1. Introduction
Mud shrimp,
Upogebia major, in the family Upogebiidae (Infraorder Gebiidea), is a deep burrowing shrimp that inhabits sandy or muddy intertidal flats along the coasts of Korea, Japan, China, and Russia [
1,
2,
3,
4,
5,
6]
U. major resides in Y-shaped burrows that can extend to more than 2.5 m below the sediment surface and often occur in very high densities [
7,
8]. They are well-known as ecosystem engineers [
3,
9,
10], and their burrows not only expand into the sediment surface areas of estuaries and supply oxygenated water deep into subsurface sediments [
11,
12,
13,
14], but they also become microhabitats for various benthic animals and influence the structure of benthic communities in these habitats [
15,
16,
17,
18,
19,
20,
21,
22,
23]. Additionally, their filter feeding improves water quality by capturing suspended particles, which in turn affects the coastal ecosystem and serves as a vital ecological function [
9,
24,
25,
26,
27,
28,
29]
U. major is common and abundant in the upper intertidal mudflats of the western coast of Korea. However, there has been a rapid expansion in its distribution since 2010, leading to reported invasions into clam culture beds along the West coast of Korea. In particular, the Manila clam culture beds in the tidal flat of Seonjaedo, Incheon, located in the middle part of the West coast of Korea, have seen a 90% reduction in clam production, and mud shrimp have invaded 3,986 ha of the clam beds of Chungcheongnam-do, which account for 76.7% of the total 5,200 ha, leading to a financial loss of 10.8 billion Korean Won (8 million US
$). However, the local media reported a recent decrease in the mud shrimp population in the clam beds [
30]. Nevertheless, the need for ecological knowledge of the
U. major population remains ford its ecological significance and effective control in the Manila clam culture.
Studies have been conducted on the larval development of
U. major [
31,
32], burrow structure [
7,
33], parasitism and commensalism [
34,
35,
36,
37], sexual dimorphismto estimate the growth curve[
38], life history, and ecological characteristics [
3,
5]. Population characteristics have been studied in Tokyo Bay, Japan, and Vostok Bay, Russia [
1,
39]. The populations in Korea have been studied in Namhae on the South coast and in Boryeong on the West coast [
40,
41]. However, no study has comprehensively observed the entire life history of the mud shrimp population from recruitment to cohort disappearance in Korea. This study focused on describing population parameters, including structure, growth, mortality, lifespan, and reproduction, by observing
U. major populations for five years inhabiting the intertidal mud flats along the West coast of Korea.
2. Materials and Methods
2.1. Study Sites
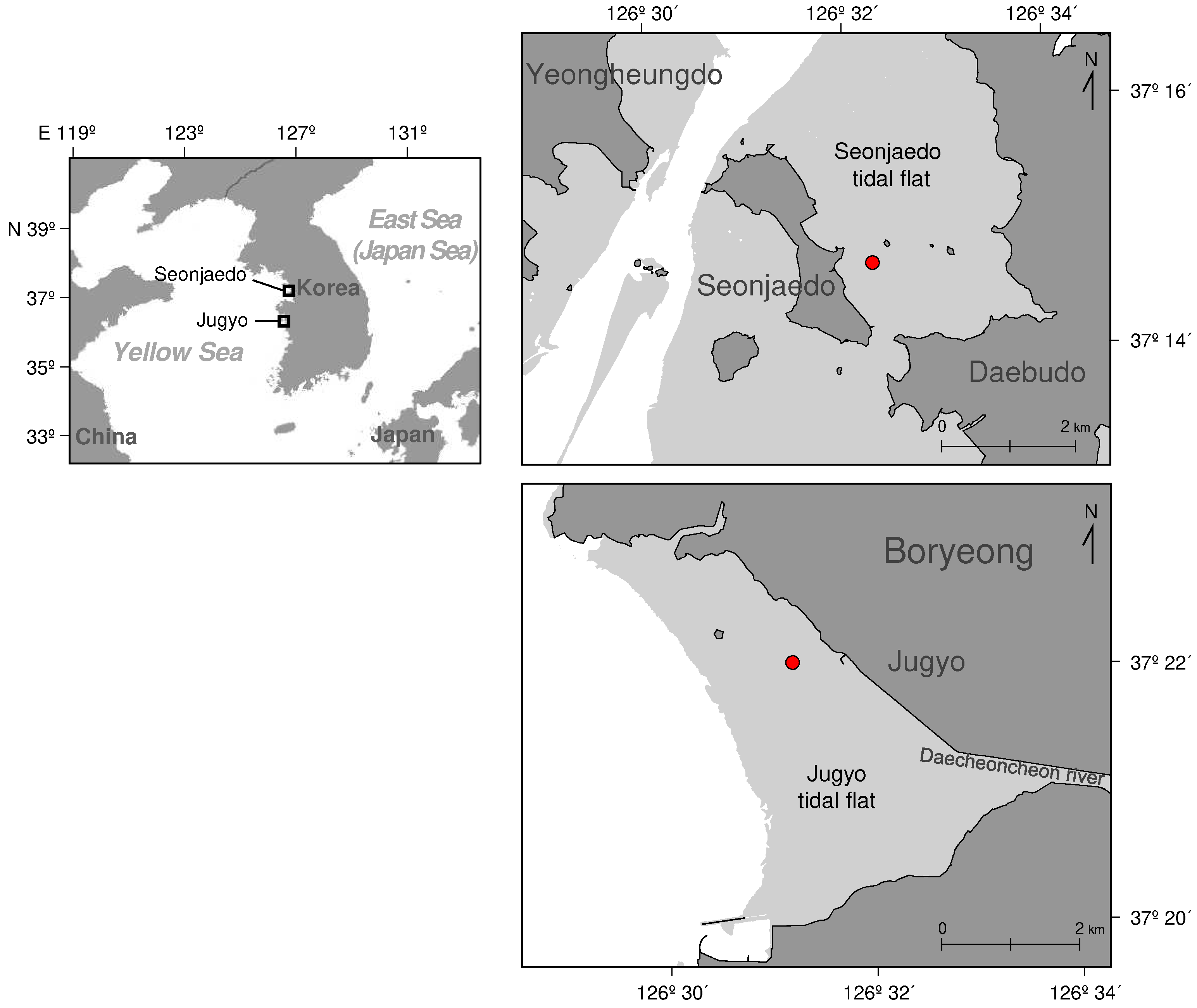
The study sites were in two regions of the West coast of South Korea: Seonjaedo in Incheon and Jugyo in Boryeong, Chungcheongnam-do. These sites have reported abnormal blooms of mud shrimp populations. Seonjaedo Island is located between two islands, Daebudo to the east and Yeongheungdo to the west (
Figure 1). With a mean tidal range of about 5.2 m [
42], expansive tidal flats about 2.5 km wide emerge during low tide. Particularly, the northeast tidal flat continues to Daebudo Island. Of the total 3.00 km² culture beds associated with the Seonjae Fishing Village Society, an area of 1.75 km² is reserved for Manila clams,
Ruditapes philippinarum. This area produces between 500 and 600 tons of Manila clams annually [
43]. The study site was on the eastern tidal flat of Seonjaedo (N 37˚ 14’ 37.3”, E 126˚ 32’ 18.0”), characterized by sandy mud (sM) to slightly gravelly muddy sand ((g)mS) with 26 – 94% sand [
44], where an abnormal bloom of mud shrimp was observed around 2010 [
45]. At this site, no efforts were made to eradicate the mud shrimp. After the bloom of the shrimp, the subsequent decline in clam production led to the cessation of fishing activities. Consequently, the mud shrimp population at the site has been maintained without any artificial disturbance. The other study site, Jugyo tidal flat (N 36° 21′ 59.5”, E 126° 31′ 10.9”), is located on the coast 100 km south of Seonjaedo (
Figure 1). The southern part of the Jugyo tidal flat is closed to the Daecheoncheon estuary. The mean tidal range is about 4.5 m [
42]. During low tide, a tidal flat with a width of 1.7 – 2.5 km is exposed along the shoreline. The tidal flat covers a total area of 5.60 km², of which 1.38 km² is dedicated to clam culture beds. The dominant type of surface sediment is sand (S), at more than 93% [
46]. The bloom of mud shrimp here was first observed by local fishermen in April 2010 [
47]. By August of the same year, it was reported that the tiny holes made by the mud shrimp had expanded to the size of a ballpoint pen. Under the guidance of the Jugyo fisheries cooperative, consistent efforts were made from 2011 until the end of the study in 2013 to remove the mud shrimp and enhance the mudflat environment for better clam production. However, the sampling station was excluded from these efforts, ensuring that the mud shrimp population persisted without any artificial intervention throughout the research period.
2.2. Field Survey
Field surveys were conducted at Seonjaedo from February 2012 to June 2014 and at Jugyo from February 2012 to December 2015. As environmental factors, the temperature of the sediment surface and the sediment 10 cm below was recorded every hour throughout the study period using a temperature logger (Thermochron iButton model DS1921G-F5#, Maxim Integrated). We used the harmonic constants obtained from the Korea Hydrographic and Oceanographic Agency (KHOA) at each site to extract elevation for the tidal datums and mean tidal range (Mean high water – Mean low water). We determined the elevation of the sampling stations using an RTK GPS (K5-PLUS, KORIDA) to estimate the inundation time. Tidal observation data from tidal stations near the study sites were obtained from the KHOA. Data at the Yeongheungdo tidal station from 2012 to 2014 was used for Seonjaedo, and data at the Boryeong station from 2012 to 2015 were used for Jugyo. The tide correction constant for the sampling stations was obtained from the KHOA, and the difference between the Incheon mean sea level (IMSL) and the local mean sea level (MSL) was calculated using the land and sea height linkage service of National Geographic Information Institute (NGII). The data were used to estimate the inundation time at each sampling station and then were converted to the daily mean.
For population analysis, mud shrimp were collected monthly at spring tides during low tide. From February 2012 to October 2012, 150 – 200 specimens were collected in a non-quantitative manner by digging into the sediment with a shovel. From November 2012 onward, all specimens within a 5 m
2 area were collected at a depth of 80 – 100 cm. The sampling depth was based on a report that approximately 97% of the total mud shrimp population was distributed at depths less than 80 cm within a 0 – 120 cm depth range in Tokyo Bay, Japan [
1]. We sieved the pooled water in the sampling puddle made by digging sediment to sample adult mud shrimp through a small hand net with a mesh size of 2 mm to collect newly settled juvenile and young shrimp with CLs less than 7 mm. The sampled shrimp were transported to the laboratory on ice under refrigerated conditions. Since burrow openings are often used to estimate the density of Gebiidean shrimp [
48,
49], we counted the burrow openings of the mud shrimp burrows in a 0.25 m² area using a 50 × 50 cm quadrat three times at each sampling. Before counting, we cleared away about 5 cm of the sediment surface to make the burrow openings more distinct. Typically, since
U. major makes Y-shaped burrows with two openings [
50], the number of openings was halved to estimate mud shrimp density.
2.3. Larboratory Work
The sampled shrimp were washed with seawater in the laboratory, the mud between their pleopods was removed using a soft brush, and the number of individuals was counted. Severely damaged specimens were converted to one per two body parts. Specimens were sexed by inspecting the following three characteristics: 1) Morphological differences in the dactylus (oblique ridges on the male medial dactyl [
51]. Position of the genital pores. Genital pores are usually found at the base of the coxae of the fifth pereopod in males but at the base of the third coxae in females [
52]. 3) Presence of the first pleopod; a pair of first pleopods is only present in females [
1,
3]. Ovigerous females were also examined. Egg development was categorized into the following three stages: 1) yellow eggs without eyespots, 2) orange eggs with eyespots, and 3) hatched eggshells. Eggs were counted for 88 randomly selected ovigerous females. Parasites in the specimens were also recorded. Infections were primarily inspected in the gill chambers and abdomen, and identified according to previous references [
3,
53,
54,
55,
56,
57,
58]. Carapace length (CL, from the tip of the rostrum to the end of the carapace) was measured in every available specimen. From those, the total length was measured (TL, from the tip of the rostrum to the posterior border of the telson) in a subset of 60 to 200 intact individuals selected each month. For specimens with a damaged carapace, we measured the telson length (from the medial anterior border to the posterior border of the telson) as an alternative. Subsequently, we used the relationship between CL and telson length
derived from randomly selected intact specimens each month to estimate the CL of damaged specimens. Length measurements were primarily conducted using a digital vernier caliper. However, we employed a stereo microscope and ocular micrometer for specimens with a CL of less than 10 mm. All measurements were accurate to 0.01 mm. Additionally, 68 specimens that had been collected and fixed in 70% ethyl alcohol by the authors from the same location, Seonjaedo in February 2011, a year prior to the present study, were processed using the same methodology and included in the analysis to get an additional population information.
2.4. Population Analysis
2.4.1. Population Structure
We analyzed shrimp density, sex ratio, parasite infection rate, the presence of ovigerous females, and the season of hatching and recruitment as characteristics of the mud shrimp population. The sex ratio was expressed as follows.
The Chi-square test (p < 0.05) was employed to ascertain if the observed sex ratios deviated from the expected 1:1 ratio overall, on a monthly basis, and by size classes.
2.4.2. Growth, Mortality and Reproduction
The parasites may influence the characteristics of the mud shrimp population [
59]. Therefore, we examined the growth, mortality, and reproduction of uninfected individuals to understand the general traits of the mud shrimp population in our study sites. Growth and mortality were analyzed based on monthly CL frequency distribution segmented into 1 mm intervals. We employed a modified von Bertalanffy growth function (VBGF), which accounts for seasonal growth curve [
60].
where
is the length at age
,
the asymptotic length,
the theoretical age when
,
is a growth constant,
is the intensity of the growth oscillations and
the onset of the first oscillation (relative to
).
Growth curves were estimated in females, males, and the overall population. For juvenile shrimps with a CL of less than 10 mm, where sexual morphological characteristics were not sufficiently developed to distinguish between sexes, half of the specimens were arbitrarily assigned to each sex for analysis. VBGF growth parameters (
,
,
,
) were estimated using Electronic Length Frequency Analysis (ELEFAN) I routine in R package TropFishR (version 1.6.3) [
61] based on CL frequency distributions [
62]. The ELEFAN_GA function, which uses the Genetic Algorithm package GA [
63], was used to fit the optimized VBGF parameters. The parameter
cannot be determined from length-frequency data and, therefore, could not be estimated using ELEFAN [
64]. Instead, based on the reported CL of post-settled juvenile in Tokyo Bay [
1], we set
, the length at age 0, to 3.5 mm. We then determined
using the
parameter outputted by ELEFAN I in TropFishR, which represents a point when yearly repeating growth curves cross a length equal to zero. In relation to this,
was also adjusted when reproducing the age-relative growth curve.
Total mortality was estimated using length-converted catch curves to account for seasonal growth [
65,
66]. The annual total mortality rate (
) and survival rate (
) were calculated from the estimated total mortality coefficient (
) as follows.
Lifespan (
) was estimated as the time required to attain 95%
, following the definition by Taylor [
67]
The recruitment patterns were obtained by backward projection of the length-frequency data set onto a year time scale with the estimated VBGF [
68] using the ELEFAN II routine in the FiSAT II package, and normal distribution was estimated with Hasselblad’s NORMSEP [
69] in the same package [
70].
