Submitted:
30 October 2023
Posted:
31 October 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. HIV Structure (Briefly)
2.1. HIV-1 Gene-Encoded Proteins and Their Functions
2.2. Mechanism of Infection
3. Preventive Vaccines
3.1. Vaccines Based on the Induction of Neutralizing Antibodies 1986–2003
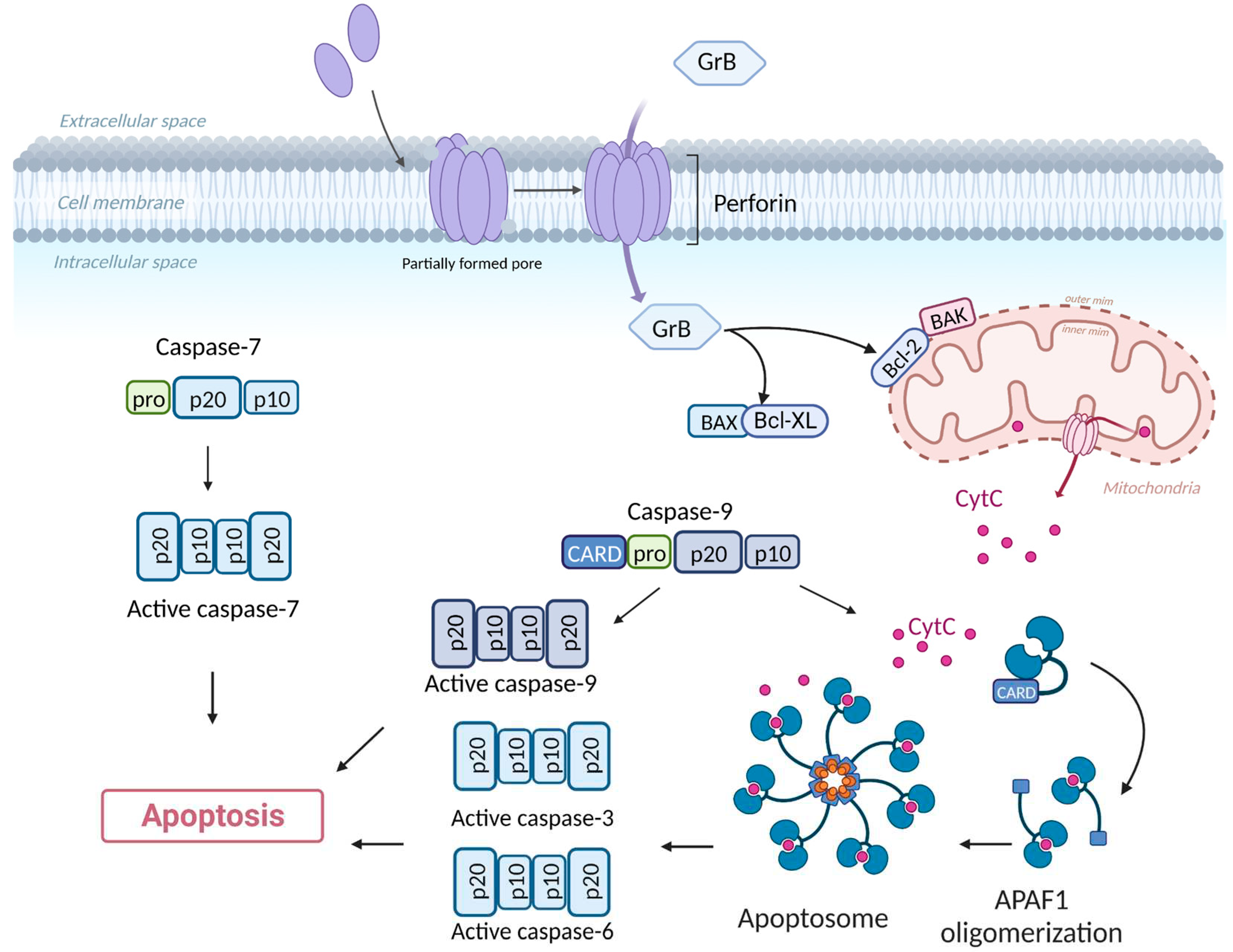
3.1.1. Direct Cytotoxicity
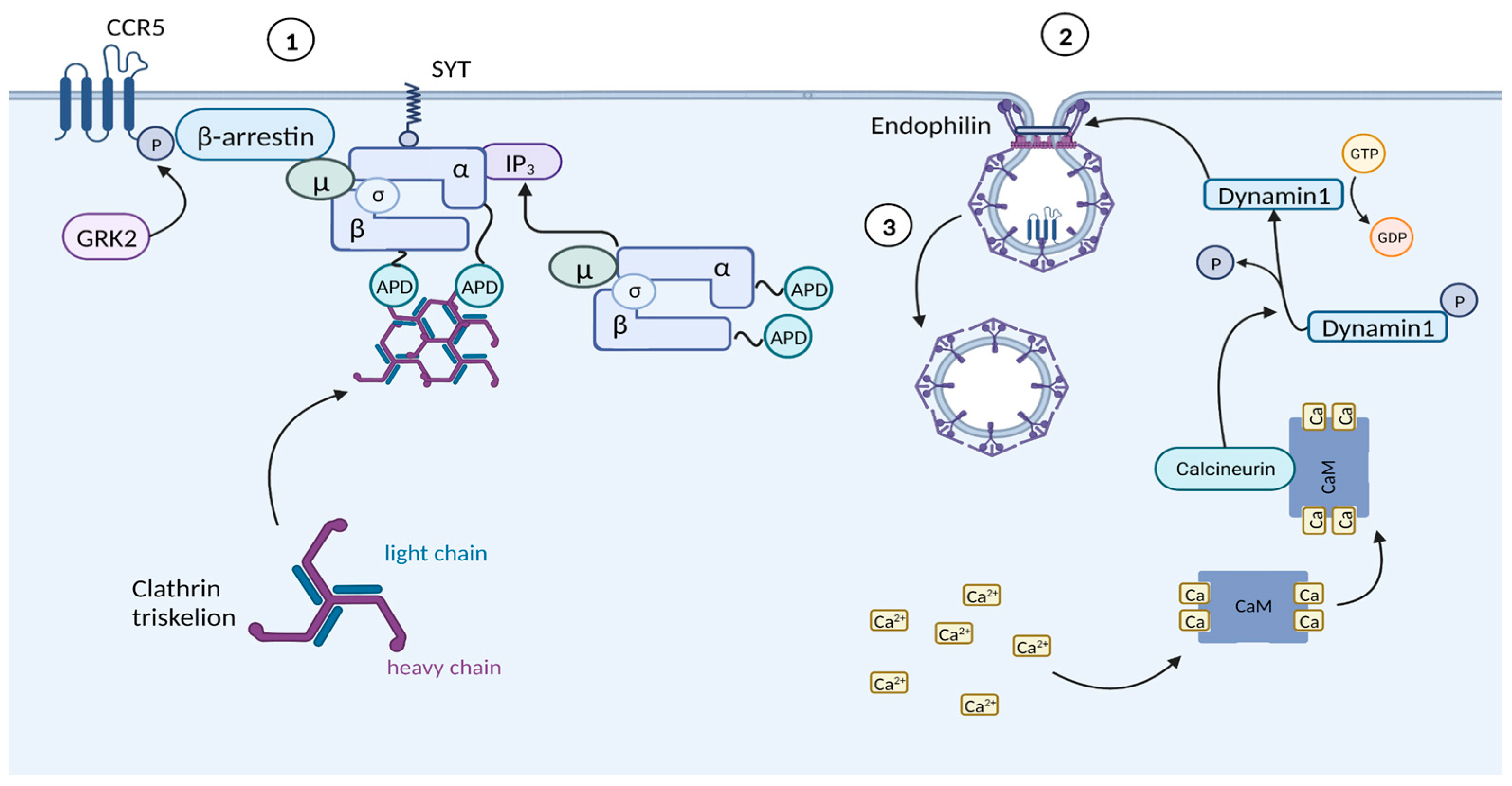
3.1.2. Chemokine-Mediated HIV Suppression
3.2. Stimulation of T-Cell Immune (1995–2007)
3.3. Mosaic HIV Vaccines
4. Therapeutic HIV Vaccines
4.1. Dendritic Cell-Based HIV Vaccines
4.2. Peptide-based HIV vaccines
4.3. DNA-Based HIV Vaccines
4.4. Viral Vector-Based HIV Vaccines
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNAIDS. Available online: https://www.unaids.org/en/Homepage (accessed on 25 October 2023).
- AVAC | Global Health Advocacy, Access & Equity. Available online: https://avac.org/ (accessed on 9 October 2023).
