Submitted:
06 November 2023
Posted:
07 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Protein Encapsulation and Characterization of the Prepared Vesicles
2.2. Efficiency of Protein Encapsulation (EE) Determined by Size Exclusion Chromatography
2.3. Antimicrobial Activity of CHAPSH3b-Loaded Vesicles
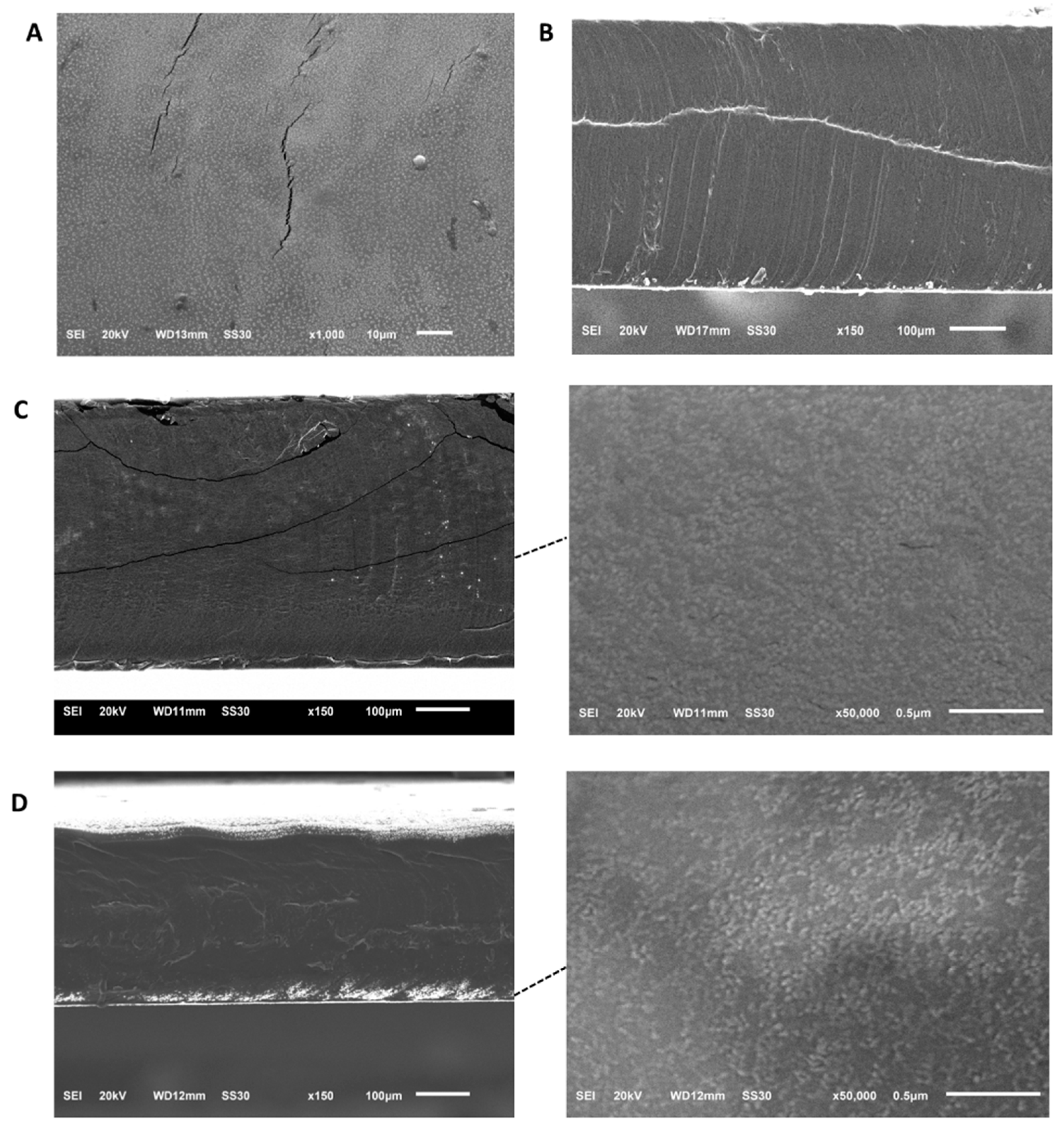
2.4. Characterization of Gelatine Films Containing Encapsulated or Free CHAPSH3b
2.5. Antimicrobial Activity of CHAPSH3b-Containing Gelatine Films
3. Discussion
4. Materials and Methods
4.1. Chemical Compounds, Bacterial Strains and Proteins
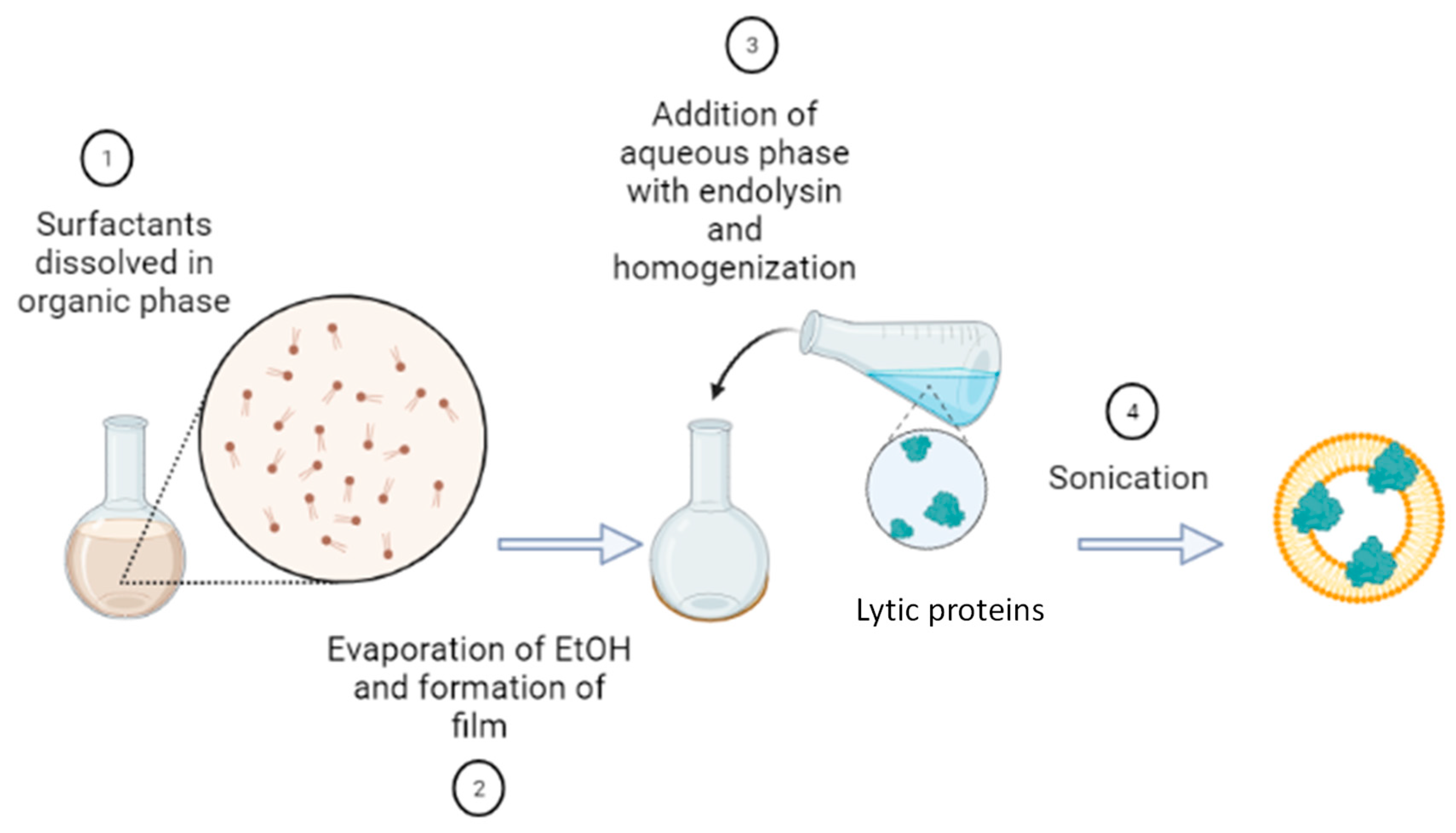
4.2. Synthesis and Characterization of Nanovesicles
4.2.1. Synthesis of Nanovesicles
4.1.2. Size and Morphology Characterization
4.1.3. Vesicles Purification
4.1.4. Determination of the Protein Encapsulation Efficiency (EE)
4.1.5. Antimicrobial Activity of Vesicles
4.2. Synthesis and Characterization of Gelatine Films
4.2.1. Films Characterization
4.2.2. Films Antimicrobial Activity
4.3. Statistical Analysis of Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- WHO Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; 2017;
- European Centre for Disease Prevention and Control Antimicrobial Resistance Surveillance in Europe; 2022;
- Pinto, R.M.; Lopes-De-Campos, D.; Martins, M.C.L.; Van Dijck, P.; Nunes, C.; Reis, S. Impact of Nanosystems in Staphylococcus Aureus Biofilms Treatment. FEMS Microbiol. Rev. 2019, 43, 622–641. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W. Biofilms and Antibiotic Therapy: Is There a Role for Combating Bacterial Resistance by the Use of Novel Drug Delivery Systems? Adv. Drug Deliv. Rev. 2005, 57, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Fernández, L.; Rodríguez, A.; García, P. Are Phage Lytic Proteins the Secret Weapon to Kill Staphylococcus Aureus? Am. Soc. Microbiol. 2018, 9, e01923–17. [Google Scholar] [CrossRef] [PubMed]
- Forier, K.; Raemdonck, K.; De Smedt, S.C.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and Polymer Nanoparticles for Drug Delivery to Bacterial Biofilms. J. Control. Release 2014, 190, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Yang, E.; Chang, P.S.; Ryu, S. Preparation and Characterization of Endolysin-Containing Liposomes and Evaluation of Their Antimicrobial Activities against Gram-Negative Bacteria. Enzyme Microb. Technol. 2019, 128, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; González, S.; Campelo, A.B.; Martínez, B.; Rodríguez, A.; García, P. Downregulation of Autolysin-Encoding Genes by Phage-Derived Lytic Proteins Inhibits Biofilm Formation in Staphylococcus Aureus. Antimicrob. Agents Chemother. 2017, 61, e02724–16. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; Donovan, D.M.; García, P. Enhanced Staphylolytic Activity of the Staphylococcus Aureus Bacteriophage VB_SauS-PhiiPla88 HydH5 Virion-Associated Peptidoglycan Hydrolase: Fusions, Deletions, and Synergy with LysH5. Appl. Environ. Microbiol. 2012, 78, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- De Maesschalck, V.; Gutiérrez, D.; Paeshuyse, J.; Lavigne, R.; Briers, Y. Advanced Engineering of Third-Generation Lysins and Formulation Strategies for Clinical Applications. Crit. Rev. Microbiol. 2020, 1–17. [Google Scholar] [CrossRef]