2.5. Comparison of Habitat Environments of U. Major Population in Other East Asian Regions
Aqua MODIS Global Mapped 11µm Daytime Sea Surface Temperature (SST, version R2019.0) and Chlorophyll-a (CHL, version R2022.0) data were obtained from NASA GIOVANNI [
71] for 2010 to 2020 to compare regional environment factors. A moderate-resolution imaging spectroradiometer (MODIS) is mounted on Aqua (EOS PM) satellites, and it scans the Earth's surface every 1 to 2 days, acquiring data in 36 spectral bands or groups of wavelengths [
72]. We evaluated the association between these environmental factors and the characteristics of the
U. major populations in different East Asian regions (
Table 1).
3. Results
3.1. Environmental Factors
3.1.1. Sediment Temperature
The annual mean temperature of Seonjaedo from January 2012 to June 2014 was 13.1 °C at the sediment surface and 13.0 °C 10 cm below the surface. The highest temperatures were recorded in August 2013, at 32.0 °C at the sediment surface and 30.5 °C 10 cm below the surface. The lowest temperatures were recorded in February 2013 at -8.5 °C at the sediment surface and -2.0 °C 10 cm below the surface. In Jugyo, the annual mean temperature from March 2013 to December 2015 was 14.4 °C at the sediment surface and 14.5 °C 10 cm below the surface. The highest temperature recorded at the sediment surface was 35.0 °C in August 2013, and the highest temperature 10 cm below the surface was 30.5 °C in August 2014. The lowest temperatures, which were recorded in February 2014, were -7.0 °C at the sediment surface and 2.0 °C 10 cm below the surface. The temperature was highest in August and lowest in February at both sites. There was no difference in the average temperature at the surface and 10 cm below in the sites. However, the range of temperature at the sediment surface was broader than that 10 cm below the surface.
3.1.2. Tidal Condition and Inundation Time
The mean tidal range of Seonjaedo was 5.393 m, whereas that of Jugyo was 4.461 m. The sampling stations were located -1.671 m from the MSL for Seonjaedo and -0.837 m for Jugyo. This corresponds to the mean low water neaps (MLWN) of the Seonjaedo and between the MSL and MLWN for Jugyo. From 2012 to 2014, the mean daily inundation time at the station in Seonjaedo was 17.3 hours per day, whereas it was 16.9 hours per day in Jugyo. The inundation time at the Seonjaedo station was approximately 30 minutes longer than that of Jugyo.
3.2. Population Structure
3.2.1. Shrimp Density
A total of 7,456 mud shrimp were collected in Seonjaedo. An average of 296 shrimp per 5 m
2 (± 65) were caught in each sampling, with a mean density of 59 individuals (ind.)/m
2 (±13) (
Figure 2a). During the same period, the average density of burrow openings was 529 openings/m
2 (± 49). Consequently, the shrimp density was 265 ind./m
2, obtained by halving the average number of burrow openings. The number of openings per catch varied monthly, ranging from 7.0 to 15.0. As a result, the ratio between the shrimp density estimated from openings and catches ranged from 3.5 to 7.5 times. On average, there were 9.4 openings per catch, and the shrimp density estimated from the openings was 4.7 times higher than that from the catches.
A total of 7,823 mud shrimp were collected in Jugyo. After November 2012, an average of 183 shrimp per 5 m
2 (± 69) were caught in each sampling, and the mean density was 37 ind./m
2 (± 14) (
Figure 2b). The opening density was an average of 236 openings/m
2 (±66). Therefore, the shrimp density estimated from openings was 118 ind./m
2. The number of openings per catch ranged from 2.4 to 16.6 monthly, and the ratio of the shrimp density estimated from openings and catches varied from 1.2 to 8.3. The average openings per catch was 7.4, and the shrimp density estimated from the openings was 3.7 times that of the catches.
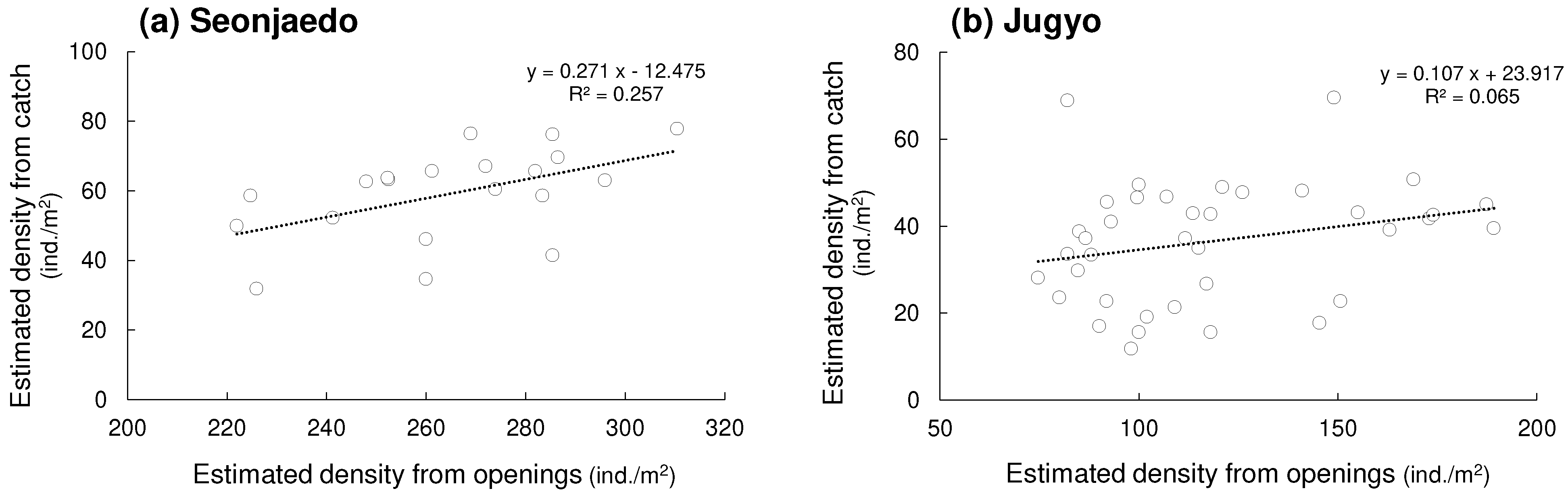
The relationship between the shrimp densities estimated from burrow openings and catches differed by the sites. A significant positive correlation between the two types of densities was found in Seonjaedo, although only 26% of the variance in shrimp density estimated from catches could be explained by opening density (
r = 0.51,
p = 0.022,
R2 = 0.26) (
Figure 3). However, no significant correlation was found in Jugyo (
r = 0.26,
p = 0.122,
R2 = 0.07).
3.2.2. Sex Ratios
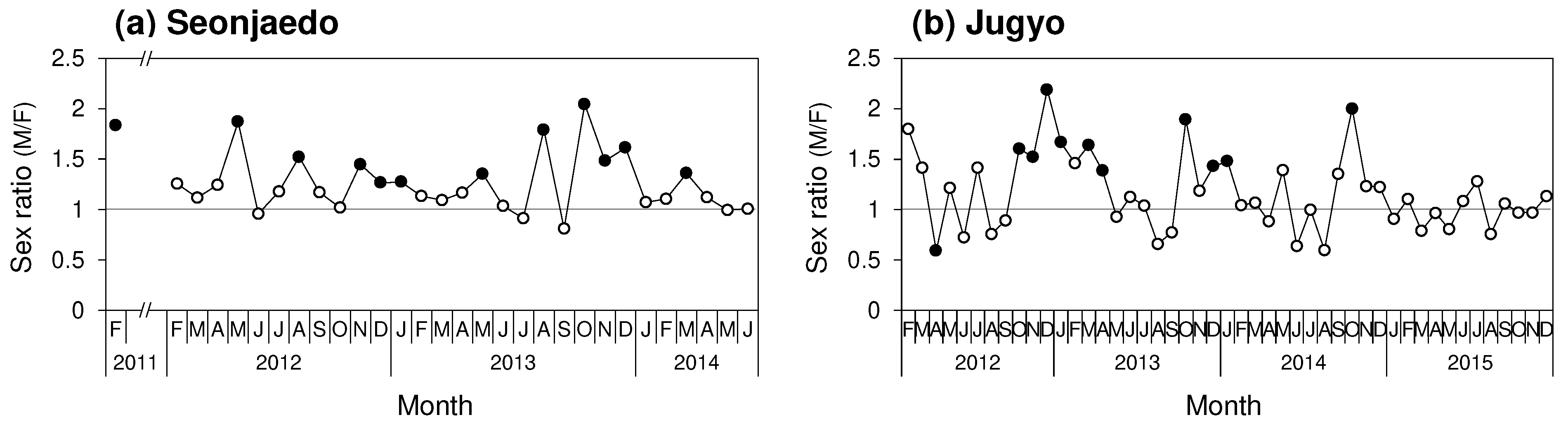
In Seonjaedo, 4,056 male and 3,361 female mud shrimp were collected, along with 39 individuals of unidentified sex. The monthly average sex ratio was 1.28 (± 0.31). Although the appearance males-to-females ratio varied monthly (Chi-squared for heterogeneity,
X2 (29,
N = 7,417) = 70.80,
p < 0.001), there was always either no difference was found between the sexes or a significantly male predominance was present (
Figure 4a). In Jugyo, 3,763 male mud shrimp, 3,370 females, and 690 individuals of unidentified sex were collected. The monthly average sex ratio was 1.17 (± 0.38). Similar to Seonjaedo, the males-to-females ratio varied monthly (
X2 (47,
N = 7133) = 139.04,
p < 0.001). Either no difference in sex was found, or males were significantly more abundant in all periods, except in April 2012, when females were more prevalent (
Figure 7b).
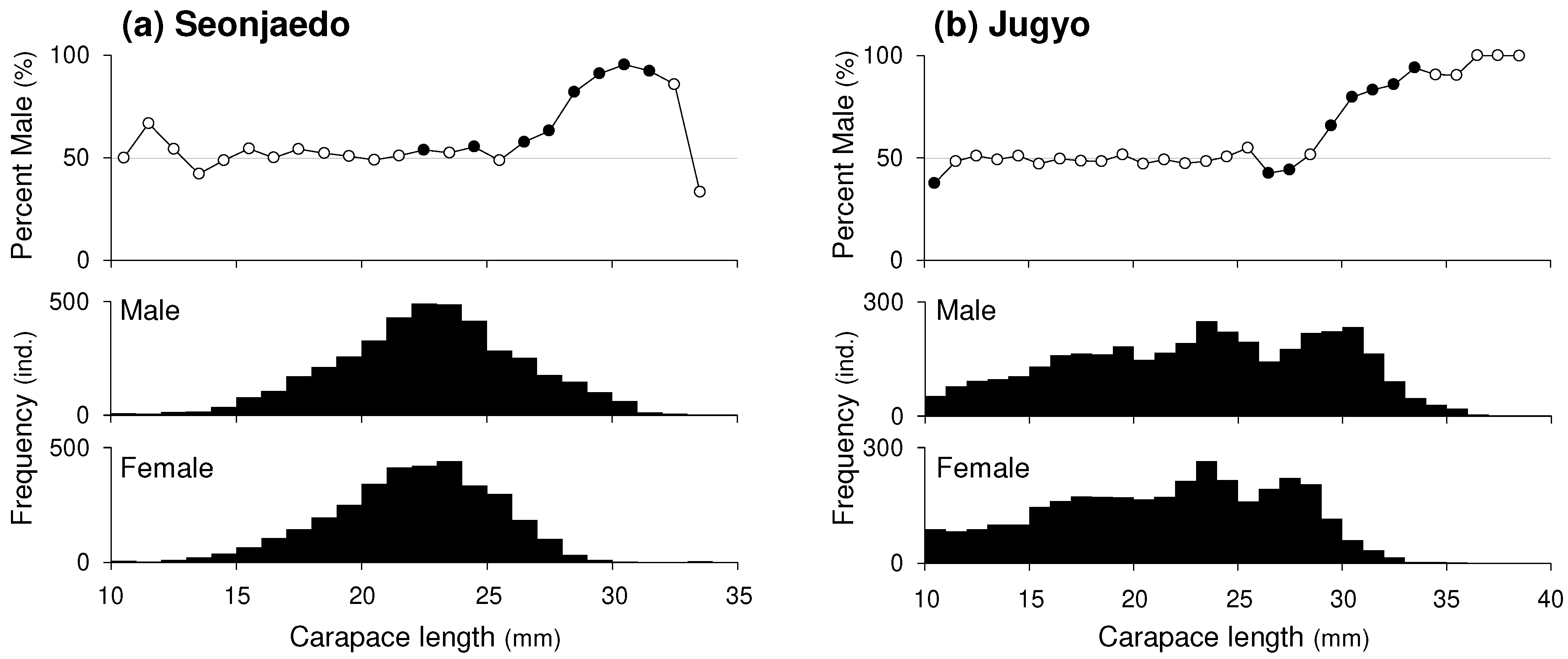
There was significant difference in CL-frequency distributions between sexes in both Seonjeado (Two-sample Kolmogorov-Smirnov test, D = 0.08769,
p < 0.001) and Jugyo (D = 0.20792,
p < 0.001). The distribution of sexual proportions by CL class was generally close to 50:50 for small-to-mid sizes. However, as the size increased, the proportion became biased toward males, with this trend becoming distinctly evident from a CL of around 29 mm and higher in both study sites (
Figure 5).
