3.1. Divalent Metal Ions as Cofactors of Cap8C and Wzb Phosphatase Activities
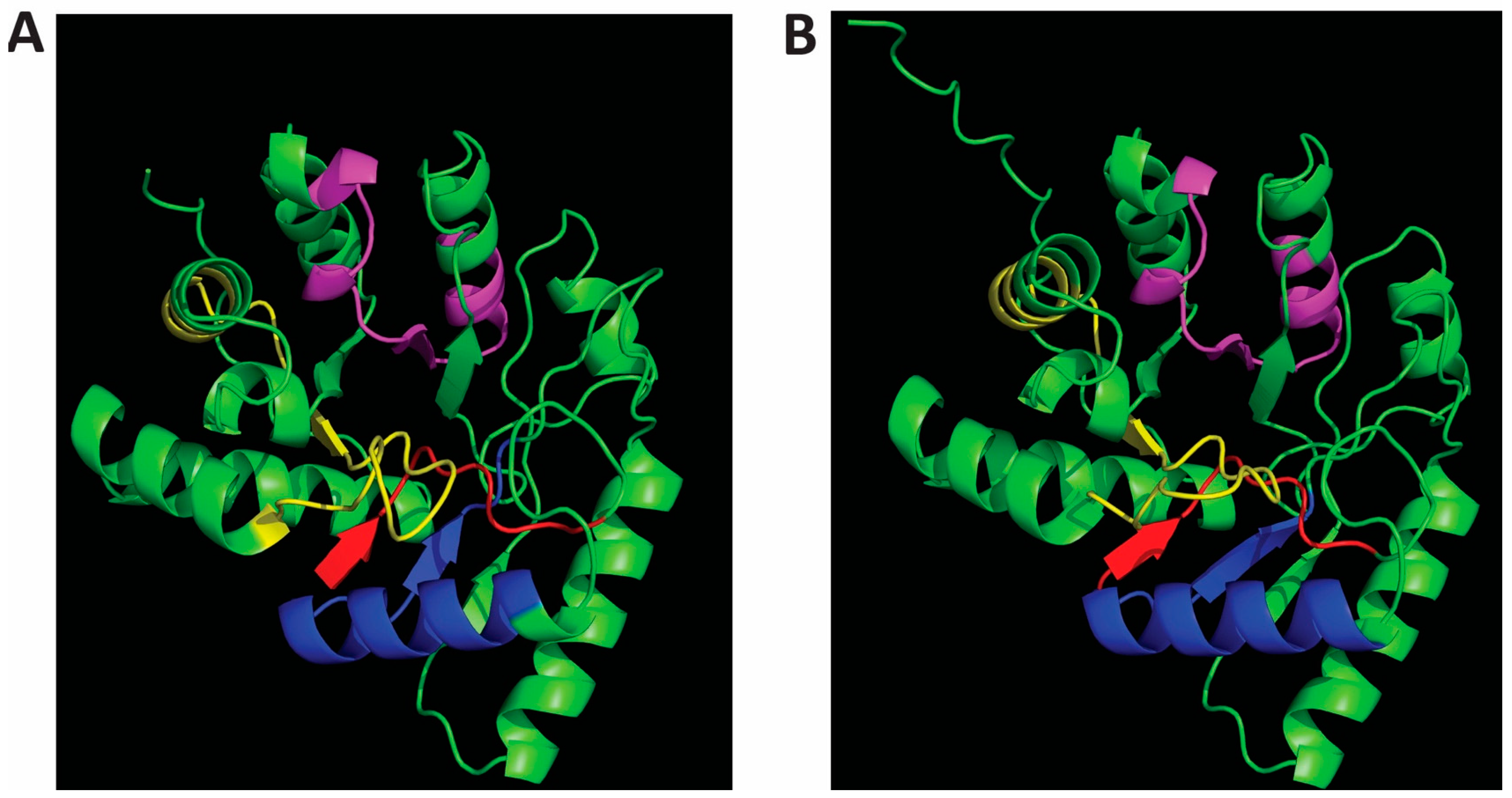
AlphaFold2-predicted structure of Cap8C and Wzb puts the two protein tyrosine phosphatases (PTPs) in the polymerase and histidinol phosphatase (PHP) family (
Figure 1). PTPs are generally not considered to have catalytic activities that are dependent on metal ions except for those in the PHP superfamily [
4,
6]. However, some PTPs have been reported to have activities modulated by the presence of some metal ions [
19,
20,
21]. Although phosphatases of the PHP superfamily are co-purified with metal ions attached to the active site, their activities in vitro in the absence of the exogenous supply of some metal ions remain barely detectable [
8,
9,
13], indicating non-structural roles for the metal ions. Hence, one of the objectives of this work was to identify the divalent metal ions that serve as activating cofactors for Cap8C and Wzb enzymes. The predicted catalytic site of PHP which consists of four motifs with conserved histidine residues have been proposed to be involved in metal dependent catalysis of phosphoester bond hydrolysis [
7,
14], through metal ion binding and coordination for enzymatic activity (14). Also, the conserved aspartate residue of the fourth motif has been predicted to be important for catalysis by participating in electron transfer [
11].
