1. Introduction
Hematopoietic cell transplantation (HCT) is an established therapeutic procedure for hematological malignancies and serious non-malignant hematological disorders1,2. It encompasses two major modalities: autologous HCT, which involves administering high doses of myelotoxic chemo- or radiotherapy followed by the reinfusion of autologous hematopoietic progenitor cells to recover the patient’s hematopoietic system and allogeneic HCT, which includes myelotoxic or immunosuppressive therapy followed by infusion of hematopoietic progenitor cells derived from a related donor, volunteer unrelated donor, or placental cord blood donor3.
Despite its curative potential, HCT is a technically complex and resource-intensive procedure, often associated with considerable toxicity. In Canada, HCT is offered by a limited number of specialized transplant centers, all based at major hospitals. Over time, advances in donor selection4, pre-transplant therapy, post-transplant supportive care5–7, and graft-versus-host disease (GVHD) management 8,9 have influenced HCT trends and outcomes. Several national10 and international11 registries have described HCT trends and outcomes and demonstrated improvements in clinical outcomes over time12,13. However, the specific quantity, trends, and clinical outcomes of HCT over time in Canada is not known.
To address this knowledge gap, we examined the characteristics of HCT in Canada using registry data. Our primary aim was to analyze the trends in HCT procedures and characteristics of recipients, as we hypothesized that the HCT landscape has evolved over time in line with other developed countries. Additionally, we examined the hypothesis that survival among transplant recipients have improved over time, potentially attributable to advances in supportive care leading to a reduction in HCT-related toxicities and associated non-relapse mortality.
2. Methods
2.1. Study Design, Participants and Setting:
We conducted a retrospective cohort analysis of autologous and allogeneic HCTs at Canadian transplant centers performed between January 1, 2000, and December 31, 2019. To determine whether patient characteristics have changed and whether outcomes have improved over time, patients were grouped into two periods: 2000 to 2009 (early era) and 2010 to 2019 (later era). We included children and adults who received a first HCT at any Canadian transplant center reporting to the Cell Therapy Transplant Canada (CTTC) registry over this period.
Separate analyses of clinical outcomes were performed on patients with non-malignant disease indications. Additionally, a subgroup analysis of allogeneic HCTs was performed on adults with indication of acute leukemia: acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), acute leukemia of ambiguous lineage, myelodysplastic syndrome (MDS) and myeloproliferative neoplasia (MPN). This subgroup analysis was intended to remove the potential confounding effect of HCT indications which were more frequently performed in the earlier HCT era and whose clinical outcomes may differ substantially from the other allogeneic HCT indications.
2.2. Data Sources:
Through a collaboration between CancerCare Manitoba and CTTC, patient, donor, and outcomes data from these centers were voluntarily collected and analyzed. The CTTC registry is a research consortium consisting of 15 adult and pediatric HCT centers across Canada. The CTTC registry collects transplant essential data (TED) forms prior to transplantation, and at 100 days, 6 months, and 1-year post-transplant and annually thereafter. Socio-demographic data, medical information regarding diagnosis, treatment, transplant, and follow-up were collected. Data on donor types, cell sources, disease prog
ression or relapse, and mortality were also recorded. The HCT database is housed by the CTTC registry at CancerCare Manitoba. Regular central auditing of the data is performed to ensure consistency and quality.
2.3. Outcomes:
Trends in the patient characteristics, transplant type, and indication of HCTs in Canada were assessed as primary outcomes. Clinically relevant outcomes including overall survival (OS) and non-relapse mortality (NRM) were also examined as primary outcomes for HCT recipients over this 20-year period. OS was defined as the time from HCT until death and NRM was defined as death from any cause other than relapse or disease progression after HCT. All data were censored at the date of last follow-up, date of last contact, date of death or date of second transplant.
2.4. Statistical Analysis:
Baseline patient, disease indication, and HCT characteristics for first transplants were summarized using descriptive statistics and reported as absolute numbers and proportions for each of the two eras. Statistical significance for comparisons were performed using Fisher's exact test for categorical data. OS and TRM were analyzed by HCT type (allogeneic or autologous), disease type (malignant or non-malignant), age groups (adult, age >18 years or pediatric, age <18 years) and by era (early or later) for first transplants. Survival probabilities were estimated using the Kaplan-Meier method and survival curves compared using the log-rank test. NRM was analyzed using cumulative incidences and Fine and Gray’s tests to accommodate competing risks of death from relapse/progression. Survival probabilities and cumulative incidences were reported with 95% confidence intervals (CI) and p-values <0.05 were considered statistically significant.
2.5. Ethics Approval:
All patients included in this study gave written consent to participate in the research database and to have their data included in observational research. This study was approved by the institutional review board of the University of Manitoba.
3. Results
A total of 18,046 first HCTs were reported from January 1, 2000, to December 31, 2019, from 15 transplant centers in Canada. Autologous transplants accounted for 10,475 (58%) of first HCTs performed. Patient characteristics for first allogeneic and autologous transplants are presented in Tables 1 and 2 respectively. Median age at HCT increased between the early (2000-2009) and later (2010-2019) eras: for allogeneic HCT, median age increased from 42 years to 50 years( p<0.0001), while for autologous transplants median age increased from 53 years to 58 years (p<0.0001). The proportion of older adults (>64 years old) receiving allogeneic HCT increased 5-fold between early and later eras (1.7% vs. 9.4%, p<0.0001). The proportion of older adults receiving autologous transplants doubled (10.4% vs. 21.5%, p<0.0001).
Donor types for allogeneic HCT are summarized in
Table 1. Matched related donors (MRDs) accounted for the largest proportion of donors in the early era (54.6%), while matched unrelated donors (MUDs) accounted for the largest proportion in the later era (56.3%). The decrease in MRDs between the two eras (54.6% early vs. 36.6% later, p<0.0001) was proportionate to the increase in MUDs (35.1% early vs. 56.3% later, p<0.0001). Use of human leukocyte antigen (HLA) mismatched related (haploidentical) donors doubled (3.4% in the early era vs. 6.1% in the later era, p<0.0001).
Regarding cell source, peripheral blood stem cells (PBSCs) were the most common source in both allogeneic and autologous transplants (
Table 1 and
Table 2). PBSC use in allogeneic transplants increased from 60.3% to 74.0% (p<0.0001) over the two eras. In contrast, bone marrow for allogeneic transplants decreased from 30.0% to 15.9% (p<0.0001). Placental cord blood represented a small proportion of allogeneic transplants over these two time periods (6.3% in the early era vs. 5.2% in later era, p=0.044).
Indications for first transplants performed are presented in
Table 1 and
Table 2. For allogeneic HCT, acute myeloid leukemia (AML) was the most common indication across both eras (36.7%), followed by ALL (14.2%) and myelodysplastic or myeloproliferative disorders (MDS/MPN) (12%). Allogeneic HCT for AML increased between early and later eras (32.1% vs. 40.5%, p<0.0001), while HCTs for chronic myeloid leukemia (CML) dropped by approximately half over this time (7.3% early vs. 3.4%, p<0.0001). The most common non-malignant disease for which HCT was undertaken was severe aplastic anemia (4.5%). The proportion of allogeneic HCT for aplastic anemia decreased from 5.4% in the early era to 3.8% in the later era (p<0.0001). In contrast, use of allogeneic HCT for other non-malignant diseases, including inherited bone marrow diseases and immune system disorders, increased from 5.5% to 8.0% (p<0.0001).
Plasma cell disorders (including multiple myeloma) were the most common indication for autologous transplants over both eras (49.4%), followed by non-Hodgkin (30.8%), and Hodgkin lymphoma (9.7%). Relative contributions for most disease indications for autologous HCT remained similar between both eras (
Table 2). Breast cancer was a rare indication for autologous HCT in the earlier era (N =31), which was no longer observed in the later era (N=0).
