Submitted:
30 October 2023
Posted:
31 October 2023
You are already at the latest version
Abstract
Keywords:
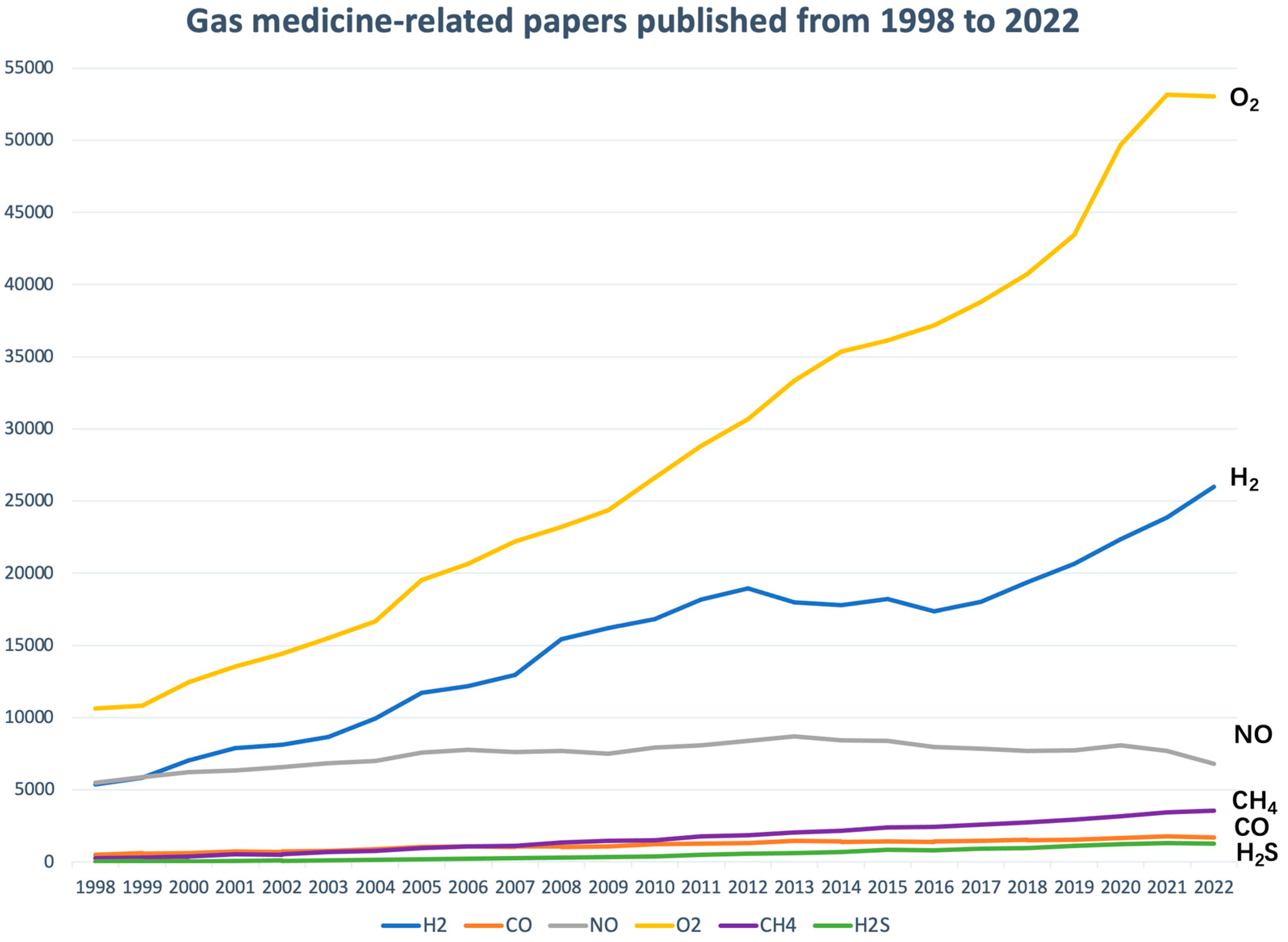
1. Introduction
2. History of H2 medicine
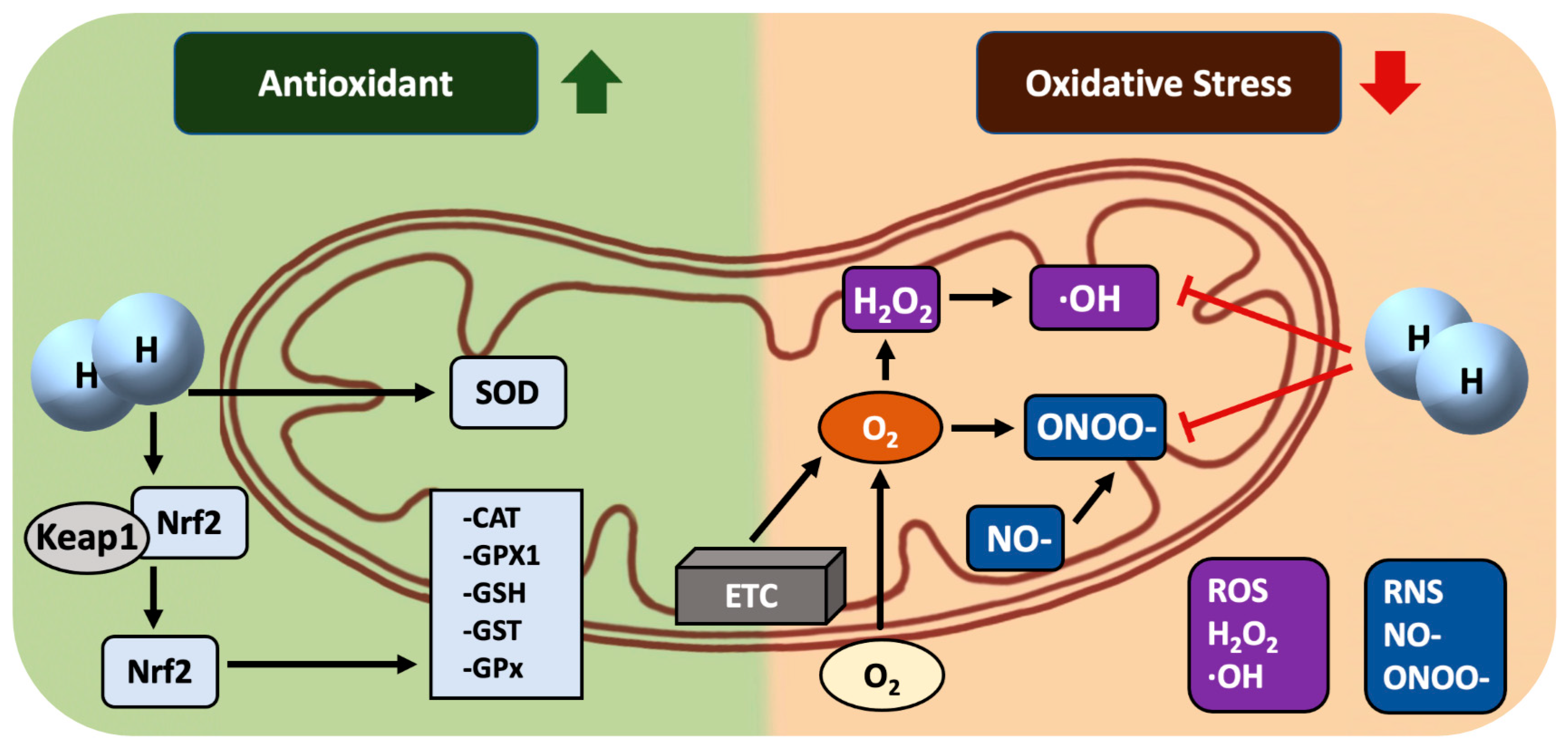
3. H2: a mitochondria-targeting molecule/nutrient, rather than a selective •OH scavenger
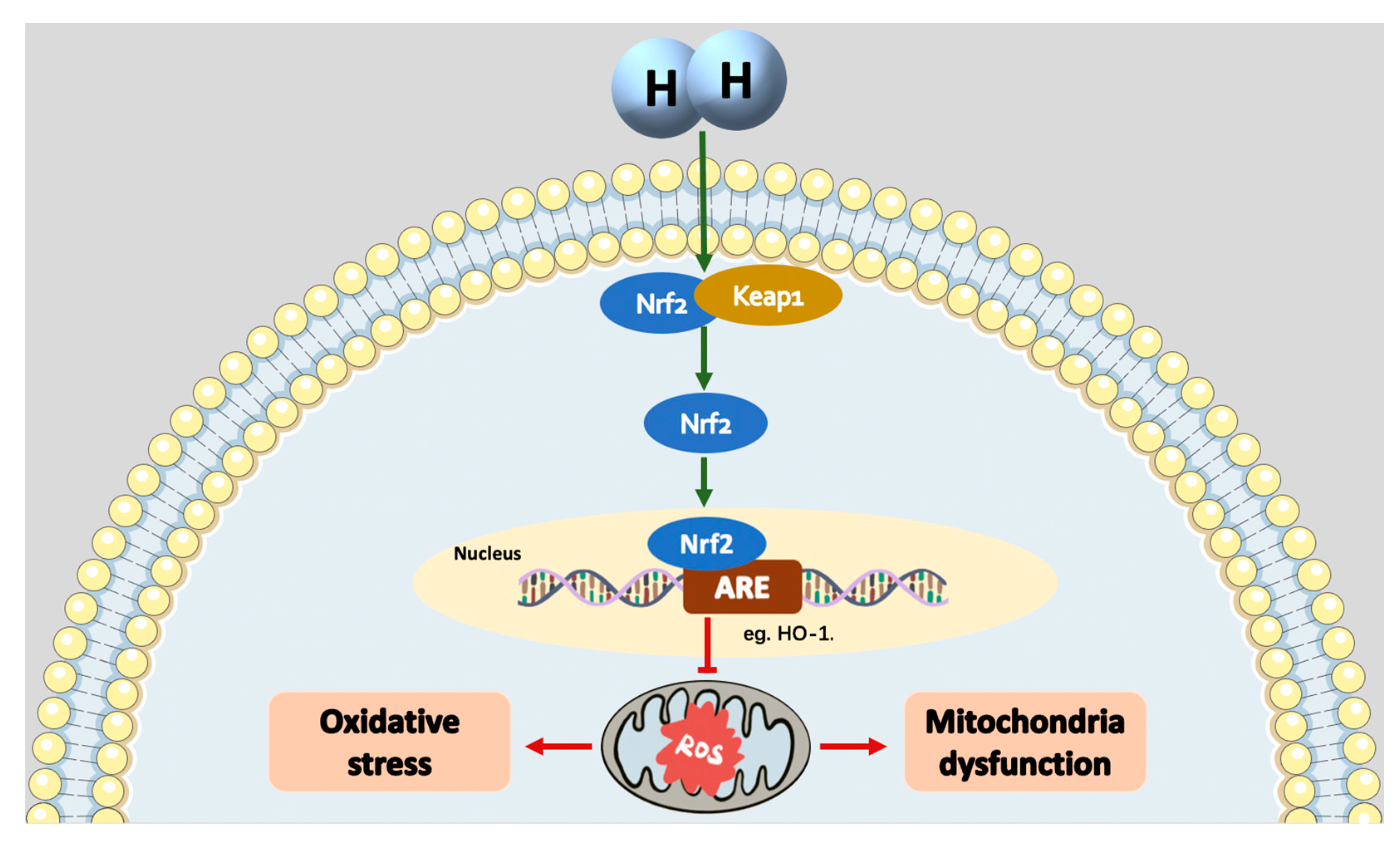
4. The mechanisms of H2 as an Nrf2 activator
5. The Medical effects of H2: Focus on the effect on mitochondria
5.1. Effects of H2 on Respiratory System Diseases
5.2. Effects of H2 on Cardiovascular System Diseases
5.3. Effects of H2 on Nervous System Diseases
5.4. Effects of H2 on Digestive System Diseases
5.5. Effects of H2 on Metabolic Syndrome
5.6. The Others
4. Conclusions and perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, J.W.; Shakir, D.; Batie, M.; Frost, M.; Rocha, S. Oxygen-sensing mechanisms in cells. The FEBS journal 2020, 287, 3888–3906. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Ichinose, F.; Bloch, D.B.; Zapol, W.M. Inhaled nitric oxide. Br J Pharmacol 2019, 176, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Roccarina, D.; Lauritano, E.C.; Gabrielli, M.; Franceschi, F.; Ojetti, V.; Gasbarrini, A. The role of methane in intestinal diseases. Am J Gastroenterol 2010, 105, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Sethi, J.; Choi, A.M. Carbon monoxide-dependent signaling. Crit Care Med 2002, 30, S12–17. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Motl, N.; Banerjee, R. H2S and its role in redox signaling. Biochimica et biophysica acta 2014, 1844, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.F.; Sun, X.J.; Xia, Z.F. Hydrogen resuscitation, a new cytoprotective approach. Clinical and