1. Introduction
Avian colibacillosis is one of the most frequent and important bacterial infections in avian pathology; it mainly affects breeding and broiler farms, causing health and welfare impairment and major economic losses [
1]. Avian pathogenic
E. coli (APEC) strains are presently considered one of the major causes of mortality in poultry, and lead to performance losses and condemnation at slaughterhouses [
2]. The disease typically manifests as a secondary infection following viral, bacterial, or parasitic primary infections. The frequent use of antibiotics in treating this condition induces the development of antimicrobial resistance, as well as the emergence of resistant strains [
3].
Over the last five years, the pathological situation in Moroccan poultry industry has undergone important and drastic changes. The outbreaks of low pathogenic avian influenza (H9N2) in 2016 and infections caused by
Mycoplasma spp. created conditions for secondary
E. coli infections, whose prevention is becoming increasingly difficult [
4]. Control of avian colibacillosis has historically been achieved through the curative and preventive use of antibiotic treatments [1, 4,]. However, careless use of antibiotics has caused an increase in the frequency of antimicrobial resistance (AMR) in clinical APEC strains. This necessitates alternative strategies to protect both animal and public health [5, 6]. Commercial APEC vaccines are available as a preventative strategy against colibacillosis; they also contribute to limiting the use of antibiotics; however, their efficacy is often limited by the lack of cross-protection between heterologous APEC strains [
7]. This has led to a paradigm change in the poultry industry, that now considers the use of autogenous vaccines an innovative solution and a part of the strategy to prevent and control avian colibacillosis, including protecting the progeny of vaccinated breeding hens [
8], leading the way to new vaccine development [
9].
The present study aimed to evaluate a tetravalent autogenous vaccine produced from APEC strains of four different serotypes (O78, O88, O91, and O185), all isolated from the Moroccan broiler breeder flocks where the autogenous vaccine was used. The impact of this immunization trial was monitored at the different stages of the poultry flow, both broiler breeders and their progeny until slaughter. It resulted in a highly significant improvement of both the health and performance of the vaccinated hens and their progeny, with a measurable effect up to slaughter for the latter.
2. Materials and Methods
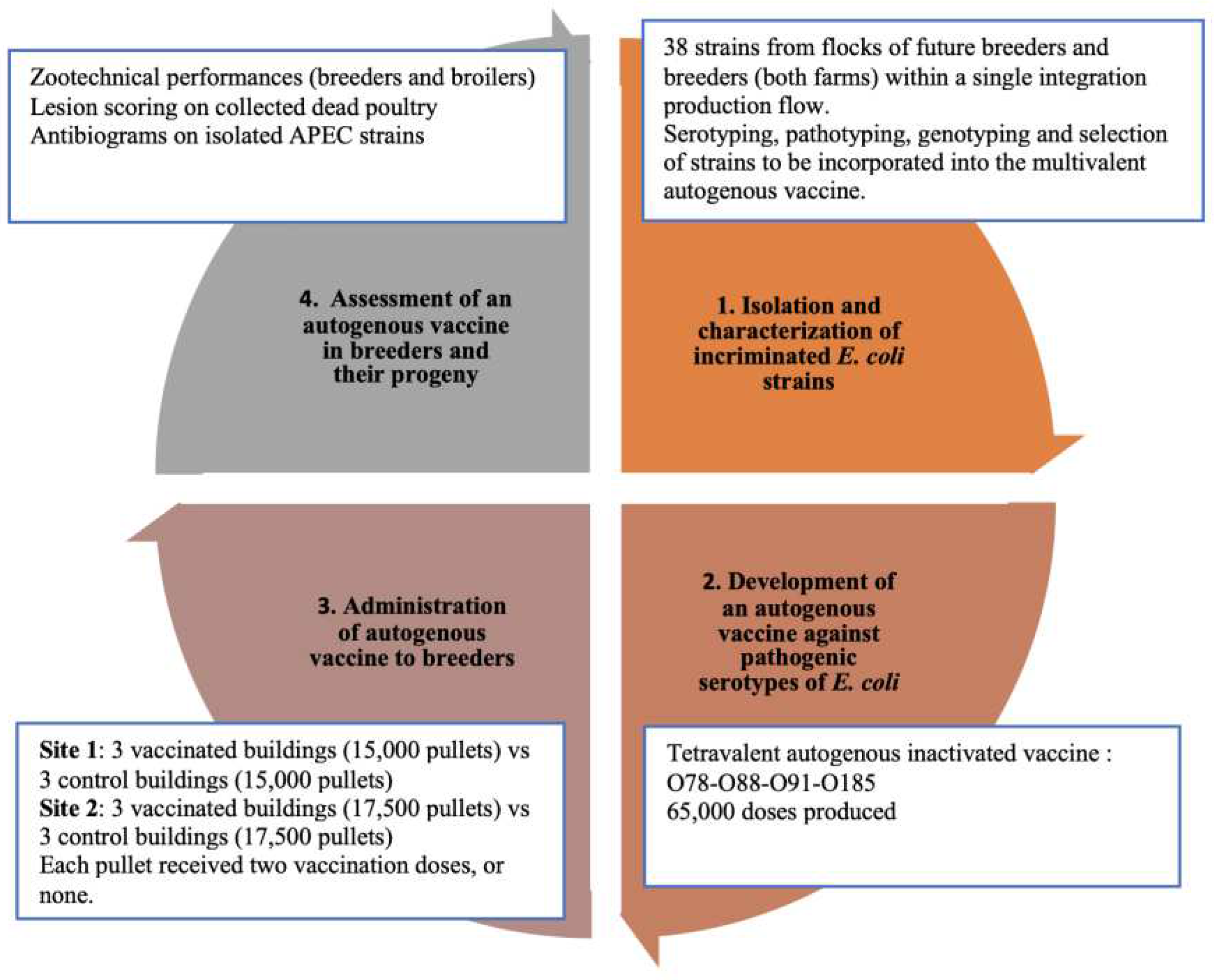
The present study was carried out from the beginning of 2020 until the end of 2022 and was performed in a single integration poultry production flow (Zalar Holding), located on the Rabat-Casablanca-El Jadida axis (Morocco). This production flow is ISO 9001 and ISO 22000 certified and maintains bird/egg traceability from pullet breeding site to broiler slaughter. The design of the study is summarized in
