1. Introduction:
Emerging infectious diseases (EIDs) continue to be a problem all across the world, but in certain areas the threat is greater than in others. The detection of new diseases will aid in the focus of surveillance efforts [
1]. Specifically, identifying a "patient zero" is critical step to help limit further transmission. Another factor to limit infectious diseases is to preserve the forestry areas. Yet, during the last 40 years, Southeast Asia has had the highest incidence of deforestation worldwide, with a degradation of 30% of woodland [
2].
In the last two decades, several high impact zoonotic disease outbreaks have been linked to bat-borne viruses. These include SARS coronavirus, Hendra virus and Nipah virus [
3]. In addition, it has been suspected that ebolaviruses and MERS coronavirus are also linked to bats [
4]. It is being increasingly accepted that bats are potential reservoirs of a large number of known and unknown viruses, many of which could spill-over into animal and human populations. However, our knowledge into basic bat biology and immunology is very limited and we have little understanding of major factors contributing to the risk of bat virus spillover events [
4].
A recent phylogenetic study has provided strong evidence that viruses isolated from bats in China are clustering by geographical location rather than by bat species [
5], suggesting that high contact rates among specific bat species favor the spread of Coronaviruses (CoVs). Notably, only a small minority of the estimated 1,240 bat species has been tested for CoVs. It is likely that many more CoVs could be discovered in bats. Although 31% of bat-borne viruses are CoVs [
6]. only 6% of all CoV sequences in GenBank are from bat CoVs. Even though the direct transmission of bat CoVs to humans has not been evidenced yet, the creation of conditions for more frequent encounters between bat CoVs, domestic animals and humans poses a significant threat for the future [
7].
The risk associated with a higher biodiversity of bats and a higher density of bat populations in close proximity to humans [
8]. Anthony and colleagues have estimated that there are at least 3,204 CoVs currently circulating in bats [
9]. Whatever the accuracy of that prediction, it remains obvious that the risk for new viruses to emerge from bats is probably very high. By being one of the regions of the world where population growth is the strongest, where sanitary conditions remain poor and where the deforestation rate is the highest, Southeast Asia meets every condition to become the place of emergence or re-emergence of infectious diseases [
2].
Unfortunately, the problem of bat-borne viruses is not restricted to CoVs. Among the 60 viral species reported to be associated with bats, 59 are RNA viruses which might possibly beresponsible for emerging and re-emerging infectious diseases in humans [
10].
The lack of understanding of how climate change and environmental degradation contribute to the emergence and spread of infectious diseases. While there is evidence that changes in temperature, precipitation, and land use can affect the distribution and abundance of disease vectors and hosts, more research is needed to fully understand the complex interactions between environmental factors and infectious disease dynamics. Additionally, there is a need for more studies that explore how human activities such as deforestation, urbanization, and global travel contribute to the emergence and spread of infectious diseases. Understanding these risk factors can inform public health strategies aimed at preventing or mitigating outbreaks of emerging infectious diseases. Thus, in this review article, we discuss the potential risk factors that contribute in emerging infectious diseases. Also, we discuss the management of disease prevention and control.
2. Deforestation:
The global rate of tropical deforestation appears to be increasing readily; between 2000 and 2005, more than 2.3% of the tropical humid forests were cleared [
11]. Infectious diseases can emarge as a result of deforestation, population growth, livestock farming, and the trade in wild animals.
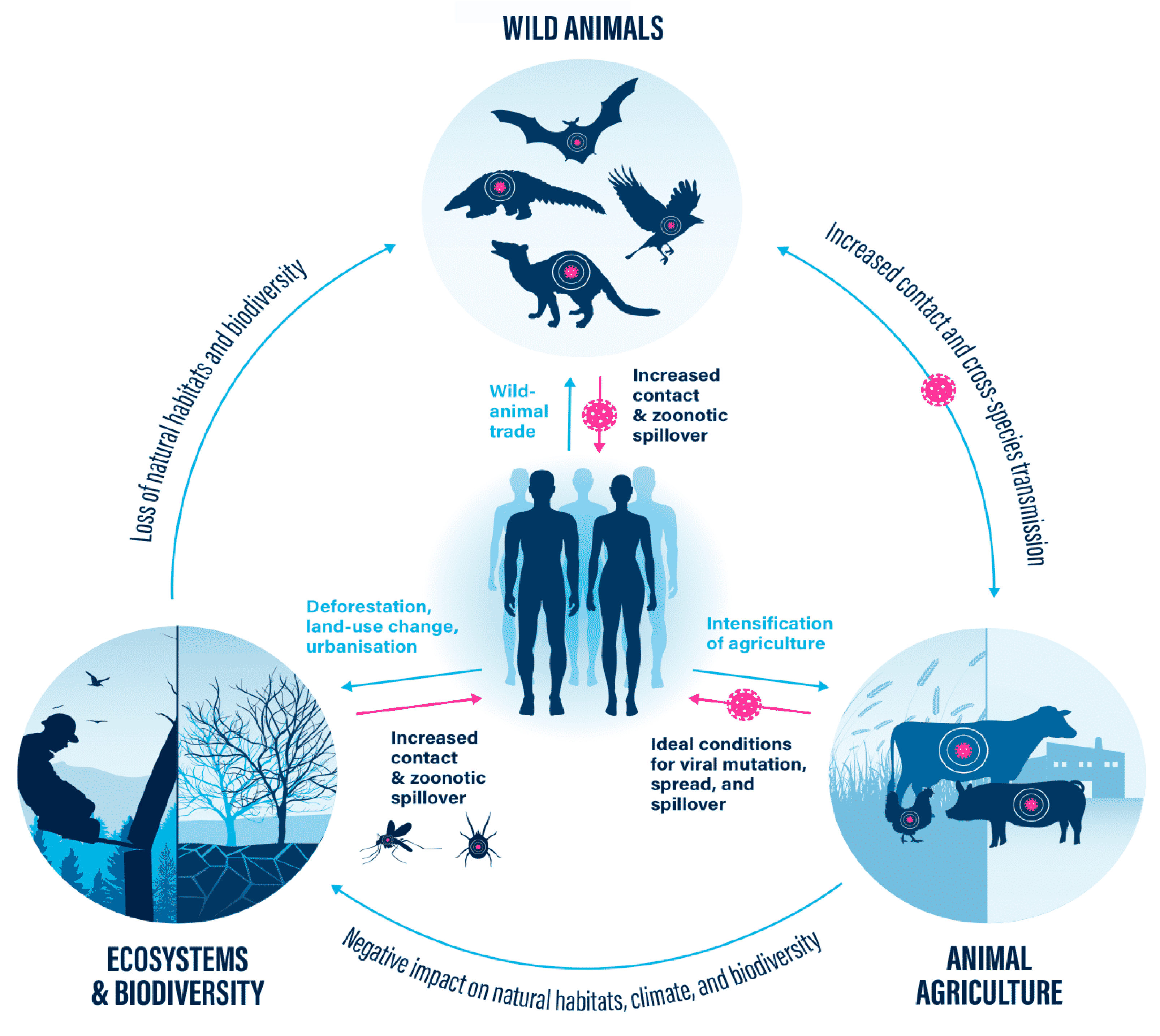
(Figure 1). The loss of natural habitats and biodiversity disrupts the balance between wildlife and ecosystems. As humans encroach upon natural habitats, animals are forced into closer proximity with domestic animals and humans, increasing the chances of pathogen transmission. Environmental degradation, deforestation, and urbanization further amplify this risk.
