1. Introduction
Sodium-potassium (Na-K) alloy is liquid-phase alloy that exists at room temperature[
1,
2]. It has been reported as a new type of anode material in sodium or potassium battery that can inhibit Na or K dendrite growth[
3]. Due to the the fluidity of Na-K alloy, it is difficult to form a stable electrode. Therefore, it is necessary to compound Na-K alloy with porous material, so as to realize the fixation of the liquid alloy.
Porous metal skeleton, such as foam copper, foam nickel and foam aluminum, are known for their good conductivity and ability to evenly distribute the metal ion flux through spatial segmentation, which can effectively suppress dendrite growth[4-8]. Among them, foam aluminum is highly potential as an electrochemical substrate material due to its light weight, high abundance[
9], and it have been used in research on deposition with sodium metal or lithium metal[
10,
11]. However, due to the high surface tension of Na-K, it is difficult to form stable and homogeneous electrode materials with typical porous metal skeleton[
12]. Therefore, to obtained a stable Na-K liquid alloy electrode, the imperative strategy is to reduce the surface tension of Na-K liquid alloy[
13,
14], and enhance the affinity of metal skeleton to Na-K liquid metal.
In this paper, graphite powder was added to sodium-potassium alloy, causing a pre-reaction that reduced the surface tension of the liquid alloy. Then the Na-K liquid alloy was fully adsorbed in the fluorinated foam aluminum by vacuum processing, forming NaKC-FAF composite electrode. This composite anode material was test in symmetrical cell and a full battery, and it demonstrated outstanding cycling performance.
2. Experiments
2.1. The foam aluminum was immersed in an aqueous solution containing 0.1% hydrogen fluoride for 10 to 20 seconds. It was then quickly transferred to a dimethyl carbonate (DMC) solvent to remove excess hydrogen fluoride solution. After that, it was dried for 2 hours in a vacuum atmosphere for the next step.
2.2 To prepare liquid sodium-potassium alloy, solid metallic potassium and sodium were mechanically stirred (at a mass ratio of 1:1) with a glass rod in an argon-filled glovebox. After stirring to form the liquid alloy, 0.2 g of graphite powder (GP) was added into 1.0 g liquid alloy and stirred for another 15 minutes to reduce the surface tension of the liquid alloy. The resulting composite was named NaKC. NaKC was mixed with fluorinated foam aluminum, ensuring that the foam aluminum (AF) was completely immersed in the liquid alloy. The mixture was put into a transition chamber for repeated vacuum operations over three times and obtained NaKC-FAF electrode.
2.3 For the cathode, sublimed sulfur was mixed with polyacrylonitrile (PAN) at a mass ratio of 4:1. The mixture was then heated at 350℃ and aged for 6 hours in flowing inert gas, resulting in a black, powdery substance called SPAN (sulfur content is 40%). The cathode was fabricated by mixing the 70 wt% SPAN, 20 wt% acetylene black, and 10 wt% guar gum. The loading amount of SPAN cathode was about 1 mg cm-2sulfur. CR2032-type cells were assembled separately with Na foil and NaKC-FAF as the anode, Celgard 3501 as the separator, and a 1.0 mol/L NaClO4 solution in a mixture of ethylene carbonate (EC) and propylene carbonate (PC) (1:1 wt.%) with 5% fluoroethylene carbonate (FEC) as the electrolyte.
2.4 Electrochemical performance was tested using a LAND-CT2001A cycler (Wuhan, China) under ambient conditions. The chemical component and morphologies of the electrodes were determined by an X-ray Photoelectron Spectroscopy (XPS, Axis-UltraDLD, Kratos Analytical, Japan), and a scanning electron microscope (SEM, Quanta FEG 250, FEI, Changsha, China).
3. Results and discussion
The prepared sodium-potassium liquid alloy (NaK) showed high surface tension (
Figure 1 (a)), while the surface tension of NaKC noticeably decreased after adding graphite powder (
Figure 1 (b)). Mixing NaKC with fluorinated foam aluminum, ensuring that the foam aluminum was completely immersed in the NaKC liquid alloy.
Figure 1 (c) showed that untreated AF did not have excellent surface affinity with NaKC. However, the NaKC alloy was uniformly filled into the pores of the treated AF (noted as NaKC-FAF), and no uneven structure was observed on the surface by macroscope observation (
Figure 1 (d)).