experimental pharmacology & physiology 2011, 38, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Chen, S.; Zhang, J.M. Hydrogen as a selective antioxidant: a review of clinical and experimental studies. The Journal of international medical research 2010, 38, 1893–1903. [Google Scholar] [CrossRef]
- Levitt, M.D. Production and excretion of hydrogen gas in man. The New England journal of medicine 1969, 281, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Zhao, L.; Shen, M.; Noda, M.; Qin, S.; Long, J.; Sun, X.; Liu, J. Hydrogen medicine: A rising star in gas medicine. 2020, 03, 153-161. [CrossRef]
- Dole, M.; Wilson, F.R.; Fife, W.P. Hyperbaric hydrogen therapy: a possible treatment for cancer. Science (New York, N.Y.) 1975, 190, 152–154. [Google Scholar] [CrossRef]
- Du, Y.; Wei, C. The reflection on the materiality of qi and its application prospect. Zibo Keji Bao (Zibo Sci & Tech News) 1996.
- Du, Y.; Wei, C. New scientific topics — a preliminary study on the significance of hydrogen in life activities.ity of qi and its application prospect. Journal of Shandong Normal University 1999, 2, 196–197. [Google Scholar]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nature medicine 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Sato, Y.; Kajiyama, S.; Amano, A.; Kondo, Y.; Sasaki, T.; Handa, S.; Takahashi, R.; Fukui, M.; Hasegawa, G.; Nakamura, N.; et al. Hydrogen-rich pure water prevents superoxide formation in brain slices of vitamin C-depleted SMP30/GNL knockout mice. Biochemical and biophysical research communications 2008, 375, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Kang, Z.; Cai, J.; Liu, W.; Liu, Y.; Zhang, J.H.; Denoble, P.J.; Tao, H.; Sun, X. Hydrogen-rich saline protects myocardium against ischemia/reperfusion injury in rats. Experimental biology and medicine (Maywood, N.J.) 2009, 234, 1212–1219. [Google Scholar] [CrossRef]
