1. Introduction
Depression is a mental disorder characterized by a persistent decrease in mood, motor, and mental activity, as well as a reduction or loss of the ability to feel pleasure (anhedonia). Concomitant symptoms may include sleep disturbances, decreased appetite, feelings of guilt and uselessness, development of suicidal thoughts [
1]. Depressive disorders require a comprehensive therapeutic approach that includes psychotherapy, lifestyle modifications, and, in most cases, the use of antidepressants [
2]. However, 30 to 60% of patients fail to respond to conventional therapy or develop pronounced adverse effects [
3,
4]. It should also be noted that the COVID-19 pandemic has significantly affected the epidemiology of the disease by increasing the number of patients with depression worldwide [
5]. Therefore, new compounds with anti-depressive properties are needed [
6].
Translational studies are widely used for testing new compounds (mainly on laboratory rodents, less often on primates). There are various methods of depression modeling in rodents that differ in their difficulties and key steps of pathogenesis [
7]. The most commonly used model of depression applied for potential antidepressants investigation is a method of chronic unpredictable mild stress (CUMS). The method is based on the exposure of animals to “subthreshold" stimuli, such as changes in the daily routine, crowding, a dirty cage, short-term food and/or water deprivation, etc. The stimuli change randomly, with a simulation lasting from two weeks to six weeks.
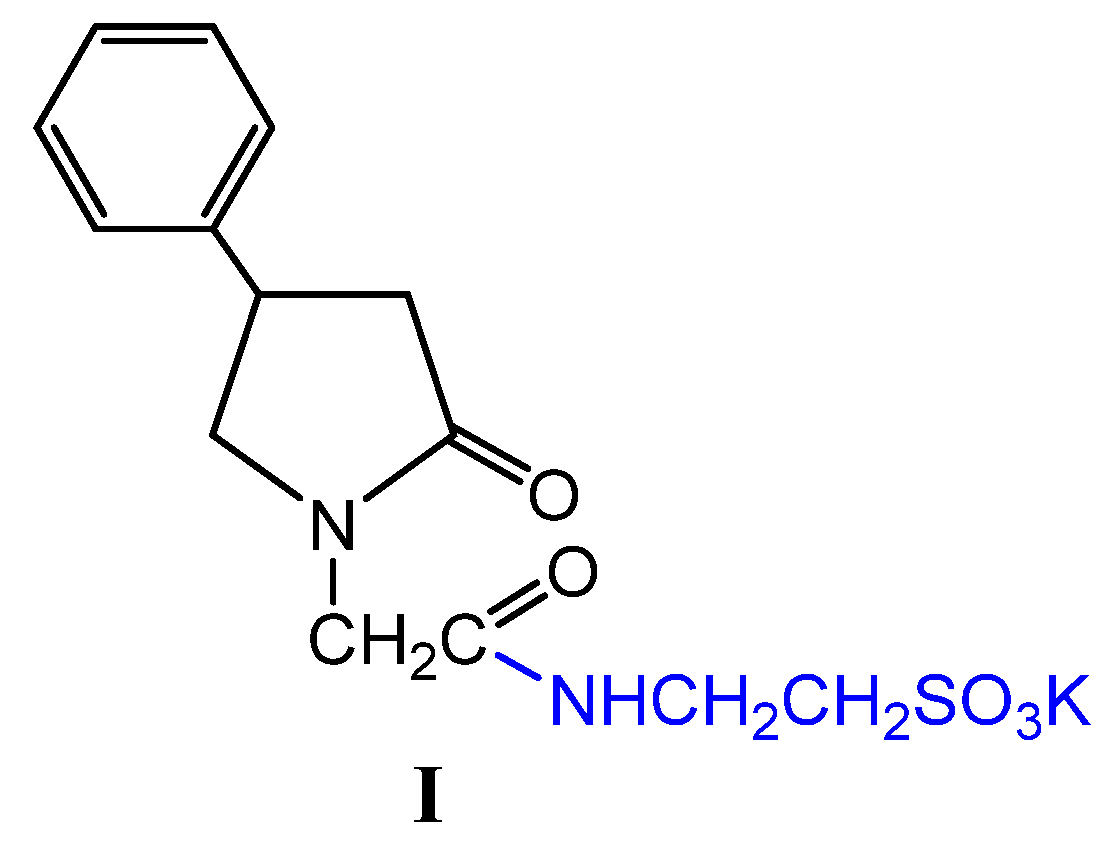
We have synthesized a new Compound
I that as a pharmacophore has taurine, this compound, according to in silico studies, has antidepressant, nootropic, and neuroprotective effects [
8]. Although taurine is a semi-essential sulfo amino acid for humans, its function in the body is very important. It is involved in membrane stabilization, osmoregulation, neuroprotection, and neuronal proliferation. We have previously shown that Compound
I crosses the blood-brain barrier, accumulates in the cerebral cortex, and positively affects the rate of neurologic deficit elimination in an animal model of ischemic stroke[
9].
The purpose of this study was to evaluate the anti-depressive properties of Compound I in a CUMS model of depression and to compare its potential efficacy with the well-known antidepressant Fluoxetine.
2. Results
The behavioral study was conducted on 52 mature male Wistar rats. Of which, 4 rats were excluded before the start of therapy, as they showed deviant behavior before randomisation. Before the experiment, all animals were weighed, and their psychoemotional state was assessed using the Hole board test (HBT)(OpenScience, Russia) [
10]. Two animals were excluded before the initiation of depression, as they showed different activity profile compared to the other animals. Then the animals were distributed into 2 groups: those without depression stimulation and those with depression (12 animals in one group and 38 animals in another) so that the groups were similar in respect of the weight and behavior activity. Then CNLS was applied to initiate the depression. Animals were exposed to various stressful influences, such as: food and water deprivation, cold stress, soft atraumatic tail clip, hot plate test, mixing of the cage animals, light/dark cycle reversal (for a detailed description of the model, see section 4.2,
Table 4).
Assessment of the development of depression in the CNLS modelTo confirm the development of depression in animals, on the 14th day of modeling, the dynamics of body weight gain, a sucrose test and a study of locomotor activity in a hole board test (HBT) and a light-dark box test (LDB) were conducted. All these tests are markers of the development of a depressive state. So, weight loss and a reduced sucrose consumption are indicators of the development of anhedonia (i.e., a decrease or loss of the ability to experience pleasure), and a decrease in locomotor activity indicates a reduced interest and curiosity. After two weeks, 2 rats from the CNLS group were excluded from the study, as they didn’t show any signs of depression.
Of the three tests confirming the development of a depressive-like state in rats, the most indicative were the test for sucrose consumption and HBT (
Table 1,
Figure 1).
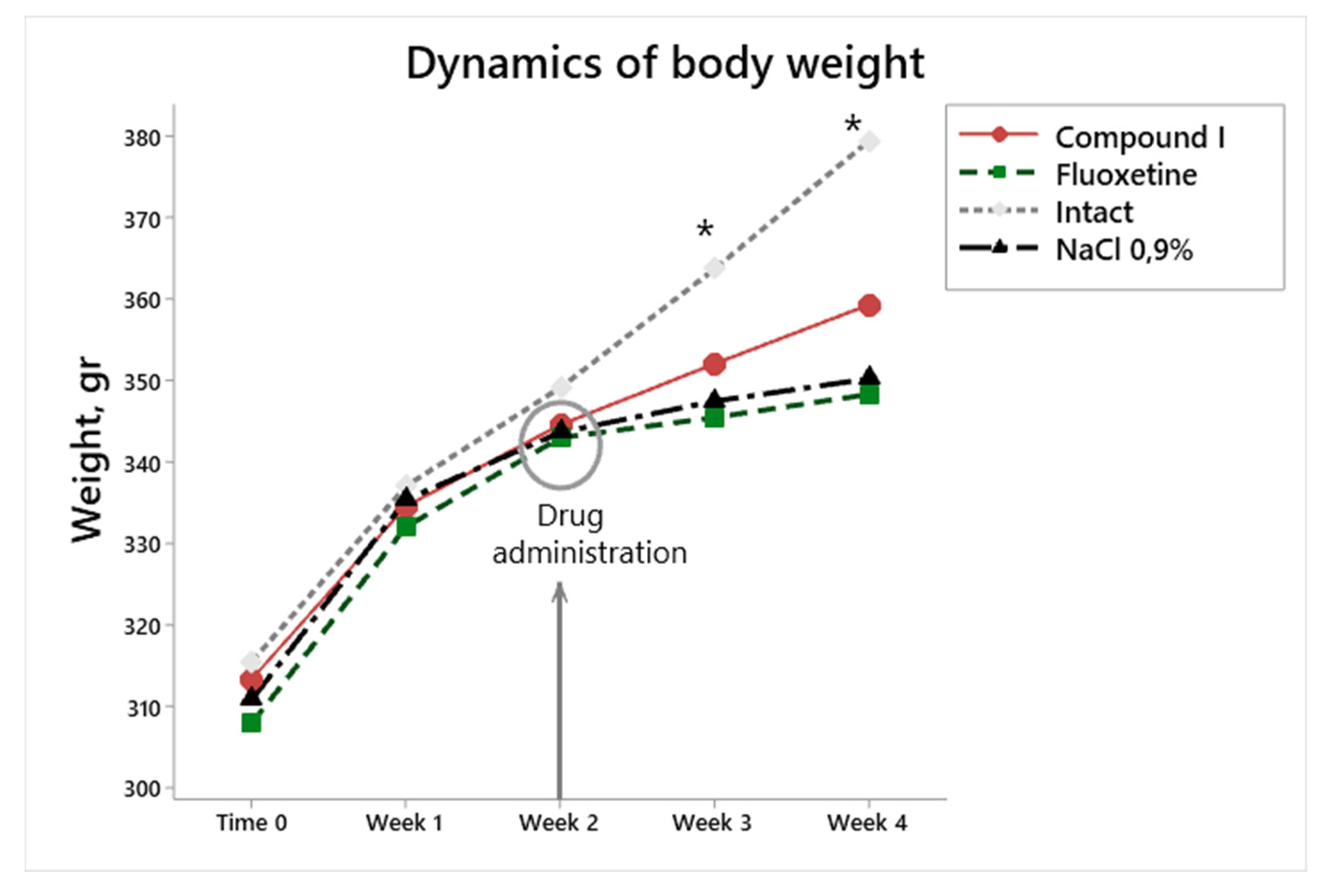
During the first two weeks of exposure to stress factors, the weight gain was similar in all groups, (
Figure 3), which may be due to the fact that 14-day observation is not enough for a noticeable weight loss.
