Introduction
Breast cancer (BC) remains the most frequent cause of cancer death among women worldwide, with over 685,000 deaths each year, accounting for almost 7% of global mortality due to cancer among female oncologic patients [
1].
Over 80% of BC related fatalities are due to development of therapy-resistant metastatic disease [
2].
In everyday clinical setting, assessment of predictive and prognostic factors relies mostly on tumor features such as size, lymph node involvement and extent of tumor spread and other histo-pathological characteristics including tumor grade, histologic type, proliferation index, biomarker expression and molecular expression profiles. These features have become core drivers in creation of prognostic algorithms (e.g., Adjuvant! Online) and indexes (e.g., the Nottingham Prognostic Index) [
3]. However, since increased diagnosis rate also meant increasing chemotherapy application, tools were necessary to mitigate this effect. Therefore, gene expression signatures were introduced into clinical practice to help guide decision for adjuvant chemotherapy [
4].
Oncotype DX Breast Recurrence Score (RS) is a genomic test based on an assay of 21 genes, with a prognostic and predictive value, initially designed for therapy planning in patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, lymph node-negative or lymph node-positive (1-3 nodes), early-stage breast cancer. The assay is designed to be able prognosticate recurrence of disease, according to increasing RS result and to be a predictive factor of chemotherapy (CHT) benefit in specific populations, as established through several clinical trials [
5,
6,
7,
8,
9].
The Oncotype DX was first published in 2004 and was implemented in clinics shortly thereafter. Its creation was based on existence of 250 genes from 447 different tumor samples from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-20 study [
10]. Sixteen genes were chosen just based on statistical association with recurrence rate, and 5 genes, responsible for housekeeping, were added to the statistical algorithm to generate a recurrence score between 0 and 100 [
11].
Data from TransATAC study on 785 ER-positive, HER-2 negative breast cancer biopsy samples showed that variation estimates of residual risk of distant recurrence between different genomic assays, including Oncotype DX, relies mostly on estrogen or proliferation gene profile. However, when compared to other available tests, Oncotype DX relies mostly (60%) on the estrogen module [
12]. Moreover, regarding its genetic module of proliferation genes, Oncotype DX uses a threshold, which makes the correlation between proliferation-related genes and its RS calculation more relevant in comparison to other signatures available on the market.
As mentioned, RS range was set between a value of 0 and 100, with higher scores representing a higher risk for disease recurrence. The RS categories were initially defined by the manufacturer as low (<18), intermediate (18−30) and high (>31), based on the results of the NSABP B-20 study [
13].
The Oncotype DX RS tool has been also subsequently validated in a retrospective analysis of tumor samples stored in the NSABP Tissue Bank from patients treated with tamoxifen in the NSABP trial B-14, a large prospective U.S. study [
14]. At 10 years, the findings showed 30.5% distant recurrence rate in the high-risk population (RS>31), significantly higher when compared with patients in the low risk (RS<18) and intermediate-risk groups, which had a recurrence rate of 6.8% and 14.3%, respectively. Based on these findings, Oncotype DX and other signatures have come to support chemotherapy administration in patients with high genomic risk [
10].
With the promotion and implementation of national screening programs across the U.S., starting in 2009, the incidence of early BC diagnosis, especially among ER-positive, HER2-negative tumors increased significantly. These tumors are usually associated with a 5-year survival rate of >94% and a recurrence rate of 15%, with unclear benefit of CHT [
15,
16]. Data analysis from the Surveillance, Epidemiology and End Results (SEER) on the impact of Oncotype DX testing on these tumors however, found an inverse correlation between the CHT administration and RS testing [
17]. RS testing increased between 2004 – 2015 from 1.5% to 34%, leading to a 6% drop in CHT applications, from 42% to 36%, among patients who underwent testing. Moreover, patients who tested for RS also tended to have improved survival when compared with those who did not, even after adjustment for clinical variables. Specific analysis indicated a reduction of approximately 80% in breast cancer-specific risk of death among those patients who underwent testing. Consistent with RS testing recommendations, the use of adjuvant CHT increased among patients in the high RS groups and decreased in the intermediate and low-risk groups. This analysis confirmed implementation of Oncotype DX testing as a risk stratification tool, especially for node negative tumors, among which its application remained double when compared to node-positive breast cancer patients [
17].
Since its introduction to clinical practice, the ASCO [
18], the National Comprehensive Cancer Network (NCCN) [
19], the European Society for Medical Oncology (ESMO) [
20], the National Institute for Health and Care Excellence [
21] and the St. Gallen Consensus Conference [
23] have included Oncotype DX testing into their breast cancer guidelines. In the U.S., Medicare started funding Oncotype DX screening in 2006. Ever since, this genomic assay has been validated through multiple prospective-retrospective, prospective non-randomized and randomized studies [
23].
One of the most relevant trials assessing benefit of CHT among specific subgroups within the predefined RS categories was the Trial Assigning Individualized Options for Treatment (TAILORx) study. In the low RS (0–10) cohort of TAILORx it was already established lack of benefit of CHT when added to endocrine therapy (Ref😊, while in the high RS (>25) category, addition of CHT was clearly associated with longer recurrence free periods. In the intermediate RS category (11–25) addition of CHT was rather uncertain and not clearly investigated.
Published in July 2018, TAILORx was a non-inferiority, prospective study in HR+/HER2- node negative patients aiming at answering benefit of CHT in intermediate RS.
In all patients, the study demonstrated no benefit of CHT in the intermediate RS category (RS 11−25) (n=6,711), in other words, endocrine therapy was non-inferior to chemo-endocrine therapy in terms of invasive disease-free survival (IDFS) (HR: 1.08 [95% CI: 0.94−1.24]; p=0.26). A further subgroup analysis emphasized a clear benefit of CHT among premenopausal women under 50 years of age, with the added IDFS benefit of 3.5% in the RS 11−15 subgroup, 9% in the RS 16−20 subgroup and 6.3% in the RS 21−25 subgroup [
6].