- Hodge, D.; Back, D.J.; Gibbons, S.; Khoo, S.H.; Marzolini, C. Pharmacokinetics and Drug-Drug Interactions of Long-Acting Intramuscular Cabotegravir and Rilpivirine. Clin. Pharmacokinet. 2021, 60, 835–853. [Google Scholar] [CrossRef]
- Temereanca, A.; Ruta, S. Strategies to Overcome HIV Drug Resistance-Current and Future Perspectives. Front. Microbiol. 2023, 14, 1133407. [Google Scholar] [CrossRef] [PubMed]
- Moskaleychik FF, Laga VY, Delgado E, Vega Y, Fernandez-Garcia A, Perez-Alvarez, Kornilaeva GV, Pronin AY, Zhernov YV, Thomson MM, Bobkova MR, Karamov EV. [Rapid spread of the HIV-1 circular recombinant CRF02-AG in Russia and neighboring countries]. Vopr Virusol. 2015;60(6):14-9. Russian.
- Karamov E., Epremyan K., Siniavin A., Zhernov Y., Cuevas M.T., Delgado E., Sánchez-Martínez M., Carrera C., Kornilaeva G., Turgiev A., Bacqué J., Pérez-Alvarez L., Thomson M.M. HIV-1 genetic diversity in recently diagnosed infections in Moscow: predominance of AFSU, frequent branching in clusters, and circulation of the Iberian subtype G variant. AIDS Res Hum Retroviruses. 2018 Jul;34(7):629-634. [CrossRef]
- Frolova OP, Butylchenko OV, Gadzhieva PG, Timofeeva MY, Basangova VA, Petrova VO, Fadeeva IA, Kashutina MI, Zabroda NN, Basov AA, Belova EV, Zhernov YV, Mitrokhin OV, Enilenis II, Severova LP. Medical Care for Tuberculosis-HIV-Coinfected Patients in Russia with Respect to a Changeable Patients’ Structure. Trop Med Infect Dis. 2022 May 31;7(6):86. [CrossRef]
- Yury V. Zhernov, Stephan Kremb, Markus Helfer, Michael Schindler, Mourad Harir, Constanze Mueller, Norbert Hertkorn, Nadezhda P. Avvakumova, Andrey I. Konstantinov, Ruth Brack-Werner, Philippe Schmitt-Kopplin and Irina V. Perminov. Supramolecular combinations of humic polyanions as potent microbicides with polymodal anti-HIV-activities. New J. Chem. 2017. 41. pp. 212-224. [CrossRef]
- Zhernov Y. Natural humic substances interfere with multiple stages of the replication cycle of human immunodeficiency virus. J Allergy Clin Immunol. 2018. V. 141. Issue 2. P. AB233.
- Zhernov Y.V., Khaitov M.R. Microbicides for topical immunoprevention of HIV infection. Bulletin of Siberian Medicine. 2019; 18(1): 49-59. [CrossRef]
- Zhernov YV, Konstantinov AI, Zherebker A, Nikolaev E, Orlov A, Savinykh MI, Kornilaeva GV, Karamov EV, Perminova IV. Antiviral activity of natural humic substances and shilajit materials against HIV-1: Relation to structure. Environ Res. 2021 Feb;193:110312. [CrossRef]
- Vassall, A.; Pickles, M.; Chandrashekar, S.; Boily, M.-C.; Shetty, G.; Guinness, L.; Lowndes, C.M.; Bradley, J.; Moses, S.; Alary, M.; et al. Cost-Effectiveness of HIV Prevention for High-Risk Groups at Scale: An Economic Evaluation of the Avahan Programme in South India. Lancet Glob. Health 2014, 2, e531–e540. [CrossRef]
- Wheatley, M.M.; Knowlton, G.S.; Butler, M.; Enns, E.A. Cost-Effectiveness of HIV Retention and Re-Engagement Interventions in High-Income Countries: A Systematic Literature Review. AIDS Behav. 2022, 26, 2159–2168. [CrossRef]
- Bozzani, F.M.; Terris-Prestholt, F.; Quaife, M.; Gafos, M.; Indravudh, P.P.; Giddings, R.; Medley, G.F.; Malhotra, S.; Torres-Rueda, S. Costs and Cost-Effectiveness of Biomedical, Non-Surgical HIV Prevention Interventions: A Systematic Literature Review. PharmacoEconomics 2023, 41, 467–480. [Google Scholar] [CrossRef] [PubMed]
- The Nobel Prize in Physiology or Medicine 2023. Available online: https://www.nobelprize.org/prizes/medicine/2023/summary/ (accessed on 25 October 2023).
- Fanales-Belasio, E.; Raimondo, M.; Suligoi, B.; Buttò, S. HIV Virology and Pathogenetic Mechanisms of Infection: A Brief Overview. Ann. Ist. Super. Sanita 2010, 46, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.; Padda, J.; Khedr, A.; Ismail, D.; Zubair, U.; Al-Ewaidat, O.A.; Padda, S.; Cooper, A.C.; Jean-Charles, G. HIV and Messenger RNA (mRNA) Vaccine. Cureus 2021, 13, e16197. [Google Scholar] [CrossRef] [PubMed]
- Nyamweya, S.; Hegedus, A.; Jaye, A.; Rowland-Jones, S.; Flanagan, K.L.; Macallan, D.C. Comparing HIV-1 and HIV-2 Infection: Lessons for Viral Immunopathogenesis. Rev. Med. Virol. 2013, 23, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Azevedo-Pereira, J.M.; Santos-Costa, Q. HIV Interaction With Human Host: HIV-2 As a Model of a Less Virulent Infection. AIDS Rev. 2016, 18, 44–53. [Google Scholar] [PubMed]
- Travers, K.; Mboup, S.; Marlink, R.; Guèye-Nidaye, A.; Siby, T.; Thior, I.; Traore, I.; Dieng-Sarr, A.; Sankalé, J.L.; Mullins, C. Natural Protection against HIV-1 Infection Provided by HIV-2. Science 1995, 268, 1612–1615. [Google Scholar] [CrossRef] [PubMed]
- Rowland-Jones, S. Protective Immunity against HIV Infection: Lessons from HIV-2 Infection. Future Microbiol. 2006, 1, 427–433. [Google Scholar] [CrossRef]
- Aberg, J.A.; Kaplan, J.E.; Libman, H.; Emmanuel, P.; Anderson, J.R.; Stone, V.E.; Oleske, J.M.; Currier, J.S.; Gallant, J.E. HIV Medicine Association of the Infectious Diseases Society of America Primary Care Guidelines for the Management of Persons Infected with Human Immunodeficiency Virus: 2009 Update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009, 49, 651–681. [Google Scholar] [CrossRef]
- Checkley, M.A.; Luttge, B.G.; Freed, E.O. HIV-1 Envelope Glycoprotein Biosynthesis, Trafficking, and Incorporation. J. Mol. Biol. 2011, 410, 582–608. [Google Scholar] [CrossRef] [PubMed]
- Ng’uni, T.; Chasara, C.; Ndhlovu, Z.M. Major Scientific Hurdles in HIV Vaccine Development: Historical Perspective and Future Directions. Front. Immunol. 2020, 11, 590780. [Google Scholar] [CrossRef] [PubMed]
- Bour, S.; Geleziunas, R.; Wainberg, M.A. The Human Immunodeficiency Virus Type 1 (HIV-1) CD4 Receptor and Its Central Role in Promotion of HIV-1 Infection. Microbiol. Rev. 1995, 59, 63–93. [Google Scholar] [CrossRef] [PubMed]