This data lead to the conclusion that there was an esteblished depression.
After confirming the presence of a depressive state in animals, rats with CNLS were divided into 3 groups for therapy (group 3 - intact animals remained unchanged).
Dep_C1 (Compound_1 Dep_C1) – n=12, therapy with Compound I 125 mg/kg (IP administration). 14 days;
Dep_Flu (Fluoxetine) – n=12, therapy with Fluoxetine 15 mg/kg (oral administration).
Int (intact animals) – n=12, not exposed to depressive stimuli and not receiving any medications
Dep_NaCl (physiological saline) – n=12, IP administration of physiological saline.
The therapy began during the stress exposure. As this better simulates the real clinical practice, where patients begin to take anti-depressants while still under stress. We chose a 14-day administration because it has been shown that this administration regimen is used for fluoxetine, a comparator drug, and gives the necessary pharmacological response in animal tests [
11].
2.1. Behavioral tests
We investigated the effects of Compound I on the development and progression of depression in the Model of CUMS in rats. The study design is shown in. (
Table 2)
Weight loss or food anhedonia is one of the most striking symptoms of the development of a depressive state. According to the literature data, in the CUMS model, under the influence of stress factors in rats, body weight significantly decreased. In our study, there were no statistically significant differences in body weight gain in animals by the beginning of the 3rd week (Fig.2). However, starting from the third week, there is a significant increase in weight gain in intact animals (
Figure 2).
Figure 2.
Changes in body weight in modeling depression, the impact of therapy. * Difference between all animal groups and intact animals according to post-hoc analysis. p<0.03.
Figure 2.
Changes in body weight in modeling depression, the impact of therapy. * Difference between all animal groups and intact animals according to post-hoc analysis. p<0.03.
Starting from the 3rd week as a result of further exposure to depressive stimuli, weight gain decreased. After the start of therapy, differences began to be observed in the group Dep_NaCl (physiological saline), with the intact group. After 2 weeks of treatment, this difference with group intact animals was observed in all groups of animals.
In the sucrose test, already a week after the start of therapy in Dep_C1 and Dep_Flu , sweet water consumption increased by 8.8% and by 14% respectively relative to sucrose consumption before therapy. For animals of Dep_C1 and Dep_Flu, the increase in consumption of sweet water continued throughout the entire therapy, although it did not reach the level of intact animals (
Table 3.). At the same time, Dep_NaCl animalconsumption of sweet water throughout the entire therapy remained at a level of depression that was 23% lower than that of intact animals (
Table 3).
Further, to evaluate the effectiveness of antidepressant therapy, the following tests were performed: Parsolt test, The light-dark box test (LDB) and The elevated plus maze test (EMT).
The Parsolt test is the main test for determining the depressive phenotype of behavior, aimed at assessing the time of immobility (immobilization), when the animal does not make active attempts to get out - jumping or diving to leave the installation) [
12]. [Dawson C.A., Horvath S.A. Swimming in small laboratory animals//Med. Sci. Sports. – 1970. Vol. 2 (2). – P. 51–78]. This test has the highest predictive validity, since the antidepressant use reduce the time of immobility, that is, they eliminate the feeling of hopelessness and inability to get out of the installation [
12].
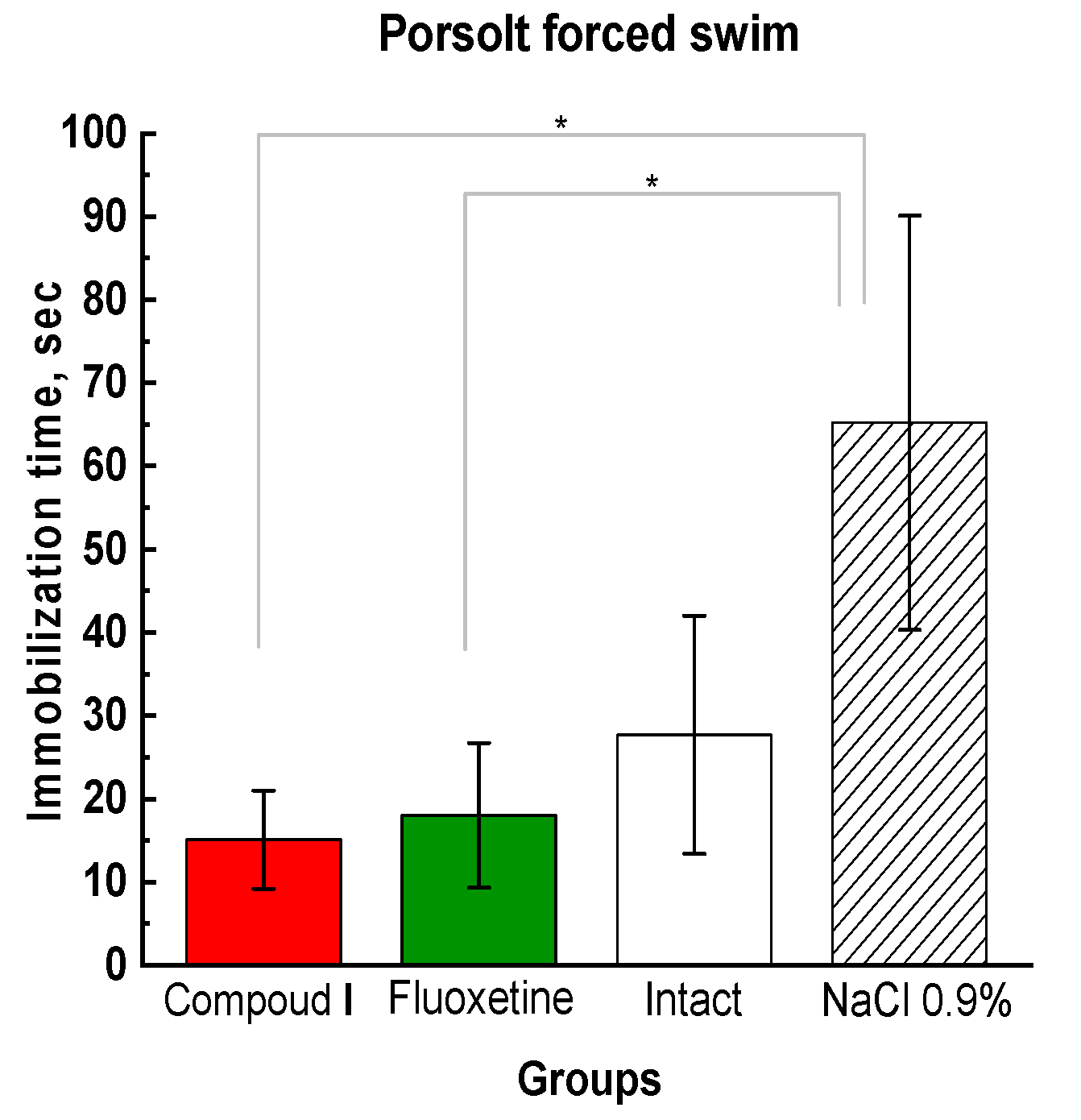
Figure 3.