Subsequent analyses of TAILORx data assessed the role of further clinical-risk stratification for identifying patients who could benefit from additional CHT [
24]. It appears that in premenopausal women with intermediate RS and low clinical risk, defined as tumor ≤3 cm and G1, 1−3 cm and G2, or ≤1 cm and G3, the estimated risk for distant recurrence was lower with CHT. Intermediate RS correlated with a higher degree of relapse in the endocrine therapy alone group, irrespective of clinical risk. In patients with higher and lower clinical risk who were randomly assigned to endocrine or chemo-endocrine therapy and in patients with high RS (≥26) who received chemo-endocrine therapy, the clinical risk was found to be prognostic of distant recurrence independent of RS. These data confirmed that both RS and clinical risk have independent predictive value on recurrence rate and should be considered separately in age-specific groups. However, clinical risk was found to not be a predictor of CHT benefit and should therefore not be used to guide CHT decision.The treatment effect linked to chemotherapy in premenopausal women with intermediate RS is comparable to what has been seen with ovarian suppression combined with an aromatase inhibitor when compared to tamoxifen. Ref. Francis PA, Pagani O, Fleming GF, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med 2018;379:122-37
A secondary analysis of TAILORx evaluated the clinical outcome in 1,389 women with BC and high RS (26−100) who received chemo-endocrine therapy [
25].The 5-year DFS was 93%, which was better than expected with endocrine therapy alone. In this cohort, the distant recurrence rate with endocrine therapy alone was estimated by combining patient-specific distant recurrence risk with chemotherapy benefit information from the NSABP B20 trial [
5]. The expected DFS rates without chemotherapy were 78.8% and 65.4% at 5 years and 9 years, whereas in the overall population, DFS was 87.6% at 5 years and 86.8% at 9 years [
25].
In this study, we looked at how data derived from TAILORx analysis affected the daily clinical therapy decision-making process in a single oncology center from Basel, Switzerland, in order to determine if any significant changes in CHT application occurred especially in the intermediate RS, node negative BC patient cohort. Secondary endpoint was to determine leading therapy decision factors. We also looked at how tumor board (TB) decision deviated from standard recommendation as based on RS results. The changes we expected to observe were mainly based on lowering of RS thresholds and therefore on change in patient number.
Methods
This is a retrospective analysis of 326 estrogen receptor positive (ER+)/HER2 negative BC patients, treated at Basel University Hospital and Cantonal Hospital Baselland from 2010-2021. The study was approved by the local ethics committee (Ethics Committee Nord-West-Schweiz,
www.eknz.ch). Patient data was anonymized and collected in a password protected file [
26].
BC patients over 18 years of age, with a HR+/HER2 negative, node positive or node negative tumor diagnosed between 2010 and 2021, with a valid RS score on file, who underwent at least one form of therapy (endocrine therapy [ET], radiotherapy [RT] and/or chemoendocrine therapy [CHT-ET]) were eligible for this study. Patients with metastatic disease were not eligible.
Primary endpoint was to assess any change in therapy (CHT-ET vs. ET alone) in the BC population before July 2018 (cohort A) and after July 2018 (timepoint of the publication of TAILORx trial findings) (cohort B), when adjusted for RS category thresholds as defined by the manufacturer and as modified in the TAILORx study protocol. Secondary endpoint was to determine any change in therapy administration in the intermediate RS, as adjusted to the TAILORx criteria for the intermediate RS category. Lastly, we looked at the main factors affecting therapy decision making for BC patients in our centers, to determine how Oncotype DX RS influences TB decision and therapy implementation.
Our first hypothesis was that CHT use decreased in RS<26 in patients >50 years. The secondary hypothesis supposed the increased of CHT in patients <50 years RS 16-25. We supposed that among other clinicopathological factors, RS and results of TAILORx study affected significantly the decision on CHT use.
Descriptive statistics were used to summarize patient characteristics in the two cohorts and, were the sample size allowed, T-test for independent distribution analysis were conducted. Statistics were based on 95% confidence intervals (CI) and hypothesis testing was made at a two-sided alpha value of 0.05.
Chi square tests were used to check for associations between various demographic and histopathological characteristics and CHT-ET use. Point biserial correlation analysis was performed to check for correlations between CHT-ET, RS result and age in A and B and logistic multiple regression analyses were made to test for the impact of age, nodal status, tumor grade, tumor size, RS score category and Ki-67% on CHT-ET treatment in A and B.
Results
Patients’ Characteristics
Cohort A included 165 and cohort B 161 patients with a mean RS of 17.72 (SD 9.59) and 17.89 (SD 9.53) (p=0.87), respectively. Demographics and the tumor characteristics are presented in
Table 1. Patients’ mean age was similar in both cohorts (58.8 (SD 11.13) in A and 57.7 (SD 12.36) years in B), however cohort B had a significantly higher percentage of patients younger than 50 years when compared to cohort A (34% vs. 24%, p<0.01), which means that Oncotype test was performed to possibly omit chemotherapy. Significantly more patients in cohort A were overweight or had obesity (55%) vs. cohort B (40%) (p<0.001). Patients in cohort B had also slightly less comorbidities (45% vs. 39%), but the difference was not statistically significant (p=0.719;
Table 1).
Tumor Characteristics
Patients in Cohort A and B were different regarding percentage of tumor with high Ki-67. Cohort B had higher percentage of high Ki-67 tumors than cohort A (39% vs. 32%, p=0.010);
Table 1. Cohort A presented with higher percentage of pT1 tumors (55% in A vs. 51% in B), although not significant (p=0.927) and higher percentage of ≥pN2 stage (4% in A vs. 1% in B), however no significant differences in pN (p=0.546). Both cohorts had the same percentage of node-negative patients (59%) and similar other characteristics. Briefly, three-quarter of tumors were of non-special type (NST) histology, and more than half were of grade 2 (54% in A and 57% in B, p=0.811), with a mean Ki-67% expression of 19% (SD 0.009) in A and 20% in B (SD 0.11) (p=0.844). TNM stage was similar in both cohorts. Interestingly, 35% and 32% of patients (cohort A vs. cohort B, respectively) were of stage IA. Over 60% of patients in both cohorts had a stage II tumor, whereas 6% in A and 8% in B had stage III tumors (p=0.602).