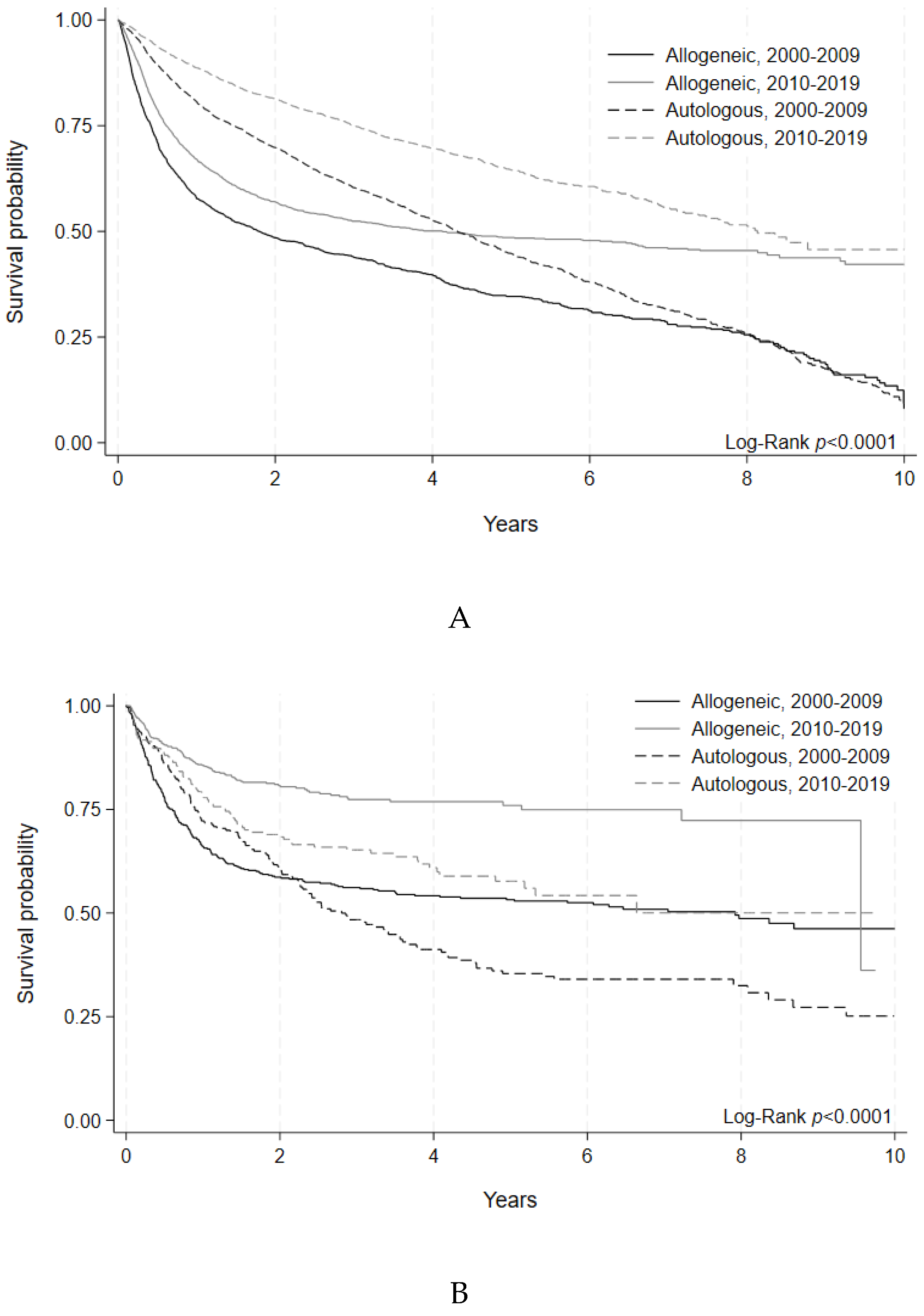
Outcomes were assessed through 5-year OS and 100-day NRM. For adults, 5-year OS after allogeneic HCT was similar between early and later eras (49% [95% C.I. 47-52%], vs. 49% [95% C.I. 47-51%], p=0.83). Autologous HCT recipients, experienced improved 5-year OS in the later era (55% [95% C.I. 54-57%] vs. 65% [95% C.I. 62-67%], p<0.0001). (
Figure 1A,
Table 3). For pediatric allogeneic HCT, there was significant improvement in 5-year OS in the later era (64% [95% C.I. 60-67%], vs. 76% [95% C.I. 71-80%], p<0.0001) while 5-year OS after pediatric autologous HCT showed a numerical increment, albeit with statistical non-significance (51% [95% C.I. 45-57%], vs. 57% [95% C.I. 49-65%], p=0.45). (
Figure 1B,
Table 3).
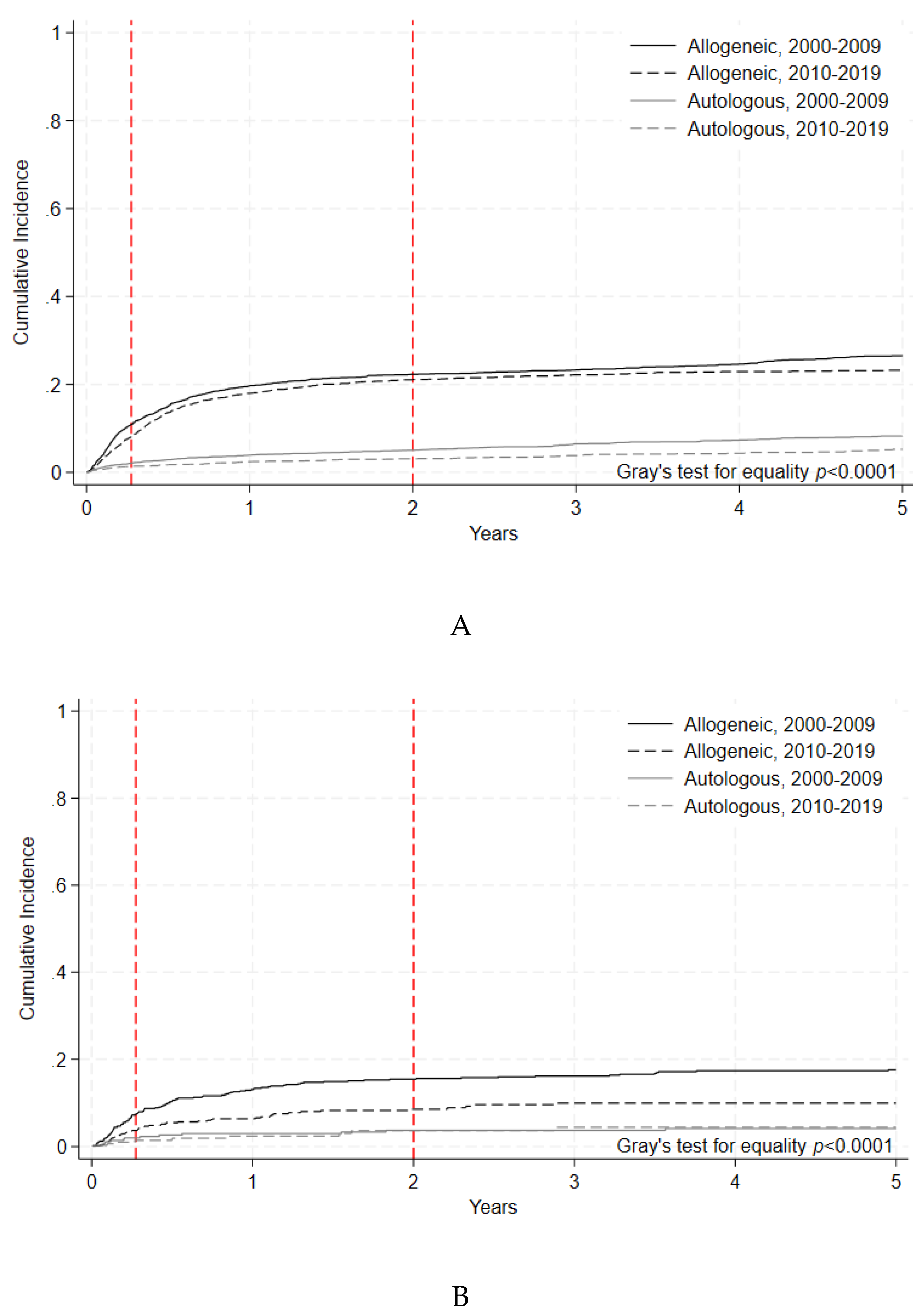
100-day NRM for allogeneic HCT improved for adults in the later era (8.1% [95% C.I. 7.1-9.0%], vs. 10.9% [95% C.I. 9.7-12.2%], p=0.0002). Improvement in 100-day NRM was also noted in pediatric allogeneic HCT patients (3.7% [95% C.I. 2.5-5.3%], vs. 7.5% [95% C.I 5.7-9.6%], p=0.0025) (
Figure 2A,
Table 4). For autologous HCT, 100-day NRM improved in the later era for adult patients (1.4 % [95% C.I. 1.0-1.7%], vs. 2.1% [95% C.I. 1.7-2.6%], p=0.0067), but for pediatric patients there was no difference in 100-day NRM between the two eras (1.4 % [95% C.I. 0.5-3.4%], vs. 1.9% [95% C.I. 0.8-3.8%], p=0.534) (
Figure 2B,
Table 4). As can be seen in these comparisons, 100-day NRM was consistently higher amongst adults compared to pediatric patients for all transplant modalities.