Figure 1. Both breeder flocks (30,000 and 35,000 head, at sites 1 and 2, respectively) had a history of recurrent colibacillosis, evidenced by the isolation and molecular characterization of
E. coli strains. For the purpose of autogenous vaccine production, organs of breeder hens and broilers from downstream production sites were sampled (liver, ovary, bone marrow) and sent to two different Moroccan diagnostic laboratories. Isolation of
E. coli strains was achieved on EMB media. Thirty-eight strains were sent to Ceva Biovac (Beaucouzé, France), which verified the species identification by Maldi-Tof mass spectrometry and performed somatic serotyping using antisera, pathotyping by PCR for detection of 18 virulence-associated genes and genotyping by Pulse Field Gel Electrophoresis (PFGE), as described elsewhere [
10]. Four dominant strains of four different serotypes — O78, O88, O91, and O185 — were selected for incorporation in the autogenous vaccine. The oily inactivated tetravalent autogenous vaccine was produced in France by Ceva Biovac and imported to Morocco for the planned field trials (see supplementary documents).
Each of the two breeder farms was composed of six buildings, with the same design, equipment, and poultry capacity per farm. All animals had ad libitum access to feed and water over the trial duration. On site 1 (30,000 Cobb 500 pullets), each building/group count was 5,000 head. On site 2 (35,000 Ross 308 pullets), each group count was 5,800 head. Each site comprised six buildings. Buildings were randomized on each site, so that pullets in three buildings would be vaccinated, and those in three other buildings would be left unvaccinated. All pullets from vaccinated groups received two doses of autogenous vaccine intra-muscularly (0.5 ml/bird) at 12 and 18 weeks of age. On each site, injection was performed on the same day; but on different dates on the two sites (26 November 2021 on site 1, 3 February 2022 on site 2). Pullets were transferred to laying facilities at 22 weeks of age, and group structure was maintained.
Performance and health parameters were recorded during the production cycle of each flock, from day-old chick arrival to culling at 60 weeks of age. From 12 weeks of age (first vaccine injection at pullet rearing site) onwards, the data were collected on a group basis. The collected parameters were mortality rate (daily collection and recording of number of dead poultry), number of hatching eggs, number saleable day-old chicks per hen. On each laying site (i.e., from 22 weeks of age onwards), necropsies were performed every 7 weeks on 10 freshly collected dead hens from vaccinated as well as control groups (10 per group). In total, six necropsy sessions were conducted by the first author and/or the five veterinarians employed by the poultry integration. Macroscopic lesions were recorded for the following organs: ovarian cluster, lungs, air sacs, heart and liver. Scoring ranged from 0 (no lesion) to 3 (severe fibrin), see supplementary table S1. Lesions were swabbed and swabs sent to the same two diagnostic laboratories, for bacterial isolation and assessment of antimicrobial resistance (see details below).