- Kwong, P.D.; Wyatt, R.; Robinson, J.; Sweet, R.W.; Sodroski, J.; Hendrickson, W.A. Structure of an HIV Gp120 Envelope Glycoprotein in Complex with the CD4 Receptor and a Neutralizing Human Antibody. Nature 1998, 393, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Patel, D.; Ma, Y.; Mann, J.F.S.; Wu, J.; Gao, Y. Employing Broadly Neutralizing Antibodies as a Human Immunodeficiency Virus Prophylactic & Therapeutic Application. Front. Immunol. 2021, 12, 697683. [Google Scholar] [CrossRef] [PubMed]
- Woodham, A.W.; Skeate, J.G.; Sanna, A.M.; Taylor, J.R.; Da Silva, D.M.; Cannon, P.M.; Kast, W.M. Human Immunodeficiency Virus Immune Cell Receptors, Coreceptors, and Cofactors: Implications for Prevention and Treatment. AIDS Patient Care STDs 2016, 30, 291–306. [Google Scholar] [CrossRef]
- Barmania, F.; Pepper, M.S. C-C Chemokine Receptor Type Five (CCR5): An Emerging Target for the Control of HIV Infection. Appl. Transl. Genomics 2013, 2, 3–16. [Google Scholar] [CrossRef] [PubMed]
- McAleer, W.J.; Buynak, E.B.; Maigetter, R.Z.; Wampler, D.E.; Miller, W.J.; Hilleman, M.R. Human Hepatitis B Vaccine from Recombinant Yeast. Nature 1984, 307, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Esparza, J. What Has 30 Years of HIV Vaccine Research Taught Us? Vaccines 2013, 1, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Dolin, R.; Graham, B.S.; Greenberg, S.B.; Tacket, C.O.; Belshe, R.B.; Midthun, K.; Clements, M.L.; Gorse, G.J.; Horgan, B.W.; Atmar, R.L.; et al. The Safety and Immunogenicity of a Human Immunodeficiency Virus Type 1 (HIV-1) Recombinant Gp160 Candidate Vaccine in Humans. Ann. Intern. Med. 1991, 114, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Perkus, M.E.; Tartaglia, J.; Paoletti, E. Poxvirus-Based Vaccine Candidates for Cancer, AIDS, and Other Infectious Diseases. J. Leukoc. Biol. 1995, 58, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Robert-Guroff, M.; Wong-Staal, F.; Gallo, R.C.; Moss, B. Expression of the HTLV-III Envelope Gene by a Recombinant Vaccinia Virus. Nature 1986, 320, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.L.; Kosowski, S.G.; Dalrymple, J.M. Expression of AIDS Virus Envelope Gene in Recombinant Vaccinia Viruses. Nature 1986, 320, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Cooney, E. Safety of and Immunological Response to a Recombinant Vaccinia Virus Vaccine Expressing HIV Envelope Glycoprotein. The Lancet 1991, 337, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Cooney, E.L.; McElrath, M.J.; Corey, L.; Hu, S.L.; Collier, A.C.; Arditti, D.; Hoffman, M.; Coombs, R.W.; Smith, G.E.; Greenberg, P.D. Enhanced Immunity to Human Immunodeficiency Virus (HIV) Envelope Elicited by a Combined Vaccine Regimen Consisting of Priming with a Vaccinia Recombinant Expressing HIV Envelope and Boosting with Gp160 Protein. Proc. Natl. Acad. Sci. USA 1993, 90, 1882–1886. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S.; Matthews, T.J.; Belshe, R.B.; Clements, M.L.; Dolin, R.; Wright, P.F.; Gorse, G.J.; Schwartz, D.H.; Keefer, M.C.; Bolognesi, D.P. Augmentation of Human Immunodeficiency Virus Type 1 Neutralizing Antibody by Priming with Gp160 Recombinant Vaccinia and Boosting with Rgp160 in Vaccinia-Naive Adults. The NIAID AIDS Vaccine Clinical Trials Network. J. Infect. Dis. 1993, 167, 533–537. [Google Scholar] [CrossRef]
- Redfield, R.R.; Wright, D.C.; James, W.D.; Jones, T.S.; Brown, C.; Burke, D.S. Disseminated Vaccinia in a Military Recruit with Human Immunodeficiency Virus (HIV) Disease. N. Engl. J. Med. 1987, 316, 673–676. [Google Scholar] [CrossRef]
- Zagury, D.; Bernard, J.; Cheynier, R.; Desportes, I.; Leonard, R.; Fouchard, M.; Reveil, B.; Ittele, D.; Lurhuma, Z.; Mbayo, K. A Group Specific Anamnestic Immune Reaction against HIV-1 Induced by a Candidate Vaccine against AIDS. Nature 1988, 332, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Cox, W.I.; Tartaglia, J.; Paoletti, E. Induction of Cytotoxic T Lymphocytes by Recombinant Canarypox (ALVAC) and Attenuated Vaccinia (NYVAC) Viruses Expressing the HIV-1 Envelope Glycoprotein. Virology 1993, 195, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Pialoux, G.; Excler, J.-L.; Rivière, Y.; Gonzalez-Canali, G.; Feuillie, V.; Coulaud, P.; Gluckman, J.-C.; Matthews, T.J.; Meignier, B.; Kieny, M.-P.; et al. A Prime-Boost Approach to HIV Preventive Vaccine Using a Recombinant Canarypox Virus Expressing Glycoprotein 160 (MN) Followed by a Recombinant Glycoprotein 160 (MN/LAI). AIDS Res. Hum. Retroviruses 1995, 11, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Marovich, M.A. ALVAC-HIV Vaccines: Clinical Trial Experience Focusing on Progress in Vaccine Development. Expert Rev. Vaccines 2004, 3, S99–S104. [Google Scholar] [CrossRef] [PubMed]
- Thongcharoen, P.; Suriyanon, V.; Paris, R.M.; Khamboonruang, C.; De Souza, M.S.; Ratto-Kim, S.; Karnasuta, C.; Polonis, V.R.; Baglyos, L.; Habib, R.E.; et al. A Phase 1/2 Comparative Vaccine Trial of the Safety and Immunogenicity of a CRF01_AE (Subtype E) Candidate Vaccine: ALVAC-HIV (vCP1521) Prime With Oligomeric Gp160 (92TH023/LAI-DID) or Bivalent Gp120 (CM235/SF2) Boost. JAIDS J. Acquir. Immune Defic. Syndr. 2007, 46, 48–55. [Google Scholar] [CrossRef] [PubMed]
- The rgp120 HIV Vaccine Study Group Placebo-Controlled Phase 3 Trial of a Recombinant Glycoprotein 120 Vaccine to Prevent HIV-1 Infection. J. Infect. Dis. 2005, 191, 654–665. [CrossRef] [PubMed]