- Irkin, R.; Esmer, O.K. Novel Food Packaging Systems with Natural Antimicrobial Agents. J. Food Sci. Technol. 2015, 52, 6095–6111. [Google Scholar] [CrossRef]
- Duarte, A.C.; Fernández, L.; De Maesschalck, V.; Gutiérrez, D.; Campelo, A.B.; Briers, Y.; Lavigne, R.; Rodríguez, A.; García, P. Synergistic Action of Phage PhiIPLA-RODI and Lytic Protein CHAPSH3b: A Combination Strategy to Target Staphylococcus Aureus Biofilms. npj Biofilms Microbiomes 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Toledo-Arana, A.; Merino, N.; Vergara-Irigaray, M.; Débarbouillé, M.; Penadés, J.R.; Lasa, I. Staphylococcus Aureus Develops an Alternative, Ica-Independent Biofilm in the Absence of the ArlRS Two-Component System †. J. Bacteriol. 2005, 187, 5318–5329. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Khanal, D.; Alreja, A.B.; Yang, H.; YK Chang, R.; Tai, W.; Li, M.; Nelson, D.C.; Britton, W.J.; Chan, H.K. Bacteriophage Endolysin Powders for Inhaled Delivery against Pulmonary Infections. Int. J. Pharm. 2023, 635, 122679. [Google Scholar] [CrossRef] [PubMed]
- Gondil, V.S.; Chhibber, S. Bacteriophage and Endolysin Encapsulation Systems: A Promising Strategy to Improve Therapeutic Outcomes. Front. Pharmacol. 