3.2.3. Cohort
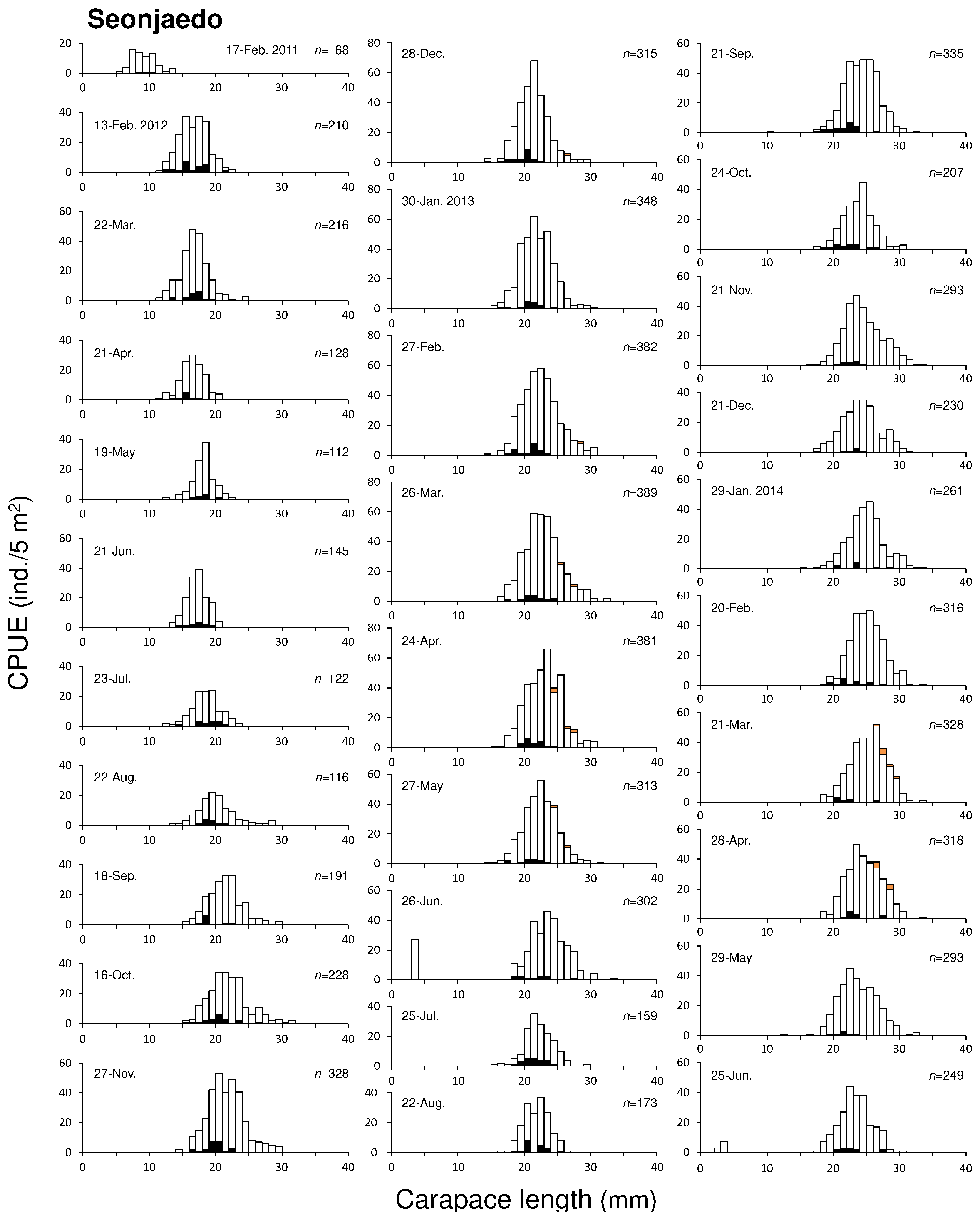
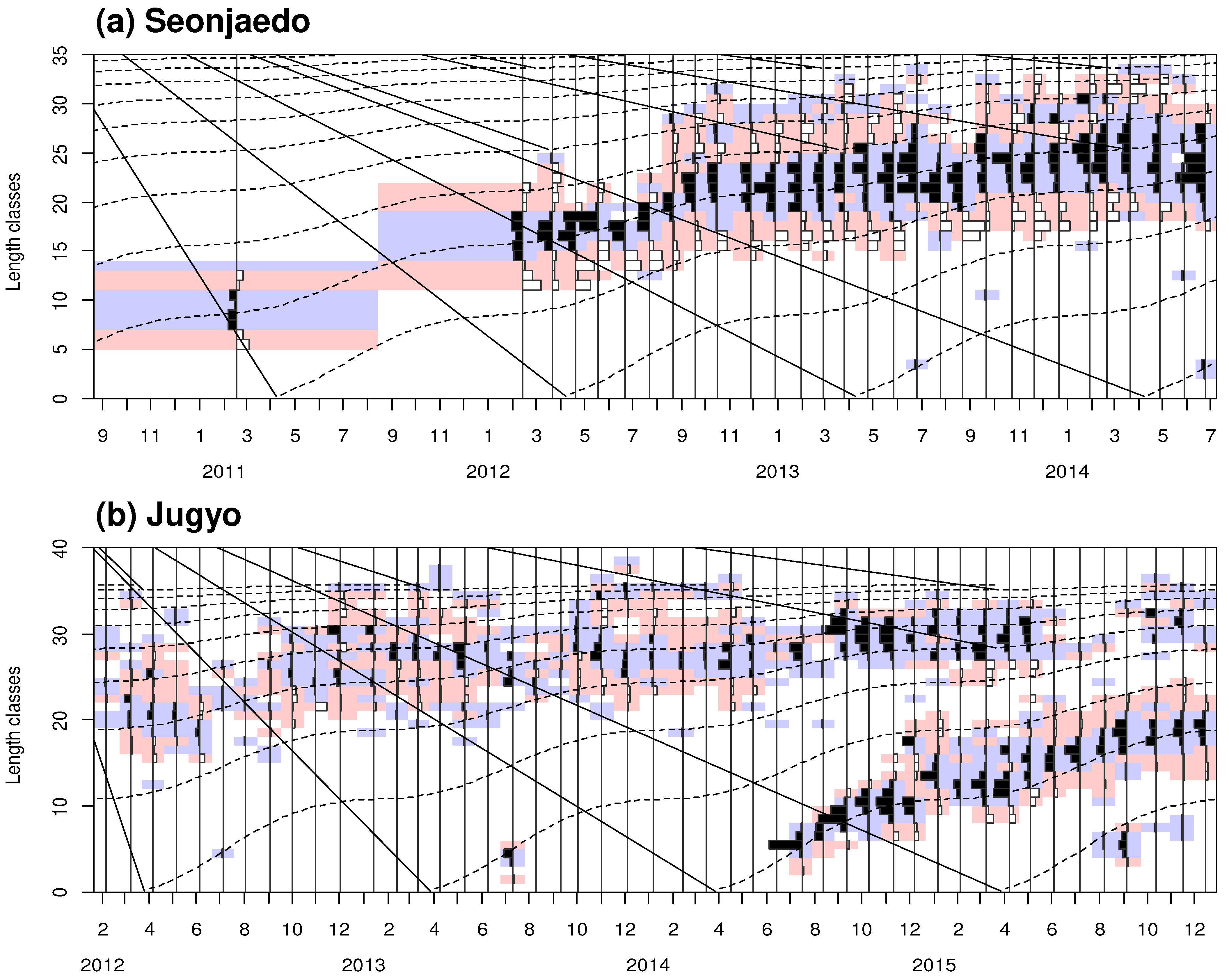
During the study period, the CL of mud shrimp collected from Seonjaedo ranged from 2.81 to 33.95 mm. The smallest and largest individuals were collected in June 2014 and June 2013, respectively. The largest male measured 33.89 mm CL (97.26 mm TL) and the largest female measured 33.95 mm CL (107.11 mm TL). From February 2011 to January 2014, we observed a single size-group that was visually distinguishable in the monthly CL-frequency distributions (
Figure 6). CL in this group ranged from 5.38 mm to 13.56 mm. Since the settlements of juvenile shrimps were observed once a year in 2013 and 2014, this size group was considered to represent a yearly cohort. Moreover, based on the growth rate of the mud shrimp population in Seonjaedo (see section
3.3.1), we estimated that this group settled in 2010. Newly settled juveniles were observed in June 2013 and June 2014. However, the former was not found in subsequent months, and the latter appeared at the end of the survey period and could not be tracked further. Larger individuals, distinct in size from the 2010 cohort, such as those with CLs of of 33.5 mm in March and 34.5 mm in June 2013, were found. However, these individuals were limited to only 1 – 2 specimens and were not consistently observed. As a result, the 2010 cohort remained the only abundant and consistently present cohort in Seonjaedo throughout the study period. The average CL of the cohort was about 8 mm when first observed in February 2011, and it grew to about 16 mm, 22 mm, and 26 mm after 1, 2, and 3 years, respectively.
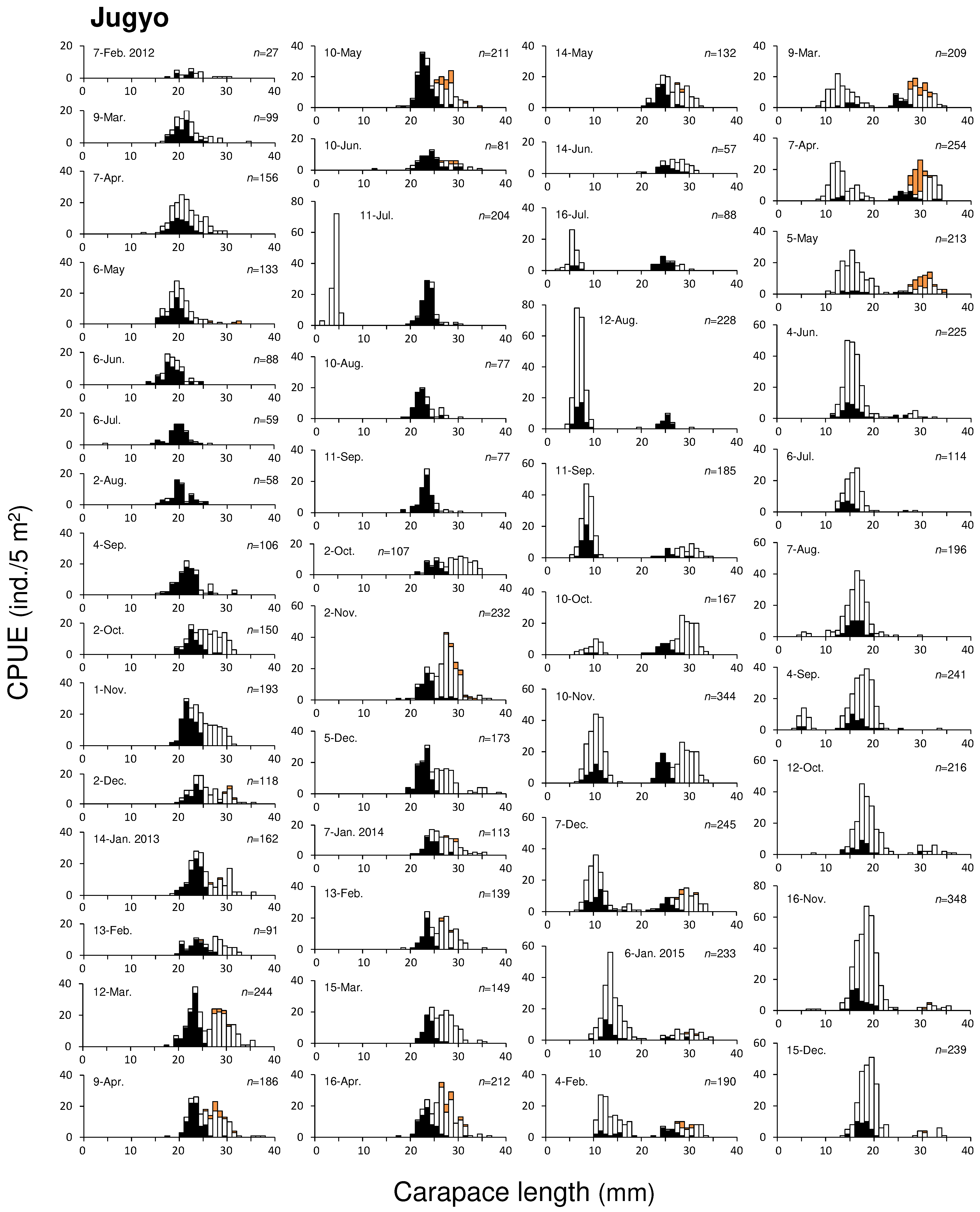
The CL of mud shrimp collected in Jugyo ranged from 1.50 mm to 38.21 mm during the study period. The smallest and largest individuals were observed in July 2013 and December 2013, respectively. The largest male and female measured 38.21 mm CL (109.78 mm TL) and 35.99 mm CL (106.55 mm TL). We identified two distinct size groups in a total of 47 CL-frequency distributions (
Figure 7). Similar to Seonjaedo, a newly settled group occurred once a year. Therefore, these size groups were considered to be yearly cohorts. In February 2012, the CL of the earlier group ranged from 17.72 mm to 24.85 mm, with an average size of about 21 mm. It grew to 26 mm by February 2013, a year later. However, after that, growth slowed, and a similar size was maintained in February 2014 as in the previous year. In February 2015, the average CL was 29 mm, and by May of the same year, it had reached approximately 30 mm, after which the number of individuals rapidly decreased. The later group first appeared in July 2014 (
Figure 7). This 2014 cohort, which settled with an average CL size of 7 mm, grew to 16 mm by July 2015, a year later. By the last survey in December 2015, 17 months after their settlement, this cohort had consistently maintained their abundance, and CLs grew to 19 mm. Newly settled groups were also observed in July 2012, July 2013, and August – November 2015, but they were not observed in the subsequent months. Given that the 2014 cohort took 17 months post-settlement to grow to an approximate CL of 19 mm, the earlier group, which had an average CL size of 21 mm in February 2012, is presumed to have settled no later than 2010. Based on the growth rate of the mud shrimp population in Jugyo (see section
3.3.1) and reports from local fishermen, we identified this size group to be the 2010 cohort.
Figure 7.
CL – frequency distributions of mud shrimp populations in Jugyo. Empty boxes (□) represent uninfected specimen, solid dark boxes (■) represent infected specimens, and a solid orange boxes (■) represent ovigerous female.
Figure 7.
CL – frequency distributions of mud shrimp populations in Jugyo. Empty boxes (□) represent uninfected specimen, solid dark boxes (■) represent infected specimens, and a solid orange boxes (■) represent ovigerous female.
3.2.4. Parasitic Infections
The monthly parasitic infection rate of the mud shrimp population in Seonjaedo ranged from 2.1% to 14.5%, with an average of 6.0% (± 2.9%). In Jugyo, it ranged from 6.1% to 91.5%, with an average of 37.1% (± 20.9%). Five species of parasites were identified in both regions: a bopyrid isopod Gyge ovalis, a sacculinid cirriped Sacculina upogebiae, a bopyrid isopod Procepon liuruiyui, a montacutid bivalve Peregrinamor ohshimai, and a bopyrid isopod Orthione griffenis. Among them, three bopyrid isopods infested the branchial chambers of the host shrimp. In contrast, S. upogebiae was an ecto-commensal and was found attached near the median line on the ventral surface of the first or second abdominal segment of the host shrimp. P. ohshimai is also an ecto-commensal but was byssally attached to the longitudinal groove of the ventral cephalothorax of U. major with the anterior part toward the head of the host. Bopyrid isopods were highly prevalent, and G. ovalis, the most dominant parasite, accounted for over 90% of the parasites in the mud shrimp populations at both sites. The impact of these parasites on the growth of the mud shrimp will be examined in more detail by another separate paper.
3.3. Growth, Mortality and Reproduction
3.3.1. Growth and Lifespan
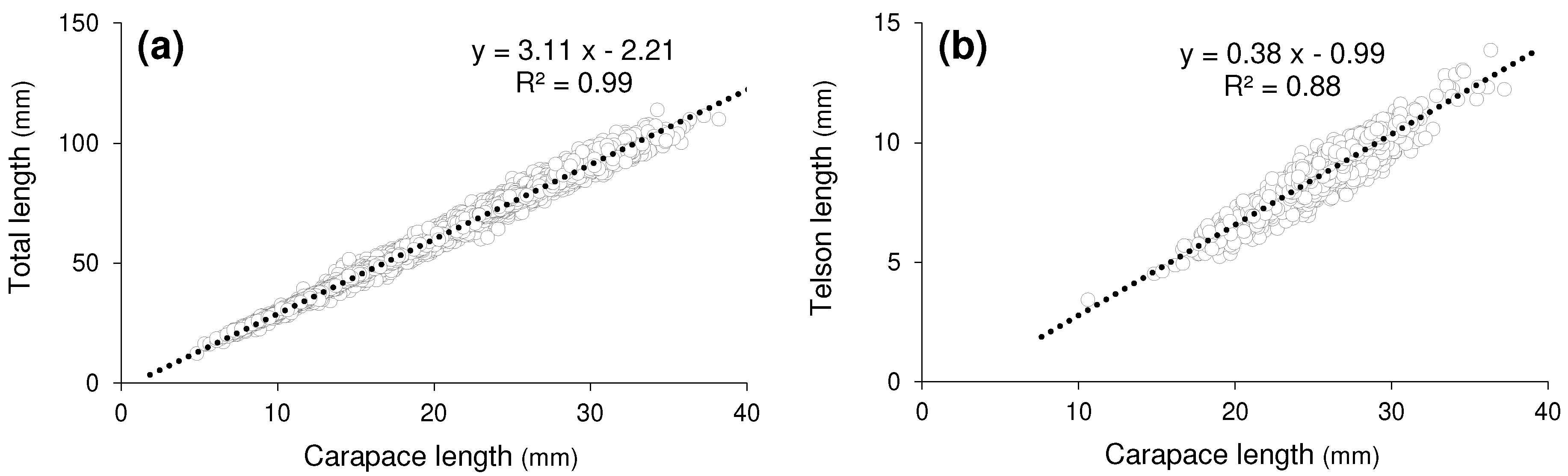
The relationship between the CL and TL, based on pooled data across both sites was described by the equation
(
Figure 8a). The ratio of CL to TL was approximately 1:3.1. The relationship between telson length and CL was given by
(
Figure 8b). The ratio was approximately 1:2.6.