The eggs laid by the breeding hens were identified by group from incubation and day-old chick delivery to downstream broiler farms (12 in total). Each broiler farm received a group of 25,000-day-old chicks hatched from vaccinated hens or a group of 25,000-day-old chicks hatched from control hens – this represented 603,000 broilers in total, whose technical performances and health data were recorded (mortality, average body weight at slaughter – day 37, feed conversion ratio, lesion score, condemnation rate and reasons for condemnation at the slaughterhouse). For
E. coli isolates from broilers, AMR testing was performed on agar plates. The results of antibiograms were interpreted as recommended by the French microbiology society [
11], with cut-off values for susceptible, intermediate, and resistant phenotypes.
The common organization and certification of the sites, their proximity, and the implementation of HACCP on farm work organization, including vaccine administration, means that environmental factors are comparable for the duration of the trial. The main outcome of the study was the health improvement of the birds, with average mortality rate and average colibacillosis-focused lesion score as explanatory variables. The hypothesis of the authors was that both variables would be significantly improved among vaccinates. Zootechnical performance parameters of laying hens and of their progeny (each being an explanatory variable) were the study’s secondary outcome. We hypothesized that these parameters would also be improved in layers and their progeny, including the slaughterhouse condemnation rate of broilers.
Each building was considered as an epidemiological unit (for breeder layers as well as for broilers). The statistical analysis of the results (analysis of variance of the effect of explicative variables) was carried out according to the generalized least squares method using the GLM (General Linear Models) procedure of the SAS (Statistical Analysis System) programme (SAS, USA).
3. Results
3.1. Breeders
Average performance obtained from vaccinated and control breeder groups are presented in