Previous reports have shown that Co
2+, Ni
2+, Mn
2+and Mg
2+ stimulate phosphatase activity to different extents, while Ca
2+ has little or no activating effect on phosphatases [
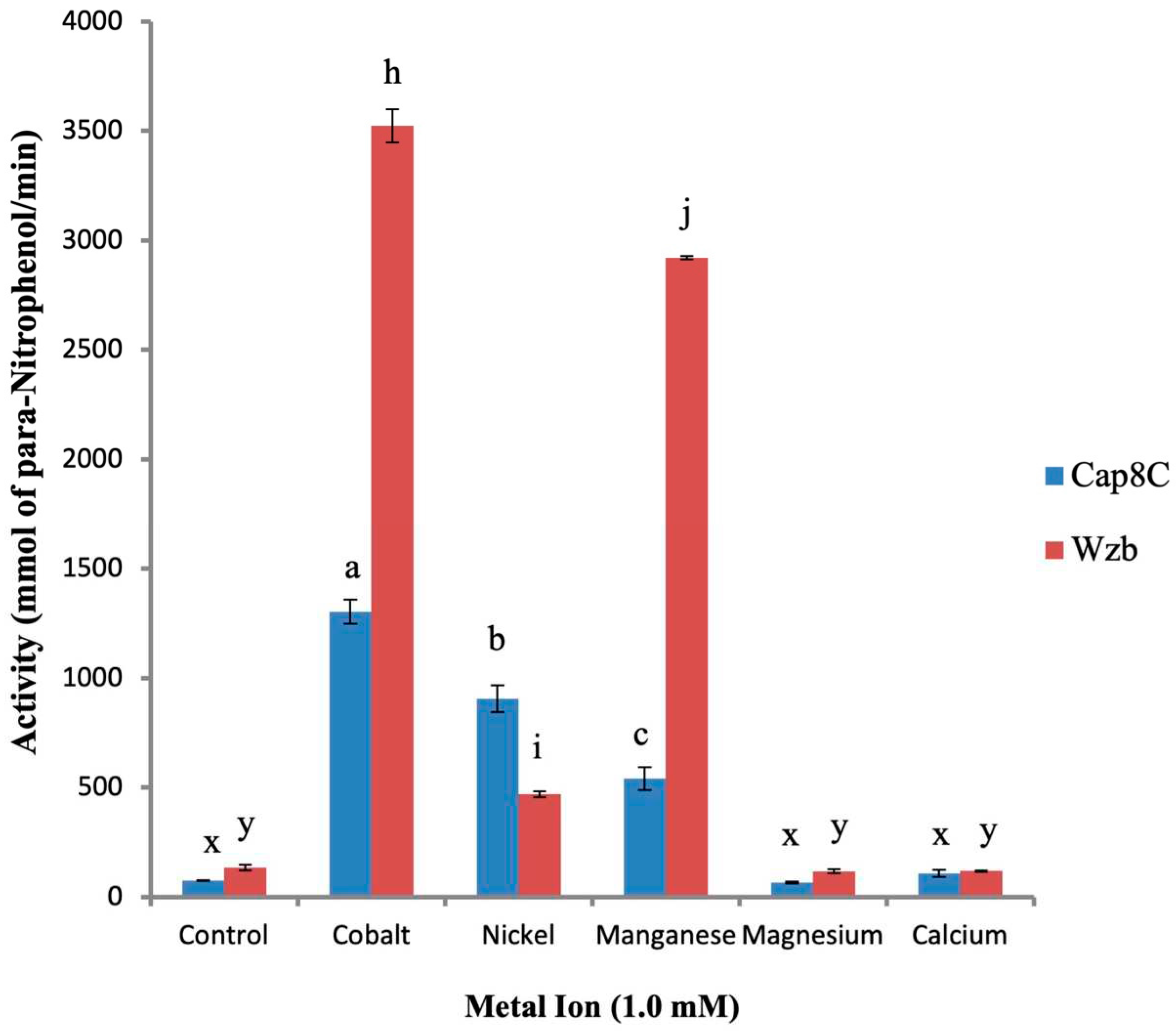
5]. The effect of these selected metal ions on the phosphatase activities of Cap8C and Wzb were investigated in this study, with the aim of establishing their dependence on these cations. Our results show that in the absence of Co
2+, Ni
2+, and Mn
2+, the activities of Cap8C and Wzb on pNPP hydrolysis were barely detectable at pH7.5 (
Figure 2). This shows both enzymes are metalloenzymes that require the presence of metal ions for full activation just as reported for members of the PHP superfamily that show low activities in the absence of metal ions [
8,
11]. Mijakovic et al. reported a metal dependent PTP of the PHP superfamily YwqE from
B. subtilis, with barely noticeable activity in the absence of 1.0 mM Mn
2+, 1.0 mM Cu
2+ and 1.0 mM Zn
2+ [
22]. A baseline level of phosphatase activity was also observed for
S. pneumoniae CpsB in the absence of 1.0 mM Co
2+, 1.0 mM Mn
2+ and 1.0 mM Mg
2+ [
11].
Our results show that Co2+ best activated both Cap8C and Wzb with 17-fold and 29-fold increase in activity, respectively. This study is the first to report Co2+ activation of Cap8C activity and shows that the PTP behaves in a similar pattern in the presence of Co2+ as other members of the PHP family. S. aureus Cap8C had been predicted to be a homologue of S. pneumoniae CpsB.
The PHP family members of PTPs have been generally described to have optimal activity at basic pH and are also sensitive to Mn
2+ [
9,
23].
Streptococcus pneumoniae CpsB, the first PTP representative of the PHP superfamily to be described, has phosphatase activity dependent on Mn
2+ (11). Like
S. pneumoniae CpsB,
B. subtilis YwqE (another member of the PHP superfamily) exhibited maximal phosphatase activity in the presence of Mn
2+ (22). Similarly, Standish et al. reported that DNA polymerase PolC exonuclease activity was significantly higher in the presence of Mn
2+ than Mg
2+ [
23]. In our study, both Cap8C and Wzb were significantly activated by Mn
2+, 7-fold and 24-fold, respectively. The characteristic activation of Cap8C and Wzb by Mn
2+ reveal a significant difference from what was described for
S. pneumoniae CpsB (manganese-dependent PTP) [
11]. This suggests that although Cap8C has been proposed to be a homologue of
S. pneumoniae CpsB, its sensitivity to Mn
2+ differs and its phosphatase activity does not seem to be dependent on the cation. The responsiveness of Cap8C to Mn
2+ in addition to earlier claim of sequence homology with the novel member of PHP superfamily,
S. pneumoniae CpsB, shows that Cap8C belongs to the PHP family.
The conserved histidine residues on the catalytic motif of phosphatases of the PHP superfamily have been suggested to be involved in metal binding, especially of Ni
2+ [
24,
25]. Although studies are yet to confirm that Ni
2+ is essential for activating phosphatase of PHP superfamily, it has however been shown to activate them to a lesser extent compared with the activating effects of Co
2+, Cu
2+ and Mn
2+ [
5,
8]. In this study, we show that Ni
2+ significantly increased the activities of Cap8C and Wzb (
Figure 2). Ni
2+ was observed to have a higher activating effect on Cap8C (12-fold) than on Wzb (4-fold). LaPointe showed that the absence of Ni
2+ in reaction mixtures containing other cations resulted in a minimal reduction in the phosphatase activity of Wzb [
7]. Also, the activity of
S. pneumoniae CpsB, the pioneer phosphatase of the PHP superfamily in the presence of Ni
2+ was observed to have increased by approximately 5-fold [
8]. Shi et al. reported that the phosphatases from the phosphoprotein phosphatase (PPP) superfamily activities were significantly activated by Ni
2+ and they include,
S. typhimurium PrpA,
S. typhimurium PrpB,
B. subtilis PrpE and
S. coelicolor SppA [
26]. However, the specific role of Ni
2+ in these systems are yet to be established.