- Pitisuttithum, P.; Gilbert, P.; Gurwith, M.; Heyward, W.; Martin, M.; van Griensven, F.; Hu, D.; Tappero, J.W.; Choopanya, K. Bangkok Vaccine Evaluation Group Randomized, Double-Blind, Placebo-Controlled Efficacy Trial of a Bivalent Recombinant Glycoprotein 120 HIV-1 Vaccine among Injection Drug Users in Bangkok, Thailand. J. Infect. Dis. 2006, 194, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Loreto, C.; La Rocca, G.; Anzalone, R.; Caltabiano, R.; Vespasiani, G.; Castorina, S.; Ralph, D.J.; Cellek, S.; Musumeci, G.; Giunta, S.; et al. The Role of Intrinsic Pathway in Apoptosis Activation and Progression in Peyronie’s Disease. BioMed Res. Int. 2014, 2014, 616149. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, R.; Kheirollahi, A.; Davoodi, J. Apaf-1: Regulation and Function in Cell Death. Biochimie 2017, 135, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Lucas, P.C.; McAllister-Lucas, L.M. Canonical and Non-Canonical Roles of GRK2 in Lymphocytes. Cells 2021, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Laporte, S.A.; Miller, W.E.; Kim, K.-M.; Caron, M.G. Beta-Arrestin/AP-2 Interaction in G Protein-Coupled Receptor Internalization: Identification of a Beta-Arrestin Binging Site in Beta 2-Adaptin. J. Biol. Chem. 2002, 277, 9247–9254. [Google Scholar] [CrossRef] [PubMed]
- Sundborger, A.; Soderblom, C.; Vorontsova, O.; Evergren, E.; Hinshaw, J.E.; Shupliakov, O. An Endophilin-Dynamin Complex Promotes Budding of Clathrin-Coated Vesicles during Synaptic Vesicle Recycling. J. Cell Sci. 2011, 124, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Royle, S.J. The Cellular Functions of Clathrin. Cell. Mol. Life Sci. CMLS 2006, 63, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.; Hanke, T. The Quest for an AIDS Vaccine: Is the CD8+ T-Cell Approach Feasible? Nat. Rev. Immunol. 2002, 2, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Mudd, P.A.; Martins, M.A.; Ericsen, A.J.; Tully, D.C.; Power, K.A.; Bean, A.T.; Piaskowski, S.M.; Duan, L.; Seese, A.; Gladden, A.D.; et al. Vaccine-Induced CD8+ T Cells Control AIDS Virus Replication. Nature 2012, 491, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Schoenly, K.A.; Weiner, D.B. Human Immunodeficiency Virus Type 1 Vaccine Development: Recent Advances in the Cytotoxic T-Lymphocyte Platform “Spotty Business. ” J. Virol. 2008, 82, 3166–3180. [Google Scholar] [CrossRef]
- Buchbinder, S.P.; Mehrotra, D.V.; Duerr, A.; Fitzgerald, D.W.; Mogg, R.; Li, D.; Gilbert, P.B.; Lama, J.R.; Marmor, M.; Del Rio, C.; et al. Efficacy Assessment of a Cell-Mediated Immunity HIV-1 Vaccine (the Step Study): A Double-Blind, Randomised, Placebo-Controlled, Test-of-Concept Trial. Lancet Lond. Engl. 2008, 372, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Duerr, A.; Huang, Y.; Buchbinder, S.; Coombs, R.W.; Sanchez, J.; Del Rio, C.; Casapia, M.; Santiago, S.; Gilbert, P.; Corey, L.; et al. Extended Follow-up Confirms Early Vaccine-Enhanced Risk of HIV Acquisition and Demonstrates Waning Effect Over Time Among Participants in a Randomized Trial of Recombinant Adenovirus HIV Vaccine (Step Study). J. Infect. Dis. 2012, 206, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, A.T.; Roederer, M.; Koup, R.A.; Bailer, R.T.; Enama, M.E.; Nason, M.C.; Martin, J.E.; Rucker, S.; Andrews, C.A.; Gomez, P.L.; et al. Phase I Clinical Evaluation of a Six-Plasmid Multiclade HIV-1 DNA Candidate Vaccine. Vaccine 2007, 25, 4085–4092. [Google Scholar] [CrossRef]
- McEnery, R. HVTN 505 Trial Expanded to See If Vaccine Candidates Can Block HIV Acquisition. IAVI Rep. Newsl. Int. AIDS Vaccine Res. 2011, 15, 17. [Google Scholar]
- Catanzaro, A.T.; Koup, R.A.; Roederer, M.; Bailer, R.T.; Enama, M.E.; Moodie, Z.; Gu, L.; Martin, J.E.; Novik, L.; Chakrabarti, B.K.; et al. Phase 1 Safety and Immunogenicity Evaluation of a Multiclade HIV-1 Candidate Vaccine Delivered by a Replication-Defective Recombinant Adenovirus Vector. J. Infect. Dis. 2006, 194, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Fischinger, S.; Shin, S.; Boudreau, C.M.; Ackerman, M.; Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kim, J.H.; Robb, M.L.; Michael, N.L.; et al. Protein-Based, but Not Viral Vector Alone, HIV Vaccine Boosting Drives an IgG1-Biased Polyfunctional Humoral Immune Response. JCI Insight 2020, 5, e135057. [Google Scholar] [CrossRef] [PubMed]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Excler, J.-L.; Nitayaphan, S.; Kaewkungwal, J.; Premsri, N.; Kunasol, P.; Karasavvas, N.; Schuetz, A.; Ngauy, V.; et al. Randomized, Double-Blind Evaluation of Late Boost Strategies for HIV-Uninfected Vaccine Recipients in the RV144 HIV Vaccine Efficacy Trial. J. Infect. Dis. 2017, 215, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Pitisuttithum, P.; Nitayaphan, S.; Chariyalertsak, S.; Kaewkungwal, J.; Dawson, P.; Dhitavat, J.; Phonrat, B.; Akapirat, S.; Karasavvas, N.; Wieczorek, L.; et al. Late Boosting of the RV144 Regimen with AIDSVAX B/E and ALVAC-HIV in HIV-Uninfected Thai Volunteers: A Double-Blind, Randomised Controlled Trial. Lancet HIV 2020, 7, e238–e248. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.E.; Huang, Y.; Grunenberg, N.; Laher, F.; Roux, S.; Andersen-Nissen, E.; De Rosa, S.C.; Flach, B.; Randhawa, A.K.; Jensen, R.; et al. Immune Correlates of the Thai RV144 HIV Vaccine Regimen in South Africa. Sci. Transl. Med. 2019, 11, eaax1880. [Google Scholar] [CrossRef]