Recurrence Score Results
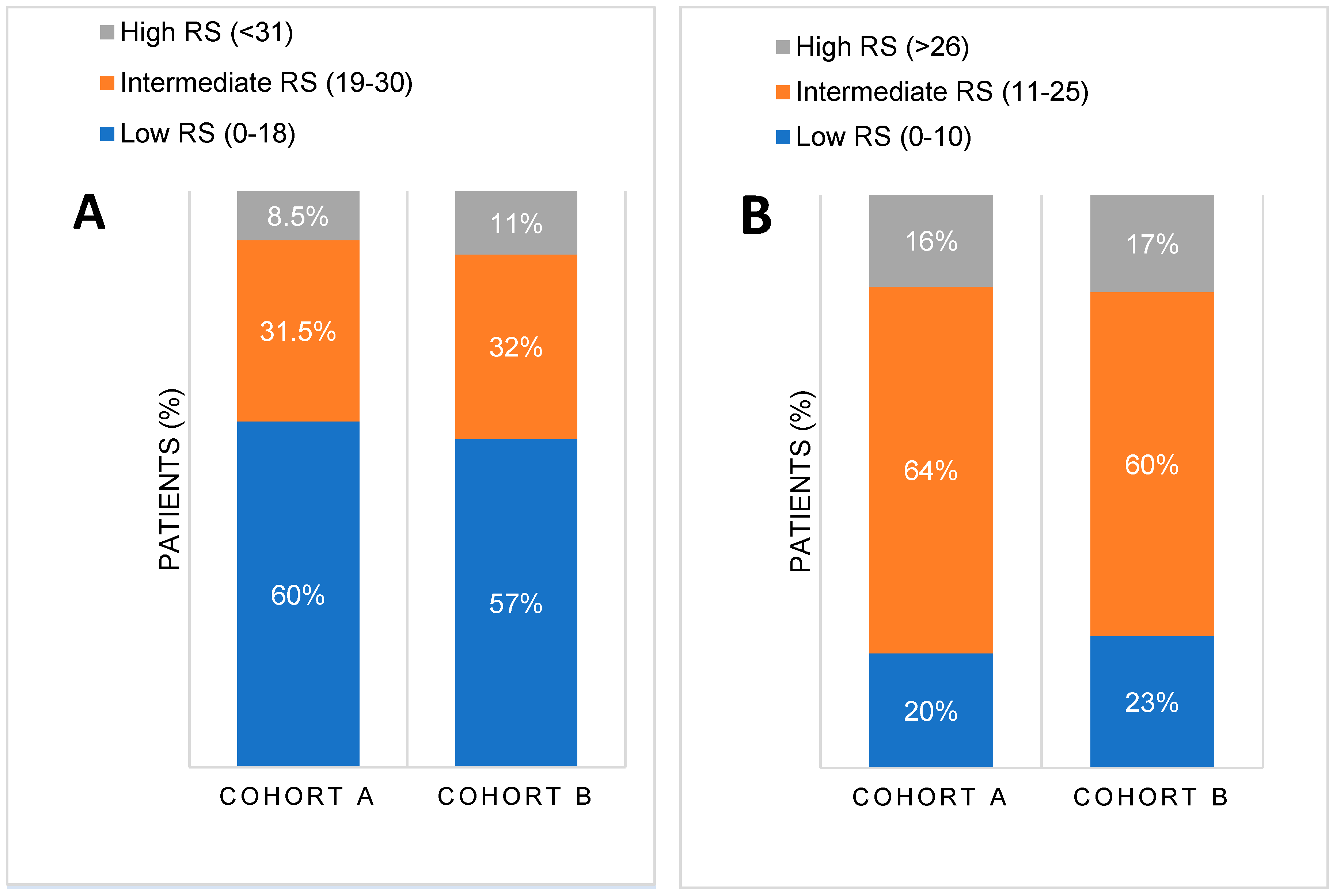
The mean RS was similar in both cohorts [17.72 (SD 9.59, IQR 10) in cohort A vs. 17.89 (SD 9.53, IQR 12) in cohort B, p=0.533]. There were no significant differences in RS distribution between the two cohorts when compared based on manufacturer’s thresholds (p=0.15 for low RS 0-18, p=0.833 for intermediate RS 19-30 and p=0.15 for high RS >31) or on TAILORx thresholds for RS categories (p=0.817 for low RS 0-10, p=0.199 for intermediate RS 11-25 and p=0.795 for high RS >26), as illustrated in
Figure 1. Importantly,
Figure 1 shows a shift in risk groups from low to intermediate one when new thresholds, based on TAILORx study results, were applied. According to the manufacturer's recommendations, up to 60% of patients had low RS (RS<18), while only around 20% had low RS according to TAILORx thresholds (RS<11). Conversely, according to the original manufacturer's guidelines, 8-10% were previously high risk (RS>30), but more (around 15%) were high risk (RS>25) according to TAILORx. Consequently, the number of patients in the intermediate risk category increased from 30% to over 60% when new RS threshold (11-25was applied.