Table 1. A significant improvement was measured for the vaccinated groups compared with the control ones for mortality rate (-7.6% and -10.2% in vaccinated flocks on sites 1 and 2, respectively). The number of saleable day-old chicks per housed hen (which is economically pivotal for the integration) was significantly improved for vaccinated hens on site one (+3.7%) and numerically on site 2 (+2.8%, p=0.06). Furthermore, a numerical improvement of +2.6 and +1.8% in the number of hatching eggs per hen was observed for the vaccinated flocks, respectively.
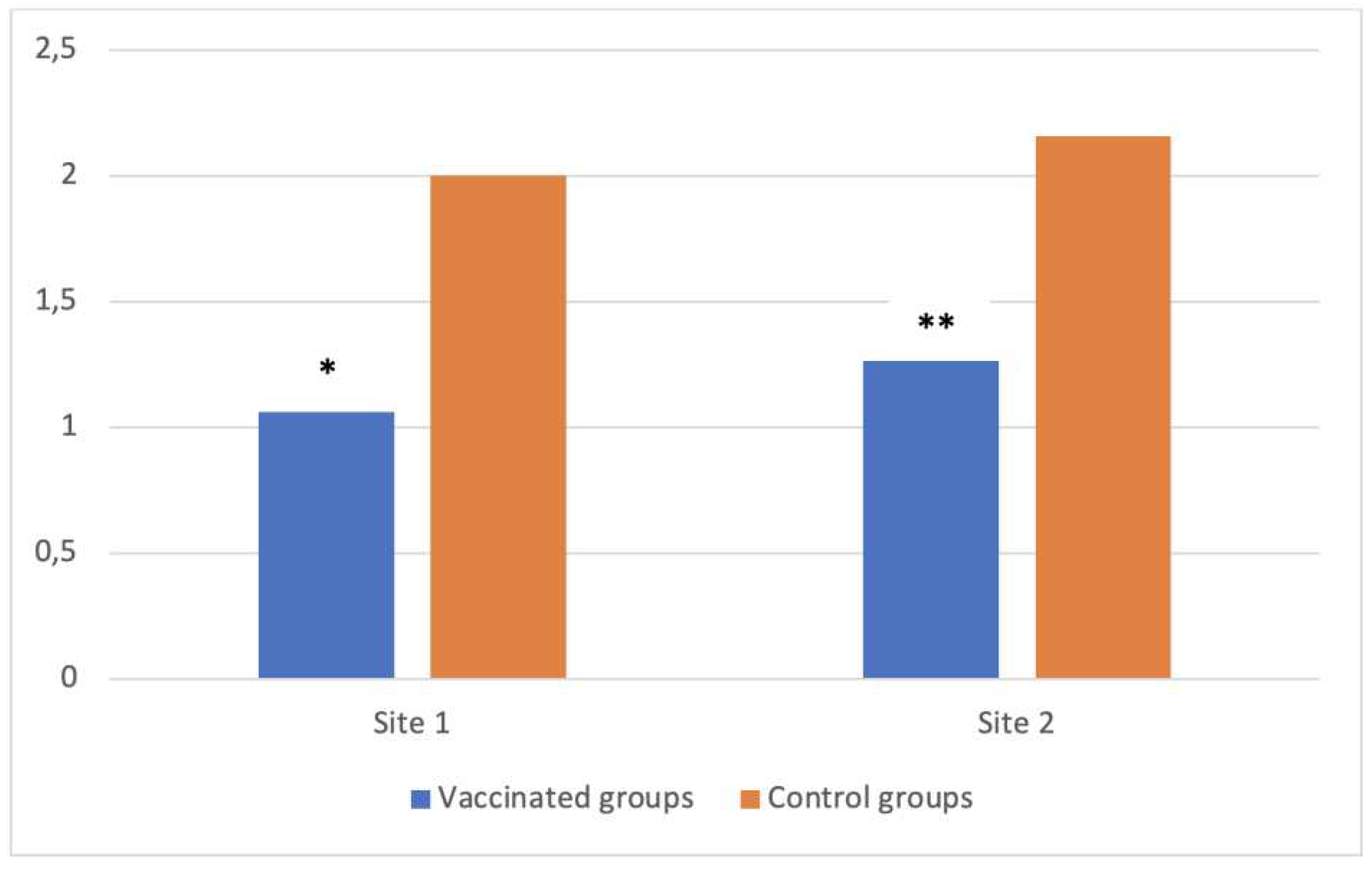
In total, six necropsy sessions of 10 cadavers were performed on dead laying breeder hens that had been collected from each building, identified and frozen. Macroscopic lesions were scored for each bird, with reference to a colibacillosis-focused lesion scoring system (see table S1), then averaged per group. These results (see table S2) show a significant reduction in the average lesion score between vaccinated and control groups, on both sites (-47.0% on site 1, p=0.04 and -41.9% on site 2, p=0.01), see
Figure 2.
3.2. Broilers
All broilers included in the trial were slaughtered at an average age of 37 days. Average live weight at slaughter was very highly significantly improved among broiler groups hatched from vaccinated layers (+2.0%; p=0.0001). Mortality rate was highly significantly reduced (-20.4%; p=0.001) while food conversion ratio (FCR) was significantly improved (-1.8%; p=0.02) for the progeny of vaccinated hens (detailed results are provided in table S3).
Weekly necropsies were performed on broilers collected as a daily routine over the whole production period (6 necropsy sessions in total, both on flocks hatched to vaccinated and control hens). The same colibacillosis-focused lesion scoring system was used, with the obvious absence of ovarian cluster among the organs scored. The average lesion score was 1.04 for broilers hatched to vaccinated hens, and 1.45 for broilers hatched to control hens (-39.4%; p=0.0075).
Regarding slaughterhouse results, both total and cellulitis condemnation rates were very highly significantly improved among broilers hatched to vaccinated hens (-14.9 and -60.0%, respectively, see
Table 2). The partial condemnation rate was also significantly improved.