Results of the animal behavioral assessment in the Porsolt forced swim test; the time of immobilization. * Compared to the animals treated with physiological saline.
Figure 3.
Results of the animal behavioral assessment in the Porsolt forced swim test; the time of immobilization. * Compared to the animals treated with physiological saline.
Since depressed rats show apathy and do not attempt to get out and freeze on the water's surface. The time of immobility is the criterion for the magnitude of anti-depressive effects of the compounds. In this study the test showed a significant reduction in the immobilization time in the Compound I and Fluoxetine groups compared to the control groups (Fig. 3).
In the Parsolt test, the time of immobilization of animals in group Dep_NaCl increases, which indicates the presence of a depressive state. We observed similar behavior in the LDB and EMT tests (Fig. 4).
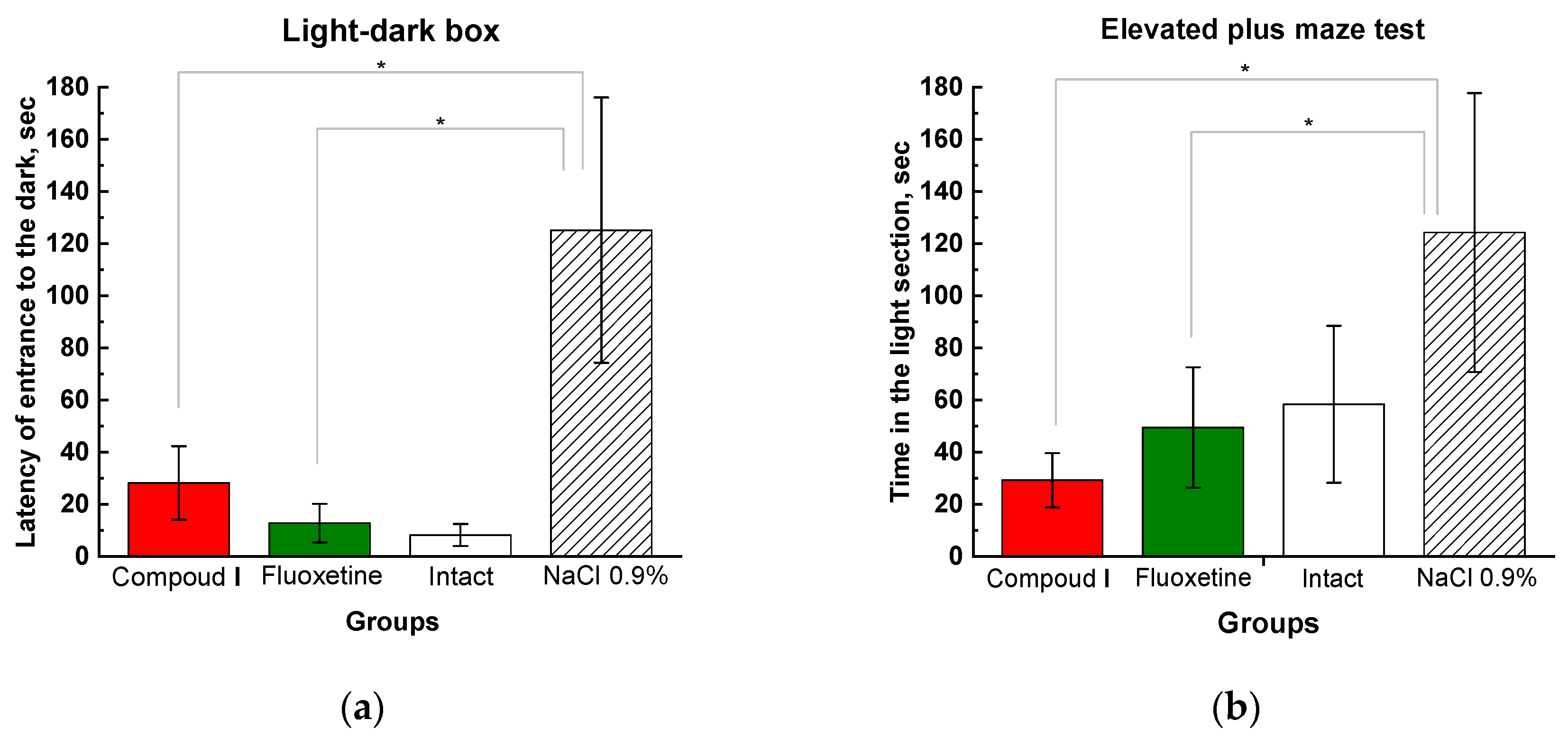
Figure 4.
Results of behavioral tests (light-dark box – (a), elevated plus maze test – (b)). The results are presented as the mean value ± standard error. * Compared to the animals treated with physiological saline.
Figure 4.
Results of behavioral tests (light-dark box – (a), elevated plus maze test – (b)). The results are presented as the mean value ± standard error. * Compared to the animals treated with physiological saline.
The light-dark box test (LDB) and the elevated plus maze test (EMT) are based on the fact that animals prefer to stay in a dark place. More severe depression is associated with the prolongation of the latency of entrance to the dark compartment and the increased time spent in the light compartment. In the Compound I and Fluoxetine groups, the entry latency and residence time in the light compartment were significantly lower than in the control group and did not differ from intact animals. Rats under the influence of the test substance and the reference antidepressant, in the presented tests, restored the normal reflex act of fear of bright spaces and also showed an increased exploratory activity (Fig. 4).
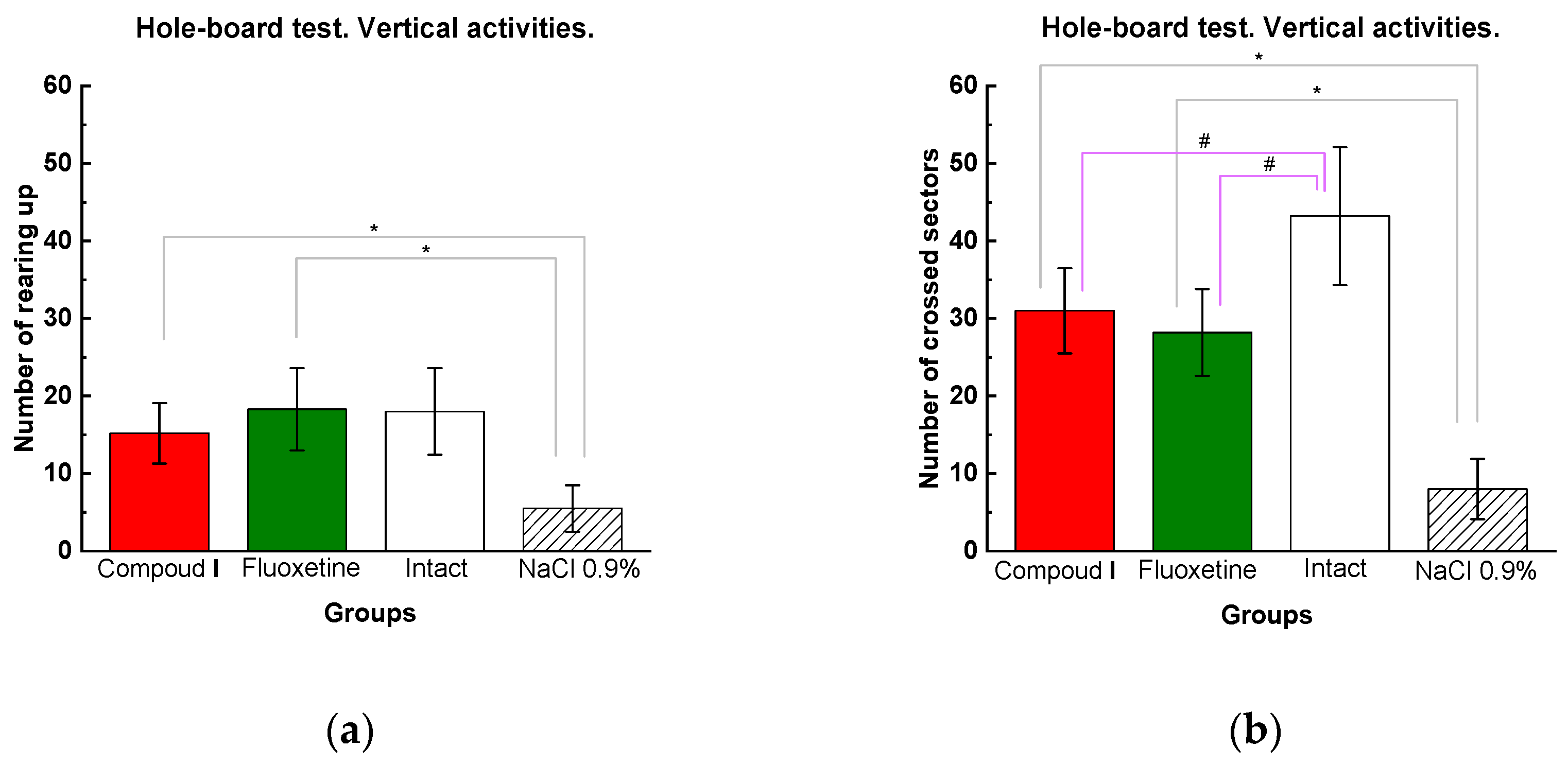
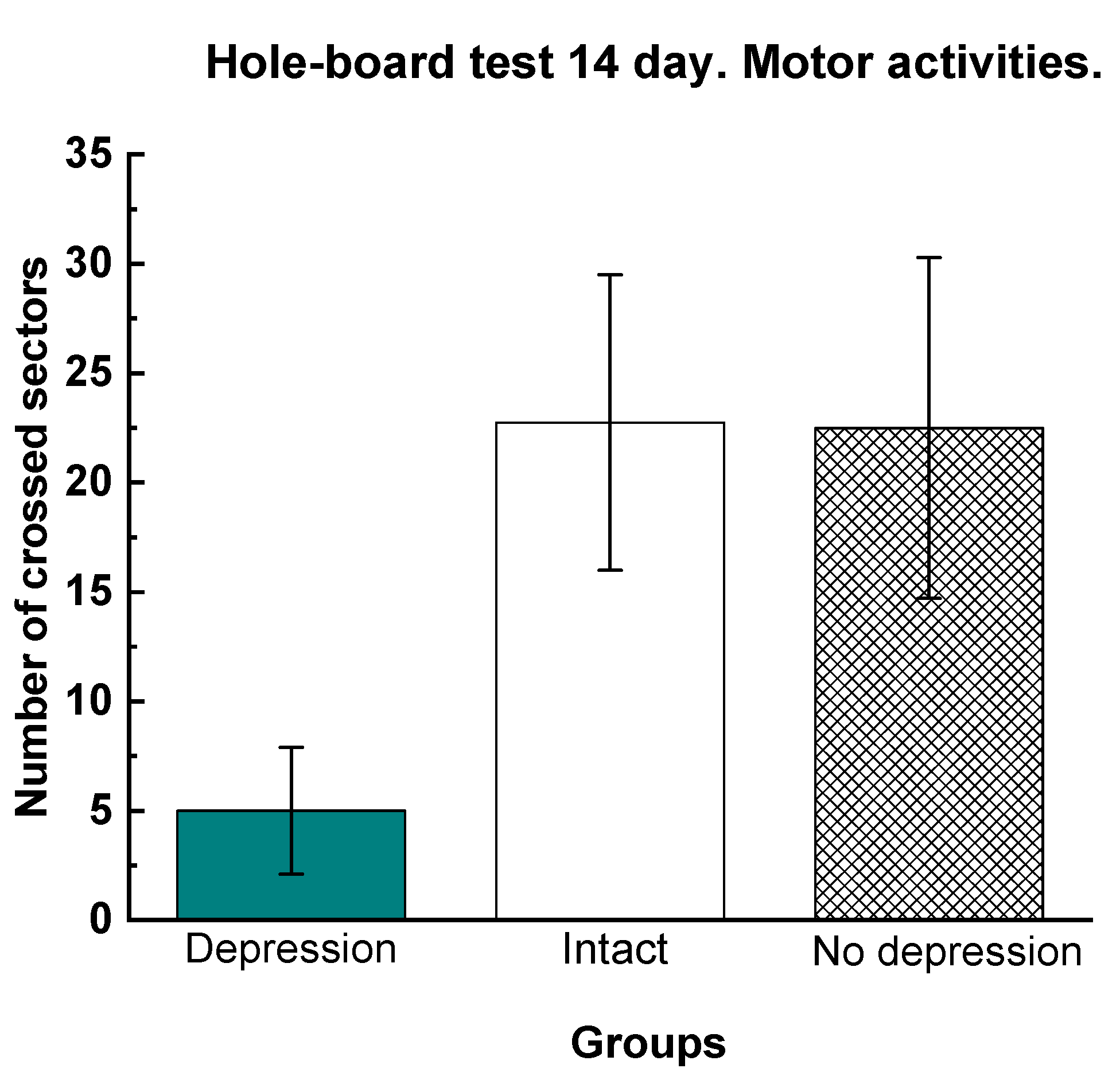
