1. Introduction
Astrocytes represent the most abundant subtype of glial cells in the central nervous system. They are characterized by a typical star-shaped morphology with numerous ramified processes and exert a huge variety of distinct functions, e.g. the support of endothelial cells at the blood-brain barrier, the maintenance of ion homeostasis around synapses and in the extracellular space, the metabolic support of neuronal survival and the regulation of synaptogenesis, among others [
1,
2,
3,
4,
5].
In rodents, the generation, expansion and maturation of astrocytes (astrogenesis) take place during the first three weeks of brain postnatal development (PND) and are for the most part completed by the end of the so-called “critical period”, a highly sensitive time window of elevated brain plasticity [
6,
7,
8]. In humans, this developmental time frame corresponds to the juvenile period of postnatal brain growth, which ends around the first decade of life, when adolescence begins [
9]. However, it is still debated whether it might further extend into adolescence and eventually early adulthood, an aspect that becomes relevant when investigating putatively detrimental effects of environmental cues on brain development or searching for the most effective therapeutic treatments for early-onset mental disorders [
10,
11].
Astrogenesis, together with the refinement and specialization of astrocyte processes [
12], coincides with a highly active period of synaptogenesis, which culminates with the establishment of properly functional neuronal networks [
13,
14]. The work of Chung and colleagues revealed that in mice an astrocyte-dependent synapse elimination (pruning), mediated by the MEGF10 phagocytic pathway and prevalently directed towards glutamatergic synapses, occurs during both developmental stages as well as in adulthood. Interestingly, they showed that MEGF10 is almost exclusively expressed in astrocytes [
15,
16] and the phagocytic pathway is essential for the establishment of the eye-specific retinogeniculate segregation and the maintenance of a functional homeostasis in the adult hippocampus [
16,
17]. Any disturbance in the sequence of these events may lead to the development of dysfunctional neuronal circuits and improper synaptic transmission, thereby contributing to the onset of brain disorders [
18].
Among additional factors that may affect brain development, sex differences have been consistently reported in epidemiological studies to be relevant for their impact on both physiological and pathological processes. However, research is lacking in deciphering the causes of these differences, thereby preventing not only a deep understanding of sex-dependent neurobiological underpinnings of synaptic network development, but also aetiological and pathogenic mechanisms of mental disorders in general. Moreover, this lack of knowledge hinders the generation of therapeutic approaches specific for either women or men [
19,
20].
The prefrontal cortex (PFC) is one of the regions with high vulnerability to environmental and endogenous stimuli, especially during sensitive early PND stages. Exposure to detrimental cues during these early growing phases might become imprinted in the developing system, ultimately shaping maladaptive adult behaviors [
9,
21]. Previous studies in humans have carefully described changes in synaptogenesis during childhood through adolescence into early adulthood [
22]. In rats, sex-dependent differences have been observed in the size of the PFC in adulthood, which started to appear during adolescence and might originate from neuronal death or pruning effects on neuronal circuits [
23].
Primary dissociated cultures have been pivotal to study molecular, morphological and biochemical features of single homogeneous cell populations, allowing the performance of animal research experiments, without excessively increasing the numbers of experimental animals or their sufferance. However, in vitro cell cultures obviously lack 3D structures to explore the functional interplays between brain cells in a more complex context, where the main cell-cell physical and mechanical interactions are preserved. Thus, several models have been developed to support the analysis of such interactions. Among them, organotypic brain slice cultures (OBSCs) have proven to be useful in investigating specific cellular and molecular processes of the brain ex vivo [
24,
25,
26]. Moreover, they preserve various aspects of structural and synaptic organization of the original tissue. With respect to other in vitro/ex vivo systems, such as the more versatile and complex brain organoids [
27,
28], OBSCs represent a useful tool with various advantages, beyond some obvious limitations [
29,
30].