2021, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.D.; Paris, J.L.; Gama, F.M.; Silva, B.F.B.; Sillankorva, S. Sustained Release of a Streptococcus Pneumoniae Endolysin from Liposomes for Potential Otitis Media Treatment. ACS Infect. Dis. 2021, 7, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Garrido, V.; Fernández, L.; Portilla, S.; Rodríguez, A.; Grilló, M.J.; García, P. Phage Lytic Protein LysRODI Prevents Staphylococcal Mastitis in Mice. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Mal, A.; Bag, S.; Ghosh, S.; Moulik, S.P. Physicochemistry of CTAB-SDS Interacted Catanionic Micelle-Vesicle Forming System: An Extended Exploration. Colloids Surfaces A 2018, 553, 633–644. [Google Scholar] [CrossRef]
- Sobral, C.N.C.; Soto, M.A.; Carmona-Ribeiro, A.M. Characterization of DODAB/DPPC Vesicles. Chem. Phys. Lipids 2008, 152, 38–45. [Google Scholar] [CrossRef]
- Qian, Y.; Hu, X.; Wang, J.; Li, Y.; Liu, Y.; Xie, L. Polyzwitterionic Micelles with Antimicrobial-Conjugation for Eradication of Drug-Resistant Bacterial Biofilms. Colloids Surfaces B Biointerfaces 2023, 231, 113542. [Google Scholar] [CrossRef]
- Marchianò, V.; Matos, M.; López, M.; Weng, S.; Serrano-Pertierra, E.; Luque, S.; Blanco-López, M.C.; Gutiérrez, G. Nanovesicles as Vanillin Carriers for Antimicrobial Applications. Membranes (Basel). 2023, 13, a93. [Google Scholar] [CrossRef]
- Estupiñan, O.; García Manrique, P.; Blanco-lopez, M.; Matos, M.; Gutierrez, G. Vitamin d3 loaded niosomes and transfersomes produced by ethanol injection method: Identification of the critical preparation step for size control. Foods 2020, 10, 1367. [Google Scholar] [CrossRef]