Viral transmission is accelerated by human-caused deforestation. The borders of tropical woods are where cases of viruses spreading from wildlife to humans most frequently occur. Here, deforestation is fostering more interactions between people and animals in their natural settings.
Fatal diseases such as yellow fever, malaria and Ebola jumped from one species to another at the margins of forests. Deforestation has frequently contributed to these incidents. Demand for four commodities—beef, soy, palm oil, and wood products—is the cause of more than 50% of the world's tropical deforestation. Deforested areas replace mature biodiverse tropical forests, with mono-crop fields and pastures that are foreign to the land [
12].
Isolated Animals living in remaining native vegetation attempt to adapt to their new habitat [
13]. Additionally, when people live in these forests, wildlife and human interaction may become more frequent. New adventurous animals could consequently move into this contact region. For instance, yellow fever is a virus spread by mosquitoes [
14]. The virus that causes it lives in primates and is spread by mosquitoes. These tend to live close to where these primates live [
14]. Perhaps, the most serious health danger posed by arboviral emergence is from widespread tropical urbanization and the colonization of this increasing territory by the extremely anthropophilic (attracted to humans) mosquito,
Aedes aegypti [
15].
Between 2016 and 2018, South America witnessed its largest number of yellow fever cases in decades. The extremely vulnerable Atlantic Forest of Brazil was mostly affected [
16]. This biodiverse area has shrunk to 7% of its original forest cover due to deforestation. One study has shown that shrinking habitat increases the concentration of primates [
17]. It demonstrates that forest fragmentation led to a greater density of primates, which in turn leads to pathogens becoming more common. Several studies have also shown that deforestation and forest fragmentation were associated with Ebola outbreaks between 2004 and 2014 [
18,
19]. Nevertheless, the development of technologies has given researchers the ability to continue studying the disease ecology caused by deforestation. Maybe, the most serious health danger of deforestation is increasing Human-wild animals contacts and zoonotic spillover.
Figure 1.
The loss of natural habitats and biodiversity, wild animal trade, and intensification of agriculture are key factors contributing to increased contact and zoonotic spillover, leading to the emergence and spread of infectious diseases. These factors are interconnected and pose significant risks to human health. Understanding the interconnectedness of these factors is crucial for implementing effective measures to mitigate the risks and prevent future outbreaks of infectious diseases. By addressing the loss of natural habitats, regulating the wild animal trade, and promoting sustainable agriculture practices, we can reduce contact and zoonotic spillover, safeguarding both human health and biodiversity.
Figure 1.
The loss of natural habitats and biodiversity, wild animal trade, and intensification of agriculture are key factors contributing to increased contact and zoonotic spillover, leading to the emergence and spread of infectious diseases. These factors are interconnected and pose significant risks to human health. Understanding the interconnectedness of these factors is crucial for implementing effective measures to mitigate the risks and prevent future outbreaks of infectious diseases. By addressing the loss of natural habitats, regulating the wild animal trade, and promoting sustainable agriculture practices, we can reduce contact and zoonotic spillover, safeguarding both human health and biodiversity.
2.1. Consequences of deforestation:
2.1.1. Transmission bottlenecks:
Transmission bottlenecks are a phenomenon in which a population of organisms experiences a sudden decrease in size due to environmental or other factors. This can have serious evolutionary consequences, as the reduced population size can lead to a decrease in genetic diversity and an increased risk of extinction [
20].
When a population of viruses experiences a transmission bottleneck, the number of individuals is severely reduced, which means that the gene pool is also reduced. Therefore, increase genetic drift. Consequently, loss of genetic variation and an increased risk of extinction due to the lack of diversity [
20].
Transmission bottlenecks can also lead to an increase in mutation rates. When a population experiences a bottleneck, there is less genetic variation and therefore fewer beneficial mutations that could potentially be passed on to future generations. This means that any mutations that do occur are more likely to be deleterious and could further reduce the fitness of the population of viruses [
20].
Overall, the data specifies that transmission usually results in a stringent bottleneck which significantly reduces the diversity of the original population. However, there are instances where transmission bottlenecks appear to be wide enough to allow for rare variants to pass through [
21]. Determining the host and viral factors that affect the transmission bottleneck is a critical step in formulating strategies to control viral spread.
2.1.2. Zoonotic spillover events:
Zoonotic spillover events are a major threat to human health, as they can cause the spread of infectious diseases from animals to humans [
22]. These events occur when a virus or bacteria that is normally found in an animal species is transferred to humans, either through direct contact or through the environment. This can lead to serious health consequences, including death [
23].
The most common zoonotic spillover event is the transmission of viruses from animals to humans. This can happen when an animal carries a virus that is not normally found in humans and then transmits it to a human through contact or by shedding the virus into the environment [
22]. Examples of this include avian influenza, SARS-CoV-2, and Ebola. In addition, some bacteria and parasites can also be transmitted from animals to humans in this way.
The consequences of zoonotic spillover events can be severe for both humans and animals alike. For humans, these events can lead to serious illnesses such as respiratory infections, gastrointestinal illnesses, and even death in some cases. In addition, these events can also have economic impacts due to lost productivity and increased healthcare costs associated with treating those affected by the disease.
To reduce the risk of zoonotic spillover events and their associated consequences for both humans and animals alike, it is important for people to practice good hygiene when handling animals or their products (such as meat or eggs). It is also important for people who work with animals (such as farmers) to take precautions such as wearing protective clothing and using disinfectants when handling animals or their products [
23]. Finally, it is important for governments and organizations around the world to invest in research into zoonotic diseases so that we can better understand how they spread and develop strategies for preventing them from occurring in the first place [
24].
3. Wild animals:
The wild animal trade involves the capture, transportation, and sale of live animals, legally and illegally. This global industry brings together diverse wildlife species, creating opportunities for pathogen exchange and adaptation. Stressful conditions during capture and transport compromise the animals' immune systems, making them more susceptible to infections. The mixing of different animal species in close proximity further increases the risk of zoonotic spillover
(Figure 1).
The public health burden that is presented by zoonoses includes outbreaks of pathogens such as Ebola virus, influenza A virus (H1N1) and Middle East respiratory syndrome coronavirus (MERS-CoV), as well as the ongoing transmission of endemic pathogens, such as
Salmonella spp., and West Nile virus [
25].