Treatment Strategies
Majority of patients underwent breast conservative surgery (68% in A and 67% in B, p=0.352), and adjuvant radiotherapy (RT) (78% vs. 76%, A vs. B, resp., p=0.223), with a mean dose which was higher in A (54.99 Gy, SD 8.31) vs. B (52.35 Gy, SD 7.88), but not significant (p=0.417). More than half underwent reconstruction (60% in cohort A, 55% in B, p=0.463),
Table S1 of the Supplement. Treatment with ET and CHT-ET is presented in
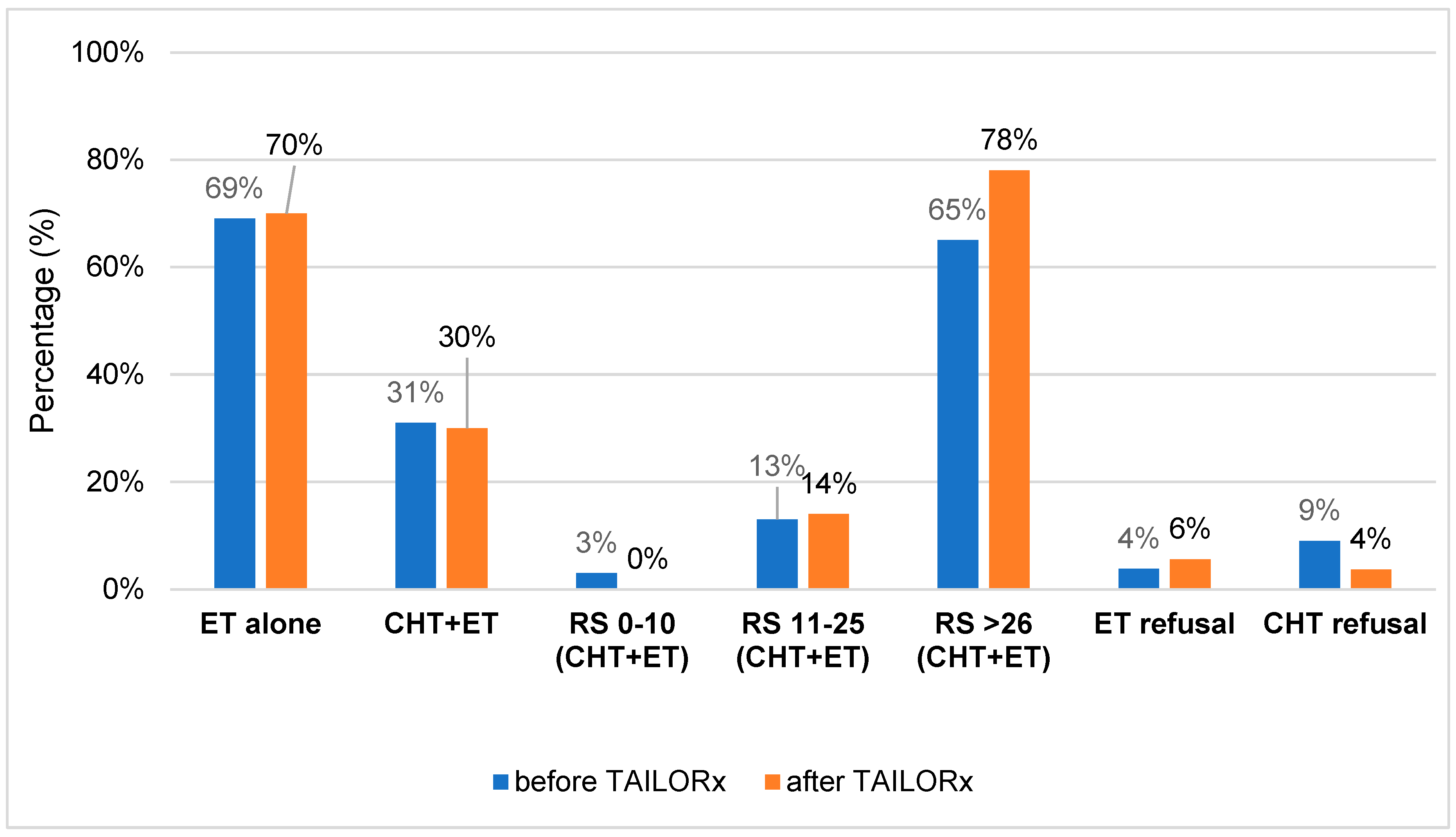
Figure 2 and
Table S1. To summarize, when comparing the percentage of patients treated with ET and CHT-ET pre- and post-TAILORx (in cohort A vs. cohort B), there was almost no difference. Around 70% of patients were treated with ET alone and 30% with CHT-ET. However, when comparing according to RS, it is obvious that no patient received CHT in low risk group vs. 3% (post- vs pre-TAILORx). CHT usage in RS 11-25 and RS ≥26 group increased by 1% and 13%, post- vs- pre-TAILORx, resp. CHT refusal was lower in cohort B. Ninety four percent of all patients were treated with ET, the majority of them with aromatase inhibitors (65.4% in A vs. 52% in B, p=0.113). Six patients (4%) in cohort A and 9 (6%) in cohort B refused ET.