Mg
2+ and Ca
2+ ions are generally not known to be essential for the activity of PTP members of the PHP superfamily but can stimulate to some extent the activity of some phosphatases [
5]. On the basis of their ionization potential, Mg
2+ and Ca
2+ are not considered to possess strong Lewis acidity when compared to other divalent metal ions such as Co
2+, Ni
2+ and Mn
2+. This might contribute to their poor activating effect during the catalysis of hydrolysis reactions [
27]. Our results show that both Mg
2+ and Ca
2+ had no significant effect on the phosphatase activity of Cap8C and Wzb, as both enzymes only yielded baseline activities in the presence of the cation (
Figure 2). These results agree with what has been reported for other PHP PTPs. Mijakovic et al. showed that Mg
2+ and Ca
2+ have no effect on the phosphatase activity of
B. subtilis YwqE [
22]. Morona et al. also reported a baseline level of phosphatase activity for
S. pneumoniae CpsB in the presence of Ca
2+ [
11].
3.2. Activation of Cap8C and Wzb by Divalent Cations is Concentration-Dependent
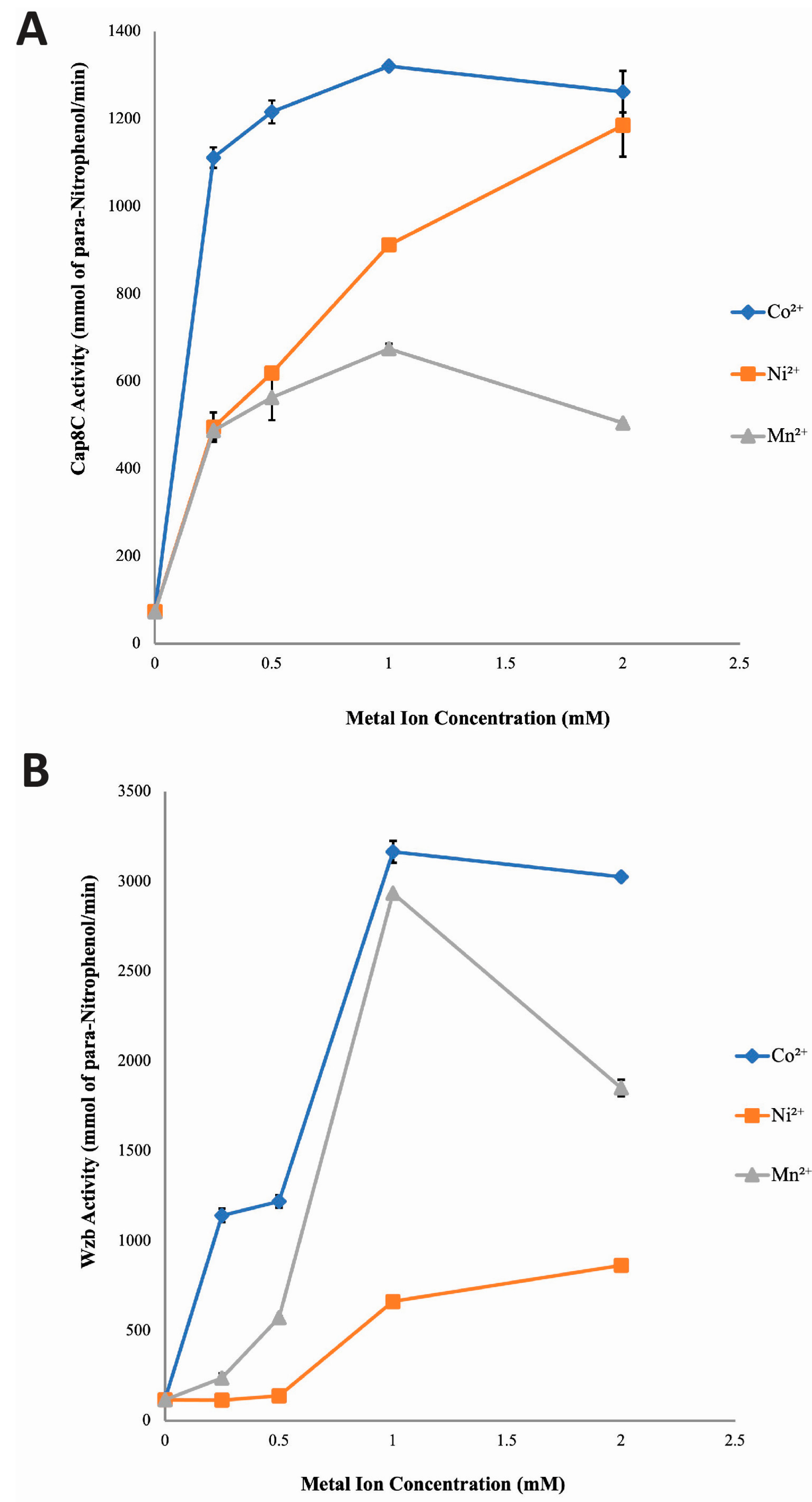
With the activating effects of Co2+, Ni2+ and Mn2+ on Cap8C and Wzb already established in the previous section, the effect of varying concentrations of these ions on the phosphatase activity of Cap8C and Wzb were investigated. The findings generally showed that the metal ions had concentration dependent activating effect on the phosphatase activities of both enzymes (Figures 3).
Protein tyrosine phosphatases of the PHP superfamily have been reported to be sensitive to Mn
2+ [
9], and the findings from this study show that Cap8C and Wzb are no exception. The findings in this study reveal that all concentrations of (0.25, 0.50, 1.00 and 2.00 mM) Mn
2+ studied had high and sustained activating effect on Cap8C. But even at its peak (1.0 mM), the activating effect of Mn
2+ was observed to be lower than what was obtained in the presence of Co
2+. This suggests that although Cap8C is sensitive to Mn
2+, the enzyme’s activity was maximal in the presence of Co
2+. On the other hand, for all the concentrations used in this study, Mn
2+ had a progressive activating effect on Wzb that peaked at 1.0 mM (
Figure 3). The findings in this study reveal that Cap8C and Wzb pose slightly different sensitivity to Mn
2+, as the cation was observed to favour the phosphatase activity of Wzb more. This finding agrees with the reports by LaPointe et al., where 0.1 mM Mn
2+ was reported to only increase the activity of
L. rhamnosus Wzb by only 2-folds [
7]. Hageleuken et al. reported that 0.5 mM Mn
2+ resulted in a 13-folds increase in the phosphatase activity of
S. pneumoniae Cps4B [
8]; this is however higher than what was obtained in our study for both enzymes.