The ecological, financial, and governmental organizations are significantly and permanently impacted by zoonotic spillover and the consequent rise of diseases. Spillover infection must be recognized and avoided in order to stop outbreaks and reduce the epidemic of infectious diseases [
22] . The study of the mechanisms that causes spillover is deeply concerned with contacts between wildlife, mankind, microbes, and the habitats in which they live. As a result, it is acknowledged that integrative techniques enable a more thorough understanding of spillover events [
26].
The vast genetic diversity and wide geographical distribution of the various bat viruses detected so far suggest that we will likely see more and more disease outbreaks caused by bat viruses in the future. Among the ‘known unknowns’, bat coronaviruses may be a particularly likely cause of future spillover into both human and livestock populations due to their already established wide genetic diversity in bats around the world [
27], their large positive strand RNA genome size with a high rate of recombination, and documented spillover events in both humans and animals[
27].
Live animal markets, also known as wet markets, are a common sight in tropical and subtropical regions of the world, particularly in Asian and African countries and those where they have migrated [
28]. These markets provide a wide variety of vertebrate and invertebrate animals (aquatic and terrestrial) to their customers, often located near other markets that sell fish, red meat, or prepared food. Unfortunately, these live animal markets have been identified as a potential source of zoonoses [
28]. The problem arises from the fact that different species of animals from various origins, ecosystems, and taxonomic groups are caged together in close quarters with unsanitary conditions. This leads to the exchange of pathogenic and parasitic microorganisms between them, which can cause zoonoses and outbreaks of EIDs [
28].
4. Animals agriculture:
Animals have been a part of human life since the dawn of time. They provide us with food, clothing, and companionship. But animals can also be a source of disease transmission to humans. This is especially true in the agricultural industry, where animals are kept in close quarters and are exposed to a variety of pathogens [
29]
(Figure 1).
The most common way that diseases are transmitted from animals to humans is through contact with animal products such as meat, milk, eggs, or hides. These products may contain bacteria or viruses that can cause illness in humans if they are not properly handled or cooked. Additionally, some viruses can be spread through the air when an infected animal coughs or sneezes near a person [
30].
In addition to direct contact with animal products or exposure to airborne viruses, some diseases can also be transmitted indirectly through contaminated water or soil. For example, E. coli bacteria can contaminate water sources used for irrigation and drinking water for both animals and humans. This contamination can lead to serious illnesses such as diarrhea and kidney failure in both species if not treated promptly [
31].
To reduce the risk of disease transmission from animals to humans in agricultural settings, it is important for farmers and ranchers to practice good hygiene and sanitation practices on their farms [
32]. This includes regularly cleaning and disinfecting all surfaces that come into contact with animals or their products as well as providing clean drinking water for both humans and animals on the farm. Additionally, farmers should ensure that all animal products are cooked thoroughly before being consumed by humans.
It is also important for farmers to vaccinate their animals against common diseases that could potentially be transmitted to humans such as coronaviruses or avian influenza [
33]. Vaccinating livestock against these diseases helps reduce the risk of transmission from infected animals to people who come into contact with them either directly or indirectly through contaminated food products or water sources [
33].
5. Management of the disease’s prevention and control:
In the current era of global health, the management of disease prevention and control is a critical component of public health. With the increasing prevalence of infectious diseases, chronic conditions, and environmental hazards [
34], it is essential that governments and healthcare organizations have effective strategies in place to manage these threats.
The first step in managing disease prevention and control is to identify the risk factors associated with each disease. This includes understanding the epidemiology of the disease, its transmission routes, and any potential interventions that can be implemented to reduce its spread [
34]. Once these risk factors are identified, public health officials can develop targeted strategies to reduce their impact [
34]. For example, if a particular disease is spread through contaminated water sources, then interventions such as water purification or improved sanitation practices can be implemented to reduce its transmission.
Once risk factors have been identified and interventions developed, it is important for public health officials to monitor their effectiveness in preventing or controlling the spread of a particular disease [
34]. This includes tracking changes in incidence rates over time as well as evaluating any new interventions that may be implemented. By monitoring these changes, public health officials can determine whether existing interventions are working or if new ones need to be developed [
34].
Finally, it is essential for public health organizations to engage with communities when developing strategies for managing disease prevention and control [
35]. This includes educating communities about the risks associated with certain diseases as well as providing resources for individuals who may be at higher risk due to their age or other factors. By engaging with communities in this way, public health organizations can ensure that their strategies are tailored to meet local needs and are more likely to be successful in preventing or controlling a particular disease [
35].
6.1. drug resistant:
The rise of drug-resistant infectious diseases is a growing global health concern. As bacteria and other pathogens become increasingly resistant to antibiotics, the ability to effectively treat and prevent infections is becoming more difficult [
36]. This has led to an increase in the spread of infectious diseases, resulting in a greater burden on public health systems around the world.
Drug resistance occurs when bacteria or other pathogens evolve to become resistant to the drugs used to treat them [
37]. This can happen naturally, but it is often accelerated by overuse or misuse of antibiotics [
38]. When antibiotics are used too frequently or inappropriately, it can lead to the development of drug-resistant strains of bacteria that are more difficult to treat. This is especially concerning for diseases such as tuberculosis, which can be fatal if not treated properly [
39].
The spread of drug-resistant infections has been exacerbated by global travel and trade, which allow for the rapid spread of pathogens from one region to another [
40]. In addition, poverty and inadequate access to healthcare can contribute to the spread of drug-resistant infections as people may not have access to proper diagnosis and treatment [
41].
In order to address this issue, it is important that governments take steps to reduce overuse and misuse of antibiotics. For example, various substitute medications have been experimented with, such as antihistamines, anesthetics, NSAIDs, antipsychotics, and cardiovascular drugs. Fecal microbiota transplantation (FMT) is a significant alternative approach being tested to combat antimicrobial resistance. By administering fresh, frozen, or encapsulated fecal matter from a suitable donor, the unhealthy gut microbiota of the patient is restored, re-establishing alpha-diversity. FMT therapy is particularly recommended for recurrent Clostridium difficile infections and has shown over 90% efficacy in randomized clinical trials. FMT is also effective in displacing vancomycin-resistant Enterococcus when they are dominant over the rest of gut microbiota and when C. difficile is present [
42]. In addition, educating healthcare providers on appropriate prescribing practices as well as ensuring that people have access to quality healthcare services [
43].
6.2. Uses of vaccines:
Vaccines are one of the most important tools in the fight against infectious diseases. They have been used to protect people from a wide range of diseases, including polio, measles, and influenza. However, there is growing concern that misuses of vaccines can lead to the spread of infectious diseases [
44].
The misuse of vaccines can occur in a variety of ways. For example, some people may not get vaccinated at all or may not receive the recommended number of doses [
45]. This can lead to a decrease in herd immunity, which is when enough people are vaccinated that it helps protect those who are not immunized from getting sick. Additionally, if a vaccine is not stored properly or administered incorrectly, it may not be effective in preventing disease [
45].