3.3. AMR phenotypes
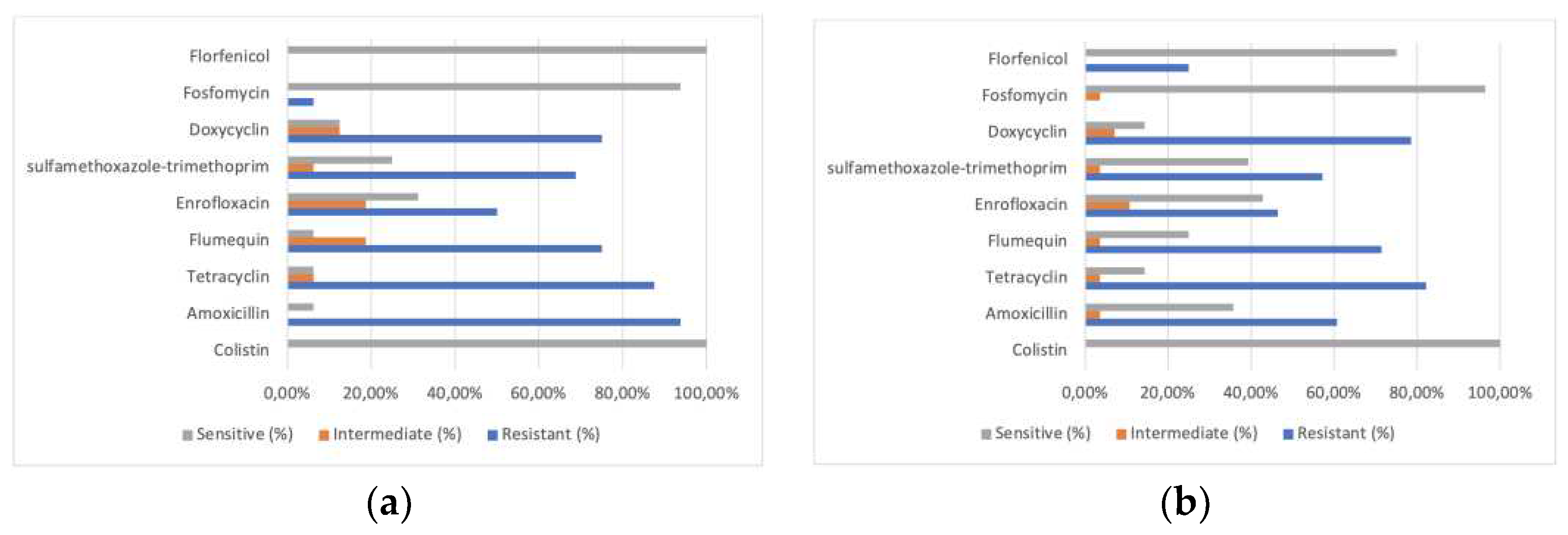
Antibiogram profiles of the 16 APEC strains isolated from dead breeding hens presenting severe colibacillosis lesions showed very high resistance frequency to most common antimicrobials prescribed in poultry medicine (see
Figure 3). Ninety-four per cent of strains were resistant to amoxicillin, 87.5% to tetracycline and 75% to doxycycline. Low resistance frequency was observed to fosfomycin (6.3%), and no resistant strain was identified towards florfenicol. Most of the 28 APEC strains isolated from broilers presented comparable phenotypes as the strains isolated from breeding hens, although amoxicillin resistance was less common (60.7%). Still, resistance to tetracycline (82.1%), doxycycline (78.6%) and flumequine (71.4%) were frequent.
4. Discussion
The significant improvement of key health (mortality rate, lesion score) and performance indicators (number of hatching eggs, and saleable day-old chicks per housed hen) in and from vaccinated groups of laying breeder hens, as compared to contemporaneous control (unvaccinated) groups, in the context of recurrent colibacillosis, can be ascribed to the protective effect of the autogenous vaccine against APEC. The improved health of the layers might also allow an improved microbial quality of the hatching eggs and day-old chicks hatched. This study confirms previous results obtained by pullet immunization with a multivalent APEC autogenous vaccine where mortality rate was reduced in the vaccinated groups, compared to contemporaneous control groups, in challenge models [12, 13]. The present study also adds to the knowledge of the impact of autogenous vaccine usage in breeders by evidencing a significant positive effect on the health and performance of progeny, which had been observed previously under experimental conditions for one of the two tested challenge strains [
14], but not in a field trial.