The hole-board test (HBT) is used in experimental pharmacology to assess orientational and motor activity. The vertical activity in the experimental group Dep_C1 was significantly higher than in the Dep_NaCl. The number of crossed sectors in the group Dep_C1 was comparable to that in the group Del_Flu and significantly higher than that in the Dep_NaCl (Fig. 5).
Figure 5.
Results of the hole-board test: (a) vertical and (b) horizontal activities. The results are presented as the mean value ± standard error. Compared to the animals treated with physiological saline, # compared to the intact animals p<0.03.
Figure 5.
Results of the hole-board test: (a) vertical and (b) horizontal activities. The results are presented as the mean value ± standard error. Compared to the animals treated with physiological saline, # compared to the intact animals p<0.03.
2.2. Monoamine concentration studies
On day 31 of the study, the OFT test was performed to assess orientation and motor activity. The results had a similar trend to the HBT Test (see SM Fig.S1 for details).
Monoamine concentration studies
According to the monoamine-related hypothesis, depression is associated with a reduced content of serotonin and an impaired ratio of other monoamines, particularly dopamine; therefore, the levels of these substances were evaluated in the hippocampus and striatum of rats. Hippocampal concentration of monoamines was accessed as in depressed patients the volume of the hippocampus decreases [
13]. Impaired neurogenesis and synaptic dysfunction in the hippocamp may play a key role in the pathophysiology of depressive disorders. In turn, the striatum was chosen for the analysis of monomamines, as the part of the brain responsible for motor functions in animals, and in depressive disorders, is also actively involved in the pathological process [
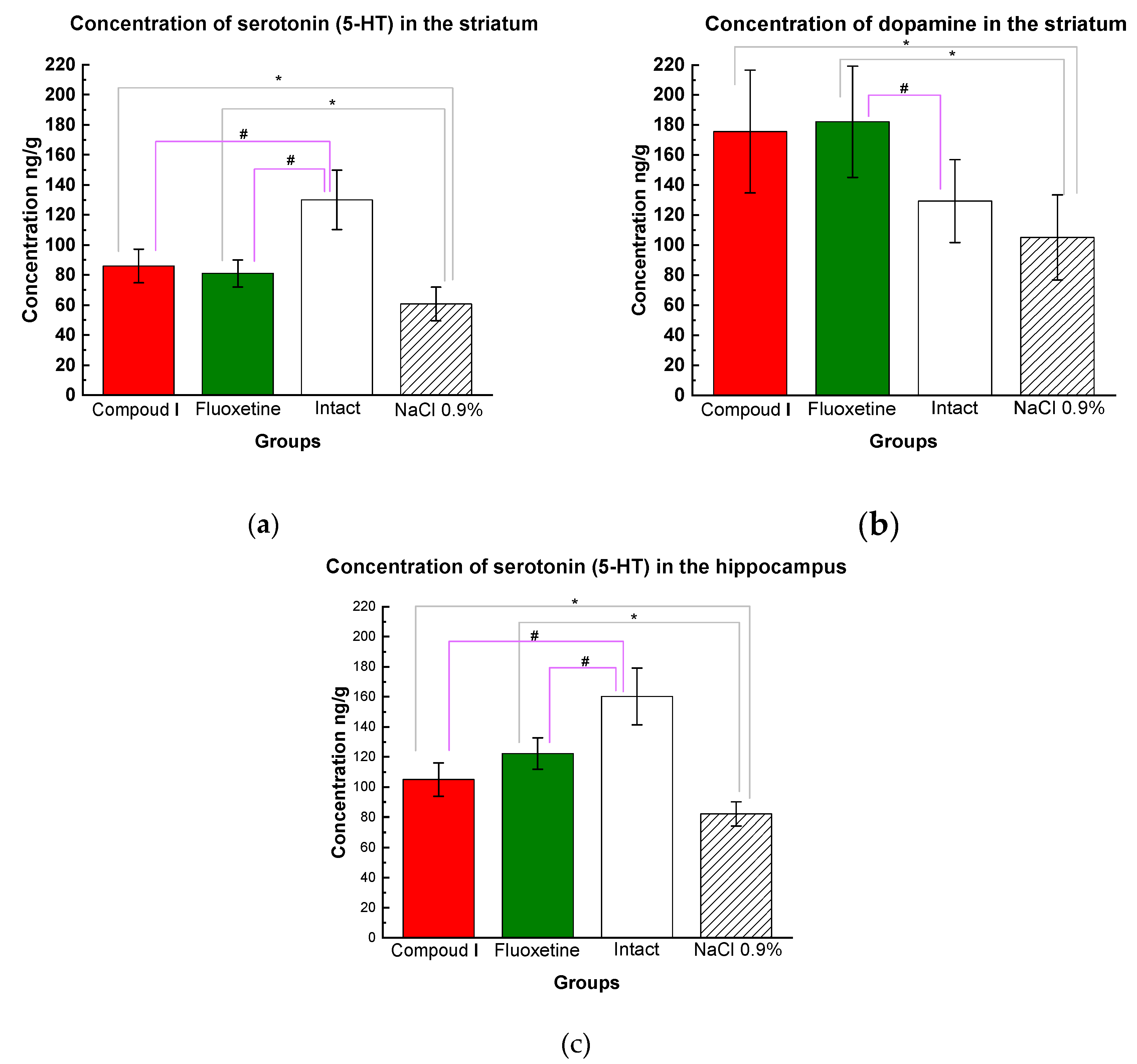
14]. Compound I therapy led to a significant elevation of serotonin in both the striatum and the hippocampus , and the striatal dopamine level tended to increase. The data obtained in the group Dep_C1 were comparable with those in the Dep_Flu (Fig. 6).