Another way that vaccines can be misused is through “vaccine tourism” – when people travel to other countries to receive vaccinations that are not available in their home country [
46]. This practice has become increasingly popular as more countries make certain vaccines available only to their citizens or residents [
47]. Unfortunately, this means that travelers may be exposed to different strains of viruses or bacteria than they would normally encounter at home, increasing their risk for infection and potentially spreading disease back home with them [
48].
Finally, some individuals may choose to delay or refuse vaccination for themselves [
49] or their children due to personal beliefs or concerns about safety and efficacy [
50]. While it is important for individuals to make informed decisions about their health care, delaying or refusing vaccination can put both the individual and those around them at risk for contracting serious illnesses like measles and whooping cough [
51].
To increase vaccine acceptance, we need to understand the reasons behind this hesitancy and develop strategies that address them. One of the main reasons for vaccine hesitancy is misinformation. False claims about vaccines' safety and efficacy have been circulating on social media platforms, leading to confusion and mistrust among some people [
52]. To counter this, health authorities need to communicate accurate information about vaccines through trusted sources such as healthcare professionals and public health agencies. They should also engage with communities to understand their concerns and address them through targeted messaging.
Another reason for vaccine hesitancy is mistrust in government and pharmaceutical companies. Some people believe that these entities prioritize profits over public health, leading them to question the safety of vaccines [
53]. To address this concern, governments should be transparent about their decision-making processes regarding vaccine development and distribution. They should also work with independent experts to ensure that vaccines are rigorously tested for safety and efficacy before being approved for use.
Access to vaccines can also be a barrier to acceptance. Some people may be hesitant because they do not have easy access to vaccination sites or cannot afford them. Governments should ensure that vaccines are widely available and accessible by setting up vaccination sites in underserved areas or providing transportation for those who cannot travel.
7. The effect of antigenic drift on vaccine effectiveness:
The effectiveness of vaccines is dependent on the ability of the vaccine to induce an immune response that can protect against infection. However, the effectiveness of vaccines can be reduced due to antigenic drift [
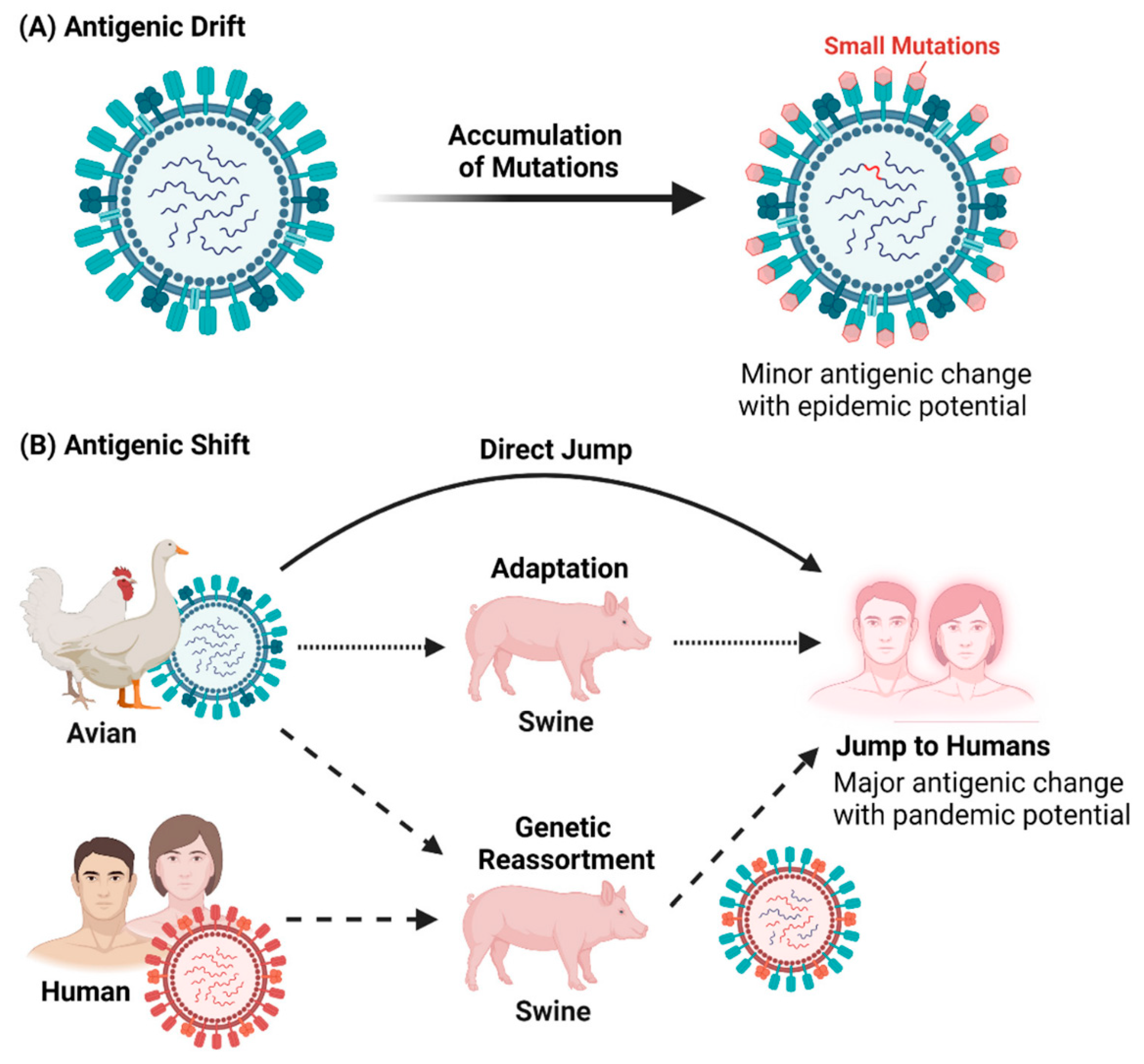
54], a process by which viruses evolve over time and become less susceptible to the vaccine.
Antigenic drift occurs when a virus undergoes small mutations in its genetic material, which can lead to changes in its surface proteins. These changes can make the virus unrecognizable to the immune system, reducing the effectiveness of the vaccine. This is especially true for viruses that mutate quickly, such as influenza
(Figure 2).
The effects of antigenic drift on vaccine effectiveness can be seen in seasonal influenza vaccines. Each year, a new vaccine is developed based on predictions about which strains of influenza will be most prevalent during that season [
54]. However, due to antigenic drift, these predictions are often inaccurate and the vaccine may not be effective against circulating strains of influenza. This means that even if a person receives a flu shot each year, they may still become infected with influenza if it has drifted away from the strain used in the vaccine [
56]. In some cases, these viruses have mutated so much that existing vaccines are no longer effective against them and new vaccines must be developed in order to provide protection.