The VBGF parameters for males, females, and the overall populations in the study sites are summarized in
Table 2. In both sites,
, representing the amplitude of seasonal growth oscillations, ranged from 0.8 to 1.0, indicating a strong amplitude in the seasonal growth oscillations of the mud shrimp populations (
Table 2,
Figure 9 and
Figure 10). The winter point (WP) indicates that the population of both sites exhibited its most attenuated growth rate between January and February (
Table 2). The growth performance index
indicates that while the growth rates between males and females were similar within each site, the population in Jugyo exhibited a higher growth rate than that in Seonjaedo (
Table 2,
Figure 10).
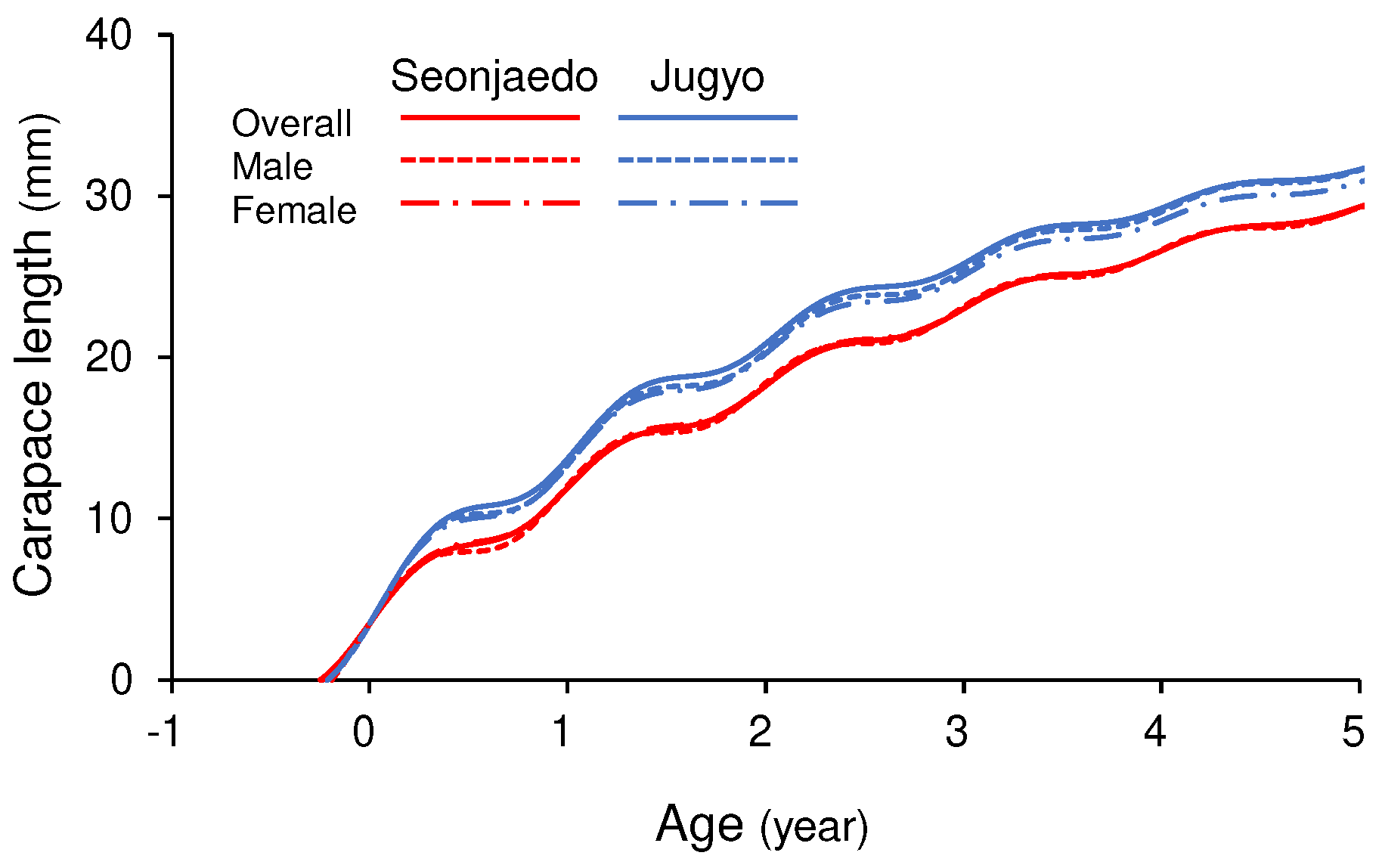
The growth curves for the pooled data of each site suggest that the Seonjaedo mud shrimp population achieved sizes of 11.90 mm, 18.24 mm, and 23.02 mm CL at 1, 2, and 3 years post-settlement (
Figure 10). In contrast, the population in Jugyo were estimated to grow to 13.73 mm, 20.86 mm, and 25.82 mm CL over the corresponding intervals, respectively. The time required for
U. major to reach its ecological minimum of 25 mm CL post-settlement was estimated to be 3.41 years for the Seonjaedo population and 2.86 years for the Jugyo population (
Figure 10). The estimated lifespan (
), based on the overall population data from each site, was 10.16 years in Seonjaedo and 8.00 years in Jugyo.
3.3.2. Mortality
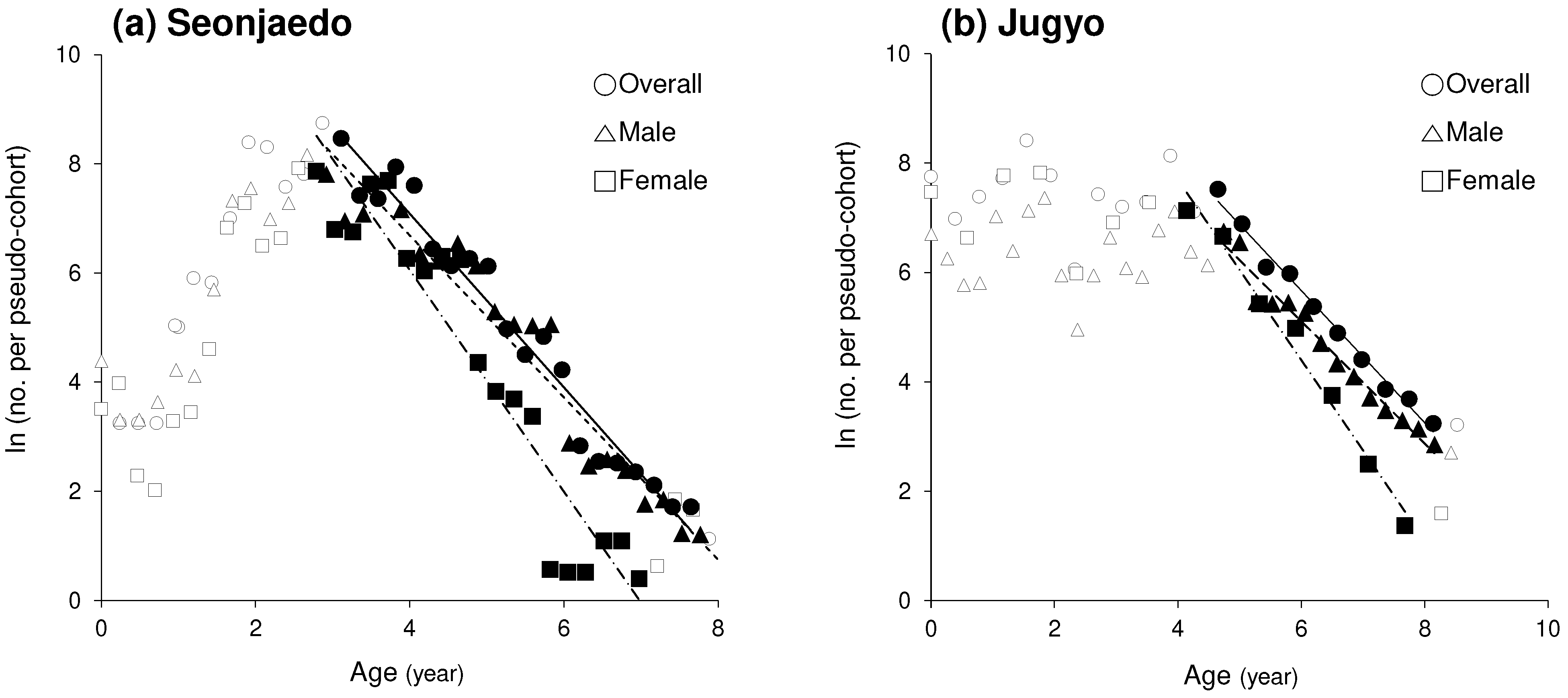
The total mortality coefficient (
) for the mud shrimp population in Seonjaedo was 1.48 for males, 2.03 for females, and 1.59 for the overall population (
Figure 11a). Correspondingly, the derived annual mortality rates (
) were 77.2%, 86.9%, and 79.6%, respectively. In Jugyo, the total mortality coefficient (
) was 1.04 for males, 1.65 for females, and 1.04 for the overall population, with annual mortality rates (
) of 67.4%, 80.8%, and 64.7%, respectively (
Figure 11b).
3.3.3. Reproduction
In Seonjaedo, ovigerous females ranging from 23.86 mm to 29.00 mm were collected. They appeared from November 2012 to May 2013 during the winter season of 2012 – 2013 and from March to April 2014 during the winter season of 2013 – 2014 (
Figure 12a). In Jugyo, the size of the ovigerous females ranged between 24.05 mm and 34.78 mm CL. The first ovigerous female was observed in May 2012, a year earlier than in Seonjaedo (
Figure 12b). Subsequently, ovigerous females appeared every winter season, typically from November or December through May or June. Therefore, the brooding season of the mud shrimp population in Seonjaedo was estimated to be from November to the following May and from November to the following June in Jugyo.
The number of eggs in egg masses ranged from 2,730 to 17,400, with an average count of 8,057 (± 3,265). Egg development, consolidated monthly across all years, showed a similar pattern in both sites (
Figure 12). From November, when ovigerous females first appeared, only yellow eggs without eyespots were found until January in Jugyo or February in Seonjaedo. Subsequently, the presence of orange eggs with eyespots gradually increased. Hatched eggshells were observed in both sites in May, and notably, in June, all the eggs found in Jugyo were hatched. The recruitment pattern in both sites indicated a single annual recruitment pulse (
Figure 12). The percentage of recruitment peaked in June, with 24.48% in Seonjaedo and 22.19% in Jugyo. The combined recruitment from May to July accounted for over 50%. Post-settled juveniles were only observed in June 2013 and June 2014 in Seonjaedo (
Figure 12a). In contrast, in Jugyo, the appearance of juveniles occurred slightly later. They were found in July 2012 and July 2013 (
Figure 12b). In 2014, they started appearing in July and were most abundant in August. They emerged even later in 2015, in August and September. Small-sized juveniles (< 7 mm CL) were also observed after October, but only a few individuals were found.
4. Discussion
4.1. Population Structure
4.1.1. Cohort
Studies on
U. major populations reported three to four cohorts with the annual recruitment of newly settled juveniles [
1,
39]. The population of other
Upogebia shrimp is typically composed of three to four cohorts, similar to that of
U. major [
52,
73,
74].
U. pugettensis populations on the West coast of the United States exhibited four to five year-classes [
48]. One the other hand, the
U. major populations were found to have only one to two cohorts in Seonjaedo and Jugyo (
Figure 6 and
Figure 7). Until June 2014, only the 2010 cohort was successfully sustained in both study sites. In other regional cases, the simultaneous presence of multiple age groups indicates that the site has been a consistently sustaining habitat for mud shrimp populations, while the occurrence of the populations in our study sites was unusual and specifically the 2010 cohort is unprecedented.
Another cause of the low number of cohorts is due to the recruitment failed. In our study, newly settled juveniles were found annually (
Figure 12). However, successful recruitment only occurred in 2014 in Jugyo (
Figure 7). Indeed, the long-term recruitment monitoring studies of burrowing shrimp (Gebiidea and Axiidea) show that even within the same area, annual recruitment can vary dramatically [
49]. Recruitment involves megalopal settlement to the benthos, subsequent survival, and potential movement thereafter [
49]. Factors affecting mud shrimp recruitment include reductions in spawning-capable females due to parasitic infections [
59,
75,
76], the transport of surface waters [
77], behavioral adaptations of larvae [
78,
79,
80], the presence of adult shrimp [
81], and subsequent mortality due to predation [
15]. In our study sites, since newly settled larvae occurred regularly every year, the recruitment failure is presumed to be due to high subsequent mortality during the benthic juvenile stages by predation. Particularly, the extinction of the settled groups in Seonjaedo in June 2013 and Jugyo in July of the same year significantly demonstrates the predation impact at post-settlement stages (
Figure 6 and
Figure 7).
4.1.2. Shrimp Density
In Tokyo Bay, Japan, the density of
U. major was an average of 36 ind./m² and a maximum of 142 ind./m² [
33], and between 7 to 11 ind./m² in Vostok Bay, Russia [
39]. The shrimp density was an average of 265 ind./m
2 (± 24) in Seonjae-do and 118 ind./m
2 (± 33) in Jugyo, with maximums of 311 ind./m
2 and 378 ind./m
2, respectively (
Figure 2). Our results are considerably higher than those reported in various studies of
U. major populations in East Asia. Notably, the density in Jugyo increased significantly due to the new settlers in 2014, and in Seonjaedo, densities were maintained by individuals older than 2 years, even in the absence of recruitment (
Figure 2). The density of other
Upogebia species in various regional seas typically ranges from 20 ind./m² to over 200 ind./m². Although most studies reported
U. africana to have a high maximum density of 200 – 600 ind./m
2 [
73,
82,
83,
84,
85], the maximum density of other
Upogebia such as
U. pusilla [
74,
86,
87],
U. omissa [
27],
U. pugettensis [
48], and
U. deltaura [
88] is generally reported to be around 100 – 140 ind./m
2. Therefore, the maximum density in our study was somewhat lower than that of
U. africana, but higher than that of Other
Upogebia species.