Figure 6.
(a) Serotonin concentrations in the striatum (HPLC). Mean values are presented, and standard deviations are used as a scatter; (b) Dopamine concentrations in the striatum (HPLC). The results are presented as the mean value ± standard error. (c) Serotonin concentrations in the hippocampus (HPLC). Compared to the animals treated with physiological saline (*p<0.05) compared to the intact animals #p<0.03 by one-way ANOVA followed by post hoc analysis by Fisher's LSD test.
Figure 6.
(a) Serotonin concentrations in the striatum (HPLC). Mean values are presented, and standard deviations are used as a scatter; (b) Dopamine concentrations in the striatum (HPLC). The results are presented as the mean value ± standard error. (c) Serotonin concentrations in the hippocampus (HPLC). Compared to the animals treated with physiological saline (*p<0.05) compared to the intact animals #p<0.03 by one-way ANOVA followed by post hoc analysis by Fisher's LSD test.
During physical or emotional stress, the hypothalamus-pituitary-adrenal (HPA) axis is activated. The HPA axis is a complex system that is activated during physical or emotional stress. This process involves the secretion of two key hormones by the hypothalamus, corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP), which then act on the pituitary gland to increase the release of adrenocorticotropic hormone (ACTH). In turn, the adrenal cortex is stimulated by ACTH to produce corticosterone, the primary stress hormone.
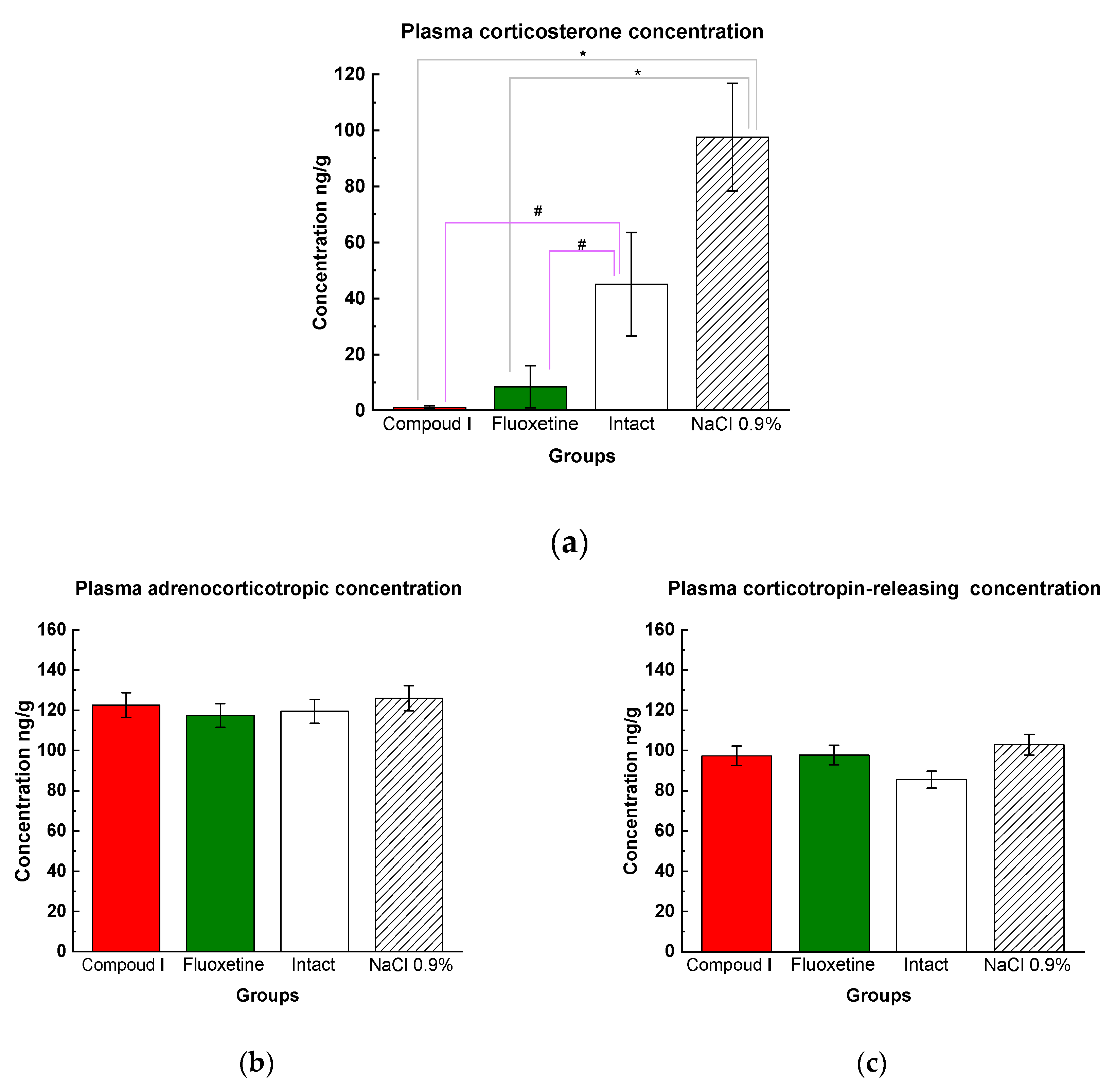
Plasma corticosterone levels were measured after completion of the entire experiment (post-mortem). In the CUMS model, rats were exposed to stress which resulted in elevated plasma levels of corticosterone in saline-treated animals ((Dep_NaCl) (Fig. 7a)). However, Compound I (Dep_C1) was found to effectively reduce the concentration of corticosterone compared to both intact (Int) and physiological saline animals (Dep_NaCl) (Fig. 7a). Interestingly, there were no significant differences in adrenocorticotropic and corticotropin-releasing hormone concentrations between the all groups (Fig. 7 b, c).
Figure 7.
(a) Results of the tests for plasma cortisol concentration (ELISA). Mean values are presented, standard deviations are used as scatter; * - p<0.01 compared with physiological saline, ** - p<0.01 compared to the intact animals by one-way ANOVA followed by post hoc analysis by Fisher's LSD test. (b) Results of the tests for adrenocorticotropic hormone concentrations in plasma (ELISA). The results are presented as the mean value ± standard error. No differences between groups of animals. (c) Results of the tests for corticotropin-releasing hormone concentrations in plasma (ELISA). The results are presented as the mean value ± standard error. No differences between groups of animals.
Figure 7.
(a) Results of the tests for plasma cortisol concentration (ELISA). Mean values are presented, standard deviations are used as scatter; * - p<0.01 compared with physiological saline, ** - p<0.01 compared to the intact animals by one-way ANOVA followed by post hoc analysis by Fisher's LSD test. (b) Results of the tests for adrenocorticotropic hormone concentrations in plasma (ELISA). The results are presented as the mean value ± standard error. No differences between groups of animals. (c) Results of the tests for corticotropin-releasing hormone concentrations in plasma (ELISA). The results are presented as the mean value ± standard error. No differences between groups of animals.