Our findings show that the activating effect of Co
2+ on Cap8C and Wzb was optimal at 1.0 mM for both enzymes. This trend of activation for Cap8C and Wzb suggests that Co
2+ might be performing the same role during catalysis by both enzymes as the patterns of activation were similar for both enzymes. Also, the role of Co
2+ in the catalysis by both enzymes may not be structural, as many metal ions that have been implicated in structural functions during catalysis are reported to be deeply buried within the active site of enzymes [
28]. The metal ions that carry out structural functions act by helping the enzyme adopt proper conformation which promotes interactions and consequently enhances enzymatic catalysis [
28]. Crystal structures that have been determined for some members of the PHP family have not shown Co
2+ to be part of the metal ion cluster at the active site of the purified enzymes [
8,
9]. Kim et al. modeled the metal ion cluster at the active site of YwqE from
Bacillus subtilis and CpsB from
S. pneumoniae as two iron (Fe) and magnesium (Mg) ions [
8]. It follows that a structural role for Co
2+ in Cap8C is a possibility that is worthy of further exploration.
Generally, Ni
2+ has been reported to be a poor activator of phosphatases especially of the polymerase and histidinol phosphatase superfamily [
5]. For Cap8C, unlike other phosphatase members of the PHP superfamily, the activating effect was observed to be high. On the other hand, Ni
2+ had the least activating effect on Wzb as expected for PTP members of the PHP superfamily. Although the activating effect of Ni
2+ was maximal at 2.0 mM, Co
2+ was a better activator of Cap8C than Ni
2+. The pattern of Co
2+ and Ni
2+ activation suggests that the cations might be influencing enzyme-substrate binding by facilitating the release of proton from bound water, yielding nucleophilic hydroxyl ion [
8,
28]. These cations may also boost Cap8C activity by interacting with negatively charged amino acid residues such as aspartic and glutamic acid at the active site, thereby stabilizing negative charges of trigonal-bipyramidal intermediate, thus translating into increased activity [
8,
9]. In agreement with our findings in this study, 0.5 mM Ni
2+ was observed to increase the activity of wild-type Cps4B from
S. pneumoniae by 5-fold [
8]. LaPointe et al. reported that the absence of Ni
2+ in a reaction mixture that contained other metal ions resulted in slight decrease in
L. rhamnosus Wzb activity [
7].
The summary of the investigation carried out on the effect of varying concentration of the metal ions (Co2+, Ni2+ and Mn2+) on the phosphatase activity of Cap8C and Wzb reveal that both enzymes had higher levels of dependence on Co2+ compared to the other two cations. These characteristics imply that though both Cap8C and Wzb belong to the PHP superfamily, they are significantly different from the phosphatase described for S. pneumoniae, CpsB.
3.3. Temperature Dependence of Cap8C and Wzb Activities
Rise in temperature is expected to exponentially increase the rate of enzyme catalysed reaction at least until a point is reached where observable decline in activity begins due to loss of the protein’s native structure [
29]. For an enzyme catalysed reaction, increase in temperature below the optimal temperature usually results in corresponding increase in activity. This is partly because the inactivation effect of temperature is not pronounced at suboptimal temperatures [
29]. However, at supraoptimal temperatures, activity decreases because of increased thermal-induced enzyme inactivation [
29]. Thermophilic and hyperthermophilic enzymes are intrinsically active and stable at high temperatures and they offer major biotechnological advantages over mesophilic or psychrophilic enzymes. They are easily purified by heat treatment, are more resistant to chemical denaturation, have higher reaction rates, and they tolerate higher substrate concentrations [
30,
31].
The effect of temperature (30–90 °C) on the phosphatase activities of Cap8C and Wzb was investigated in the presence of Co2+, Ni2+ and Mn2+. This was done to understand the catalytic behaviour and stability of these enzymes in the presence of the divalent ions at various temperatures.
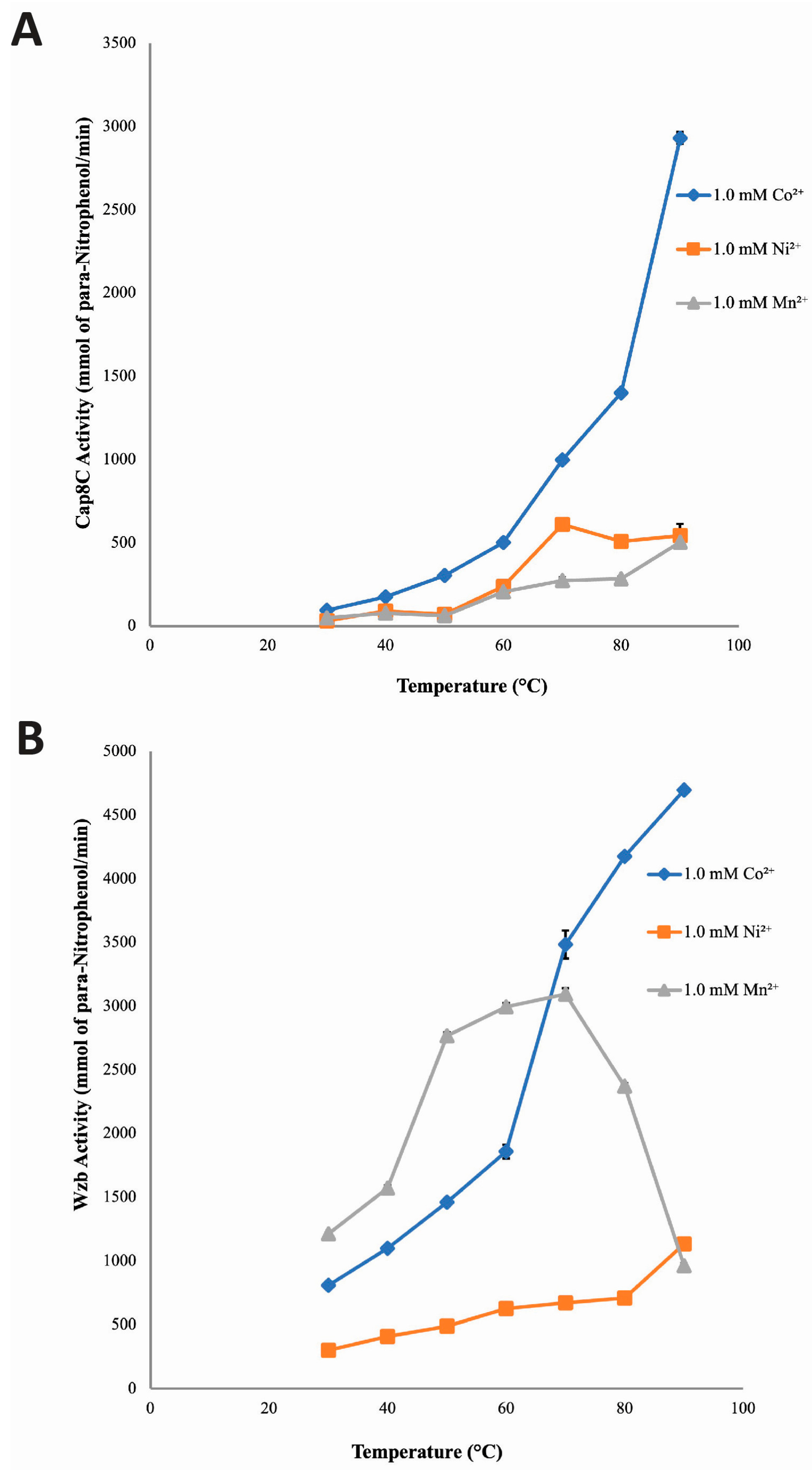
Results of this study showed that in the presence of Co
2+, Ni
2+ and Mn
2+, Cap8C showed thermostable tendency as activities were observed to increase with temperature beyond what is observed for enzymes of mesophilic origin. In the presence of Co
2+, Cap8C had optimal activity at 90 °C which is significantly higher than the optimal growth temperature (37 °C) of
S. aureus. This indicates that in the presence of the cation, increase in Cap8C activity was maintained with increase in temperature. This suggests that as stabilizing bonds of Cap8C break and reform rapidly, Co
2+ was able to promote and maintain conformational changes in the protein needed for catalysis to occur at a faster pace [
32]. The results also showed that in the presence of Co
2+, Cap8C was more resistant to heat–induced inactivation that might have taken place at high temperatures.