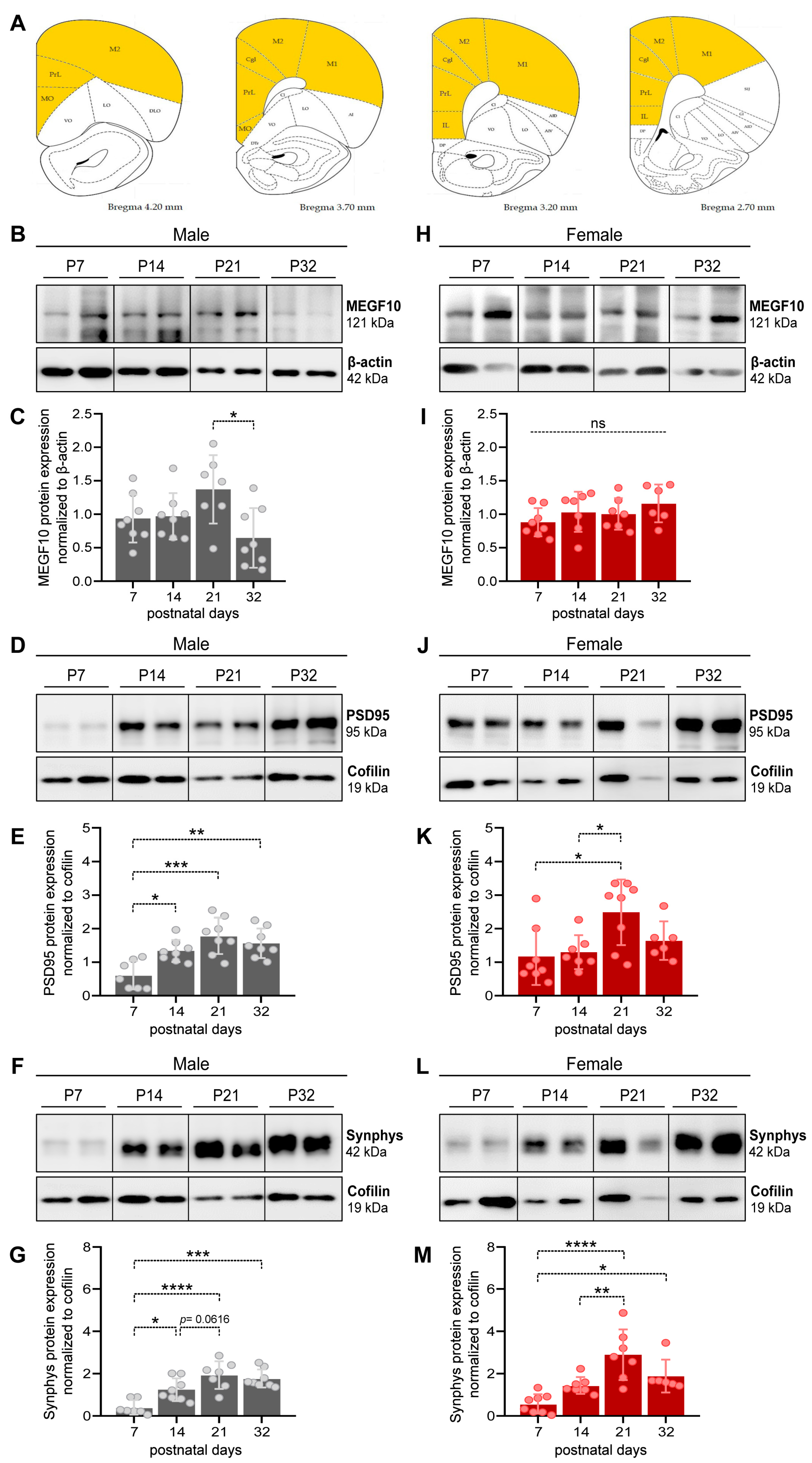
Here, we first measured via Western blot the expression of MEGF10 and the glutamatergic synaptic markers synaptophysin (presynaptic) and postsynaptic density-95 (PSD-95; postsynaptic) during the critical period of brain development, at postnatal (P) day 7, P14 and P21, and after its closure, at P32, in the cortex of male and female Wistar rats. In parallel, we used immunofluorescent-immunohistochemistry combined with the 3D reconstruction of single astrocytes to examine age-dependent and sex-specific differences in astrocyte-mediated synapse elimination more closely into the infralimbic/prelimbic areas of PFC. Furthermore, we established and validated an OBSC model, which might serve to evaluate how the MEGF10 phagocytic pathway supports the shaping of glutamatergic synaptic networks in the PFC during the critical period and whether it might be implicated in brain disorders with synaptic aberrancies. The application of pharmacological and/or genetic manipulations in OBSCs may be helpful to identify and investigate novel, sex-specific, disease trajectories and to potentially develop alternative treatment options tailored to the needs of males and females.
3. Results
3.1. MEGF10, PSD95 and synaptophysin expression in the PFC of male and female developing rat pups
We first examined differences in the expression of the phagocytic protein MEGF10 and the synaptic markers synaptophysin (synphys) and PSD95 in the cortex of male and female rat pups from P7 to P32. We observed that in males MEGF10 total protein levels remained unaltered through P7-P21 and decreased at P32. Contrarily, in female pups MEGF10 total protein levels did not change at any of the developmental stages examined (
Figure 1B,C, males, one-way ANOVA, F(3,27)=3,787, p<0.0218, Tukey’s posthoc test: P21-P32, *p<0.05;
Figure 1H,I, females, one-way ANOVA, F(3,24)=1.399, p=0.2673, ns, not significant).