3. Discussion
Depressive disorder is a complex and multifactorial illness with an uncertain pathogenesis. One theory suggests that it is caused by abnormal levels of monoamines (such as serotonin, dopamine, norepinephrine, and their precursors) in various parts of the brain [
15]. This hypothesis has been the basis for developing many of the current antidepressant drugs. However, even with progress in treatment, current antidepressants do not always alleviate all depression symptoms, such as sleep disorders, anxiety, and fatigue [
16]. The involvement of the hypothalamus-pituitary axis has also been shown, as patients with depressive disorders often have elevated cortisol levels [
17]. Moreover, there is evidence that the activation of peripheral immune response and neuroinflammation can contribute to the development of depressive disorders [
18]. Based on the available data, it can be assumed that depression pathogenesis involves disruptions in the interactions between the nervous, endocrine, and immune systems, and that a comprehensive therapeutic approach is required, which has been clinically proven. For instance, a depressive disorder may develop after a viral infection [
19], malignancies [
20], or diabetes mellitus [
21], even in patients without a family history of depression.
There is evidence that compounds based on the pyrrolidine ring have an anticonvulsant effect with anxiolytic and antidepressant properties [
22]. The biological activity of compounds based on the pyrrolidin-2-one heterocycle is highly dependent on conformational changes in the chiral center and on the introduced pharmacophores [
23,
24,
25]. To enhance the potential therapeutic properties of pyrrolidin-2-one, we proposed6 the introduction of a taurine residue, which has neuroprotective properties and can improve exploratory activity. Several studies have suggested that taurine may have antidepressant and anxiolytic effects. For example, in rats with ethanol-induced CNS depression, taurine was found to increase sleep time, and in a CUMS model, taurine administration reduced anxiety and restored sucrose intake [
26,
27]. Using a "silyl" method for N-alkylation of lactams, we synthesized Compound
I, which incorporates a taurine residue [
9,
28].
The objective of this study was to determine whether taurine modification of the pyrrolidin-2-one heterocycle (Compound I) has antidepressant activity and compare its effects to the widely used antidepressant Fluoxetine. We selected stimuli to model depression using the chronic unpredictable mild stress method, which proved effective in inducing depressive behavior by the second week of the experiment. In our CUMS model 95% of the animals developed depression.This was confirmed by the HBT test, where rats exhibited reduced motor activity, and the LDB which showed increased anxiety levels (as evidenced by a longer latency to enter the dark compartment). Additionally, there was a trend towards weight loss compared to the intact group after 21 days of modeling. To assess the depressive state of the animals, we used simple and quick behavioral tests such as the sucrose preference test, the dark-light box, and the hole-board test. These tests proved effective in identifying depressive behavior in most of the rats, except for two particularly active rats that did not exhibit any signs of depression.
The primary behavioral test demonstrating antidepressant properties of the compounds was the forced swimming test. In our study, rats treated with Compound I and Fluoxetine significantly reduced their immobility time and did not stop trying to escape until the end of the test. Based on the results of several behavioral tests, the tested compound exhibited antidepressant activity by increasing motor reactions and exploratory behavior while decreasing apathy and anxiety. The antidepressant effect was comparable to that of the selective serotonin reuptake inhibitor Fluoxetine (fig. 3-6).
When evaluating the behavioral responses of animals treated with Compound I and Fluoxetine, they exhibited greater motor activity and exploratory behavior compared to control groups (fig 5). These results suggest that Compound I can mitigate depression symptoms such as apathy and hypokinesia. In the "Light-dark box" and "elevated plus maze" tests, depressed rats lose their preference for the dark side due to apathy, indicating a lack of survival reflex. However, animals treated with Compound I and Fluoxetine regained their ability to hide, as demonstrated by a decrease in the latency period for entering the dark compartment and reduced time spent in the lighted arm of the maze during the EPM test. In contrast, animals treated with saline solution remained apathetic and stayed in the lighted compartments.
Postmortem data from animal and human studies have shown that dysfunction in the monoaminergic neurotransmitter systems, such as serotonin (5-HT), norepinephrine (NE), and dopamine (DA), plays a key role in the development of depression [
29]. However, conflicting results exist regarding the effects of chronic stress on the levels of these neurotransmitters. Our study supports the findings that chronic stress leads to a decrease in 5-HT levels in the hippocampus [
30,
31].
Our behavioral test results showed that treatment with a serotonin reuptake inhibitor (SSRI) fluoxetine had a positive effect in this model, indicating that the balance of serotonin plays a crucial role in the development of depression in CUMS. We also demonstrated that administration of Compound
I resulted in the restoration of serotonin levels in both the striatum and the hippocampus in rats, which was comparable to the levels observed in rats treated with Fluoxetine. This suggests that the two drugs may share similar mechanisms of action. In future studies, we plan to investigate the levels of homovanillic acid and the precursors of dopamine and serotonin. For a long time, the activation of the hypothalamic-pituitary system was considered the key step in the pathogenesis of depressive behavior in the CUMS model, rather than neuroinflammatory changes [
32]. In our study, we demonstrated an increase in corticosterone levels in the blood plasma of animals subjected to CUMS without therapy, which was significantly reduced upon treatment with Compound
I and Fluoxetine. The ability to influence depressive behavior by normalizing the balance of monoamines and reducing stress hormone levels has been confirmed by some studies on Fluoxetine [
33], but not all researchers agree, as data are suggesting that Fluoxetine may actually increase corticosterone levels in some cases [
34,
35]. Our study provides direct evidence of a link between the reduction of depressive behavior, the increase in monoamine concentrations, and the decrease in corticosterone levels upon treatment with Compound
I and Fluoxetine.
4. Materials and Methods
4.1. Chemistry
Compound
I was synthesized according to the previously proposed method [
36] The method involves a four-step approach starting with the alkylation of lactam 2 with ethyl chloroacetate, followed by alkaline hydrolysis, acid esterification, and treatment with taurine to obtain Compound
I. This method is suggested for industrial production due to higher yields and less contamination.
Scheme 2.
Representative synthetic routes for Compound I.
Scheme 2.
Representative synthetic routes for Compound I.
The yields of the products were 80 – 90%.
4.2. Animals models
The behavioral study was conducted on 48 mature male Wistar rats with an average weight of 220 ± 12 g, obtained from the animal nursery of FSBI Federal Research Center for Cytology and Genetics of the Siberian Branch of the Russian Academy of Sciences in Novosibirsk. The animals were housed in a standard facility at N.I. Pirogov Russian National Research Medical University with automatic day and night cycles (08:00-20:00 – "day", 20:00-08:00 – "night"), optimal temperature (20-24° C), and humidity levels (45-65%), ensuring their comfort and meeting the standards of Directive 2010/63/EU on the protection of animals used for scientific purposes. The experiments were approved by the Commission for the Care and Use of Animals of Pirogov Russian National Research Medical University (Protocol No. 08/2021).
The study design is presented in table 2.
Table 4.
Stress factors and induction times for depression modeling.
Table 4.
Stress factors and induction times for depression modeling.
| Exposure |
Study day |
| Food deprivation for 24 hours |
10, 14, 17 |
| Water deprivation for 12 hours |
6, 12, 19 |
| Cold stress for 30 min (+6 °C) |
2, 9, 16 |
| Soft atraumatic tail clip (for 1 min; the lateral part of the tail 2 cm from the tip). |
3, 8, 20 |
| Hot plate test (for 1 min, T 50°C) |
5, 13, 15 |
| Mixing of the cage animals (2 "new" and 2 "old" rats in the cage) |
4, 7, 21 |
| Light/dark cycle reversal (turning on the light from 20.00 to 8.00 and turning off the light source from 8.00 to 20.00) |
1, 11, 18 |
Group of intact animals were not exposed to stressful conditions. To confirm the depressive state, a sucrose test was performed, and on Day 14, the hole-board test was repeated.
4.4. Treatment (administration of compounds)
The dose of Compound
I (125 mg/kg) was chosen based on the results of our previous studies: pharmacokinetic studies, and the studies of the beneficial effects of the substance on neurologic deficits [
9,
37]. The intraperitoneal route of administration was chosen as an equivalent of iv infusion (for longer administration - more than 5 days). Fluoxetine was selected as the comparator substance because selective serotonin reuptake inhibitors are currently used as first-line drugs for the treatment of depression and are widely applied in in vivo studies. The therapeutic dose for rats with short-term treatment was 15 mg/kg [
36].
4.5. Behavioral test for the efficassy measurment.
Anhedonia, a significant reduction or loss of the ability to experience pleasure, is a hallmark symptom of depressive disorder. In rat studies, the sucrose test is the most commonly utilized method to evaluate the severity of anhedonia [
37]. To conduct the test, animals are subjected to water (6 h) and food deprivation (12 h) and then placed into cages (2 rats per cage). The test is performed from 9:00 to 21:00, during which time the animals are only allowed access to drinking water or a 2% sucrose solution for 12 hours. The bottles are weighed before and immediately after the test.