Analysis of Chemoendocrine and Endocrine Therapy Use
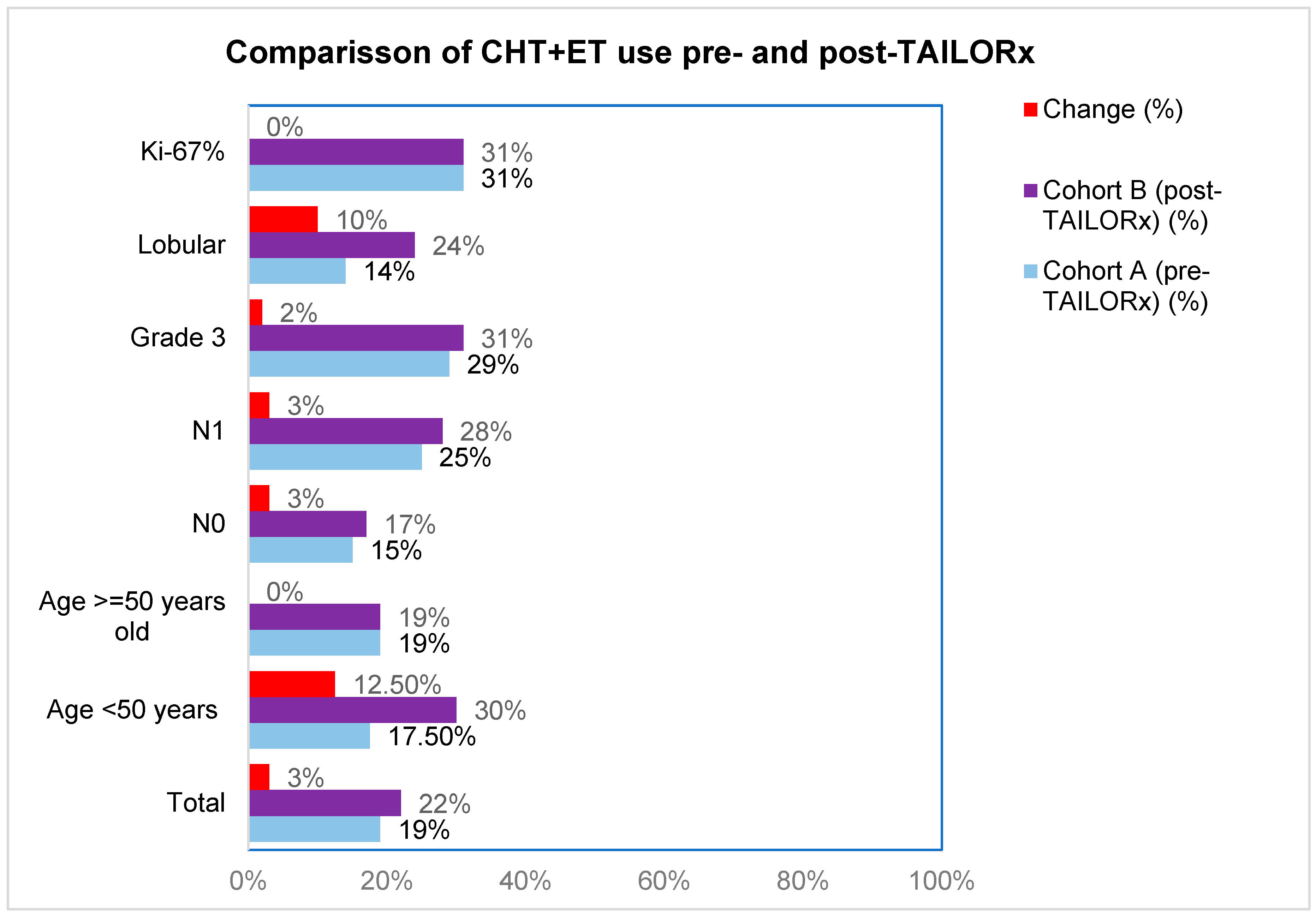
First, we analyzed differences in CHT-ET use between cohorts according to RS groups 0-10, 11-20, 21-25, 21-25, 26-20 and >30, in order to see which RS intervals were the mostly affected by the change brought about by TAILORx (
Table 2). The greatest change for less CHT use was in RS 21-25 (from 35% to 17%). We also analyzed the impact of other characteristics on CHT-ET use In cohort B, we observed an increase in use of CHT in patients <50 years by 12.5%, in lobular carcinoma by 10% and in node-negative, node-positive and grade 3 tumors by 2-3% (
Figure 3,
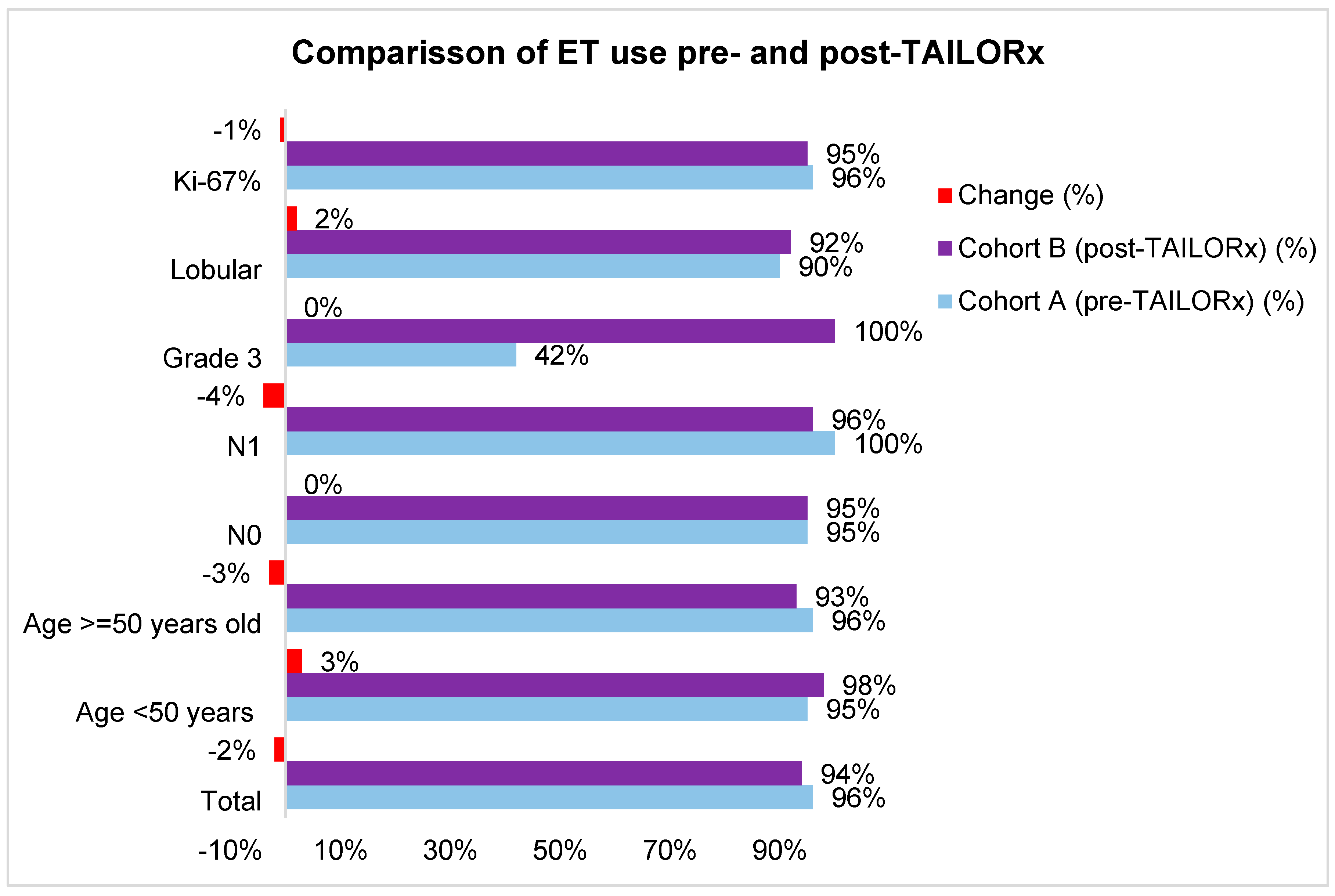
Table S2). The use of ET was high in both cohorts (96% in cohort A and 95% in cohort B). The difference according to tumor and patients’ characteristics were small (-4% to +3%), except for grade 3 tumors, where ET use increased by 58% (
Figure 4,
Table S2).