We found additional differences between sexes when we evaluated the expression of synphys and PSD95. In males, we observed a significant rise in the amount of PSD95 between P7 and P14, which reached a peak and remained elevated throughout the subsequent stages (Fig1D,E, one-way ANOVA, F(3,27)=9.714, p=0.0002; Tukey’s posthoc test: P7-P14, *p<0.05, P7-P21, ***p<0.001, P7-P32, **p<0.01). In females, levels of PSD95 were already slightly higher than in males at P7 and showed a peak of expression at P21, which went down again at P32 (Fig1J,K, one-way ANOVA, F(3,25)=4.646, p=0.0103; Tukey’s posthoc test: P7-P21, *p<0.05, P14-P21, *p<0.05).
When we examined synphys, we saw that, in males, changes in expression levels followed a similar pattern than for PSD95, with a rise between P7 and P14, which plateaued throughout P21 and P32 (
Figure 1F,G, one-way ANOVA, F(3.26)=13.28, p<0.0001; Tukey’s posthoc test: P7-P14, *p<0.05, P7-P21, ****p<0.0001, P7-P32, ***p<0.001). In females, the rise in protein expression was delayed in a similar way as for PSD95, with a peak at P21, which was however still slightly elevated at P32, although less prominent than at P21 (
Figure 1L,M, one-way ANOVA, F(3,24)=11.82, p<0.0001; Tukey’s posthoc test: P7-P21, ****p<0.0001, P14-P21, **p<0.001, P7-P32, *p<0.05).
3.2. Astrocyte-dependent synapse elimination in the PFC of male and female developing rat pups
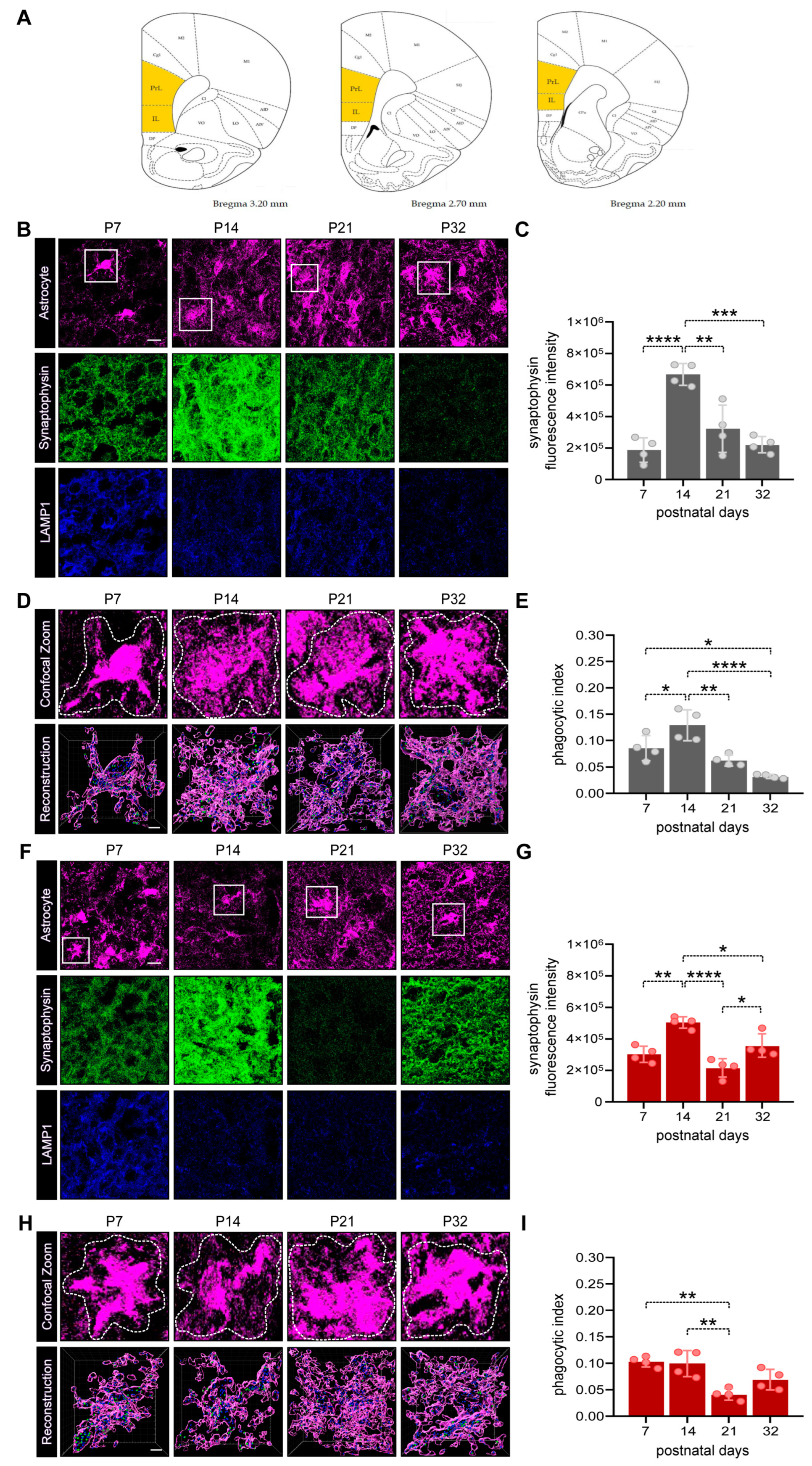
Although markers for adult astrocytes have been identified and successfully used so far [
6], it is still under debate which markers can unequivocally label early-stage postnatal astrocytes. Based on the work of Raponi and colleagues [
7], we decided to use a mixture of GFAP and S100ß antibodies to identify early-stage mature astrocytes of the PFC. To characterize changes in synphys protein in a more precise and localized way than previously done with Western blot, we performed immunofluorescent-immunohistochemistry in slices and quantified differences in our parameters of interest in the infralimbic/prelimbic areas of the PFC (
Figure 2A).