Burrow opening counts is frequently used as surrogate measures of mud shrimp abundance [
13,
48]. In
Upogebia burrows, a shrimp exhibits a solitary habit, and 2 to 4 openings per
Upogebia burrow have been observed [
7,
13,
27,
89,
90,
91]. In our study, the average openings per shrimp in Seonjaedo and Jugyo were 9.4 and 7.4, respectively (
Figure 2), which were higher than the reported value of 2 for
U. major in Tokyo Bay, based on burrow casting [
7].
U. major may be one of the deepest burrowers in soft bottom, and their burrows may reach a depth of 2.5 m [
7]. However, our sampling depth was limited to a maximum of 1 m.
Seasonal variations in number of openings per shrimp, with a decrease during the autumn and winter, have been reported in
U. pugettensis population in Willapa Bay [
48]. Similar seasonal variations were also found in our study and in another report surveyed at Jugyo, being higher during the summer season [
92]. Regarding the former, Dumbauld et al. (1996) explained it as a result of the collapse of openings with increased wave exposure, with a decrease in shrimp activity due to lower salinity and cooler temperatures. On the other hand, the latter was suggested to be a result of shrimp behavior, burrowing more deeply to avoid the high temperatures during the summer season [
92]. Considering the temperature conditions at our study sites, where the sediment surface temperature rises to 32 – 35°C during the summer season, this suggestion seems reasonable at Seonjaedo and Jugyo as well. We assumed that shrimp burrow deeper to avoid the sampling shock and high temperatures in summer. This is related to the suggestion by Kinoshita [
7] that burrows may provide mud shrimp with refuge from biological and physical stresses.
4.1.3. Sex Ratio
Reports on the sex ratio of the
U. major population are scarce, but it appears to be close to 1:1. Analyses based on the sampling data revealed that the sex ratio varied monthly in Tokyo Bay and Vostok Bay (Tokyo Bay:
X2 (33,
N = 1,183) = 62.02,
p < 0.01; Vostok Bay:
X2 (10,
N = 2,432) = 34.85,
p < 0.001) and the results of the Chi-square test for goodness of fit to a 1:1 sex ratio for each sampling occasion indicated that significant sex bias was observed in only 18% of the total sampling in Tokyo Bay and 13% in Vostok Bay (
p < 0.05) (data from [
1,
39]). A 1:1 sex ratio was also reported in Namhae [
40]. In our study, sex ratio was often male-biased, although varied monthly. A significant female-bias was observed only once (
Figure 4). This differs somewhat from the 1:1 sex ratio observed in other East Asian
U. major populations. However, female bias has been more commonly reported in other
Upogebia species (e.g.,
U. africana [
73,
93];
U. pugettensis [
48];
U. omissa [
94];
U. issaeffi [
95];
U. deltaura [
88]; and
U. pusilla [
96]). Although the general pattern for Gebiidean and Axiidean shrimps is equality for the overall, among adult, female predominance is common [
97]. Aggressive intraspecific behavior and competition among males for females are considered reasons for the skewed sex ratio [
27,
98]. Therefore, reports of a 1:1 (e.g.
U. africana [
83];
U. issaeffi [
95]) or male-biased (e.g.
U. pusilla [
86,
99]) is not common. The sex ratio in mud shrimp was found to vary spatiotemporally among conspecifics [
87,
100]. The potential efficiency of sampling gear or methodology has been consistently presented [
83,
96,
101,
102]. Thus, the ecological significance of observed unequal sex ratios remains still unclear [
96,
101]
.
The sex ratio in marine crustaceans was more a function of animal size than other factors [
103]. The present study showed a near 1:1 sex ratio up to middle-size, followed by a clear male bias, with the proportion of males increasing with size (
Figure 5). This pattern coincides with the "standard pattern" described by Wenner [
103], where the sex ratio is equal in most size classes, but prominent deviations can occur in certain size classes due to gender-different behaviors or other reasons. The sex ratio-size pattern in
Upogebia shrimp populations is highly variable [
40,
48,
88,
94,
95,
99,
100]. Biases in primary sex ratio are thought to be influenced by evolutionary mechanisms, whereas skewed sex ratios during growth and development are considered as a result of sex-specific ecology [
104].
The equal sex ratio in initial sizes and male bias in larger sizes indicate that the skewed sex ratio in the populations in Seonjaedo and Jugyo are affected by sex-specific ecology such as growth rate and development, longevity and mortality, parasites, sex reversal, and migration [
103,
104]. No migration has been reported for
U. major [
3], and given the clear sexual dimorphism, sex reversal is not considered to occur [
1]. Parasitic infection has been suggested as only a potential cause for intersex [
36]. In our study, and in those from Tokyo Bay and Vostok Bay, there were no differences in the lifespan or growth rate of
U. major between sexes, except in Namhae [
1,
39,
40]. Female mortality was somewhat higher only in our study (
Figure 11). The limited ecological characteristics of sex differences do not offer clear insight into the sex ratio characteristics in our study population. Moreover, the potential impact from diverse and limited sampling methodologies [
96,
101,
103] may be the cause of the various sex ratio-size patterns reported in
Upogebia populations. Further studies are necessary with more diverse populations and refined sampling methods to understand the ecological characteristics and causes of sex differences.
4.2. Growth, Mortality and Longevity
4.2.1. Growth
The growth pattern and rate of
U. major were reported to be similar in both sexes in Tokyo Bay and Vostok Bay [
1,
39], which is consistent with our study in Seonjaedo and Jugyo (
Figure 10). In contrast, males were reported to have a faster growth rate than females in Namhae on the South coast of Korea [
40]. In addition, the seasonal growth oscillation (C) of the shrimp population in Namhae ranged between 0.4 and 0.61, and there was a difference in WP, the slowest growth period, between males and females [
40]. However, our study revealed that the seasonal oscillation (C > 0.7) was higher than in Namhae and WP occurred between December and January regardless of sex (
Table 2). The values of C correlate strongly with fluctuations in habitat temperature or temperature-related environmental factors [
105]. Therefore, the higher C in Seonjaedo and Jugyo compared to Namhae could be explained by wider annual fluctuations in water temperature (
Table 1). Lee [
40] suggested that the WP for males and females differed because they were influenced by the coolest water temperature and reproduction cycle, respectively. The present study showed that the seasonal sex-specific growth related to reproduction is not apparent. It may be due to the difference in size composition; the Namhae samples were predominantly composed of matured shrimp, but our samples included relatively a larger portion of immature shrimp (
Figure 6 and
Figure 7).
The growth of
U. major has been studied using various methods including estimation through the VBGF, and cohort analysis, therefore, we utilized the yearly growth of CL after settlement for comparison purposes [
1,
39,
40,
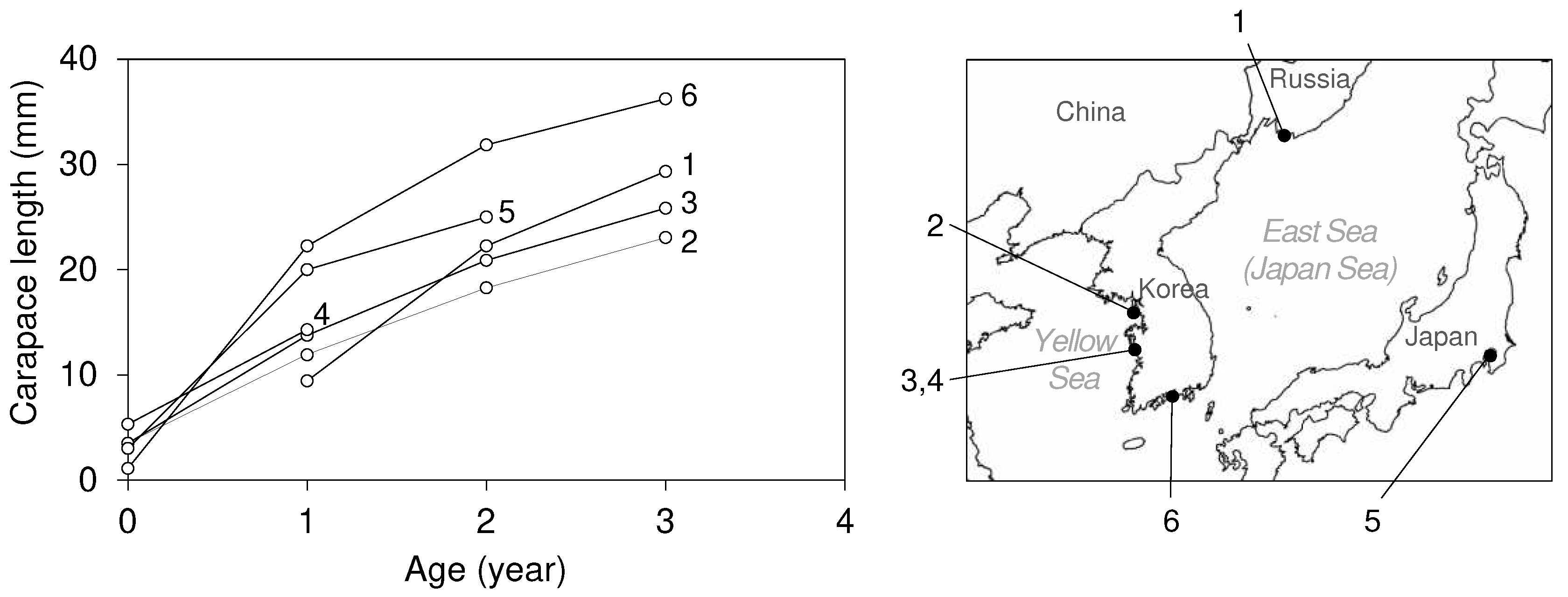
41]. The growth rate of the mud shrimp population in East Asia increases from higher to lower latitudes, which is especially clear in the latitude-specific CL distribution of +1 year old shrimps after settlement (
Figure 13).
The most important external factors influencing the growth of marine crustaceans are temperature and food supply [
106]. Hence, the latitudinal distribution of growth rates can first be explained by the distribution of sea water temperature along latitude (
Table 1). For food supply, upogebiid shrimps, including
U. major, are primarily suspension feeders [
24,
29,
107], and also directly consume deposits in their burrowing habits [
108,
109,
110]. Therefore, variable factors such as chlorophyll-a, organic content, and suspended particles can all be indicators of potential food. As information on chlorophyll-a obtained from satellite remote sensing have been widely used as one of the factors for growth modeling of filter feeder bivalves [
111,
112,
113], we also adopt chlorophyll-a as the mud shrimp growth factor because of their suspension feeding. Although chlorophyll-a does not fully represent the total particulate organic matter (POM) as a food source for filter feeders, it is act as a proxy for the living organics available [
114]. The annual average distribution of chlorophyll-a in the habitats of
U. major increases from higher to lower latitudes (
Table 1). This also appears to partially explain the growth distribution of
U. major across latitudes from the perspective of food supply. Population density is also one factor enabling quantitative comparisons of habitat conditions in terms of food supply. A negative relationship is found between
Upogebia population density and growth [
52]. This is presumed to be due to high population density inducing competition for food and space resources. However, the shrimp CL in Vostok Bay, the northernmost region are larger than those of Seonjaedo and Jugyo from the +2 year after settlement (
Figure 13). Therefore, the regional growth differences in
U. major that cannot be explained solely by the latitudinal distribution of SST or chlorophyll-a, may be attributed to the significantly higher shrimp density in Seonjaedo and Jugyo compared to other regions of
U. major habitats.
4.2.2. Mortality and Longevity
The lifespan of
Upogebia shrimp is reported to be three to five years in other studies. [
48,
73,
74,
86,
99,
100]. The theoretical maximum lifespan (A
95) of individual mud shrimp of the Seonjaedo and Jugyo, estimated using the VBGF, was 10.2 years and 8.0 years, respectively. Although the estimated lifespan can indicate the theoretical maximum lifespan of an individual, it is difficult to consider it as the average lifespan of the shrimp cohort. The lifespan is usually defined as the time from the first recognition of a cohort to its disappearance from the samples [
115]. However, in the mud shrimp population in Tokyo Bay, the largest of the three size groups could consist of individuals from various age groups [
1]. This seems to be due to the characteristics of the
U. major population, which grows rapidly until reaching maturity, spawns and then lives for several years with slow growth [
1], indicating the difficulty of applying age determination based on length-frequency data. This length-frequency-based model requires additional data or assumptions, leading to possible alternative outcomes [
106]. Fortunately, in our study, only the 2010 cohort existed until 2014 in both two study sites (
Figure 6 and
Figure 7). Recruitment was successful only in 2014 at Jugyo, allowing for a clear distinction between the two groups (
Figure 7).
The annual mortality rate was 77.2% at Seonjaedo and 67.4% at Jugyo. However, the 2010 cohort in Jugyo disappeared rapidly after June 2015, 5 years post-settlement, with only a few individuals observed until the last survey period in December 2015 (
Figure 7). Therefore, we assumed that the lifespan of the shrimp cohort on the West coast of Korea is approximately five to six years after settlement. This is somewhat longer than the three to four year lifespan of
U. major reported in Tokyo Bay [
7] and Vostok Bay [
39].
4.3. Reproduction
The sizes of ovigerous females of
U. major were reported as 25.1 mm to 38.9 mm CL [
1,
39,
40]. The smallest ovigerous females at Seonjaedo and Jugyo measured 23.86 mm and 24.05 mm CL, respectively, and the largest with a size of 34.8 mm CL was found at Jugyo. This range is not significantly different from the size range of ovigerous
U. major females reported (
Table 3).
The
U. major in Tokyo Bay and Vostok Bay were suggested to have a maturation time of over two years [
1,
39]. Although the estimated time required to grow mud shrimp to 25 mm CL was 3.41 years in Seonjaedo and 2.86 years in Jugyo (
Figure 10), some faster-growing individuals from the 2010 cohort in Seonjaedo began to be observed breeding as early as the winter season of 2012–2013 (
Figure 6). Thus, it appears that in both Seonjaedo and Jugyo, spawning can start as early as 2.5 years after settlement.
Shrimp populations in Seonjaedo and Jugyo spawned once a year (
Figure 12), somewhat consistent with results from other studies on
U. major populations in East Asia [
1,
39,
40]. The breeding season for
U. major is winter to spring (
Table 3). In this aspect,
U. major interestingly is similar to
U. pugettensis found on the West coast of the USA but different from
U. yokoyai and
U. pusilla, which breed in the summer,
U. africana, with a long breeding period, and
U. omissa, which spawns year-round. No distinct regional variations in
U. major population breeding seasons related to temperature distribution have been found from Tokyo Bay to Vostok Bay including Korean coast (
Table 3).
4.4. Consideration on the Life Cycle of Korean Mud Shrimp
Gebiideans typically have five or six planktonic zoeal larval stages, ending in metamorphosis into a stage that settles and is called a megalopa. Some species show abbreviated larval development with as few as two zoeal stages [
10]. Here,
U. major hatches out from March to May as the first zoea and subsequently spend about 14 to 16 days in a planktonic stage, passing through another two zoeal stages prior to metamorphosis into post-larvae, megalopa [
1,
31,
32]. The size of megalopa during the settlement period is 1.6 mm – 2.0 mm CL, and the time from hatching to settlement can be one month or longer [
1,
32,
117]. The size of post-settlement larvae ranges from 1.5 mm to 3.0 mm CL [
1], present study]. They can construct their own independent burrows and the size of the burrow increases with the growth of the inhabiting shrimp [
7].