- Kajiya, M.; Sato, K.; Silva, M.J.; Ouhara, K.; Do, P.M.; Shanmugam, K.T.; Kawai, T. Hydrogen from intestinal bacteria is protective for Concanavalin A-induced hepatitis. Biochemical and biophysical research communications 2009, 386, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Kissner, R.; Koppenol, W.H. ONOOH does not react with H2: Potential beneficial effects of H2 as an antioxidant by selective reaction with hydroxyl radicals and peroxynitrite. Free radical biology & medicine 2014, 75, 191–194. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Y.; Bian, Y.; Li, Y.; Liu, L.; Zhang, H.; Xie, K.; Wang, G.; Yu, Y. Hydrogen Gas Protects Against Intestinal Injury in Wild Type But Not NRF2 Knockout Mice With Severe Sepsis by Regulating HO-1 and HMGB1 Release. Shock (Augusta, Ga.) 2017, 48, 364–370. [Google Scholar] [CrossRef]
- Hyspler, R.; Ticha, A.; Schierbeek, H.; Galkin, A.; Zadak, Z. The Evaluation and Quantitation of Dihydrogen Metabolism Using Deuterium Isotope in Rats. PLoS One 2015, 10, e0130687. [Google Scholar] [CrossRef]
- Itoh, T.; Fujita, Y.; Ito, M.; Masuda, A.; Ohno, K.; Ichihara, M.; Kojima, T.; Nozawa, Y.; Ito, M. Molecular hydrogen suppresses FcepsilonRI-mediated signal transduction and prevents degranulation of mast cells. Biochemical and biophysical research communications 2009, 389, 651–656. [Google Scholar] [CrossRef]
- Jiang Xue; Liu Boyan; Wu Fenglin; Xue Yazhuo; Shucun, Q. Progress in clinical research on the use of hydrogen molecules in preventive health care. Chinese Journal of Geriatric Care 2023, 117-122.