Using a combination of antibodies against synaptophysin and the lysosomal associated membrane protein 1 (LAMP1), we measured the phagocytic index (for details, see §2.2.3 of the Materials and Methods section) of single astrocytes in the the PFC of male and female rat pups from P7 to P32.
The analysis of synphys fluorescence intensity showed that, in males, a rapid increase of the signal occurred between P7 and P14, followed by an equally rapid signal decline by P21, which stabilized at P32 (
Figure 2B,C, one-way ANOVA, F(3.12)=21.36, p<0.0001; Tukey’s posthoc test: P7-P14, ****p<0.0001, P14-P21, **p<0.001, P14-P32, ***p<0.001). Similar to males, synphys fluorescent intensity in females showed a rapid rise from P7 to P14 and a decline between P14 and P21. However, differently than in males, the signal increased again by P32, suggesting that a second wave of synaptogenesis may occur in female pups (
Figure 2F,G, one-way ANOVA, F(3.12)=18.01, p<0.0001; Tukey’s posthoc test: P7-P14, **p<0.01, P14-P21, ****p<0.0001, P14-P32, *p<0.05, P21-P32, *p<0.05).
When we examined the changes in the rates of synapse elimination, we observed again sex-dependent differences, which were comparable to the respective synphys intensity patterns. In males, we measured a rise in synapse elimination between P7 and P14, which decreased by P21 and dropped down at P32 (Fig2 D,E, one-way ANOVA, F(3.12)=17.17, p=0.0001; Tukey’s posthoc test: P7-P14, *p<0.05, P7-P32, *p<0.05, P14-P21, **p<0.001, P14-P32, ****p<0.0001). In females, the pattern of synapse elimination displayed higher levels of phagocytic index already detectable at P7, which remained elevated at P14, went down at P21 and slightly increased again at P32 (Fig2 H,I, one-way ANOVA, F(3.12)=11.12, p=0.0009; Tukey’s posthoc test: P7-P21, **p<0.001, P14-P21, **p<0.001).