Although with lower levels of activities, in the presence of Ni
2+ and Mn
2+, Cap8C activity increased steadily with increase in temperature with optimal activity at 70 and 90 °C, respectively (
Figure 4a), as against 37 °C optimal growth temperature of
S. aureus. The result implies that the binding of Ni
2+ and Mn
2+ to Cap8C helped to some extent in stabilizing the conformational changes necessary for catalysis to take place at high temperature. In summary, our findings showed that in the presence of the metal ions, Cap8C displayed thermostable potentials in the order Co
2+>Ni
2+>Mn
2+ and the thermostability is higher than what was reported for other PTPs from the same species [
33]. Earlier, PTPa and PTPb from
S. aureus were characterised and both enzymes had optimal activity at 40 °C [
33]. A protein tyrosine phosphatase from mesophilic fungi,
Metarhizium anisopliae, has been reported to show thermostable properties in the absence of metal ions, with optimum activity between 70 to 75 °C [
34].
Like Cap8C, Wzb had thermostable properties in the presence of the metal ions, although to different extent as its activity remarkably increased with temperature. With Co
2+, Wzb had progressive increase in activity with increase in temperature which peaked at 90 °C; this is significantly higher than the optimal growth temperature (6 to 41 °C) of
L. rhamnosus. This implies that Co
2+ was able to initiate and stabilize conformational changes of Wzb that was required for catalysis to happen faster. Also, in the presence of Co
2+, more energy would be required to denature Wzb. In a like manner, Ni
2+ might also have shielded Wzb from the deleterious effect of high temperature by ensuring that the continuous breaking and reforming of Wzb stabilizing bonds were not disrupted as temperature increased [
32,
35].
The findings that Cap8C and Wzb are PTPs found in mesophilic organisms with thermophilic properties in the presence of Co
2+, Ni
2+ and Mn
2+ is not unprecedented. LaPointe et al. (7) reported the thermostable properties of
L. rhamnosus Wzb in the presence of metal ions with activity peaking at 75 °C; however, the thermostability was studied in the presence of Cu
2+, Co
2+, Fe
3+, Mn
2+ and Mg
2+ combination. John et al. have also characterised a thermostable protein tyrosine phosphatase from
Trypanosoma evansi with optimal temperature at 70 °C [
36]. This thermostable PTP as described for
T. evansi was suggested to play important role in the adaptation of the organism to extreme temperatures
in vivo. In addition to the claim that thermostable protein tyrosine phosphatase can be found in mesophiles is the purification and characterization of thermostable PTP from
Metarhizium anisopliae which showed optimal activity at 70 °C [
37]. Zhang and colleagues suggested that the high percentage of polar amino acid and proline content of
Metarhizium anisopliae PTP might be responsible for its thermostability as these features play significant role in protein stability [
37].
3.4. Effect of Metal Ion Cofactors on Thermostability of Cap8C and Wzb
The factors that confer thermostability on enzymes have been carefully studied and the general conclusion is that no single universal mechanism can promote stability at high temperatures [
31]. However, some structural features have been proposed to contribute to enzyme thermostability. These features include more interactions (like hydrophobic, electrostatic, hydrogen bonds, disulfide bonds and metal binding) than those found in less stable enzymes. Others include superior conformational structure such as higher packing efficiency, more rigidity, reduced entropy of unfolding, conformational strain release and stability of α-helix [
30,
31].
Having established that Cap8C and Wzb showed thermostable properties in the presence of the cofactors, to further understand this, the stability profiles of these enzymes at different temperatures (50, 60, 70, 80 and 90 °C) were investigated at different pre-incubation times (0, 2, 5, 10, 15, 20 and 30 minutes) in the presence of metal ion cofactors. The objective of this investigation was to further probe into the predicted thermostable properties of both enzymes.
Since most technological processes require elevated bioprocessing temperatures, interest has been drawn into the development of “thermoactive and thermostable” enzymes that would achieve higher catalytic rates and provide significant processing advantages [
30]. Thermophilic enzymes have been reported to be stable and perform optimally within the temperature range of 60 to 80 °C and typically do not perform well below 40 °C [
30].
The thermophilic behaviour of Cap8C in the presence of Co
2+ may imply that the cation acts to lock the active site in a stable conformation thereby ensuring increase in activity as temperature increases. This is an interesting finding that might require further investigations into the stability of Cap8C at high temperatures in the presence of the cation for longer duration to fully establish its thermostability. The stability of Cap8C at 90 °C in the presence of Co
2+ as cofactor was observed to be higher than that of PTP from
Thermus thermophiles, an extreme thermophile as reported [
29]. Vieille et al. reported that the presence of Co
2+ remarkably stabilized
Bacillus licheniformis xylose isomerase, as both
Ea of thermal denaturation and inactivation temperature were significantly higher than those of the apoenzyme [
35].
