Submitted:
02 November 2023
Posted:
03 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Population.
2.2. Study Design
2.3. Disease Activity
2.4. Pulmonary Function Tests
2.5. FeNO and CANO Measurements
2.6. CRP Collection
2.7. Endpoints and Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Participants
3.2. Pulmonary Function Tests
3.3. FeNO Measurements
3.4. Lung Function, FeNO, CRP, and Correlation with Clinical Disease Activity
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernstein CN, Blanchard JF, et al. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol 2001, Apr;96(4):1116-22. [CrossRef]
- Nikolaus S, Schreiber S. Diagnostics of Inflammatory Bowel Disease. Gastroenterology 2007, 133:1670–1689. [CrossRef]
- Rothfuss KS, Stange EF, et al. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol. 2006, 12:4819-4831. [CrossRef]
- Georgakopoulou V.E, Tarantinos K, et al. Role of pulmonary function testing in inflammatory bowel diseases (Review). Med Int (Lond). 2022, Jul-Aug; 2(4): 25. [CrossRef]
- Herrlinger KR, Noftz MK, et al. Alterations in pulmonary function in inflammatory bowel disease are frequent and persist during remission. Am J Gastroenterol, 2002, 97:377-81. [CrossRef]
- Ragnoli B, Radaeli A, et al. Fractional nitric oxide measurement in exhaled air (FeNO): perspectives in the management of respiratory diseases. Ther Adv Chronic Dis 2023, Vol. 14: 1–17. [CrossRef]
- ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. American Journal of Respiratory and Critical Care Medicine, 2005. [CrossRef]
- Horváth I, Barnes PJ, et al. A european respiratory society technical standard: exhaled biomarkers in lung disease. Eur Respir J 2017, 49: 1600965. [CrossRef]
- Högman M, Malinovschi A, et al. Added value with extended NO analysis in atopy and asthma. Clin Physiol Funct Imaging 2011, 31:294–299. [CrossRef]
- George SC, Hogman M, et al. Modeling pulmonary nitric oxide exchange. J Appl Physiol (1985) 2004, 96:831-9. [CrossRef]
- Lewis JD, Chuai S, et al. Use of the noninvasive components of the mayo score to assess clinical response in Ulcerative Colitis. Inflamm Bowel Dis 2008, 14(12):1660–1666. [CrossRef]
- Miller, M.R.; Hankinson, J. et al. Standardization of spirometry. Eur. Respir. J. 2005, 26, 319–338.
- Wanger J, Clausen JL, et al. Standardization of the measurement of lung volumes. Eur Respir J. 2005, 26: 511–522. [CrossRef]
- MacIntyre N, Crapo RO, et al. Standardization of the single breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005, 26: 720– 735. [CrossRef]
- Marrades RM, Diaz O, et al. Adjustment of DLCO for hemoglobin concentration. Am J Respir Crit Care Med 1997, 155: 236–241. [CrossRef]
- de Jongste JC, Merkus PJFM, et al. Lung diffusing capacity; in Hammer J, Eber E (eds): Paediatric Pulmonary Function Testing. Progr Respir Res. Basel, Karger, 2005, vol 33, pp 157–165.
- Nikolaus M, Tsoukias M, et al. A two-compartment model of pulmonary nitric oxide exchange dynamics. Appl Physiol 1998, 85(2):65366. [CrossRef]
- Olivieri M, Talamini G, et al. Reference values for exhaled nitric oxide (reveno) study. Respir Res 2006, 7: 94. [CrossRef]
- Olin A-C, Bake B et al. Fraction of exhaled nitric oxide at 50 mL/s: reference values for adult lifelong never-smokers. Chest 2007, 131: 1852–1856. [CrossRef]
- Kovesi T, Kulka R et al. Exhaled nitric oxide concentration is affected by age, height, and race in healthy 9- to 12-year-old children. Chest 2008, 133: 169–175. [CrossRef]
- Dweik, R.A.; Boggs, P.B. et al. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [CrossRef]
- Malerba M, Damiani G, et al. Values in elderly people for exhaled nitric oxide study. Rejuvenation Res 2016, 19: 233–238. [CrossRef]
- See KC and Christiani DC. Normal values and thresholds for the clinical interpretation of exhaled nitric oxide levels in the US general population: results from the national health and nutrition examination survey 2007-2010. Chest 2013, 143: 107–116. [CrossRef]
- Guida G, Bagnasco D, et al. Critical evaluation of asthma biomarkers in clinical practice. Front Med 2022, 9: 969243. [CrossRef]
- Storch I, Sachar D et al. Pulmonary manifestations of inflammatory bowel disease. Inflamm Bowel Dis 2003, 9: 104-115. [CrossRef]
- Songür N, Songür Y, et al. Pulmonary function tests and high-resolution CT in the detection of pulmonary involvement in inflammatory bowel disease. J Clin Gastroenterol 2003, 37: 292-298. [CrossRef]
- Black H, Mendoza M, et al. Thoracic manifestations of inflammatory bowel disease. Chest 2007, 131:524-32. [CrossRef]
- Gupta SJ, Gupta VL, et al. Assessment of occult pulmonary involvement in ulcerative colitis. Inflamm Intest Dis 2020, 5: 144-150. [CrossRef]
- Godet PG, Cowie R, et al. Pulmonary function abnormalities in patients with ulcerative colitis. Am J Gastroenterol. 1997, 92:1154-1156.