3.3. Establishing organotypic brain slice cultures (OBSCs) to examine synapse elimination in the PFC during early postnatal developmental stages
3.3.1. Viability assay
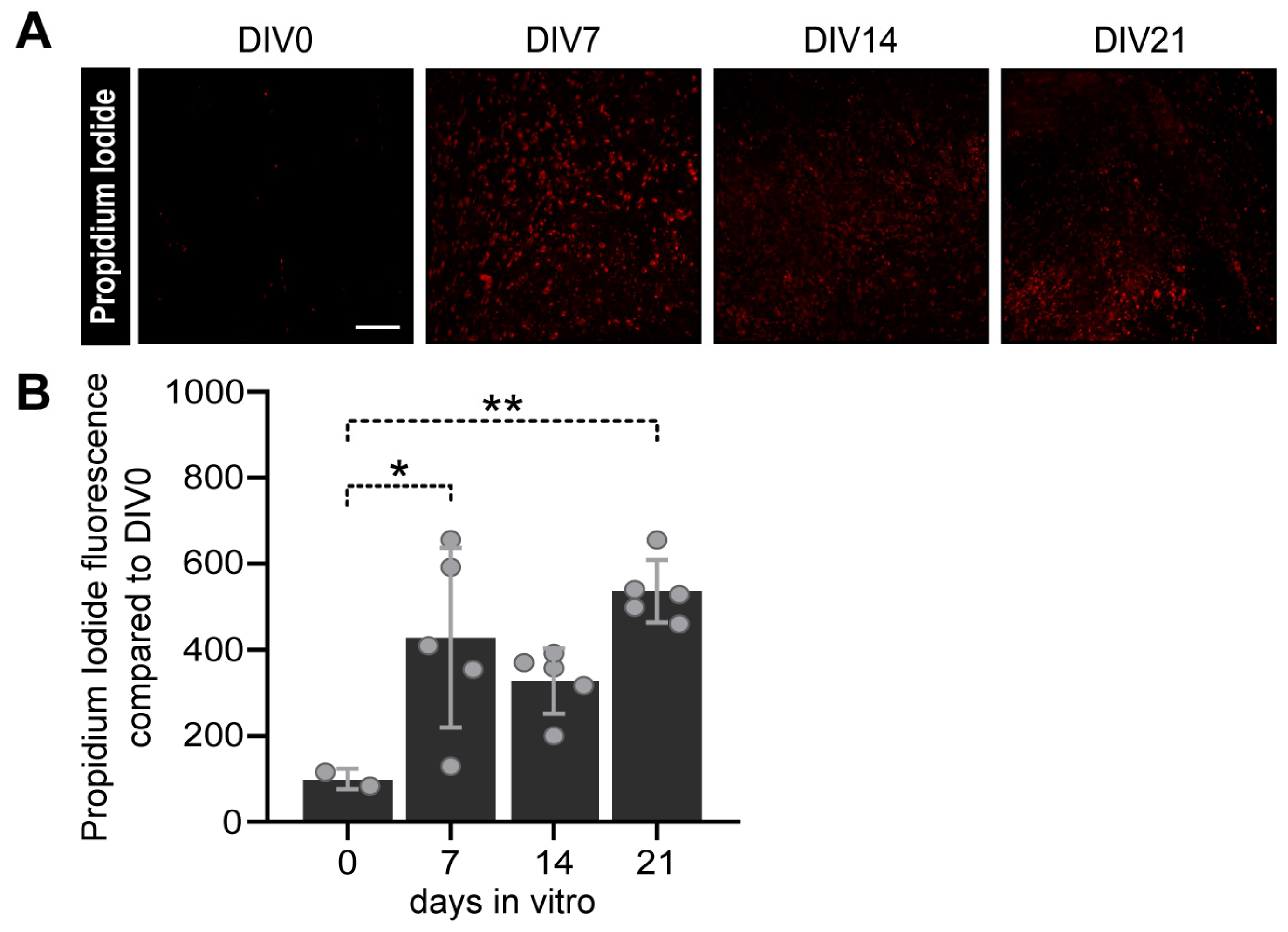
The procedure used to prepare organotypic slices may cause tissue damage, which in turn potentially affects their viability (33). To assess the amount of cellular damage over the culturing period, OBSCs were labeled with Propidium Iodide (PI), which is only able to penetrate compromised cell membranes and therefore marks dead or dying cells. DAPI was additionally used to counterstain cell nuclei and precisely identify single cells. The intensity of PI staining was then used as a correlate measure of cell death and data from 7 days-in-vitro (DIV7) to DIV21 were normalized to DIV0, the time point taken immediately after cutting.
We could observe an increase of PI staining intensity from DIV0 to DIV7 (
Figure 3), which remained stable afterwards (
Figure 3A; one-way ANOVA, F(3, 13)= 5.984; p=0.0086), thereby suggesting that the viability of slices was maintained along all experimental stages.