In order to reduce the effects of antigenic drift on vaccine effectiveness, scientists are working on developing more effective vaccines that are better able to recognize mutated viruses and provide protection against them [
57]. In addition, researchers are exploring ways to improve existing vaccines by making them more resistant to mutation or by using multiple strains in one vaccine in order to provide broader protection against different variants of a virus [
58].
8. Climate change and Pollution:
The world is facing a global health crisis. Climate change and pollution are two of the most significant contributors to the spread of infectious diseases [
59,
60]. The emission of GHGs is causing a wide range of climatic hazards that can worsen 277 diseases that affect humans. These diseases make up 58% of all infectious diseases that have affected humanity [
61]. C. Mora et al identified over 1,000 pathways through which these climatic hazards can lead to disease outbreaks caused by a diverse range of pathogens [
61]. The large number of diseases and transmission pathways affected by climate change highlights the urgent need to take aggressive action to reduce GHG emissions and mitigate the threat to human health.
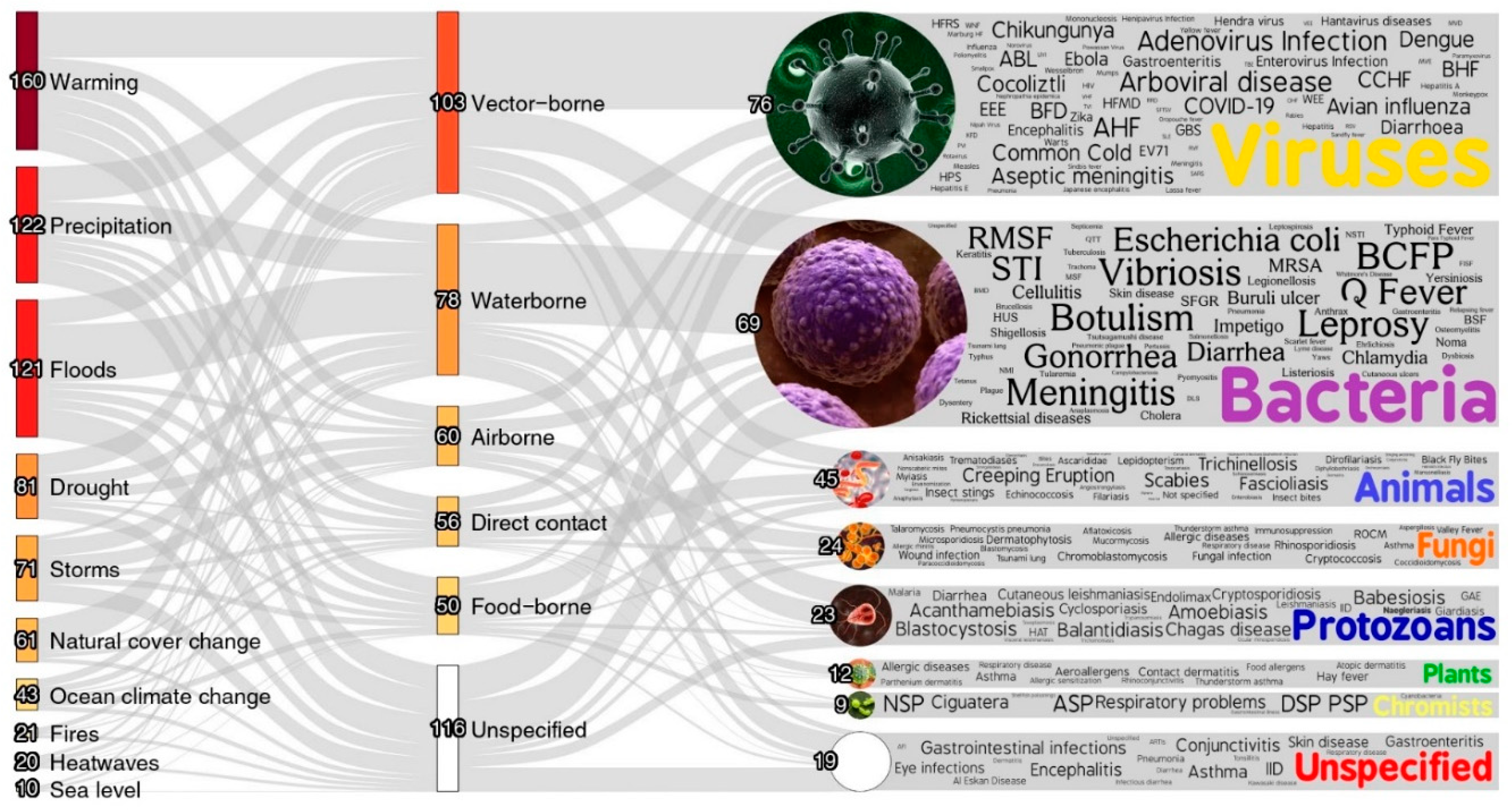
Figure 3.
Interactions between Climatic Hazards and Pathogenic Diseases: Visualizing Aggravation Pathways. The thickness of the lines in the figure reflects the number of unique pathogenic diseases influenced by various transmission types associated with climatic hazards. By observing the color gradient, which ranges from darker to lighter shades, one can discern the relative quantity of diseases affected. Darker colors indicate a larger number of diseases, while lighter colors suggest a smaller number. The numbers displayed at each node serve as an indicator of the distinct pathogenic diseases associated with that specific node [
61].
Figure 3.
Interactions between Climatic Hazards and Pathogenic Diseases: Visualizing Aggravation Pathways. The thickness of the lines in the figure reflects the number of unique pathogenic diseases influenced by various transmission types associated with climatic hazards. By observing the color gradient, which ranges from darker to lighter shades, one can discern the relative quantity of diseases affected. Darker colors indicate a larger number of diseases, while lighter colors suggest a smaller number. The numbers displayed at each node serve as an indicator of the distinct pathogenic diseases associated with that specific node [
61].
The effects of climate change on human health are far-reaching and complex. Warmer temperatures can lead to an increase in air pollution, which can worsen existing respiratory conditions or trigger new ones [
62]. In addition, extreme weather events can cause flooding and displacement of people, leading to overcrowding in shelters and other areas where disease transmission is more likely [
63].
Pollution is another major factor in the spread of infectious diseases. Air pollution has been linked to an increased risk of respiratory illnesses such as asthma and COPD [
64]. In addition, water pollution can lead to waterborne illnesses such as cholera and typhoid fever. The World Health Organization estimates that nearly one million people die each year from water-related diseases caused by polluted drinking water sources [
65].
Governments must invest in clean energy sources such as solar and wind power to reduce our reliance on fossil fuels that contribute to climate change and air pollution [
66]. In addition, we must work together to reduce waste production and improve waste management systems so that pollutants do not end up in our waterways or airways [
67]. Finally, we must ensure access to clean drinking water for all people so that they do not fall ill due to contaminated sources [
68]. By taking action now, we can protect ourselves from the devastating effects of climate change and pollution on our health – both now and for future generations [
69].