- Dierkes-Globisch A and Mohr H: Pulmonary function abnormalities in respiratory asymptomatic patients with inflammatory bowel disease. Eur J Intern Med 2002, 13: 385. [CrossRef]
- Mohamed-Hussein AA, Mohamed NA et al. Changes in pulmonary function in patients with ulcerative colitis. Respir Med 2007, 101: 977-982. [CrossRef]
- Ji XQ, Wang LX et al. Pulmonary manifestations of inflammatory bowel disease. World J Gastroenterol 2014, 20:13501-13511. [CrossRef]
- Zhao Y, Wang J, et al. Pulmonary dysfunction in 114 patients with inflammatory bowel disease. Medicine (Baltimore) 2017, 96: e6808. [CrossRef]
- Yilmaz A, Yilmaz Demirci N, et al. A Pulmonary involvement in inflammatory bowel disease. World J Gastroenterol 2010, 16: 4952-4957. [CrossRef]
- Ellrichmann M, Bethge J, et al. Subclinical pulmonary involvement in active IBD responds to biologic therapy. J Crohns Colitis 2021, 15: 1339-1345. [CrossRef]
- Tunc B, Filik L, et al. Pulmonary function tests, high-resolution computed tomography findings and inflammatory bowel disease. Acta Gastroenterol Belg 2006, 69: 255-260.
- Wallaert B, Colombel JF, et al. Evidence of lymphocyte alveolitis in Crohn's disease. Chest 1985, 87: 363-367. [CrossRef]
- Lee MK, Yoon HK, et al. Nonspecific bronchoprovocation test. Tuberc Respir Dis (Seoul) 2017, 80: 344-350. [CrossRef]
- Chatzimichael A and Paraskakis E: Elevated levels of alveolar nitric oxide may indicate presence of small airway inflammation in patients with inflammatory bowel disease. Lung 2019, 197: 663-670. [CrossRef]
- Christie PM and Hill GL: Effect of intravenous nutrition on nutrition and function in acute attacks of inflammatory bowel disease. Gastroenterology 1990, 90: 730-736. [CrossRef]
- Tzanakis NE, Tsiligianni IG, et al. Pulmonary involvement and allergic disorders in inflammatory bowel disease. World J Gastroenterol 2010, 16:299–305. [CrossRef]
- Keely S, Talley NJ, et al. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol 2012, 5:7–18. [CrossRef]
- D’Andrea N, Vigliarolo R, et al. Respiratory involvement in inflammatory bowel diseases. Multidiscip Respir Med 2010, 5:173–82. [CrossRef]
- Fireman E, Masarwy F, et al. Induced sputum eosinophilia in ulcerative colitis patients: the lung as a mirror image of intestine? Respir Med 2009, 103:1025–32. [CrossRef]
- Marvisi M, Borrello PD, Brianti M, et al. Changes in the carbon monoxide diffusing capacity of the lung in ulcerative colitis. Eur Respir J. 2000, 16:965-968. [CrossRef]
- Sethy PK, Dutta U, et al. Pulmonary and hematological alterations in idiopathic ulcerative colitis. Indian J Gastroenterol 2003, 22: 176-179. [PubMed]
- Ateş F, Karincaoğlu M, Hacievlıyagıl SS, et al. Alterations in the pulmonary function tests of inflammatory bowel diseases. Turk J Gastroenterol 2011, 22: 293-299. [CrossRef]
- Xavier RJ, Podolsky DK: Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448: 427–434. [CrossRef]
- Abraham C, Cho JH: Inflammatory bowel disease. Mechanisms of disease. N Engl J Med 2009, 361: 2066–2078.