3.3.2. MEGF10 expression and astrocyte-dependent synapse elimination in the PFC of OBSCs derived from male and female rat pups
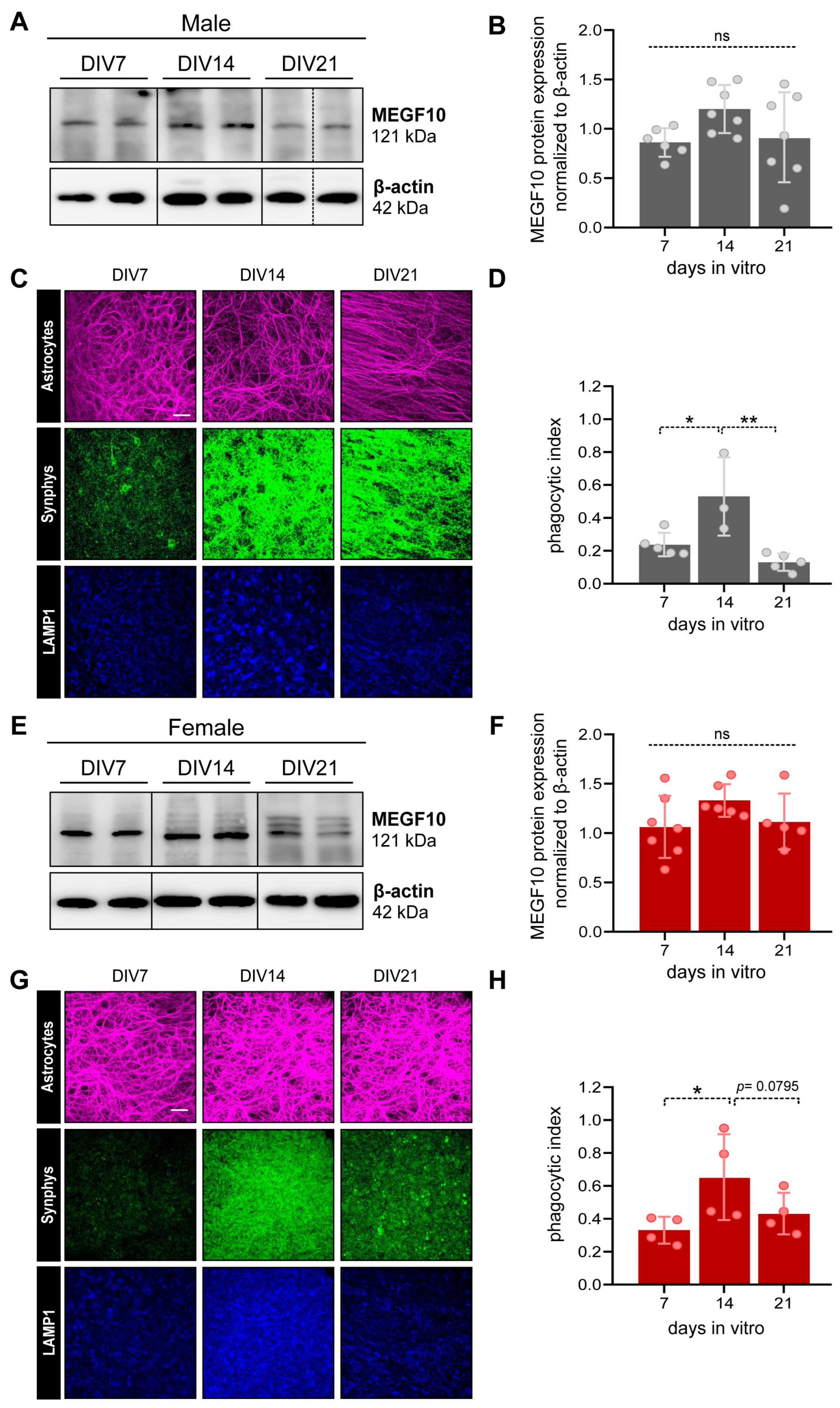
To validate OBSCs for the examination of differences in astrocyte-mediated age- and sex-dependent phagocytosis, we first analyzed MEGF10 expression levels in the PFC of OBSCs prepared from male and female rat pups at DIV7, 14 and 21.
In accordance with results obtained from rat pups (
Figure 1B,H), we observed no differences in the expression of MEGF10 levels in male OBSCs between DIV7 and DIV21 (
Figure 4A,B, one-way ANOVA with mixed-effects model, F(2, 11)= 2.563, ns, not significant). The same was true for female OBSCs, which also showed no differences detectable at any of the stages examined (
Figure 4E,F, one-way ANOVA with repeated measures, F(2, 9)=1.937, ns, not significant).
We then evaluated the astrocyte-dependent phagocytosis, using GFAP to label astrocytes and avoid difficulties in the analysis of single astrocytes in this type of ex vivo system, where the double staining with S100ß might impair the identification and examination of single cells ([
33] and our data). The examination revealed that in OBSCs derived from male rat pups, the rate of astrocyte-mediated synapse elimination (measured as described in §2.3.5 of the Materials and Methods section) increased between DIV7 and DIV14 to reach a peak and drop down again at DIV21, as we previously saw in rat pups at comparable developmental stages (
Figure 4C,D, one-way ANOVA with repeated measures, F(2,6)= 11.14, p=0.0096, Tukey’s posthoc test: DIV7- DIV14, * p<0.05, DIV14- DIV21, ** p<0.001). In females, however, differently than from female rat pups, we observed an analogous trend to change as in males, with a significant increase in the rate of phagocytosis between DIV7 and DIV14, which declined again between DIV14 and DIV21 (
Figure 4G,H, one-way ANOVA with repeated measures, F(2,6)= 8.067, p=0.0199, Tukey’s posthoc test: DIV7- DIV14, * p<0.05, DIV14- DIV21, p=0.0795).