Implementation of RS Guidelines into TB Decision
Comparison of TB decision with RS guidelines post-TAILORx showed that in cohort A 90% (n=46/51) of patients, and in B 85% (n=41/48) of patients were assigned to CHT-ET. This speaks for a relative modest reduction of TB recommendation for CHT-ET, of only 5%. However, since in cohort B there are statistically more patients aged <50 years (54/161 patients in cohort B vs. 40/165 in cohort A, p<0.001), decision could have been affected by that. Consequently, TB decision for ET alone increased from 70% in cohort A to 73% in cohort B (
Table S1).
Implementation of TB Decision in Clinical Practice
The decision of the TB on optimal adjuvant systemic therapy (CHT-ET or ET) was not fully implemented. Implementation was numerically higher in cohort B vs. A (83% vs. 75%), but this was not statistically significant (p=0.522). However, implementation of CHT-ET in cohort B was significantly higher when compared to cohort A (73% vs. 61%, p<0.001). Recommendation of TB for ET was implemented in most cases (98% in A and 97% in B, p=0.824). All the relevant information pertaining to therapy administration in the two populations is presented in
Table S1.
Logistic Regression
In cohort A, decision for CHT administration was influenced by younger age (p=0.03), intermediate and/or high RS result (p<0.001), node positive status (p=0.003) and higher tumor stage (p=0.043). In cohort B on the other side, decision for CHT-ET was influenced only by younger age (p<0.001) and node positive status (p=0.013) (
Table S3).
Finally, in the entire population, logistic regression analysis of factors affecting CHT-ET administration shows that the model is significant Chi
2 (2) = 13, p<0.001 and that CHT-ET is significantly influenced by age
(p=0.001), pN status (p<0.001) and Oncotype RS score in all categories (low 0-10, intermediate 11-25, high ≥26, p<0.001), as shown in Table S4. Interestingly, it was not influenced by cohort.
Discussion
Our findings show a very modest reduction in TBrecommendation for therapy with CHT-ET from 31% before TAILORx to 30% in the post TAILORx era,. Recommendation for CHT increased in RS≥26.. According to logistic regression, RS group, nodal status and age significantly impact the decision for or against CHT administration. pur findings 10% less patients, than otherwise recommended by RS guidelines, were undertreated and did not receive chemotherapy in cohort A. However, since results from the TAILORx trial had not been published back then, one can argue that among 23 patients ≤ 50 years old with intermediate RS 16-25, a decision for chemotherapy at the moment would not have been necessarily warranted. This trend seems be maintained and deepened over time, as 85% of patients which should receive CHT-ET per RS guidelines, are actually recommended by TB to receive it in the post TAILORx cohort.
When selecting our data according to the RS categories, we observed a reduction of CHT-ET recommendation in the intermediate RS category 11-25 where we noted a 5% reduction from 22% (n=23) to 17% (n=16). However, due to high refusal rate in A (33% vs. 15% in B), CHT-ET was administered in 19% of the whole population in A, and in 25% in B, showing therefore no significant change in treatment applications over time (p=0.763). While a reduction of 18% (from 35% in cohort A to 17% in cohort B) was noted in the intermediate RS interval 21-25, it was balanced by an increase of 7% (from 6% in cohort A to 13% in cohort B) in CHT-ET application among patients with intermediate RS 11-20, contrary to other study findings, like those reported by Tesch et al. [
27]. However, an increase in CHT-ET was noted among patients with higher RS (>30), where CHT applications raised from 57% to 78%, a finding which was also reported across several other similar studies [
27].
Moreover, there was no relevant change in CHT-ET administration to be reported according to age under or equal to 50 years old (p=0.59) or over 51 years old (p=0.066), although we noted an increase of 12.5% in CHT-ET administration from A to B among patients under 50 years of age. Among older patients (>50 years of age), an equal percentage of CHT-ET (19%) was administered in A and in B, confirming maintenance of older practices in this age group. When analyzing according to age 50 or younger and 51 and older, age did not significantly affect CHT-ET application in A (p=0.835) nor B (p=0.552), but older age had more of an impact (p=0.009 in A and p<0.001 in B). This should explain why older patients tended to ET be spared the burden of CHT. RS result was however significant when deciding for CHT among younger women in A (p=0.009) and in B (p=0.001), which can explain the relative (+12.5%) increase in CHT-ET applications among younger women.
Interestingly, our logistic regression analysis looking at possible factors which might have influenced CHT-ET applications showed a significant impact of age, when not split for under or over 50 years of age (p=0.03 in A and p<0.001 in B) and nodal status (p=0.034 in A and p=0.013 in B). Also, physicians before TAILORx seemed to have been driven more by tumor size (p=0.043) and contrary to expectations, by intermediate and high RS result (p<0.001) than after TAILORx publication (p=0.678).
In terms of other treatments, there are no differences in surgery (68.4% in A and 67% in B, p=0.114), lack of surgical breast reconstruction (52% in A, 48.4% in B, p=0.253), ET (94% in A vs. 94.4%% in p=0.229), radiotherapy (78% in A and 76% in B, p=0.223) and osteo-oncologic therapy (41% of patients in A and 45% in B, p=0.695).