Different electrolytes namely 1.0 M NaClO
4/EC-PC-5% FEC and 1.0 M NaPF
6/Diglyme, were separately dropped onto the NaKC-FAF electrode. From
Figure 1 (e), it can be seen that when the carbonate-based electrolyte was dropped onto the NaKC-FAF electrode, the electrode color remains unchanged and there is no significant change for the liquid sodium-potassium metal inside the electrode. However, when the ether-based electrolyte is applied, as shown in
Figure 1 (f), the part of the electrolyte in contact with the surface of NaKC-FAF electrode changes from silver to black. Additionally, a small amount of liquid sodium-potassium metal escaped to the electrode surface, forming spherical droplets on the NaKC-FAF electrode (the inserted picture of
Figure 1 (f)). A similar phenomenon occurred with the literature [
15], which speculated that the slight dissolution of the Na–K in ethers may destroy the physical bonding between the liquid Na–K and the porous material. The NaKC-FAF electrode was found to be more structurally stable in the carbonate-based electrolyte (1.0 M NaClO
4/EC-PC-5% FEC), and thus this electrolyte was used throughout the subsequent experiments.
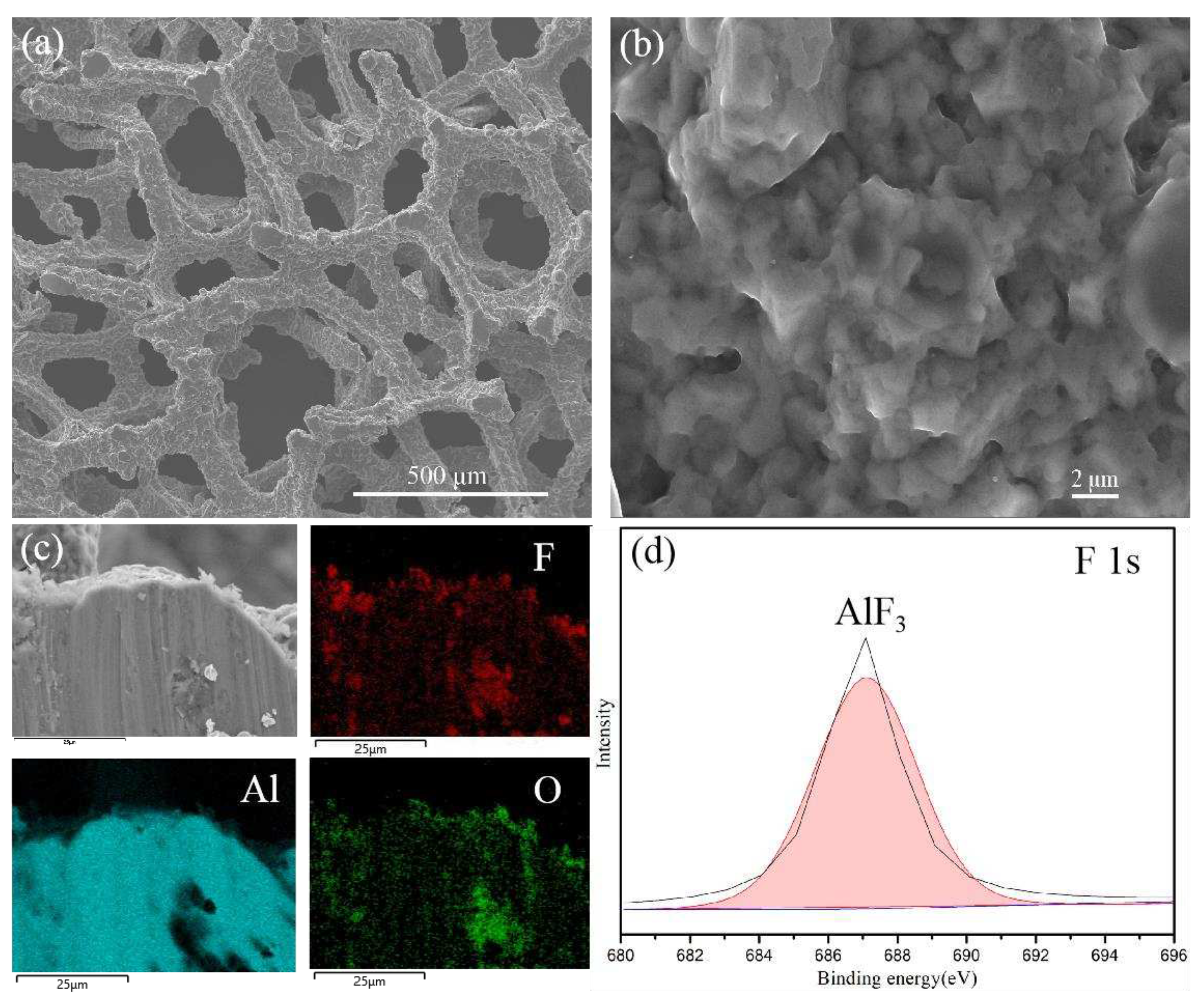
The SEM image of fluorinated aluminum foam (FAF) (
Figure 2 (a) and (b)) reveals that the foam aluminum treated with HF (FAF) still retains a similar morphology to that of the raw material structure, and there exist some micropores on the surface. The elemental overlay results obtained from EDAX illustrate that the fluorine (F) elements are distributed homogeneously on the surface of porous aluminum foam (
Figure 2 (c)). The ingredients analysis carried out by XPS fitting spectrum (shown in
Figure 2 (d)) highlights that the F elements exist in the compound of AlF
3 within the surface of aluminum foam, and the aluminum element at the interface is mainly composed of Al
2O
3 and AlF
3 (Figure S1)
. It indicated that the fluorinated treatment was successfully conducted.