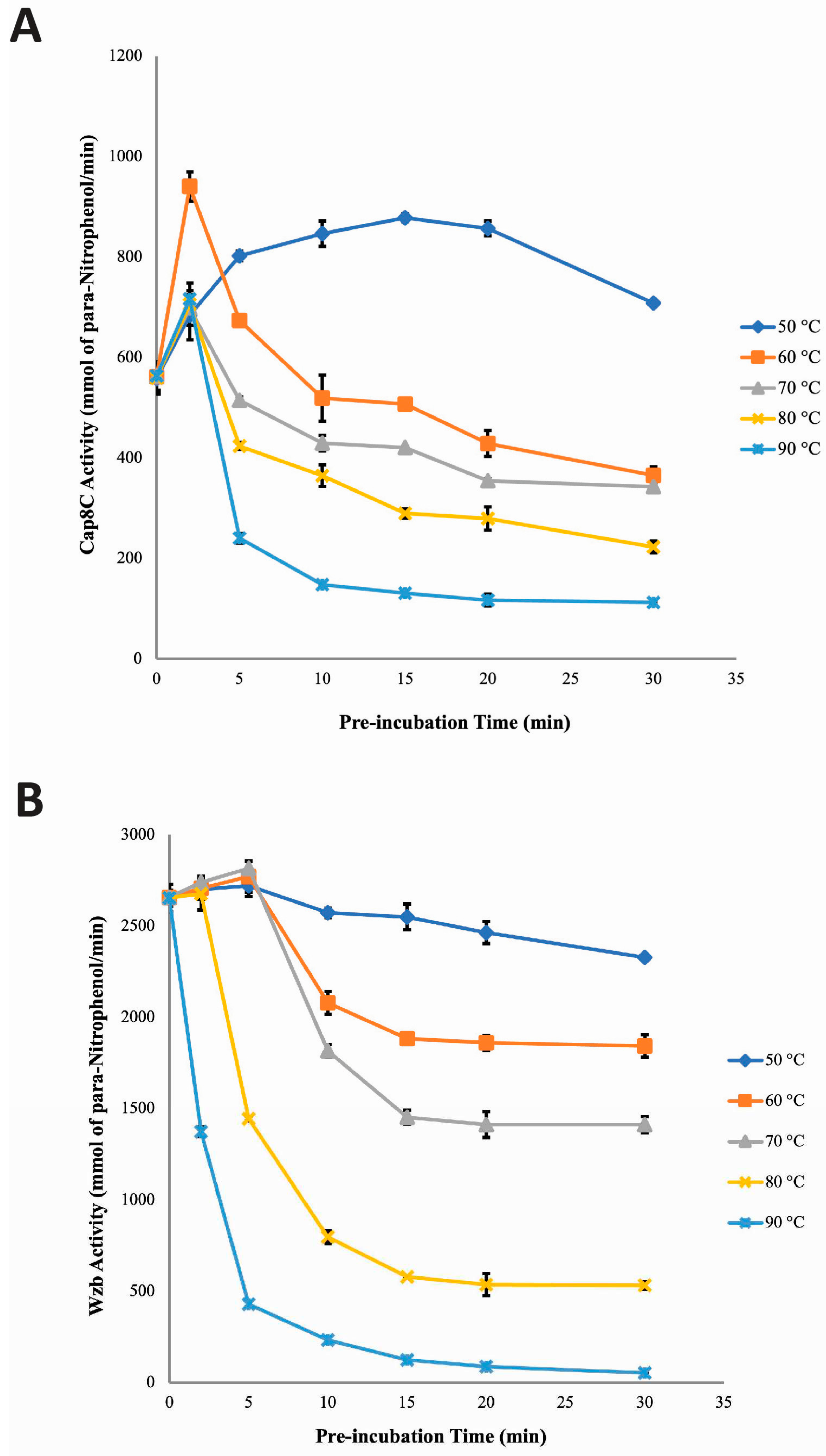
The Wzb stability profile study done in the presence of Co
2+ (
Figure 6) revealed that Wzb was less stable in the presence of the cofactor when compared with Cap8C. Findings of this investigation indicated that pre-incubation of Wzb for 2–30 minutes at high temperature (90 °C) resulted in loss of activity which might be due to loss of the enzyme’s native conformation. It could be that lesser thermal energy would be required for Wzb to lose tightly bound Co
2+, ultimately leading to unfolding thereby exposing the enzyme to chemical modifications (such as cysteine oxidation, deamidation and peptide bond hydrolysis) that eventually result in irreversible inactivation [
30,
32].