Our finding did show a slight reduction of 3% in CHT-ET TB recommendation after TAILORx and due to more patient compliance also a broader implementation of TB recommendations from 67% in A to 85% in B (p<0.001).
Our analysis did show a trend of undertreatment among older Swiss BC patients, which seems to be maintained in the post TAILORx era and which seems to be due to an interplay of patient refusal and personalized medicine.
When compared to the population selected for the TAILORx study, median age in our cohorts was 59 years old (SD 11.73, 29-85), slightly older than in TAILORx (55-28 years old) [
27], with 74% being postmenopausal patients vs. TAILORx where postmenopausal patients accounted for 64% to 71% [
28]. Results are also comparable to those reported in a similar prospective study conducted in Brazil. Only 9% of our patients had grade 1 tumors vs. 26% in TAILORx, a finding similar to others reported in similar analyses. Average tumor size in our cohort was also larger than that reported in TAILORx (2.5 cm vs. 1.7 cm in TAILORx cohort), while nodal status was positive for 40% of patients, similar to the cohort analysed by Mattar et al. in which 32% of tumors were node positive [
28]. This may explain the relative maintenance of therapy practices before and after TAILORx publication, however it speaks against the decision to recommend less CHT-ET than otherwise recommended by RS based guidelines.
Our results also contradict those reported in the Italian study conducted by Cognetti et al. which rshowed significant changes in treatment recommendation for 1683 patients after physicians were presented with RS result. As such, CHT-ET recommendation dropped by 51%, while hormone therapy increased by 35%. According to these findings, 12% of patients would have otherwise been undertreated and 49% of patients assigned for CHT-ET would have been overtreated [
29]. In our study, 10% of patients in A and 15% in B would have been, according to TB decision, undertreated, however these results are further inflated through patient refusal, so that 39% patients in A and 33% in B were in fact, according to RS guidelines, undertreated. A tendency of undertreatment in older BC patients, a is increasing, the fact that need inclusion of geriatric assessment ad patient informing about benefits of treatment in everyday practice. HT-ET administration in our node positive cohort seems to be similar to that reported by Cognetti et al. (28%). Similar results are reported when CHT is analyzed in lobular BC (23% vs. 24% in our B cohort) and in women under 51 years old (32% vs. 30% in our B cohort). However, results differ for older patients with much less CHT applications in our population (19% vs. 32% in the Italian cohort), grade 3 tumors (31% vs. 53% in results reported by Cognetti et al.) and Ki-67 (31% in our B cohort vs. 40% in the Italian study) [
29].
Mattar et al. also report a significant reduction in CHT-ET recommendation of 66% at two hospitals in Brazil, and compare it to other results reported in Europe (38% reduction) and Mexico (28%). However, this is prospective study conducted by means of survey with 6 licensed oncologists before and after knowing RS results. Per design itself, physician decision might be biased [
29].
On the contrary, by being a retrospective analysis looking at interdisciplinary TB decision in a relatively heterogenous BC patient population, our analysis reflects more the everyday clinical reality pertaining to Swiss patients and physicians.
The strenght of our study…Our institution has long tradition of experience with Oncotype DX test. Here we present data on over 350 patients, treated at a single oncological center divided between two hospitals in a relatively homogenous population – mostly white, postmenopausal women. While this might reflect the realities of the region, it might not be representative for the entire Swiss BC population. It provides us with a realistic information of what is actually happening in the daily clinical setting especially at interdisciplinary tumor board meetings
The limitations of our study is the retrospective design. However, contrary to most findings the Swiss BC population does not seem to be plagued by overtreatment, which might point to better financial practices among Swiss oncologists.
Another limitation is driven from the relatively short follow up period, which prevented us for analyzing the outcome of non-compliance to TB recommendation. For study validation, such analysis is highly recommended and should be pursued in the future. Future implications: to analyse the performing of Oncotype in N+ patients, similar to Rx PONDER study……
Conclusion
Our analysis showed maintenance of therapy practices and recommendations in our Swiss cohort post TAILORx publication, with relative tendency for undertreatment especially when considering patients over 50 years of age. When looking at the intermediate RS category there are several changes that emerged post TAILORx, most of them stemming from the simple shift in threshold for each category, with relative increase in CHT-ET administration in RS 11-20, but decrease in patients with RS 21-25.
According to new RS categories, there is a 40% reduction in the low RS category, with redistribution of patients in the intermediate RS which gains 32.5% and high RS category which gains 8% more patients, findings which resemble those reported in the literature.
Post TAILORx there is a slight reduction in CH-ET recommendation according to TB, and CHT-ET application, however in our Swiss cohort about 10-15% of patients remain undertreated based solely on TB decision. When accounting for patient refusal the difference becomes even more poignant with 61% concordance in CHT-ET administration and RS based recommendation before TAILORx publication and 73% after TAILORx publication. RS result seems to influence CHT application in patients over and under 50 years of age, as does age over 50 independently. More specifically, in patients over 50 years of age, CHT-ET remains relatively unchanged, while CHT in younger patients raises by 12.5%. Overall, when not divided in categories ( ≤ 50 years old and ≥ 51 years old), age and nodal status seem to be important factors when deciding therapy, before, as well as after TAILORx publication.
Our findings point to a tendency for undertreatment of Swiss BC patients, which are similar to those reported in an Italian prospective study conducted on 1724 patients [
29], however no significant change in CHT-ET clinical recommendation and application, especially among patients over 51 years of age.