Additionally, in assessing depression using the behavioral hole-board test, decreased vertical activity (rearing and hiding episodes - not more than 15) and horizontal activity (not more than 20 crossed sectors) are used as criteria.
4.6. Behavioral tests
The following tests were used for the assessment of general motor activity and orientation and exploratory behavior (see
Table 2).
The Hole board test (HBT) (OpenScience, Russia) was conducted using a modified technique [
38] before the study to create groups with identical behavior; on Day 14 of the experiment - to confirm the development of a depressive state; and on Day 30 of the experiment, to assess the psycho-emotional state. The number of crossed sectors, vertical activity, speed, and the distance traveled were monitored. The open-field test (OpenScience, Russia) was carried out on Day 31 of experiment using the method described in [
39] for 5 min. The parameters tested were the same as in the HBT. Two similar tests were used to exclude memorization and habituation of animals to the settings. The light-dark box test (LDB) (Neurobotics, Russia) was conducted as described earlier [
40]: the latency of entrance to the dark compartment, the time spent in the light compartment, the number of peeking out from the dark compartment, the number of rearing episodes were recorded over a period of 3 min. The test was performed on Day 14 of depression modeling to assess the development of depression and on Day 33 of experiment. The elevated plus maze test (EMT) (OpenScience, Russia) was conducted using previously described the method [
41]. The time spent and the number of visits to the light compartment, the number of rearing episodes, hanging, peeking out, and the number and time of grooming and fading episodes, were recorded on Day 34 of the experiment. The Porsolt forced swim test (FST) was conducted for 5 min. The time and the number of episodes of active swimming and immobility, the number of jumps and dives according to [
42] were recorded. The test was carried out on the last day of the experiment.
4.7. Monoamine concentration studies (HPLC)
To evaluate serotonin and dopamine concentrations in the brain structures, the rats were decapitated with a guillotine and the hippocampus and striatum were isolated on ice. The samples were studied on a high-performance liquid chromatography apparatus (Shimadzu LC-20AD). The samples were stored at -80 °C. To carry out the analysis, the weighed samples were placed in 2 mL test tubes with seal caps. The weights of the samples were 35±10 mg for the striatum and 41±14 mg for the hippocampus. Caffeic acid was used as an internal standard.
240 mL of deionized water, 30 mL of caffeic acid solution (500 ng/mL), and 30 mL of formic acid were added to the test samples. The resulting mixture was homogenized on a Precellys Evolution apparatus (France) at 5000 rpm for 1.5 min. 300 µL of chloroform was added to the resulting solution and mixed on Multi-Vortex Biosan (Latvia) for 30 seconds, followed by centrifugation (CM-50; ELMI; Latvia) for 5 minutes at 12,000 g. 300 µL of chloroform was added to the resulting supernatant and mixed on a Multi-Vortex for 30 seconds, followed by centrifugation (CM-50; ELMI; Latvia) for 3 min at 12,000 g. The supernatant was transferred to 300 µL vials for subsequent testing.
Two calibration curves were used for the determination of monoamine concentrations - for the hippocampal and striatal samples. The brain tissue samples used to construct the calibration curve were kept at ambient temperature for several hours to ensure complete degradation of dopamine and serotonin. To construct calibration curves, 210 mL of deionized water, 30 mL of caffeic acid solution (500 ng/mL), 30 µL of a mixture of serotonin and dopamine reference standards, and 30 µL of formic acid were added to the suspension. Further sample preparation was identical to that described above.
Supelco Ascentis (Sigma-Aldrich; USA) C18 25 cm 4.6 mm column. Mobile phase A; 0.2% formic acid in deionized water; mobile phase B: 0.2% formic acid solution in methanol. 0 – 0.5 min A - 95%, B - 5%; 0.5 – 7 min linear gradient to A - 20%, B – 80%; 7.01 – 10 min B – 100%; 10.01 – 17 min A - 95%, B – 5%. The volume of the pumped mobile phase was 0.8 mL/min. The temperature of the sample cell was 9°C. The temperature of the thermostat was 40 °C. The injection volume was 20 mL.
The compounds were analyzed using the electrospray ionization (ESI) method. Positive ionization was used for testing. The interface voltage was 3.5 kV. The temperature of the heating unit was 400 °C, the desolvation temperature was 250 °C, the spray gas flow rate was 3 L/min, the drying gas flow rate was 15 L/min, and the gas CID pressure was 60 kPa. The MRM transitions used were: 154.05→137.10 for dopamine and 177.10→160.10 for serotonin, 178.95→134.95 for caffeic acid.
Sample collection. Blood, hippocampus and striatum were collected 24 hours after the last behavioral test, on the 36th day of the experiment. Serum was separated after centrifuging at 3000 rpm for 10 minutes at 4 °C, and was kept at −20 °C for further analysis. Hippocampus samples were dissected immediately after the rats were sacrificed, frozen in liquid nitrogen, and then stored under −80 °C.
4.8. Measurement of hormones and inflammatory factors.
Enzyme-linked immunosorbent assay (ELISA) was used to measure serum hormone levels. Serum concentrations of corticosterone (Cort), corticotropin-releasing hormone (CRH), and adrenocorticotropic hormone (ACTH) (#ELK8633, #ELK 2606, #ELK2414, Wuhan, China) were measuredusing commercial ELISA kits. Serum values were determined without any dilution. All experimental steps were performed according to the manufacturers instructions. The plates were precoated with Cort, CRH and antibodies to PRL, respectively. Optical density values were recorded at 450 nm and concentrations were calculated from the standard curve.
Statistical analysis was performed using STATICTICA software version 12.0. The Kolmogorov-Smirnov test was used to assess the assumption of normal behavioral and biochemical findings. All values were presented as mean ± standard error of the mean (SEM), and p values less than 0.05 were considered significant. One-way analysis of variance was used to analyze all behavioral and biochemical data, followed by post hoc analysis using the Fisher test for LSD.
Supplementary Materials
The following supporting information can be downloaded at: Figure S1: The results of the Open-field test; Table S1: Data of HBT conducted on the zero and 14th days of the experiment.
Author Contributions
Conceptualization, V.V.N. and Yu.I.B.; methodology, D.A.B, D.I.G., V.I.B., S.S.K., Ya.V.G., T.A.S., A.A.E., K.A.S.,T.D.V. and K.E.P. software, D.A.B; validation, Ya.V.G., D.A.B., and D.I.G..; formal analysis, D.A.B.; investigation D.A.B, D.I.G. and T.A.S; V.V.N; resources, X.X.; data curation, T.A.S; writing—original draft preparation D.A.B., T.A.S and A.A.E.; writing—review and editing, T.A.S and A.A.E.; supervision, Yu.I.B.; project administration, N.M.K. and V.V.N.; funding acquisition, Yu.I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 21-73-20250.