9. The Emerging Infectious Diseases burden:
The world is facing a growing burden of EIDs and it is a problem that needs to be addressed. The emergence of new infectious diseases is a global health concern due to the potential for rapid spread and the lack of effective treatments or vaccines [
60].
In recent years, we have seen the emergence of novel viruses such as SARS-CoV-2 as well as other pathogens such as Zika virus, MERS-CoV, and Nipah virus. These diseases can cause severe illness and death in humans, but they can also have devastating economic impacts due to disruption of trade and travel [
70].
The burden of EIDs is particularly concerning in low-income countries where healthcare systems are often weak or nonexistent. These countries often lack the resources to detect new pathogens quickly or respond effectively to outbreaks. This leaves them vulnerable to rapid spread of disease with potentially devastating consequences [
71].
In order to address this growing burden of EIDs, there needs to be an increased focus on prevention and preparedness measures at both the global and local levels. This includes strengthening healthcare systems in low-income countries so they are better able to detect new pathogens quickly and respond effectively to outbreaks [
72]. It also includes investing in research into new treatments and vaccines for emerging infections so that we are better prepared for future outbreaks [
73]. Finally, it requires increased collaboration between countries so that information about new pathogens can be shared quickly and effective responses can be coordinated across borders [
74]. By taking these steps now, we can reduce the burden of EIDs on our world’s population now and in the future.
10. Concluding remarks and future perspectives:
The risk of novel human infections is higher in certain areas than others, and identifying these 'hotspots' can help direct surveillance efforts. In the last two decades, a number of high-impact zoonotic outbreaks have been linked to bat-borne viruses, such as SARS coronavirus, Hendra virus and Nipah virus. It is believed that ebolaviruses and MERS coronavirus may also be connected to bats. It is becoming increasingly accepted that bats are potential hosts for a wide range of known and unknown viruses, many of which could spread to animal and human populations. With 31% of CoVs described in bats, the possibility of newly emerging CoVs-associated diseases should be taken seriously.
We are still in the early stages when it comes to understanding bat biology and how some of the most dangerous viruses can coexist with them. The emergence of a disease is an unpredictable event, making it impossible to predict future scenarios or dynamics for EIDs.
It has been demonstrated that live animals in markets can act as potential carriers of various viruses, increasing the risk of transmission to humans. The diversity and rapid evolution of virus lineages in bats and other wild animals demonstrate how difficult it will be to identify viruses with the potential to cause major human outbreaks before they emerge.
To prevent future outbreaks, it is essential to improve regulations regarding live animal markets and wildlife trade, as well as establishing high hygiene standards. Laws should be implemented to prohibit the trade of wild animals. If we are to discover therapeutic options and vaccines, it is even more important to educate people on the risks associated with anthropized environments.
Several factors related to human behavior, pathogen evolution, environmental and ecological issues have been found to contribute to ID emergence and transmission. Antigenic drift is an important factor that affects vaccine effectiveness and it is important for scientists and healthcare providers alike to understand how this process works in order to ensure that people receive effective vaccinations against infectious diseases. However, recent advances in biotechnology, immunology and artificial intelligence have enabled us to make progress in diagnosis, treatment, prevention and control of infectious diseases. The fight against infectious diseases will always be ongoing, but the advancement of bioscience offers a promising outlook for the near future.
Authors’ Contributions
MIM: wrote initial and final draft, conceptualization, Illustration, collected and organize data. AMM: wrote initial and final draft, supervision, conceptualization. All authors have critically reviewed and approved the final draft.
Ethical approval
There is no ethical issue.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgment
The authors acknowledge the Deanship of Scientific Research at University of Bahri for the supportive cooperation.
Conflict of interest
The authors declare that they have no competing interests.
References
- Woolhouse, M.E.J. Emerging diseases go global. Nature 2008, 451, 898–899. [Google Scholar] [CrossRef] [PubMed]
- Afelt, A.; Frutos, R.; Devaux, C. Bats, Coronaviruses, and Deforestation: Toward the Emergence of Novel Infectious Diseases? Front. Microbiol. 2018, 9, 702. [Google Scholar] [CrossRef]
- Daniels, P.W.; Halpin, K.; Hyatt, A.; Middleton, D. Infection and disease in reservoir and spillover hosts: determinants of pathogen emergence. Curr. Top. Microbiol. Immunol. 2007, 315, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Anderson, D.E. Viruses in bats and potential spillover to animals and humans. Curr. Opin. Virol. 2019, 34, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.D.; Wang, W.; Hao, Z.Y.; Wang, Z.X.; Guo, W.P.; Guan, X.Q.; Wang, M.R.; Wang, H.W.; Zhou, R.H.; Li, M.H.; et al. Extensive diversity of coronaviruses in bats from China. Virology 2017, 507, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef]
- Chan, J.F.; To, K.K.; Tse, H.; Jin, D.Y.; Yuen, K.Y. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 2013, 21, 544–555. [Google Scholar] [CrossRef]
- Peel, A.J.; Sargan, D.R.; Baker, K.S.; Hayman, D.T.S.; Barr, J.A.; Crameri, G.; Suu-Ire, R.; Broder, C.C.; Lembo, T.; Wang, L.F.; et al. Continent-wide panmixia of an African fruit bat facilitates transmission of potentially zoonotic viruses. Nat. Commun. 2013, 4, 2770. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.J.; Johnson, C.K.; Greig, D.J.; Kramer, S.; Che, X.; Wells, H.; Hicks, A.L.; Joly, D.O.; Wolfe, N.D.; Daszak, P.; et al. Global patterns in coronavirus diversity. Virus Evol. 2017, 3, vex012. [Google Scholar] [CrossRef]