- Salzman NH, Bevins CL: Negative interactions with the microbiota: IBD. Adv Exp Med Biol 2008, 635: 67–78. [CrossRef]
- Salmi M, Jalkanen S: Lymphocyte homing to the gut: attraction, adhesion, and commitment. Immunol Rev 2005; 206: 100–113. [CrossRef]
- Keely S, Talley NJ, Hansbro PM: Pulmonary intestinal cross-talk in mucosal inflammatory disease. Immunology 2012; 5: 7–18. [CrossRef]
- Ozyilmaz E, Yildirim B, et al. Is there any link between oxidative stress and lung involvement due to inflammatory bowel disease: an experimental study. Hepatogastroenterology 2011;58: 1898–903. [CrossRef]
- Lundberg JO, Hellstrom PM, et al. Greatly increased luminal nitric oxide in ulcerative colitis. Lancet 1994; 344(8938): 1673-74. [CrossRef]
- Koek GH, Verleden GM, et al. Activity related increase of exhaled nitric oxide in Crohn's disease and ulcerative colitis: A manifestation of systemic involvement? Respir Med 2002 96: 530-535. [CrossRef]
- Malerba M, Ragnoli B. Exhaled nitric oxide as a marker of lung involvement in Crohn’s disease. International Journal of Immunology and Immunopathology. 2011, 24(4):1119-1124. [CrossRef]
- Quenon L, Hindryckx P, et al. Hand-held fractional exhaled nitric oxide measurements as a non-invasive indicator of systemic inflammation in Crohn's disease. J Crohns Colitis 2013, 7: 644-648. [CrossRef]
- Ozyilmaz E, Yildirim B, et al. Value of fractional exhaled nitric oxide (FE NO) for the diagnosis of pulmonary involvement due to inflammatory bowel disease. Inflamm Bowel Dis 2010, 16: 670-676. [CrossRef]