- Estupiñan, O.R.; Garcia-Manrique, P.; del Carmen Blanco-Lopez, M.; Matos, M.; Gutiérrez, G. Vitamin D3 Loaded Niosomes and Transfersomes Produced by Ethanol Injection Method: Identification of the Critical Preparation Step for Size Control. Foods 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- García-Manrique, P.; Machado, N.D.; Fernández, M.A.; Blanco-López, M.C.; Matos, M.; Gutiérrez, G. Effect of Drug Molecular Weight on Niosomes Size and Encapsulation Efficiency. Colloids Surfaces B Biointerfaces 2020, 186, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Marchianò, V.; Matos, M.; Serrano, E.; Álvarez, J.R.; Marcet, I.; Carmen Blanco-López, M.; Gutiérrez, G. Lyophilised Nanovesicles Loaded with Vitamin B12. J. Mol. Liq. 2022, 365, 120129. [Google Scholar] [CrossRef]
- Melis, V.; Letizia Manca, M.; Bullita, E.; Tamburini, E.; Castangia, I.; Cardia, M.C.; Valenti, D.; Fadda, A.M.; Peris, J.E.; Manconi, M. Inhalable Polymer-Glycerosomes as Safe and Effective Carriers for Rifampicin Delivery to the Lungs. Colloids Surfaces B Biointerfaces 2016, 143, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Audia Triani, O.; Akhmad Kharis, Nugroho Ronny, M. ; Sugeng, R. Effect of Ratio Span 60 - Cholesterol on the Characteristic of Niosomes Vitamin D3. Res. J. Pharm. Technol. 2022, 15, 5551–5554. [Google Scholar] [CrossRef]
- Bucci, A.R.; Marcelino, L.; Mendes, R.K.; Etchegaray, A. The Antimicrobial and Antiadhesion Activities of Micellar Solutions of Surfactin, CTAB and CPCl with Terpinen-4-Ol: Applications to Control Oral Pathogens. World J. Microbiol. Biotechnol. 2018, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Qiao, J.; Xiong, M.P. Antibacterial and Biofilm-Eradicating Activities of PH-Responsive Vesicles against Pseudomonas Aeruginosa. Mol. Pharm. 2022, 19, 2406–2417. [Google Scholar] [CrossRef] [PubMed]