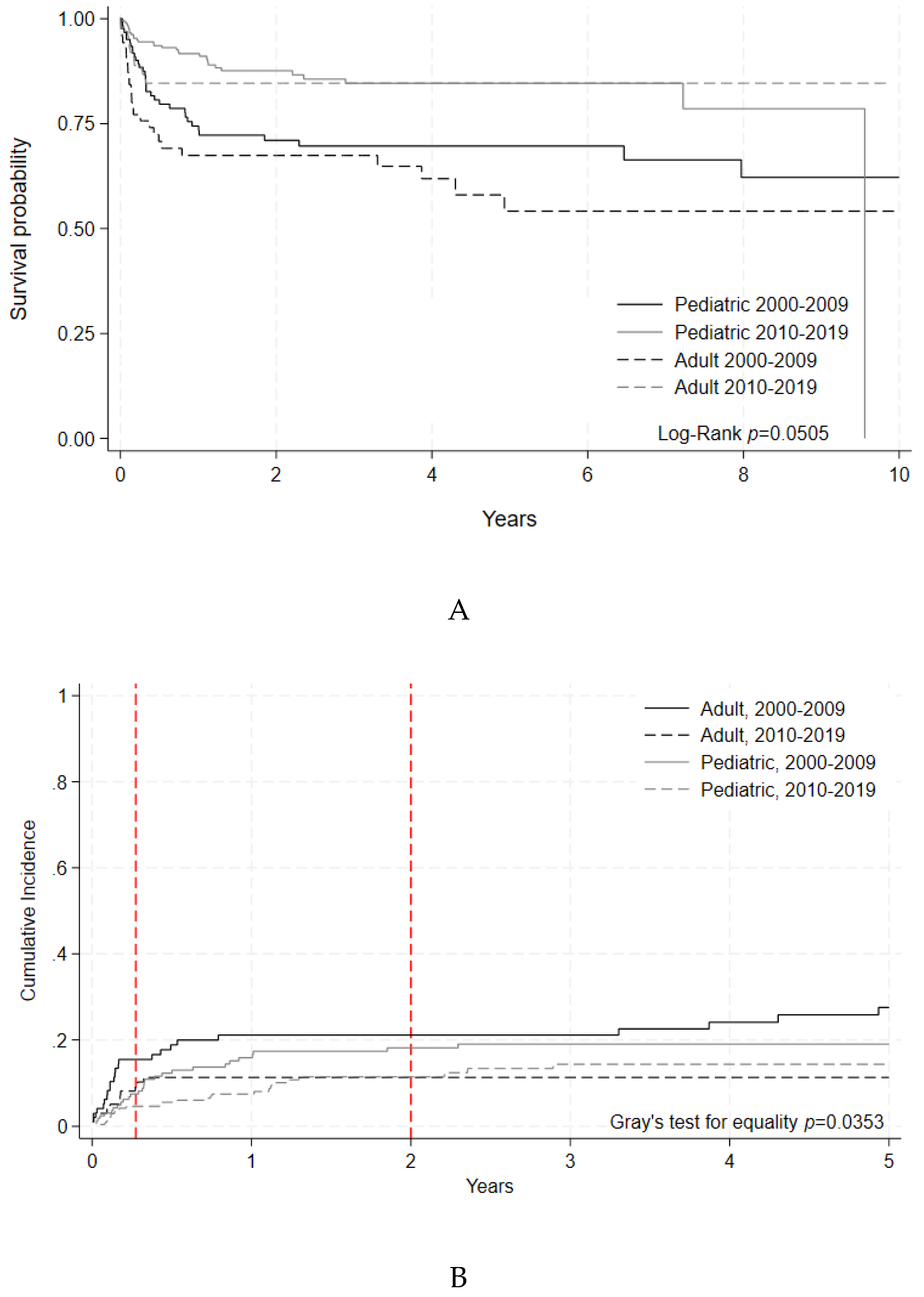
To better evaluate changes in clinically relevant outcomes, OS and NRM were separately assessed for non-malignant and select malignant disease indications. For non-malignant disease indications, 5-year OS was numerically improved in the later era for adult patients (85 % [95% C.I. 75-90%] vs. 70% [95% C.I. 59-79%], p=0.1529) with a statistically significant improvement for pediatric patients (85 % [5% C.I. 78-89%] vs. 78% [95% C.I. 70-84%] , p=0.0393) (
Figure 3A,
Table 5A). 100-day NRM for non-malignant diseases decreased numerically in the later era for adult patients (p=0.1686). For pediatric patients, 100-day NRM similarly showed a downward trend (p=0.2425) between early and later eras (
Figure 3B,
Table 5B).
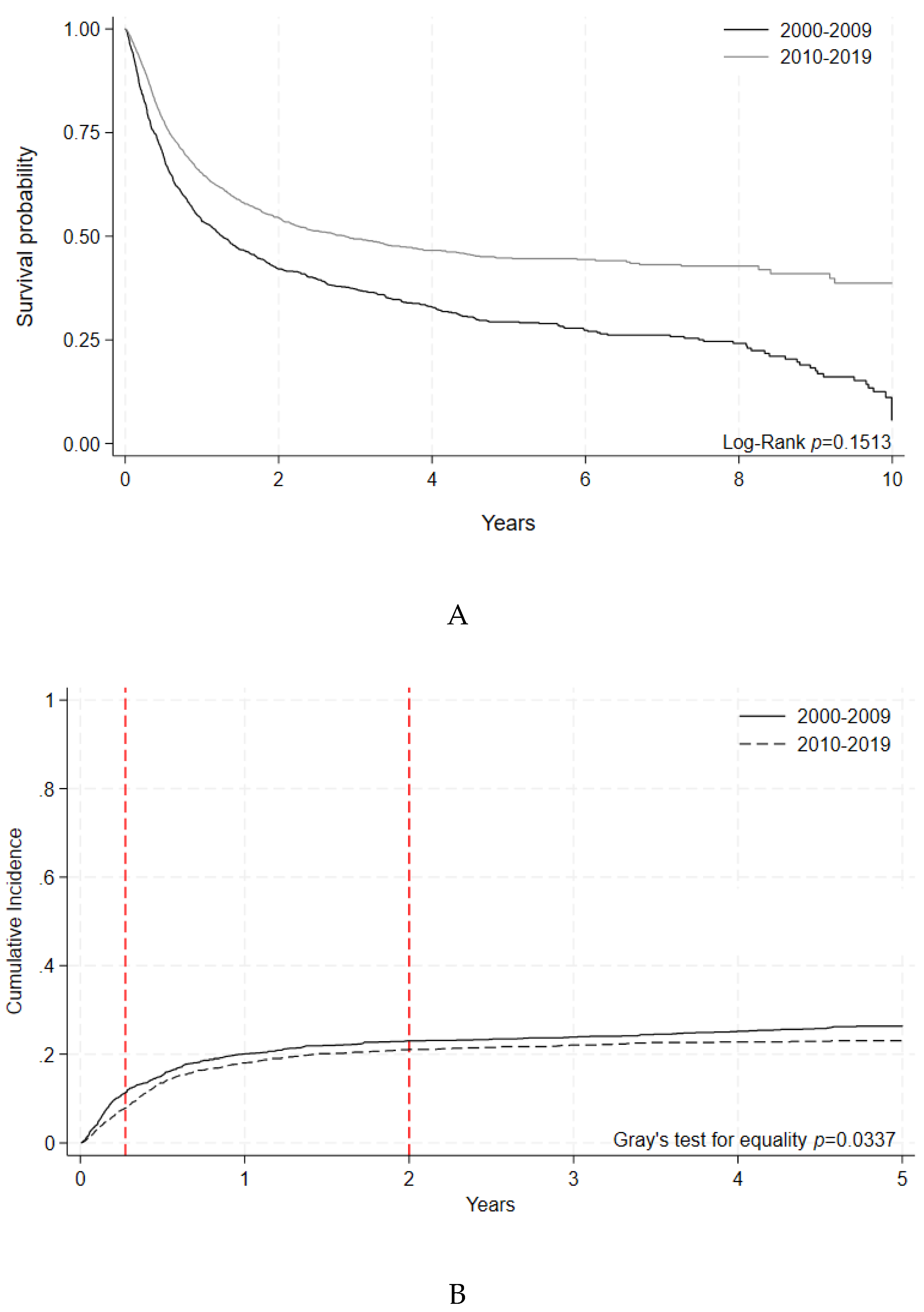
A subgroup analysis of allogeneic HCTs was performed on adults with indications of acute leukemias, myelodysplastic syndromes, and myeloproliferative neoplasms (AML, ALL, AL of ambiguous lineage and MDS or MPN), excluding chronic myeloid leukemia. Within this subgroup, 5-year OS was stable: 44.8% [95% C.I 42.1 - 47.5 %] in the early era vs. 45.7 [95% C.I. 43.1-48.2%], in the later era, p=0.1693 (
Figure 4A,
Table 6A). For these patients, 100-day NRM decreased significantly in the later era (7.85% [95% C.I 6.8-8.9] vs. 11.4% [95% C.I 9.9-13.1], p=0.0002) (
Figure 4B,
Table 6B).