The life cycle of mud shrimp based on the two study sites can be described from post-settlement through breeding, hatching, and until the disappearance of a cohort and summarized as follows: The shrimp that hatch in May or June spend about a month in a planktonic larval stage and recruit in June or July. Post-settled larvae continue to grow, reaching a CLs of 11.9 mm to 13.73 mm in their first year after settlement, and 18.24 mm to 20.86 mm in the second year. The fastest-growing individuals in a cohort experience their first breeding in the winter season of the second year after settlement. In the third year, shrimp grow to CLs of 23.02 mm to 25.82 mm, mature, continue to reproduce with about 8,000 eggs, and live for about five to six years after settlement.
Author Contributions
Conceptualization, S.K. and J.-S.H.; methodology, S.K. and J.-S.H.; validation, S.K. and J.-S.H.; formal analysis, S.K., C.Y., C.-L.L., S.N. and J.-S.H.; investigation, S.K., C.Y., C.-L.L., S.N. and J.-S.H.; resources, S.K., C.Y., C.-L.L., S.N. and J.-S.H.; data curation, S.K., C.Y. and C.-L.L.; writing—original draft preparation, S.K. and J.-S.H.; writing—review and editing, J.-S.H.; visualization, S.K., C.-L.L. and J.-S.H.; supervision, J.-S.H.; project administration, S.K. and J.-S.H.; funding acquisition, J.-S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korea Ministry of Oceans and Fisheries through the Gyeonggi-Incheon Sea Grant Center, 2010-2012; The Korea Ministry of Oceans and Fisheries through the Chungcheong Sea Grant Center, 2012-2014; The National Marine Biodiversity Institute of Korea (MABIK), 2030M00100
Acknowledgments
This work reported in this publication was partly supported by the Korea Ministry of Oceans and Fisheries through the Gyeong-gi-Incheon Sea Grant Center (2010-2012) and the Chungcheong Sea Grant Center (2012-2014). Preparation of this manuscript was supported by the National Marine Biodiversity Institute of Korea (MABIK; 2030M00100). Our thanks also go to Mr. Yoo-Jun Kim for the support of laboratory works.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kinoshita, K.; Furota, T.; Nakayama, S. Life Cycle Characteristics of the Deep-Burrowing Mud Shrimp Upogebia major (Thalassinidea: Upogebiidae) on a Tidal Flat Along the Northern Coast of Tokyo Bay. J. Crust. Biol. 2003, 23, 318–327. [Google Scholar] [CrossRef]
- Itani, G. Distribution of Intertidal Upogebiid Shrimp (Crustacea: Decapoda: Thalassinidea) in Japan. Cont. Biol. Lab. Kyoto Univ. 2004, 29, 383–399. [Google Scholar]
- Hong, J.-S. Biology of the Mud Shrimp Upogebia major (de Haan, 1841), with Particular Reference to Pest Management for Shrimp Control in Manila Clam Bed in the West Coast of Korea. Ocean Polar Res. 2013, 35, 323–349. [Google Scholar] [CrossRef]
- Kornienko, E.S. Burrowing Shrimp of the Infraorders Gebiidea and Axiidea (Crustacea: Decapoda). Russ. J. Mar. Biol. 2013, 39, 1–14. [Google Scholar] [CrossRef]
- Kinoshita, K. Life History Characteristics and Burrow Structure of the Mud Shrimp (Decapoda: Upogebiidae). Plankton Benthos Res. 2022, 17, B170402. [Google Scholar] [CrossRef]
- Kitabatake, K.; Izumi, K.; Kondo, N.I.; Okoshi, K. Phylogeography and Genetic Diversity of the Japanese Mud Shrimp Upogebia major (Crustacea, Decapoda, Upogebiidae): Natural or Anthropogenic Dispersal? Zookeys 2023, 1182, 259–287. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K. Burrow Structure of the Mud Shrimp Upogebia major (Decapoda: Thalassinidea: Upogebiidae). J. Crust. Biol. 2002, 22, 474–480. [Google Scholar] [CrossRef]
- Li, H.Y.; Lin, F.-J.; Chan, B.K.K.; Chan, T.-Y. Burrow Morphology and Dynamics of Mudshrimp in Asian Soft Shores. J. Zool. 2008, 274, 301–311. [Google Scholar] [CrossRef]
- D’Andrea, A.F.; DeWitt, T.H. Geochemical Ecosystem Engineering by the Mud Shrimp Upogebia pugettensis (Crustacea: Thalassinidae) in Yaquina Bay, Oregon: Density-dependent Effects on Organic Matter Remineralization and Nutrient Cycling. Limnol. Oceanogr. 2009, 54, 1911–1932. [Google Scholar] [CrossRef]
- Pillay, D.; Branch, G. Bioengineering Effects of Burrowing Thalassinidean Shrimps on Marine Soft-Bottom Ecosystems; Oceanogr. Mar. Biol.: Annu., Rev., Gibson, R.N., Atkinson, R.J.A., Gordon, J.D.M., Eds.; CRC Press, 2011; Volume 49, pp. 137–191. ISBN 9781439853658. [Google Scholar]
- Thompson, R.K.; Pritchard, A.W. Respiratory Adaptations of Two Burrowing Crustaceans, Callianassa Californiensis and Upogebia pugettensis (Decapoda, Thalassinidea). Biol. Bull. 1969, 136, 274–287. [Google Scholar] [CrossRef]
- Thompson, R.K. Functional Morphology of the Hindgut Gland of Upogebia pugettensis (Crustacea, Thalassinidea) and Its Role in Burrow Construction. Ph.D. thesis, University of California Berkeley, California, 1972. [Google Scholar]
- Dworschak, P.C. The Biology of Upogebia pusilla (PETAGNA) (Decapoda, Thalassinidea) I. The Burrows. Mar. Ecol. 1983, 4, 19–43. [Google Scholar] [CrossRef]
- Chapman, J.W.; Carter, C.S. A Rapid Intertidal Megafauna Survey Method Applied to Upogebia pugettensis, and Its Introduced Parasite, Orthione Griffensis. J. Crust. Biol. 2014, 34, 349–356. [Google Scholar] [CrossRef]
- Posey, M.H. Predation on a Burrowing Shrimp : Distribution and Community Consequences. J. Exp. Mar. Biol. Ecol. 1986, 103, 143–161. [Google Scholar] [CrossRef]
- Simenstad, C.A.; Fresh, K.L. Influence of Intertidal Aquaculture on Benthic Communities in Pacific Northwest Estuaries: Scales of Disturbance. Estuaries 1995, 18, 43. [Google Scholar] [CrossRef]
- Kim, I.-H. Poecilostomatoid Copepods from an Intertidal Mud Flat in the Yellow Sea. J. Nat. Hist. 2000, 34, 367–432. [Google Scholar] [CrossRef]
- Itani, G. Silent Biodiversity in the Burrows - Animals Associated with the Mud Shrimp. Benthos Res. 2001, 56, 50–53. [Google Scholar]
- Itani, G. Diverse Commensalism in the Burrows of the Tidal Flat. In Parasitism and Symbiosis; Ishibashi, N., Nawa, Y., Eds.; Tokai University Press: Japan, 2008; pp. 217–237. [Google Scholar]
- Itoh, H.; Nishida, S. A New Species of Hemicyclops (Copepoda, Poecilostomatoida) from Burrows of the Mud Shrimp Upogebia major in an Estuarine Mud-Flat in Tokyo Bay, Japan. Hydrobiologia 2002, 474, 139–146. [Google Scholar] [CrossRef]
- Marin, I. Redescription of the Alpheid Shrimp Betaeus levifrons Vinogradov, 1950 (Crustacea, Decapoda, Alpheidae) from Peter the Great Bay, Russian Coast of the Sea of Japan. Zootaxa 2010, 2613, 51. [Google Scholar] [CrossRef]
- Marin, I.N.; Korn, O.M.M.; Kornienko, E.S. Symbiotic Crab Sestrostoma balssi (Shen, 1932) (Varunidae: Gaeticinae) from Vostok Bay, Sea of Japan: A New Species for the Fauna of Russia. Russ. J. Mar. Biol. 2011, 37, 509–511. [Google Scholar] [CrossRef]
- Hong, J.-S.; Lee, C.-L.; Kim, S.; Yu, C. Macro-Invertebrate Ectoparasites Associated with the Mud Shrimp, Upogebia major from the West Coast of Korea. In Proceedings of the Abstracts, Fishery Sciences Association of Korea 2012; Korean Society of Fisheries and Aquatic Science: Busan, 2013. [Google Scholar]
- Dworschak, P.C. The Pumping Rates of the Burrowing Shrimp Upogebia pusilla (Petagna) (Decapoda: Thalassinidea). J. Exp. Mar. Biol. Ecol. 1981, 52, 25–35. [Google Scholar] [CrossRef]
- Griffis, R.B.; Suchanek, T.H. A Model of Burrow Architecture and Trophic Modes in Thalassinidean Shrimp (Decapoda: Thalassinidea). Mar. Ecol. Prog. Ser. 1991, 79, 171–183. [Google Scholar] [CrossRef]
- Felder, D.L.; Griffis, R. Dominant Infaunal Communities at Risk in Shoreline Habitats: Burrowing Thalassinid Crustacea. OCS Reports. 1994. [Google Scholar]
- Coelho, V.R.; Cooper, R.A.; De, S.; Rodrigues, A. Burrow Morphology and Behavior of the Mud Shrimp Upogebia omissa (Decapoda: Thalassinidea: Upogebiidae). Mar. Ecol. Prog. Ser. 2000, 200, 229–240. [Google Scholar] [CrossRef]
- DeWitt, T.H.; D’Andrea, A.F.; Brown, C.A.; Griffen, B.D.; Eldridge, P.M. Impact of Burrowing Shrimp Population on Nitrogen Cyclling and Water Quality in Western North American Temperature Estuaries. In Proceedings of the Symposium on Ecology of Large Bioturbators in Tidal Flats and Shallow Sublittoral Sediments – from Individual Behavior to Their Role as Ecosystem Engineers; Tamaki, A., Ed.; University of Nagasaki: Japan, 2004; pp. 107–118. [Google Scholar]
- Griffen, B.D.; DeWitt, T.H.; Langdon, C. Particle Removal Rates by the Mud Shrimp Upogebia pugettensis, Its Burrow, and a Commensal Clam: Effects on Estuarine Phytoplankton Abundance. Mar. Ecol. Prog. Ser. 2004, 269, 223–236. [Google Scholar] [CrossRef]
- Financial News Clam Beds Recover after 10 Years in Seonjaedo, Yeongheung-Myeon, Incheon. Available online: https://www.fnnews.com/news/201805150852578762 (accessed on 5 January 2023).
- Konishi, K. Larval Development of the Mud Shrimp Upogebia (Upogebia) major (De Haan) (Crustacea: Thalassinidea: Upogebiidae) under Laboratory Conditions, with Comments on Larval Characters of Thalassinid Families. Bull. Nat. Res. Inst. Aqua. 1989, 15, 1–17. [Google Scholar]
- Jo, Q.-T. Redescription of Larval Development of Upogebia major (De Haan). Master's thesis, National Fisheries University of Pusan, Busan, 1990. [Google Scholar]
- Kinoshita, K.; Furota, T. Burrow Structure and Life-History Characteristics of the Mud Shrimp, Upogebia major. In Proceedings of the Proceedings of the symposium on "Ecology df large bioturbators in tidal flats and shallow sublittoral sediments - from individual behavior to their role as ecosystem engineers; Vol. 24; Tamaki, A., Ed.; Nagasaki University: Nagasaki, March 24, 2004; pp. 7–13. [Google Scholar]
- Kato, M.; Itani, G. Commensalism of a Bivalve, Peregrinamor ohshimai, With a Thalassinidean Burrowing Shrimp, Upogebia major. J. Mar. Biol. Ass. UK. 1995, 75, 941–947. [Google Scholar] [CrossRef]
- Sato, M.; Uchida, H.; Itani, G.; Yamashita, H. Taxonomy and Life History of the Scale Worm Hesperonoe hwanghaiensis (Polychaeta: Polynoidae), Newly Recorded in Japan, with Special Reference to Commensalism to a Burrowing Shrimp, Upogebia major. Zool. Sci. 2001, 18, 981–991. [Google Scholar] [CrossRef]
- Ubaldo, J.P.; Nanri, T.; Takada, Y.; Saigusa, M. Prevalence and Patterns of Infection by the Epicaridean Parasite, Gyge ovalis and the Emergence of Intersex in the Estuarine Mud Shrimp, Upogebia major. J. Mar. Biol. Ass. UK. 2014, 94, 557–566. [Google Scholar] [CrossRef]
- Selin, N.I. The Prevalence of Macroparasite Infection in the Mud Shrimp Upogebia major (De Haan, 1841) (Decapoda: Gebiidea) from Peter the Great Bay, Sea of Japan. Russ. J. Mar. Biol. 2019, 45, 355–362. [Google Scholar] [CrossRef]
- Nanri, T.; Fukushige, M.; Ubaldo, J.P.; Kang, B.-J.; Masunari, N.; Takada, Y.; Hatakeyama, M.; Saigusa, M. Occurrence of Abnormal Sexual Dimorphic Structures in the Gonochoristic Crustacean, Upogebia major (Thalassinidea: Decapoda), Inhabiting Mud Tidal Flats in Japan. J. Mar. Biol. Ass. UK. 2011, 91, 1049–1057. [Google Scholar] [CrossRef]
- Selin, N.I. The Population Dynamics and Growth of the Mud Shrimp Upogebia major (De Haan, 1841) (Crustacea: Decapoda) from Peter the Great Bay, Sea of Japan. Russ. J. Mar. Biol. 2017, 43, 270–275. [Google Scholar] [CrossRef]
- Lee, K.T. Growth and Reproduction of the Mud Shrimp, Upogebia major (de Haan, 1841) (Thalassinidea: Upogebiidae) on the Intertidal Mudflat of the Southern Sea, Korea. Master of Science, Pukyong National University, 2016. [Google Scholar]
- Song, J.-H.; Ahn, H.-M.; Jeung, H.-D.; Chung, S.-O.; Kang, H.-W. Growth of Two Mud Shrimps (Upogebia major and Austinogebia wuhsienweni) Settled in Boryeong and Hongseong Tidal Flat. Env. Biol. Res. 2019, 37, 217–227. [Google Scholar] [CrossRef]
-
KHOA Annual Report of Korea Oceanographic Observation Network (2022); Busan, 2022.
- Yeongheung branch of Fisheries Cooperatives. In Status of Fishing Rights in Incheon; 2010.
- Kim, S.; Hong, J.-S. Comparison of Mass Mortality Rates in Spring of Manila Clams (Ruditapes philippinarum) in Sunjae Island, Incheon, According to Differences in Aquaculture Environment. In Proceedings of the Proceedings of joint symposium of The korean Association of Ocean Science and Technology Societies in 2011; The korean Association of Ocean Science and Technology: Busan, 2011; pp. 165–166. [Google Scholar]
- Dong-a Ilbo, Clam Disappearance Incident in Sunjaedo. Available online: https://www.donga.com/news/article/all/20120316/44832514/1 (accessed on 5 November 2023).
- MIFAFF Survey on the Status of Tidal Flat Fisheries in Oil Spill Affected Areas. Republic of Korea, 2012.