A previous field study has been conducted in broiler breeder hens vaccinated with Poulvac
® (O78) and additionally with a bivalent autogenous
E. coli vaccine [
15]. The latter contained two strains belonging to the clonal complex 95, that are both closely related to an O2 reference strain, hence providing protection against a single serotype. In the present field trial, the tetravalent autogenous vaccine was based on four
E. coli isolates of different, well-defined serotypes (O78, O88, O91, and O185).
The significant improvement of the average colibacillosis-focused lesion score among vaccinated groups in our trial finds logical explanation in the protective effect of autogenous vaccines against severe colibacillosis. Previous work has obtained comparable results upon experimental aerogenic APEC infection, where the overall gross pathology scores were 2.8 unvaccinated and 1.95 in the vaccinated
E. coli challenged groups, respectively [
16]. The recent trial reporting on the efficacy of a bivalent autogenous vaccine (where both valences correspond to a single serotype) similarly evidenced a significant reduction in the average lesion scores among vaccinates, as compared to control groups [
15]. Taken together, these results suggest that autogenous vaccination is efficacious at limiting the severity of colibacillosis in breeding laying hens, provided the clinical strains included in the vaccine have been adequately selected.
Regarding the progeny of the immunized hens, the standards implemented at the integration level (ISO 9001 and ISO 22000) provide a reliable framework for the traceability of the different groups (pullets, breeding layers, egg collection and hatching, day-old chicks and broilers up to slaughter). Also, the comparable design of the broiler house facilities, management and feed/water origin, and their relative proximity allow for a comparison of the two types of progenies. The significant improvement of health (mortality rate, average colibacillosis-focused lesion score) and technical performance indicators (FCR, and average body weight at slaughter) in broiler flocks hatched from immunized laying breeder hens, compared to those hatched from control hens imply that chicks acquired substantial passive maternal immunity. It has also been previously documented that an autogenous vaccine induces specific antibodies in the serum of laying hens [
12]. The transfer of IgY induced by immunization from a hens’ serum to the vitellus of her eggs is well described and industrially used for egg-yolk antibody production (for a review, see [
17]). Also, the transfer of anti-APEC maternal antibodies to their progeny after vaccination has been demonstrated under experimental conditions, with the use of a candidate enterobactin conjugate vaccine [
9]. Furthermore, broiler chickens were proven protected by passive immunity further to the immunization of their female ascendants with an autogenous inactivated vaccine under experimental conditions [
14]. It is then reasonable to conclude that, in the present field trial, APEC-specific antibodies induced by the multivalent inactivated autogenous vaccine induced a significant protection in the progeny of the laying hens via passive transfer of maternal antibodies, and that this protection was significant. Another – non exclusive – possibility is that immunization of the breeder hens reduced the level of vertical transmission of APEC strains to the progeny, as suggested by [
8]. However, the present trial was not designed to provide data in support to this hypothesis.
Regarding condemnation at the slaughterhouse, the significant reduction in condemnation rates, both for total condemnation and for those related to cellulitis (a clinical expression of colibacillosis in broilers, [
18]) among broiler flocks hatched from immunized breeder laying hens, as compared to those hatched from control hens, suggests a prolonged protection by passive maternal immunity, up to slaughter (mean of 37 days of age in the present trial). To the authors’ knowledge, this had not been evidenced under field conditions before. If this result was to be confirmed in other trials, this would benefit the poultry industry since condemnation at the slaughterhouse is a significant source of economic loss [
19]. The Moroccan poultry industry recently estimated the yearly economic loss from colibacillosis-related condemnation at over 4.5 million US dollars [
20].
APEC strains isolated during the trial all show a high frequency of resistance to several antimicrobial families, whether they originate from the breeder or the broiler flocks. These results are in line with those from a previous study on breeders in Morocco between 2018 and 2021, 75
E. coli clinical strains were resistant to more than half of the tested antibiotics, excepting fosfomycin (6.1% of resistant strains), colistin and florfenicol (0%, [
21]). These last two antibiotics are not used in breeders in practice (colistin is only available in an injectable presentation, and there is no florfenicol-based veterinary medicinal product that is registered for usage in laying hens). Fosfomycin is rarely prescribed in the authors’ experience because of field reports of limited efficacy on recurrent colibacillosis, even when the APEC strains prove to be sensitive
in vitro. In broilers, a previous Moroccan study also reported an
E. coli resistance rate above 50% for most antibiotics, but for fosfomycin (16.1%) and colistin (2.94%) [
22] and the same remarks apply to both of these antibiotics in field practice. The most concerning aspect of our results is that all tested APEC strains (16 from breeders and 28 from broilers) were multi-resistant (i.e., resistant to 3 antibiotic families or more). Although not unexpected (a previous study on 5 million Moroccan broilers showed that 93% of them had received at least one antibiotic treatment [
23]), this result is a clear call for action to drastically reduce antibiotic usage in the Moroccan poultry industry. Furthermore, the possible contamination of poultry meat by
E. coli strains with a clear zoonotic potential [24, 25] is also a cause for public health vigilance. A wider usage of autogenous vaccines has the potential to contribute to the reduction of antibiotic usage, limit the selection of AMR on farms, and improve poultry production [
13], while providing measurable added value in the downstream segment of broiler production.