- Liu, M.Y.; Xie, F.; Zhang, Y.; Wang, T.T.; Ma, S.N.; Zhao, P.X.; Zhang, X.; Lebaron, T.W.; Yan, X.L.; Ma, X.M. Molecular hydrogen suppresses glioblastoma growth via inducing the glioma stem-like cell differentiation. Stem Cell Res Ther 2019, 10, 145. [Google Scholar] [CrossRef]
- Smith, R.A.J.; Hartley, R.C.; Cochemé, H.M.; Murphy, M.P. Mitochondrial pharmacology. Trends in Pharmacological Sciences 2012, 33, 341–352. [Google Scholar] [CrossRef]
- Chen, X.; Cui, J.; Zhai, X.; Zhang, J.; Gu, Z.; Zhi, X.; Weng, W.; Pan, P.; Cao, L.; Ji, F.; et al. Inhalation of Hydrogen of Different Concentrations Ameliorates Spinal Cord Injury in Mice by Protecting Spinal Cord Neurons from Apoptosis, Oxidative Injury and Mitochondrial Structure Damages. Cell Physiol Biochem 2018, 47, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Song, R.; Pang, Q.; Liu, Y.; Zhuang, J.; Chen, Y.; Hu, J.; Hu, J.; Liu, Y.; Liu, Z.; et al. Propofol inhibits parthanatos via ROS-ER-calcium-mitochondria signal pathway in vivo and vitro. Cell Death Dis 2018, 9, 932. [Google Scholar] [CrossRef]
- Ostojic, S.M. Targeting molecular hydrogen to mitochondria: barriers and gateways. Pharmacological research 2015, 94, 51–53. [Google Scholar] [CrossRef]
- Ito, M.; Ibi, T.; Sahashi, K.; Ichihara, M.; Ito, M.; Ohno, K. Open-label trial and randomized, double-blind, placebo-controlled, crossover trial of hydrogen-enriched water for mitochondrial and inflammatory myopathies. Medical gas research 2011, 1, 24. [Google Scholar] [CrossRef]
- Murphy, M.P.; Smith, R.A. Drug delivery to mitochondria: the key to mitochondrial medicine. Advanced drug delivery reviews 2000, 41, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.M.; Neupert, W. Protein transport into mitochondria. Current Opinion in Microbiology 2000, 3, 210–214. [Google Scholar] [CrossRef]
- Ohta, S. Recent progress toward hydrogen medicine: potential of molecular hydrogen for preventive and therapeutic applications. Current pharmaceutical design 2011, 17, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochimica et Biophysica Acta (BBA) - General Subjects 2012, 1820, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Chen, X.; Ohta, S.; Sun, X. Review and prospect of the biomedical effects of hydrogen. Medical gas research 2014, 4, 19. [Google Scholar] [CrossRef]
- Sun, X.; Ohta, S.; Zhang, J.H. Discovery of a hydrogen molecular target. Medical gas research 2023, 13, 41–42. [Google Scholar] [CrossRef]
- Liu, J.; Ames, B.N. Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer's disease, and Parkinson's disease. Nutritional neuroscience 2005, 8, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, W.; Zhao, B.; Wang, Y.; Wertz, K.; Weber, P.; Zhang, P. Targeting mitochondrial biogenesis for preventing and treating insulin resistance in diabetes and obesity: Hope from natural mitochondrial nutrients. Advanced drug delivery reviews 2009, 61, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Gao, P.; Shi, L.; Chen, L.; Liu, J.; Long, J. Central and Peripheral Metabolic Defects Contribute to the Pathogenesis of Alzheimer's Disease: Targeting Mitochondria for Diagnosis and Prevention. Antioxidants & redox signaling 2020, 32, 1188–1236. [Google Scholar] [CrossRef]
- Hou, C.; Wang, Y.; Zhu, E.; Yan, C.; Zhao, L.; Wang, X.; Qiu, Y.; Shen, H.; Sun, X.; Feng, Z.; et al. Coral calcium hydride prevents hepatic steatosis in high fat diet-induced obese rats: A potent mitochondrial nutrient and phase II enzyme inducer. Biochem Pharmacol 2016, 103, 85–97. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, J.; Feng, Z.; Cao, K.; Liu, J.; Xu, H. Hydrogen-rich and hyperoxygenate saline inhibits lipopolysaccharide-induced lung injury through mediating NF-κB/NLRP3 signaling pathway in C57BL/6 mice. Environ Toxicol 2022, 37, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Liu, C.; Zhou, L.; Qu, K.; Wang, R.; Tai, M.H.; Lei Lei, J.C.; Wu, Q.F.; Wang, Z.X. A review of hydrogen as a new medical therapy. Hepato-gastroenterology 2012, 59, 1026–1032. [Google Scholar] [CrossRef]
- Hirano, S.I.; Ichikawa, Y.; Kurokawa, R.; Takefuji, Y.; Satoh, F. A "philosophical molecule," hydrogen may overcome senescence and intractable diseases. Medical gas research 2020, 10, 47–49. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox biology 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochimica et biophysica acta. Molecular cell research 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Lu, Y.; Li, C.F.; Ping, N.N.; Sun, Y.Y.; Wang, Z.; Zhao, G.X.; Yuan, S.H.; Zibrila, A.I.; Soong, L.; Liu, J.J. Hydrogen-rich water alleviates cyclosporine A-induced nephrotoxicity via the Keap1/Nrf2 signaling pathway. Journal of biochemical and molecular toxicology 2020, 34, e22467. [Google Scholar] [CrossRef]