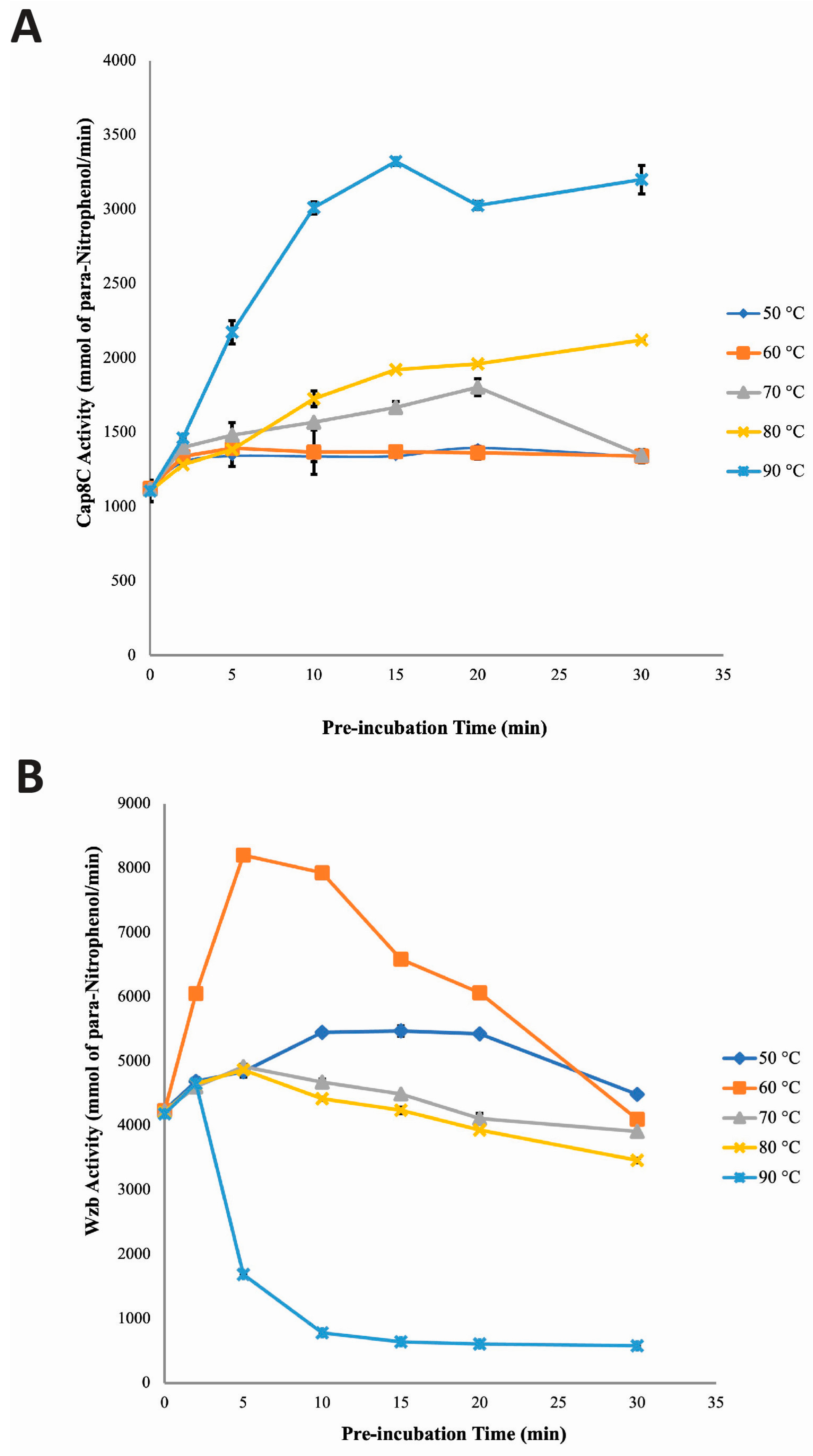
The study of the stability profile of Cap8C in the presence of Ni
2+ revealed that the enzyme was stable within the temperature range of 50 to 70 °C (
Figure 7). Loss of Wzb activity was visible at 80 and 90 °C with increased pre-incubation time. Findings gathered from this study revealed that at 50, 60 and 70 °C, Ni
2+ was able to maintain a shielding effect on Cap8C against the deleterious effect of the high temperatures likely due to ensuring that the active site conformation for activity was maintained.
Like Cap8C, Wzb was observed to be less stable at high temperatures in the presence of Ni
2+ with increase in pre-incubation time (
Figure 7). The poor stabilizing effect of Ni
2+ on Wzb as observed in this section was expected, as it had already been established that the cofactor is a poor activator of the enzyme. One possible explanation is that the cation was unable to lock the active site of Wzb in a stable conformation for a long period of time as temperature increased [
32].
Protein tyrosine phosphatases of the PHP superfamily have been reported to be significantly activated by Mn
2+, with some members such as
Streptococcus pneumoniae CpsB having activity dependent on the cofactor [
11]. This study has shown that Mn
2+ significantly activated Cap8C but to a lesser extent when compared with other metal ions like Co
2+ and Ni
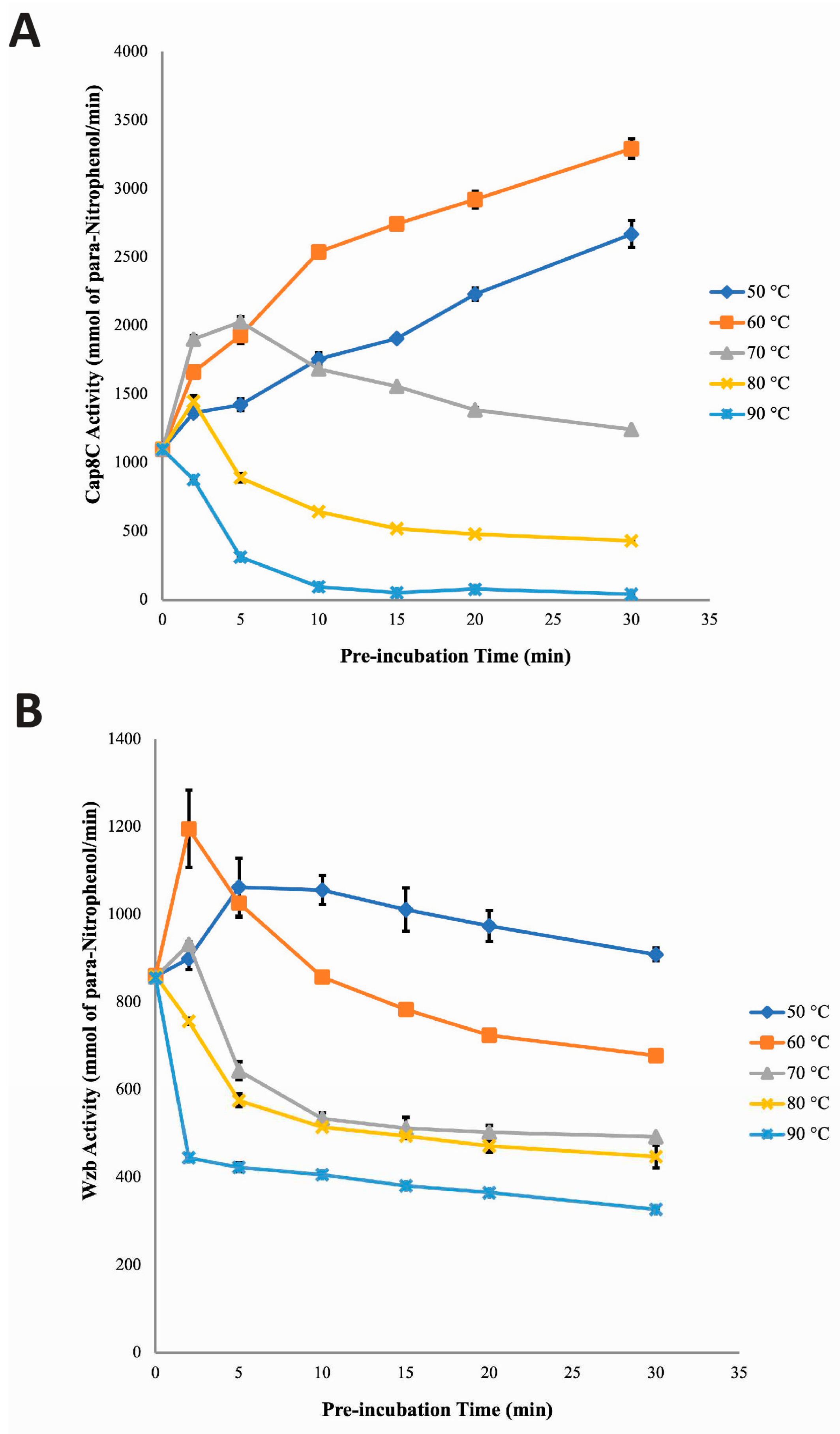
2+. Based on the results from this investigation, it was observed that Cap8C was less stable at high temperatures in the presence of Mn
2+ (
Figure 5). Cap8C was observed in this study to be most stable at 50 °C. Similar pattern of initial activation followed by rapid loss of Cap8C activity was recorded at 60, 70, 80 and 90 °C as pre-incubation time approached 30 minutes. The initial increase in Cap8C activity that was observed at 60, 70, 80 and 90 °C supports earlier findings that Mn
2+ activates the enzyme at high temperatures. However, after prolonged pre-incubation, Mn
2+ was unable to maintain the protein structure at high temperatures, thereby leading to the disruption of the stabilizing interactions and in turn loss of Cap8C activity.