- Portilla, S.; Fernández, L.; Gutiérrez, D.; Rodríguez, A.; García, P. Encapsulation of the Antistaphylococcal Endolysin Lysrodi in Ph-Sensitive Liposomes. Antibiotics 2020, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dai, X.; Wei, X.; Yu, Y.; Chen, X.; Zhang, X.; Li, C. Near-Infrared Light-Activated Thermosensitive Liposomes as Efficient Agents for Photothermal and Antibiotic Synergistic Therapy of Bacterial Biofilm. ACS Appl. Mater. Interfaces 2018, 10, 14426–14437. [Google Scholar] [CrossRef]
- Selvamani, V. Stability Studies on Nanomaterials Used in Drugs. Charact. Biol. Nanomater. Drug Deliv. 2019, 425–444. [Google Scholar]
- Morais, D.; Tanoeiro, L.; Marques, A.T.; Gonçalves, T.; Duarte, A.; Matos, A.P.A.; Vital, J.S.; Cruz, M.E.M.; Carvalheiro, M.C.; Anes, E.; et al. Liposomal Delivery of Newly Identified Prophage Lysins in a Pseudomonas Aeruginosa Model. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Kour, A.; Panda, J.J.; Harjai, K.; Chhibber, S. Exploring Endolysin-Loaded Alginate-Chitosan Nanoparticles as Future Remedy for Staphylococcal Infections. AAPS PharmSciTech 2020, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, R.; Caro-León, F.J.; Nakal, A.; Ruiz, S.; Doñoro, C.; García-Fernández, L.; Vázquez-Lasa, B.; San Román, J.; Sanz, J.; García, P.; et al. DEAE-Chitosan Nanoparticles as a Pneumococcus-Biomimetic Material for the Development of Antipneumococcal Therapeutics. Carbohydr. Polym. 