- Sun, R.; Zhao, N.; Wang, Y.; Su, Y.; Zhang, J.; Wang, Y.; Yu, Y.; Wang, G.; Wang, Z.; Xie, K. High concentration of hydrogen gas alleviates Lipopolysaccharide-induced lung injury via activating Nrf2 signaling pathway in mice. International immunopharmacology 2021, 101, 108198. [Google Scholar] [CrossRef] [PubMed]
- Gatbonton-Schwager, T.; Yagishita, Y.; Joshi, T.; Wakabayashi, N.; Srinivasan, H.; Suzuki, T.; Yamamoto, M.; Kensler, T.W. A Point Mutation at C151 of Keap1 of Mice Abrogates NRF2 Signaling, Cytoprotection in Vitro, and Hepatoprotection in Vivo by Bardoxolone Methyl (CDDO-Me). Molecular pharmacology 2023, 104, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhang, G.; Zheng, Y.; Yang, Y.; Chen, C.; Xia, J.; Liang, L.; Lei, C.; Hu, Y.; Cai, X.; et al. SLC27A5 deficiency activates NRF2/TXNRD1 pathway by increased lipid peroxidation in HCC. Cell death and differentiation 2020, 27, 1086–1104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Hannink, M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 2003, 23, 8137–8151. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Chun, K.S.; Kim, D.H.; Kim, S.J.; Kim, S.H.; Cho, N.C.; Na, H.K.; Surh, Y.J. Curcumin induces stabilization of Nrf2 protein through Keap1 cysteine modification. Biochem Pharmacol 2020, 173, 113820. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Muramatsu, A.; Saito, R.; Iso, T.; Shibata, T.; Kuwata, K.; Kawaguchi, S.I.; Iwawaki, T.; Adachi, S.; Suda, H.; et al. Molecular Mechanism of Cellular Oxidative Stress Sensing by Keap1. Cell reports 2019, 28, 746–758.e744. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol Cell Biol 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Suzuki, T.; Inoue, J.; Iso, T.; Wells, G.; Moore, T.W.; Mizushima, T.; Dinkova-Kostova, A.T.; Kasai, T.; Kamei, T.; et al. Molecular basis for the disruption of Keap1-Nrf2 interaction via Hinge & Latch mechanism. Commun Biol 2021, 4, 576. [Google Scholar] [CrossRef]
- Liu, B.; Xue, J.; Zhang, M.; Wang, M.; Ma, T.; Zhao, M.; Gu, Q.; Qin, S. Hydrogen inhalation alleviates nonalcoholic fatty liver disease in metabolic syndrome rats. Mol Med Rep 2020, 22, 2860–2868. [Google Scholar] [CrossRef]
- Murakami, Y.; Ito, M.; Ohsawa, I. Molecular hydrogen protects against oxidative stress-induced SH-SY5Y neuroblastoma cell death through the process of mitohormesis. PLoS One 2017, 12, e0176992. [Google Scholar] [CrossRef]
- Iketani, M.; Sakane, I.; Fujita, Y.; Ito, M.; Ohsawa, I. H(2)-induced transient upregulation of phospholipids with suppression of energy metabolism. Medical gas research 2023, 13, 133–141. [Google Scholar] [CrossRef]
- Jesus, A.A.; Passaglia, P.; Santos, B.M.; Rodrigues-Santos, I.; Flores, R.A.; Batalhão, M.E.; Stabile, A.M.; Cárnio, E.C. Chronic molecular hydrogen inhalation mitigates short and long-term memory loss in polymicrobial sepsis. Brain research 2020, 1739, 146857. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Feng, J.; Lian, N.; Yang, M.; Xie, K.; Wang, G.; Wang, C.; Yu, Y. Hydrogen gas alleviates blood-brain barrier impairment and cognitive dysfunction of septic mice in an Nrf2-dependent pathway. International immunopharmacology 2020, 85, 106585. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zheng, Y.; Zhu, K.; Cai, Z.; Liu, W.; Sun, X.; Liu, J.; Zhu, D. Hydrogen gas protects against delayed encephalopathy after acute carbon monoxide poisoning in a rat model. Neurol Res 2020, 42, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Zhang, S.; Wu, L.; Huan, L.; Huang, F.; Cui, Y.; Lin, Z. Hydrogen Gas Inhalation Attenuates Seawater Instillation-Induced Acute Lung Injury via the Nrf2 Pathway in Rabbits. Inflammation 2016, 39, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.G.; Xie, K.L.; Han, H.Z.; Wang, W.N.; Liu, D.Q.; Wang, G.L.; Yu, Y.H. Heme oxygenase-1 mediates the anti-inflammatory effect of molecular hydrogen in LPS-stimulated RAW 264.7 macrophages. International journal of surgery (London, England) 2013, 11, 1060–1066. [Google Scholar] [CrossRef]
- Kawamura, T.; Wakabayashi, N.; Shigemura, N.; Huang, C.S.; Masutani, K.; Tanaka, Y.; Noda, K.; Peng, X.; Takahashi, T.; Billiar, T.R.; et al. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am J Physiol Lung Cell Mol Physiol 2013, 304, L646–656. [Google Scholar] [CrossRef]