Due to the simple preparation and stable performance of SPAN, and the fact that both SPAN and NaKC-FAF are more stable in carbonates, we chose NaKC-FAF-SPAN batteries as the focus of our research.
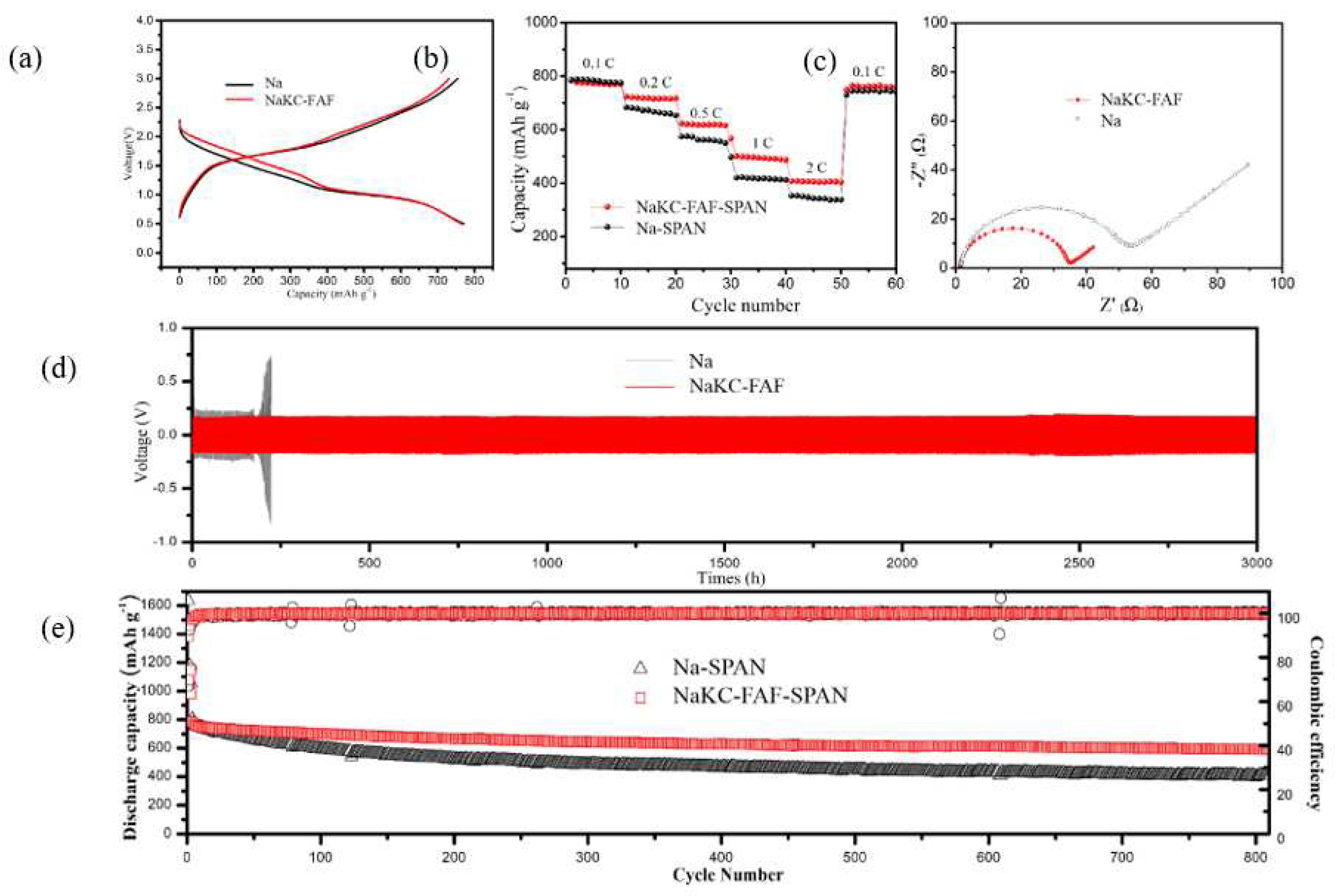
Figure 3 (a) displays the typical cyclic galvanostatic discharge-charge curves of Na-SPAN and NaKC-FAF-SPAN full cells. After the capacity normalization during the 20th cycle of the discharge process, it was observed that the voltage plateau of the NaKC-FAF-SPAN battery was approximately 0.2 V higher than that of the Na-SPAN battery, which is consistent with the results in Figure S2. This suggests that the dissolved potassium ions participate in the reaction of the cathode sulfurized polyacrylonitrile (SPAN) during the discharge process. Potassium ions have a lower redox potential (-2.931 V
vs. Standard Hydrogen Electrode(SHE)) compared to Na/Na
+ (-2.71 V
vs. SHE), which results in a battery with potassium ions having a higher voltage plateau during discharge[
16]. The voltage difference between the two discharge curves being equal to the difference in SHE potentials also confirms the participation of potassium ions in the entire discharge process.
Figure 3 (b) illustrates the cycling performance of two compared batteries at different current densities. It is evident that the NaKC-AF-SPAN battery (red line) exhibits better rate performance than the Na-SPAN battery (black line), especially at high current density of 2.0 C (1 C = 500 mAh g
-1). This is due to the more stable interface of the NaKC-FAF electrode, and the NaKC-FAF-SPAN battery showing faster kinetics with increasing current densities, producing relatively high capacities at 0.1 C, 0.2 C, 0.4 C, 0.5 C, 1.0 C, and 2.0 C, which are 793 mAh g
-1, 715 mAh g
-1, 550 mAh g
-1, 501 mAh g
-1, and 402 mAh g
-1, respectively. In contrast, the capacity of the Na-SPAN battery under the same conditions is lower, which are 798 mAh g