2021, 273, 118605. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Ma, Q.; Li, S.; Wang, W.; Ma, Y.; Zhao, H.; Sun, J.; Wang, J. Preparation and Characterization of Biodegradable Composited Films Based on Potato Starch/Glycerol/Gelatin. J. Food Qual. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- de Dicastillo, C.L.; Settier-Ramírez, L.; Gavara, R.; Hernández-Muñoz, P.; Carballo, G.L. Development of Biodegradable Films Loaded with Phages with Antilisterial Properties. Polymers (Basel). 2021, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; López, A.; Sáez-Orviz, S.; Marcet, I.; García, P.; Rendueles, M.; Díaz, M. Effectiveness of Bacteriophages Incorporated in Gelatine Films against Staphylococcus Aureus. Food Control 2021, 121, 1–8. [Google Scholar] [CrossRef]
- Kim, S.; Chang, Y. Anti-Salmonella Polyvinyl Alcohol Coating Containing a Virulent Phage PBSE191 and Its Application on Chicken Eggshell. Food Res. Int. 2022, 162, 111971. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Toledo-Arana, A.; Berasain, C.; Ghigo, J.-M.; Amorena, B.; Penadés, J.R.; Lasa, I. SarA and Not ΣB Is Essential for Biofilm Development by Staphylococcus Aureus. Mol. Microbiol. 2003, 48, 1075–1087. [Google Scholar] [CrossRef]
- Obeso, J.M.; Martínez, B.; Rodríguez, A.; García, P. Lytic Activity of the Recombinant Staphylococcal Bacteriophage ΦH5 Endolysin Active against Staphylococcus Aureus in Milk. Int. J. Food Microbiol. 2008, 128, 212–218. [Google Scholar] [CrossRef]