- Bekker, L.-G.; Moodie, Z.; Grunenberg, N.; Laher, F.; Tomaras, G.D.; Cohen, K.W.; Allen, M.; Malahleha, M.; Mngadi, K.; Daniels, B.; et al. Subtype C ALVAC-HIV and Bivalent Subtype C Gp120/MF59 HIV-1 Vaccine in Low-Risk, HIV-Uninfected, South African Adults: A Phase 1/2 Trial. Lancet HIV 2018, 5, e366–e378. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F.; Gilbert, P.B.; McElrath, M.J.; Zolla-Pazner, S.; Tomaras, G.D.; Alam, S.M.; Evans, D.T.; Montefiori, D.C.; Karnasuta, C.; Sutthent, R.; et al. Immune-Correlates Analysis of an HIV-1 Vaccine Efficacy Trial. N. Engl. J. Med. 2012, 366, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Zolla-Pazner, S.; Gilbert, P.B. Revisiting the Correlate of Reduced HIV Infection Risk in the Rv144 Vaccine Trial. J. Virol. 2019, 93, e00629–19. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; D’Apice, L.; Prisco, A.; De Berardinis, P. HIV Vaccination: A Roadmap among Advancements and Concerns. Int. J. Mol. Sci. 2018, 19, 1241. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Haynes, B.F.; Cain, D.W. HIV mRNA Vaccines-Progress and Future Paths. Vaccines 2021, 9, 134. [Google Scholar] [CrossRef] [PubMed]
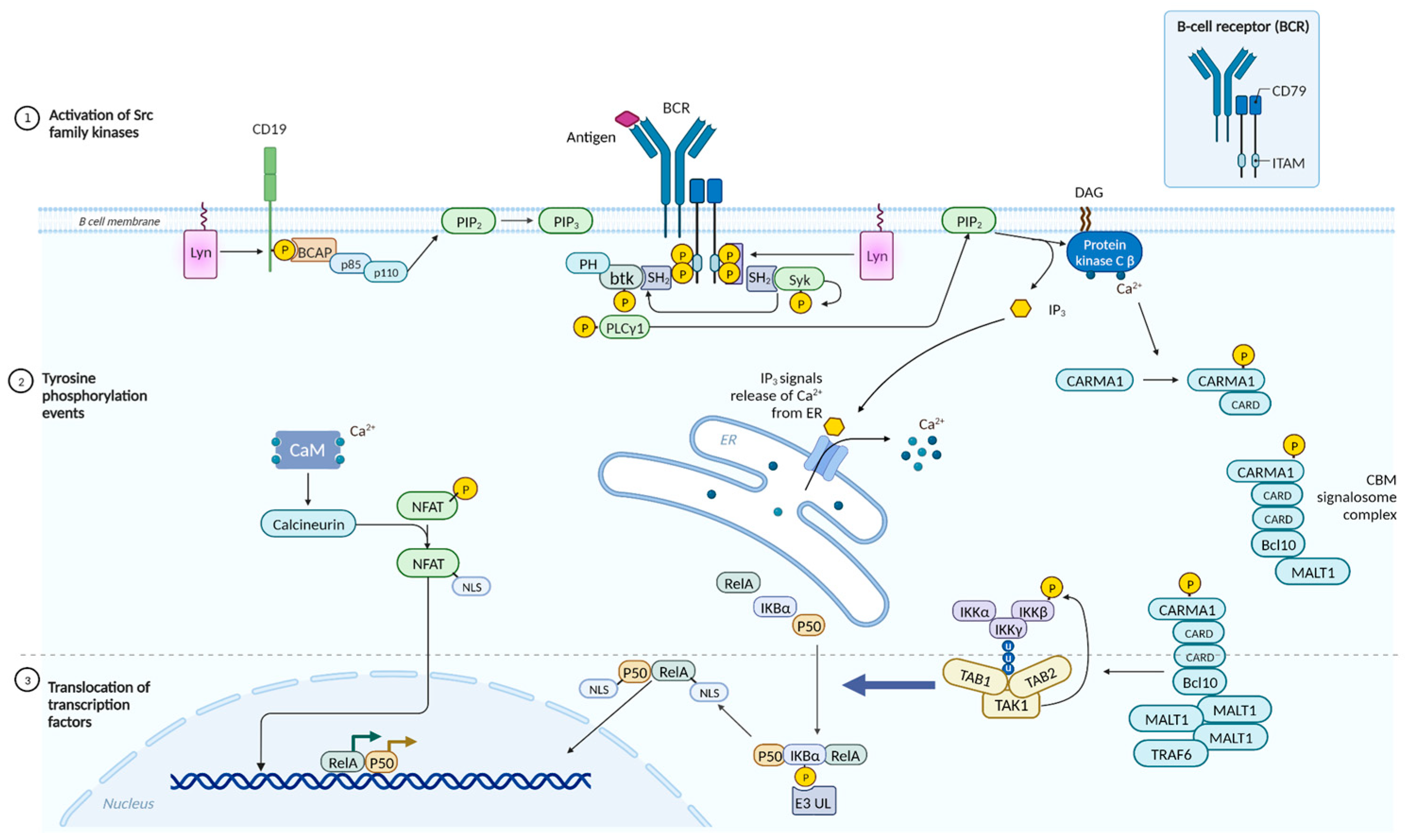
- Hobeika, E.; Nielsen, P.J.; Medgyesi, D. Signaling Mechanisms Regulating B-Lymphocyte Activation and Tolerance. J. Mol. Med. 2015, 93, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Debant, M.; Hemon, P.; Brigaudeau, C.; Renaudineau, Y.; Mignen, O. Calcium Signaling and Cell Fate: How Can Ca2+ Signals Contribute to Wrong Decisions for Chronic Lymphocytic Leukemic B Lymphocyte Outcome? Int. J. Dev. Biol. 2015, 59, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Grondona, P.; Bucher, P.; Schulze-Osthoff, K.; Hailfinger, S.; Schmitt, A. NF-κB Activation in Lymphoid Malignancies: Genetics, Signaling, and Targeted Therapy. Biomedicines 2018, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.D.; Huang, X.; Jørgensen, H.G.; Michie, A.M. Transcriptional Regulation by the NFAT Family in Acute Myeloid Leukaemia. Hemato 2021, 2, 556–571. [Google Scholar] [CrossRef]
- Larijani, M.S.; Ramezani, A.; Sadat, S.M. Updated Studies on the Development of HIV Therapeutic Vaccine. Curr. HIV Res. 2019, 17, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Julg, B. Therapeutic Vaccines for the Treatment of HIV. Transl. Res. J. Lab. Clin. Med. 2020, 223, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Bhardwaj, N. Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment. Trends Cancer 2018, 4, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Sabado, R.L.; Bhardwaj, N. Cancer Immunotherapy: Dendritic-Cell Vaccines on the Move. Nature 2015, 519, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Espinar-Buitrago, M.; Muñoz-Fernández, M.A. New Approaches to Dendritic Cell-Based Therapeutic Vaccines Against HIV-1 Infection. Front. Immunol. 2022, 12, 719664. [Google Scholar] [CrossRef]
- Jacobson, J.M.; Routy, J.-P.; Welles, S.; DeBenedette, M.; Tcherepanova, I.; Angel, J.B.; Asmuth, D.M.; Stein, D.K.; Baril, J.-G.; McKellar, M.; et al. Dendritic Cell Immunotherapy for HIV-1 Infection Using Autologous HIV-1 RNA: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Acquir. Immune Defic. Syndr. 1999 2016, 72, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Macatangay, B.J.C.; Riddler, S.A.; Wheeler, N.D.; Spindler, J.; Lawani, M.; Hong, F.; Buffo, M.J.; Whiteside, T.L.; Kearney, M.F.; Mellors, J.W.; et al. Therapeutic Vaccination With Dendritic Cells Loaded With Autologous HIV Type 1-Infected Apoptotic Cells. J. Infect. Dis. 2016, 213, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- García, F.; Plana, M.; Climent, N.; León, A.; Gatell, J.M.; Gallart, T. Dendritic Cell Based Vaccines for HIV Infection: The Way Ahead. Hum. Vaccines Immunother. 2013, 9, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- García, F.; Climent, N.; Guardo, A.C.; Gil, C.; León, A.; Autran, B.; Lifson, J.D.; Martínez-Picado, J.; Dalmau, J.; Clotet, B.; et al. A Dendritic Cell-Based Vaccine Elicits T Cell Responses Associated with Control of HIV-1 Replication. Sci. Transl. Med. 2013, 5, 166ra2. [Google Scholar] [CrossRef] [PubMed]
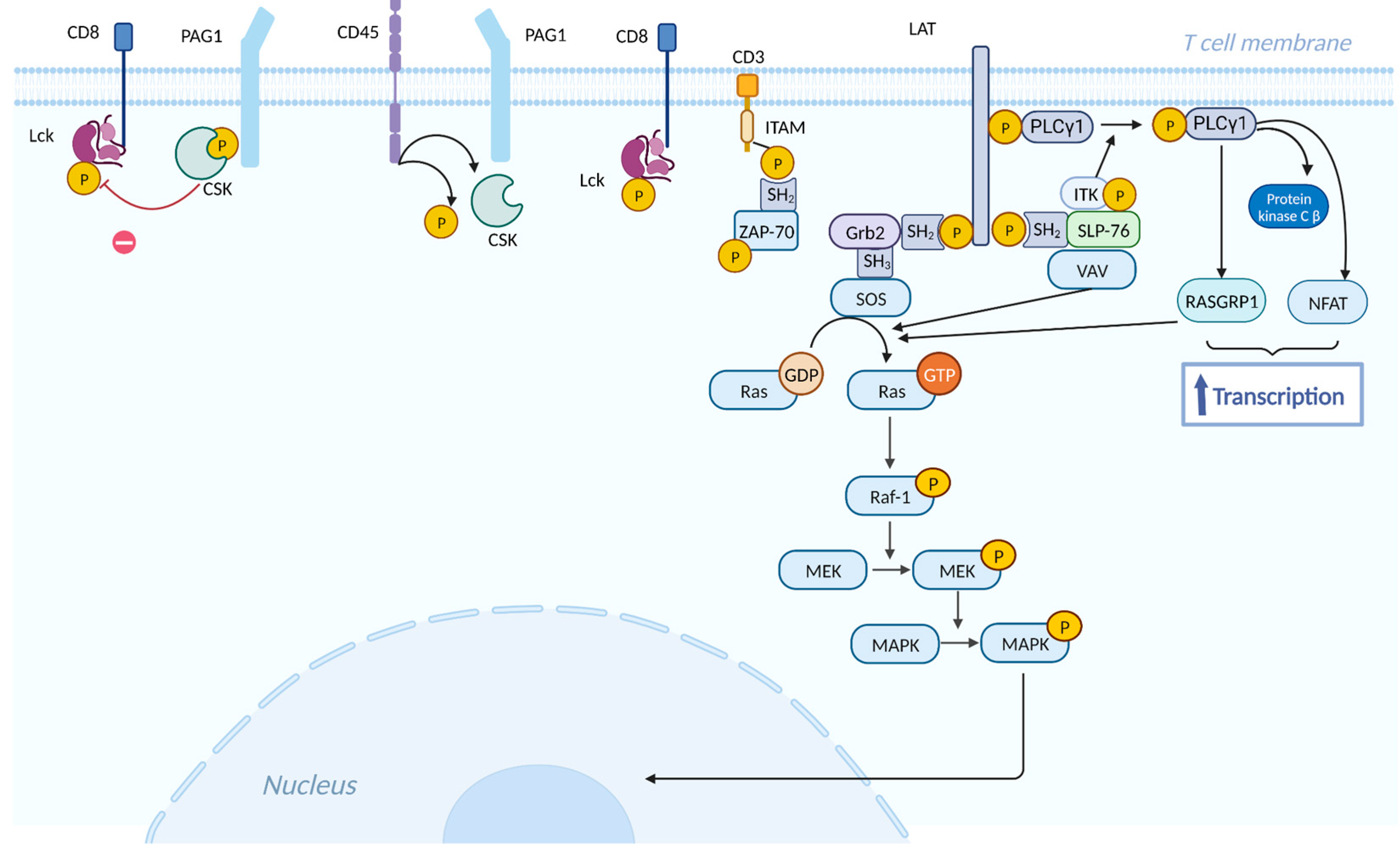
- Muro, R.; Takayanagi, H.; Nitta, T. T Cell Receptor Signaling for γδT Cell Development. Inflamm. Regen. 2019, 39, 6. [Google Scholar] [CrossRef]
- Park, I.; Yun, Y. Transmembrane Adaptor Proteins Positively Regulating the Activation of Lymphocytes. Immune Netw. 2009, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, J.D. The Ras-MAPK Signal Transduction Pathway. Sci. Signal. 2010, 3. [Google Scholar] [CrossRef]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef] [PubMed]
- Larijani, M.S.; Sadat, S.M.; Bolhassani, A.; Pouriayevali, M.H.; Bahramali, G.; Ramezani, A. In Silico Design and Immunologic Evaluation of HIV-1 P24-Nef Fusion Protein to Approach a Therapeutic Vaccine Candidate. Curr. HIV Res. 2018, 16, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynski, M.; Toth, I. Peptide-Based Synthetic Vaccines. Chem. Sci. 2016, 7, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Rockstroh, J.K.; Asmuth, D.; Pantaleo, G.; Clotet, B.; Podzamczer, D.; van Lunzen, J.; Arastéh, K.; Mitsuyasu, R.; Peters, B.; Silvia, N.; et al. Re-Boost Immunizations with the Peptide-Based Therapeutic HIV Vaccine, Vacc-4x, Restores Geometric Mean Viral Load Set-Point during Treatment Interruption. PLoS ONE 2019, 14, e0210965. [Google Scholar] [CrossRef] [PubMed]
- Pollard, R.B.; Rockstroh, J.K.; Pantaleo, G.; Asmuth, D.M.; Peters, B.; Lazzarin, A.; Garcia, F.; Ellefsen, K.; Podzamczer, D.; van Lunzen, J.; et al. Safety and Efficacy of the Peptide-Based Therapeutic Vaccine for HIV-1, Vacc-4x: A Phase 2 Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Infect. Dis. 2014, 14, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Gharakhanian, S.; Katlama, C.; Launay, O.; Bodilis, H.; Calin, R.; Ho, R.; Fang, T.; Marcu, M.; Autran, B.; Vieillard, V.; et al. VAC-3S, an Immunoprotective HIV Vaccine Directed to the 3S Motif of Gp41, in Patients Receiving ART: Safety, Dose & Immunization Schedule Assessment. Phase I Study Results: Immunologic Endpoints & Safety I ● Introduction and Background Phase I Study: Methods Phase I Study Results: Immunologic Endpoints & Safety.; October 7 2013.