- Ikonomi E, Rothstein RD, et al. Measurement of fractional exhaled nitric oxide as a marker of disease activity in inflammatory bowel disease. J Gastroenterol Pancreatol Liver Disord 2016. 3. [CrossRef]
- Protopapas AA, Vradelis S, et al. Elevated levels of alveolar nitric oxide may indicate presence of small airway inflammation in patients with inflammatory bowel disease. Lung 2019, 197: 663-670. [CrossRef]
- Paraskakis E, Brindicci C, et alMeasurement of bronchial and alveolar nitric oxide production in normal children and children with asthma. Am J Respir Crit Care Med. 2006, 174(3):260–267. [CrossRef]
- Lázár Z, Horváth P, et al. A suitable protocol for measuring alveolar nitric oxide in asthma with differing severity to assess peripheral airways inflammation. J Asthma 2019; 56: 584–593. [CrossRef]
- Berry M, Hargadon B, et al. Alveolar nitric oxide in adults with asthma: evidence of distal lung inflammation in refractory asthma. Eur Respir J 2005, 25(6):986–991. [CrossRef]
- van Veen IH, Sterk PJ, et al. Alveolar nitric oxide versus measures of peripheral airway dysfunction in severe asthma. Eur Respir J 2006, 27(5):951–956. [CrossRef]
- Williamson PA, Clearie K, et al. Assessment of small-airways disease using alveolar nitric oxide and impulse oscillometry in asthma and COPD. Lung 2011, 189(2):121–129. [CrossRef]