- Xie, K.; Yu, Y.; Pei, Y.; Hou, L.; Chen, S.; Xiong, L.; Wang, G. Protective effects of hydrogen gas on murine polymicrobial sepsis via reducing oxidative stress and HMGB1 release. Shock (Augusta, Ga.) 2010, 34, 90–97. [Google Scholar] [CrossRef]
- Li, Y.; Xie, K.; Chen, H.; Wang, G.; Yu, Y. Hydrogen gas inhibits high-mobility group box 1 release in septic mice by upregulation of heme oxygenase 1. The Journal of surgical research 2015, 196, 136–148. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Fu, Z. Molecular hydrogen is a potential protective agent in the management of acute lung injury. Molecular medicine (Cambridge, Mass.) 2022, 28, 27. [Google Scholar] [CrossRef]
- Xie, Q.; Li, X.X.; Zhang, P.; Li, J.C.; Cheng, Y.; Feng, Y.L.; Huang, B.S.; Zhuo, Y.F.; Xu, G.H. Hydrogen gas protects against serum and glucose deprivation-induced myocardial injury in H9c2 cells through activation of the NF-E2-related factor 2/heme oxygenase 1 signaling pathway. Mol Med Rep 2014, 10, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xie, K.; Han, H.; Li, Y.; Liu, L.; Yang, T.; Yu, Y. Molecular hydrogen protects mice against polymicrobial sepsis by ameliorating endothelial dysfunction via an Nrf2/HO-1 signaling pathway. International immunopharmacology 2015, 28, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Chen, H.; Wang, G.; Yu, Y.; Xie, K. Hydrogen-rich saline attenuates chemotherapy-induced ovarian injury via regulation of oxidative stress. Exp Ther Med 2015, 10, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.C.; Devendran, S.; Méndez-García, C.; Mythen, S.M.; Wright, C.L.; Fields, C.J.; Hernandez, A.G.; Cann, I.; Hylemon, P.B.; Ridlon, J.M. Bile acid oxidation by Eggerthella lenta strains C592 and DSM 2243(T). Gut microbes 2018, 9, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Gallenga, C.E.; Tetè, G.; Caraffa, A.; Ronconi, G.; Younes, A.; Toniato, E.; Ross, R.; Kritas, S.K. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. Journal of biological regulators and homeostatic agents 2020, 34, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, J.; Feng, Z.; Cao, K.; Liu, J.; Xu, H. Hydrogen-rich and hyperoxygenate saline inhibits lipopolysaccharide-induced lung injury through mediating NF-kappaB/NLRP3 signaling pathway in C57BL/6 mice. Environ Toxicol 2022, 37, 1575–1586. [Google Scholar] [CrossRef]
- Dong, A.; Yu, Y.; Wang, Y.; Li, C.; Chen, H.; Bian, Y.; Zhang, P.; Zhao, Y.; Yu, Y.; Xie, K. Protective effects of hydrogen gas against sepsis-induced acute lung injury via regulation of mitochondrial function and dynamics. International immunopharmacology 2018, 65, 366–372. [Google Scholar] [CrossRef]
- Yuan, L.; Shen, J. Hydrogen, a potential safeguard for graft-versus-host disease and graft ischemia-reperfusion injury? Clinics (Sao Paulo, Brazil) 2016, 71, 544–549. [Google Scholar] [CrossRef]
- Kawamura, T.; Huang, C.S.; Tochigi, N.; Lee, S.; Shigemura, N.; Billiar, T.R.; Okumura, M.; Nakao, A.; Toyoda, Y. Inhaled hydrogen gas therapy for prevention of lung transplant-induced ischemia/reperfusion injury in rats. Transplantation 2010, 90, 1344–1351. [Google Scholar] [CrossRef]
- Liu, R.; Fang, X.; Meng, C.; Xing, J.; Liu, J.; Yang, W.; Li, W.; Zhou, H. Lung inflation with hydrogen during the cold ischemia phase decreases lung graft injury in rats. Experimental biology and medicine (Maywood, N.J.) 2015, 240, 1214–1222. [Google Scholar] [CrossRef]
- Tanaka, Y.; Shigemura, N.; Kawamura, T.; Noda, K.; Isse, K.; Stolz, D.B.; Billiar, T.R.; Toyoda, Y.; Bermudez, C.A.; Lyons-Weiler, J.; et al. Profiling molecular changes induced by hydrogen treatment of lung allografts prior to procurement. Biochemical and biophysical research communications 2012, 425, 873–879. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, Y.; Wu, S.; Wu, W.; Deng, Y.; Shao, A. Molecular hydrogen: A potential radioprotective agent. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2020, 130, 110589. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, Y.; Ohsawa, I.; Terasaki, M.; Takahashi, M.; Kunugi, S.; Dedong, K.; Urushiyama, H.; Amenomori, S.; Kaneko-Togashi, M.; Kuwahara, N.; et al. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. Am J Physiol Lung Cell Mol Physiol 2011, 301, L415–426. [Google Scholar] [CrossRef] [PubMed]