- Brook, C.E.; Dobson, A.P. Bats as 'special' reservoirs for emerging zoonotic pathogens. Trends Microbiol. 2015, 23, 172–180. [Google Scholar] [CrossRef]
- Hansen, M.C.; Stehman, S.V.; Potapov, P.V.; Loveland, T.R.; Townshend, J.R.; DeFries, R.S.; Pittman, K.W.; Arunarwati, B.; Stolle, F.; Steininger, M.K.; et al. Humid tropical forest clearing from 2000 to 2005 quantified by using multitemporal and multiresolution remotely sensed data. Proc. Natl. Acad. Sci. United States Am. 2008, 105, 9439–9444. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Kulmann-Leal, B.; Kaminski, V.L.; Valverde-Villegas, J.M.; Veiga, A.; Spilki, F.R.; Fearnside, P.M.; Caesar, L.; Giatti, L.L.; Wallau, G.L.; et al. Beyond diversity loss and climate change: Impacts of Amazon deforestation on infectious diseases and public health. An. Da Acad. Bras. De Cienc. 2020, 92, e20191375. [Google Scholar] [CrossRef]
- Chapman, C.A.; Bicca-Marques, J.C.; Dunham, A.E.; Fan, P.; Fashing, P.J.; Gogarten, J.F.; Guo, S.; Huffman, M.A.; Kalbitzer, U.; Li, B.; et al. Primates Can Be a Rallying Symbol to Promote Tropical Forest Restoration. Folia Primatol. ; Int. J. Primatol. 2020, 91, 669–687. [Google Scholar] [CrossRef]
- Tuells, J.; Henao-Martínez, A.F.; Franco-Paredes, C. Yellow Fever: A Perennial Threat. Arch. Med. Res. 2022, 53, 649–657. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed]
- de Thoisy, B.; Silva, N.I.O.; Sacchetto, L.; de Souza Trindade, G.; Drumond, B.P. Spatial epidemiology of yellow fever: Identification of determinants of the 2016-2018 epidemics and at-risk areas in Brazil. PLoS Neglected Trop. Dis. 2020, 14, e0008691. [Google Scholar] [CrossRef] [PubMed]
- Orkin, J.D.; Kuderna, L.F.K.; Marques-Bonet, T. The Diversity of Primates: From Biomedicine to Conservation Genomics. Annu. Rev. Anim. Biosci. 2021, 9, 103–124. [Google Scholar] [CrossRef]
- Rulli, M.C.; Santini, M.; Hayman, D.T.; D'Odorico, P. The nexus between forest fragmentation in Africa and Ebola virus disease outbreaks. Sci. Rep. 2017, 7, 41613. [Google Scholar] [CrossRef] [PubMed]
- Olivero, J.; Fa, J.E.; Real, R.; Márquez, A.L.; Farfán, M.A.; Vargas, J.M.; Gaveau, D.; Salim, M.A.; Park, D.; Suter, J.; et al. Recent loss of closed forests is associated with Ebola virus disease outbreaks. Sci. Rep. 2017, 7, 14291. [Google Scholar] [CrossRef]
- McCrone, J.T.; Lauring, A.S. Genetic bottlenecks in intraspecies virus transmission. Curr. Opin. Virol. 2018, 28, 20–25. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Sun, W.; Zhang, L.; Ji, J.; Zhang, Z.; Cheng, X.; Li, Y.; Xiao, F.; Zhu, A.; et al. Population Bottlenecks and Intra-host Evolution During Human-to-Human Transmission of SARS-CoV-2. Front. Med. 2021, 8, 585358. [Google Scholar] [CrossRef] [PubMed]
- Plowright, R.K.; Parrish, C.R.; McCallum, H.; Hudson, P.J.; Ko, A.I.; Graham, A.L.; Lloyd-Smith, J.O. Pathways to zoonotic spillover. Nat. Rev.. Microbiol. 2017, 15, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Chies, J.A.B. Zoonotic spillover: Understanding basic aspects for better prevention. Genet. Mol. Biol. 2021, 44, e20200355. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.A.; Venkatachalam-Vaz, J.; Drake, J.M. Spillover of zoonotic pathogens: A review of reviews. Zoonoses Public Health 2021, 68, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.R.; Baldwin, V.M.; Roy, S.; Essex-Lopresti, A.E.; Prior, J.L.; Harmer, N.J. Zoonoses under our noses. Microbes Infect. 2019, 21, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife--threats to biodiversity and human health. Sci. (New York N.Y.) 2000, 287, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhao, K.; Shi, Z.L.; Zhou, P. Bat Coronaviruses in China. Viruses 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Galindo-González, J. Live animal markets: Identifying the origins of emerging infectious diseases. Curr. Opin. Environ. Sci. Health 2022, 25, 100310. [Google Scholar] [CrossRef] [PubMed]
- Hollenbeck, J.E. Interaction of the role of Concentrated Animal Feeding Operations (CAFOs) in Emerging Infectious Diseases (EIDS). Infect. Genet. Evol. : J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2016, 38, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Collin, É.; Périé, P.; Touboul, H. [Livestock-related zoonoses]. La Rev. Du Prat. 2019, 69, 328–332. [Google Scholar]
- Ashbolt, N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004, 198, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Bondeson, L. Assessment of measures to prevent disease associated with animals in agricultural fairs--Maine, 2008. Am. J. Infect. Control 2009, 37, 665–667. [Google Scholar] [CrossRef]
- Tizard, I.R. Vaccination against coronaviruses in domestic animals. Vaccine 2020, 38, 5123–5130. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.D.; Jarolimova, J.; Elnaiem, A.; Huang, C.X.; Richterman, A.; Ivers, L.C. Effectiveness of contact tracing in the control of infectious diseases: a systematic review. Lancet Public Health 2022. [Google Scholar] [CrossRef] [PubMed]
- Destoumieux-Garzón, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S. The one health concept: 10 years old and a long road ahead. Front. Vet. Sci. 2018, 14. [Google Scholar] [CrossRef]
- Gow, N.A.; Johnson, C.; Berman, J.; Coste, A.T.; Cuomo, C.A.; Perlin, D.S.; Bicanic, T.; Harrison, T.S.; Wiederhold, N.; Bromley, M. The importance of antimicrobial resistance in medical mycology. Nat. Commun. 2022, 13, 5352. [Google Scholar] [CrossRef] [PubMed]
- Chebbac, K.; Ghneim, H.K.; El Moussaoui, A.; Bourhia, M.; El Barnossi, A.; Benziane Ouaritini, Z.; Salamatullah, A.M.; Alzahrani, A.; Aboul-Soud, M.A.; Giesy, J.P. Antioxidant and antimicrobial activities of chemically-characterized essential oil from Artemisia aragonensis Lam. against drug-resistant microbes. Molecules 2022, 27, 1136. [Google Scholar] [CrossRef] [PubMed]
- Mallah, N.; Orsini, N.; Figueiras, A.; Takkouche, B. Education level and misuse of antibiotics in the general population: a systematic review and dose–response meta-analysis. Antimicrob. Resist. Infect. Control 2022, 11, 1–22. [Google Scholar] [CrossRef]
- Shah, S.; Kasarla, R.R.; Yadav, N.S.; Shrestha, A.; Gautam, S.; Chaudhary, S.K. Antibiotic Resistance in Clinical Medicine. J. Univers. Coll. Med. Sci. 2022, 10, 66–71. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, D.; Singh, A.; Sunita, K. Colistin Resistance and Management of Drug Resistant Infections. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022. [Google Scholar] [CrossRef]
- Jama, K. Socioeconomic Determinants of Antibiotic Resistance. 2022.