Table 6A.
Overall Survival by Era following Allogeneic HCT indicated for Adult Acute Leukemias. Malignant disease indications included were AML, ALL, AL of ambiguous lineage, and MDS/MPN. Number of individuals at risk and overall survival with 95% confidence intervals are indicated at 1, 2 ,5 and 10 years post transplant.
Table 6A.
Overall Survival by Era following Allogeneic HCT indicated for Adult Acute Leukemias. Malignant disease indications included were AML, ALL, AL of ambiguous lineage, and MDS/MPN. Number of individuals at risk and overall survival with 95% confidence intervals are indicated at 1, 2 ,5 and 10 years post transplant.
| |
2000-2009 |
2010-2019 |
P-value |
Overall P-value |
| No. at risk |
OS, % (95% CI) |
No. at risk |
OS, % (95% CI) |
| 1-yr |
831 |
62.4 (59.8 - 64.9) |
1222 |
65.6 (63.6 – 67.6) |
0.014 |
0.1513 |
| 2-yr |
672 |
53.6 (50.9 – 56.2) |
762 |
55.0 (52.8 - 57.3) |
0.0911 |
| 5-yr |
423 |
44.8 (42.1 - 47.5) |
307 |
45.7 (43.1 - 48.2) |
0.1693 |
| 10-yr |
268 |
40.1 (37.3 - 42.9) |
24 |
41.3 (37.6 – 44.9) |
0.1522 |
Table 6B.
Non-Relapse Mortality by Era following Allogeneic HCT indicated for Adult Acute Leukemias. Malignant disease indications included were AML, ALL, AL of ambiguous lineage, and MDS/MPN. Number of individuals at risk and cumulative incidence of non-relapse mortality with 95% confidence intervals are indicated at 100 days, 1 year, 2 years, and 5 years post transplant.
Table 6B.
Non-Relapse Mortality by Era following Allogeneic HCT indicated for Adult Acute Leukemias. Malignant disease indications included were AML, ALL, AL of ambiguous lineage, and MDS/MPN. Number of individuals at risk and cumulative incidence of non-relapse mortality with 95% confidence intervals are indicated at 100 days, 1 year, 2 years, and 5 years post transplant.
| |
2000-2009 |
2010-2019 |
P-value |
Overall P-value |
| No. at risk |
Cumulative Incidence, % (95% CI) |
No. at risk |
Cumulative Incidence, % (95% CI) |
| 100 days |
1216 |
11.4 (9.9 - 13.1) |
1925 |
7.85 (6.8 - 8.9) |
0.0002 |
0.0337 |
| 1-yr |
781 |
20.1 (18.1 - 22.2) |
1157 |
18.1 (16.5 – 19.7) |
0.0642 |
| 2-yr |
630 |
23.0 (20.8 - 25.3) |
724 |
21.1 (19.3 - 22.9) |
0.0815 |
| 5-yr |
413 |
26.3 (23.9 - 28.7) |
293 |
23.0 (21.1 – 25.0) |
0.0349 |
4. Interpretation
This national level study provides insights into the trends and outcomes of HCT in Canada over a 20-year period. We observed an increasing number of transplants being offered to Canadians, which is likely to be reflective improvements in health care access, greater awareness of the potential benefits of HCT, and higher number of medically eligible patients as HCT can be delivered more safely. We noted aa shift towards older adults receiving both allogeneic and autologous HCTs, with a 5-fold increase in older adults (>64 years old) accessing allogeneic transplants. We also observed that MUDs displaced MRDs as the dominant donor type in allogeneic HCT, suggesting increased reliance on national and international stem cell donor registries to match Canadian patients.
Regarding key clinical outcomes, there were significant temporal improvements in OS that benefited both pediatric and adult allogeneic HCT recipients. We highlight the ongoing challenge of higher NRM in allogeneic HCT compared to autologous HCT, likely due to deeper and more prolonged immune deficiency after allogeneic HCT, often compounded by GVHD. Age-related differences in NRM after allogeneic HCT were apparent, with higher NRM in adults compared to children; suggesting that adult patients are more vulnerable to the adverse effects of allogeneic HCT. However, there were temporal improvements in NRM in the 2010-2019 (later) era for both adult autologous and allogeneic HCT recipients as well as for children receiving allogeneic HCT.
The rise in both allogeneic and autologous HCT among older adults in the later era is consistent with trends in other jurisdictions 10 and poses unique challenges. While older adults may benefit from allogeneic HCT with appropriate selection15, they experience inferior outcomes in OS and NRM compared to younger adults16. This is due to the associated increase incidence in frailty, medical co-morbidities, and inherently more treatment resistant hematological diseases in older adults17,18. We observed a 5-fold increase in allogeneic HCT among older adults during the later era, which potentially impacted OS in these patients. However, there was a measurable reduction in 100-day NRM of allogeneic HCT patients treated for acute leukemia and related myeloid malignancies, which attests to temporal improvements in both patient selection and hospital-based care of transplant recipients despite their older age. Advances in pre- and post-transplant care, such as the of reduced intensity conditioning, more effective graft-vs-host disease prophylaxis, and optimal use of anti-microbial agents also likely contributed to these positive changes19.
In the later era , we observed that MUDs became the dominant donor type for allogeneic HCT with a proportionate reduction in the use of matched related donors. We also noted a rise in haploidentical (HLA mismatched) related donors. While similar trends have been reported in other registries10,11, the proportion of haploidentical related transplants in Canada remains relatively low compared to other registries10,11, while cord blood transplant usage remains stable. The shift towards matched unrelated donors may be attributed to decreasing family sizes in Canada20,21, stem cell registry expansion, and greater acceptance of HLA mismatching associated with improved GVHD prophylaxis and treatment22,23. Additionally, the preference for PBSCs over bone marrow as the donor cell source in the later era highlights improvements in procedural logistics, relative ease of PBSC collection, and improved donor experiences associated with this apheresis-based collection technique24.