- Saengsin, K.; Sittiwangkul, R.; Chattipakorn, S.C.; Chattipakorn, N. Hydrogen therapy as a potential therapeutic intervention in heart disease: from the past evidence to future application. Cellular and molecular life sciences: CMLS 2023, 80, 174. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Zhang, Z.; Liu, L.; Zhou, Y.; Liu, F. Protective Mechanism and Clinical Application of Hydrogen in Myocardial Ischemia-reperfusion Injury. Pakistan journal of biological sciences: PJBS 2020, 23, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jia, L.; Chen, B.; Zhang, L.; Liu, J.; Long, J.; Li, Y. Hydrogen Inhalation is Superior to Mild Hypothermia in Improving Cardiac Function and Neurological Outcome in an Asphyxial Cardiac Arrest Model of Rats. Shock (Augusta, Ga.) 2016, 46, 312–318. [Google Scholar] [CrossRef]
- Luo, Z.L.; Cheng, L.; Ren, J.D.; Fang, C.; Xiang, K.; Xu, H.T.; Tang, L.J.; Wang, T.; Tian, F.Z. Hydrogen-rich saline protects against ischemia/reperfusion injury in grafts after pancreas transplantations by reducing oxidative stress in rats. Mediators of inflammation 2015, 2015, 281985. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Chen, H.; Wu, Q.; Xie, K. Hydrogen-rich saline alleviates inflammation and apoptosis in myocardial I/R injury via PINK-mediated autophagy. International journal of molecular medicine 2019, 44, 1048–1062. [Google Scholar] [CrossRef]
- Feng, R.; Cai, M.; Wang, X.; Zhang, J.; Tian, Z. Early Aerobic Exercise Combined with Hydrogen-Rich Saline as Preconditioning Protects Myocardial Injury Induced by Acute Myocardial Infarction in Rats. Applied biochemistry and biotechnology 2019, 187, 663–676. [Google Scholar] [CrossRef]
- Noda, K.; Tanaka, Y.; Shigemura, N.; Kawamura, T.; Wang, Y.; Masutani, K.; Sun, X.; Toyoda, Y.; Bermudez, C.A.; Nakao, A. Hydrogen-supplemented drinking water protects cardiac allografts from inflammation-associated deterioration. Transplant international: official journal of the European Society for Organ Transplantation 2012, 25, 1213–1222. [Google Scholar] [CrossRef]
- Qiu, P.; Liu, Y.; Zhang, J. Recent Advances in Studies of Molecular Hydrogen against Sepsis. International journal of biological sciences 2019, 15, 1261–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, A.; Xie, K.; Yu, Y. Protective Effects of Hydrogen on Myocardial Mitochondrial Functions in Septic Mice. Biomed Res Int 2020, 2020, 1568209. [Google Scholar] [CrossRef]
- Tao, B.; Liu, L.; Wang, N.; Tong, D.; Wang, W.; Zhang, J. Hydrogen-Rich Saline Attenuates Lipopolysaccharide-Induced Heart Dysfunction by Restoring Fatty Acid Oxidation in Rats by Mitigating C-Jun N-Terminal Kinase Activation. Shock (Augusta, Ga.) 2015, 44, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.S.; Zheng, H. Chronic hydrogen-rich saline treatment reduces oxidative stress and attenuates left ventricular hypertrophy in spontaneous hypertensive rats. Molecular and cellular biochemistry 2012, 365, 233–242. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; He, B.; Xiao, J.; Wang, Z.; Sun, X. Anti-inflammatory effect of hydrogen-rich saline in a rat model of regional myocardial ischemia and reperfusion. International journal of cardiology 2011, 148, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Htun, Y.; Nakamura, S.; Kusaka, T. Hydrogen and therapeutic gases for neonatal hypoxic-ischemic encephalopathy: potential neuroprotective adjuncts in translational research. Pediatric research 2021, 89, 753–759. [Google Scholar] [CrossRef]
- Noda, M.; Liu, J.; Long, J. Neuroprotective and Preventative Effects of Molecular Hydrogen. Current pharmaceutical design 2021, 27, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Peng, Y.; Qin, C.; Fan, F.; Liu, J.; Long, J. Hydrogen-rich water improves cognitive impairment gender-dependently in APP/PS1 mice without affecting Aβ clearance. Free radical research 2018, 52, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Yoritaka, A.; Takanashi, M.; Hirayama, M.; Nakahara, T.; Ohta, S.; Hattori, N. Pilot study of H₂ therapy in Parkinson's disease: a randomized double-blind placebo-controlled trial. Movement disorders: official journal of the Movement Disorder Society 2013, 28, 836–839. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, H.; Ji, M.; Jia, M.; Chen, H.; Yang, J.; Duan, M. Hydrogen-rich saline attenuates neuronal ischemia--reperfusion injury by protecting mitochondrial function in rats. The Journal of surgical research 2014, 192, 564–572. [Google Scholar] [CrossRef]