- Chungcheong Sea Grant Community Meeting in Jugyo-Myeon, Boryeong-Si (Ecology and Control of Mud Shrimp). Republic of Korea, 2014.
- Dumbauld, B.R.; Armstrong, D.A.; Feldman, K.L. Life-History Characteristics of Two Sympatric Thalassinidean Shrimps, Neotrypaea Californiensis and Upogebia pugettensis, with Implications for Oyster Culture. J. Crust. Biol. 1996, 16, 689–708. [Google Scholar] [CrossRef]
- Dumbauld, B.R.; Bosley, K.M. Recruitment Ecology of Burrowing Shrimps in US Pacific Coast Estuaries. Estuaries Coast. 2018, 41, 1848–1867. [Google Scholar] [CrossRef]
- Kinoshita, K.; Itani, G. Interspecific Differences in the Burrow Morphology between the Sympatric Mud Shrimps, Austinogebia narutensis and Upogebia Issaeffi (Crustacea: Thalassinidea: Upogebiidae). J. Mar. Biol. Ass. UK. 2005, 85, 943–947. [Google Scholar] [CrossRef]
- Sakai, K. Three Species of the Genus Upogebia (Decapoda, Crustacea) in Japan. J. Seika Women’s Jun. Coll. 1968, 1, 45–50. [Google Scholar]
- Yamasaki, M.; Nanri, T.; Taguchi, S.; Takada, Y.; Saigusa, M. Latitudinal and Local Variations of the Life History Characteristics of the Thalassinidean Decapod, Upogebia yokoyai: A Hypothesis Based on Trophic Conditions. Estuar. Coast Shelf Sci. 2010, 87, 346–356. [Google Scholar] [CrossRef]
- Shoji, K. A New Commensal Bivalve Attached to a Burrowing Shrimp. Venus 1938, 8, 119–128. [Google Scholar]
- Shiino, S.M. Bopyrids from Kyusyu and Ryukyu. Rec. Oceanogr. Works Japan 1939, 10, 79–99. [Google Scholar]
- Shiino, S.M. Rhizocephala of Japan. J. Sigenkagaku Kenkyusyo 1943, 1, 1–36. [Google Scholar]
- Markham, J.C. New Species and Records of Bopyridae (Crustacea: Isopoda) Infesting Species Fo the Genus Upogebia (Crustacea: Decapoda: Upogebiidae): The Genera Orthione Markham, 1988, and Gyge Cornalia & Panceri, 1861. In Proceedings of the Biological society of Washington; Oregon; 2004; pp. 186–198. [Google Scholar]
- Kil, H.J.; Park, T.S. First Record of Peregrinamor ohshimai (Mollusca Bivalvia) from Korea. Korean J. Syst. Zool. 2009, 25, 205–207. [Google Scholar] [CrossRef]
- Lee, C.-L. Bopyrid Isopods (Crustacea: Isopoda) Parasitic on Thalassinideans (Crustacea: Decapoda) in Korean Waters. Master of Science, Inha university, Incheon, 2014. [Google Scholar]
- Dumbauld, B.R.; Chapman, J.W.; Torchin, M.E.; Kuris, A.M. Is the Collapse of Mud Shrimp (Upogebia pugettensis) Populations Along the Pacific Coast of North America Caused by Outbreaks of a Previously Unknown Bopyrid Isopod Parasite (Orthione griffenis)? Estuaries Coast. 2011, 34, 336–350. [Google Scholar] [CrossRef]
- Somers, I.F. On a Seasonally Oscillating Growth Function. Fishbyte 1988, 6, 8–11. [Google Scholar]
- Mildenberger, T.K.; Taylor, M.H.; Wolff, M. TropFishR : An R Package for Fisheries Analysis with Length-frequency Data. Methods Ecol. Evol. 2017, 8, 1520–1527. [Google Scholar] [CrossRef]
- Pauly, D.; David, N. ELEFAN I, a BASIC Program for the Objective Extraction of Growth Parameters from Length-Frequency Datal. Hamburg, 1981; Volume 28. [Google Scholar]
- Scrucca, L. GA: A Package for Genetic Algorithms in R. J. Stat. Softw. 2013, 53. [Google Scholar] [CrossRef]
- Pauly, D. A Review of the ELEFAN System for Analysis of Length-Frequency Data in Fish and Aquatic Invertebrates. 1987. [Google Scholar]
- Pauly, D. Length-Converted Catch Curves and the Seasonal Growth of Fishes. Fishbyte 1990, 8, 24–29. [Google Scholar]
- Sparre, P. Can We Use Traditional Length-Based Fish Stock Assessment When Growth Is Seasonal? Fishbyte 1990, 8, 29–32. [Google Scholar]
- Taylor, C.C. Cod Growth and Temperature. ICES J. Mar. Sci. 1958, 23, 336–370. [Google Scholar] [CrossRef]
- Pauly, D. Studying Single-Species Dynamics in a Tropical Multispecies Context. In Proceedings of the Theory and management of tropical fisheries. ICLARM Conference Proceedings; Pauly, D., Murphy, G. i., Eds.; Manila, 1982; 9, pp. 33–70. [Google Scholar]
- Hasselblad, V. Estimation of Parameters for a Mixture of Normal Distributions. Technometrics 1966, 8, 431–444. [Google Scholar] [CrossRef]
- Gayanilo, F.C.; Pauly, D. Fao Computerized Information Series Fisheries 8 Fao-Iclarm Stock Assessment Tools Reference Manual. Rome, 1997. [Google Scholar]
- NASA GIOVANNi. Available online: http://disc.sci.gsfc.nasa.gov/giovanni (accessed on 5 November 2023).
- NASA Aqua MODIS Global Mapped Chlorophyll (CHL) Data, Version R2022. Available online: https://cmr.earthdata.nasa.gov/search/concepts/C2330512018-OB_DAAC (accessed on 5 November 2023).
- Hanekom, N.; Baird, D. Growth, Production and Consumption of the Thalassinid Prawn Upogebia africana (Ortmann) in the Swartkops Estuary. Afr. Zool. 1992, 27, 130–139. [Google Scholar]
- Conides, A.J.; Nicolaidou, A.; Apostolopoulou, M.; Thessalou-Legaki, M. Growth, Mortality and Yield of the Mudprawn Upogebia pusilla (Petagna, 1792) (Crustacea: Decapoda: Gebiidea) from Western Greece. Acta Adriat. 2012, 53, 87–103. [Google Scholar]
- Repetto, M.; Griffen, B.D. Physiological Consequences of Parasite Infection in the Burrowing Mud Shrimp, Upogebia pugettensis, a Widespread Ecosystem Engineer. Mar. Freshw. Res. 2012, 63, 60. [Google Scholar] [CrossRef]
- Asson, D.; Chapman, J.; Dumbauld, B. No Evidence That the Introduced Parasite Orthione griffenis Markham, 2004 Causes Sex Change or Differential Mortality in the Native Mud Shrimp, Upogebia pugettensis (Dana, 1852). Aquat. Invasions 2017, 12, 213–224. [Google Scholar] [CrossRef]
- Dudas, S.E.; Grantham, B.A.; Kirincich, A.R.; Menge, B.A.; Lubchenco, J.; Barth, J.A. Current Reversals as Determinants of Intertidal Recruitment on the Central Oregon Coast. ICES J. Mar. Sci. 2009, 66, 396–407. [Google Scholar] [CrossRef]
- Morgan, S.G.; Fisher, J.L.; Miller, S.H.; McAfee, S.T.; Largier, J.L. Nearshore Larval Retention in a Region of Strong Upwelling and Recruitment Limitation. Ecol. 2009, 90, 3489–3502. [Google Scholar] [CrossRef]
- Morgan, S.G.; Fisher, J.L.; McAfee, S.T.; Largier, J.L.; Halle, C.M. Limited Recruitment during Relaxation Events: Larval Advection and Behavior in an Upwelling System. Limnol. Oceanogr. 2012, 57, 457–470. [Google Scholar] [CrossRef]
- Shanks, A.; Morgan, S.; Macmahan, J.; Reniers, A.; Jarvis, M.; Brown, J.; Fujimura, A.; Griesemer, C. Onshore Transport of Plankton by Internal Tides and Upwelling-Relaxation Events. Mar. Ecol. Prog. Ser. 2014, 502, 39–51. [Google Scholar] [CrossRef]
- Tamaki, A.; Ingole, B. Distribution Of Juvenile And Adult Ghost Shrimps, Callianassa japonica Ortmann (Thalassinidea), On An Intertidal Sand Flat: Intraspecific Facilitation As A Possible Pattern-Generating Factor. J. Crust. Biol. 1993, 13, 175–183. [Google Scholar] [CrossRef]
- Hodgson, A.N. Distribution and Abundance of the Macrobenthic Fauna of the Kariega Estuary. 1987; Volume 22. [Google Scholar]
- Hodgson, A.N.; Allanson, B.R.; Cretchley, R. An Estimation of the Standing Stock and Population Structure of Upogebia africana (Crustacea: Thalassinidae) in the Knysna Estuary. Trans. Roy. Soc. South Afr. 2000, 55, 187–196. [Google Scholar] [CrossRef]
- Sumida, P.Y.G.; Güth, A.Z.; Quintana, C.O.; Pires-Vanin, A.M.S. Distribution and Sediment Selection by the Mud Shrimp Upogebia Noronhensis (Crustacea: Thalassinidea) and the Potential Effects on the Associated Macroinfaunal Community. J. Mar. Sci. Eng. 2020, 8, 1032. [Google Scholar] [CrossRef]
- Day, J.H. Estuarine Ecology - with Particular Reference to Southern Africa; Day, J.H., Ed.; CRC Press: Rotterdam, Nertherlands, 1981; ISBN 9789061912057. [Google Scholar]
- Gouvis, N.; Kevrekidis, T.; Koukouras, A. Population Dynamics, Reproduction and Growth of Upogebia pusilla (Decapoda, Thalassinidea) in the Evros Delta (North Aegean Sea). Crustaceana 1997, 70, 799–812. [Google Scholar] [CrossRef]
- Jugovic, J.; Horvat, E.; Lipej, L. Seasonal Abundance, Vertical Distribution and Life History Traits of Mediterranean Mud Shrimp Upogebia pusilla (Decapoda: Gebiidea) on the Slovenian Coast. Acta Adriat. 2018, 58, 297–312. [Google Scholar] [CrossRef]
- Tunberg, B. Studies on the Population Ecology of Upogebia deltaura (Leach) (Crustacea, Thalassinidea). Estuar. Coast Shelf Sci. 1986, 22, 753–765. [Google Scholar] [CrossRef]
- Stevens, B.A. Ecological Observations on Callianassidae of Puget Sound. Ecology 1929, 10, 399–405. [Google Scholar] [CrossRef]
- Swinbanks, D.D.; Murray, J.W. Biosedimentological Zonation of Boundary Bay Tidal Flats, Fraser River Delta, British Columbia. Sedimentology 1981, 28, 201–237. [Google Scholar] [CrossRef]
- Kinoshita, K.; Itani, G.; Uchino, T. Burrow Morphology and Associated Animals of the Mud Shrimp Upogebia yokoyai (Crustacea: Thalassinidea: Upogebiidae). J. Mar. Biol. Ass. UK. 2010, 90, 947–952. [Google Scholar] [CrossRef]
-
NIFS Development of the Best Management Strategies for Manila Clam Aquaculture in Tidal Flat; 2017.
- Hill, B.J. The Effect Of Heated Effluent On Egg Production In The Estuarine Prawn Upogebza Afrzcana (Ortmann). 1977; Volume 29. [Google Scholar]
- Santos, R. de C.; Santos Silva, L.F.; Dos Santos, B.; Motta, J.M.; Rodrigues Alves, D.F. Population Structure and Fecundity of Upogebia omissa (Decapoda: Gebiidea: Upogebiidae) in an Estuarine Region in Sergipe, Northeastern Brazil. Pesquisa e Ensino em Ciências Exatas e da Natureza 2018, 2, 95. [Google Scholar] [CrossRef]
- Selin, N.I. Some Features of the Biology of the Mud Shrimp Upogebia issaeffi (Balls, 1913) (Decapoda: Upogebiidae) from the Subtidal Zone of Vostok Bay, Sea of Japan. Russ. J. Mar. Biol. 2014, 40, 24–29. [Google Scholar] [CrossRef]
- Tucker, B.W. On the Effects of an Epicaridan Parasite, Gyge branchialis, on Upogebia littoralis. J. Cell. Sci. 1930, S2-74, 1–118. [Google Scholar] [CrossRef]
- Botter-Carvalho, M.L.; Costa, L.B.; Gomes, L.L.; Clemente, C.C.C.; Carvalho, P.V.V.D.C. Reproductive Biology and Population Structure of Axianassa australis (Crustacea, Axianassidae) on a Sand-Mud Flat in North-Eastern Brazil. J. Mar. Biol. Ass. UK. 2015, 95, 735–745. [Google Scholar] [CrossRef]
- Felder, D.L.; Lovett, D.L. Relative Growth And Sexual Maturation In The Estuarine Ghost Shrimp Callianassa louisianensis Schmitt, 1935. J. Crust. Biol. 1989, 9, 540–553. [Google Scholar] [CrossRef]
- Costa, L.B.; Marinho, N.M.; Carvalho, P.V.V.C.; Botter-Carvalho, M.L. Population Dynamics of the Mud Shrimp Upogebia omissa (Crustacea: Gebiidea: Upogebiidae) from the Southwestern Atlantic Coast of Brazil. Reg. Stud. Mar. Sci. 2020, 36, 101281. [Google Scholar] [CrossRef]
- Dworschak, P.C. The Biology of Upogebia pusilla (PETAGNA) (Decapoda, Thalassinidea) III. Growth and Production. Mar. Ecol. 1988, 9, 51–77. [Google Scholar] [CrossRef]
- Rowden, A.A.; Jones, M.B. A Contribution to the Biology of the Burrowing Mud Shrimp, Callianassa subterranea (Decapoda: Thalassinidea). J. Mar. Biol. Ass. UK. 1994, 74, 623–635. [Google Scholar] [CrossRef]
- Botter-Carvalho, M.L.; Santos, P.J.P.; Carvalho, P.V.V.C. Population Dynamics of Callichirus major (Say, 1818) (Crustacea, Thalassinidea) on a Beach in Northeastern Brazil. Estuar. Coast. Shelf Sci. 2007, 71, 508–516. [Google Scholar] [CrossRef]
- Wenner, A.M. Sex Ratio as a Function of Size in Marine Crustacea. Amer. Soc. Naturalists 1972, 106, 321–350. [Google Scholar] [CrossRef]
- Ewers-Saucedo, C. Evaluating Reasons for Biased Sex Ratios in Crustacea. Invertebr. Reprod. Dev. 2019, 63, 222–230. [Google Scholar] [CrossRef]
- Pauly, D.; Ingles, J. Aspects of the Growth and Natural Mortality of Exploited Coral Reef Fishes. In Proceedings of the Fourth International Coral Reef Symposium; Gomez, E., Birkeland, C.E., Buddemeyer, R.W., Johannes, R.E., Marsh, J.A., Jr., Tsuda, R.T., Eds.; Manila, 1981; Vol. 1; pp. 89–98. [Google Scholar]
- Hartnoll, R.G. Growth in Crustacea-Twenty Years On. Hydrobiologia 2001, 449, 111–122. [Google Scholar] [CrossRef]
- Yokoyama, H.; Tamaki, A.; Koyama, K.; Ishihi, Y.; Shimoda, K.; Harada, K. Isotopic Evidence for Phytoplankton as a Major Food Source for Macrobenthos on an Intertidal Sandflat in Ariake Sound, Japan. Mar. Ecol. Prog. Ser. 2005, 304, 101–116. [Google Scholar] [CrossRef]
- Dworschak, P.C. Feeding Behaviour of Upogebia pusilla and Callianassa Tyrrhena (Crustacea, Decapoda, Thalassinidea). Invest. Pesquera 1987, 51, 421–429. [Google Scholar]
- Bosley, K.M.; Copeman, L.A.; Dumbauld, B.R.; Bosley, K.L. Identification of Burrowing Shrimp Food Sources Along an Estuarine Gradient Using Fatty Acid Analysis and Stable Isotope Ratios. Estuaries Coast. 2017, 40, 1113–1130. [Google Scholar] [CrossRef]
- Coelho, V. Trophic Strategies and Functional Morphology of Feeding Appendages, with Emphasis on Setae, of Upogebia omissa and Pomatogebia operculata (Decapoda: Thalassinidea: Upogebiidae). Zool. J. Linn. Soc. 2000, 130, 567–602. [Google Scholar] [CrossRef]
- Radiarta, I.N.; Saitoh, S.-I.; Miyazono, A. GIS-Based Multi-Criteria Evaluation Models for Identifying Suitable Sites for Japanese Scallop (Mizuhopecten yessoensis) Aquaculture in Funka Bay, Southwestern Hokkaido, Japan. Aquaculture 2008, 284, 127–135. [Google Scholar] [CrossRef]
- Thomas, Y.; Mazurié, J.; Alunno-Bruscia, M.; Bacher, C.; Bouget, J.-F.; Gohin, F.; Pouvreau, S.; Struski, C. Modelling Spatio-Temporal Variability of Mytilus edulis (L.) Growth by Forcing a Dynamic Energy Budget Model with Satellite-Derived Environmental Data. J Sea Res 2011, 66, 308–317. [Google Scholar] [CrossRef]
- Snyder, J.; Boss, E.; Weatherbee, R.; Thomas, A.C.; Brady, D.; Newell, C. Oyster Aquaculture Site Selection Using Landsat 8-Derived Sea Surface Temperature, Turbidity, and Chlorophyll a. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Newell, C.R.; Hawkins, A.J.S.; Morris, K.; Boss, E.; Thomas, A.C.; Kiffney, T.J.; Brady, D.C. Using High-Resolution Remote Sensing to Characterize Suspended Particulate Organic Matter as Bivalve Food for Aquaculture Site Selection. J. Shellfish Res. 2021, 40, 113–118. [Google Scholar] [CrossRef]
- Baldwin, A.P.; Bauer, R.T. Growth, Survivorship, Life-Span, and Sex Change in the Hermaphroditic Shrimp Lysmata wurdemanni (Decapoda: Caridea: Hippolytidae). Mar. Biol. 2003, 143, 157–166. [Google Scholar] [CrossRef]
- de Oliveira, D.B.; Silva, D.C.; Martinelli, J.M. Density of Larval and Adult Forms of the Burrowing Crustaceans Lepidophthalmus siriboia (Callianassidae) and Upogebia vasquezi (Upogebiidae) in an Amazon Estuary, Northern Brazil. J. Mar. Biol. Ass. UK. 2012, 92, 295–303. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Yamamoto, S.; Iwamura, S. Propagation Experiment of Shellfishes (Mud Shrimp Control Experiment); 1987. [Google Scholar]
Figure 1.