- Suri R, Paraskakis E, et alAlveolar, but not bronchial nitric oxide production is elevated in cystic fibrosis. Pediatr Pulmonol. 2007, 42(12):1215–1221. [CrossRef]
- Brindicci C, Ito K, et al. Exhaled nitric oxide from lung periphery is increased in COPD. Eur Respir J 2005, 26(1):52–59. [CrossRef]
- Lázár Z, Kelemen Gálffy G, et al. Central and peripheral airway nitric oxide in patients with stable and exacerbated chronic obstructive pulmonary disease. J Breath Res 2018; 12: 036017. [CrossRef]
- Cameli P, Bargagli E, et al. Extended exhaled nitric oxide analysis in interstitial lung diseases: a systematic review. Int J Mol Sci 2020; 21: 6187. [CrossRef]
- Malerba M, Ragnoli B et al. Exhaled nitric oxide levels in alpha-1-antitrypsin PiMZ subjects. J Intern Med 2009; 265: 382–387. [CrossRef]
- Vincken S, Sylvia V, et al. The role of FeNO in stable COPD patients with eosinophilic airway inflammation. Respir Med 2021; 181: 106377. [CrossRef]
- Yamaji Y, Oishi K, et al. Detection of type 2 biomarkers for response in COPD. J Breath Res 2020; 14: 026007. [CrossRef]
- Malerba M, Radaeli A, et al. Exhaled Nitric Oxide as a Biomarker in COPD and Related Comorbidities. BioMed Res Int 2014;2014: 271918. [CrossRef]
| Variable | All n= 83 | UC n=42 | Controls n=41 |
|---|---|---|---|
| Age, years (IQR) | 32 (28-37) | 36 (31-43) | 29 (27-32) |
| Male sex, n (%) | 39 (46.9) | 22 (52.4) | 17 (41.4) |
| BMI, (IQR) | 22.7 (21-24.2) | 22 (20.7-24.9) | 22.8 (21.7-23.8) |
| Disease activity, n (%) | 42 (100) | ||
| Mild (1) | 3 (7.1) | ||