Our study highlights the persistent challenge of higher NRM in allogeneic HCT compared to autologous HCT. This difference is likely attributable to greater immune suppression associated with allogeneic HCT, as well as the inherent risk of GVHD and its potentially severe complications25. We also observed a higher NRM in adult allogeneic recipients compared to pediatric recipients. NRM rises for patients aged 21-40 compared to those under 2026. Similar findings from a national study in South Korea reported increased early NRM following allogeneic HCT in AML patients over 20 years of age compared to younger patients27. Moreover, a recent review highlighted studies reporting a lower incidence of severe acute GVHD among pediatric recipients compared to adults28, with GVHD incidence increasing with age within pediatric cohorts29. Taken together, these insights demonstrate age-related differences in HCT outcomes and emphasize the importance of tailored approaches for distinct patient groups.
Amongst all age groups and time periods that we studied, outcomes after allogeneic HCT for non-malignant diseases were better than those for patients with malignancies. Amongst non-malignant hematological diseases, the principal indication in Canada was severe aplastic anemia, a disease that is expected to be cured after allogeneic HCT in most patients. As the ancestry of the Canadian population becomes enriched for individuals of Asian and African ancestry, we expect that sickle cell disease and thalassemia may be become more frequent reasons for allogeneic HCT, especially in children and young adults. Future outcomes studies should assess trends and outcomes for these emerging conditions.
This study has several limitations. Firstly, this analysis is based on transplants voluntarily reported to the CTTC registry, and thus, does not account for all HCTs performed in Canada during that time. While CTTC registry data underrepresents the entirety of transplant activity in Canada, the potential for geographical bias is low given that individual HCT centers do not specialize in specific patient populations or treatment modalities Thus, potential under-reporting of HCT data from some centres does not represent a source of differential bias in transplant type or outcome. Regarding outcomes reported in this study, it is important to consider the decreasing reliability of the data with time due to follow-up loss, especially beyond 10 years post-transplant, and the increased likelihood of other, unrelated factors influencing OS and NRM. However, transplant centres are generally highly committed to the long-term care of their patients, especially in the allogeneic HCT setting, where follow-up with recipients is often indefinite in duration. This close follow-up of patients ensures higher quality of long-term outcome data. Additionally, we were unable to report on trends in the use of reduced intensity or non-myeloablative conditioning regimens for allogeneic HCT, which gained popularity in the recent era. Despite this limitation, we acknowledge the increased use of these novel conditioning regimens likely contributed to the observed improvements in outcomes in allogeneic HCT during the later era.
5. Conclusion:
In conclusion, over the 20-year study period, transplant activity has increased, and key clinical outcomes have generally improved for adult and pediatric populations receiving HCT in Canada. The landscape of HCT in Canada has also evolved to serve older patients, with increased reliance on volunteer unrelated donors. Despite offering allogeneic HCT to older patients, OS rates in adults remained stable. These data serve as a benchmark for quality management in HCT centers across the country and should be helpful for resource planning by health providers and funding authorities.
Author Contributions
MS: IP: KP conceived the project, analyzed data, searched literature, wrote and edited the article. DA, OB, GM, OI, ER created and analyzed data, did statistical analyses, and edited the article. GP performed statistical analyses, searched literature, wrote and edited the article. SB, TT, KH, AP interpreted data, searched literature, and edited the article.
Funding
The CTTC registry is supported by unrestricted research grants from: Kite, a Gilead Company, Bristol Myers Squibb, Medexus, and Sanofi
Data Availability Statement
The data that support the findings of this study are not publicly available to ensure and maintain the privacy and confidentiality of individuals’ health information. Requests for data may be made to the appropriate data stewards (CancerCare Manitoba’s Research and Resource Impact Committee) who may be contacted via the corresponding author.
Conflicts of Interest
Dr Hay: ad hoc participation on advisory boards for BMS, Kite, Novartis, and Janssen, as well as Research funding from Janssen. Dr Seftel: ad hoc participation on an advisory board for Beigene.
References
- Kanate AS, Majhail NS, Savani BN, et al. Indications for Hematopoietic Cell Transplantation and Immune Effector Cell Therapy: Guidelines from the American Society for Transplantation and Cellular Therapy. Biol blood marrow Transplant J Am Soc Blood Marrow Transplant 2020, 26, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Snowden JA, Sánchez-Ortega I, Corbacioglu S, et al.Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant 2022, 57, 1217–1239. [CrossRef] [PubMed]
- Balassa K, Danby R, Rocha V. Haematopoietic stem cell transplants: principles and indications. Br J Hosp Med (Lond) 2019, 80, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hurley CK, Oudshoorn M, Setterholm M. Donor registries and search strategies. Methods Mol Biol 2012, 882, 531–547. [Google Scholar]
- Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol blood marrow Transplant J Am Soc Blood Marrow Transplant 2012, 18, 348–371. [Google Scholar] [CrossRef] [PubMed]
- Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol blood marrow Transplant J Am Soc Blood Marrow Transplant 2009, 15, 1143–1238. [Google Scholar] [CrossRef] [PubMed]
- Saad A, de Lima M, Anand S, et al. Hematopoietic Cell Transplantation, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020, 18, 599–634. [Google Scholar] [CrossRef] [PubMed]
- Luznik L, O’Donnell P V, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol blood marrow Transplant J Am Soc Blood Marrow Transplant 2008, 14, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Huang X-J, Liu D-H, Liu K-Y, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 2006, 38, 291–297. [Google Scholar] [CrossRef] [PubMed]
- 10. Bolon Y, Atshan R, Allbee-Johnson M, Estrada-Merly N, Lee S. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR summary slides. 2022.
- Passweg JR, Baldomero H, Chabannon C, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant 2021, 56, 1651–1664. [Google Scholar] [CrossRef]
- Penack O, Peczynski C, Mohty M, et al. How much has allogeneic stem cell transplant-related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv 2020, 4, 6283–6290. [Google Scholar] [CrossRef] [PubMed]
- Hahn T, McCarthy PLJ, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol Off J Am Soc Clin Oncol 2013, 31, 2437–2449. [Google Scholar] [CrossRef] [PubMed]
- Paulson K, Brazauskas R, Khera N, et al. Inferior Access to Allogeneic Transplant in Disadvantaged Populations: A Center for International Blood and Marrow Transplant Research Analysis. Biol blood marrow Transplant J Am Soc Blood Marrow Transplant 2019, 25, 2086–2090. [Google Scholar] [CrossRef] [PubMed]
- Rashidi A, Ebadi M, Colditz GA, DiPersio JF. Outcomes of Allogeneic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia: A Systematic Review and Meta-analysis. Biol blood marrow Transplant J Am Soc Blood Marrow Transplant 2016, 22, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Ringdén O, Boumendil A, Labopin M, et al. Outcome of Allogeneic Hematopoietic Stem Cell Transplantation in Patients Age >69 Years with Acute Myelogenous Leukemia: On Behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Biol blood marrow Transplant J Am Soc Blood Marrow Transplant 2019, 25, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Muffly LS, Kocherginsky M, Stock W, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica 2014, 99, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Olin RL, Fretham C, Pasquini MC, et al. Geriatric assessment in older alloHCT recipients: association of functional and cognitive impairment with outcomes. Blood Adv 2020, 4, 2810–2820. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt MCB, Ciurea SO. Recent Advances in Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia. Biol blood marrow Transplant J Am Soc Blood Marrow Transplant 2020, 26, e215–21. [Google Scholar] [CrossRef] [PubMed]
- Allan DS, Takach S, Smith S, Goldman M. Impact of Declining Fertility Rates in Canada on Donor Options in Blood and Marrow Transplantation. Biol Blood Marrow Transplant [Internet] 2009, 15, 1634–1637, Available from: https://www.sciencedirect.com/science/article/pii/S1083879109003395. [Google Scholar] [CrossRef] [PubMed]
- Besse K, Maiers M, Confer D, Albrecht M. On Modeling Human Leukocyte Antigen–Identical Sibling Match Probability for Allogeneic Hematopoietic Cell Transplantation: Estimating the Need for an Unrelated Donor Source. Biol Blood Marrow Transplant [Internet] 2016, 22, 410–417, Available from: https://www.sciencedirect.com/science/article/pii/S1083879115006187. [Google Scholar] [CrossRef] [PubMed]
- Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol 2009, 10, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Mehta RS, Saliba RM, Rondon G, et al. Post-Transplantation Cyclophosphamide Versus Tacrolimus and Methotrexate Graft-Versus-Host Disease Prophylaxis for HLA-Matched Donor Transplantation. Transplant Cell Ther 2022, 28, 695.e1–695.e10. [Google Scholar]
- Amouzegar A, Dey BR, Spitzer TR. Peripheral Blood or Bone Marrow Stem Cells? Practical Considerations in Hematopoietic Stem Cell Transplantation. Transfus Med Rev 2019, 33, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Khoury HJ, Wang T, Hemmer MT, et al. Improved survival after acute graft-versus-host disease diagnosis in the modern era. Haematologica 2017, 102, 958–966. [Google Scholar] [CrossRef] [PubMed]
- MacMillan ML, Robin M, Harris AC, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol blood marrow Transplant J Am Soc Blood Marrow Transplant 2015, 21, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Kong SG, Jeong S, Lee S, Jeong J-Y, Kim DJ, Lee HS. Early transplantation-related mortality after allogeneic hematopoietic cell transplantation in patients with acute leukemia. BMC Cancer [Internet] 2021, 21, 177, Available from: https://doi.org/10.1186/s12885-021-07897-3. [Google Scholar] [CrossRef]
- Wölfl M, Qayed M, Benitez Carabante MI, et al. Current Prophylaxis and Treatment Approaches for Acute Graft-Versus-Host Disease in Haematopoietic Stem Cell Transplantation for Children With Acute Lymphoblastic Leukaemia. Front Pediatr 2021, 9, 784377. [Google Scholar]
- Qayed M, Wang T, Hemmer MT, et al. Influence of Age on Acute and Chronic GVHD in Children Undergoing HLA-Identical Sibling Bone Marrow Transplantation for Acute Leukemia: Implications for Prophylaxis. Biol blood marrow Transplant J Am Soc Blood Marrow Transplant 2018, 24, 521–528. [Google Scholar] [CrossRef]
Figure 1A.