The study areas, Seonjaedo and Jugyo tidal flats, located on the West coast of Korea. The circular red marks (●) indicate sampling station in the Seonjaedo (N 37˚ 14’ 37.3”, E 126˚ 32’ 18.0”) and Jugyo (N 36° 21′ 59.5”, E 126° 31′ 10.9”).
Figure 1.
The study areas, Seonjaedo and Jugyo tidal flats, located on the West coast of Korea. The circular red marks (●) indicate sampling station in the Seonjaedo (N 37˚ 14’ 37.3”, E 126˚ 32’ 18.0”) and Jugyo (N 36° 21′ 59.5”, E 126° 31′ 10.9”).
Figure 2.
Monthly variations of the mud shrimp densities estimated from the burrow openings and catch in the study sites. (a): Seonjaedo, (b): Jugyo. A higher number of openings per catch of around 15, was mainly observed during the summer season in both study sites. The shrimp density estimated from both openings and catches was higher in Seonjaedo than in Jugyo.
Figure 2.
Monthly variations of the mud shrimp densities estimated from the burrow openings and catch in the study sites. (a): Seonjaedo, (b): Jugyo. A higher number of openings per catch of around 15, was mainly observed during the summer season in both study sites. The shrimp density estimated from both openings and catches was higher in Seonjaedo than in Jugyo.
Figure 3.
Relationship between the shrimp density estimated from burrows and catch in study sites. (a): Seonjaedo tidal flat, (b): Jugyo tidal flat.
Figure 3.
Relationship between the shrimp density estimated from burrows and catch in study sites. (a): Seonjaedo tidal flat, (b): Jugyo tidal flat.
Figure 4.
Monthly variations of the sexual proportion and sex ratio of the mud shrimp populations in the study sites. (a): Seonjaedo, (b): Jugyo. The empty circles (○) represent no bias and the solid circles (●) represent a sex bias.
Figure 4.
Monthly variations of the sexual proportion and sex ratio of the mud shrimp populations in the study sites. (a): Seonjaedo, (b): Jugyo. The empty circles (○) represent no bias and the solid circles (●) represent a sex bias.
Figure 5.
Sex distribution in mud shrimp populations by CL class. (a): Seonjaedo, (b): Jugyo.
Figure 5.
Sex distribution in mud shrimp populations by CL class. (a): Seonjaedo, (b): Jugyo.
Figure 6.
CL-frequency distributions of mud shrimp populations in Seonjaedo. Empty boxes (□) represent uninfected specimens, solid dark boxes (■) represent infected specimens, and solid grey boxes (■) represent ovigerous females.
Figure 6.
CL-frequency distributions of mud shrimp populations in Seonjaedo. Empty boxes (□) represent uninfected specimens, solid dark boxes (■) represent infected specimens, and solid grey boxes (■) represent ovigerous females.
Figure 8.
Linear regressions – (a): between CL (mm) and TL (mm), (b): between CL (mm) and Telson lengths (mm). Data were pooled from two study sites.
Figure 8.
Linear regressions – (a): between CL (mm) and TL (mm), (b): between CL (mm) and Telson lengths (mm). Data were pooled from two study sites.
Figure 9.
Restructured length frequency distribution (MA = 7) of the overall uninfected mud shrimp populations in the study sites, shown with yearly repeating growth curves from the seasonally oscillating VBGF. (a): Seonjaedo, (b): Jugyo.
Figure 9.
Restructured length frequency distribution (MA = 7) of the overall uninfected mud shrimp populations in the study sites, shown with yearly repeating growth curves from the seasonally oscillating VBGF. (a): Seonjaedo, (b): Jugyo.
Figure 10.
Growth curves of male, female, and overall populations of mud shrimp in the study sites. For all curves, L_0, which is the length of individuals at t = 0, was set at 3.5 mm CL, which was the reported size of the post-settled mud shrimp population in Tokyo Bay, Japan (Kinoshita et al., 2003).
Figure 10.
Growth curves of male, female, and overall populations of mud shrimp in the study sites. For all curves, L_0, which is the length of individuals at t = 0, was set at 3.5 mm CL, which was the reported size of the post-settled mud shrimp population in Tokyo Bay, Japan (Kinoshita et al., 2003).
Figure 11.
Length-converted catch curve of mud shrimp populations in the study sites. a: Seonjaedo tidal flat, b: Jugyo tidal flat.
Figure 11.
Length-converted catch curve of mud shrimp populations in the study sites. a: Seonjaedo tidal flat, b: Jugyo tidal flat.
Figure 12.
Monthly occurrence of ovigerous females in mud shrimp populations, development of egg stages, recruitment pattern and abundance of post-settled juveniles in (a) Seonjaedo, (b) Jugyo.
Figure 12.
Monthly occurrence of ovigerous females in mud shrimp populations, development of egg stages, recruitment pattern and abundance of post-settled juveniles in (a) Seonjaedo, (b) Jugyo.
Figure 13.
The yearly growth of CL (mm) after settlement of
U. major in East Asian regions: 1. Vostok Bay, Russia [
39]; 2. Seonjaedo (the present study); 3. Jugyo (the present study); 4. Jugyo [
41]; 5. Tokyo Bay, Japan [
1]; 6. Namhae (Male) [
40]. Note: The growth of CL in Namhae was obtained from [
40] (Figure 15a), due to an error of the VBGF in that study.
Figure 13.
The yearly growth of CL (mm) after settlement of
U. major in East Asian regions: 1. Vostok Bay, Russia [
39]; 2. Seonjaedo (the present study); 3. Jugyo (the present study); 4. Jugyo [
41]; 5. Tokyo Bay, Japan [
1]; 6. Namhae (Male) [
40]. Note: The growth of CL in Namhae was obtained from [
40] (Figure 15a), due to an error of the VBGF in that study.
| |
Vostok Bay
Russia |
Seonjaedo
West coast of Korea |
Jugyo
West coast of Korea |
Tokyo Bay
Japan |
Namhae
South coast of Korea |
| Geographical position |
42°54’N 132°43'E |
37˚14'N 126˚32'E |
36°22'N 126°31'E |
35°40'N 139°56'E |
34°54'N 127°55'E |
Monthly maximum
SST (°C) |
21.90 |
26.04 |
26.96 |
29.08 |
28.52 |
Monthly minimum
SST (°C) |
-0.23 |
2.65 |
4.08 |
9.73 |
6.92 |
| Mean SST (°C) |
10.1 |
14.2 |
14.8 |
19.0 |
17.6 |
| Chlorophyll-a (mg/m3) |
3.5 |
5.4 |
7.8 |
12.6 |
7.6 |
Table 2.
Parameter estimates obtained from the ELEFAN using the seasonally oscillating VBGF.
Table 2.
Parameter estimates obtained from the ELEFAN using the seasonally oscillating VBGF.
| Parameter |
Seonjaedo |
|
Jugyo |
| Pooled |
Male |
Female |
|
Pooled |
Male |
Female |
|
37.70 |
37.01 |
37.10 |
|
37.18 |
36.94 |
36.02 |
|
0.28 |
0.29 |
0.29 |
|
0.36 |
0.37 |
0.36 |
|
0.25 |
0.27 |
0.27 |
|
0.22 |
0.21 |
0.24 |
|
0.76 |
0.96 |
0.81 |
|
1.00 |
1.00 |
0.76 |
|
0.53 |
0.46 |
0.54 |
|
0.55 |
0.52 |
0.52 |
(date) |
0.03(11-Jan.) |
0.96(15-Dec.) |
0.04(15-Jan.) |
|
0.05(19-Jan.) |
0.05(6-Jan.) |
0.09(9-Jan.) |
|
0.258 |
0.244 |
0.344 |
|
0.305 |
0.345 |
0.351 |
|
2.63 |
2.62 |
2.60 |
|
2.70 |
2.70 |
2.67 |
Table 3.
The breeding season, size of ovigerous female and maturation time of
Upogebia shrimp (modified from Kinoshita [
5]).
Table 3.
The breeding season, size of ovigerous female and maturation time of
Upogebia shrimp (modified from Kinoshita [
5]).
Species
/ Locality |
Geographical position
(Lat., Lon.) |
Breeding season |
Size of ovigerous
female (mm CL) |
Maturation
(year) |
Reference |
| J |
F |
M |
A |
M |
J |
J |
A |
S |
O |
N |
D |
| U. pugettensis |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Willapa Bay, USA |
46°40’N, 123°57’W |
▬ |
▬ |
▬ |
▬ |
▬ |
|
|
|
|
▬ |
▬ |
▬ |
20 – 30 |
3 |
[48] |
| U. major |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Vostok Bay, Russia |
42°54’N, 132°43’E |
|
|
|
▬ |
▬ |
|
|
|
|
|
|
|
73 (TL) |
> 2 |
[39] |
| Seonjaedo, West coast of Korea |
37˚14’N, 126˚31’E |
▬ |
▬ |
▬ |
▬ |
▬ |
|
|
|
|
|
|
▬ |
23.9 – 29.0
71.3 – 90.5 (TL) |
2.5 |
Present
study |
| Jugyo, West coast of Korea |
36°22’N, 126°30’E |
▬ |
▬ |
▬ |
▬ |
▬ |
▬ |
|
|
|
|
|
▬ |
24.1 – 34.8
74.2 – 101.4 (TL) |
2.5 |
Present
study |
| Tokyo Bay, Japan |
35°40’N, 139°56’E |
▬ |
▬ |
▬ |
▬ |
▬ |
|
|
|
|
|
|
▬ |
25.1 – 34.4 |
> 2 |
[1] |
| Namhae, South coast of Korea |
34°91’N, 127°92’E |
▬ |
▬ |
▬ |
▬ |
|
|
|
|
|
|
▬ |
▬ |
25.8 – 38.9 |
NA |
[40] |
| U. vasquezi |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Northern Brazil |
0°40’S, 47°37’W |
▬ |
▬ |
▬ |
|
▬ |
▬ |
▬ |
|
|
|
|
▬ |
NA |
NA |
[116] |
| U. yokoyai |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Wakyama, Japan |
34°11’N, 135°10’E |
|
|
|
|
|
|
|
▬ |
▬ |
▬ |
|
|
NA |
NA |
[18] |
| Hiroshima, Japan |
34°21’N, 132°21’E |
|
|
|
|
▬ |
▬ |
▬ |
▬ |
▬ |
|
|
|
50 – 87 (TL) |
1 |
[52] |
| Kochi, Japan |
33°28’N, 133°30’E |
|
|
|
|
▬ |
▬ |
▬ |
▬ |
|
|
|
|
35 – 52 (TL) |
1 |
[52] |
| U. pusilla |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lagoon of Grado, Itary |
45°43'N, 13°16'E |
|
|
▬ |
▬ |
▬ |
▬ |
▬ |
▬ |
▬ |
|
|
|
36 (TL) |
2 – 3 |
[100] |
| Evros Delta, Greece |
40°46'N, 26°02'E |
|
|
|
▬ |
▬ |
▬ |
▬ |
|
|
|
|
|
39 (TL) |
1 |
[86] |
| Lazaret and Strunjan, Slovenia |
45°33'N, 13°46'E |
|
|
|
▬ |
▬ |
▬ |
|
|
|
|
|
|
32 – 40 (TL) |
NA |
[87] |
| U. africana |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Swartkops estuary, South Africa |
33°51'S 25°36'E |
▬ |
▬ |
▬ |
|
|
|
▬ |
▬ |
▬ |
▬ |
▬ |
▬ |
12 – 17 |
1.5 |
[73] |
| U. omissa |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Casa Caiada Beach, Brazil |
7°59'S, 34°50'W |
▬ |
▬ |
▬ |
▬ |
▬ |
▬ |
▬ |
▬ |
▬ |
▬ |
▬ |
▬ |
6.33 |
1 |
[99] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).