The differences in the stabilization efficacy of Co
2+, Ni
2+ and Mn
2+ observed might be due to their ability to adopt different geometries in the same site in the absence of substrate. In comparison with Co
2+ and Ni
2+, Mn
2+ had lesser affinity for Cap8C as increasing pre-incubation time at 60 to 90 °C might lead to steady dissociation of the enzyme-metal complex. This steady dissociation of the holoenzyme would result in the corresponding increase in exposure of Cap8C to the inactivating effects of high temperatures. Mn
2+ was reported to significantly shield and stabilize
Bacillus licheniformis xylose isomerase (BLXI) at high temperature slightly more than Co
2+ [
35]. Mutations of metal binding sites on xylose isomerase (XI) have been reported to result in destabilization of the enzyme [
35].
Figures 6 shows that Co
2+ best activated Wzb, while Mn
2+ was only slightly less activating. Results from the investigation of the effect of Mn
2+ on the stability profile of Wzb showed that the cofactor was less efficient than Co
2+ and Ni
2+ in stabilizing Wzb at high temperatures (
Figure 6 and
Figure 7). In the presence of Mn
2+, Wzb was less stable as increasing temperature and pre-incubation time had corresponding inactivating effect on Wzb. Initial pre-incubation of Wzb at 50, 60, 70 and 80 °C led to the activation of the enzyme. These were immediately followed by decline in activity. The initial increase in Wzb activity showed that for short time exposure, Mn
2+ was able to effect activation and prevent denaturation at these temperatures. At 90 °C, Wzb was observed to be completely inactivated as activity after 30 minutes pre-incubation was barely detectable (
Figure 5). This suggests that increasing pre-incubation time at high temperatures may lead to quick dissociation of the Wzb- Mn
2+ complex, resulting in the release of the bound cofactor, exposing Wzb to the deleterious effects of high temperatures. Contrary to our findings, LaPointe et al. reported that Wzb activity increased with temperature (up to 75 °C) after 30 minutes pre-incubation in the presence of a combination of metal ion Cu
2+, Co
2+, Fe
3+, Mn
2+ and Mg
2+ [
7]. The disparity in the findings here and that of LaPointe et al. [
7] implies that for Wzb to be stable at high temperatures, it may require the concerted action of multiple activating metal ions. The inactivation pattern of Wzb after 30 minutes pre-incubation in this study was observed to be similar to what was reported by Wang et al. for a PTP purified from
Thermus thermophiles (an extreme thermophilic bacteria) [
29].
An apparent theme that has been described for thermostable enzymes is the sequence feature with high percentage of small amino acid residues (Gly, Ala, Ser, and Val). The packing of small residues lowers the entropy cost of packing at high temperature and promotes intimate interactions between helices. Additionally, relative scarcity of cysteine and destabilizing polar residues (36Asp, Asn, Glu, Gln, and Arg) has also been suggested to improve thermostability [
38]. Interestingly, both Cap8C and Wzb do not seem to possess most of these sequence features due to their mesophilic origin. This further suggests that their thermostable tendencies may be dependent on the presence of the metal ions. The stability profiles of Cap8C and Wzb in the presence of the 1.0 mM metal ions can be summarized in the order Co
2+>Ni
2+>Mn
2+.
Bacteria can be classified based on their optimal growth temperature into four groups; psychrophiles (-5 to 15°C), mesophiles (15 to 45°C), thermophiles (45 to 80°C), and hyperthermophiles (≥80°C) [
39]. Generally, enzymes that are synthesized by thermophiles and hyperthermophiles are regarded as thermozymes, they are usually thermostable, or resistant to irreversible inactivation at high temperature and are thermophilic (optimally active at temperatures between 60 and 125°C) [
31]. Psychrozymes originate from psychrophiles, while mesophiles produce enzymes known as mesozymes [
40].
S. aureus and
L. rhamnosus are typical examples of mesophiles with optimum growth temperature (OGT) of (7–48°C) and (6–41°C) respectively [
41].
Lactobacillus rhamnosus Wzb is important for extracellular polysaccharide production and polymerization [
42], and
Staphylococcus aureus Cap8C is part of a group of enzymes required for the biosynthesis of Capsular polysaccharide [
43]. There are reports of mesozymes with thermophilic potentials. For example, two thermophilic restriction endonucleases PtaI and PpaAII from mesophilic cyanobacteria strains have been reported with optimum activity at 65–80°C, which is far above the lethal temperature of the parent organism (40°C) [
44]. Lapointe et al. showed that
L. rhamnosus Wzb had optimal activity at 75°C which is almost 30 °C higher than its OGT [
7]. These interesting albeit unusual patterns are very good and require further investigation.
Merone et al. reported a thermostable PHP phopshotriesterase from
Sulfolobus solfataricus that is activated by Mg
2+, Co
2+ and Ni
2+ ions [
45]. Further studies on this and other systems could help provide insight into the structural and mechanistic basis for the thermophilic properties of these enzymes. Baros et al. also showed that mutations in the degenerate metal-binding site of
E. coli DNA polymerase III PHP domain decrease the overall stability of the polymerase and reduce its activity [
46]. This suggest that the observed thermophilic properties of the PHP PTPs might be linked to the unique distorted (α/β)7 barrel of the PHP domain.