-1, 693 mAh g
-1, 598 mAh g
-1, 423 mAh g
-1, and 338 mAh g
-1, respectively. These results indicate that the NaKC-FAF composite electrode can effectively improve the rapid charge and discharge capability of the battery.
To investigate the cycle stability of the NaKC-FAF electrode, NaKC-FAF|NaKC-FAF symmetric cells were tested and compared with Na|Na symmetric cells. After 150 hours of charge/discharge cycling, the symmetric cells underwent AC impedance spectroscopy testing. As shown in
Figure 3 (c), the interface resistance of the NaKC-FAF electrode was much lower than that of the Na electrode in carbonate-based electrolytes. Additionally, at a current density of 1 mA cm
-2/1 mAh cm
-2 , the NaKC-FAF|NaKC-FAF battery exhibited stable cycling for over 3000 hours at lower polarization voltages (
Figure 3 (d)), while the cycling stability of the Na|Na battery was only about 200 hours, indicating that the NaKC-FAF|NaKC-FAF electrode has better cycling stability.
Figure 3 (e) presents a comparison of the long-term cycling stability of the full battery. In the first five cycles, the coulombic efficiency was relatively high due to the irreversible consumption of Na
+/K
+ by SPAN. During the first 20 cycles, the specific capacities of Na-SPAN and NaKC-FAF-SPAN batteries were maintained at 750 mAh g
-1 without significant difference, indicating that Na and NaKC-FAF electrodes had almost no effect on the cycling performance in the initial stage. However, as the cycling continued, the specific capacity of Na-SPAN battery decreased significantly, while NaKC-FAF-SPAN showed better stability over 800 cycles. The capacity retention of NaKC-FAF-SPAN reached 72%, while that of Na-SPAN was only 49%. This indicates that the NaKC-FAF liquid alloy electrode can effectively improve the cycling performance of sodium-sulfur batteries.
