiee, S., Kholdi, S., Salehian, M., and Nasrolahei, M. "Bacterial assessment of food handlers in Sari City, Mazandaran province, north of Iran." J Infect Public Health 10, no 2(2017): 171-176. [CrossRef]
-
Okareh, O.T., and Erhahon, O.O. "Microbiological assessment of food and hand-swabs samples of school food vendors in Benin city, Nigeria." Food Public Health 5, no 1 (2015): 23–28.
-
Osman, M., Kamal-Dine, K., El Omari, K., Rafei, R., Dabboussi, F., and Hamze, M. "Prevalence of Staphylococcus aureus methicillin-sensitive and methicillin-resistant nasal carriage in food handlers in Lebanon: a potential source of transmission of virulent strains in the community." Access microbiology 1, no 6(2019): e000043.
-
Ritchie, S.R., Isdale, E., Priest, P., Rainey, P.B., and Thomas, M.G. "The turnover of strains in intermittent and persistent nasal carriers of Staphylococcus aureus." Journal of Infection 72, no 3(2016): 295–301. [CrossRef]
-
Saeed, H.A, and Hamid, H.H. "Bacteriological and parasitological assessment of food handlers in the Omdurman area of Sudan." J. Microbiol. Immunol Infect 43, no 1 (2010): 70–73. [CrossRef]
-
Sakr, A., Bregeon, F., M`ege, J.L., Rolain, J.M., and Blin, O. "Staphylococcus aureus nasal colonization: An update on mechanisms, epidemiology, risk factors, and subsequent infections." Frontiers in Microbiology 9, no OCT (2018): 1–15. [CrossRef]
- Šegalo S, Maestro D, Obradović Z, and Jogunčić A. "Nasal carriage rate and antimicrobial resistance pattern of Staphylococcus aureus among the food handlers in Canton Sarajevo, Bosnia and Herzegovina." Journal of Health Sciences 10, no 2(2020): 139–146. [CrossRef]
- Seow, W-L., Mahyudin, N.A., Amin-Nordin, S., Radu, S., and Abdul-Mutalib, N.A. "Antimicrobial resistance of Staphylococcus aureus among cooked food and food handlers associated with their occupational information in Klang Valley, Malaysia." Food Control 124 (2021): 107872. [CrossRef]
-
Skramm, I., Moen, A.E., and Bukholm, G. "Nasal carriage of Staphylococcus aureus: Frequency and molecular diversity in a randomly sampled Norwegian community population." Journal of Pathology, Microbiology and Immunology 119, no 8(2011): 522–528.
-
Vicar, E.K., Alo, D.B., Koyiri, V.C., Opare-Asamoah, K., Obeng-Bempong, M., and Mensah, G.I. "Carriage of antibiotic resistant bacteria and associated factors among food handlers in Tamale Metropolis, Ghana: Implications for food safety." Microbiology Insights 28, no 16 (2023): 11786361221150695. [CrossRef]
-
World Health Organization (WHO). "Food safety. 2022. Available at: https://www.who.int/news-room/fact-sheets/detail/food-safety. Accessed 10 May 2023.
-
World Health Organization (WHO). "Estimates of the global burden of foodborne diseases: Food borne disease burden epidemiology reference group 2007–2015. 2015. (Available from: https://www.who.int/foodsafety/areas_work/foodborne-disease/ferg/en. Accessed 5 May 2023.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).