- Brekke, K.; Sommerfelt, M.; Ökvist, M.; Dyrhol-Riise, A.M.; Kvale, D. The Therapeutic HIV Env C5/Gp41 Vaccine Candidate Vacc-C5 Induces Specific T Cell Regulation in a Phase I/II Clinical Study. BMC Infect. Dis. 2017, 17, 228. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.J.; Gómez Román, V.R.; Jensen, S.S.; Leo-Hansen, C.; Karlsson, I.; Katzenstein, T.L.; Rodrigues, C.M.; Jespersen, S.; Janitzek, C.M.; Té, D. da S.; et al. Clade A HIV-1 Gag-Specific T Cell Responses Are Frequent but Do Not Correlate with Viral Loads in a Cohort of Treatment-Naive HIV-Infected Individuals Living in Guinea-Bissau. Clin. Vaccine Immunol. CVI 2012, 19, 1999–2001. [Google Scholar] [CrossRef] [PubMed]
- Boffito, M.; Fox, J.; Bowman, C.; Fisher, M.; Orkin, C.; Wilkins, E.; Jackson, A.; Pleguezuelos, O.; Robinson, S.; Stoloff, G.A.; et al. Safety, Immunogenicity and Efficacy Assessment of HIV Immunotherapy in a Multi-Centre, Double-Blind, Randomised, Placebo-Controlled Phase Ib Human Trial. Vaccine 2013, 31, 5680–5686. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, B.; Morrow, M.P.; Hutnick, N.A.; Shin, T.H.; Lucke, C.E.; Weiner, D.B. Clinical Applications of DNA Vaccines: Current Progress. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 53, 296–302. [Google Scholar] [CrossRef] [PubMed]
- van Diepen, M.T.; Chapman, R.; Douglass, N.; Galant, S.; Moore, P.L.; Margolin, E.; Ximba, P.; Morris, L.; Rybicki, E.P.; Williamson, A.-L. Prime-Boost Immunizations with DNA, Modified Vaccinia Virus Ankara, and Protein-Based Vaccines Elicit Robust HIV-1 Tier 2 Neutralizing Antibodies against the CAP256 Superinfecting Virus. J. Virol. 2019, 93, e02155-18. [Google Scholar] [CrossRef] [PubMed]
- Lisziewicz, J.; Calarota, S.A.; Lori, F. The Potential of Topical DNA Vaccines Adjuvanted by Cytokines. Expert Opin. Biol. Ther. 2007, 7, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Felber, B.K.; Valentin, A.; Rosati, M.; Bergamaschi, C.; Pavlakis, G.N. HIV DNA Vaccine: Stepwise Improvements Make a Difference. Vaccines 2014, 2, 354–379. [Google Scholar] [CrossRef] [PubMed]
- Munson, P.; Liu, Y.; Bratt, D.; Fuller, J.T.; Hu, X.; Pavlakis, G.N.; Felber, B.K.; Mullins, J.I.; Fuller, D.H. Therapeutic Conserved Elements (CE) DNA Vaccine Induces Strong T-Cell Responses against Highly Conserved Viral Sequences during Simian-Human Immunodeficiency Virus Infection. Hum. Vaccines Immunother. 2018, 14, 1820–1831. [Google Scholar] [CrossRef] [PubMed]
- Janssen Vaccines & Prevention B.V. A Combined Phase 1/2a, Exploratory Study of a Therapeutic Vaccine Using an Adenovirus Type 26 Vector Prime and Modified Vaccinia Ankara Boost Combination With Mosaic Inserts in HIV-1 Infected Adults Who Initiated Antiretroviral Treatment During Acute HIV Infection; clinicaltrials.gov, 2021.
- Jacobson, J.M.; Zheng, L.; Wilson, C.C.; Tebas, P.; Matining, R.M.; Egan, M.A.; Eldridge, J.; Landay, A.L.; Clifford, D.B.; Luetkemeyer, A.F.; et al. The Safety and Immunogenicity of an Interleukin-12-Enhanced Multiantigen DNA Vaccine Delivered by Electroporation for the Treatment of HIV-1 Infection. J. Acquir. Immune Defic. Syndr. 1999 2016, 71, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Tebas, P.; Ramirez, L.; Morrow, M.; Yan, J.; Shah, D.; Lee, J.; Weiner, D.; Boyer, J.; Bagarazzi, M.; Sardesai, N. Potent Cellular Immune Responses after Therapeutic Immunization of HIV-Positive Patients with the PENNVAX®-B DNA Vaccine in a Phase I Trial. Retrovirology 2012, 9, P276–1742. [Google Scholar] [CrossRef]
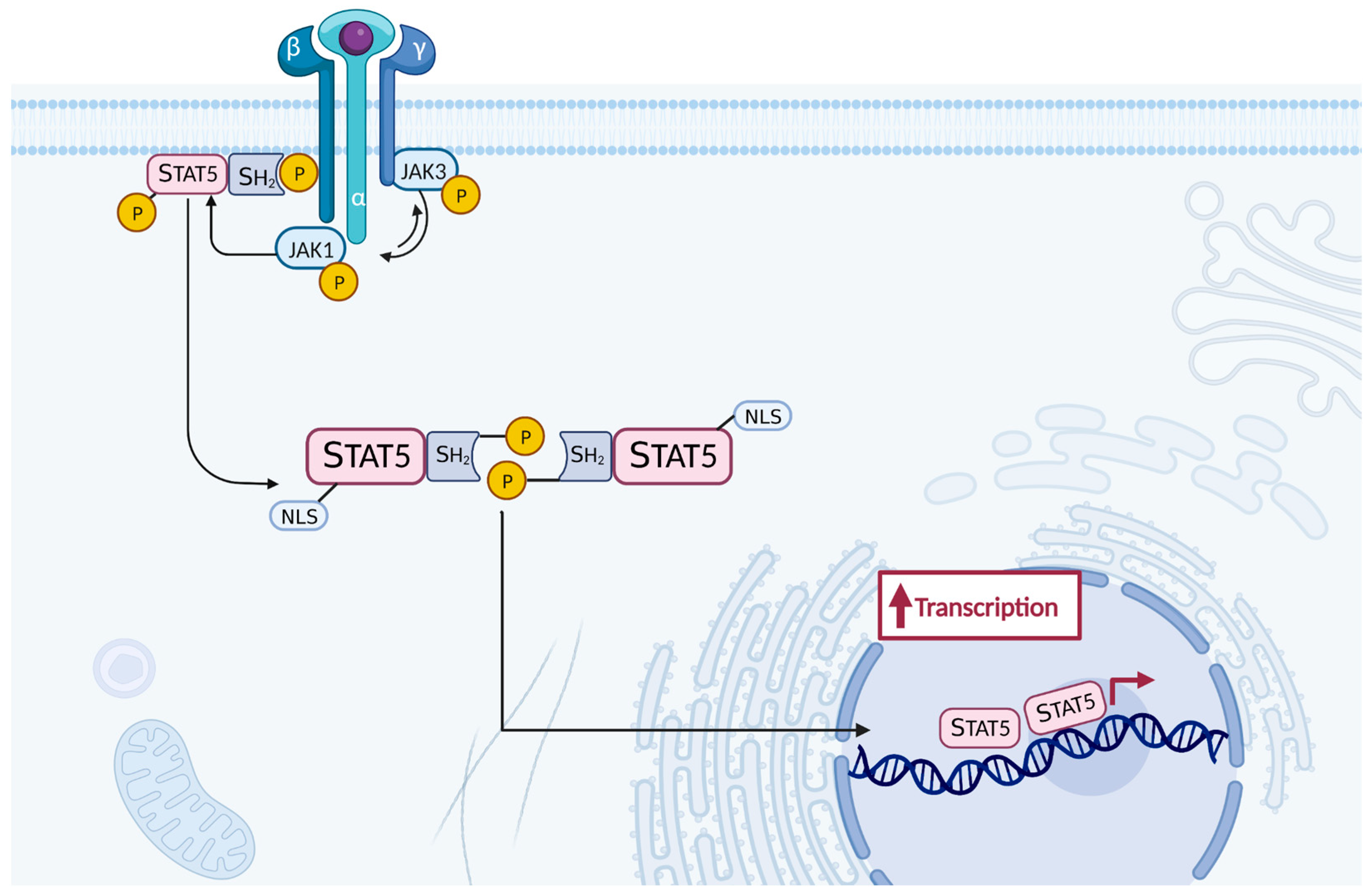
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef]
- Riddler, S. A Phase I Study to Evaluate the Safety, Tolerability and Immunogenicity of a Therapeutic HIV Vaccine Composed of Autologous Dendritic Cells Loaded With Autologous Inactivated Whole Virus or Conserved Peptides in ART-Treated HIV-Infected Adults; clinicaltrials.gov, 2023.