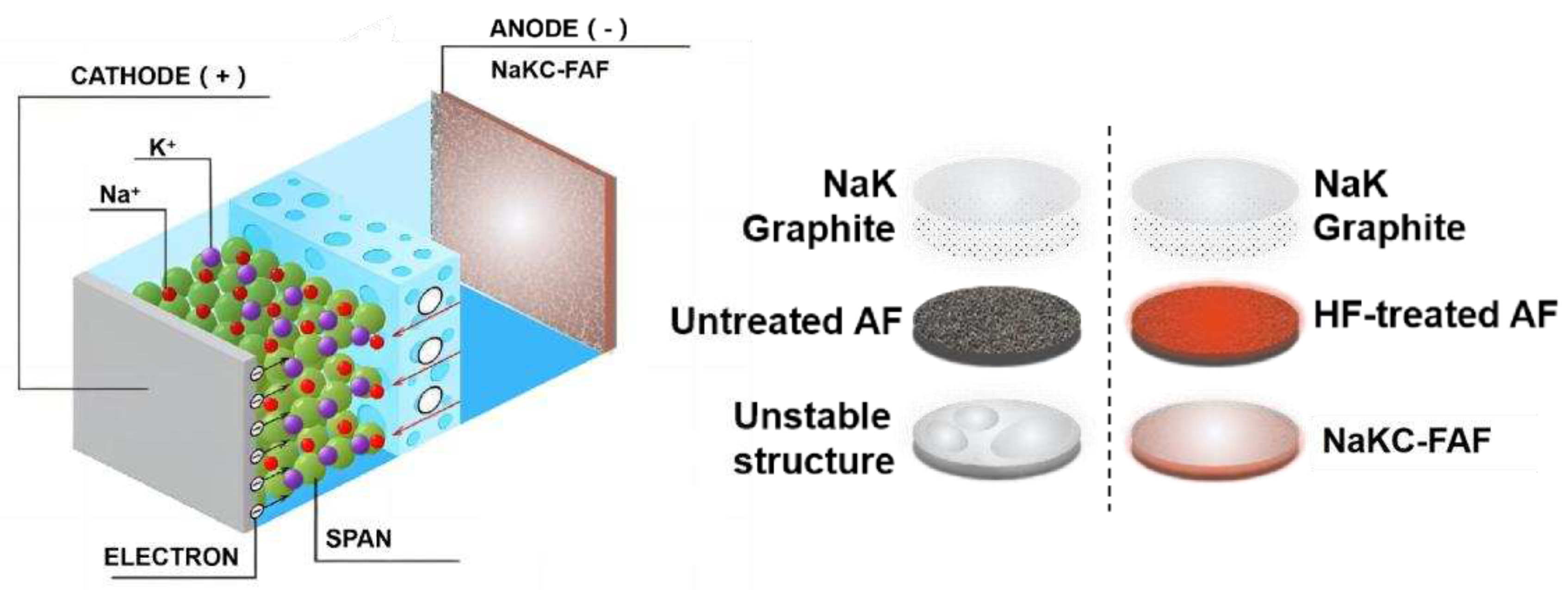
Figure 4 shows the schematic diagram of the NaKC-FAF electrode. By adding graphite powder to the NaK liquid alloy, the surface tension of NaKC alloy can be reduced, while fluorinated foam aluminum exhibits higher affinity for the NaKC alloy. Therefore, the NaKC alloy can be well combined with fluorinated foam aluminum, allowing the NaKC alloy to be uniformly immobilized in the fluorinated foam aluminum. This provides a fast electron transfer pathway for Na
+ and K
+ in foam aluminum, whereas the non-fluorinated AF electrode has weaker affinity with NaKC alloy and poorer uniformity after being combined with the sodium-potassium alloy. Additionally, the NaKC composite has a certain fluidity and the interface has self-repairing properties, which can prevent the formation of sodium and potassium dendrites. Therefore, through the synergistic effect between the FAF and the NAKC, the NaKC-FAF composite anode effectively suppresses the formation of dendrites and improves the cycling stability of the electrode interface.
4. Conclusions
In summary, we have reported a novel anode material (NaKC-FAF) by combining sodium-potassium liquid alloy and fluorinated aluminum foam. According to the statistics, the NaKC-FAF symmetrical cell provides a stable circuit exceed 3000 hours in 1.0 M NaClO4/EC-PC-5% FEC electrolyte, and a full battery with a NaKC-FAF anode and SPAN cathode exhibited approximately 72% capacity retention after 800 cycles. It is worth mentioning that aluminum foam is a normal and eco-friendly material with low density. We believe that this facile and novel design will contribute to the development of next-generation high-energy-density electrochemical systems.