4. Discussion
Postmortem studies in human and non-human primates have shown that in cortical areas all synaptogenic events, which lead to supernumerary synapses and the subsequent age-dependent synapse elimination, increase after birth and reach a peak in early childhood to decline during later developmental stages and finally become refined in late adolescence/early adulthood [
22,
34,
35,
36,
37,
38]. However, brain scan imaging methods have shown that dynamic changes in the density of the gray matter may last longer than the adolescence, opening the question whether such events continue beyond it and even through the third decade of life, before reaching adult levels [
39]. Such long developmental range of neuronal network reorganization might also explain the high impact of environmental challenges on the formation of human cognitive and emotional capacities, as well as their potent detrimental effects, which may underlie the onset of neuropsychiatric disorders [
21].
In addition to these neurobiological modifications based on intrinsic factors, it is also clear that sex-specific hormonal changes may influence synaptic formation/elimination to favor the development of adaptive behavioral responses during sexual maturation in both males and females. However, only few studies consider sex differences among the critical factors that should be considered when interpreting experimental findings and measured parameters [
23,
37]. Therefore, research in this direction is highly warranted to better understand sex-dependent maturational trajectories of brain development in healthy contexts, which can in turn guide medical interventions in disease states with a focus on sex differences.
Here, we sought to offer an alternative tool to examine age- and sex-dependent changes in astrocyte-mediated synapse elimination in the prefrontal cortex (PFC) at early postnatal stages, from birth until adolescence. We compared developing brains from rat pups with organotypic brain slices (OBSCs), a 3D system so far used to investigate hippocampal developmental processes [
26,
31,
33,
40], which is more amenable to pharmacological, genetic and other types of manipulations and is less expensive than other models. In rat pups, we examined age- and sex-dependent rates of synapse formation and elimination and evaluated whether putative sex-dependent differences in astrocyte-mediated synapse elimination occurred. We found that MEGF10 showed a slight decline in the average of its protein levels between P21 and P32 in males (
Figure 1B,C), which marks the end of the juvenile period, correspondent to what is so far consensually considered the time of closure for the cortical developmental critical period [
41]. On the contrary, MEGF10 levels did not change in females at any developmental stage (
Figure 1I,J). These findings were effectively reproducible in OBSCs, which did not show any relevant modifications in the levels of MEGF10 between DIV7 and DIV21 (
Figure 4A,B and E,F). These first results suggested that OBSCs might undergo a sort of developmental programmed “reset” after cutting and show similarities between P7 and DIV7 stages.
To next evaluate whether this system represented a useful platform to examine changes in synaptic elimination during the critical period of cortical development, we first measured and correlated changes in synaptic proteins with the astrocyte-mediated phagocytosis in pups from P7 to P32.
Here, we observed clear age- but also sex-dependent developmental patterns, which suggested that sex-specific determinants influence both the rates of synaptogenesis and astrocyte-mediated phagocytosis (
Figure 1 D-G, J-M and
Figure 2 D,E and H,I).
We initially observed an apparent inconsistency between results obtained on the expression levels of synaptophysin by Western blot (
Figure 1F-M) and immunohistochemistry (IHC,
Figure 2A,D). On the one hand, the expression of this protein in Western blot increased from P7 to P14 and remained elevated until P32 in males (Fig1 F,G), while it peaked with a delayed increase in females between P14 and P21 (Fig1 L,M). On the other hand, the IHC showed a different pattern, with an apparent peak at P14 which decreased afterwards in males and decreased in females at P21 to increase again by P32 (Fig2 B,C and F,G). This could be explained by the different methods used to prepare tissues for the two experiments: as mentioned in the Method section, brain tissue for Western blots might have contained tissue from other brain cortical areas around the PFC (Fig1 A), whereas the IHC analysis was restricted to the infralimbic/prelimbic areas of the PFC (Fig2 A). Only with sophisticated methods such as laser microdissection, we may be able to execute the experiments in the exact same way.
Our results in pups showed that, at the end of the juvenile period around P32, also the pattern of astrocyte-dependent phagocytosis followed a different trend in females when compared to males in the PFC (
Figure 2 D,E and H,I). We observed a second slight rise in the rate of synapse pruning at P32, which correlated with a significant elevation in synaptophysin levels only in females at this time point (
Figure 2 F,G). These results suggest that in females multiple waves of synaptogenesis and synaptic refinement through astrocyte-dependent synapse elimination might occur (at P7/P14 and at P32), which could also be examined in human and non-human primates to possibly explain the reduced sizes of the PFC found in adult females with respect to males [
23,
37]. However, further experiments should be done to validate this hypothesis, such as examining later developmental stages and eventually increasing samples sizes. Moreover, we could argue that a detrimental environmental hit, such as stress or a shock, during the late adolescence in females may have long-lasting effects, as the system seems to be still in a developmental phase with respect to the male counterparts. This is in fact what has been observed in female-related disorders, which may have such a preferential incidence due to the different maturational trajectories of the neuronal circuits in the two sexes.