- GeneCure Biotechnologies A Phase I Dose-Escalation Clinical Trial to Evaluate the Safety and Immunogenicity of a Replication-Defective HIV-1 Vaccine (HIVAXTM) in HIV-1 Infected Subjects Receiving Highly Active Antiretroviral Therapy; clinicaltrials.gov, 2020.
- ANRS, Emerging Infectious Diseases A Phase I/II Randomised Therapeutic HIV Vaccine Trial in Individuals Who Started Antiretrovirals During Primary or Chronic Infection; clinicaltrials.gov, 2019.
- Theravectys, S.A. A Multi-Center, Randomized, Double-Blind, Placebo-Controlled Phase I/II Trial to Compare the Safety, Tolerability and Immunogenicity of the Therapeutic THV01 Vaccination at 5.10E+6 TU (Transducing Unit), 5.10E+7 TU (Transducing Unit) or 5.10E+8 TU (Transducing Unit) Doses to Placebo in HIV-1 Clade B Infected Patients Under Highly Active Antiretroviral Therapy (HAART); clinicaltrials.gov, 2019.
- Janssen Vaccines & Prevention, B.V. A Safety, Tolerability and Immunogenicity Study of 2 Different Regimens of Tetravalent Ad26.Mos4.HIV Prime Followed by Boost With MVA-Mosaic OR Ad26.Mos4.HIV Plus a Combination of Mosaic and Clade C Gp140 Protein in HIV-1 Infected Adults on Suppressive ART; clinicaltrials.gov, 2022.
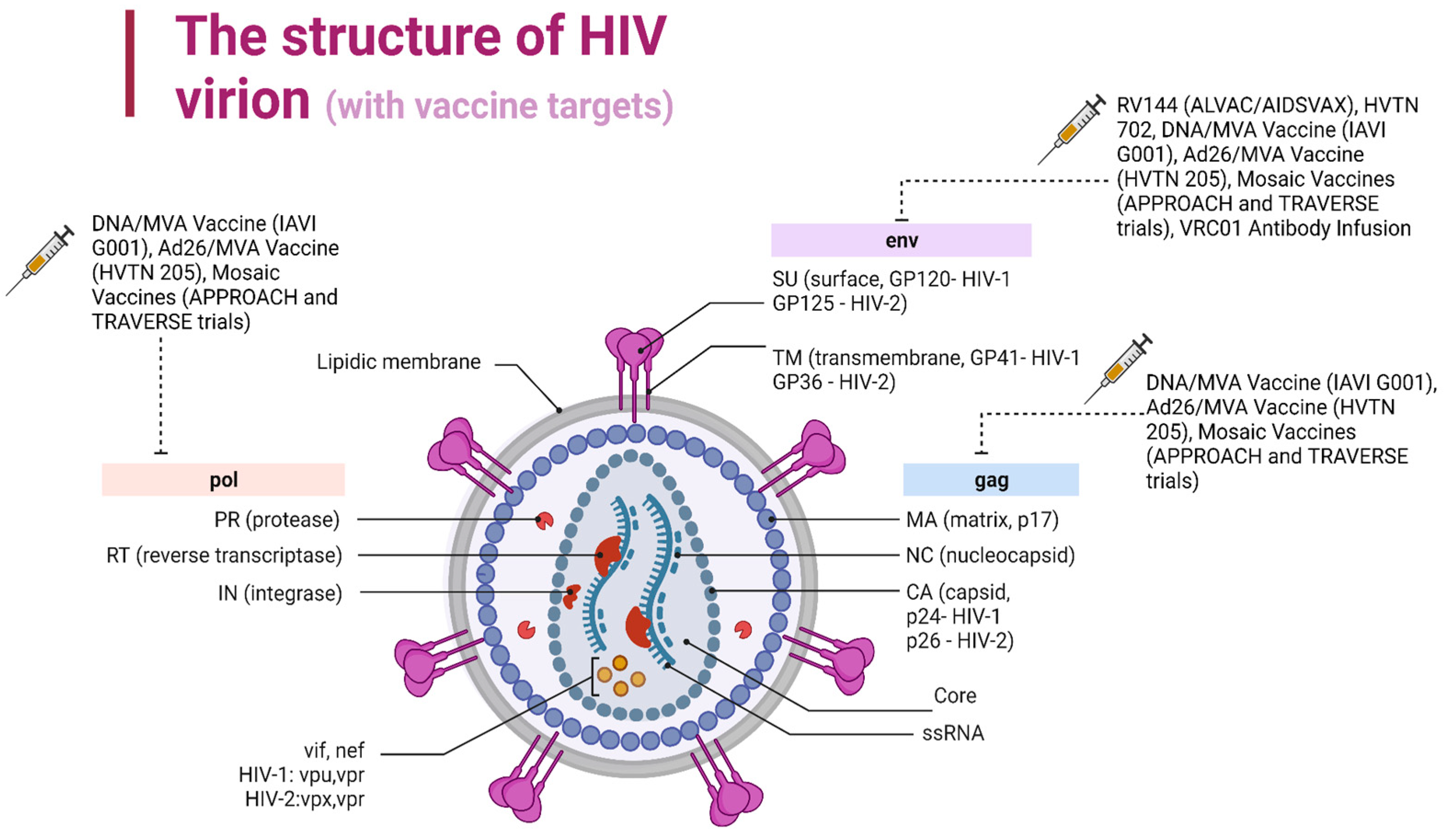
| Gene | Function |
|---|---|
| Gag (structural) | Encodes p24 (capsid), p7, and p6 core proteins and p17 matrix protein |
| Pol (structural) | Encodes for reverse transcriptase, integrase, and protease; Reverse transcriptase transforms viral RNA into DNA, integrase incorporates viral DNA into the chromosomal DNA of the host, and protease cleaves huge gag and Pol protein precursors into their components, all of which are required for viral replication. |
| Env (structural) | Encodes for gp120 and gp41, the glycoproteins of the viral envelope which target the receptors of the cell surface |
| Tat (regulatory) | Encodes for the Tat protein, which is produced early after infection and increases HIV gene expression |
| Vif (auxiliary) | Encodes for a small protein called Vif that promotes the infectivity of the viral particles |
| Vpu (auxiliary) | Encodes for a protein called Vpu that takes part in the arrest of the cell cycle |
| Rev (regulatory) | Encodes for a protein called Rev that regulates the nuclear export of the mRNA |
| Nef (auxiliary) | Encodes for the Nef protein, which modulates cellular signaling and increases the downregulation of the cell surface’s CD4 receptors, allowing viral replication. |
| Study | Vaccine (Immunogen) | Location (site) | Target group | Date | Efficacy |
|---|---|---|---|---|---|
| HVTN 305 (NCT05781542) | ALVAC-HIV and AIDSVAX B/E | Thailand (Clade B) | 162 women and men | 2012-2017 | No |
| HVTN 306 | ALVAC-HIV and AIDSVAX B/E | Thailand (Clade B) | 360 men and women aged 20–40 years | 2012-2017 | No |
| HVTN 097 (NCT02109354) | ALVAC-HIV (vCP1521) and AIDSVAX B/E | South Africa (Clade B/E) | 100 black Africans (men and women) aged 18–40 years | 2012-2013 | No |
| HVTN 100 (NCT02404311) | ALVAC-HIV (vCP2438) and bivalent subtype C gp120/MF59 | South Africa (Clade C) | 252 men and women | 2015-2018 | No |
| Study | Vaccine (Immunogen) | Location (site) | Target group | Date | Efficacy |
|---|---|---|---|---|---|
| VaxSyn | Recombinant envelope glycoprotein subunit (rgp160) of HIV | Canada (Clade B) | 72 adults | 1987 | No |
| HIVAC-1e | Recombinant vaccinia virus designed to express HIV gp160 | USA (Clade B) | 35 male adults | 1988 | No |
| VAX003 (VaxGen) (NCT00006327) | AIDSVAX B/E (subtype B - MN; subtype AE - A244 rgp120) | Thailand (Clade B/E) | 2,545 men and women IDUs |
1998-2002 | No |
| VAX004 (VaxGen) (NCT00002441) | AIDSVAX B/B (subtype B - MN and GNE8 rgp120) | North America (Clade B) | 5,417 MSM and 300 women |
1999-2003 | No |
| STEP HVTN502 | Ad5 expressing subtype B Gag (CAM-1), Pol (IIIB), Nef (JR-FL) | North America the Caribbean South America, and Australia (Clade B) | 3,000 MSM and heterosexual men and women |
2004-2007 | No |
| Phambili HVTN 503 (NCT00413725) | Ad5 expressing subtype B Gag (CAM-1), Pol (IIIB), Nef (JR-FL) | South Africa (Clade C) | 801 adults | 2003-2007 | No |
| RV 144 (NCT00223080) | ALVAC-HIV (vCP1521) expressing Gag and Pro (subtype B LAI), CRF01_AE gp120 (92TH023) linked to transmembrane anchoring portion of gp41 (LAI) AIDSVAX B/E Aluminium hydroxide |
Thailand (Clade B) | 16,402 community-risk men and women |
2003-2009 | Yes 31% |
| HVTN 505 (NCT00865566) | 6 DNA plasmids - subtype B Gag, Pol, Nef and subtypes A, B and C Env 4 rAd5 vectors - subtype B Gag/Pol and subtypes A, B and C Env |
United States (Clade B) | 2,504 men or transgender women who have sex with men | 2009-2017 | No |
| Uhambo HVTN 702 (NCT02968849) | ALVAC-HIV (vCP2438) expressing Gag and Pro (subtype B LAI), subtype C gp120 (ZM96.C) linked to transmembrane anchoring portion of gp41 (LAI) | South Africa (Clade C) | 5,400 men and women | 2016-2021 | No |
| IMBOKODO HVTN 705 (NCT03060629) | Ad26.Mos4.HIV Subtype C gp140 |
Sub-Saharan Africa (Clade C) | 2600 women | 2017-2022 | No data |
| MOSAICO HVTN 706 (NCT03964415) | Ad26.Mos4.HIV Subtype C gp140 or bivalent gp140 (subtype C/Mosaic) |
Europe North America and South America (Clade C) | 3800 MSM and transgender persons | 2019-2024 | No data |
| PrEPVacc (NCT04066881) | DNA-HIV-PT123 plasmid and AIDSVAX B/E or DNA-HIV-PT123 plasmid with trimeric CN54gp140; MVA-CMDR (Chang Mai double recombinant) and trimeric CN54gp140 Concurrent PrEP administration of either TAF/FTC or TDF/FTC |