5. Conclusions
This field trial provides additional confirmation of the interest of E. coli autogenous vaccines in the improvement of health and technical performance of broiler breeders and their progeny. It also confirms under field conditions that maternal passive immunity confers a measurable and lasting protection regarding colibacillosis in the progeny of laying hens. Antibiotic resistance profiles obtained from APEC strains isolated from breeders and their progeny show a high level of AMR in the Moroccan poultry industry, which should be tackled urgently. In this regard, autogenous vaccines have the potential to represent an innovative and customized solution to limit the antimicrobial resistance problem and improve productivity throughout the poultry value chain.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Colibacillosis-focused lesion scoring system used by all veterinarians performing necropsies on breeding and broiler farms during the trial; Table S2: Detailed average lesion scores from breeders, as noted over necropsy sessions (10 dead poultry per session); Table S3: Average zootechnical performance and mortality rate of the broilers hatched to the vaccinated and non-vaccinated flocks involved in the trial; Table S4: Detailed average lesion scores from broilers hatched to vaccinated or control laying hens, as noted over necropsy sessions (10 dead poultry per session).
Author Contributions
Lhoussaine Oubouyahia, Saadia Nassik, and Ouafaa Fassi Fihri conceived and planned the experiments; Lhoussaine Oubouyahia carried out the experiments. Amal Essalah-Bennani, Eric Badin and Hanane Balil contributed to sample preparation. Lhoussaine Oubouyahia, Eric Thibault and Hubert Gantelet contributed to the interpretation of the results. Lhoussaine Oubouyahia took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed by the Zalar Holding poultry group and Ceva Animal Health, with the contribution of the Hassan II Agronomic and Veterinary Institute in part of the laboratory analyses at the unit of avian pathology.
Institutional Review Board Statement
All animal procedures in the present trial were conducted in agreement with the recommendations of the Hassan II Agronomy and Veterinary Institute of Rabat and the Moroccan Ministry of Agriculture, which are in accordance with international ethical legislation (European Union Directive 2010/63/EU) and complied with ARRIVE (Animal Research Reporting of
In Vivo Experiments) guidelines (
https://arriveguidelines.org/).
Data Availability Statement
The data presented in this study are the propriety of Zalar Holding poultry group, and may be available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank the senior management and veterinarians of Zalar Holding, especially Dr. Kamal Legrari, Dr. Yahya Hader, and Dr. Mouhcine Faouzi, as well as the Ceva Moroccan team and Ceva Biovac for their excellent technical support. We also thank Dr Mouahid and his laboratory teams for providing analysis and sampling. Our many thanks also to Mr. Abderrahmane Jannoune from the Agronomic Research National Institute of Rabat for valuable support in the statistical data analysis.
Conflicts of Interest
The authors declare they have no conflict of interest. Eric Thibault and Hubert Gantelet are employed by Ceva Biovac, the manufacturer of the autogenous vaccine; they played no role in sample preparation, protocol design and data collection.
References
- Barnes, H.J.; Nolan, L.K. Colibacillosis in poultry. In Diseases of Poultry, 12th ed. In: Saif, Y.M., Fadly. Blackwell Publishing: Ames, IA, USA, 2008; pp. 691–732.