Overall Survival of Allogeneic and Autologous HCT by Era in Adult Patients. Survival
probability indicated by year and transplant type for HCTs performed during each time period. Logrank
(p<0.0001). B. Overall Survival of Allogeneic and Autologous HCT by Era in Pediatric Patients.
Survival probability indicated by year and transplant type for HCTs performed during each time
period. Log-rank (p<0.0001).
Figure 1A.
Overall Survival of Allogeneic and Autologous HCT by Era in Adult Patients. Survival
probability indicated by year and transplant type for HCTs performed during each time period. Logrank
(p<0.0001). B. Overall Survival of Allogeneic and Autologous HCT by Era in Pediatric Patients.
Survival probability indicated by year and transplant type for HCTs performed during each time
period. Log-rank (p<0.0001).
Figure 2A.
Non-Relapse Mortality of Adult Allogeneic and Autologous HCT by Era. Cumulative incidence of non-relapse mortality events indicated by year and transplant type during each time period. Gray’s test for equality (p<.0001). B. Non-Relapse Mortality of Pediatric Allogeneic and Autologous HCT by Era. Cumulative incidence of non-relapse mortality events indicated by year and transplant type during each time period. Gray’s test for equality (p<.0001).
Figure 2A.
Non-Relapse Mortality of Adult Allogeneic and Autologous HCT by Era. Cumulative incidence of non-relapse mortality events indicated by year and transplant type during each time period. Gray’s test for equality (p<.0001). B. Non-Relapse Mortality of Pediatric Allogeneic and Autologous HCT by Era. Cumulative incidence of non-relapse mortality events indicated by year and transplant type during each time period. Gray’s test for equality (p<.0001).
Figure 3A.
Overall Survival of HCT by Era for Non-Malignant Disease Indications. Survival
probability indicated by year and age group for HCTs performed during each time period. Log-rank
(p= 0.0505).B. Non-Relapse Mortality of HCT by Era for Non-Malignant Disease Indications.
Cumulative incidence of non-relapse mortality events indicated by year and age group for HCTs
performed during each time period. Gray’s test for equality (p=0.0353).
Figure 3A.
Overall Survival of HCT by Era for Non-Malignant Disease Indications. Survival
probability indicated by year and age group for HCTs performed during each time period. Log-rank
(p= 0.0505).B. Non-Relapse Mortality of HCT by Era for Non-Malignant Disease Indications.
Cumulative incidence of non-relapse mortality events indicated by year and age group for HCTs
performed during each time period. Gray’s test for equality (p=0.0353).
Figure 4A.
Overall Survival by Era following Allogeneic HCT indicated for Adult Acute Leukemias. Malignant disease indications included were AML, ALL, AL of ambiguous lineage, and MDS/MPN. Survival probabilities indicated by year for HCTs performed during each time period. Log-rank (p=0.1513). B. Non-Relapse Mortality by Era following Allogeneic HCT indicated for Adult Acute Leukemias. Malignant disease indications included were AML, ALL, AL of ambiguous lineage, and MDS/MPN. Cumulative incidence of non-relapse mortality events indicated by year for HCTs performed during each time period. Gray’s test for equality (p= 0.0337).
Figure 4A.
Overall Survival by Era following Allogeneic HCT indicated for Adult Acute Leukemias. Malignant disease indications included were AML, ALL, AL of ambiguous lineage, and MDS/MPN. Survival probabilities indicated by year for HCTs performed during each time period. Log-rank (p=0.1513). B. Non-Relapse Mortality by Era following Allogeneic HCT indicated for Adult Acute Leukemias. Malignant disease indications included were AML, ALL, AL of ambiguous lineage, and MDS/MPN. Cumulative incidence of non-relapse mortality events indicated by year for HCTs performed during each time period. Gray’s test for equality (p= 0.0337).
Table 1.
Baseline Population Characteristics for First Allogeneic Transplants. Analysis of patients receiving a first allogeneic HCT between January 1, 2000, and December 31, 2019.
Table 1.
Baseline Population Characteristics for First Allogeneic Transplants. Analysis of patients receiving a first allogeneic HCT between January 1, 2000, and December 31, 2019.