Uganda, Tanzania, Mozambique, Republic of South Africa (Clade C) | 1668 Adults Men and Women | 2020-2023 | No data |
| Trial | Name | Hypothesis | Year | Target group | Site | Vaccine Candidates | Immunogene design | Vaccine Manufacturer |
|---|---|---|---|---|---|---|---|---|
| IAVI G002 NCT05001373 |
A Phase 1 Study to Evaluate the Safety A Phase I Trial to and Immunogenicity of eOD-GT8 and Immunogenic 60mer mRNA Vaccine (mRNA-1644) delivered by an m and Core-g28v2 60mer mRNA Vaccine negative adults (mRNA-1644v2-Core |
Sequential vaccination by a germline-targeting prime followed by directional boost immunogens can induce specific classes of B-cell responses and guide their early maturation toward broadly neutralizing antibody (bnAb) development through an mRNA platform |
2021- 2023 | 56 adults ages 18 to 50 | 4 sites in the US (Atlanta; San Antonio; Seattle; Washington, DC) | Two experimental HIV vaccines based on messenger RNA (mRNA) platform: 1. eOD-GT8 60mer mRNA Vaccine (mRNA-1644) 2. Core-g28v2 60mer mRNA Vaccine (mRNA-1644v2-Core) |
IAVI Neutralizing Antibody left (NAC) at Scripps Research |
Moderna |
| IAVI G003 NCT05414786 |
A Phase I Trial to Evaluate the Safety and Immunogenicity of eOD-GT8 60mer delivered by an mRNA platform in HIV negative adults | eOD-GT8 60mer delivered by an mRNA platform in HIV negative adults will induce immune responses in African populations as was seen in IAVI G001, which demonstrated this recombinant protein (eOD-GT8 60mer) safely induced immune responses in 97% of recipients, who were healthy U.S. adults | 2022- 2023 | 18 healthy, HIV- negative adults | 2 sites: Kigali, Rwanda, and Tembisa, South Africa | One experimental HIV vaccine based on messenger RNA (mRNA) platform: 1. eOD-GT8 60mer delivered by an mRNA Vaccine platform (mRNA- 1644) |
IAVI Neutralizing Antibody left (NAC) at Scripps Research |
Moderna |
| HVTN 302 NCT05217641 |
A Clinical Trial to Evaluate the Safety and Immunogenicity of BG505 MD39.3, BG505 MD39.3 gp151, and BG505 MD39.3 gp151 CD4KO HIV Trimer mRNA Vaccines in Healthy, HIV-uninfected Adult Participants | The BG505 MD39.3 soluble and membrane-bound trimer mRNA vaccines will be safe and well-tolerated among HIV-uninfected individuals and will elicit autologous neutralizing antibodies | 2022- 2023 | 108 adults ages 18 to 55 years | 11 sites in the US (Birmingham; Boston; Los Angeles; New York City; Philadelphia; Pittsburgh; Rochester; Seattle) |
Three experimental HIV vaccines based on messenger RNA (mRNA) platform: 1. BG505 MD39.3 mRNA 2. BG505 MD39.3 gp151 mRNA 3. BG505 MD39.3 gp151 CD4K0 mRNA |
Scripps Consortium for HIV/AIDS Vaccine Development (CHAVD) and IAVI Neutralizing Antibody left (NAC) at Scripps Research |
Moderna |
| Trial | Phase | Registry Identifier | Result | Status | Last Update | Ref. |
|---|---|---|---|---|---|---|
| AGS-004 (personalized therapeutic vaccine utilizing patient-derived dendritic cells and HIV antigens) | IIb | NCT00672191 | Induction of CD4 and CD8, reduction of VL, One severe adverse event | Completed | 2013 | [79] |
| Autologous HIV-1 ApB DC Vaccine | I/II | NCT00510497 | Safe and immunogenic Reduction of HIV blood reservoir | Completed | 2016 | [80] |
| Dendritic cell vaccine (DCV-2) | I/II | NCT00402142 | Safely induced marginal immune responses, whereas markedly increased Vacc-C5-induced regulatory T cell | Completed | 2014 | [81] |
| Dendritic cells loaded with HIV-1 lipopeptides | I | NCT00796770 | Safe and showed few CD8 T cell responses | Completed | 2017 | [82] |
| Trial | Phase | Registry Identifier | Result | Status | Last Update | Ref. |
|---|---|---|---|---|---|---|
| Vacc-4x | II | NCT00659789 | Induction of CD4 and CD8, reduction of VL, One severe adverse event | Completed | 2017 | [90] |
| VAC-3S | I/II | NCT01549119 | Safe and immunogenic Reduction of HIV blood reservoir | Completed | 2015 | [91] |
| Vacc-C5 | I/II | NCT01627678 | Safely induced marginal immune responses, whereas markedly increased Vacc-C5-induced regulatory T cell | Completed | 2014 | [92] |
| AFO-18 | I | NCT01141205 | Safe and showed few CD8 T cell responses | Completed | 2013 | [93] |
| HIV-v | I | NCT01071031 | Safe and can elicit T- and B-cell responses that significantly reduce viral load. | Completed | 2012 | [94] |
| Trial | Phase | Registry Identifier | Result | Status | Last Update | Ref. |
|---|---|---|---|---|---|---|
| Ad26.Mos.HIV + MVA-Mosaic | II | NCT02919306 | Not reported | Completed | 2018 | [100] |
| MAG pDNA vaccine +/- IL-12 | I | NCT01266616 | Elicted CD4+ but not CD8+ T-cell responses to multiple HIV-1 antigens. | Completed | 2015 | [101] |
| PENNVAX-B (Gag, Pol, Env) + electroporation | I | NCT01082692 | Strong induction of CD8 T cell responses | Completed | 2012 | [102] |
| Trial | Phase | Registry Identifier | Result | Status | Last Update | Ref. |
|---|---|---|---|---|---|---|
| DC-HIV04 Comparison of Dendritic Cell-Based Therapeutic Vaccine Strategies for HIV Functional Cure |
I | NCT03758625 | Not reported | Recruting | 2018 | [104] |
| GCHT01 | I | NCT01428596 | Not reported | Active | 2019 | [105] |
| GTU-MultiHIV B-clade + MVA HIV-B (DNA + viral vector vaccines) | II | NCT02972450 | Not reported | Not yet recruting | 2018 | [106] |
| THV01 (lentiviral vector-based therapeutic vaccine) | I/II | NCT02054286 | Not reported | Active | 2019 | [107] |
| Ad26.Mos4.HIV + MVA-Mosaic or clade C gp140 + mosaic gp140 |
I | NCT03307915 | Not reported | Recruting | 2019 | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