This points to the fact that despite TAILORx results, decision for or against CHT-ET among Swiss BC oncologists is rather personalized to each case and taken in an interdisciplinary fashion, something which was not reported in previous studies that focused more on the decision of medical oncology physicians. While this points to an undertreatment trend in our cohort, with all its implications – less toxicity for patients, less financial burden, higher risk - it raises questions about what is it that influences TB to decide against CHT-ET, when should they reconsider and what are the practical implications for patient survival and event free survival, for which a separate analysis of this cohort is highly recommended.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. [CrossRef]
- Quaglino E, Conti L, Cavallo F. Breast cancer stem cell antigens as targets for immunotherapy. Semin Immunol. 2020;47:101386. [CrossRef]
- Rakha EA, Ellis IO. Modern classification of breast cancer: should we stick with morphology or convert to molecular profile characteristics. Adv Anat Pathol. 2011;18(4):255-67. [CrossRef]
- Sestak I, Cuzick J, Dowsett M, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol. 2015;33(8):916-22. [CrossRef]
- P. B. Fisher et al., “Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: Long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials,” Lancet, vol. 364, no. 9437, pp. 858–868, Sep. 2004. [CrossRef]
- J. A. Sparano et al., “Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer.,” N. Engl. J. Med., vol. 379, no. 2, pp. 111–121, Jul. 2018. [CrossRef]
- F. Cardoso et al., “70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer.,” N. Engl. J. Med., vol. 375, no. 8, pp. 717–29, Aug. 2016. [CrossRef]
- F. Fitzal et al., “The genomic expression test EndoPredict is a prognostic tool for identifying risk of local recurrence in postmenopausal endocrine receptor-positive, her2neu-negative breast cancer patients randomised within the prospective ABCSG 8 trial,” Br. J. Cancer, vol. 112, no. 8, pp. 1405–1410, Apr. 2015. [CrossRef]
- I. Sestak et al., “Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk,” J. Clin. Oncol., vol. 33, no. 8, pp. 916–22, Mar. 2015. [CrossRef]
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817-26. [CrossRef]
- Matikas A, Foukakis T, Swain S, et al. Avoiding over- and undertreatment in patients with resected node-positive breast cancer with the use of gene expression signatures: are we there yet? Ann Oncol. 2019;30(7):1044-1050. [CrossRef]
- Buus R, Sestak I, Kronenwett R, et al. Molecular Drivers of Oncotype DX, Prosigna, EndoPredict, and the Breast Cancer Index: A TransATAC Study. J Clin Oncol. 2021;39(2):126-135. [CrossRef]
- Fisher B, Dignam J, Wolmark N, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89(22):1673-82. [CrossRef]
- Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93(9):684-90. [CrossRef]
- Cancer Stat Facts: Female Breast Cancer Subtypes. [Accessed April 2023]. Available from: https://seer.cancer.gov/statfacts/html/breast-subtypes.html.
- Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432-44. [CrossRef]
- Schaafsma E, Zhang B, Schaafsma M, et al. Impact of Oncotype DX testing on ER+ breast cancer treatment and survival in the first decade of use. Breast Cancer Res. 2021;23(1):74. [CrossRef]
- Andre F, Ismaila N, Allison KH, et al. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J Clin Oncol. 2022;40(16):1816-1837. [CrossRef]
- Telli ML, Gradishar WJ, Ward JH. NCCN Guidelines Updates: Breast Cancer. J Natl Compr Canc Netw. 2019;17(5.5):552-555.
- Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194-1220. [CrossRef]
- Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer. National Institute for Health and Clinical Excellence (NICE) 2018. [Accessed April 2023]. Available from: https://www.nice.org.uk/guidance/dg34/resources/tumour-profiling-tests-to-guide-adjuvant-chemotherapy-decisions-in-early-breast-cancer-pdf-1053750722245.
- Burstein HJ, Curigliano G, Thürlimann B, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32(10):1216-1235. [CrossRef]
- Kizy S, Altman AM, Marmor S, et al. 21-gene recurrence score testing in the older population with estrogen receptor-positive breast cancer. J Geriatr Oncol. 2019;10(2):322-329. [CrossRef]
- Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and Genomic Risk to Guide the Use of Adjuvant Therapy for Breast Cancer. N Engl J Med. 2019;380(25):2395-2405. [CrossRef]
- Sparano JA, Gray RJ, Makower DF, et al. Clinical Outcomes in Early Breast Cancer With a High 21-Gene Recurrence Score of 26 to 100 Assigned to Adjuvant Chemotherapy Plus Endocrine Therapy: A Secondary Analysis of the TAILORx Randomized Clinical Trial. JAMA Oncol. 2020;6(3):367-374. [CrossRef]
- Chiru ED, Grasic Kuhar C, Oseledchyk A, et al. SIOG2022-0204 - 21-Gene Oncotype DXRecurrence-Score benefits and application in elderly breast cancer patients. J Geriatr Oncol. 2022;13(8, Supplement 1):S18-S19. [CrossRef]
- Tesch ME, Speers C, Diocee RM, Gondara L, Peacock SJ, Nichol A, Lohrisch CA. Impact of TAILORx on chemotherapy prescribing and 21-gene recurrence score-guided treatment costs in a population-based cohort of patients with breast cancer. Cancer. 2022 Feb 15;128(4):665-674. [CrossRef] [PubMed]
- Mattar A, Fonseca GR, Romão MBA, Shida JY, de Oliveira VM, Bastos MCS, Bagnoli F, Rinaldi JF, Stiepcich MMÁ, da Silva MALG, Jakubowski DM, Chao C, Oliveira SC, Gebrim LH. Substantial Reduction in Adjuvant Chemotherapy With the Use of the 21-Gene Test to Manage Early Breast Cancer in a Public Hospital in Brazil. JCO Glob Oncol. 2021 Jun;7:1003-1011. [CrossRef]
- Cognetti F, Masetti R, Fabi A, Bianchi G, Santini D, Rognone A, Catania G, Angelucci D, Naso G, Giuliano M, Vassalli L, Vici P, Scognamiglio G, Generali D, Zambelli A, Colleoni M, Tinterri C, Scanzi F, Vigna L, Scavina P, Gamucci T, Marrazzo E, Scinto AF, Berardi R, Fabbri MA, Pinotti G, Franco D, Terribile DA, Tonini G, Cianniello D, Barni S. PONDx: real-life utilization and decision impact of the 21-gene assay on clinical practice in Italy. NPJ Breast Cancer. 2021 May 5;7(1):47. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).