| |
Total
N (%) |
2000-2009
N (%) |
2010-2019
N (%) |
P-value |
| Total |
7571 (100) |
3407 (45) |
4164 (55) |
<0.0001 |
| Age at HCT in years (median, range) |
45 (0 – 75) |
42 (0 - 75) |
50 (0 -74) |
<0.0001 |
| Age groups (N, %) |
| Pediatric [<18 years] |
1479 (19.5) |
765 (22.5) |
714 (17.2) |
<0.0001 |
| Young adult [18-39 years] |
1565 (20.7) |
823 (24.12) |
742 (17.8) |
<0.0001 |
| Middle-aged adult [40-64 years] |
4078 (53.9) |
1760 (51.7) |
2318 (55.7) |
0.0005 |
| Older adult [65+ years] |
448 (5.9) |
58 (1.7) |
390 (9.4) |
<0.0001 |
| Unknown |
1 (0.01) |
1 (0.01) |
0 (0) |
0.45 |
| Sex (N, %) |
| Male |
4397 (58.3) |
1987 (58.9) |
2410 (57.9) |
0.3979 |
| Female |
3143 (41.7) |
1389 (41.1) |
1754 (42.1) |
0.3979 |
| Unknown |
31 (0.4) |
31 (0.4) |
0 (0) |
<0.0001 |
| Donor type (N, %) |
| Matched related |
3386 (44.7) |
1861 (54.6) |
1525 (36.6) |
<0.0001 |
| Syngeneic (monozygotic twin) |
50 (0.7) |
34 (1.0) |
16 (0.4) |
0.0014 |
| Haplo-identical |
369 (4.9) |
115 (3.4) |
254 (6.1) |
<0.0001 |
| Other relative |
14 (0.2) |
10 (0.3) |
4 (0.1) |
0.0594 |
| Matched unrelated |
3542 (46.8) |
1197 (35.1) |
2345 (56.3) |
<0.0001 |
| Mismatched unrelated |
209 (2.8) |
189 (5.6) |
20 (0.5) |
<0.0001 |
| Multiple donor |
1 (0.01) |
1 (0.03) |
0 (0) |
0.4426 |
| Cell source (N, %) |
|
|
|
|
| Bone marrow |
1734 (22.2) |
1043 (30.0) |
691 (15.9) |
<0.0001 |
| Peripheral blood |
5304 (67.9) |
2093 (60.3) |
3211 (74.0) |
<0.0001 |
| Cord blood |
444 (5.7) |
220 (6.3) |
224 (5.2) |
0.0436 |
| Unknown |
331 (4.2) |
117 (3.4) |
214 (4.9) |
0.0007 |
| Indication for HCT (N, %) |
| Acute myelogenous leukemia |
2781 (36.7) |
1093 (32.1) |
1688 (40.5) |
<0.0001 |
| Acute lymphoblastic leukemia |
1074 (14.2) |
530 (15.6) |
544 (13.1) |
0.0021 |
| Myelodysplastic/myeloproliferative disorders (+ preleukemia) |
912 (12.0) |
376 (11.0) |
536 (12.9) |
0.0158 |
| Non-Hodgkin lymphoma |
720 (9.5) |
428 (12.6) |
292 (7.0) |
<0.0001 |
| Chronic myelogenous leukemia |
389 (5.1) |
247 (7.3) |
142 (3.4) |
<0.0001 |
| Chronic lymphocytic leukemia |
344 (4.5) |
186 (5.5) |
158 (3.8) |
0.0006 |
| Plasma cell disorder (+ multiple myeloma) |
37 (0.5) |
31 (0.9) |
6 (0.1) |
<0.0001 |
| Hodgkin lymphoma |
20 (0.3) |
17 (0.5) |
3 (0.1) |
0.0004 |
| Other malignancies |
417 (5.5) |
120 (3.5) |
297 (7.1) |
<0.0001 |
| Severe aplastic anemia |
341 (4.5) |
183 (5.4) |
158 (3.8) |
0.0012 |
| Other non-malignant disease |
520 (6.9) |
187 (5.5) |
333 (8.0) |
<0.0001 |
| Other |
16 (0.2) |
9 (0.3) |
7 (0.2) |
0.453 |
Table 2.
Baseline Population Characteristics for First Autologous Transplants. Analysis of patients receiving a first autologous HCT between January 1, 2000, and December 31, 2019.
Table 2.
Baseline Population Characteristics for First Autologous Transplants. Analysis of patients receiving a first autologous HCT between January 1, 2000, and December 31, 2019.
| |
Total
N (%) |
2000-2009
N (%) |
2010-2019
N (%) |
P-value |
| Total |
10475 (100) |
4966 (47.4) |
5509 (52.6) |
<0.0001 |
| Age at HCT in years (median, range) |
55 (0 – 81) |
53 (0 - 81) |
58 (0 - 79) |
<0.0001 |
| Age groups (N, %) |
|
| Pediatric [<18 years] |
785 (7.5) |
394 (7.9) |
391 (7.1) |
0.11 |
| Young adult [18-39 years] |
1235 (11.8) |
720 (14.5) |
515 (9.4) |
<0.0001 |
| Middle-aged adult [40-64 years] |
6755 (64.5) |
3336 (67.2) |
3419 (62.1) |
<0.0001 |
| Older adult [65+ years] |
1699 (16.2) |
515 (10.4) |
1184 (21.5) |
<0.0001 |
| Unknown |
1 (0.01) |
1 (0.02) |
0 (0) |
0.4741 |
| Sex (N, %) |
|
| Male |
6321 (60.4) |
2956 (59.6) |
3365 (61.1) |
0.1187 |
| Female |
4149 (39.6) |
2005 (40.4) |
2144 (38.9) |
0.1187 |
| Unknown |
5 (0.05) |
5 (0.1) |
0 (0) |
0.0239 |
| Cell source (N, %) |
|
|
|
|
| Bone marrow |
95 (0.9) |
83 (1.6) |
12 (0.2) |
<0.0001 |
| Peripheral blood |
10349 (95.6) |
4848 (95.5) |
5501 (95.7) |
<0.0001 |
| Cord blood |
0 (0) |
0 (0) |
0 (0) |
N/A |
| Unknown |
381 (3.5) |
148 (2.9) |
233 (4.1) |
0.0014 |
| Indication for HCT (N, %) |
|
| Plasma cell disorder (+ multiple myeloma) |
5176 (49.4) |
2303 (46.4) |
2873 (52.2) |
<0.0001 |
| Non-Hodgkin lymphoma |
3224 (30.8) |
1515 (30.5) |
1709 (31) |
0.5816 |
| Hodgkin lymphoma |
1016 (9.7) |
575 (11.6) |
441 (8.0) |
<0.0001 |
| Acute myelogenous leukemia |
77 (0.7) |
72 (1.4) |
5 (0.1) |
<0.0001 |
| Chronic lymphocytic leukemia |
7 (0.1) |
4 (0.1) |
3 (0.1) |
0.7146 |
| Acute lymphoblastic leukemia |
6 (0.1) |
4 (0.1) |
2 (0.04) |
0.432 |
| Myelodysplastic/myeloproliferative disorders (+ preleukemia) |
1 (0.01) |
1 (0.02) |
0 (0) |
0.4741 |
| Chronic myelogenous leukemia |
1 (0.01) |
1 (0.02) |
0 (0) |
0.4741 |
| Other malignancies |
868 (8.3) |
454 (0.1) |
414 (7.5) |
0.0606 |
| Other Non-Malignant disease |
82 (0.8) |
26 (0.5) |
56 (1.0) |
0.0052 |
| Other |
17 (0.2) |
11 (0.2) |
6 (0.1) |
0.2234 |
Table 3.
Overall Survival of Allogeneic and Autologous HCT by Era and Transplant Type. Adult and pediatric patients receiving a first HCT between January 1, 2000, and December 31, 2019 were categorized by transplant type and time period. Number of individuals at risk and overall survival with 95% confidence intervals are indicated at 1, 2 ,5 and 10 years post transplant.
Table 3.
Overall Survival of Allogeneic and Autologous HCT by Era and Transplant Type. Adult and pediatric patients receiving a first HCT between January 1, 2000, and December 31, 2019 were categorized by transplant type and time period. Number of individuals at risk and overall survival with 95% confidence intervals are indicated at 1, 2 ,5 and 10 years post transplant.
| |
Allogeneic HCT |
Autologous HCT |
Overall
P-value
|
| 2000-2009 |
2010-2019 |
P-value |
2000-2009 |
2010-2019 |
P-value |
| No. at risk |
OS, % (95% CI) |
No. at risk |
OS, %
(95% CI) |
No. at risk |
OS, %
(95% CI) |
No. at risk |
OS, %
(95% CI) |
| Adult |
1-yr |
1428 |
66 (64 - 68) |
1621 |
67 (65 - 69) |
0.1293 |
2838 |
83 (82 - 85) |
2901 |
88 (87 - 89) |
<0.0001 |
|
| 2-yr |
1207 |
59 (57 - 61) |
1050 |
57 (55 - 59) |
0.9466 |
2278 |
75 (73 - 76) |
2051 |
82 (80 - 83) |
<0.0001 |
|
| 5-yr |
757 |
49 (47 - 52) |
454 |
49 (47 - 51) |
0.8347 |
1196 |
55 (54 - 57) |
735 |
65 (62 - 67) |
<0.0001 |
<0.0001 |
| 10-yr |
458 |
43 (41 - 45) |
35 |
45 (41 - 47) |
0.6482 |
556 |
40 (38 - 42) |
28 |
49 (45 - 53) |
<0.0001 |
|
| Pediatric |
1-yr |
459 |
73 (69 - 76) |
424 |
85 (82 - 88) |
<0.0001 |
234 |
78 (73 - 82) |
159 |
78 (72 - 83) |
0.972 |
|
| 2-yr |
392 |
67 (64 - 71) |
289 |
80 (77 - 83) |
<0.0001 |
194 |
69 (63 - 73) |
119 |
68 (61 – 7`4) |
0.8041 |
|
| 5-yr |
299 |
64 (60 - 67) |
78 |
76 (71 - 80) |
<0.0001 |
121 |
51 (45 - 57) |
45 |
57 (49 - 65) |
0.4523 |
<0.0001 |
| 10-yr |
142 |
61 (57 - 65) |
2 |
55 (21 - 78) |
<0.0001 |
67 |
47 (41 - 53) |
1 |
50 (39 - 60) |
0.612 |
|
Table 4.