Author Contributions
Conceptualization, J.L. and K.C.; methodology, J.L.; software, J.L.; validation, J.L., J.Z. and X.M.; formal analysis, S.C.; investigation, J.L.; resources, K.C.; data curation, J.L.; writing—original draft preparation, J.L.; writing—review and editing, J.L.; visualization, J.Z.; supervision, K.C.; project administration, S.C.; funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luo Y, Xu J, Mou P, et al. Cobalt/Nitrogen Co-Doped Carbon Materials Enhance the Reaction Rate of Sodium-Potassium Alloy Electrodes[J]. Small, 2023, 2304981. [CrossRef]
- Xue, L.; Gao, H.; Zhou, W.; Xin, S.; Park, K.; Li, Y.; Goodenough, J.B. Liquid K-Na Alloy Anode Enables Dendrite-Free Potassium Batteries. Adv. Mater. 2016, 28, 9608–9612. [Google Scholar] [CrossRef]
- Shao M, Wu N, Chen T, et al. Amorphous hollow carbon film as a flexible host for liquid Na-K alloy anode[J]. Chinese Chemical Letters, 2023, 34, 107767. [CrossRef]
- Wang, J.; Chen, M.; Lu, Z.; Chen, Z.; Si, L. Radical Covalent Organic Frameworks Associated with Liquid Na-K toward Dendrite-Free Alkali Metal Anodes. Adv. Sci. 2022, 9, 2203058. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, M.; Yang, X.; Lu, X.; Wu, D.; Zhang, Q.; Zhu, Y.; Gu, M. Na–K Alloy Anode for High-Performance Solid-State Sodium Metal Batteries. Nano Lett. 2022, 22, 9614–9620. [Google Scholar] [CrossRef]
- Yuan, W.; Ding, T.; Mou, P.; Luo, Y.; Li, L.; Chen, Y.; Chen, X.; Shu, J.; Zhang, L. Semi-Solid CNT@NaK Anode for Potassium Metal Battery. Adv. Funct. Mater. 2023, 33, 2209774. [Google Scholar] [CrossRef]
- Huang, G.; Chen, S.; Guo, P.; Tao, R.; Cao, Y. In situ constructing lithiophilic NiFx nanosheets on Ni foam current collector for stable lithium metal anode via a succinct fluorination strategy. Chem. Eng. J. 2020, 395, 125122. [Google Scholar] [CrossRef]
- Adewale, K.; Kamel, E.; Aboubakr, M. Porous transition metal-based nanostructures as efficient cathodes for aluminium-air batteries, Curr. Opin. Electrochem. 2023, 37, 1011981. [Google Scholar] [CrossRef]
- Yang Z, Wang J, Cui C, et al. High power density & energy density Li-ion battery with aluminum foam enhanced electrode: Fabrication and simulation[J]. Journal of Power Sources, 2022, 524: 230977. [CrossRef]
- Chen J, Wang Y, Li S; et al. Porous metal current collectors for alkali metal batteries[J]. Advanced Science 2023, 10, 2205695. [Google Scholar] [CrossRef] [PubMed]
- Li Z, Zhu K, Liu P; et al. 3D Confinement Strategy for Dendrite-Free Sodium Metal Batteries[J]. Advanced Energy Materials 2022, 12, 2100359. [Google Scholar] [CrossRef]
- Xue, L.; Zhou, W.; Xin, S.; Gao, H.; Li, Y.; Zhou, A. Room-temperature liquid Na-K anode membranes. Angew. Chem. 2018, 57, 14184–14187. [Google Scholar] [CrossRef] [PubMed]
- Yuan W, Ding T, Mou P; et al. Semi-Solid CNT@ NaK Anode for Potassium Metal Battery[J]. Advanced Functional Materials 2023, 33, 2209774. [Google Scholar] [CrossRef]
- Zhang L, Li Y, Zhang S; et al. Non㎞ewtonian Fluid State K-Na Alloy for a Stretchable Energy Storage Device[J]. Small Methods 2019, 3, 1900383. [Google Scholar] [CrossRef]
- Xue L, Zhou W, Xin S; et al. Room-temperature liquid Na–K anode membranes[J]. Angewandte Chemie 2018, 130, 14380–14383. [Google Scholar] [CrossRef]
- Lei Y J, Yang H L, Liang Y; et al. Progress and Prospects of Emerging Potassium-Sulfur Batteries[J]. Advanced Energy Materials 2022, 12, 2202523. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).