- Apostolakos, I.; Laconi, A. Occurrence of Colibacillosis in Broilers and Its Relationship With Avian Pathogenic Escherichia coli (APEC) Population Structure and Molecular Characteristics. Front Vet Sci. 2021, 8, 737720. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Murray CJL. Global burden of antimicrobial resistance: essential pieces of a global puzzle - Authors' reply. Lancet 2022, 399, 2349–2350. [Google Scholar] [CrossRef] [PubMed]
- Oubouyahia, L.; Nassik, S. Colibacillose aviaire au Maroc : Infection redoutable à double impact. Rev. Maroc. Sci. Agron. Vét. 2021, 9, 383–389. [Google Scholar]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect Drug Resist 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q. Assessment of global health risk of antibiotic resistance genes. Nat Commun. 2022, 13, 1553. [Google Scholar] [CrossRef] [PubMed]
- Guabiraba, R.; Schouler, C. Avian colibacillosis: still many black holes. FEMS Microbiol Lett. 2015, 362, fnv118. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.; Bachmeier, J. New strategies to prevent and control avian pathogenic Escherichia coli (APEC). Avian Pathol. 2021, 50, 370–381. [Google Scholar] [CrossRef]
- Wang, H.; Logue, CM. Assessment of an Enterobactin Conjugate Vaccine in Layers to Protect Their Offspring from Colibacillosis. Pathogens 2023, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Fratamico PM, DebRoy, C. Advances in Molecular Serotyping and Subtyping of Escherichia coli. Front Microbiol. 2016, 7, 644. [Google Scholar] [CrossRef]
- Comité de l’Antibiogramme de la SFM (CA-SFM) V1.0 Juin 2023. Available online: https://www.sfm-microbiologie.org/boutique/comite-de-lantibiograme-de-la-sfm-casfm/ (accessed on 13 October 2023).
- Li, L.; Thøfner, I. Evaluation of the efficacy of an autogenous Escherichia coli vaccine in broiler breeders. Avian Pathol. 2017, 46, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Koutsianos, D.; Gantelet, H. An Assessment of the Level of Protection Against Colibacillosis Conferred by Several Autogenous and/or Commercial Vaccination Programs in Conventional Pullets upon Experimental Challenge. Vet Sci. 2020, 7, E80. [Google Scholar] [CrossRef] [PubMed]
- Keita, A.; Le Devendec, L. Efficacy of passive immunization in broiler chicks via an inactivated Escherichia coli autogenous vaccine administered to broiler breeder hens. Avian Pathol. 2022, 51, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Šenk, D.; Papoušková, A. Impact of commercial and autogenous Escherichia coli vaccine combination on broiler breeder stock performance, gross pathology, and diversity of Escherichia coli isolates. Acta Vet. Brno, 2022, 91, 383–390. [Google Scholar] [CrossRef]
- Kromann, S.; Olsen, RH. Protective Potential of an Autogenous Vaccine in an Aerogenous Model of Escherichia coli Infection in Broiler Breeders. Vaccines (Basel) 2021, 9, 1233. [Google Scholar] [CrossRef] [PubMed]
- Yakhkeshi, S.; Wu, R. Trends in industrialization and commercialization of IgY technology. Front Immunol. 2022, 13, 991931. [Google Scholar] [CrossRef] [PubMed]
- Amer MM, Mekky HM. Molecular identification, genotyping of virulence-associated genes, and pathogenicity of cellulitis-derived Escherichia coli. Vet World. 2020, 13, 2703–2712. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Souza, W.; Debesa Belizário Granjeiro, M. Analysis of the economic loss and the main causes of total condemnation of poultry carcasses under Brazilian federal inspection between 2013 and 2017. Arch. Vet. Sci. 2019, 24, 36–49. [Google Scholar] [CrossRef]
- Alaoui, A. Impact économique de la colibacillose dans une intégration avicole. Veterinary thesis, Hassan II Agronomic and Veterinary Institute, Rabat, Morocco; 2021.
- Oubouyahia, L.; Fassi-Fihri, O. Colibacillosis and antimicrobial resistance from breeders in Morocco. WVPA First Africa meeting, June 2022, Marrakesch, proceeding, p. 78.
- Rahmatallah, N. La résistance aux antibactériens dans les élevages de poulets au Maroc : évaluation de l’utilisation des antibiotiques et mesure des résistances d’une bactérie sentinelle : Escherichia coli. Veterinary thesis, Hassan II Agronomic and Veterinary Institute, Rabat, Morocco; 2020.
- Rahmatallah, N.; El Rhaffouli, H. Consumption of antibacterial molecules in broiler production in Morocco. Vet Med Sci. 2018, 4, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Manges AR, Geum HM. Global Extraintestinal Pathogenic Escherichia coli (ExPEC) Lineages. Clin Microbiol Rev. 2019, 32, e00135–18. [Google Scholar] [CrossRef]
- Hend, M.Y.; Yousef, Mahmoud, E. Hashad. Surveillance of Escherichia coli in different types of chicken and duck hatcheries: One health outlook. Poult. Sci. 2023, 103108. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).