Non-Relapse Mortality of Allogeneic and Autologous HCT by Era and Transplant Type. Adult and pediatric patients receiving a first HCT between January 1, 2000, and December 31, 2019 were categorized by transplant type and time period. Number of individuals at risk and cumulative incidence of non-relapse mortality with 95% confidence intervals are indicated at 100 days, 1 year, 2 years, and 5 years post transplant.
Table 4.
Non-Relapse Mortality of Allogeneic and Autologous HCT by Era and Transplant Type. Adult and pediatric patients receiving a first HCT between January 1, 2000, and December 31, 2019 were categorized by transplant type and time period. Number of individuals at risk and cumulative incidence of non-relapse mortality with 95% confidence intervals are indicated at 100 days, 1 year, 2 years, and 5 years post transplant.
| |
Allogeneic HCT |
Autologous HCT |
Overall P-value |
| 2000-2009 |
2010-2019 |
P-value |
2000-2009 |
2010-2019 |
P-value |
| No. at risk |
Cumulative Incidence, % (95% CI) |
No. at risk |
Cumulative Incidence, % (95% CI) |
No. at risk |
Cumulative Incidence, % (95% CI) |
No. at risk |
Cumulative Incidence, % (95% CI) |
| Adult |
100 days |
1980 |
10.9 (9.7 - 12.2) |
2454 |
8.1 (7.1 - 9.0) |
0.0002 |
3624 |
2.1 (1.7 - 2.6) |
3479 |
1.4 (1.0 - 1.7) |
0.0067 |
<0.0001 |
| 1-yr |
1316 |
19.7 (18.1 - 21.3) |
1501 |
17.9 (16.5 - 19.4) |
0.0598 |
2443 |
3.9 (3.3 - 4.5) |
2430 |
2.5 (1.9 - 2.9) |
0.0002 |
| 2-yr |
1099 |
22.3 (20.6 - 24.0) |
975 |
21.1 (19.5 - 22.7) |
0.1359 |
1746 |
5.1 (4.3 - 5.8) |
1598 |
3.1 (2.5 - 3.7) |
<0.0001 |
| 5-yr |
690 |
26.5 (24.6 - 28.4) |
422 |
23.2 (21.5 - 24.9) |
0.0281 |
822 |
8.3 (7.2 - 9.3) |
494 |
5.2 (4.2 - 6.3) |
<0.0001 |
| Pediatric |
100 days |
612 |
7.5 (5.7 - 9.6) |
587 |
3.7 (2.5 - 5.3) |
0.0025 |
299 |
1.9 (0.8 - 3.8) |
205 |
1.4 (0.5 - 3.4) |
0.5304 |
<0.0001 |
| 1-yr |
443 |
13.0 (10.6 - 15.6) |
417 |
6.4 (4.6 - 8.4) |
<0.0001 |
203 |
2.9 (1.5 - 5.2) |
132 |
2.4 (0.9 - 4.9) |
0.567 |
| 2-yr |
384 |
15.4 (12.7 - 18.2) |
283 |
8.6 (6.4 - 11.1) |
0.0001 |
158 |
3.7 (2.0 - 6.8) |
97 |
3.7 (1.6 - 6.9) |
0.7837 |
| 5-yr |
291 |
17.6 (14.7 - 20.6) |
79 |
9.9 (7.5 - 12.8) |
0.0001 |
108 |
4.2 (2.3 - 6.8) |
40 |
4.5 (2.1 - 8.1) |
0.9081 |
Table 5.
A. Overall Survival of HCT by Era for Non-Malignant Disease Indications. Adult and pediatric patients receiving a first HCT for non-malignant disease indications between January 1, 2000, and December 31, 2019 were categorized by transplant type and time period. Number of individuals at risk and overall survival with 95% confidence intervals are indicated at 1, 2 ,5 and 10 years post transplant.
Table 5.
A. Overall Survival of HCT by Era for Non-Malignant Disease Indications. Adult and pediatric patients receiving a first HCT for non-malignant disease indications between January 1, 2000, and December 31, 2019 were categorized by transplant type and time period. Number of individuals at risk and overall survival with 95% confidence intervals are indicated at 1, 2 ,5 and 10 years post transplant.
| |
2000-2009 |
2010-2019 |
P-value |
Overall P-value |
| No. at risk |
OS, %
(95% CI) |
No. at risk |
OS, %
(95% CI) |
| Adult |
1-yr |
66 |
76 (66 - 84) |
59 |
85 (75 - 90) |
0.3199 |
0.1529 |
| 2-yr |
62 |
76 (66 - 84) |
47 |
85 (75 - 90) |
0.3199 |
| 5-yr |
41 |
70 (59 - 79) |
24 |
85 (75 - 90) |
0.1529 |
| 10-yr |
27 |
70 (59 - 79) |
0 |
- |
0.1529 |
| Pediatric |
1-yr |
111 |
81 (74 - 86) |
165 |
91 (87 - 95) |
0.0037 |
0.0923 |
| 2-yr |
97 |
79 (71 - 85) |
106 |
87 (82 - 91) |
0.0153 |
| 5-yr |
77 |
78 (70 - 84) |
30 |
85 (78 - 89) |
0.0393 |
| 10-yr |
41 |
75 (67 - 82) |
2 |
53 (10 - 83) |
0.0923 |
Table 5.
B. Non-Relapse Mortality of HCT by Era for Non-malignant Disease Indications. Adult and pediatric patients receiving a first HCT for a non-malignant disease indication between January 1, 2000, and December 31, 2019 were categorized by transplant type and time period. Number of individuals at risk and cumulative incidence of non-relapse mortality with 95% confidence intervals are indicated at 100 days, 1 year, 2 years, and 5 years post transplant.
Table 5.
B. Non-Relapse Mortality of HCT by Era for Non-malignant Disease Indications. Adult and pediatric patients receiving a first HCT for a non-malignant disease indication between January 1, 2000, and December 31, 2019 were categorized by transplant type and time period. Number of individuals at risk and cumulative incidence of non-relapse mortality with 95% confidence intervals are indicated at 100 days, 1 year, 2 years, and 5 years post transplant.
| |
2000-2009 |
2010-2019 |
P-value |
Overall P-value |
| No. at risk |
Cumulative Incidence, % (95% CI) |
No. at risk |
Cumulative Incidence, % (95% CI) |
| Adult |
100 days |
78 |
15.5 (9.0 - 23.3) |
85 |
9.2 (4.5 - 15.8) |
0.1686 |
0.0245 |
| 1-yr |
67 |
21.1 (13.5 - 29.8) |
60 |
11.3 (5.9 - 18.4) |
0.0704 |
| 2-yr |
63 |
21.1 (13.5 - 29.8) |
48 |
11.3 (5.9 - 18.4) |
0.0704 |
| 5-yr |
42 |
27.5 (18.3 - 37.5) |
26 |
11.3 (5.9 - 18.4) |
0.0245 |
| Pediatric |
100 days |
142 |
7.4 (4.0 - 12.1) |
219 |
4.6 (2.4 - 7.8) |
0.2425 |
0.1434 |
| 1-yr |
112 |
15.9 (10.5 - 22.1) |
170 |
7.4 (4.5 - 11.2) |
0.0109 |
| 2-yr |
98 |
18.2 (12.3 - 24.8) |
107 |
11.4 (7.3 - 16.4) |
0.0406 |
| 5-yr |
78 |
19.0 (13.0 - 25.8) |
31 |
14.3 (9.4 - 20.2) |
0.0946 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).