- Marchianò, V.; Matos, M.; Serrano-Pertierra, E.; Gutiérrez, G.; Blanco-López, M.C. Vesicles as Antibiotic Carrier: State of Art. Int. J. Pharm. 2020, 585, 119478. [Google Scholar] [CrossRef]
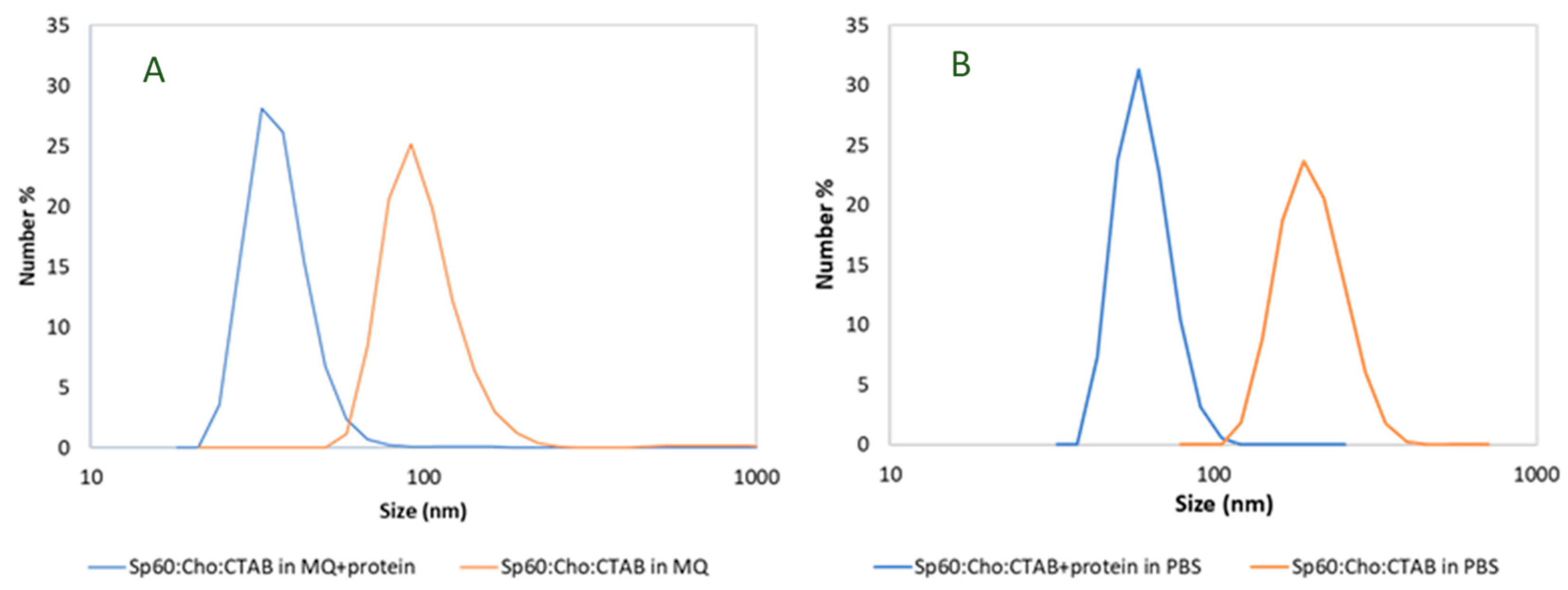
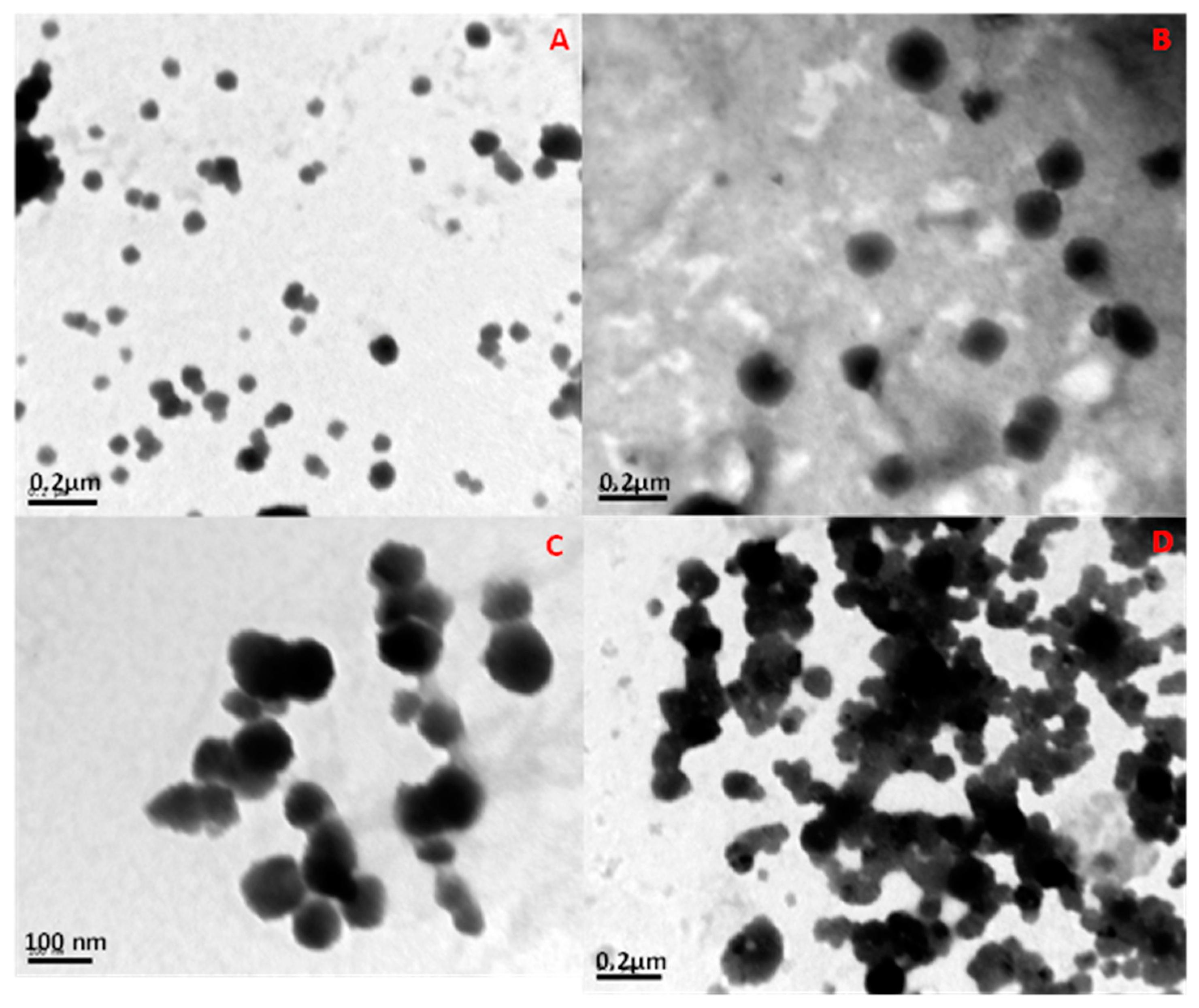
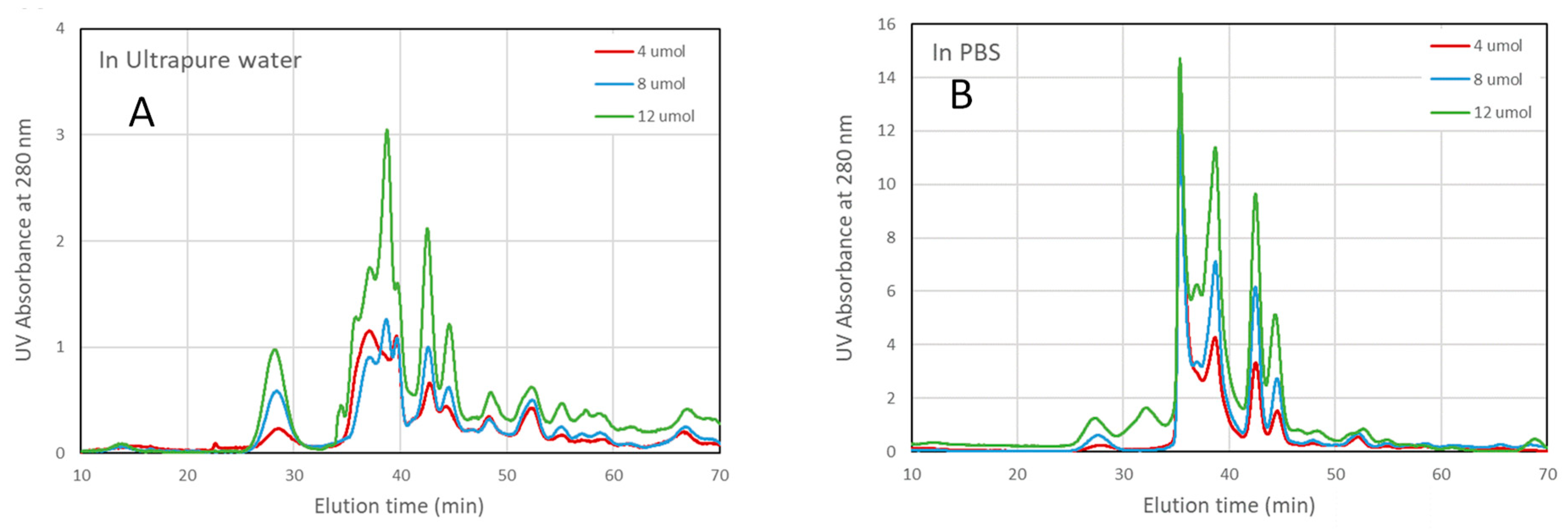
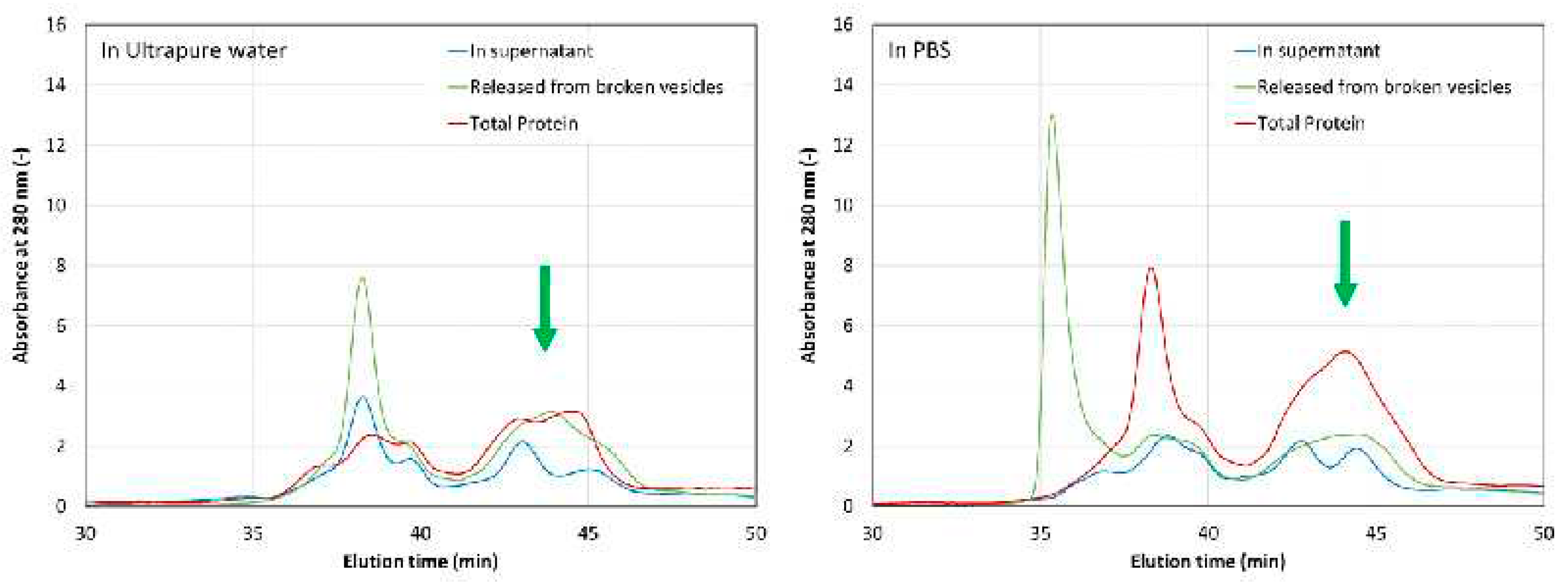

| Formulation | Aqueous phase | Size (nm) | Zeta potential (mV) |
| Sp60 : Cho : CTAB (no protein) | MQ water | 100±27 | 55±2 |
| Sp60 : Cho : CTAB + CHAPSH3b (8 µM) | MQ water | 38±18 | 46±5 |
| Sp60 : Cho : CTAB (no protein) | PBS buffer | 205±46 | 28±2 |
| Sp60 : Cho : CTAB + CHAPSH3b (8 µM) | PBS buffer | 77±21 | 30±4 |
| Time | Control | CHAPSH3b (8 µM) | Empty vesicles | CHAPSH3b (8 µM) loaded vesicles |
| 1 h | 8.42 ± 0.06 | 7.85 ± 0.02 | 5.27 ± 0.90 | 3.74 ± 1.71** |
| 2 h | 8.24 ± 0.13 | 7.28 ± 0.60 | 4.13 ± 3.60* | 3.90 ± 3.40** |
| 4 h | 7.62 ± 0.71 | 6.50 ± 0.46 | 1.33 ± 2.30**** | 0.00 ± 0.00**** |
| 6 h | 8.72 ± 0.58 | 7.32 ± 0.06 | 1.15 ± 1.99**** | 0.00 ± 0.00**** |
| 24 h | 6.81 ± 0.30 | 6.36 ± 0.68 | 3.22 ± 2.79* | 3.07 ± 2.66* |
| Incubation Time | Control | CHAPSH3b (8 μM) | CHAPSH3b (8 µM) loaded vesicles | Empty vesicles |
|---|---|---|---|---|
| 4 h | 8.6±0.30 | 5.18±0.17* | 0.00±0.00* | 0.00±0.00* |
| 24 h | 9.50±0.17 | 7.43±0.32* | 0.00±0.00* | 0.00±0.00* |
| After 14 days of storage | ||||
| 4 h | 9.02±0.12 | 8.57±0.22 | 0.00±0.00* | 0.00±0.00* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