Institutional Review Board Statement
“The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Pirogov Russian National Research Medical University Animal Care code No. 08/2021 and date of approval 25.02.2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health, O. Depression and other common mental disorders: global health estimates; World Health Organization: Geneva, 2017, 2017. [Google Scholar]
- Wise, J. NICE guidance on depression: 35 health organisations demand “full and proper” revision. BMJ 2019, 365, l2356. [Google Scholar] [CrossRef]
- Artigas, F.; Bortolozzi, A.; Celada, P. Can we increase speed and efficacy of antidepressant treatments? Part I: General aspects and monoamine-based strategies. European Neuropsychopharmacology 2018, 28, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, K.S. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef]
- Kostev, K.; Weber, K.; Riedel-Heller, S.; von Vultée, C.; Bohlken, J. Increase in depression and anxiety disorder diagnoses during the COVID-19 pandemic in children and adolescents followed in pediatric practices in Germany. Eur Child Adolesc Psychiatry 2023, 32, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Rafeyan, R.; Papakostas, G.I.; Jackson, W.C.; Trivedi, M.H. Inadequate Response to Treatment in Major Depressive Disorder: Augmentation and Adjunctive Strategies. J Clin Psychiatry 2020, 81. [Google Scholar] [CrossRef]
- Borozdenko, D.A.; Khovanova, S.S.; Kiseleva, N.M.; ., N. V.V. MODELING DEPRESSION. Medical Journal of the Russian Federation 2019, 25, 176–180. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Druzhilovskiy, D.S.; Lagunin, A.; Gloriozova, T.; Rudik, A.V.; Dmitriev, A.V.; Pogodin, P.; Poroikov, V. Computer-aided prediction of biological activity spectra for chemical compounds: opportunities and limitation. Biomedical Chemistry: Research and Methods 2018, 1, e00004. [Google Scholar] [CrossRef]
- Borozdenko, D.; Lyakhmun, D.N.; Golubev, Y.V.; Tarasenko, D.; Negrebetsky, V. Study of the new 4-phenylpyrrolidinone-2 derivative pharmacokinetics and neuroprotective effect in the ischemic stroke animal model. Bulletin of Russian State Medical University, 2020. [Google Scholar] [CrossRef]
- Sarkar, D. A Review of Behavioral Tests to Evaluate Different Types of Anxiety and Anti-anxiety Effects. Clin Psychopharmacol Neurosci 2020, 18, 341–351. [Google Scholar] [CrossRef]
- Dulawa, S.C.; Holick, K.A.; Gundersen, B.; Hen, R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 2004, 29, 1321–1330. [Google Scholar] [CrossRef]
- Dawson, C.A.; Horvath, S.M. Swimming in small laboratory animals. Med Sci Sports 1970, 2, 51–78. [Google Scholar] [CrossRef]
- Opel, N.; Redlich, R.; Zwanzger, P.; Grotegerd, D.; Arolt, V.; Heindel, W.; Konrad, C.; Kugel, H.; Dannlowski, U. Hippocampal Atrophy in Major Depression: a Function of Childhood Maltreatment Rather than Diagnosis? Neuropsychopharmacology 2014, 39, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, Y.; Pu, J.; Gui, S.; Zhong, X.; Tian, L.; Song, X.; Qi, X.; Wang, H.; Xie, P. Chronic Stress in a Rat Model of Depression Disturbs the Glutamine-Glutamate-GABA Cycle in the Striatum, Hippocampus, and Cerebellum. Neuropsychiatr Dis Treat 2020, 16, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Hillhouse, T.M.; Porter, J.H. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol 2015, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, T.P. Depressive disorders: Treatment failures and poor prognosis over the last 50 years. Pharmacol Res Perspect 2019, 7, e00472. [Google Scholar] [CrossRef] [PubMed]
- Kvarta, M.D.; Bradbrook, K.E.; Dantrassy, H.M.; Bailey, A.M.; Thompson, S.M. Corticosterone mediates the synaptic and behavioral effects of chronic stress at rat hippocampal temporoammonic synapses. J Neurophysiol 2015, 114, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Amruta, N.; Chastain, W.H.; Paz, M.; Solch, R.J.; Murray-Brown, I.C.; Befeler, J.B.; Gressett, T.E.; Longo, M.T.; Engler-Chiurazzi, E.B.; Bix, G. SARS-CoV-2 mediated neuroinflammation and the impact of COVID-19 in neurological disorders. Cytokine Growth Factor Rev 2021, 58, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Singh, G. Biological Mechanisms of Cancer-Induced Depression. Front Psychiatry 2018, 9, 299. [Google Scholar] [CrossRef]
- Holt, R.I.; de Groot, M.; Golden, S.H. Diabetes and depression. Curr Diab Rep 2014, 14, 491. [Google Scholar] [CrossRef]
- Malykh, A.G.; Sadaie, M.R. Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders. Drugs 2010, 70, 287–312. [Google Scholar] [CrossRef]
- Li Petri, G.; Raimondi, M.V.; Spanò, V.; Holl, R.; Barraja, P.; Montalbano, A. Pyrrolidine in Drug Discovery: A Versatile Scaffold for Novel Biologically Active Compounds. Topics in Current Chemistry 2021, 379, 34. [Google Scholar] [CrossRef] [PubMed]
- Veinberg, G.; Vorona, M.; Zvejniece, L.; Vilskersts, R.; Vavers, E.; Liepinsh, E.; Kazoka, H.; Belyakov, S.; Mishnev, A.; Kuznecovs, J.; et al. Synthesis and biological evaluation of 2-(5-methyl-4-phenyl-2-oxopyrrolidin-1-yl)-acetamide stereoisomers as novel positive allosteric modulators of sigma-1 receptor. Bioorganic & Medicinal Chemistry 2013, 21, 2764–2771. [Google Scholar]
- Genton, P.; Van Vleymen, B. Piracetam and levetiracetam: close structural similarities but different pharmacological and clinical profiles. Epileptic Disord 2000, 2, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.F.; Ren, S.; Tang, R.Y.; Xu, C.; Zhou, J.Q.; Lin, S.M.; Feng, Y.; Yang, Q.H.; Hu, J.M.; Yang, J.C. Antidepressant effect of taurine in chronic unpredictable mild stress-induced depressive rats. Sci Rep 2017, 7, 4989. [Google Scholar] [CrossRef] [PubMed]
- Mattucci-Schiavone, L.; Ferko, A.P. Acute effects of taurine and a taurine antagonist on ethanol-induced central nervous system depression. Eur J Pharmacol 1985, 113, 275–278. [Google Scholar] [CrossRef]
- Shipov, A.; Kramarova, E.; Negrebetsky, V.; Pogozhikh, S.; Yu, I. Synthesis, Molecular and Crystal Structure of PHE Notropyl. Bull. RSMU 2006, 1, 56–61. [Google Scholar]
- Hamon, M.; Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry 2013, 45, 54–63. [Google Scholar] [CrossRef] [PubMed]
- O'Mahony, C.M.; Clarke, G.; Gibney, S.; Dinan, T.G.; Cryan, J.F. Strain differences in the neurochemical response to chronic restraint stress in the rat: Relevance to depression. Pharmacology Biochemistry and Behavior 2011, 97, 690–699. [Google Scholar] [CrossRef]
- Torres, I.L.S.; Gamaro, G.D.; Vasconcellos, A.P.; Silveira, R.; Dalmaz, C. Effects of Chronic Restraint Stress on Feeding Behavior and on Monoamine Levels in Different Brain Structures in Rats. Neurochemical Research 2002, 27, 519–525. [Google Scholar] [CrossRef]
- Willner, P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol Stress 2017, 6, 78–93. [Google Scholar] [CrossRef]
- Jayakumar, S.; Raghunath, G.; Ilango, S.; Vijayakumar, J.; Vijayaraghavan, R. Effect of Fluoxetine on the Hippocampus of Wistar Albino Rats in Cold Restraint Stress Model. J Clin Diagn Res 2017, 11, Af01–af06. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.G.; Cunha, M.P.; Neis, V.B.; Balen, G.O.; Colla, A.; Grando, J.; Brocardo, P.S.; Bettio, L.E.B.; Capra, J.C.; Rodrigues, A.L.S. Fluoxetine reverses depressive-like behaviors and increases hippocampal acetylcholinesterase activity induced by olfactory bulbectomy. Pharmacology Biochemistry and Behavior 2012, 103, 220–229. [Google Scholar] [CrossRef]
- Young, E.A.; Altemus, M.; Lopez, J.F.; Kocsis, J.H.; Schatzberg, A.F.; deBattista, C.; Zubieta, J.-K. HPA axis activation in major depression and response to fluoxetine: a pilot study. Psychoneuroendocrinology 2004, 29, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Borozdenko, D.A.; Ezdoglian, A.A.; Shmigol, T.A.; Gonchar, D.I.; Lyakhmun, D.N.; Tarasenko, D.V.; Golubev, Y.V.; Cherkashova, E.A.; Namestnikova, D.D.; Gubskiy, I.L.; et al. A Novel Phenylpyrrolidine Derivative: Synthesis and Effect on Cognitive Functions in Rats with Experimental Ishemic Stroke. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Poirier, G.L.; Hitora-Imamura, N.; Sandi, C. Emergence in extinction of enhanced and persistent responding to ambiguous aversive cues is associated with high MAOA activity in the prelimbic cortex. Neurobiol Stress 2016, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rebai, R.; Jasmin, L.; Boudah, A. The antidepressant effect of melatonin and fluoxetine in diabetic rats is associated with a reduction of the oxidative stress in the prefrontal and hippocampal cortices. Brain Res Bull 2017, 134, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Labots, M.; Van Lith, H.A.; Ohl, F.; Arndt, S.S. The modified hole board--measuring behavior, cognition and social interaction in mice and rats. J Vis Exp, 10.3791/52529. [CrossRef]
- Sestakova, N.; Puzserova, A.; Kluknavsky, M.; Bernatova, I. Determination of motor activity and anxiety-related behaviour in rodents: methodological aspects and role of nitric oxide. Interdiscip Toxicol 2013, 6, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Arrant, A.E.; Schramm-Sapyta, N.L.; Kuhn, C.M. Use of the light/dark test for anxiety in adult and adolescent male rats. Behav Brain Res 2013, 256, 119–127. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2007, 2, 322–328. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).