These differences could depend on long-range synaptic innervation, but also local cellular mechanisms, which might both contribute to the sex-specific synaptic refinements in the infralimbic/prelimbic areas of the PFC. Therefore, we established a 3D culturing system to examine developmentally regulated, but also sex-dependent mechanisms, which control synaptic elimination during the juvenile stages of postnatal brain growth. As discussed before, the comparison of the early PND stages of pups’ brain growth with OBSCs revealed that slices prepared from P4-P6, when sensory inputs have already reached cortical areas [
42] and astrocyte proliferation/differentiation processes are occurring most exclusively locally [
6], might retain enough autonomous information to display very similar patterns of MEGF10 expression in the cortex of both sexes in pups and OBSCs (
Figure 1 B,C and H,I and
Figure 4 A,B and E,F). More strikingly, we noticed that in males the astrocyte-mediated synaptic phagocytosis was preserved in OBSCs and followed the same dynamics showed by the developing brains (
Figure 2 D,E and
Figure 4 C,D). However, the findings were quite different in female OBSCs, with the change in the rate of phagocytosis resembling the male-derived OBSCs, with a peak at DIV14 and reduction afterwards, than the female developing brains, where the levels of phagocytosis at P7 were as high as at P14 and dropped down at P21 (
Figure 4 D,G,H and
Figure 2 D,E and H,I). These results suggested that this parameter is not sufficiently preserved in our model system to claim yet its validity for the examination of sex-specific changes in astrocyte-dependent synapse elimination. We hypothesized that this factor may be affected by the cutting procedure [
29], which is preventing putatively essential long-range, most likely peripheral, sex-specific determinants, such as sexual hormones, to reach the cortex and specify the sex-dependent reshaping of this distinct astrocyte-mediated function. More experiments should be performed to assess whether i.e. the administration of sexual hormones in OBSCs might help to phenocopy the phagocytic rate found in the developing female brains. However, even though cultures can be maintained for long times [
29,
40], studies of hormonal interferences with neuronal network formation might be limited in this system.
Another possibility to explain the differences observed might rely on the method we used to quantify the rate of synaptic pruning in OBSCs, because the immunohistochemical stainings used for brains had to be slightly adapted in slices to allow the characterization of astrocyte-associated synaptic engulfment. To rule out possible confounding factors, the use of virally-mediated astrocyte labelling may help to isolate and characterize the rate of synaptic material engulfment in a more refined way in OBSCs, too. However, this procedure may increase again the inter-sample variability and has to be carefully established to reach high reproducibility and reliability of experimental findings.
Finally, in view of the known limitations of the OBSCs to perform studies on synaptic changes in cortical areas because of the disruption of both short- and long-range axonal inputs during the cutting procedure [
29], we expected reduced rates of synaptic elimination in brain slices. Moreover, we were aware of a possible increase in astrocyte gliosis, which has been described in OBSCs and might have affected our results [
43]. However, it has been reported that especially the lysosomal-dependent phagocytosis is atually reduced and not increased in reactive astrocytes [
44]. This further strongly supports the specificity of our results obtained in OBSCs on dynamically changing rates of phagocytosis in the PFC along PND stages and lack of artifactual effects due to the tissue damage derived from the dissection.
Of course, other limitations exist, such as the impaired capability of directly correlating any type of induced manipulation with complex changes like behavioral parameters.
However, overall this system offers a lot of advantages which should be considered when planning animal research experiments. For example, they enable to directly correlate these effects with selected molecular or biochemical processes or cell-type specific responses occurring in situ in distinct brain regions. This is surely also in line with animal welfare’s regulations, which encourage the use of alternative model systems to sensibly reduce the number(s) of animals used to perform multiple experiments and measure various parameters in biomedical research, without reducing the acquisition of strong and reliable data from molecular or histochemical examinations [
45].
Here, we showed that OBSCs can be a useful support to examine age- and sex-dependent changes in astrocyte-dependent synapse elimination in the rat PFC during postnatal developmental stages. This model might be very useful to evaluate especially male-related differences in astrocyte responses to environmental or endogenous insults, which might induce synaptic aberrancies. Therefore, they may become an ideal model to study the neurobiological underpinnings of neuropsychiatric or neurodevelopmental disorders with synaptic deficits correlated with an astrocyte pathology, such as schizophrenia, autism disorder or major depressive disorder.