| Intermediate (2) | 13 (31) | ||
| Severe (3) | 26 (61.9) | ||
| Variable | All n= 83 | UC n=42 | Controls n=41 | p* |
|---|---|---|---|---|
| Lung function | ||||
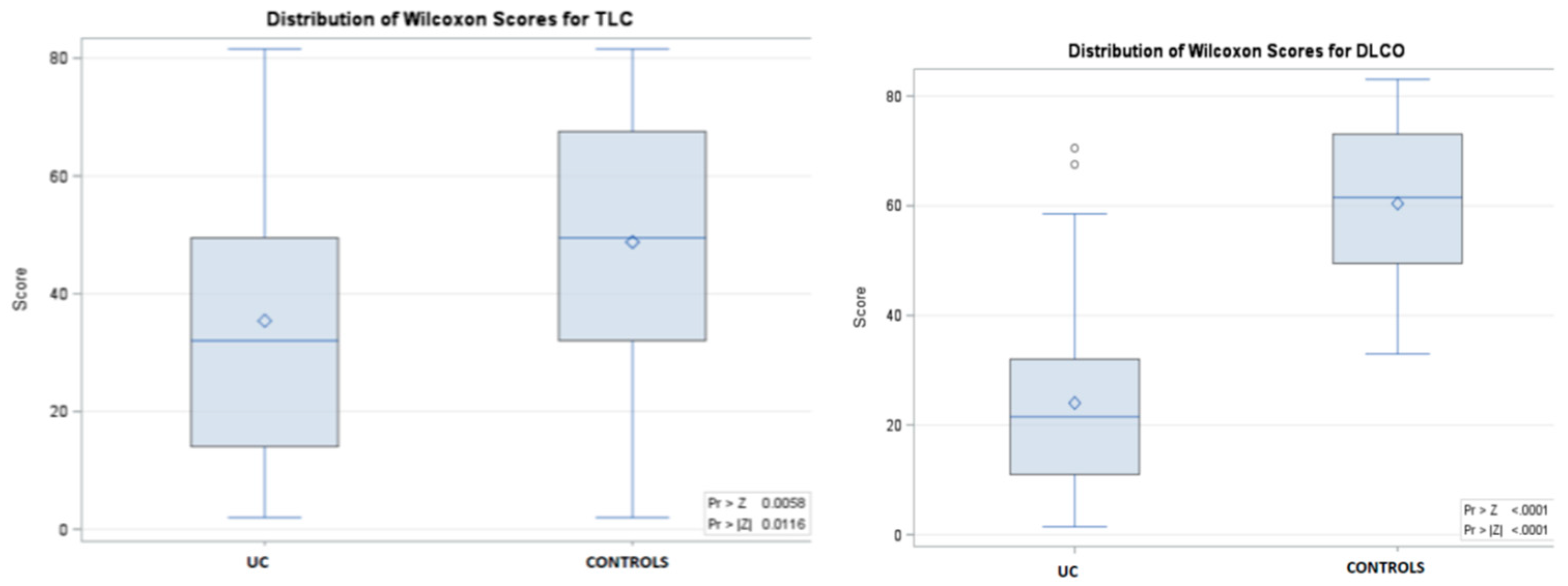
| FVC - %, (IQR) | 94 (90-98) | 92 (88-96) * | 96 (92-99) | 0.0028 |
| FEV1/FVC - %, (IQR) | 84 (81-87) | 84 (78-88) | 84 (82-87) | 0.1634 |
| FEV1 - %, (IQR) | 97 93-103) | 95 (92-99) | 97 (94-104) | 0.0694 |
| TLC - %, (IQR) | 96 (86-104) | 90 (82-98) * | 98 (90-106) | 0.0116 |
| DLCO - %, (IQR) | 90 (78-103) | 78 (73-82) * | 102 (96-112) | < 0.0001 |
| Inflammatory markers | ||||
| CRP - mg/dl, (IQR) | NA | 12.9 (6.1-35.5) | NA | |
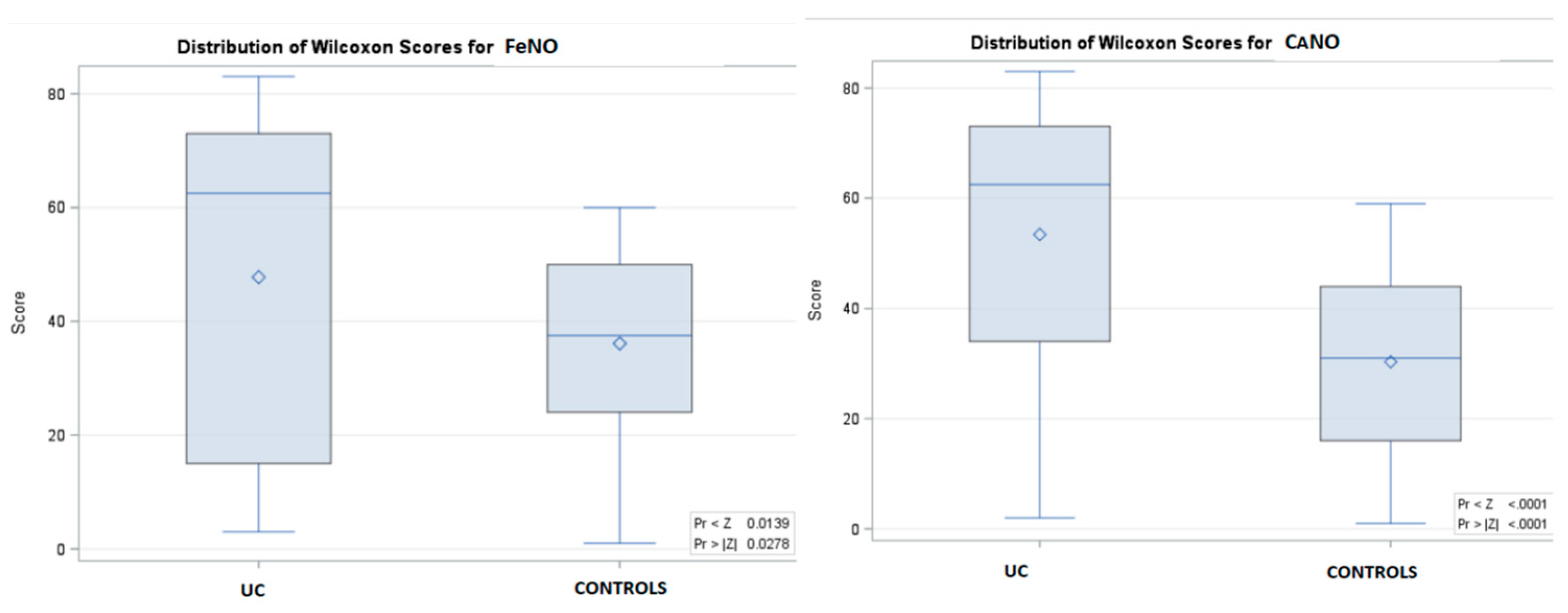
| FeNO - ppb, (IQR) | 10.8 (7.9-18.4) | 18.3 (6.7-34.1) * | 10.1 (8.2-12) | 0.0278 |
| CANO - ppb, (IQR) | 2.4 (1.8-6.1) | 5.9 (2.1-10) * | 2.1(1.5-2.5) | < 0.0001 |
| Variable | Mild (1-2) n= 16 | Severe (3) n=26 | p |
|---|---|---|---|
| Age, years (IQR) | 35.0 (32.5-38.0) | 37.0 (30.0-43.0) | N.S. |
| BMI, (IQR) | 21.5 (19.3-25.9) | 21.5 (19.3-25.9) | N.S. |
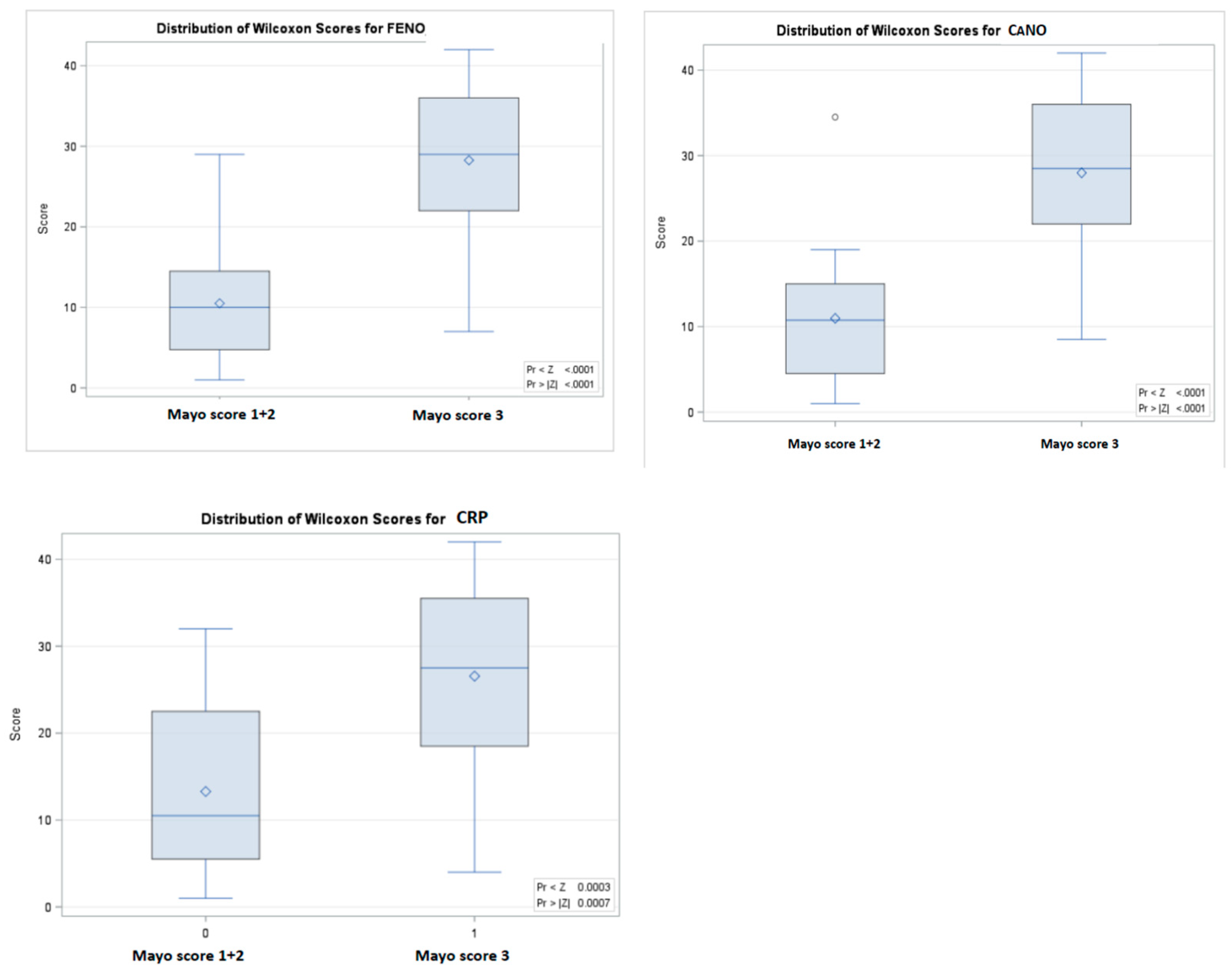
| CANO – ppb, (IQR) | 2.0 (1.4-2.8) | 8.1 (6.1-11.0) * | < 0.0001 |
| FeNO – ppb, (IQR) | 6.2 (4.4-8.7) | 27.1 (18.4-38.4) * | < 0.0001 |
| CRP - mg/dl, (IQR) | 5.8 (2.0-16.1) | 23.0 (12.0-45.0) * | 0.0007 |
| TLC - %, (IQR) | 98.0 (89.5-102.0) | 88.0 (80.0-98.0) | 0.0769 |
| DLCO - %, (IQR) | 84.5 (76.0-93.5) | 77.5 (72.0-80.0) | 0.0624 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