- Li, Q.; Yu, P.; Zeng, Q.; Luo, B.; Cai, S.; Hui, K.; Yu, G.; Zhu, C.; Chen, X.; Duan, M.; et al. Neuroprotective Effect of Hydrogen-Rich Saline in Global Cerebral Ischemia/Reperfusion Rats: Up-Regulated Tregs and Down-Regulated miR-21, miR-210 and NF-κB Expression. Neurochemical research 2016, 41, 2655–2665. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hou, L.; Chen, D.; Lin, F.; Chang, T.; Li, M.; Zhang, L.; Niu, X.; Wang, H.; Fu, S.; et al. Hydrogen-rich saline attenuates isoflurane-induced caspase-3 activation and cognitive impairment via inhibition of isoflurane-induced oxidative stress, mitochondrial dysfunction, and reduction in ATP levels. American journal of translational research 2017, 9, 1162–1172. [Google Scholar] [PubMed]
- Xia, C.; Liu, W.; Zeng, D.; Zhu, L.; Sun, X.; Sun, X. Effect of hydrogen-rich water on oxidative stress, liver function, and viral load in patients with chronic hepatitis B. Clinical and translational science 2013, 6, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chiang, J.Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacological reviews 2014, 66, 948–983. [Google Scholar] [CrossRef]
- Hu, H.L.; Gao, J.; Guo, W.J.; Zhou, F.H.; Liu, H.Y.; Su, C.C. Anti-injury effect of hydrogen-enriched water in a rat model of liver injury induced by aflatoxin B(1). Sheng Li Xue Bao 2019, 71, 725–731. [Google Scholar] [PubMed]
- Liang, B.; Shi, L.; Du, D.; Li, H.; Yi, N.; Xi, Y.; Cui, J.; Li, P.; Kang, H.; Noda, M.; et al. Hydrogen-Rich Water Ameliorates Metabolic Disorder via Modifying Gut Microbiota in Impaired Fasting Glucose Patients: A Randomized Controlled Study. Antioxidants (Basel, Switzerland) 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, S.; Qin, M.; Mao, Y.; Jin, L.; Che, N.; Liu, S.; Ge, R. Hydrogen-Rich Saline Ameliorates Allergic Rhinitis by Reversing the Imbalance of Th1/Th2 and Up-Regulation of CD4+CD25+Foxp3+Regulatory T Cells, Interleukin-10, and Membrane-Bound Transforming Growth Factor-β in Guinea Pigs. Inflammation 2018, 41, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Guo, A.; Han, X.; Wu, S.; Chen, C.; Luo, C.; Li, H.; Li, S.; Hei, Z. Aerosol inhalation of a hydrogen-rich solution restored septic renal function. Aging 2019, 11, 12097–12113. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Hu, J.; Gu, Y.; Shen, Z.; Xu, L.; Jia, X.; Zhang, X.; Ding, X. Hydrogen-Rich Saline Alleviates Kidney Fibrosis Following AKI and Retains Klotho Expression. Frontiers in pharmacology 2017, 8, 499. [Google Scholar] [CrossRef]
- Shi, Q.; Liao, K.S.; Zhao, K.L.; Wang, W.X.; Zuo, T.; Deng, W.H.; Chen, C.; Yu, J.; Guo, W.Y.; He, X.B.; et al. Hydrogen-rich saline attenuates acute renal injury in sodium taurocholate-induced severe acute pancreatitis by inhibiting ROS and NF-κB pathway. Mediators of inflammation 2015, 2015, 685043. [Google Scholar] [CrossRef]
- Xie, F.; Song, Y.; Yi, Y.; Jiang, X.; Ma, S.; Ma, C.; Li, J.; Zhanghuang, Z.; Liu, M.; Zhao, P.; et al. Therapeutic Potential of Molecular Hydrogen in Metabolic Diseases from Bench to Bedside. Pharmaceuticals (Basel) 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Adzavon, Y.M.; Xie, F.; Yi, Y.; Jiang, X.; Zhang, X.; He, J.; Zhao, P.; Liu, M.; Ma, S.; Ma, X. Long-term and daily use of molecular hydrogen induces reprogramming of liver metabolism in rats by modulating NADP/NADPH redox pathways. Scientific reports 2022, 12, 3904. [Google Scholar] [CrossRef] [PubMed]
- Korovljev, D.; Trivic, T.; Drid, P.; Ostojic, S.M. Molecular hydrogen affects body composition, metabolic profiles, and mitochondrial function in middle-aged overweight women. Irish journal of medical science 2018, 187, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, K.; Nishimaki, K.; Kamimura, N.; Ohta, S. Molecular hydrogen suppresses free-radical-induced cell death by mitigating fatty acid peroxidation and mitochondrial dysfunction. Canadian journal of physiology and pharmacology 2019, 97, 999–1005. [Google Scholar] [CrossRef]
- Ito, M.; Ibi, T.; Sahashi, K.; Ichihara, M.; Ito, M.; Ohno, K. Open-label trial and randomized, double-blind, placebo-controlled, crossover trial of hydrogen-enriched water for mitochondrial and inflammatory myopathies. Medical gas research 2011, 1, 24. [Google Scholar] [CrossRef]
| Mechanism | Example | ||
|---|---|---|---|
| Class I | Elec-trophilic | Cys151-dependent compounds | Bardoxolone methyl Sulforaphane, Dimethyl-fumarate |
| Class II | Target Cys288 | 15d-PGJ2 | |
| Class III | React with any of the three sensor cysteines Cys151/Cys273/Cys288 | 4-HNE, NaAsO2, 9-nitro-octadec-9-enoic acid |
|
| Class IV | Target cysteines Cys77/Cys434 | Pubescenoside A | |
| Class V | Non-Electrophilic | Target Cys226/Cys613/Cys622/Cys624 | H2O2, Cadmium chloride, Zinc chloride, Prostaglandin A2 |
| Class VI | Protein-protein interaction inhibitors (PPI) | CPUY192018 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