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M. Progress in alternative strategies to combat antimicrobial resistance: Focus on antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Tiberi, S.; Utjesanovic, N.; Galvin, J.; Centis, R.; D'Ambrosio, L.; van den Boom, M.; Zumla, A.; Migliori, G.B. Drug resistant TB–latest developments in epidemiology, diagnostics and management. Int. J. Infect. Dis. 2022, 124, S20–S25. [Google Scholar] [CrossRef] [PubMed]
- Delany, I.; Rappuoli, R.; De Gregorio, E. Vaccines for the 21st century. EMBO Mol. Med. 2014, 6, 708–720. [Google Scholar] [CrossRef]
- Ophir, Y.; Walter, N.; Walter, D.; Velho, R.M.; Lokmanoglu, A.D.; Pruden, M.L.; Andrews, E.A. Vaccine hesitancy under the magnifying glass: A systematic review of the uses and misuses of an increasingly popular construct. Health Commun. 2022, 1–15. [Google Scholar] [CrossRef]
- Espindola, J.; Vaca, M. On the morality of vaccination tourism. Bioethics 2022, 36, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Wu, C.; Zhao, Q.; Sun, Y.; Zhang, Y.J.; Zhou, E.M. Vaccine Development against Zoonotic Hepatitis E Virus: Open Questions and Remaining Challenges. Front. Microbiol. 2018, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Al-Ansi, A.; Ariza-Montes, A.; Arraño-Muñoz, M.; Giorgi, G.; Han, H. Vaccine Passport and Traveler Behaviors in the New Market of the Domestic and International Tourism Industry Facing the With-Corona Era. Front. Psychol. 2022, 13, 900976. [Google Scholar] [CrossRef]
- Yuan, J.; Lam, W.T.W.; Xiao, J.; Ni, Y.M.; Cowling, B.J.; Liao, Q. Why do Chinese older adults in Hong Kong delay or refuse COVID-19 vaccination? A qualitative study based on Grounded Theory. J. Gerontol.. Ser. B Psychol. Sci. Soc. Sci. 1093. [Google Scholar] [CrossRef]
- Nurmi, J.; Harman, B. Why do parents refuse childhood vaccination? Reasons reported in Finland. Scand. J. Public Health 2022, 50, 490–496. [Google Scholar] [CrossRef]
- Wiysonge, C.S.; Ndwandwe, D.; Ryan, J.; Jaca, A.; Batouré, O.; Anya, B.M.; Cooper, S. Vaccine hesitancy in the era of COVID-19: could lessons from the past help in divining the future? Hum. Vaccines Immunother. 2022, 18, 1–3. [Google Scholar] [CrossRef]
- Kricorian, K.; Civen, R.; Equils, O. COVID-19 vaccine hesitancy: misinformation and perceptions of vaccine safety. Hum. Vaccines Immunother. 2022, 18, 1950504. [Google Scholar] [CrossRef]
- Patwary, M.M.; Alam, M.A.; Bardhan, M.; Disha, A.S.; Haque, M.Z.; Billah, S.M.; Kabir, M.P.; Browning, M.H.; Rahman, M.M.; Parsa, A.D. COVID-19 vaccine acceptance among low-and lower-middle-income countries: a rapid systematic review and meta-analysis. Vaccines 2022, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Bartoszko, J.; Loeb, M. The burden of influenza in older adults: meeting the challenge. Aging Clin. Exp. Res. 2021, 33, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.Y.; Gan, S.K. Peering into Avian Influenza A(H5N8) for a Framework towards Pandemic Preparedness. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Choi, M.J.; Yun, J.W.; Song, J.Y.; Ko, K.; Mould, J.F.; Cheong, H.J. A Comparative Analysis of Influenza-Associated Disease Burden with Different Influenza Vaccination Strategies for the Elderly Population in South Korea. Vaccines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Hung, I.F. Approaches in broadening the neutralizing antibody response of the influenza vaccine. Expert Rev. Vaccines 2021, 20, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, T.; Ben-Yedidia, T. Epitope-based approaches to a universal influenza vaccine. J. Autoimmun. 2014, 54, 15–20. [Google Scholar] [CrossRef]
- Semenza, J.C.; Rocklöv, J.; Ebi, K.L. Climate change and cascading risks from infectious disease. Infect. Dis. Ther. 2022, 11, 1371–1390. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; McKenzie, T.; Gaw, I.M.; Dean, J.M.; von Hammerstein, H.; Knudson, T.A.; Setter, R.O.; Smith, C.Z.; Webster, K.M.; Patz, J.A. Over half of known human pathogenic diseases can be aggravated by climate change. Nat. Clim. Change 2022, 12, 869–875. [Google Scholar] [CrossRef]
- Rupasinghe, R.; Chomel, B.B.; Martínez-López, B. Climate change and zoonoses: A review of the current status, knowledge gaps, and future trends. Acta Trop. 2022, 226, 106225. [Google Scholar] [CrossRef]
- Wang, J.; Wah Yu, C.; Cao, S.-J. Urban development in the context of extreme flooding events. SAGE Publications Sage UK: London, England: 2022; Vol. 31, pp 3-6.
- Nazar, W.; Niedoszytko, M. Air pollution in Poland: A 2022 narrative review with focus on respiratory diseases. Int. J. Environ. Res. Public Health 2022, 19, 895. [Google Scholar] [CrossRef] [PubMed]
- Organization, W.H. Water for health: taking charge; World Health Organization (WHO): 2001.
- Holechek, J.L.; Geli, H.M.; Sawalhah, M.N.; Valdez, R. A global assessment: can renewable energy replace fossil fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Yu, Z.; Khan, S.A.R.; Ponce, P.; Muhammad Zia-ul-haq, H.; Ponce, K. Exploring essential factors to improve waste-to-resource recovery: A roadmap towards sustainability. J. Clean. Prod. 2022, 350, 131305. [Google Scholar] [CrossRef]
- O. E. Olusegun, A. O. Anthony, O. E. O. R. CatherineTomilola, and C. U. Ogiesoba-Eguakun, "Public health concerns and water quality integrity of selected water sources in a peri-urban community," Sustainable Water Resources Management, vol. 8, no. 5, p. 150, 2022.
- Perera, F.; Nadeau, K. Climate change, fossil-fuel pollution, and children’s health. New Engl. J. Med. 2022, 386, 2303–2314. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.E.; Kuhn, M.; Prettner, K. Modern infectious diseases: macroeconomic impacts and policy responses. J. Econ. Lit. 2022, 60, 85–131. [Google Scholar] [CrossRef]
- Dhai, A. Are we preparing for the next pandemic? Health and Medical Publishing Group (HMPG): 2022; Vol. 15, pp 2-3.
- Kretzschmar, M.; Mangen, M.-J.J.; Pinheiro, P.; Jahn, B.; Fevre, E.M.; Longhi, S.; Lai, T.; Havelaar, A.H.; Stein, C.; Cassini, A. New methodology for estimating the burden of infectious diseases in Europe. PLoS Med. 2012, 9, e1001205. [Google Scholar] [CrossRef]
- Damaso, C.R. The 2022 monkeypox outbreak alert: Who is carrying the burden of emerging infectious disease outbreaks? Lancet Reg. Health–Am. 2022, 13. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Sommerfeld, J.; Lassi, Z.S.; Salam, R.A.; Das, J.K. Global burden, distribution, and interventions for infectious diseases of poverty. Infect. Dis. Poverty 2014, 3, 1–7. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).